Hypoglycemic Effect of Pleurotus citrinopileatus and Hericium erinaceus Buccal Tablets on Diabetic Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Optimization of Fermentation Medium
2.2.1. Single-Factor Experiments
2.2.2. Orthogonal Experiment
2.3. Preparation and Detection of Buccal Tablets
2.4. Mice Culture and Parameter Measurements
2.4.1. Modeling of Diabetes Mice
2.4.2. Experimental Design
2.4.3. Measurement of Body Weight, FBG, and Glucose Tolerance in Diabetic Mice
2.4.4. Determination of Blood Lipids
2.4.5. Measurement of Antioxidant Enzymes
2.4.6. NovaSeq Sequencing
2.4.7. Sequencing Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Optimization of Fermentation Conditions in P. citrinopileatus and H. erinaceus
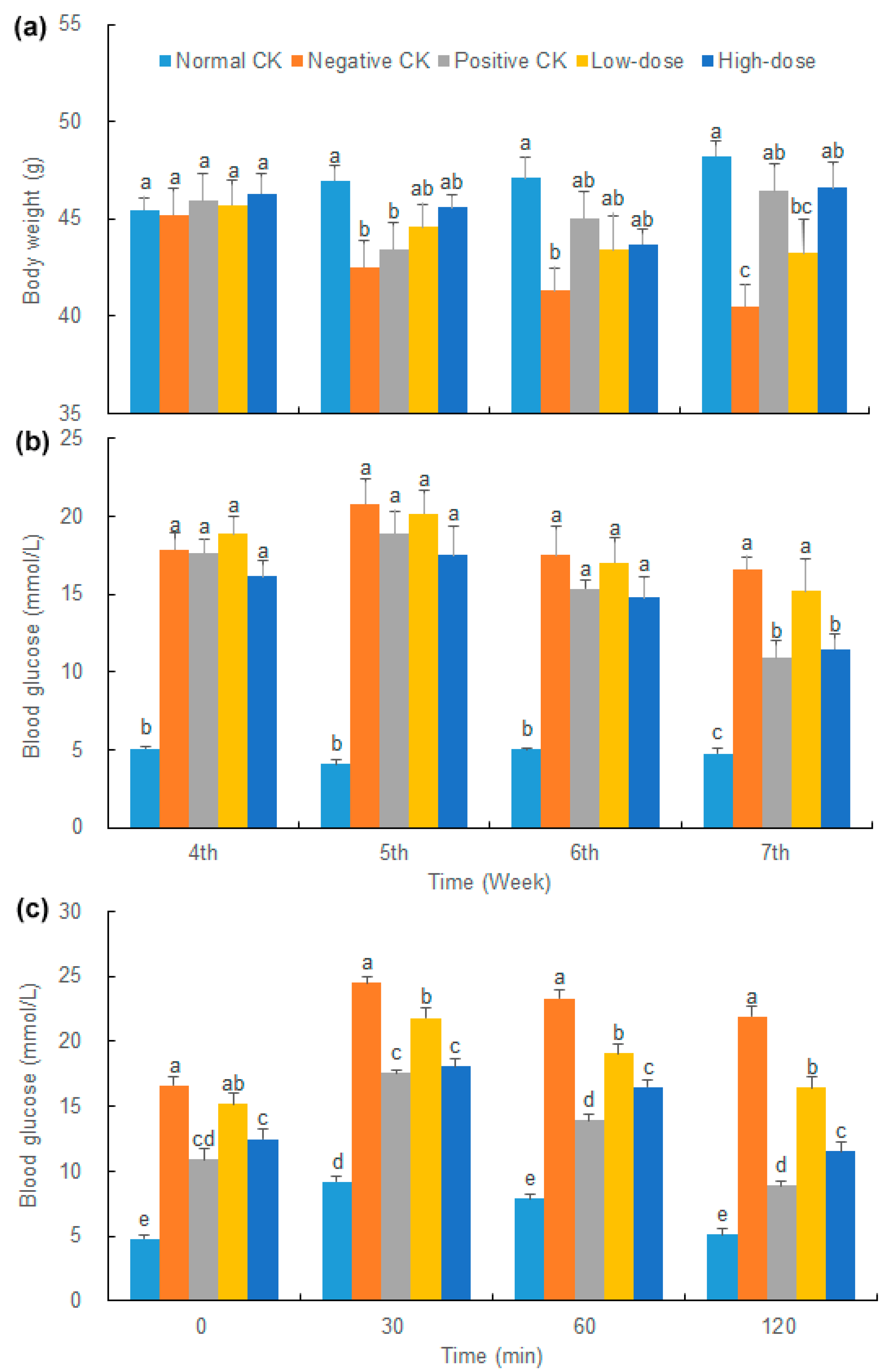
3.2. Effect of Buccal Tablets on Body Weight of Diabetic Mice
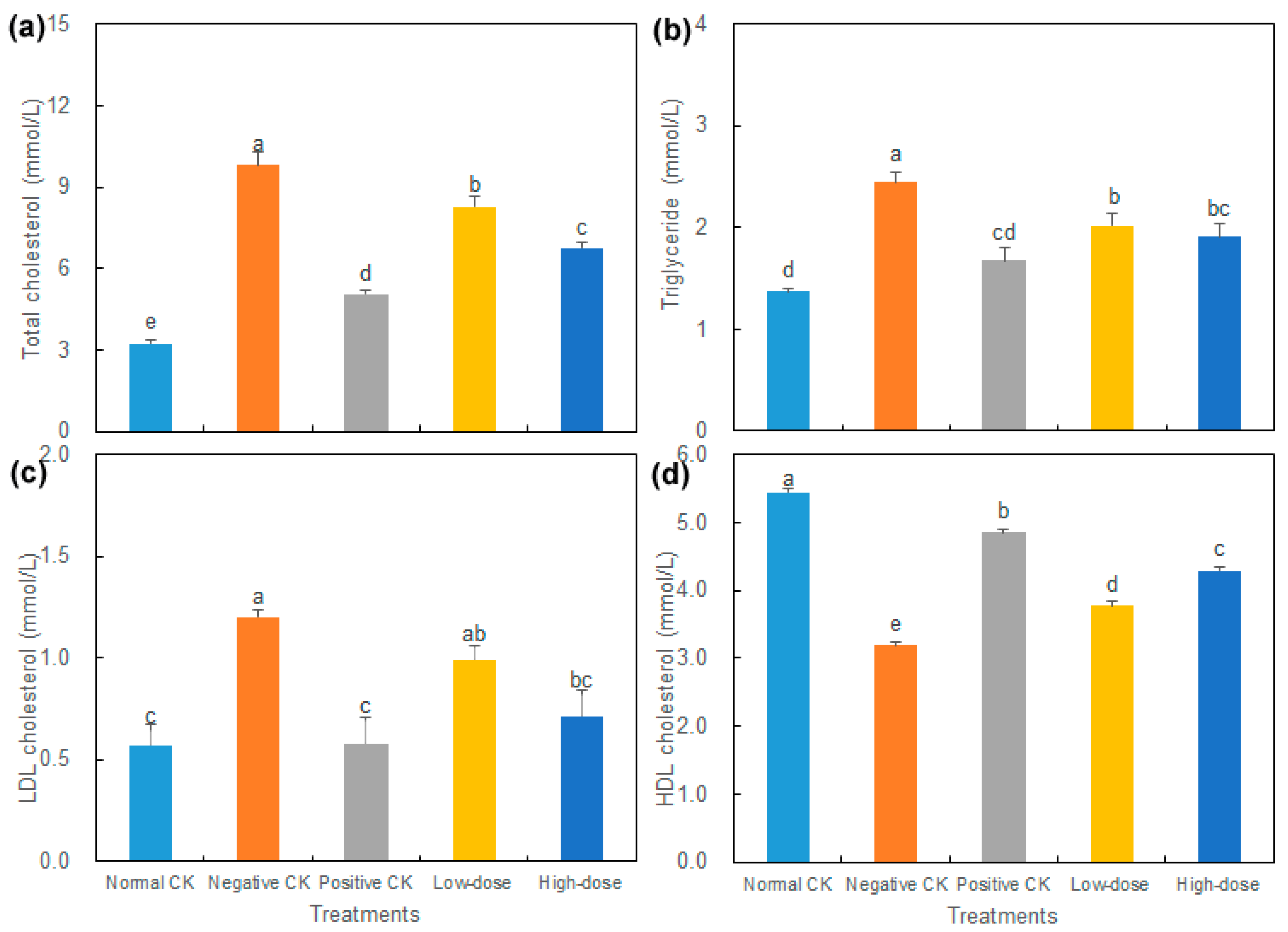
3.3. Effect of Buccal Tablets on Blood Glucose and Lipids of Diabetic Mice
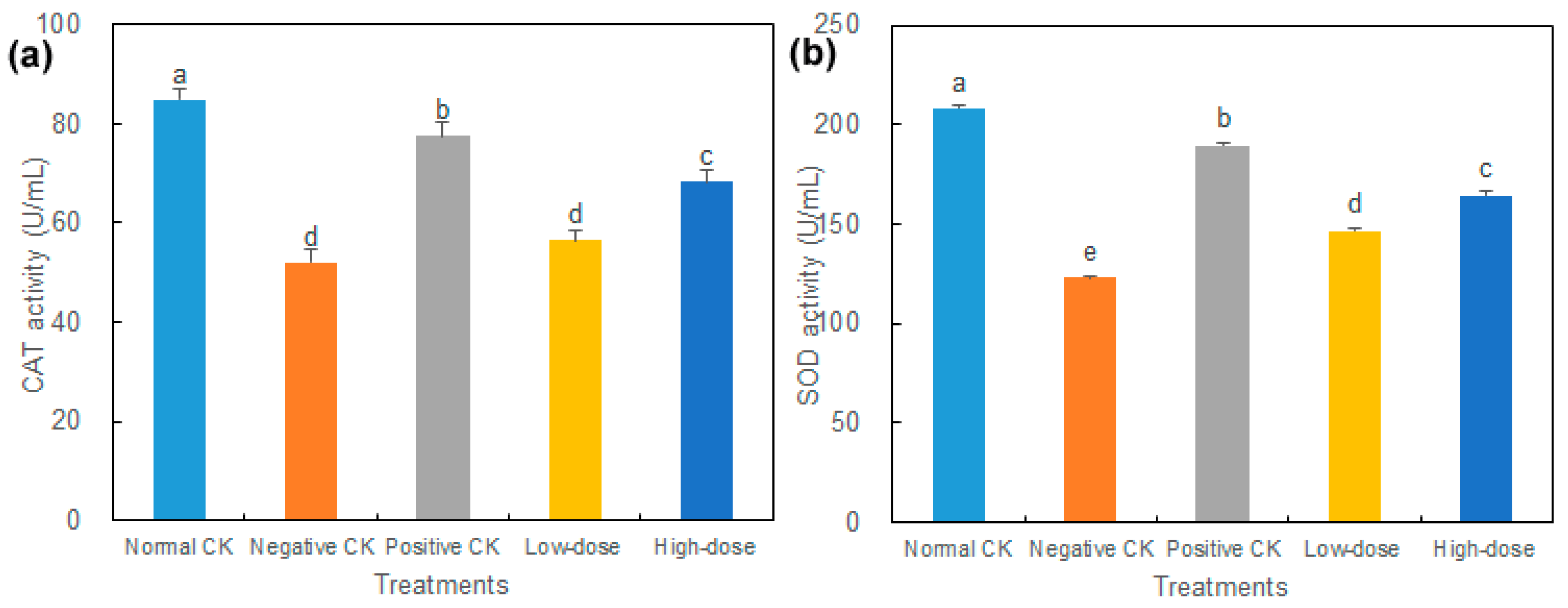
3.4. Effect of Buccal Tablets on Antioxidant Enzymes of Diabetic Mice
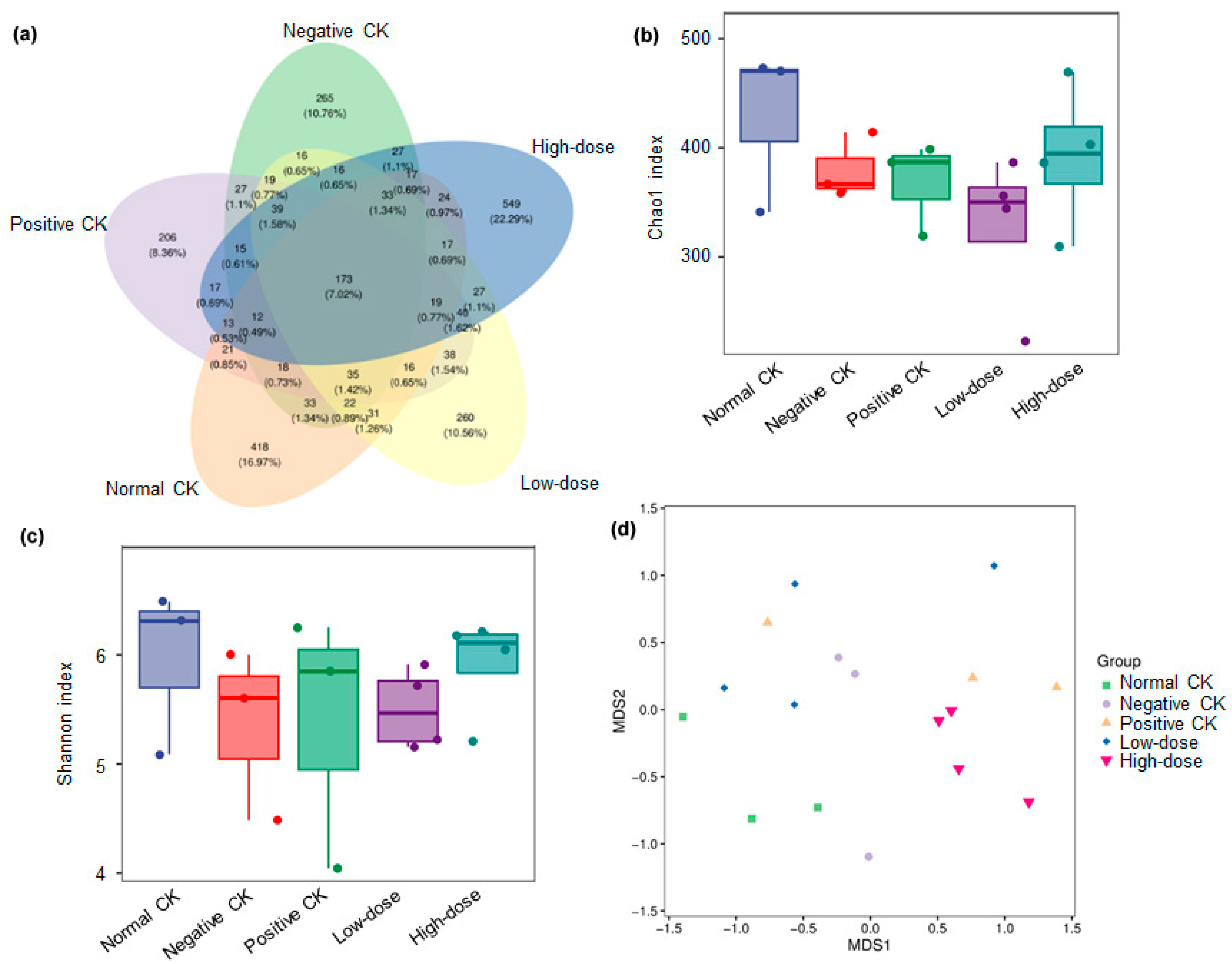
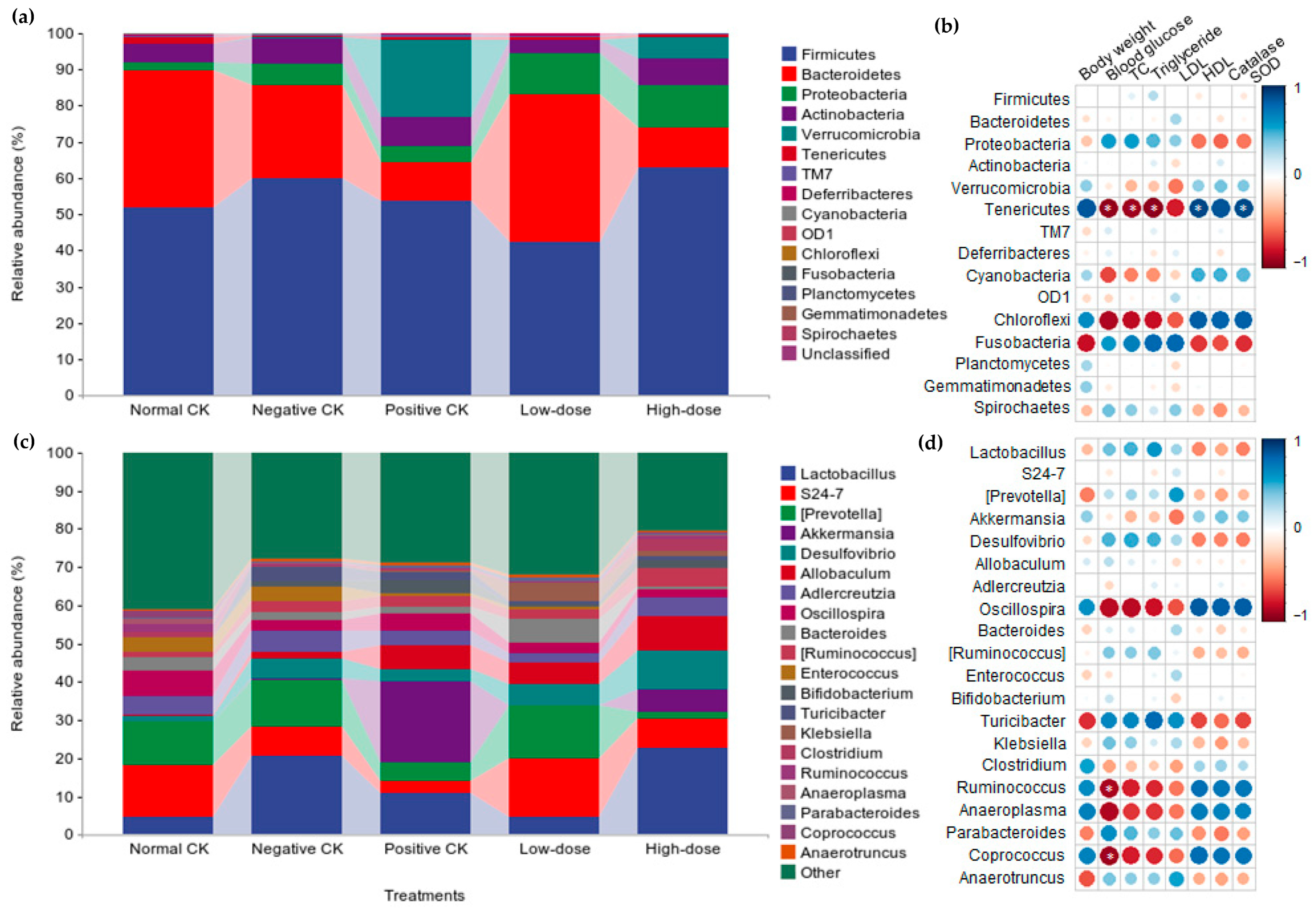
3.5. Effect of Buccal Tablets on Microbial Community Diversity
3.6. Effect of Buccal Tablets on Microbial Community Abundance
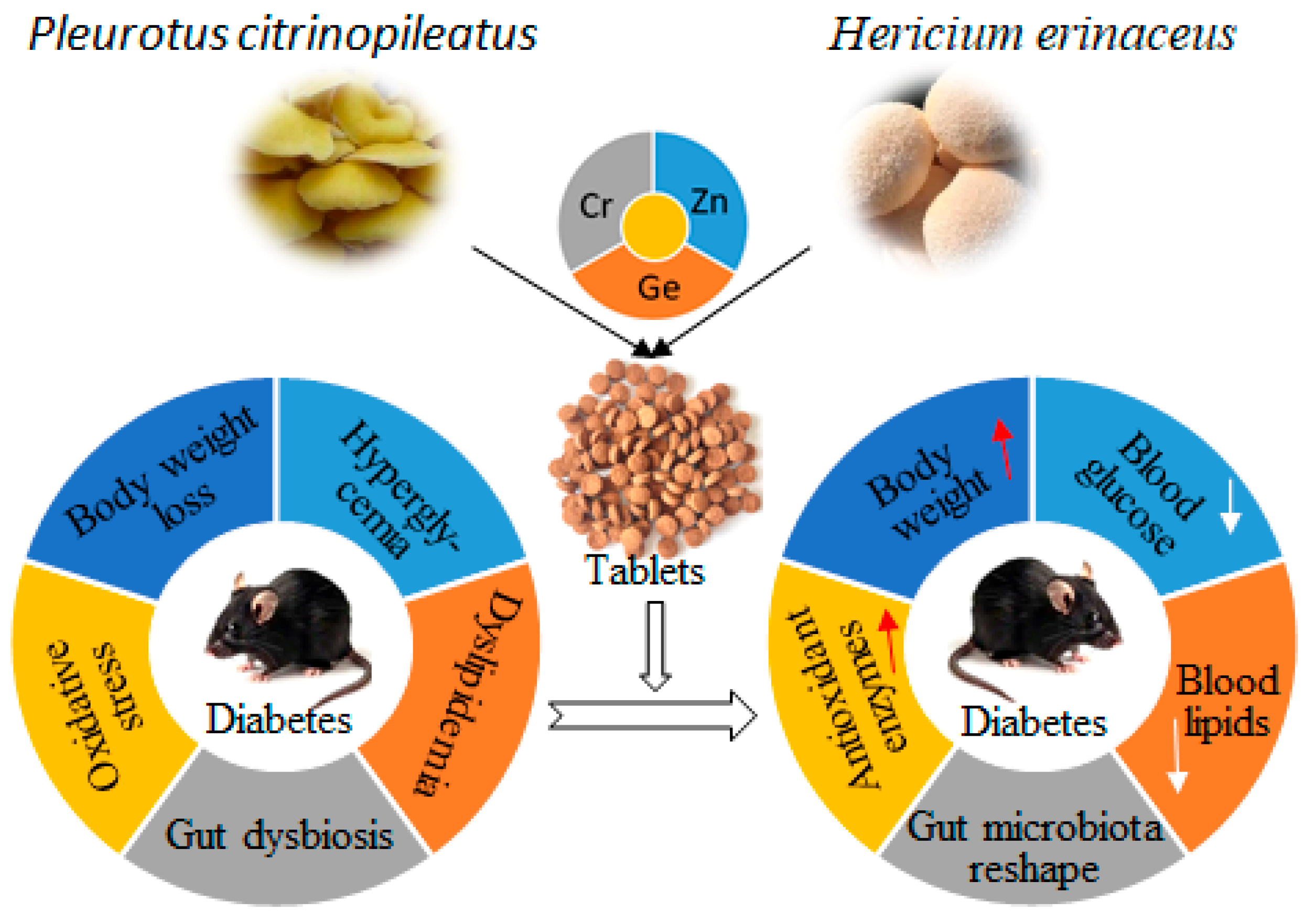
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, N.P.; Magalhaes, G.C.; Mayrink, P.H.; Verano-Braga, T. Omics to unveil diabetes mellitus pathogenesis and biomarkers: Focus on proteomics, lipidomics, and metabolomics. In Mass Spectrometry-Based Approaches for Treating Human Diseases and Diagnostics; Verano-Braga, T., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2024; Volume 1443, pp. 211–220. [Google Scholar]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Mzimela, N.; Dimba, N.; Sosibo, A.; Khathi, A. Evaluating the impact of type 2 diabetes mellitus on pulmonary vascular function and the development of pulmonary fibrosis. Front. Endocrinol. 2024, 15, 1431405. [Google Scholar] [CrossRef]
- Kour, N.; Bhagat, G.; Singh, S.; Bhatti, S.S.; Arora, S.; Singh, B.; Bhatia, A. Polyphenols mediated attenuation of diabetes associated cardiovascular complications: A comprehensive review. J. Diabetes Metab. Dis. 2024, 23, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Gargari, B.P.; Tajfar, P.; Tarighat-Esfanjani, A. A systematic review of whey protein supplementation effects on human glycemic control: A mechanistic insight. Diabetes Metab. Synd. 2022, 16, 102540. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, J.; Cao, T.; Zhang, T.; Liu, Y.; Yan, Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Well. 2023, 12, 1969–1980. [Google Scholar] [CrossRef]
- Andrade, G.M.; de Souza, E.L.; Zárate-Salazar, J.R.; de Oliveira, J.N.; Tavares, J.F.; Lima, M.S.; de Medeiros, R.L.; de Albuquerque, T.M.; Pereira, F.O. Unveiling the potential prebiotic effects of edible mushroom Pleurotus djamor during in vitro colonic fermentation. J. Agric. Food Chem. 2024, 72, 26722–26732. [Google Scholar] [CrossRef]
- Ge, Y.; Qiu, H.; Zheng, J. Physicochemical characteristics and anti-hyperlipidemic effect of polysaccharide from BaChu mushroom (Helvella leucopus). Food Chem. X 2022, 15, 100443. [Google Scholar] [CrossRef]
- Yang, M.; Qin, X.; Liu, X. A review of polysaccharides from Ganoderma lucidum: Preparation methods, structural characteristics, bioactivities, structure-activity relationships and potential applications. Int. J. Biol. Macromol. 2025, 303, 140645. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Ke, D.; Wang, W.; Ye, F.; Zeng, J.; Zong, Y. High fatty acid accumulation and coloration molecular mechanism of the elm mushroom (Pleurotus citrinopileatus). Biosci. Biotechnol. Biochem. 2024, 88, 437–444. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Yin, J.; Nie, S.; Xie, M. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59, S96–S115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Li, H.; Xiao, Y.; Ying, H. Recent advancements in the application of multi-elemental profiling and ionomics in cardiovascular diseases. J. Trace Elem. Med. Biol. 2025, 88, 127616. [Google Scholar] [CrossRef] [PubMed]
- Mehri, A. Trace elements in human nutrition (II)—An update. Int. J. Prev. Med. 2020, 11, 2–19. [Google Scholar] [CrossRef]
- MacKenzie, S.; Bergdahl, A. Zinc homeostasis in diabetes mellitus and vascular complications. Biomedicines 2022, 10, 139. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. eBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lv, B.; Zhi, L.; Shao, Y.; Liu, X.; Mitteregger, M.; Chakaroun, R.; Tremaroli, V.; Hazen, S.T.; Wang, R.; et al. Microbiome–metabolome dynamics associated with impaired glucose control and responses to lifestyle changes. Nat. Med. 2025, 31, 2222–2231. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, J.; Sun, M.; Jiang, Y.; Wang, S.; Wang, L.; Yu, N.; Peng, D.; Wang, Y.; Chen, W. Poria cocos polysaccharide improves intestinal barrier function and maintains intestinal homeostasis in mice. Int. J. Biol. Macromol. 2023, 249, 125953–126064. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, H.; Jin, Z.; Wang, C.; Xia, M.; Chen, B.; Lv, B.; Diaz, L.P.; Li, X.; Feng, R.; et al. Hydrogen sulfide produced by the gut microbiota impairs host metabolism via reducing GLP-1 levels in male mice. Nat. Metab. 2024, 6, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Saini, R.K.; Kumar, A.; Chawla, P.; Kaushik, R. Mushrooms as nutritional powerhouses: A review of their bioactive compounds, health benefits, and value-added products. Foods 2025, 14, 741. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, R.; Li, H. Current progress on truffle submerged fermentation: A promising alternative to its fruiting bodies. Appl. Microbiol. Biotechnol. 2015, 99, 2041–2053. [Google Scholar] [CrossRef]
- Habibi, E.; Hemmati, P.; Arabnozari, H.; Khalili, H.A.; Sharifianjazi, F.; Enderami, S.E.; Sarker, S.D.; Hassannia, H.; Nahar, L. Phytochemical analysis and immune-modulatory potential of Trichaptum biforme polysaccharides: Implications for cancer. Int. J. Biol. Macromol. 2024, 280, 135691. [Google Scholar] [CrossRef]
- Cai, W.; Ding, Z.; Wang, Y.; Yang, Y.; Zhang, H.; Yan, J. Hypoglycemic benefit and potential mechanism of a polysaccharide from Hericium erinaceus in streptozotoxin-induced diabetic rats. Process Biochem. 2020, 88, 180–188. [Google Scholar] [CrossRef]
- The National Pharmacopoeia Commission. Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2010. [Google Scholar]
- GB/Z21922-2008; Fundermental Terminology and Definition of Nutritional Component in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2008.
- GB5009.5-2016; Determination of Protein in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB5009.6-2016; Determination of Lipid in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB5009.91-2017; Determination of Potassium and Sodium in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2017.
- GB4789.2-2022; Food Microbiology Examination—Total Bacteria Count. National Health Commission of the People’s Republic of China: Beijing, China, 2022.
- GB4789.3-2016; Food Microbiology Examination—Coliform bacteria Count. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB4789.15-2016; Food Microbiology Examination—Mold and Yeast Count. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB4789.4-2016; Food microbiology examination—Salmonella Count. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB4789.10-2016; Food Microbiology Examination—Staphylococcus aureus Count. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB5009.12-2017; Determination of Lead in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2017.
- GB5009.11-2014; Determination of Total Arsenic in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2014.
- GB5009.17-2021; Determination of Total Mercury in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2021.
- GB5009.123-2014; Determination of Chromium in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2014.
- GB5009.14-2017; Determination of Zinc in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2017.
- Zhang, Y.; Wang, H.; Hu, M.; Cai, R.; Miao, Y.; Zhu, X. Heavy metals potentially drive co-selection of antibiotic resistance genes by shifting soil bacterial communities in paddy soils along middle and lower Yangtze River. Pedosphere 2024, 34, 606–619. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Zhao, R.; Luo, L.; Zhao, Z.; Ma, H. Effects of four bivalent mineral ions on the production of crude polysaccharides from liquid fermentation of Pleurotus djamor. Tianjin Agric. Sci. 2017, 23, 9–12. [Google Scholar]
- Liu, Y. Study on Physiological and Biochemical Mechanism of Chromium Enrichment in Cordyceps militaris. Master’s Thesis, Ludong University, Yantai, China, 2023. [Google Scholar]
- Zhu, Y.; Wang, W.; Wang, J.; Zhu, Y.; Zhang, Y.; Xu, X.; Dai, Y. Antioxidant capacity of different solvent extracts from Ge-riched Auricularia auricula mycelium in vitro. Sci. Technol. Food Ind. 2024, 45, 21–28. [Google Scholar]
- Nishimura, K.; Iitaka, S.; Nakagawa, H. Effect of trivalent chromium on erythropoietin production and the prevention of insulin resistance in HepG2 cells. Arch. Biochem. Biophys. 2021, 708, 108960. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, E.H.; Yeo, M.A.; Kim, S.J.; Seo, M.; Moon, H.I. Antidiabetic effects of three Korean sorghum phenolic extracts in normal and streptozotocin-induced diabetic rats. Food Res. Int. 2011, 44, 127–132. [Google Scholar] [CrossRef]
- Song, Q.; Wu, H.; Weng, S.; Wang, Y.; Kong, L.; Liu, Z.; Zhang, K. Artemisia argyi polysaccharide ameliorates hyperglycemia through modulating gut microbiota and serum metabolism in type 2 diabetic mice. J. Funct. Foods 2024, 122, 106525. [Google Scholar] [CrossRef]
- Tao, Z.; Zou, Y.; Ye, Z.; Lin, J.; Zheng, Q. The intervention effects of Pleurotus citrinopileatus polysaccharides with different molecular weights on high-fat diet mice. Int. J. Biol. Macromol. 2025, 310, 143085. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.; Guan, J.; Chen, J.; Xu, X. Mushroom polysaccharides with potential in anti-diabetes: Biological mechanisms, extraction, and future perspectives: A review. Front. Nutr. 2022, 9, 1087826. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Nie, S.; Tan, L.; Li, C.; Gong, D.; Xie, M. A polysaccharide from Ganoderma atrum improves liver function in type 2 diabetic rats via antioxidant action and short-chain fatty acids excretion. J. Agric. Food Chem. 2016, 64, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Jiang, J.; Zeng, Y.; Zhu, J.; Wang, S.; Huang, D.; Cao, C. Polysaccharide NAP-3 synergistically enhances the efficiency of metformin in type 2 diabetes via bile acid/GLP-1 axis through gut microbiota remodeling. J. Agric. Food Chem. 2024, 72, 21077–21088. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Lei, J.; He, C.; Peng, Z.; Liu, R.; Pan, X.; Meng, J.; Feng, C.; Xu, L.; Cheng, Y.; et al. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from Clitocybe squamulose. Int. J. Biol. Macromol. 2022, 208, 343–355. [Google Scholar] [CrossRef]
- Su, L.; Xin, C.; Yang, J.; Dong, L.; Mei, H.; Dai, X.; Wang, Q. A polysaccharide from Inonotus obliquus ameliorates intestinal barrier dysfunction in mice with type 2 diabetes mellitus. Int. J. Biol. Macromol. 2022, 214, 312–323. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, A.; Berkhout, M.D.; Geerlings, S.Y.; Belzer, C. Akkermansia muciniphila: Biology, microbial ecology, host interactions and therapeutic potential. Nat. Rev. Microbiol. 2025, 23, 162–177. [Google Scholar] [CrossRef]
- Behary, J.; Amorim, N.; Jiang, X.; Raposo, A.; Gong, L.; Mcgovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Huang, C.; He, C.; Yang, T.; Ren, R.; Xin, Z.; Wang, X. Quercetin-driven Akkermansia muciniphila alleviates obesity by modulating bile acidmetabolism via an ILA/m6A/CYP8B1 signaling. Adv. Sci. 2025, 12, 2412865. [Google Scholar] [CrossRef]
- Ansaldo, E.; Slayden, L.C.; Ching, K.L.; Koch, M.A.; Wolf, N.K.; Plichta, D.R.; Brown, E.M.; Graham, D.B.; Xavier, R.J.; Moon, J.J.; et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 2019, 364, 1179–1184. [Google Scholar] [CrossRef]
- Shoer, S.; Shilo, S.; Godneva, A.; Ben-Yacov, O.; Rein, M.; Wolf, B.C.; Lotan-Pompan, M.; Bar, N.; Weiss, E.I.; Houri-Haddad, Y.; et al. Impact of dietary interventions on pre-diabetic oral and gut microbiome, metabolites and cytokines. Nat. Commun. 2023, 14, 5384. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zou, Y.; Tang, H.; Zhuang, J.; Ye, Z.; Wei, T.; Lin, J.; Zheng, Q. Cordyceps militaris polysaccharides modulate gut microbiota and improve metabolic disorders in mice with diet-induced obesity. J. Sci. Food Agric. 2023, 103, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
| Inspection Items | Inspection Findings | Testing Criterion | Stipulated by the Standard | Determination Outcome |
|---|---|---|---|---|
| Energy content (kJ/100 g) | 1748 | GB/Z21922-2008 [25] | / | / |
| Protein content (g/100 g) | 14.2 | GB5009.5-2016 [26] | / | / |
| Lipid content (g/100 g) | 5.2 | GB5009.6-2016 [27] | / | / |
| Carbohydrate composition (g/100 g) | 77.3 | GB/Z21922-2008 | / | / |
| Sodium content (mg/100 g) | 3.06 | GB5009.91-2017 [28] | / | / |
| Total aerobic plate count (CFU/g) | 220 | GB4789.2-2022 [29] | ≤3 × 104 | Compliant |
| Coliform group (MPN/g) | <0.3 | GB4789.3-2016 [30] | ≤0.92 | Compliant |
| Molds and yeasts (CFU/g) | <10 | GB4789.15-2016 [31] | ≤50 | Compliant |
| Salmonella (/25 g) | Undetected | GB4789.4-2016 [32] | ≤0 | Compliant |
| Staphylococcus aureus (/25 g) | Undetected | GB4789.10-2016 [33] | ≤0 | Compliant |
| Lead (mg/kg) | 0.095 | GB5009.12-2017 [34] | ≤2.0 | Compliant |
| Total arsenic (mg/kg) | Undetected (<0.01) | GB5009.11-2014 [35] | ≤1.0 | Compliant |
| Total mercury (mg/kg) | Undetected (<0.003) | GB5009.17-2021 [36] | ≤0.3 | Compliant |
| Chromium (mg/kg) | Undetected (<0.01) | GB5009.123-2014 [37] | ≤0.5 | Compliant |
| Zinc (mg/kg) | 440 | GB5009.14-2017 [38] | / | / |
| Protocol | Ion Content | Polysaccharide Content (mg/g) | |||
|---|---|---|---|---|---|
| Cr3+ | Zn2+ | Ge4+ | P. citrinopileatus | H. erinaceus | |
| 1 | 1 | 1 | 1 | 19.487 | 26.626 |
| 2 | 1 | 2 | 3 | 16.686 | 13.375 |
| 3 | 1 | 3 | 2 | 23.358 | 11.159 |
| 4 | 2 | 1 | 2 | 26.936 | 21.764 |
| 5 | 2 | 2 | 1 | 12.487 | 19.720 |
| 6 | 2 | 3 | 3 | 25.671 | 18.729 |
| 7 | 3 | 1 | 3 | 21.100 | 26.005 |
| 8 | 3 | 2 | 2 | 15.772 | 19.220 |
| 9 | 3 | 3 | 1 | 14.358 | 23.419 |
| P. citrinopileatus | |||||
| K1 | 59.531 | 67.522 | 46.332 | ||
| K2 | 65.094 | 44.945 | 66.066 | ||
| K3 | 51.229 | 63.387 | 63.456 | ||
| R | 13.864 | 22.577 | 19.734 | ||
| H. erinaceus | |||||
| K1 | 51.161 | 74.395 | 69.765 | ||
| K2 | 60.213 | 52.316 | 52.143 | ||
| K3 | 68.644 | 53.307 | 58.109 | ||
| R | 17.484 | 22.079 | 17.622 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhang, K.; Liang, Y.; Shi, K.; Ma, J.; Yu, J.; Lu, C.; Liu, A.; Zhu, X. Hypoglycemic Effect of Pleurotus citrinopileatus and Hericium erinaceus Buccal Tablets on Diabetic Mice. Biology 2025, 14, 1591. https://doi.org/10.3390/biology14111591
Yang Z, Zhang K, Liang Y, Shi K, Ma J, Yu J, Lu C, Liu A, Zhu X. Hypoglycemic Effect of Pleurotus citrinopileatus and Hericium erinaceus Buccal Tablets on Diabetic Mice. Biology. 2025; 14(11):1591. https://doi.org/10.3390/biology14111591
Chicago/Turabian StyleYang, Zhongyi, Kailu Zhang, Yan Liang, Kexin Shi, Jinqiang Ma, Juan Yu, Cunlong Lu, Aimin Liu, and Xiancan Zhu. 2025. "Hypoglycemic Effect of Pleurotus citrinopileatus and Hericium erinaceus Buccal Tablets on Diabetic Mice" Biology 14, no. 11: 1591. https://doi.org/10.3390/biology14111591
APA StyleYang, Z., Zhang, K., Liang, Y., Shi, K., Ma, J., Yu, J., Lu, C., Liu, A., & Zhu, X. (2025). Hypoglycemic Effect of Pleurotus citrinopileatus and Hericium erinaceus Buccal Tablets on Diabetic Mice. Biology, 14(11), 1591. https://doi.org/10.3390/biology14111591





