Simple Summary
This study investigates the bacterial strain Lactococcus sp. KTH0-1S as a potential alternative expression host and microbial cell factory. We confirmed that it is safe, carrying no harmful genes, and possesses traits that support survival in the digestive system and fermentation environments. Uniquely, the strain naturally produces nisin, which can act as an inducer for the NICE system, providing auto-induction properties that offer a simpler and more cost-effective alternative to conventional hosts. The strain also grows efficiently with nutrient support and can utilize a variety of sugars, including those from agricultural by-products. These characteristics make Lactococcus sp. KTH0-1S a promising next-generation probiotic and a versatile, food-grade microbial host for producing valuable proteins.
Abstract
The safety, genetic distinctiveness, and functional capabilities of Lactococcus sp. KTH0-1S, a strain isolated from Thai fermented shrimp (Kung-Som), were investigated to assess its potential as a next-generation probiotic and microbial cell factory. Whole-genome sequencing and multilocus sequence typing (MLST) analysis revealed that Lactococcus sp. KTH0-1S is a novel, phylogenetically distinct strain within the Lactococcus genus. Comprehensive in silico safety evaluation confirmed the absence of antimicrobial resistance genes and major virulence factors, supporting its suitability for food-grade applications. The genome encodes multiple probiotic-relevant traits, including stress tolerance (e.g., dnaK, clpP), adhesion and biofilm formation (e.g., gapA, luxS, glf2), and nutrient acquisition genes, enabling adaptation to gastrointestinal and fermentation environments. Notably, Lactococcus sp. KTH0-1S harbors a chromosomally encoded nisin Z biosynthesis gene cluster with auto-induction capability, providing a self-regulated and stable alternative to conventional plasmid-based NICE systems in Lactococcus lactis. The strain also exhibits nisin immunity, allowing tolerance to high nisin concentrations, thus supporting robust protein production. Genomic evidence and phenotypic assays confirmed a functional respiration metabolism activated by heme supplementation, enhancing biomass yield and culture stability. Furthermore, the presence of diverse CAZyme families (GHs, GTs, CEs) enables utilization of various carbohydrate substrates, including lignocellulosic and starchy agro-industrial residues. These properties collectively underscore Lactococcus sp. KTH0-1S as a safe, stable, and metabolically versatile candidate for probiotic applications and as a cost-effective, food-grade expression host for biotechnological production.
1. Introduction
Lactic acid bacteria (LAB) are essential in fermented foods and recognized for their technological, nutritional, and health-promoting properties. Their historical safe consumption has earned many LAB strains “Generally Recognized as Safe” (GRAS) status from regulatory bodies like the U.S. FDA [1]. Beyond their role as starter cultures in dairy and plant-based fermentations, some LAB also function as probiotics, contributing to gut health, immune modulation, and pathogen inhibition [2,3]. This growing demand for functional foods and microbial therapeutics has spurred interest in identifying novel LAB strains with enhanced probiotic and technological traits.
Among LAB, Lactococcus lactis is a well-established model organism, extensively used in food biotechnology and synthetic biology. Its advantages include a small, well-annotated genome, non-pathogenic status, food-grade compatibility, and ease of genetic manipulation [4], which have facilitated the development of sophisticated expression systems. These systems make L. lactis a versatile microbial cell factory for producing heterologous proteins, bioactive compounds, and therapeutic molecules [5,6].
A key innovation in LAB expression technology is the Nisin-Controlled Expression (NICE) system, which uses the bacteriocin nisin as an inducer for gene expression. While the NICE system is tightly regulated and widely employed, its reliance on exogenous nisin supplementation creates challenges for industrial scalability. Adding nisin not only increases production costs but can also inhibit host cell growth due to its antimicrobial activity [7,8,9,10,11]. An appealing solution lies in using naturally nisin-producing Lactococcus strains. These strains carry the full nisin biosynthetic gene cluster, including immunity genes such as nisI and nisFEG, which protect the host from self-produced nisin [10]. Such strains offer the potential for auto-induction, eliminating the need for external inducers and enabling more cost-effective and scalable recombinant protein production. Furthermore, their inherent nisin immunity allows them to tolerate higher intracellular nisin concentrations, potentially enhancing expression levels without compromising cell viability. These features are particularly valuable when combined with the selection of novel Lactococcus strains that possess distinct genetic backgrounds, potentially offering new biosynthetic capabilities or stress-resistance traits not found in model strains.
For any LAB strain to be suitable for industrial, food, or therapeutic applications, comprehensive genomic and functional characterization is critical. This includes in silico screening for antimicrobial resistance (AMR) genes, virulence factors, and mobile genetic elements to assess biosafety [12], alongside functional analyses of probiotic traits such as stress tolerance, adhesion to intestinal cells, and biofilm formation. This integrated approach aligns with the concept of next-generation probiotics (NGPs), which are identified through genomic screening for defined, beneficial traits rather than empirical selection [13]. This allows for the discovery of novel LAB strains that combine safety, functionality, and technological potential, particularly those capable of dual roles as probiotics and hosts for recombinant protein production.
This study explores the potential of novel, auto nisin-producing Lactococcus strain that integrate probiotic characteristics, nisin immunity, and genetic stability. This strategy represents a promising avenue for developing next-generation microbial cell factories as alternative expression hosts suitable for both food-grade and therapeutic applications.
2. Material and Methods
2.1. Bacterial Growth, DNA Extraction, and Whole-Genome Sequencing (WGS)
Lactococcus sp. KTH0-1S was originally isolated from Kung-Som, a traditional Thai fermented shrimp product [14]. For routine cultivation, the strain was grown on M17 agar (Sigma-Aldrich, St. Louis, MO, USA) at 30 °C for 24 h. A single pure colony was subsequently transferred to M17 broth and cultivated at 30 °C for 18–24 h under static conditions (no agitation). Genomic DNA was extracted using GenElute™ Bacterial Genomic DNA kit (Sigma-Aldrich, St. Louis, MO, USA), following the manufacturer’s protocol. Briefly, 3 mL of overnight culture was harvested and resuspended in lysozyme solution, followed by incubation at 37 °C for 1 h to disrupt the peptidoglycan layer. RNase treatment was performed to remove RNA, after which proteinase K and lysis solution were added, and the sample was incubated at 55 °C for 10 min. Ethanol was then added to the lysate, which was transferred to a spin column for DNA binding. The column was washed twice with provided wash buffers, and DNA was finally eluted in nuclease-free water. DNA concentration and purity were assessed using NanoDrop spectrophotometer (ThermoFisher, Waltham, MA, USA).
Prior to whole-genome sequencing, species identification was confirmed by amplifying the 16S rRNA gene using PCR. The amplification was carried out using NEB Q5® High-Fidelity 2X Master Mix (New England Biolabs, Ipswich, MA, USA) with universal primers 27F and 1492R (Table 1) as described by [15]. The amplified product (~1500 bp) was visualized via agarose gel electrophoresis and purified using the Monarch® DNA Gel Extraction Kit (New England Biolabs, USA). The purified PCR product was quantified using NanoDrop spectrophotometer and submitted for Sanger sequencing. The resulting sequence was analyzed using NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi accessed on 15 May 2024) to confirm species identity.

Table 1.
Primers designed and used in this study.
The confirmed genomic DNA of Lactococcus sp. KTH0-1S was sequenced using a hybrid long-read approach combining both PacBio Single-Molecule Real-Time (SMRT) sequencing (Newark, CA, USA) and Oxford Nanopore Technologies (ONT), (Oxford, UK). PacBio sequencing was carried out to obtain highly accurate long reads, while ONT sequencing was performed using the Rapid Barcoding Kit 24 V14 (SQK-RBK114.24) (Oxford, UK) following the manufacturer’s standard protocol. ONT sequencing was conducted in high-accuracy base-calling mode using the R10.4.1 flow cell, generating real-time long-read data. The datasets from both platforms were integrated to enhance genome assembly completeness and accuracy.
2.2. Genome Assembly and Annotation
The raw reads were assembled de novo using Flye version 2.9.5 [16]. The quality of the assembled genome was evaluated with QUAST version 5.3 [17]. Genome annotation was carried out using both Prokka version 1.14.6 [18] and the RAST web server [19]. A circular genome map was created for visualization and annotation using Proksee server [20]. In addition, functional annotation was performed using eggNOG-mapper v2.1.12 [21], which classified the predicted proteins into clusters of orthologous groups (COGs) [22]. Additionally, BlastKOALA v3.1 was used to assign proteins to Kyoto Encyclopedia of Genes and Genomes (KEGG) Orthology (KO) groups and to generate corresponding KEGG pathway maps [23].
2.3. Multilocus Sequence Typing (MLST) Phylogenetic Tree Construction
The autoMLST 2.0 (Automated Multi-Locus Species Tree) tool [24] was applied to the genome of Lactococcus sp. KTH0-1S, along with available Lactococcus type strains. The phylogenetic analysis utilized core housekeeping genes derived from the genomes using default settings with 1000 bootstrap replicates. Streptomyces alboniger NRRL B-1832 was used as the outgroup.
2.4. In Silico Safety Evaluation
The safety profile of Lactococcus sp. KTH0-1S was assessed through in silico analysis. Antibiotic resistance genes (ARGs) were identified using ResFinder v4.1 [25] and the Comprehensive Antibiotic Resistance Database (CARD) (https://card.mcmaster.ca accessed on 30 June 2025), implemented via ABRicate v1.0.1 with default parameters [26]. To explore potential virulence factors, the genome was queried against the Virulence Factor Database (VFDB) (https://www.mgc.ac.cn/VFs/ accessed on 7 July 2025) [27]. Homology comparisons were carried out using BLASTp with an identity cutoff of >80% against known pathogenic virulence genes. Moreover, mobile genetic elements (MGEs) and prophage regions were identified by the MobileOG database (https://mobileogdb.flsi.cloud.vt.edu accessed on 11 September 2025) and PHASTEST v3.0, respectively [28]. In addition, secondary metabolite biosynthetic gene clusters (BGCs) in the Lactococcus sp. KTH0-1S genome were identified using the antiSMASH 8.0 platform [24].
2.5. Cell Factory Potential Genes Analysis for Alternative Expression Host of Lactococcus sp. KTH0-1S
2.5.1. Prediction of Bacteriocin (Nisin Z) Cluster
In this study the potential genes required for protein expression in Lactococcus sp. KTH0-1S, as the alternative cell factory, were identified. The nisin Z cluster genes were analyzed to check the possibility of using Lactococcus sp. KTH0-1S as the expression host for Nisin-Controlled Expression system (NICE), which has high efficiency for homologous and heterologous protein expression in Lactococcus. The Bacteriocin-related genes were predicted using BAGEL4 [29] and confirm the integrity of the nisin biosynthetic cluster identified in the genome sequence, PCR was carried out using primers targeting key genes (nisZ, nisK, nisR and nisI) (Table 1). These genes are particularly important for the functionality of the NICE system and the potential for auto-induction. PCR validation ensured that no sequencing or assembly artifacts occurred and provided a reliable basis for subsequent applications, such as amplification of the genes for construction of new expression plasmids tailored to Lactococcus sp. KTH0-1S. The amplified DNA sequences were then analyzed on 1% agarose gel electrophoresis to determine the presence of the 4 genes based on size.
2.5.2. Respiration Metabolism Verification
Some L. lactis showed the respiration growth which resulted in much higher cell biomass formation and cell robustness [30,31], which is beneficial for cell factory applications. Lactococcus sp. KTH0-1S was analyzed for genes encoding all enzymes involved in the electron transport chain of L. lactis, including NADH dehydrogenase as electron donor, menaquinones as electron carrier, and cytochrome oxidase as the electron acceptor, using annotations based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg/ accessed on 20 October 2024) [32,33,34].
The respiration growth characteristic of Lactococcus sp. KTH0-1S was also verified. Lactococcus sp. KTH0-1S was cultivated in GM17 medium and incubated at 30 °C. The respiration metabolism behavior was investigated under facultative anaerobic (static) and aerobic (200 rpm) conditions, and the overnight cultures were transferred into GM17 medium with or without heme and menaquinone supplementation. The growth (OD600) in all culture conditions was collected after 24 h of incubation. The nisin activity of the cell-free supernatant from each sample was checked against a Lactiplantibacillus plantarum WCFS1 indicator strain, and the inhibition activity was confirmed and calculated as AU/mL using a broth microdilution assay.
2.5.3. Carbohydrate-Active Enzyme Analysis
The genome of Lactococcus sp. KTH0-1S was analyzed for carbohydrate-active enzymes (CAZymes) using the dbCAN2 meta server, with annotations based on the CAZy database [35]. This database classifies CAZymes into six main categories: glycoside hydrolases (GHs), glycosyltransferases (GTs), carbohydrate esterases (CEs), carbohydrate-binding modules (CBMs), auxiliary activity enzymes (AAs), and polysaccharide lyases (PLs).
3. Results
3.1. Overview of the Genome in Lactococcus sp. KTH0-1S
The whole genome sequencing analysis identified the obtained strain KYH-01S as Lactococcus sp. The genome of this strain consists of a chromosome spanning 2,379,149 bp with a GC content of 35.1%, and a plasmid, pKTH0-1S, which is 43,284 bp with a GC content of 33.2%. A total of 2342 coding sequences (CDSs) were identified in the chromosome, while 62 CDSs were found in the plasmid. The RAST annotation system identified 236 and 3, respectively. Moreover, this strain showed the highest sequence similarity to Lactococcus cremoris (Accession number: GCA_004354515.1), with an average nucleotide identity (ANI) of 88.57% (Table 2). The genome visualization of Lactococcus sp. KTH0-1S was illustrated in Figure 1.

Table 2.
Genome features and information of Lactococcus sp. KTH0-1S.
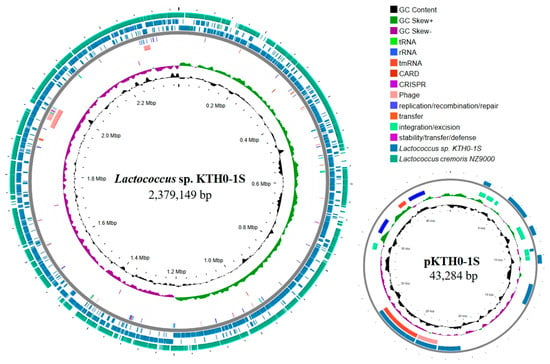
Figure 1.
Circular genome maps of Lactococcus sp. KTH0-1S and its plasmid pKTH0-1S. The outermost circle represents the comparison with Lactococcus cremoris NZ9000, Lactococcus sp. KTH0-1S genome, mobile genetic elements (MGEs), phage, antimicrobial resistance genes (ARGs), CRISPR-Cas, GC skew (positive and negative), and GC content.
3.2. Multilocus Sequence Typing (MLST) Phylogenetic Tree
The MLST phylogenetic tree depicts the genetic relationship of Lactococcus sp. KTH0-1S with other Lactococcus strains using multilocus sequence typing based on conserved housekeeping genes. Although ANI analysis clearly indicates its taxonomic placement, MLST was additionally performed to support strain-level phylogenetic comparison with publicly available references. The resulting tree shows that Lactococcus sp. KTH0-1S occupies a distinct clade, genetically distant from established type strains, reinforcing its divergence within the genus (Figure 2).
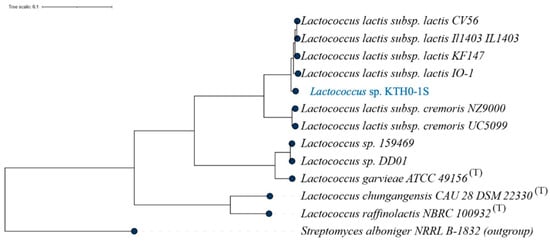
Figure 2.
MLST phylogenetic tree showing the genetic relationship of Lactococcus sp. KTH0-1S with other strains based on multilocus sequence typing analysis.
3.3. Functional Characterization and Prediction
The functional classifications of Lactococcus sp. KTH0-1S were performed based on three distinct categories, including RAST subsystems, COG categories, and KO pathways. The RAST subsystems show the distribution of genomic functions, with a significant emphasis on metabolism, especially in the categories of carbohydrates, amino acids and derivatives, and protein metabolism. Other prominent categories include DNA and RNA metabolism, fatty acids, lipids, and isoprenoids, and respiration. The genetic elements category, particularly involving phages, prophages, transposable elements, and plasmids, also stands out, showing the presence of mobile genetic elements. Furthermore, smaller groups such as stress response, regulation, cell signaling, and cellular processes highlight the mechanisms for managing environmental and internal stressors of the strain (Figure 3A). However, 71% of the genome was unassigned in the RAST subsystem.
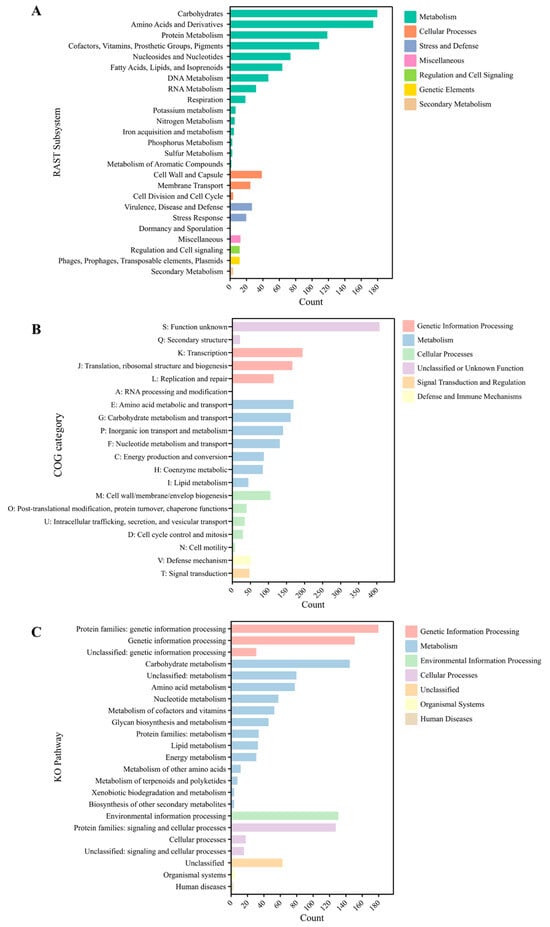
Figure 3.
Functional classification of KTH0-1S genomic features. (A) RAST subsystems, (B) COG categories, and (C) KO pathways.
In terms of COG categories, this analysis categorizes data based on functional families, with the highest counts found in categories related to genetic information processing, such as RNA processing, translation, and transcription. Other significant categories include metabolism, particularly amino acid metabolism and energy production, and cellular processes, such as cell cycle control and intracellular trafficking. Defense mechanisms also appear, reflecting the capacity of this strain for adaptive responses to threats (Figure 3B). Moreover, KO pathways further organize the functional data, where genetic information processing and metabolism dominate the landscape. Particularly notable are pathways related to amino acid metabolism, energy metabolism, and lipid metabolism, indicating key functional roles in cellular biosynthesis and energy management. The environmental information processing and cellular processes categories also appear, while human diseases and organismal systems are represented with fewer counts, highlighting a lesser focus on these functions (Figure 3C).
3.4. Safety Assessment and Secondary Metabolite Biosynthetic Gene Clusters Analysis
The safety of Lactococcus sp. KTH0-1S was evaluated by predicting genes associated with antimicrobial resistance (AMR) and virulence factors. The strain was not found to harbor the ARG in its genome. Moreover, several virulence factor genes were identified in Lactococcus sp. KTH0-1S. The cpsI gene, which is involved in immune modulation, can help the strain modulate the immune system of host, potentially providing protective effects against pathogens. The tufA gene, encoding elongation factor Tu, plays a role in adhesion, which is important for the bacteria to effectively colonize the gut and outcompete harmful microbes. Similarly, htpB, a heat shock protein, aids in adherence, improving the stability and persistence of the strain in the gastrointestinal tract. Furthermore, genes like cps4I, which are involved in the synthesis of capsular polysaccharides, contribute to immune modulation and protect the strain from host defenses. The fbp54 gene, encoding fibronectin-binding protein, also supports adherence to intestinal cells. Genes, such as hasC, involved in the synthesis of N-acetylglucosamine, promote immune modulation, potentially benefiting gut health by supporting the natural immune response of the host (Table 3).

Table 3.
Identification of virulence factor-associated genes found in the Lactococcus sp. KTH0-1S genome.
The antiSMASH analysis revealed multiple biosynthesis gene clusters for secondary metabolite production across seven regions, which are crucial for its antimicrobial properties. Terpene precursor biosynthesis clusters were identified in regions 1, 5, and 6, while region 2 encodes the RiPP Recognition Element (RRE). Region 3 is associated with betalactone synthesis, region 4 with lanthipeptide-class-I production, and region 7 with type III polyketide synthase (T3PK) biosynthesis, as shown in Figure 4.
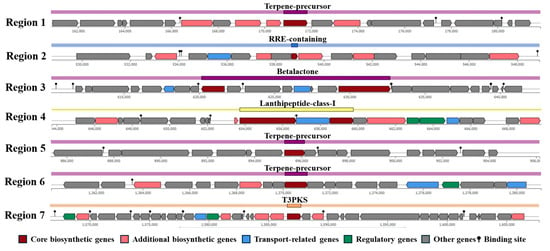
Figure 4.
Identification of secondary metabolites biosynthesis gene clusters in Lactococcus sp. KTH0-1S genome.
3.5. Identification of Mobile Genetic Element (MGE) and Prophage Region
The MGE analysis of Lactococcus sp. KTH0-1S identified 88 genes classified into distinct functional categories. The majority of genes were associated with replication, recombination, and repair (29 genes), followed by integration and excision (22 genes), including integrases and recombinases. Additional categories included phage and transfer-related genes, which found 19 and 12 genes, respectively. A smaller number of genes were linked to stability, transfer, and defense mechanisms (6 genes) (Figure 5). Among these, integration-related genes included integrases (int2, int-Tn), a transposase (tnpA), and a site-specific recombinase (xerS), which are involved in the movement and integration of genetic elements. Phage-associated genes such as clpB, clpP, clpX, dnaK, cro, kilA, xhlB, orf45, tmk, and oppA suggest the presence of prophage elements. Replication and repair functions were represented by core genes including dnaB, dnaH, dnaJ, ftsZ, gyrA, gyrB, mutS, radA, rarA, recA, recR, recU, rnhB, rnj, ruvB, ssb, topA, umuC, uvrA, uvrB, xseA, and xth, which are essential for genome maintenance. Stability and defense-related genes such as ardA, dcm, hsdM, hsdR, rex, and ycbY were also detected, along with transfer-related genes including copR, dut, groL, oppB, oppC, oppD, and oppF, which are involved in metal resistance, DNA metabolism, and transport systems (Table S1). However, several genes were unclassified (unknown) in the MGE category, indicating mobile element-related sequences that could not be confidently assigned to a specific functional group based on current databases. Moreover, two prophage regions were identified in the genome of Lactococcus sp. KTH0-1S, both classified under the Siphoviridae family and marked as non-transposable, representing various phage functions, including structural, replication, lysis, and regulatory proteins (Table S2).
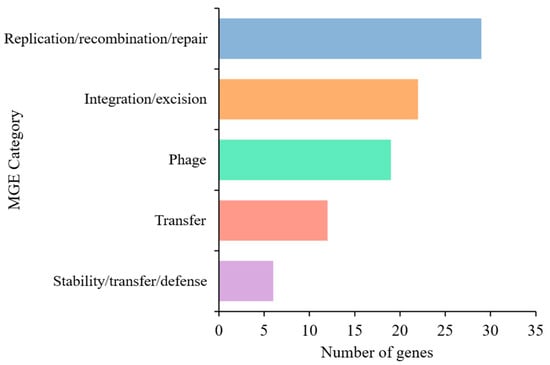
Figure 5.
Distribution of MGE categories identified in the Lactococcus sp. KTH0-1S genome using the mobileOG-db tool.
3.6. Identification of Probiotic Marker Genes
The identification of probiotic genes in the Lactococcus sp. KTH0-1S genome revealed several key functions associated with stress tolerance, adhesion, biofilm formation, and nutrient acquisition. Genes such as dnaK, clpP, groL, and dnaJ are involved in stress response mechanisms, ensuring survival under adverse conditions of the strain. The gapA, pgi, and tpiA genes support energy metabolism and adhesion, both essential for colonization in the gut. While luxS and glf2 genes play roles in biofilm formation, with luxS contributing to quorum sensing and glf2 to exopolysaccharide production. The eno aids in metabolism, supporting biofilm development. Moreover, lepA, bglH, pepT, tuf, padC, gapA, and fusA are involved in nutrient acquisition, enabling the strain to utilize various substrates, enhancing its ability to thrive in diverse environments (Table 4).

Table 4.
Key genes associated with probiotic properties identified in Lactococcus sp. KTH0-1S genome.
3.7. Exopolysaccharide (EPS)-Associated Gene Identification
The Lactococcus sp. KTH-01S genome contains several genes associated with EPS biosynthesis and modification. epsA functions as a transcriptional activator for EPS biosynthesis. epsB is a manganese-dependent protein–tyrosine phosphatase, while epsC and epsD encode tyrosine-protein kinases involved in modulating EPS production. epsE is a galactosephosphotransferase responsible for transferring galactose to undecaprenyl-phosphate, a key step in EPS synthesis. epsF and epsH are involved in glycosyltransferase and acetyltransferase activities, respectively. lytR acts as an antiterminator, regulating the transcription of EPS genes. Genes related to capsular polysaccharide synthesis, including cpsA, cpsB, cpsC, cpsD, cpsH, and cpsE, are involved in sugar transfer, polysaccharide export, and exopolysaccharide synthesis. The glt2 and glt1 encode glycosyltransferases, while licD3 is involved in lipopolysaccharide modification (Table 5).

Table 5.
EPS biosynthesis genes found in Lactococcus sp. KTH-01S genome.
3.8. Identification of Bacteriocin Biosynthetic Clusters
Comprehensive genome analysis of Lactococcus sp. KTH0-1S using the BAGEL4 tool identified multiple gene clusters associated with bacteriocin biosynthesis, including those responsible for the production of lantibiotic-class antimicrobial peptides (Figure 6). Notably, a nisin Z biosynthetic gene cluster was detected (green), encompassing key genes involved in precursor modification, such as lanB and lanC (blue), which mediate dehydration and cyclization reactions essential for nisin maturation.
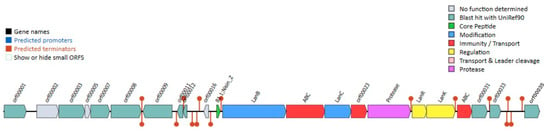
Figure 6.
Identification of bacteriocin biosynthesis gene clusters in Lactococcus sp. KTH0-1S genome.
Regulatory elements (yellow) within the cluster include the two-component system comprising the response regulator (lanR) and the histidine kinase sensor (lanK), which coordinately regulate transcription of the nisin operon in response to environmental stimuli. A gene encoding a nisin leader peptide-processing serine protease (purple) was also identified, facilitating the cleavage of the leader sequence to yield the mature, bioactive peptide.
In addition, genes involved in immunity and export mechanisms were annotated. These include the ABC-type nisin transport ATP-binding protein, the nisin immunity protein (lipopeptide; orf00023), and an ABC transporter-like system (orf00031, orf00033), marked in red (Figure 6). Collectively, these findings highlight the genetic potential of Lactococcus sp. KTH0-1S for the production, regulation, and self-protection against the lantibiotic nisin Z.
PCR amplification was performed to confirm the presence of key nisin-associated genes (nisZ, lanK, lanR, and lanI) in the genome of Lactococcus sp. KTH0-1S. All four genes were successfully amplified, verifying their presence in the chromosomal DNA (Supplementary Figure S1). The confirmed presence of these genes supports the strain’s potential for nisin production and its suitability as a candidate for use in biotechnological applications.
3.9. Respiration Metabolism Verification
Functional annotation of the Lactococcus sp. KTH0-1S genome using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database revealed the presence of genes encoding the core components required for respiration metabolism in L. lactis. Specifically, the strain harbors the ndh gene encoding NADH dehydrogenase, which initiates the electron transport process by transferring electrons from NADH to quinones (Table 6). In addition, a complete set of genes involved in the biosynthesis of menaquinone (vitamin K2), including menA, menB, menC, menD, menE, menF, and menH were identified. Menaquinone functions as a key electron carrier in the electron transport chain (ETC) of Gram-positive bacteria, including lactic acid bacteria [30], linking dehydrogenase activity to terminal oxidases [31].

Table 6.
Relevant genes related with respiration metabolism identified in Lactococcus sp. KTH0-1S genome.
Moreover, genes encoding the cytochrome bd-type oxidase complex (cydA, cydB, cydC, cydD) were also detected (Table 6). This oxidase complex serves as the terminal electron acceptor in the ETC, facilitating oxygen reduction under low-oxygen (microaerobic) conditions. The presence of these genes strongly suggests that Lactococcus sp. KTH0-1S possesses the genetic capacity for respiration metabolism, provided that essential cofactors such as heme and menaquinone are available similar to the respiration-enabled L. lactis strains described previously [30,31].
3.10. Growth Behavior and Respiratory Activation
The growth of Lactococcus sp. KTH0-1S was evaluated under different cultivation conditions to assess its respiratory potential. Under anaerobic conditions, the strain exhibited moderate growth with a final OD600 of 1.257 ± 0.07 and a decrease in pH to 5.02 ± 0.03 (Figure 7). Aerobic conditions alone increased the final OD600 to 1.823 ± 0.02. Supplementation with heme under aerobic conditions resulted in the highest biomass accumulation (OD600 = 2.413 ± 0.07), while the addition of menaquinone (MK-4) along with heme did not lead to a significant further increase (OD600 = 2.408 ± 0.07), indicating that heme was the primary limiting factor for respiratory activation on this strain. Similar trends were observed in the final culture pH, with the highest values recorded in aerobic cultures supplemented with heme or heme + MK-4 were pH = 5.52 ± 0.01 and 5.54 ± 0.2, indicating reduced acidification.
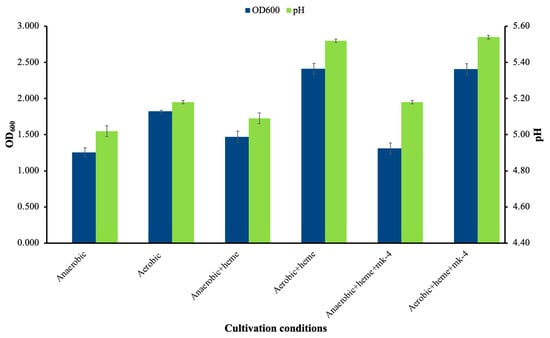
Figure 7.
Cell growth and pH of Lactococcus sp. KTH-01S under different growth conditions.
Nisin activity from Lactococcus sp. KTH0-1S was measured in various cultured conditions. The highest antimicrobial activity was observed in anaerobic + heme (14.00 ± 0.50 mm) and anaerobic (13.50 ± 0.87 mm) conditions (Table 7). Slightly reduced inhibition zones were observed under respiratory conditions, particularly in aerobic + heme + MK-4 (11.00 ± 0.50 mm), but the strain remained active in nisin production across all tested growth modes.

Table 7.
The nisin activity of Lactococcus sp. KTH0-1S from various cultivation conditions.
3.11. Carbohydrate-Active Enzyme (CAZyme) Prediction
CAZyme families were then identified in the Lactococcus sp. KTH-01S genome by mapping to the CAZy database using the dbCAN2 server. The results showed that the glycosyltransferase (GT) family was the most abundant, with families GT2, GT1, GT4, GT51, GT5, GT35, and GT28. Several glycoside hydrolase (GH) families were also present, including GH13, GH31, GH65, GH8, GH43, GH38, GH32, GH20, and GH18. The genome also contained carbohydrate esterase (CE) family CE1, carbohydrate-binding modules (CBM) families CBM50, CBM48, and CBM73, and auxiliary activities (AA) family AA10 (Figure 8).
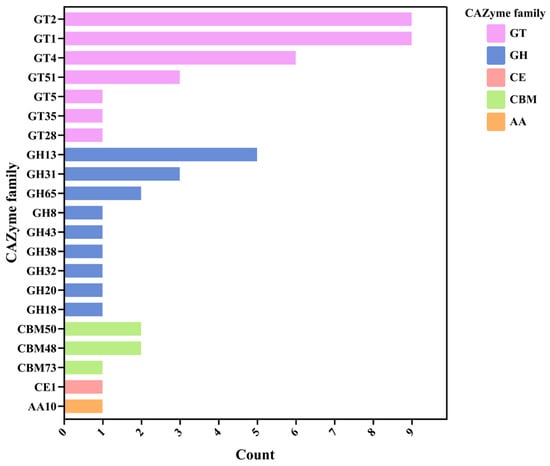
Figure 8.
Identification of CAZyme families in the Lactococcus sp. KTH-01S genome. The bar chart shows the distribution of various CAZyme families, including GT (glycosyltransferase), GH (glycoside hydrolase), CE (carbohydrate esterase), CBM (carbohydrate-binding modules), and AA (auxiliary activities).
4. Discussion
The MLST analysis of Lactococcus sp. KTH0-1S revealed that it is genetically distinct from other reference strains within the Lactococcus genus. The phylogenetic tree showed that Lactococcus sp. KTH0-1S occupies a unique position, which suggests that it represents a novel strain with distinct genetic characteristics compared to other type strains. This genetic uniqueness highlights its potential for novel applications in the probiotic and biotechnological fields.
The genome of Lactococcus sp. KTH0-1S was extensively analyzed to assess its potential as a probiotic, including its antimicrobial properties, safety profile, and ability to perform essential probiotic functions such as adhesion, biofilm formation, and stress tolerance. The functional annotation of Lactococcus sp. KTH0-1S revealed a genomic landscape rich in genes related to core metabolic and cellular processes, suggesting the robust metabolic flexibility and adaptive capabilities of this strain. RAST subsystem classification emphasized a predominant role of metabolism, especially in carbohydrate, amino acid, and protein metabolism, which are essential for energy production, biosynthesis, and survival under varying environmental conditions. The presence of genes involved in DNA and RNA metabolism, fatty acid and lipid biosynthesis, and respiration further underscores the strain’s potential for growth and maintenance in diverse niches. Notably, a substantial proportion of the genome (71%) remained unassigned to known subsystems, implying the existence of many hypothetical or uncharacterized genes that may contribute to unique strain-specific features or probiotic traits not yet captured in reference databases. Compared to the closest strain, L. cremoris (GCA_004354515.1), which exhibited 75% of unassigned CDSs in the RAST subsystem, Lactococcus sp. KTH0-1S showed a similar trend, indicating that a large portion of the genome in both strains may consist of hypothetical or uncharacterized genes, reflecting limited functional annotation and potential strain-specific features. In addition, COG and KO pathway analyses further confirmed dominant roles in genetic information processing, energy metabolism, and cellular processes, supporting the strain’s potential resilience, adaptability, and suitability as a probiotic.
In silico safety analysis was performed to evaluate the presence of antimicrobial resistance (AMR) genes and virulence factors. The results showed that Lactococcus sp. KTH0-1S does not harbor any significant AMR genes, indicating that it poses a minimal risk of transferring resistance to harmful pathogens [36,37,38]. In addition, the strain was found to possess a low number of virulence-associated genes, with no significant genes linked to pathogenicity.
Genes identified in Lactococcus sp. KTH0-1S genome, are increasingly recognized in the context of probiotic function due to their roles in host-microbe interactions. The cpsI and cps4I genes, involved in capsular polysaccharide biosynthesis, may contribute to immune modulation and provide protection against environmental stressors within the gastrointestinal tract, enhancing bacterial persistence [39]. Moreover, the tufA gene, encoding elongation factor Tu, and htpB, a heat shock protein, have been reported to function as surface-associated adhesion factors in non-pathogenic lactic acid bacteria, facilitating colonization of the intestinal mucosa [40]. Similarly, fbp54, encoding a fibronectin-binding protein, may support adhesion to epithelial cells, a key feature for probiotic efficacy [41], while the hasC gene, implicated in the synthesis of N-acetylglucosamine, may also play a role in modulating host immune responses and maintaining mucosal integrity [42]. These genes are annotated and more consistent with attributes beneficial for probiotic activity, including adhesion, immune interaction, and environmental resilience, rather than pathogenicity. This suggests that Lactococcus sp. KTH0-1S is safe for consumption and does not have the genetic makeup that would pose a risk to human health. The identification of 88 MGE-associated genes in Lactococcus sp. KTH0-1S highlights the dynamic nature of its genome and reflects its evolutionary potential. The high proportion of genes related to replication, recombination, and repair suggests that the strain maintains a robust system for preserving genome integrity and accommodating genomic rearrangements. The presence of integrases, transposases, and recombinases within the integration/excision category indicates the potential for site-specific recombination and the mobility of genetic elements, which may facilitate adaptation to changing environments [43]. Furthermore, the detection of numerous phage-related genes, along with two integrated prophage regions, supports the notion that phage interactions have played a role in shaping the genome structure. The prophage elements, particularly those classified under the Siphoviridae family, carry a variety of functional components, including structural proteins, lysis enzymes, and transcriptional regulators, reflecting their potential involvement in lysogenic conversion [44]. The presence of defense- and restriction–modification-related genes, such as hsdR, hsdM, ardA, and dcm, suggests that the strain possesses systems for protecting against foreign DNA, while transfer-associated genes such as copR and oligopeptide transporter components (oppB-F) may contribute to environmental sensing and nutrient uptake [45]. The detection of several unclassified MGE-associated genes further indicates the presence of potentially novel or poorly characterized mobile elements, underscoring the need for continued database expansion and functional validation. The MGE profile of Lactococcus sp. KTH0-1S reflects a flexible and adaptive genome structure that may support its ecological fitness and functional roles in complex environments.
A comprehensive analysis of the Lactococcus sp. KTH0-1S genome revealed several genes linked to key probiotic properties, including stress tolerance, adhesion, biofilm formation, and nutrient acquisition. Stress tolerance is facilitated by genes like dnaK, clpP, groL, and dnaJ [46], which help the strain withstand environmental stresses such as heat, oxidative stress, and low pH [47]. These genes encode chaperone proteins and proteases that assist in maintaining protein stability and promoting protein folding, which are essential for bacterial survival under the harsh conditions encountered in the gastrointestinal tract, particularly in the stomach and small intestine [48]. For adhesion to intestinal cells, genes like gapA, pgi, and tpiA [49], involved in glycolysis and energy production, indirectly support adhesion by providing the metabolic energy needed for this process [49,50]. Adhesion is crucial for probiotics to establish a stable colony in the gut, and these genes contribute to the strain’s ability to bind to intestinal epithelial cells, ensuring that it can adhere to the gut lining and resist being washed out during intestinal transit [51]. Biofilm formation, an important feature for probiotics, is supported by the luxS gene, which is involved in quorum sensing, enabling bacteria to communicate and regulate gene expression in response to population density [52]. This mechanism is essential for biofilm formation, which offers protection from environmental stresses like bile salts and antimicrobial agents [53]. The glf2 gene encodes UDP-galactopyranose mutase, which is involved in the synthesis of polysaccharides for biofilm formation [54], suggesting that Lactococcus sp. KTH0-1S can form biofilms, enhancing its stability and survival in the gastrointestinal tract. Regarding nutrient acquisition and metabolism, genes such as lepA, bglH, pepT, tuf, padC, gapA, and fusA contribute to various aspects of nutrient acquisition, transport, and metabolism. The lepA encodes a divalent copper transporter, essential for maintaining metal ion homeostasis, which is crucial for enzymatic activities and overall cellular function [55]. The bglH is involved in utilizing beta-glucosides, which are abundant in plant-based foods, allowing the strain to thrive in diverse dietary environments [56]. pepT aids in the breakdown of peptides, facilitating nutrient acquisition [56,57], while padC is involved in phenolic acid metabolism, relevant for the strain’s survival on plant-based substrates in the gut [58]. The ability to metabolize phenolic compounds could further enhance the strain’s fitness in the gastrointestinal tract, where such compounds are commonly found in the diet.
The identification of a complete nisin Z biosynthesis gene cluster in Lactococcus sp. KTH0-1S supports its potential not only as a natural producer of antimicrobial peptides but also as a promising alternative host for the Nisin-Controlled Expression (NICE) system, which is widely used in L. lactis for regulated expression of heterologous proteins, relies on a two-component quorum-sensing mechanism involving the nisR and nisK regulatory genes and external supplementation of nisin as an inducer [10]. In Lactococcus sp. KTH0-1S, the native presence of nisZ (structural gene), lanB and lanC (post-translational modification enzymes), nisP (leader peptidase), as well as the regulatory (lanK, lanR) and immunity genes (orf00023, orf00031, orf00033) suggests that this strain can autonomously produce, regulate, and tolerate nisin expression [10]. This auto-induction capability offers significant advantages for biotechnological applications. Unlike the model NICE host L. lactis NZ9000, which requires precise external dosing of purified nisin to initiate gene expression, Lactococcus sp. KTH0-1S can likely initiate expression endogenously as cell density increases and nisin accumulates in the culture medium. This reduces the need for expensive nisin supplementation, simplifies process development, and enables more scalable and reproducible expression workflows. In cost-sensitive industries such as food, feed, and nutraceutical production, auto-induction significantly enhances process economics.
Furthermore, the presence of complete nisin immunity mechanisms in Lactococcus sp. KTH0-1S strengthens its candidacy for recombinant expression. In traditional NICE hosts, high-level nisin exposure can lead to growth inhibition or cell lysis due to the lack of strong resistance elements. In contrast, KTH0-1S encodes the lipoprotein nisI (orf00023) and the ATP-binding cassette (ABC) transporter complex nisFEG (ABC, orf00031, orf00033), which together protect the host from the pore-forming activity of nisin by sequestering it and actively exporting it [59]. This allows KTH0-1S to tolerate higher nisin concentrations, facilitating stronger induction signals and higher target gene expression without compromising cell viability. This feature is especially critical when overexpressing toxic proteins or aiming for high volumetric productivity in industrial fermenters. Another important advantage of KTH0-1S is the chromosomal localization of the nisin biosynthetic operon. Unlike plasmid-based systems, which require antibiotic selection and are subject to genetic instability during prolonged or large-scale cultivation, chromosomally integrated expression cassettes offer greater genetic stability. This makes the strain more suitable for food-grade applications, where the use of antibiotic resistance markers and plasmid maintenance systems is restricted or undesirable.
In addition to its suitability as a production chassis, genome analysis of KTH0-1S revealed a diverse set of carbohydrate-active enzymes (CAZymes), including glycoside hydrolases (GHs), glycosyltransferases (GTs), and carbohydrate esterases (CEs). These enzymes confer the ability to degrade and utilize a wide variety of complex carbohydrates, positioning the strain for flexible growth on diverse carbon sources, including inexpensive agricultural or food industry by-products. For instance, GH families such as α-amylases and glucoamylases enable the hydrolysis of starchy wastes like potato peels and cassava pulp into fermentable sugars [60]. Similarly, the presence of cellulases, xylanases, and acetylxylan esterases allows for partial utilization of lignocellulosic substrates such as wheat straw, rice husks, or sugarcane bagasse provided appropriate pre-treatment is performed to reduce recalcitrance [61].
The combination of metabolic flexibility and robust expression capabilities makes KTH0-1S highly suitable for low-cost, high-density fermentations, which are critical for commercial biomanufacturing. Its ability to grow under both fermentative and respiratory conditions (as shown in earlier results) further enhances its robustness, stress tolerance, and biomass yield, particularly when supplemented with heme. This opens opportunities for aerobic or semi-aerobic fed-batch strategies, which are often preferred in industrial fermenters for improved process control and oxygen availability.
5. Conclusions
Lactococcus sp. KTH0-1S is a promising alternative to conventional L. lactis expression hosts. Its naturally integrated nisin gene cluster provides autonomous induction and immunity; its genomic features support broad carbohydrate metabolism; and its stable chromosomal traits reduce operational complexity. Together, these attributes support the development of Lactococcus sp. KTH0-1S as a next-generation, food-grade cell factory and efficient expression host for sustainable and scalable bioproduction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14101323/s1. Figure S1: PCR amplification result of nisZ, LanK, LanR, and LanI gene from Lactococcus sp. KTH0-1S genomic DNA; Table S1: Mobile genetic elements (MGEs) predicted in the Lactococcus sp. KTH0-1S genome; TableS2: Identification of prophage regions in Lactococcus sp. KTH0-1S genome.
Author Contributions
N.W.: Conceptualization; N.W., P.P., C.K., N.C., K.S., S.K. and H.A.: Data curation; N.W., H.A., C.K., N.C. and K.S.: Formal analysis, Investigation, Methodology; N.W. and S.K.: Project administration; N.W., S.K. and K.S.: Supervision; N.W., N.N., C.K., N.C. and K.S.: Validation, Visualization; N.W., C.K., N.C., K.S., H.A. and N.N.: Roles/Writing—original draft; N.W., P.P., C.K., N.C. and K.S.: Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work (Grant No. RGNS 65-032) was supported by Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (OPS MHESI), Thailand Science Research and Innovation (TSRI).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The genome of Lactococcus sp. KTH0-1S has been submitted to the BioProject in the accession number PRJNA1274712 and the BioSample accession number SAMN48999503.
Acknowledgments
Authors would like to thank Department of Biotechnology, Faculty of Ago-Industry, Kasersart University. Thank you Suppasil Maneerat for kindly provided Lactococcus lactis KTH0-1S.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klein, G.; Pack, A.; Bonaparte, C.; Reuter, G. Taxonomy and Physiology of Probiotic Lactic Acid Bacteria. Int. J. Food Microbiol. 1998, 41, 103–125. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic Acid Bacteria as Functional Starter Cultures for the Food Fermentation Industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Watthanasakphuban, N.; Tani, A.; Benjakul, S.; Maneerat, S. Detection and Preliminary Characterization of a Narrow Spectrum Bacteriocin Produced by Lactobacillus Pentosus K2N7 from Thai Traditional Fermented Shrimp (Kung-Som). Songklanakarin J. Sci. Technol. 2015, 38, 47–55. [Google Scholar]
- Kunji, E.R.S.; Slotboom, D.J.; Poolman, B. Lactococcus Lactis as Host for Overproduction of Functional Membrane Proteins. Biochim. Biophys. Acta Biomembr. 2003, 1610, 97–108. [Google Scholar] [CrossRef] [PubMed]
- García-Fruitós, E. Lactic Acid Bacteria: A Promising Alternative for Recombinant Protein Production. Microb. Cell Fact. 2012, 11, 157. [Google Scholar] [CrossRef]
- Tavares, L.M.; de Jesus, L.C.L.; da Silva, T.F.; Barroso, F.A.L.; Batista, V.L.; Coelho-Rocha, N.D.; Azevedo, V.; Drumond, M.M.; Mancha-Agresti, P. Novel Strategies for Efficient Production and Delivery of Live Biotherapeutics and Biotechnological Uses of Lactococcus Lactis: The Lactic Acid Bacterium Model. Front. Bioeng. Biotechnol. 2020, 8, 1269. [Google Scholar] [CrossRef]
- De Ruyter, P.G.; Kuipers, O.P.; De Vos, W.M. Controlled Gene Expression Systems for Lactococcus Lactis with the Food-Grade Inducer Nisin. Appl. Environ. Microbiol. 1996, 62, 3662–3667. [Google Scholar] [CrossRef]
- Kuipers, O.P.; De Ruyter, P.G.G.A.; Kleerebezem, M.; De Vos, W.M. Controlled Overproduction of Proteins by Lactic Acid Bacteria. Trends Biotechnol. 1997, 15, 135–140. [Google Scholar] [CrossRef]
- Kuipers, O.P.; de Ruyter, P.G.G.A.; Kleerebezem, M.; de Vos, W.M. Quorum Sensing-Controlled Gene Expression in Lactic Acid Bacteria. J. Biotechnol. 1998, 64, 15–21. [Google Scholar] [CrossRef]
- Mierau, I.; Kleerebezem, M. 10 Years of the Nisin-Controlled Gene Expression System (NICE) in Lactococcus Lactis. Appl. Microbiol. Biotechnol. 2005, 68, 705–717. [Google Scholar] [CrossRef]
- Kleerebezem, M.; de Vos, W.M.; Kuipers, O.P. The Lantibiotics Nisin in Subtilin Act as Extracellular Regulators of Their Own Biosynthesis. In Cell-Cell Signalling in Bacteria; Dunny, G., Winans, S.C., Eds.; American Society for Microbiology: Washington, DC, USA, 1998; pp. 159–173. [Google Scholar]
- Plavec, T.V.; Berlec, A. Safety Aspects of Genetically Modified Lactic Acid Bacteria. Microorganisms 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-Generation Probiotics: The Spectrum from Probiotics to Live Biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef]
- Saelao, S.; Maneerat, S.; Thongruck, K.; Watthanasakphuban, N.; Wiriyagulopas, S.; Chobert, J.M.; Haertlé, T. Reduction of Tyramine Accumulation in Thai Fermented Shrimp (Kung-Som) by Nisin Z-Producing Lactococcus Lactis KTH0-1S as Starter Culture. Food Control 2018, 90, 249–258. [Google Scholar] [CrossRef]
- Lane, D.J. Sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackebrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: New York, NY, USA, 1991; pp. 115–175. [Google Scholar]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-Prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality Assessment Tool for Genome Assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-Depth Characterization and Visualization of Bacterial Genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hern̗andez-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. EggNOG-Mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Vera Alvarez, R.; Karamycheva, S.; Makarova, K.S.; Wolf, Y.I.; Landsman, D.; Koonin, E.V. COG Database Update 2024. Nucleic Acids Res. 2025, 53, D356–D363. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. AntiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Florensa, A.F.; Kaas, R.S.; Clausen, P.T.L.C.; Aytan-Aktug, D.; Aarestrup, F.M. ResFinder–an Open Online Resource for Identification of Antimicrobial Resistance Genes in next-Generation Sequencing Data and Prediction of Phenotypes from Genotypes. Microb. Genom. 2022, 8, 000748. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded Curation, Support for Machine Learning, and Resistome Prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A General Classification Scheme for Bacterial Virulence Factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Brown, C.L.; Mullet, J.; Hindi, F.; Stoll, J.E.; Gupta, S.; Choi, M.; Keenum, I.; Vikesland, P.; Pruden, A.; Zhang, L. MobileOG-Db: A Manually Curated Database of Protein Families Mediating the Life Cycle of Bacterial Mobile Genetic Elements. Appl. Environ. Microbiol. 2022, 88, e0099122. [Google Scholar] [CrossRef]
- Van Heel, A.J.; De Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A User-Friendly Web Server to Thoroughly Mine RiPPs and Bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef]
- Watthanasakphuban, N.; Virginia, L.J.; Haltrich, D.; Peterbauer, C. Analysis and Reconstitution of the Menaquinone Biosynthesis Pathway in Lactiplantibacillus Plantarum and Lentilactibacillus Buchneri. Microorganisms 2021, 9, 1476. [Google Scholar] [CrossRef]
- Watthanasakphuban, N.; Srila, P.; Pinmanee, P.; Sompinit, K.; Rattanaporn, K.; Peterbauer, C. Development of High Cell Density Limosilactobacillus Reuteri KUB-AC5 for Cell Factory Using Oxidative Stress Reduction Approach. Microb. Cell Factories 2023, 22, 86. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG Resource for Deciphering the Genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Aoki-Kinoshita, K.F.; Kanehisa, M. Gene Annotation and Pathway Mapping in KEGG. In Comparative Genomics; Humana Press: Totowa, NJ, USA, 2007; pp. 71–91. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Hattori, M.; Aoki-Kinoshita, K.F.; Itoh, M.; Kawashima, S.; Katayama, T.; Araki, M.; Hirakawa, M. From Genomics to Chemical Genomics: New Developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. DbCAN2: A Meta Server for Automated Carbohydrate-Active Enzyme Annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef]
- Tóth, A.G.; Csabai, I.; Judge, M.F.; Maróti, G.; Becsei, Á.; Spisák, S.; Solymosi, N. Mobile Antimicrobial Resistance Genes in Probiotics. Antibiotics 2021, 10, 1287. [Google Scholar] [CrossRef]
- Sylvere, N.; Mustopa, A.Z.; Budiarti, S.; Meilina, L.; Hertati, A.; Handayani, I. Whole-Genome Sequence Analysis and Probiotic Characteristics of Lactococcus lactis subsp. lactis Strain Lac3 Isolated from Traditional Fermented Buffalo Milk (Dadih). J. Genet. Eng. Biotechnol. 2023, 21, 49. [Google Scholar] [CrossRef]
- Bennedsen, M.; Stuer-Lauridsen, B.; Danielsen, M.; Johansen, E. Screening for Antimicrobial Resistance Genes and Virulence Factors via Genome Sequencing. Appl. Environ. Microbiol. 2011, 77, 2785–2787. [Google Scholar] [CrossRef]
- An, J.; Zhang, Y.; Zhao, Z.; Huan, R.; Yi, H.; Wang, H.; Luan, C.; Feng, S.; Huang, H.; Li, S.; et al. Molecular Organization and Functional Analysis of a Novel Plasmid-Borne Cps Gene Cluster from Lactiplantibacillus Plantarum YC41. Microbiol. Spectr. 2023, 11, e0415022. [Google Scholar] [CrossRef]
- García, G.; Soto, J.; Díaz, A.; Barreto, J.; Soto, C.; Pérez, A.B.; Boffill, S.; Cano, R.D.J. Randomized Clinical Trials Demonstrate the Safety Assessment of Alkalihalobacillus Clausii AO1125 for Use as a Probiotic in Humans. Microorganisms 2024, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Muscariello, L.; De Siena, B.; Marasco, R. Lactobacillus Cell Surface Proteins Involved in Interaction with Mucus and Extracellular Matrix Components. Curr. Microbiol. 2020, 77, 3831–3841. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, W.; Tao, W.; Xing, J.; Li, J.; Yang, Y.; Guo, Y. Whole-Genome Sequencing of Lactiplantibacillus Plantarum YY-112 and Investigation of Its Immune-Modulating Abilities In Vivo. Fermentation 2023, 9, 996. [Google Scholar] [CrossRef]
- Shikov, A.E.; Savina, I.A.; Nizhnikov, A.A.; Antonets, K.S. Recombination in Bacterial Genomes: Evolutionary Trends. Toxins 2023, 15, 568. [Google Scholar] [CrossRef] [PubMed]
- Bujak, K.; Decewicz, P.; Rosinska, J.M.; Radlinska, M. Genome Study of a Novel Virulent Phage VB_SspS_KASIA and Mu-like Prophages of Shewanella Sp. M16 Provides Insights into the Genetic Diversity of the Shewanella Virome. Int. J. Mol. Sci. 2021, 22, 11070. [Google Scholar] [CrossRef]
- Rocha, E.P.C.; Bikard, D. Microbial Defenses against Mobile Genetic Elements and Viruses: Who Defends Whom from What? PLoS Biol. 2022, 20, e3001514. [Google Scholar] [CrossRef]
- Lee, M.G.; Kang, M.J.; Cha, S.; Kim, T.R.; Park, Y.S. Acid Tolerance Responses and Their Mechanisms in Lactiplantibacillus Plantarum LM1001. Food Sci. Biotechnol. 2024, 33, 2213–2222. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837. [Google Scholar] [CrossRef]
- Zzaman, S.; Reddy, J.M.; Bastia, D. The DnaK-DnaJ-GrpE Chaperone System Activates Inert Wild Type π Initiator Protein of R6K into a Form Active in Replication Initiation. J. Biol. Chem. 2004, 279, 50886–50894. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Guo, C.; Zhang, H.; Liang, Y.; Cheng, Y.; Qu, H.; Wa, Y.; Zhang, C.; Guan, C.; et al. The Regulation of Simulated Artificial Oro-Gastrointestinal Transit Stress on the Adhesion of Lactobacillus Plantarum S7. Microb. Cell Fact. 2023, 22, 170. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Wang, S.W.; Tao, Y.; Ye, J.Z.; Liang, Y.; Peng, X.X.; Yang, L.F.; Li, H. A Glucose-Mediated Antibiotic Resistance Metabolic Flux from Glycolysis, the Pyruvate Cycle, and Glutamate Metabolism to Purine Metabolism. Front. Microbiol. 2023, 14, 1267729. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Wen, Y.; Chen, S.; Zhang, X.; Zhang, C.; Liu, X. Unraveling the Secrets of Probiotic Adhesion: An Overview of Adhesion-Associated Cell Surface Components, Adhesion Mechanisms, and the Effects of Food Composition. Trends Food Sci. Technol. 2025, 159, 104945. [Google Scholar] [CrossRef]
- Xavier, K.B.; Bassler, B.L. LuxS Quorum Sensing: More than Just a Numbers Game. Curr. Opin. Microbiol. 2003, 6, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, R.; Zhang, J.; Li, P. Overexpression of LuxSPromotes Stress Resistance and Biofilm Formation of Lactobacillus ParaplantarumL-ZS9 by Regulating the Expression of Multiple Genes. Front. Microbiol. 2018, 9, 423652. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, D.; Tang, N.; Rui, X.; Zhang, Q.; Chen, X.; Dong, M.; Li, W. Biosynthesis of Exopolysaccharide and Structural Characterization by Lacticaseibacillus Paracasei ZY-1 Isolated from Tibetan Kefir. Food Chem. Mol. Sci. 2021, 3, 100054. [Google Scholar] [CrossRef]
- Kumari, K.; Rawat, V.; Shadan, A.; Sharma, P.K.; Deb, S.; Singh, R.P. In-Depth Genome and Pan-Genome Analysis of a Metal-Resistant Bacterium Pseudomonas Parafulva OS-1. Front. Microbiol. 2023, 14, 1140249. [Google Scholar] [CrossRef]
- Marasco, R.; Muscariello, L.; Varcamonti, M.; De Felice, M.; Sacco, M. Expression of the BglH Gene of Lactobacillus Plantarum Is Controlled by Carbon Catabolite Repression. J. Bacteriol. 1998, 180, 3400. [Google Scholar] [CrossRef]
- Wegmann, U.; Klein, J.R.; Drumm, I.; Kuipers, O.P.; Henrich, B. Introduction of Peptidase Genes from Lactobacillus Delbrueckii Subsp. Lactis into Lactococcus Lactis and Controlled Expression. Appl. Environ. Microbiol. 1999, 65, 4729–4733. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, P.T.; Gury, J.; Dartois, V.; Nguyen, T.K.C.; Seraut, H.; Barthelmebs, L.; Gervais, P.; Cavin, J.F. Phenolic Acid-Mediated Regulation of the PadC Gene, Encoding the Phenolic Acid Decarboxylase of Bacillus Subtilis. J. Bacteriol. 2008, 190, 3213. [Google Scholar] [CrossRef]
- Oddone, G.M.; Mills, D.A.; Block, D.E. Incorporation of NisI-Mediated Nisin Immunity Improves Vector-Based Nisin-Controlled Gene Expression in Lactic Acid Bacteria. Plasmid 2009, 61, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Singh, R.; Shrivastava, M.; Darjee, S.; Mageshwaran, V.; Phurailtpam, L.; Rohatgi, B. Microbial Production of α-Amylase from Agro-Waste: An Approach towards Biorefinery and Bio-Economy. Energy Nexus 2024, 14, 100293. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The Carbohydrate-Active Enzymes Database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).