Simple Summary
High salt stress impairs the growth and metabolite production of industrial microorganisms, including the tea-fermenting fungus Eurotium cristatum. This study explored the role of long non-coding RNAs (lncRNAs) in the salt stress response of E. cristatum. We identified 203 lncRNAs, 120 of which were differentially expressed under salt stress conditions. These stress-responsive lncRNAs are linked to key phenotypic changes, such as changes in the morphology and biosynthesis of valuable metabolites such as polysaccharides and melanin. Further analysis indicated that specific lncRNAs may act as molecular switches, modulating critical pathways in carbohydrate metabolism and stress responses. Our findings provide novel insights into fungal adaptation mechanisms and highlight potential genetic targets for engineering robust industrial strains with enhanced productivity, offering promising applications in the food and biotechnology industries.
Abstract
Long non-coding RNAs (lncRNAs) are crucial regulators in eukaryotic organisms, yet their roles in filamentous fungi, particularly in environmental adaptation and metabolic changes, remain largely unexplored. Here, we investigated the roles of lncRNAs in salt stress response, morphological differentiation, and metabolic regulation in Eurotium cristatum. Using strand-specific RNA sequencing, we identified lncRNAs in sexual and asexual mycelia of E. cristatum and analyzed their expression profiles. We identified 203 lncRNAs, with 120 significantly differentially expressed (FDR < 0.01; |log2 (fold change)| ≥ 1) under salt stress, including 57 upregulated and 63 downregulated in the asexual morph compared to the sexual morph. These lncRNAs correlated with physiological indicators like mycelial biomass, polysaccharide content, and melanin production. Target gene prediction and functional enrichment analysis revealed that these lncRNAs influenced morphogenesis and secondary metabolite synthesis in E. cristatum by regulating pathways including carbohydrate metabolism, peroxisome function, and protein ubiquitination. The lncRNA MSTRG.10627.3 showed the highest upregulation (log2FC = 10.53, FDR < 1 × 10−105), while MSTRG.3124.1 was significantly downregulated in the sexual morph (log2FC = −4.94, FDR < 1 × 10−88). A regulatory network of lncRNAs involved in salt stress responses was constructed, providing insights into fungal environmental adaptation mechanisms and potential targets for industrial strain improvement.
1. Introduction
Long non-coding RNAs (lncRNAs) are a class of RNA molecules longer than 200 nucleotides that lack significant open reading frames (ORFs) and typically do not encode proteins []. Long non-coding RNAs were once dismissed as mere transcriptional “noise” or “junk DNA” []. However, advancements in high-throughput sequencing and bioinformatic tools have uncovered their role as key regulators in essential eukaryotic processes, such as differentiation, cell cycle, and metabolism []. Furthermore, the dysregulation of lncRNAs is linked to numerous diseases. Although most lncRNAs exhibit lower sequence conservation than their protein-coding counterparts [], their expression patterns show strong spatiotemporal specificity and are highly responsive to environmental conditions [], suggesting a potential role in the fine-tuned regulation of developmental signals and responses to environmental stimuli.
Regulatory mechanisms of lncRNAs are complex and diverse, primarily including the following [,]: (1) Chromatin modification and epigenetic regulation (recruiting modification complexes to influence histone marks or DNA methylation), (2) transcriptional regulation (acting as co-factors to interfere with or enhance transcription factor activity), (3) post-transcriptional regulation [binding to messenger RNAs (mRNAs) to affect their stability, splicing, or translation and functioning as competing endogenous RNAs to sequester microRNAs (miRNAs), thereby suppressing miRNA-mediated repression of target genes], and (4) protein function regulation (serving as scaffold molecules to mediate the formation of protein complexes or alter protein localization). Based on their mode of action, these mechanisms can be classified as cis-acting (regulating genes near their own genomic loci) or trans-acting (regulating distant genes through diffusion) [].
LncRNAs play diverse roles in various processes, including growth and development, stress responses, and secondary metabolite synthesis in animals and plants [,,]. However, compared to animal and plant lncRNAs, research on fungal lncRNAs remains limited. Their functions and mechanisms remain unclear, with current research predominantly focusing on model fungi such as Saccharomyces cerevisiae [], Aspergillus nidulans [], and some pathogenic fungi [,]. Moreover, systematic identification and functional exploration of lncRNAs in filamentous fungi of significant economic and ecological importance, particularly those involved in specialized food fermentation, such as Eurotium cristatum, are currently lacking.
Eurotium cristatum, commonly referred to as the “Jinhua fungus”, plays a crucial role in determining the quality of fermented teas, such as the Fuzhuan brick tea [,]. This microorganism undergoes two distinct morphological stages during its life cycle: the conidial (asexual) and ascospore (sexual) stages []. As a homothallic fungus, E. cristatum can self-fertilize and complete its sexual cycle independently. The shift between these stages is influenced by environmental factors, with osmotic pressure being a key regulator. High osmotic conditions suppress sexual reproduction and favor the asexual phase, while standard or hypotonic media encourage the development of sexual morphs [,]. This physiological response guided our experimental approach. We cultivated the sexual morph (G01) in a modified medium with reduced salt concentration and induced an asexual morph (G02) under high-salt stress. It is crucial to recognize that the phenotypic and transcriptomic differences analyzed in this study reflect an integrated response to environmental conditions. Specifically, the “salt stress response” of asexual morphs is inherently linked to salt-induced developmental reprogramming away from the sexual phase. Consequently, the observed differences likely encompass both direct osmotic adaptation and the effects of the morphological shift itself. Osmotic pressure influences the physiological state and metabolic profile of E. cristatum. For instance, the yield of the secondary metabolite lovastatin can be up to 20 times higher in the sexual form than in the asexual form [], and extracts of sexual morphs exhibit significantly stronger antimicrobial activity than those of asexual morphs [], indicating substantial differences in their metabolic pathways. However, the key molecular switches linking external environmental signals to the internal regulatory networks governing morphological transitions and metabolism, particularly the potential regulatory roles of lncRNAs, remain unclear.
The present study focused on the conidial and ascospore morphs of E. cristatum. Strand-specific lncRNA sequencing was performed to systematically identify lncRNAs in these two distinct physiological states and to analyze their basic sequence characteristics. Association analyses were conducted by integrating physiological indicators, such as mycelial biomass, melanin content, and polysaccharide content, to identify key candidate lncRNAs involved in regulating morphological differentiation and melanin and polysaccharide biosynthesis in E. cristatum. By investigating the potential roles of lncRNAs in salt stress responses and the regulation of morphological transition and secondary metabolism in E. cristatum, this study enhances our understanding of the post-transcriptional regulatory networks underlying various biological activities in filamentous fungi and provides a theoretical foundation and genetic resource for microbial strain improvement and directed regulation of fermentation using advanced molecular techniques.
2. Materials and Methods
2.1. Strain Culture and Sample Preparation
The E. cristatum strain (JH1805) utilized in this study was sourced from Hunan City University. Initially, the strain was activated on potato dextrose agar and then inoculated into a liquid medium with an inoculation amount of 1% under aseptic conditions for cultivation. The methods for cultivating the sexual (designated G01) and asexual (designated G02) morphs of E. cristatum are detailed below. The sexual morph (G01) was cultured statically (without shaking) in a modified potato dextrose liquid medium (300 g/L potato, 100 g/L glucose, and 0.5 mol/L NaCl) at 28 °C. Similarly, the asexual morph (G02) was cultured statically (without shaking) in a high-salt potato dextrose liquid medium [] (300 g/L potato, 100 g/L glucose, and 3 mol/L NaCl) at 28 °C. For each morph, three independent biological replicates were cultivated under identical conditions. After 168 h of cultivation, mycelia were collected using sterile filter meshes, and their fresh weight was recorded. The mycelia were then thoroughly rinsed with sterile physiological saline, immediately frozen in liquid nitrogen, and subsequently stored at −80 °C.
2.2. Scanning Electron Microscopy
Samples of both sexual and asexual morphs of E. cristatum were prepared for SEM observation through the following process. Initially, the mycelial pellets were fixed in 2.5% glutaraldehyde overnight at 4 °C and then rinsed with a phosphate buffer (0.1 M, pH 7.0). The samples underwent progressive dehydration using a graded ethanol series (30%, 50%, 70%, 80%, 90%, 95%, and 100% × 3), with each step lasting 15 min. To preserve the delicate fungal structures, the dehydrated samples were subjected to critical point drying using liquid CO2. Subsequently, the dried samples were mounted on aluminum stubs, and their surfaces were sputter-coated with gold using an ion sputter coater to enhance conductivity []. Finally, the specimens were observed using a scanning electron microscope (SEM; HITACHI S-3000N; Hitachi High-Tech Corp., Tokyo, Japan). The dimensions of the fungal structures were measured using the ImageJ software (v1.53) [].
2.3. Total RNA Extraction, Quality Control, and Library Construction
Strand-specific RNA sequencing was conducted on individual sexual (G01) and asexual (G02) mycelial samples (without biological replicates) to facilitate initial transcriptome assembly and lncRNA identification. Additionally, the expression patterns of key differentially expressed lncRNAs (DE-lncRNAs) were validated using reverse transcription polymerase chain reaction (RT-qPCR) (see Section 2.8).
Total RNA was isolated from 500 mg of mycelia (fresh weight) per sample using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
RNA Quality Control: The concentration and purity of RNA were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). RNA integrity was measured using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
rRNA depletion and library construction: The Ribo-Zero rRNA Removal Kit (Illumina, San Diego, CA, USA) was used to eliminate ribosomal RNA (rRNA) from the total RNA sample. Strand-specific transcriptome sequencing libraries were fabricated using the NEBNext Ultra Directional RNA Library Prep Kit (New England Biolabs, Ipswich, MA, USA). The NEBNext Ultra Directional RNA Library Prep Kit employs the dUTP second-strand marking method to preserve the strand orientation information. Briefly, during the first-strand cDNA synthesis, random hexamer primers were used. Subsequently, during the second-strand synthesis, dTTP was replaced with dUTP. Following adapter ligation and polymerase chain reaction (PCR) amplification, the uracil-containing second strand was selectively degraded using the enzyme Uracil-Specific Excision Reagent (USER). This process ensures that only the first strand (which is complementary to the original RNA template) is amplified and sequenced, thereby allowing unambiguous determination of the transcriptional strand of origin for each resulting read. Library construction involved several steps. First, the RNA was fragmented. Complementary DNA (cDNA) was then synthesized. End repair was subsequently performed, followed by dA-tailing. Adapter ligation was the next step, which was succeeded by size selection. Finally, the libraries were enriched using PCR.
Library quality control and sequencing was performed using an Agilent 2100 Bioanalyzer to evaluate the quality of the constructed libraries, and the effective concentration was precisely measured using qPCR. Subsequently, libraries that met these requirements were sequenced on an Illumina HiSeq 2500 platform (Illumina, San Diego, CA, USA) to obtain PE150 paired-end reads.
2.4. Sequencing Data Quality Control and Alignment
The raw sequencing data underwent thorough quality control (QC). Initial QC reports were generated using FastQC (v0.11.9). Subsequently, adapter sequences, reads with an excessive number of ambiguous bases (N’s), and low-quality reads were filtered out using fastp (v0.23.4) []. MultiQC (v1.14) was used to aggregate and summarize the QC results from FastQC and Fastp into a single report. The resulting high-quality clean data were aligned to the E. cristatum reference genome (National Center for Biotechnology Information accession: ASM171748v1) using HISAT2 (v2.0.4) []. The strand-specific library parameter “--rna-strandness RF” was applied during alignment to improve the transcript strand orientation accuracy []. Alignment metrics, including overall and unique alignment rates, were calculated to ensure data suitability for subsequent analyses.
2.5. Transcript Assembly, LncRNA Identification, and Quantification
De novo transcript assembly for each sample was performed on BAM files generated using StringTie (v1.3.1) []. The transcripts of all the samples were merged using StringTie’s merge mode to create a unified set. Transcripts were compared with the reference genome annotation file using GFFcompare (v0.9.8) for transcript classification [].
Next, lncRNA transcripts were identified by first filtering transcripts based on size (200+ nt), having at least two exons, and fragments per kilobase per million mapped reads (FPKM) ≥ 0.1. Transcripts with coding potential were assessed and filtered using CPC2, CNCI, CPAT, and Pfam scans []. This yielded the final set. StringTie was used to estimate the standardized expression levels of all genes and transcripts.
2.6. Differential Expression Analysis
Differential expression in E. cristatum samples was analyzed using edgeR (v 4.0) []. Genes or lncRNAs were regarded as differentially expressed if they met the criteria of an absolute value of log2(fold change) equal to or greater than one and a false discovery rate (FDR) of less than 0.01.
2.7. LncRNA Target Gene Prediction and Functional Enrichment Analysis
Cis-Acting Target Prediction: The Perl script was used to predict possible cis-acting target genes of the lncRNAs. This script pinpoints protein-coding genes situated within a 100-kilobase span either upstream or downstream of the genomic locus of the lncRNA [].
Prediction of Trans-Acting Targets: The LncTarD (v 2.0) tool was used to predict the potential trans-acting target genes. This prediction was based on the free energy associated with base complementary pairing between lncRNAs and mRNAs. A normalized free energy threshold of less than −0.1 was applied for these predictions [].
Functional Annotation and Enrichment Analysis: The Groups of mRNAs showing differential expression and the anticipated target genes of DE-lncRNAs were examined as described below. Gene Ontology (GO) enrichment analysis was performed using clusterProfiler 4.0 with a hypergeometric test to identify significant enrichment []. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was performed via KOBAS (version 3.0) employing a hypergeometric test, to pinpoint notable enrichment within metabolic and signaling pathways []. A significance threshold of FDR < 0.05 was set for all enrichment results.
2.8. RT-qPCR Validation of DE-LncRNAs
RT-qPCR with three independent biological replicates was employed to validate DE-lncRNAs detected via high-throughput sequencing. TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract RNA from mycelial samples G01 and G02. The PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Shiga, Japan) was used to eliminate genomic DNA and generate first-strand cDNA. Ten randomly selected differentially expressed lncRNAs were identified (Table 1). Primer Premier 5.0 software [] was used to design specific primers. The relative expression level of DE-lncRNAs was calculated using the 2−ΔΔCT method []. The expression of each target lncRNA was normalized to the stable internal reference gene β-Actin, and the sexual morph sample (G01) was designated as the calibrator. Therefore, the calculated relative expression represents the fold change in the asexual morph (G02) relative to G01.

Table 1.
Primer sequences of DE-lncRNAs used for RT-qPCR.
2.9. Evaluation of Physiological Indicators in the Fermentation Broth
Three physiological indicators (mycelial growth rate, melanin content, and polysaccharide content) were quantitatively analyzed in the E. cristatum fermentation broth after seven days of cultivation.
Mycelial growth rate was assessed by measuring mycelial dry weight []. Briefly, after fermentation, the mycelia were collected, washed with distilled water, dried at 60 °C to a constant weight, and precisely weighed. The mycelial dry weight per unit volume of culture broth (g/mL) was then calculated.
Extracellular melanin in the fermentation broth was extracted using an alkaline extraction method []. Briefly, 50 mL of the fermentation broth was mixed with 70 mL of 1 mol/L NaOH solution and stirred in a 74 °C water bath for extraction. The mixture was then filtered and heated. The filtrate was acidified to pH 2.5 using 6 mol/L HCl, and allowed to stand in a 74 °C water bath for 4 h to precipitate melanin. The precipitate was collected by centrifugation at 8000× g for 15 min at 4 °C, washed to neutralize the pH with distilled water, and freeze-dried to obtain crude melanin. Crude melanin was purified via sequential extraction using ethyl acetate, chloroform, and ethanol. Purified melanin was freeze-dried and weighed, and the melanin content per unit volume of culture broth was calculated.
Polysaccharides in the fermentation broth were extracted using water extraction and alcohol precipitation []. Briefly, 50 mL of the fermentation broth was filtered to remove mycelia and insoluble impurities. Activated carbon was then added to the filtrate to adsorb the pigments. The decolorized filtrate was concentrated using a rotary evaporator at 55 °C. Proteins were removed from the concentrate by shaking with Sevag reagent at 160 rpm for 30 min, followed by centrifugation. Polysaccharides were precipitated from the remaining liquid by adding anhydrous ethanol and incubation at 4 °C for 24 h. The precipitate was collected via centrifugation at 8000 rpm, thoroughly dried in a vacuum dryer at 40 °C, and precisely weighed. Finally, the polysaccharide content per unit volume of culture broth was calculated.
2.10. Statistical Analysis
The experimental data were analyzed using the SPSS 23 software []. Standard deviations were used to determine the sample variability. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Cultivation of E. cristatum Mycelia
The liquid fermentation characteristics of the two morphs of E. cristatum are shown in Figure 1. The sexual morph pellicle appeared golden yellow with a rough and dense surface, and dark brown oil-like exudates were occasionally observed in the central region (Figure 1A). In contrast, the asexual morph pellicle exhibited a relatively uniform gray-green color with a smoother, tighter, and finer surface than the sexual morph. Moreover, no exudates were produced, indicating a high degree of distinction between sexual morphs (Figure 1B).
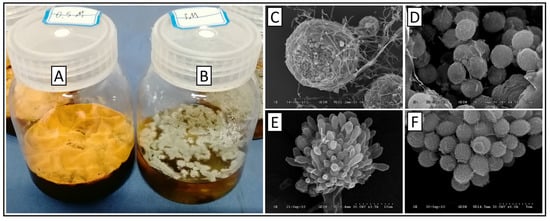
Figure 1.
Fermentation broths and microscopic characteristics of the sexual and asexual morphs of Eurotium cristatum. (A) Sexual fermentation broth. (B) Asexual fermentation broth. (C) Cleistothecia. (D) Ascospores. (E) Conidial heads. (F) Conidia.
Scanning electron microscopy revealed the sexual and asexual reproductive structures of E. cristatum.
Sexual reproductive structures: Cleistothecia exhibited a rough surface with numerous grooves, appearing spherical or ellipsoidal with a diameter of 80–160 µm (Figure 1C). Ascospores measured 3.5–5 µm × 4.5–6 µm, with distinct surface ornamentation characterized by rough sharp warts and two prominent “coronary” projections at the equatorial region of the spore (Figure 1D).
3.2. Sequencing Data Quality and Alignment Statistics
Strand-specific lncRNA sequencing of sexual (G01) and asexual (G02) mycelial samples of E. cristatum was performed. A total of 34.40 Gb of clean data were obtained. The data volume for each sample exceeded 17 Gb, and the percentage of Q30 bases was >93.71%, indicating high-quality sequencing data. Clean reads were aligned to the A. cristatus GZAAS20.1005 (PRJNA271918) reference genome. The alignment rates for G01 and G02 were 97.74% and 96.99%, respectively, demonstrating high data utility and suitability for subsequent analyses.
3.3. LncRNA Identification and Classification
Through comprehensive screening using the StringTie assembly and four potential coding prediction tools (CPC2, CNCI, CPAT, and Pfam), 203 high-confidence lncRNAs were identified. Based on their genomic locations relative to known genes, these lncRNAs were classified into four categories. Long intergenic non-coding RNAs (lincRNAs) were the most abundant lncRNAs (70.9%), followed by antisense (26.6%) and sense (2.5%) lncRNAs. No intronic lncRNAs were detected.
A comparison of the sequenced lncRNAs and mRNAs revealed that the lncRNA length was primarily between 400 and 1200 nt (Figure 2A), whereas the mRNA length was mainly between 400 and 2000 nt and above 3000 nt (Figure 2B). The predicted ORF lengths of the lncRNAs were mainly between 50 and 150 nt (Figure 2C), whereas those of mRNAs were predominantly between 200 and 400 nt (Figure 2D). Compared with mRNAs, lncRNAs of E. cristatum exhibited shorter transcript lengths, fewer exons, and shorter ORFs, which is consistent with the typical structural characteristics of lncRNAs. The classification of the 203 lncRNAs is summarized in Supplementary Table S1. Their nucleotide sequences are provided in Supplementary File S1, and the corresponding genomic annotations are available in Supplementary File S2.
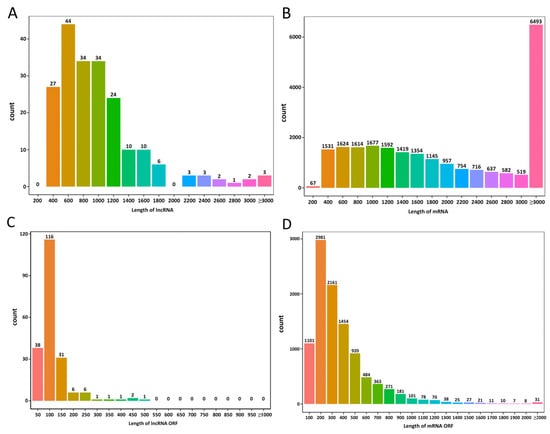
Figure 2.
Characteristics of long non-coding (lncRNAs) and messenger RNAs (mRNAs) in Eurotium cristatum. (A) LncRNA length statistics. (B) mRNA length statistics. (C) LncRNA open reading frame (ORF) length statistics. (D) mRNA ORF length statistics. The horizontal axis represents length, while the vertical axis represents the number of RNAs within that length range.
3.4. DE-LncRNA Analysis
Using thresholds of |log2(fold change)| ≥ 1 and FDR < 0.01, 120 DE-lncRNAs were identified (Full details of the 120 DE-lncRNAs are provided in Supplementary Table S2). Among these, 57 were upregulated and 63 were downregulated in G02 (asexual morphs) compared to those in G01 (sexual morphs). The clustering heatmap of the DE-lncRNAs in Figure 3 clearly shows their expression patterns, indicating significant differences in lncRNA expression profiles under different salt stress conditions.
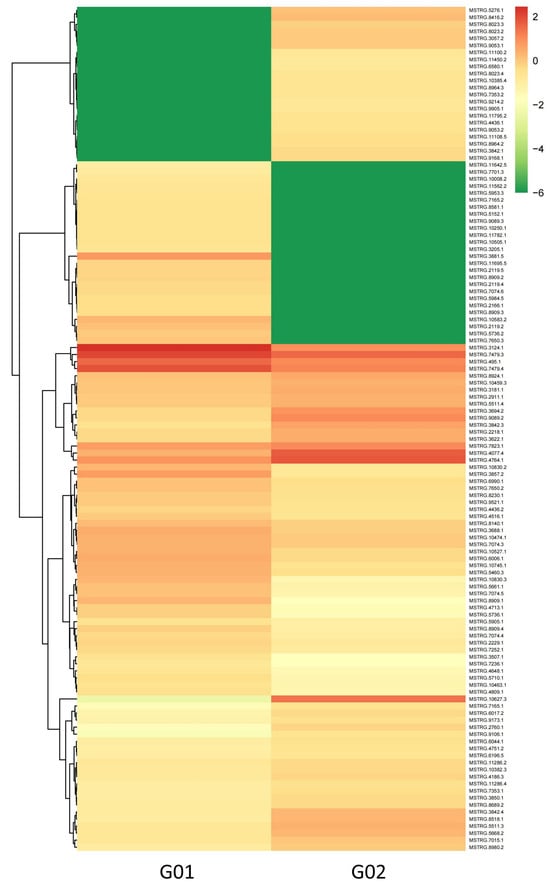
Figure 3.
Clustering heatmap of differentially expressed long non-coding RNAs (DE-lncRNAs).
3.5. Target Gene Prediction and Functional Enrichment Analysis
Predictions were made for cis-acting target genes situated within a 100-kilobase range either upstream or downstream of the DE-lncRNAs. GO enrichment analysis was carried out, and the results indicated that these target genes were notably enriched in several biological processes. These included oxidation-reduction reactions (GO: 0055114), transmembrane transport (GO: 0055085), and carbohydrate metabolic pathways (GO: 0005975). KEGG pathway analysis (Figure 4) indicated that these target genes were primarily involved in many pathways, including carbon metabolism (ko01200), urine metabolism (ko00230), and peroxisome metabolism (ko04146).
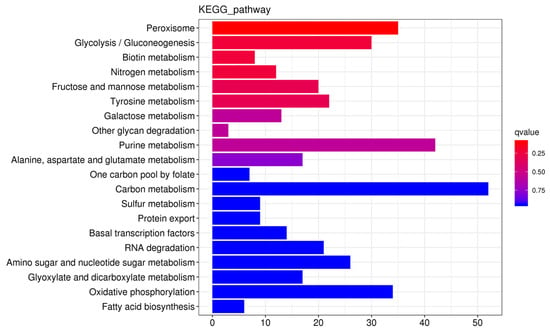
Figure 4.
Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment bar plot of the cis-acting target genes of DE-lncRNAs. Note: The x-axis represents the gene number (number of genes of interest annotated to a specific term). The y-axis represents each pathway term. The bar color indicates the p value determined via the hypergeometric test.
Trans-acting target genes based on complementary base pairing were predicted using LncTar software. GO enrichment analysis showed that these genes were significantly involved in processes, such as DNA integration (GO: 0015074) and protein ubiquitination (GO: 0071947 and 2000060). KEGG analysis revealed enrichment in various pathways, including ribosome and ribosome biogenesis, in eukaryotes (Figure 5).
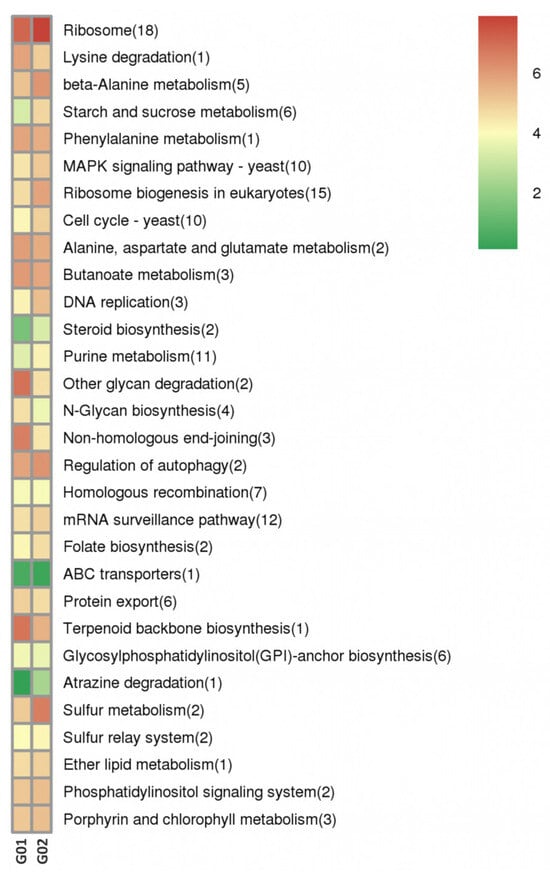
Figure 5.
KEGG enrichment cluster plot of the trans-acting target genes of DE-lncRNAs.
3.6. RT-qPCR Validation
To assess the accuracy of the RNA sequencing data, ten randomly selected DE-lncRNAs with significant differences in expression levels were validated using RT-qPCR. The results are shown in Figure 6. Gene expression levels determined via Illumina sequencing were largely consistent with those measured via RT-qPCR, as the same genes analyzed via both methods exhibited similar upregulation and downregulation trends. These results confirm that our transcriptome sequencing data were accurate and reliable for further analyses.
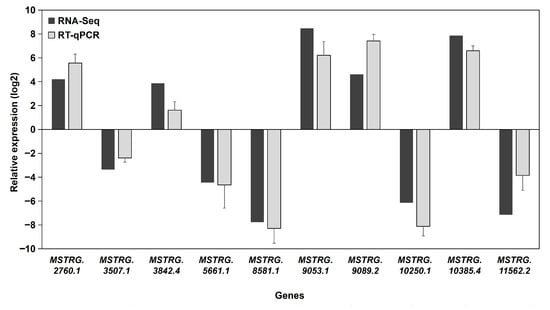
Figure 6.
The relative expression of ten DE-lncRNAs was determined by RT-qPCR. Data are presented as the fold change in the asexual morph (G02) relative to the sexual morph (G01, which served as the calibrator), shown as mean ± SD (n = 3). The corresponding FPKM values from RNA-seq are presented for qualitative trend comparison. The concordant direction of change (up- or down-regulation) between the methodologies supports the reliability of the RNA-seq data.
3.7. Association Analysis Between LncRNAs and Phenotypes
Physiological indicator measurements revealed significant differences in the growth and metabolite accumulation of E. cristatum under different culture conditions (Figure 7). Compared to the asexual mycelia (G02) fermented in the high-salt potato glucose liquid medium, sexual mycelia (G01) fermented in the standard potato glucose liquid medium exhibited superior physiological phenotypes, with 219.7% higher mycelial biomass (6.17 vs. 1.93 g/L, G01 vs. G02), 135% higher extracellular melanin content (3.22 vs. 1.37 g/L, G01 vs. G02), and 53.3% higher extracellular polysaccharide content (0.23 vs. 0.15 g/L, G01 vs. G02).
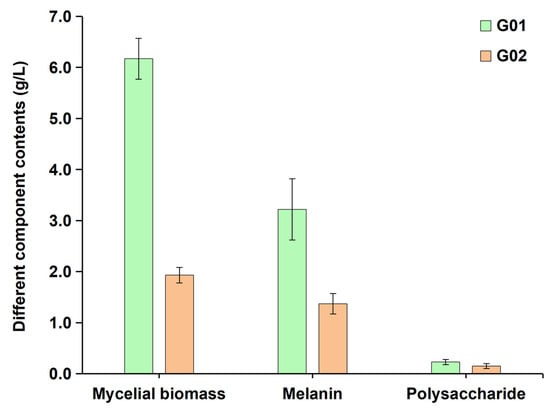
Figure 7.
Contents of different components in the Eurotium cristatum fermentation broth.
To elucidate the molecular regulatory mechanisms underlying these significant phenotypic differences, we analyzed the DE-lncRNAs and found that their expression patterns were highly associated with the phenotypic traits of E. cristatum. In G01, the lncRNA MSTRG.3124.1 (an antisense lncRNA) was abundant (FPKM = 281.48). However, its expression was significantly lower in G01 than in G02 (log2FC = −4.94), making it one of the most highly expressed lncRNAs that was downregulated in sexual morphs compared to asexual morphs.
In G02 cells, the three lncRNAs showed high expression levels. Among them, MSTRG.10627.3 (a lincRNA) exhibited the most significant expression difference (FDR = 2.55 × 10−106; log2FC = 10.53), with its expression level in G02 (FPKM = 25.57) far exceeding that in G01 (FPKM ≈ 0). MSTRG.4077.4 (a lincRNA lncRNA) also showed high expression in G02 cells (FPKM = 57.59) and significant differential expression (FDR = 2.95 × 10−76; log2FC = 4.78). Furthermore, MSTRG.4764.1 (a lincRNA lncRNA) maintained a relatively high expression in G02 (FPKM = 65.61), showing significant differential expression (FDR = 2.94 × 10−41; log2FC = 3.14). The expression patterns of these DE-lncRNAs showed a high correlation with phenotypic indicators such as mycelial biomass, polysaccharide yield, and melanin yield, suggesting their important regulatory roles in these physiological processes.
4. Discussion
In the present study, we integrated transcriptomic and phenotypic analyses to investigate the roles of lncRNAs in E. cristatum under two contrasting physiological conditions: a sexual morph cultivated under standard conditions and an asexual morph induced by high-salinity stress. We emphasize that these states result from a complex interplay, in which salt stress serves as an environmental signal that triggers developmental transitions. Consequently, the differential expression of lncRNAs and the associated metabolic profiles reported here reflect a combined response involving direct osmotic stress adaptation and extensive reprogramming inherent to alternative developmental programs (asexual vs. sexual). Our results suggested that lncRNAs function as key regulatory molecules in this integrated response network.
Salt stress significantly altered the lncRNA expression profiles of E. cristatum, resulting in the identification of 120 DE lncRNAs. This finding suggests the broad involvement of lncRNAs in the transcriptional regulatory responses of E. cristatum to adversity, which is consistent with previous reports of abiotic stress inducing extensive lncRNA expression in other fungal species. LncRNAs and associated transcription factors regulate tolerance to high salt (5.13 mol/L NaCl) in Aspergillus sydowii []. Notably, antisense lncRNAs accounted for a substantial proportion (26.6%) of DE-lncRNAs in E. cristatum in this study. This class of lncRNAs typically forms complementary pairs with coding genes on their sense strands, enabling the precise regulation of target gene expression at the transcriptional or post-transcriptional levels []. This mechanism may form the molecular basis for the rapid and precise adjustment of gene expression in E. cristatum under stressful conditions.
Functional enrichment analysis of cis-acting target genes associated with DE-lncRNAs offers valuable insights into phenotypic disparities. These target genes are significantly enriched in various biological processes. For instance, they were notably involved in the “oxidation–reduction process” (GO: 0055114), “transmembrane transport” (GO: 0055085), and “carbohydrate metabolic process” (GO: 0005975). High-salt environments cause cellular osmotic imbalances and reactive oxygen species bursts; therefore, the regulation of redox homeostasis and ion transport is crucial for cellular adaptation to stress. The expression patterns of related lncRNAs/target genes in the G01 strain were more conducive to maintaining intracellular homeostasis, thereby explaining its growth advantage, as evidenced by its high mycelial biomass (6.17 g/L). More importantly, enrichment of target genes in pathways, such as the “carbon metabolism” (ko01200) and “purine metabolism” (ko00230) pathways, strongly correlated with high polysaccharide yield (0.23 g/L) in the G01 strain. We hypothesized that highly expressed lncRNAs (e.g., MSTRG.3124.1) in G01 promote polysaccharide biosynthesis by regulating adjacent sugar metabolism-related genes under normal conditions.
To further investigate the association between the DE-lncRNAs, mycelial morphological differentiation, and metabolite synthesis phenotypes, several key lncRNAs were screened. These molecules were categorized into two groups: those specifically highly expressed in G01 (high-yield sexual mycelia), which may positively regulate metabolite synthesis, and those specifically highly expressed in G02 (low-yield asexual mycelia), which may negatively regulate related pathways. In interpreting these results, we noted that the regulatory significance of an lncRNA was not solely dictated by its abundance. In living organisms, lncRNAs with low copy numbers can exert critical and precise regulatory effects [,]. In this study, we prioritized DE-lncRNAs as strong functional candidates because their pronounced changes showed a statistically robust correlation with major phenotypic shifts in morphology and metabolite production. This approach provides a practical basis for generating mechanistic hypotheses. Therefore, the following discussion focuses on the high-priority candidates within this context. Among these candidates, lncRNA MSTRG.3124.1 was significantly more abundant in G01 (FPKM = 281.48) than in G02 (log2FC = −4.94; FDR < 1 × 10−88). We predicted that it drives the efficient synthesis of melanin and polysaccharides in G01 by regulating the expression of target genes such as glycosyltransferases. In contrast, several lncRNAs were specifically activated in G02, showing strong potential for negative regulation. Among them, MSTRG.10627.3 showed the most considerable differential expression (FDR < 1 × 10−105; log2FC = 10.53), and its high expression in G02 suggests that it is a key inhibitory switch in response to high salt stress conditions. MSTRG.4077.4 also exhibited very high differential expression (FPKM = 57.59; FDR < 1 × 10−75), and possibly acted as a primary inhibitory molecule in G02. MSTRG.4764.1 showed relatively high expression in G02 (FPKM = 65.61) and basal expression in both groups, and was possibly responsible for basal inhibition. These lncRNAs collectively reduced the metabolite content in G02 by inhibiting the expression of key enzyme genes involved in pathways such as glycolysis/gluconeogenesis (ko00010) and tyrosine metabolism (ko00350).
High melanin synthesis in G01 (3.22 g/L) strongly indicated the potent activation of the melanin synthesis pathway. Although the classical melanin synthesis pathway was not directly detected in the KEGG enrichment results, significant enrichment of target genes in the “peroxisome” pathway (ko04146) provided a critical clue. Peroxisomes are important intracellular oxidative metabolic sites that are involved in the degradation and synthesis of various substances, including melanin precursors [,]. Early enzymes involved in melanin biosynthesis (e.g., BcPKS12/13 and BcYGH1) are localized in peroxisomes []. Therefore, we hypothesized that specific lncRNAs in G01 create an oxidative microenvironment favorable for melanin synthesis, or directly regulate the expression of key melanin synthesis enzyme genes by cis-regulating the expression of peroxisome-related genes, thereby driving substantial melanin accumulation. Conversely, significant upregulation of lncRNAs, such as MSTRG.10627.3, and MSTRG.4077.4, under high salt stress conditions (G02) may inhibit these pathways, leading to decreased melanin production.
Analysis of the trans-acting target genes revealed another layer of a broad regulatory network. Significant enrichment in processes such as “DNA integration” and “protein ubiquitination” indicated that some lncRNAs were widely involved in maintaining genome stability and protein degradation processes via base complementary pairing. Protein ubiquitination is an important post-translational modification involved in various processes such as the cell cycle, signal transduction, and stress responses [,]. These results suggest that some highly expressed lncRNAs in G02 affect the activity of key salt stress response factors at the protein level via trans-acting mechanisms, thereby indirectly influencing morphogenesis and metabolism.
In conclusion, we constructed a preliminary regulatory model for lncRNAs involved in the salt stress response in E. cristatum. Under high-salt stress conditions, lncRNA expression profiles were reprogrammed. Some lncRNAs (e.g., MSTRG.3124.1) directly regulate adjacent metabolic genes related to sugar metabolism and melanin synthesis via cis-acting mechanisms, whereas others (e.g., MSTRG.10627.3 and MSTRG.4077.4) affect broad stress responses and protein degradation pathways via trans-acting mechanisms. The combined effects of these regulatory mechanisms may lead to the morphological transition of mycelia from the sexual to the asexual form and inhibit the synthesis of secondary metabolites such as polysaccharides and melanin.
5. Conclusions
Through integrated lncRNA sequencing and physiological phenotype analysis, this study systematically revealed the expression characteristics and potential regulatory functions of lncRNAs in E. cristatum under salt stress. A total of 203 lncRNAs were identified, 120 of which were closely associated with mycelial morphological differentiation and metabolite accumulation. The high expressed MSTRG.3124.1 in the high-yield G01 strain possibly promoted polysaccharide and melanin synthesis by regulating carbohydrate metabolism and the peroxisome pathway. In contrast, lncRNAs highly expressed in the G02 strain, such as MSTRG.10627.3 and MSTRG.4077.4, may negatively regulate secondary metabolism by inhibiting metabolic pathways and activating protein ubiquitination. These results suggest that lncRNAs play important roles in salt stress response and metabolic process regulation in E. cristatum, providing new insights into the environmental adaptation mechanisms of filamentous fungi and establishing a theoretical foundation for improving industrial strain performance via genetic modifications.
However, the proposed model requires further validation. Future work should not only seek to confirm the roles of the highly expressed candidate lncRNAs identified here but also explore the potential functions of lower-abundance DE-lncRNAs. In particular, the functions of key lncRNAs should be confirmed through gene knockout or overexpression experiments to observe their direct effects on target gene expression, mycelial morphology, and metabolite production. Furthermore, an integrated analysis of lncRNAs with other regulatory molecules, such as miRNAs and circular RNAs, would help construct a comprehensive competing endogenous RNA network, thereby systematically elucidating the molecular mechanisms governing the morphology and metabolism of E. cristatum.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14111592/s1, Table S1: Distribution of lncRNA categories; Table S2: Expression data and statistics for the 120 DE-lncRNAs; File S1: LncRNA sequence information; File S2: Eurotium cristatum lncRNA annotations.
Author Contributions
Conceptualization, Y.W.; methodology, X.Q. and Y.W.; software, Z.X.; validation, M.D. and X.Q.; formal analysis, Z.H. and Z.X.; investigation, M.D., X.Q. and Z.L.; resources, Z.H.; data curation, Z.X. and M.D.; writing—original draft, Y.W. and Q.Z.; writing—review and editing, Z.H., Z.X. and M.D.; visualization, Q.Z.; supervision, Z.H.; project administration, Z.L.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Undergraduate Innovation and Entrepreneurship Training Program (S202411527026), Scientific Research Project of Hunan Provincial Education Department (24A0574), Project of Science and Technology Commissioners Serving Rural Revitalization (2024RC8220), Hunan Province College Students Entrepreneurship Guidance Studio Platform Project (2025CKX021), and Hunan Provincial Natural Science Foundation (2025JJ70402).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw transcriptome sequencing data generated in this study have been deposited in the NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra/ accessed on 11 November 2025) under accession number PRJNA827193. All other supporting data are contained within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Ruffo, P.; Traynor, B.J.; Conforti, F.L. Unveiling the regulatory potential of the non-coding genome: Insights from the Human Genome Project to precision medicine. Genes Dis. 2025, 12, 101652. [Google Scholar] [CrossRef]
- Luna-Arias, J.P.; Castro-Muñozledo, F. Participation of the TBP-associated factors (TAFs) in cell differentiation. J. Cell. Physiol. 2024, 239, e31167. [Google Scholar] [CrossRef]
- Nitsche, A.; Stadler, P.F. Evolutionary clues in lncRNAs. Wiley Interdiscip. Rev. RNA 2017, 8, e1376. [Google Scholar] [CrossRef]
- Poloni, J.d.F.; Oliveira, F.H.S.d.; Feltes, B.C. Localization is the key to action: Regulatory peculiarities of lncRNAs. Front. Genet. 2024, 15, 1478352. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.B.; Tsitsipatis, D.; Gorospe, M. Integrated lncRNA function upon genomic and epigenomic regulation. Mol. Cell 2022, 82, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Bure, I.V.; Nemtsova, M.V.; Kuznetsova, E.B. Histone modifications and non-coding RNAs: Mutual epigenetic regulation and role in pathogenesis. Int. J. Mol. Sci. 2022, 23, 5801. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, T.; Zhu, G.; Wu, B.; Zhang, C.; Zhu, H. LncRNAs exert indispensable roles in orchestrating the interaction among diverse noncoding RNAs and enrich the regulatory network of plant growth and its adaptive environmental stress response. Hortic. Res. 2023, 10, uhad234. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, H.; Wang, B.; Yuan, F. Research progress on the roles of lncRNAs in plant development and stress responses. Front. Plant Sci. 2023, 14, 1138901. [Google Scholar] [CrossRef]
- Do, D.N.; Suravajhala, P. Editorial: Role of non-coding RNAs in animals. Animals 2023, 13, 805. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Li, D.; Zhang, Y.; Bai, B.; Chai, B.; Wen, Z. A novel lncRNA YIL163C enhances genomic stability and antifungal resistance via the DNA damage response in Saccharomyces cerevisiae. Front. Microbiol. 2025, 16, 1571797. [Google Scholar] [CrossRef]
- Tang, J.; Chen, X.; Yan, Y.; Huang, J.; Luo, C.; Tom, H.; Zheng, L. Comprehensive transcriptome profiling reveals abundant long non-coding RNAs associated with development of the rice false smut fungus, Ustilaginoidea virens. Environ. Microbiol. 2021, 23, 4998–5013. [Google Scholar] [CrossRef] [PubMed]
- Glad, H.M.; Tralamazza, S.M.; Croll, D. The expression landscape and pangenome of long non-coding RNA in the fungal wheat pathogen Zymoseptoria tritici. Microb. Genom. 2023, 9, 001136. [Google Scholar] [CrossRef]
- Gervais, N.C.; Shapiro, R.S. Discovering the hidden function in fungal genomes. Nat. Commun. 2024, 15, 8219. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Lei, L.; Li, X.; Hu, W.; Zhang, T.; Yang, W.; Ma, B.; Si, S.; Xu, Y.; Yu, L. Secondary Metabolites Produced by the Dominant Fungus Eurotium cristatum in Liupao Tea and Their Hypolipidemic Activities by Regulating Liver Lipid Metabolism and Remodeling Gut Microbiota. J. Agric. Food Chem. 2024, 72, 27978–27990. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Kang, M.; Quan, W.; Qiu, G.; Tao, T.; Li, C.; Zhu, S.; Lu, B.; Liu, Z. Soluble Dietary Fiber from Fermentation of Tea Residues by Eurotium cristatum and the Effects on DSS-Induced Ulcerative Colitis. J. Agric. Food Chem. 2025, 73, 15717–15732. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, T.; Lin, L.; Xu, W.; Hu, Y.; Huang, T.; Xiao, Y.; Xiao, W.; Gong, Z. Luminescence and fahua-fermentation qualities of an autofluorescent microorganism from Fu brick tea. J. Food Process. Preserv. 2022, 46, e16202. [Google Scholar] [CrossRef]
- Ren, C.; Tan, Y.; Ren, X.; Liu, Y.; Liu, Z. Metabolomics reveals changes in metabolite concentrations and correlations during sexual development of Eurotium cristatum (synonym: Aspergillus cristatus). Mycosphere 2017, 8, 1626–1639. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, K.; Bai, J.R.; Wu, Y.P.; Gao, H. Insight into effects of isolated Eurotium cristatum from Pingwu Fuzhuan brick tea on the fermentation process and quality characteristics of Fuzhuan brick tea. J. Sci. Food Agric. 2020, 100, 3598–3607. [Google Scholar] [CrossRef]
- Li, T.; Liu, Z.; Li, J.; Zheng, Y.; Liu, Z.; Ling, P. Lovastatin production by wild Eurotium cristatum isolated from fuzhuan brick tea produced using forest resources in Auhua. Forests 2023, 14, 1409. [Google Scholar] [CrossRef]
- Xiao, H.; Li, T.; Tang, H.; Chen, Y.; Wang, W.; Liu, C.; Hu, Z.; Yu, S.; Wen, R.; Liu, S. Antibacterial Activity of Propagule Extracts of Eurotium cristatum. Food Ferment. Ind. 2020, 46, 65–69. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Y.; Liu, Y.; Tan, Y.; Ren, X.; Zhang, X.; Hyde, K.D.; Liu, Y.; Liu, Z. Comparative genomic and transcriptomic analyses of the Fuzhuan brick tea-fermentation fungus Aspergillus cristatus. BMC Genom. 2016, 17, 428. [Google Scholar] [CrossRef] [PubMed]
- Vakili-Ghartavol, M.; Arouiee, H.; Golmohammadzadeh, S.; Naseri, M. Optimal methods for the preparation of fungal mycelium for examination with the scanning electron microscope. Plant Pathol. 2022, 11, 2. [Google Scholar] [CrossRef]
- Lam, J.; Katti, P.; Biete, M.; Mungai, M.; AshShareef, S.; Neikirk, K.; Garza Lopez, E.; Vue, Z.; Christensen, T.A.; Beasley, H.K. A universal approach to analyzing transmission electron microscopy with ImageJ. Cells 2021, 10, 2177. [Google Scholar] [CrossRef]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. Imeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Thakur, V. RNA-seq data analysis for differential gene expression using HISAT2–stringtie–ballgown pipeline. In Transcriptome Data Analysis; Springer: New York, NY, USA, 2024; pp. 101–113. [Google Scholar]
- Feng, Z.; Peng, F.; Xie, F.; Liu, Y.; Zhang, H.; Ma, J.; Xing, J.; Guo, X. Comparison of capture-based mtDNA sequencing performance between MGI and illumina sequencing platforms in various sample types. BMC Genom. 2024, 25, 41. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Xue, L.; Zhao, T.; Ding, W.; Han, Y.; Ye, H. A comparison of transcriptome analysis methods with reference genome. BMC Genom. 2022, 23, 232. [Google Scholar] [CrossRef]
- Pertea, G.; Pertea, M. GFF utilities: GffRead and GffCompare. F1000Research 2020, 9, ISCB Comm J-304. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Yang, D.-C.; Kong, L.; Hou, M.; Meng, Y.-Q.; Wei, L.; Gao, G. CPC2: A fast and accurate coding potential calculator based on sequence intrinsic features. Nucleic Acids Res. 2017, 45, W12–W16. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Lun, A.T.; Baldoni, P.L.; Smyth, G.K. edgeR v4: Powerful differential analysis of sequencing data with expanded functionality and improved support for small counts and larger datasets. Nucleic Acids Res. 2025, 53, gkaf018. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dou, M.; Liu, R.; Jiao, Y.; Hao, Z.; Xu, Y. Identification of long non-coding RNAs in response to downy mildew stress in grape. Fruit Res. 2022, 2, 1–14. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Qi, C.; Mo, Q.; Zhong, K.; Liu, J.; Cai, H.; Li, J.; Chen, J.; Yang, J. lncRNA-Encoded Small Peptide Promotes Viral Infection. Mol. Plant Pathol. 2025, 26, e70084. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, J.; Lan, X.; Jia, R.; Hu, L.; Wang, Y. Response surface methodology (RSM) mediated optimization of medium components for mycelial growth and metabolites production of Streptomyces alfalfae XN-04. Microorganisms 2022, 10, 1854. [Google Scholar] [CrossRef]
- Yang, N.; Liu, S.; Wang, J.; Li, X.; Liu, X. Alkaline extraction process of extracellular melanin from Eurotium cristatum. China Brew. 2020, 39, 5. [Google Scholar]
- Tao, A.; Feng, X.; Sheng, Y.; Song, Z. Optimization of the Artemisia polysaccharide fermentation process by Aspergillus niger. Front. Nutr. 2022, 9, 842766. [Google Scholar] [CrossRef]
- George, D.; Mallery, P. IBM SPSS Statistics 29 Step by Step: A Simple Guide and Reference; Routledge: Abingdon, UK, 2024. [Google Scholar]
- Jiménez-Gómez, I.; Valdés-Muñoz, G.; Moreno-Ulloa, A.; Pérez-Llano, Y.; Moreno-Perlín, T.; Silva-Jiménez, H.; Barreto-Curiel, F.; Sánchez-Carbente, M.d.R.; Folch-Mallol, J.L.; Gunde-Cimerman, N. Surviving in the brine: A multi-omics approach for understanding the physiology of the halophile fungus Aspergillus sydowii at saturated NaCl concentration. Front. Microbiol. 2022, 13, 840408. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, T.; Wu, X.; Yang, Z.; Sun, Y. Identification cloning and functional analysis of novel natural antisense lncRNA CFL1-AS1 in cattle. Epigenetics 2023, 18, 2231707. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, Y.; Li, Q.; Cao, Y.; Yang, D.; Liu, S.; Wang, X.; Mi, Y.; Liu, Y.; Ding, C. A lncRNA fine-tunes salicylic acid biosynthesis to balance plant immunity and growth. Cell Host Microbe 2022, 30, 1124–1138.e1128. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, L.-Z.; Chen, L.-L. Long noncoding RNA and protein abundance in lncRNPs. RNA 2021, 27, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lu, K.; Yang, L.; Jiang, J.; Li, L. Peroxin MoPex22 Regulates the Import of Peroxisomal Matrix Proteins and Appressorium-Mediated Plant Infection in Magnaporthe oryzae. J. Fungi 2024, 10, 143. [Google Scholar] [CrossRef]
- Falter, C.; Reumann, S. The essential role of fungal peroxisomes in plant infection. Mol. Plant Pathol. 2022, 23, 781–794. [Google Scholar] [CrossRef]
- Jia, H.; Liu, N.; Zhang, L.; Li, P.; Meng, Y.; Yuan, W.; Li, H.; Tantai, D.; Qu, Q.; Cao, Z. Fungal Melanin in Plant Pathogens: Complex Biosynthesis Pathways and Diverse Biological Functions. Plants 2025, 14, 2121. [Google Scholar] [CrossRef]
- Wang, R.; You, X.; Zhang, C.; Fang, H.; Wang, M.; Zhang, F.; Kang, H.; Xu, X.; Liu, Z.; Wang, J. An ORFeome of rice E3 ubiquitin ligases for global analysis of the ubiquitination interactome. Genome Biol. 2022, 23, 154. [Google Scholar] [CrossRef]
- Ma, P.; Mao, B. The many faces of the E3 ubiquitin ligase, RNF220, in neural development and beyond. Dev. Growth Differ. 2022, 64, 98–105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).