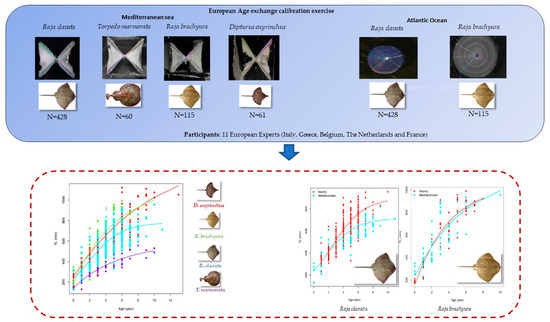
Measurement of the Growth of the Main Commercial Rays (Raja clavata, Raja brachyura, Torpedo marmorata, Dipturus oxyrinchus) in European Waters Using Intercalibration Methods
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Ageing Procedures
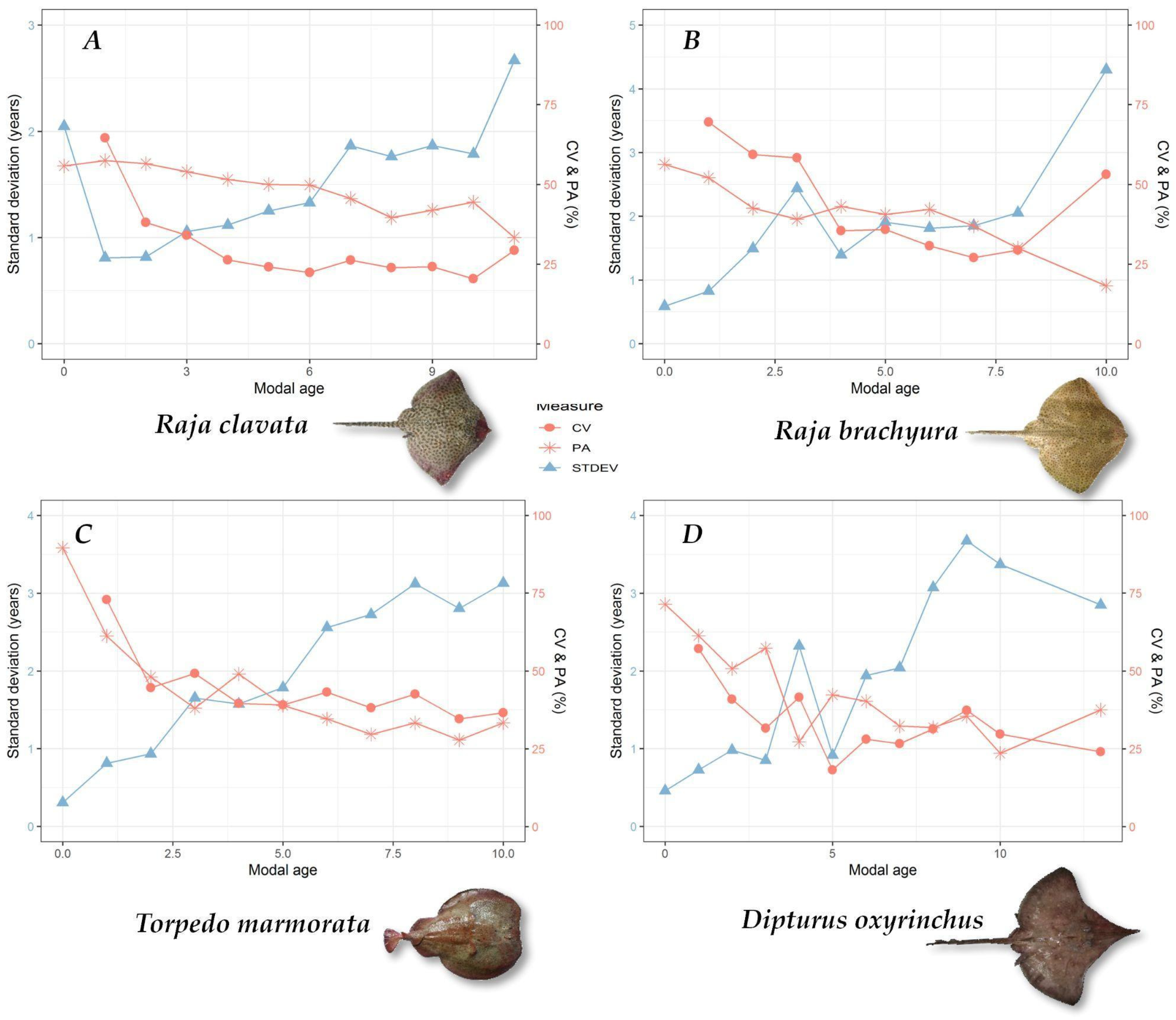
2.3. Ageing Data Precision
2.4. Growth Model Estimation
- the unconstrained von Bertalanffy model [34] (vbp):
- the von Bertalanffy model with forced t0 = 0 (vbt0p):
- the Gompertz model [35] (vbL1p):
- the logistic model [36] (log.p):
2.5. Data Analysis
3. Results
3.1. Sample Composition
3.2. Ageing Precision
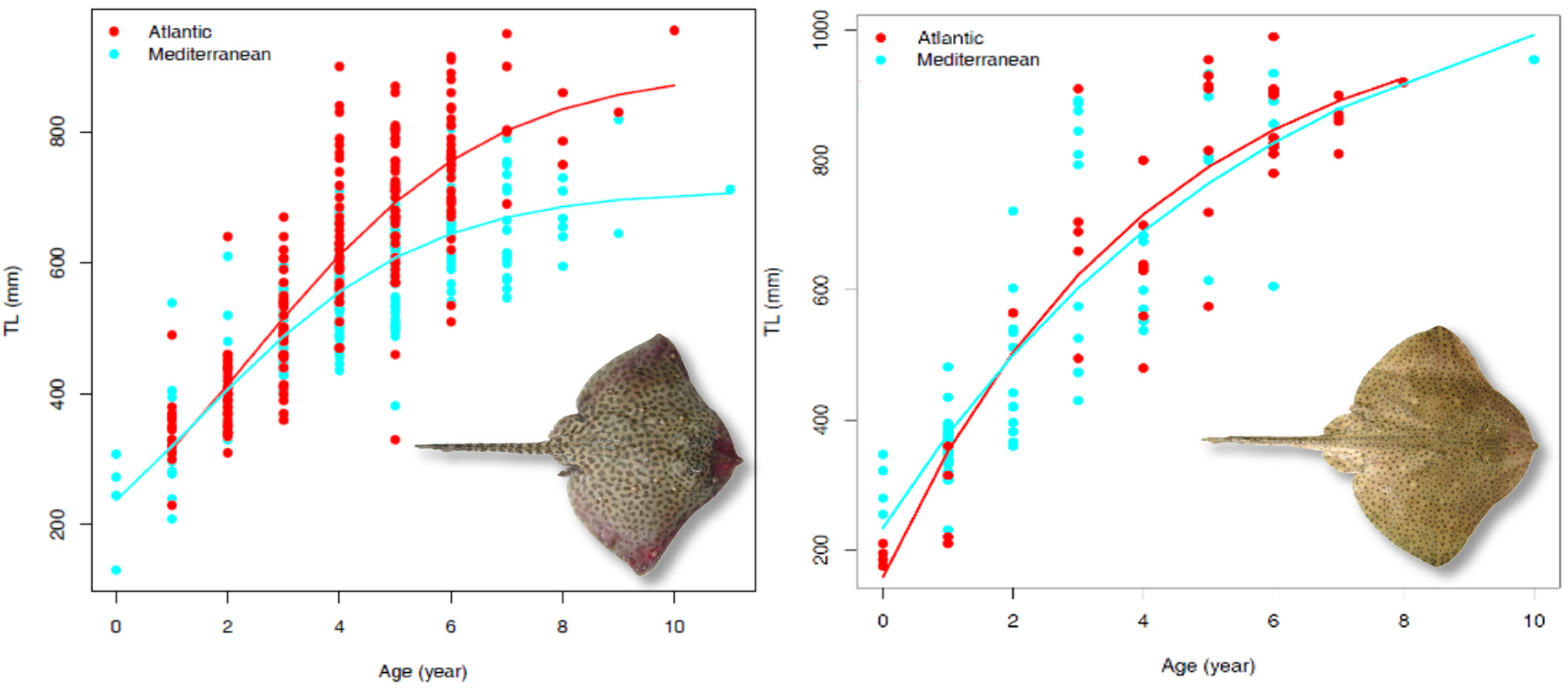
3.3. Growth Parameters
4. Discussion
| Species | Sector | Geographical Area | Sampling | Growth Model | Lifespan (Years tmax) | ϕ | Sources | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | N | TL (mm) Max | Age (Years) Max | Vertebrae Preparation/Ageing Method | Growth Model | TL∞ | k | t0 | TL1 | ||||||
| Raja brachyura | Atlantic Ocean | North-east Atlantic | F + M | 45 | 990 | 8 | whole | vbt0p | 1052.19 | 0.25 | −0.72 | - | 12 | 5.43 | this study |
| Atlantic Ocean | Irish seas | F | 910 | whole | vbp | 1443.00 | 0.19 | −0.31 | 5.60 | [56] | |||||
| M | 777 | 1194.00 | 0.26 | −0.31 | 5.57 | ||||||||||
| Atlantic Ocean | Irish seas | F | 141 | 1080 | sectioned | 1547.00 | 0.13 | −0.84 | 5.49 | [57] | |||||
| M | 127 | 1090 | 1458.00 | 0.15 | −0.93 | 5.49 | |||||||||
| Atlantic Ocean | Irish seas | F | 1120 | tagging | vbt | 1184.00 | 0.19 | −0.80 | 5.43 | [44] | |||||
| M | 1150 | 1150.00 | 0.19 | −0.18 | 5.40 | ||||||||||
| Atlantic Ocean | Portugal waters | F + M | 139 | 1106 | caudal thorns | vbt | 133.50 | 0.12 | 0.29 | 3.33 | [45] | ||||
| Atlantic Ocean | North-east Atlantic | F | 31 | 911 | whole | log | 1020 | 0.24 | - | [29] | |||||
| M | 25 | 876 | vbp | 857 | 0.18 | ||||||||||
| Mediterranean Sea | Sardinian seas | F + M | 60 | 955 | 10 | sectioned | vbt0p | 1166.32 | 0.17 | −1.33 | - | 18 | 5.36 | this study | |
| Mediterranean Sea | Sardinian seas | F + M | 168 | 955 | 16 | sectioned | vbt | 1113.4 | 0.1 | −1.19 | - | 5.09 | [58] | ||
| R. clavata | Atlantic Ocean | North-east Atlantic | F + M | 214 | 955 | 10 | whole | log.p | 897.25 | 0.46 | - | 229.03 | 23 | 5.57 | this study |
| Atlantic Ocean | Irish seas | F | 93 | sectioned | vbt | 1395.00 | 0.09 | −1.74 | 5.26 | [57] | |||||
| M | 165 | 1065.00 | 0.14 | −1.74 | 5.19 | ||||||||||
| Atlantic Ocean | Welsh seas | F | 135 | whole | vbt | 1176.00 | 0.16 | −0.71 | 5.34 | [59] | |||||
| M | 54 | 1009.00 | 0.18 | −0.95 | 5.26 | ||||||||||
| Atlantic Ocean | Portuguese waters | F + M | 251 | 913 | 10 | caudal thorns | vbt | 1280.00 | 0.12 | −0.61 | 5.28 | [46] | |||
| Atlantic Ocean | North-East Atlantic | F | 45 | 906 | whole | log | 831 | 0.354 | - | [29] | |||||
| M | 42 | 785 | vbp | 807 | 0.17 | ||||||||||
| Mediterranean Sea | Central-western Mediterranean | F + M | 212 | 819 | 11 | sectioned | log.p | 713.14 | 0.49 | - | 237.48 | 15 | 5.40 | this study | |
| Mediterranean Sea | South Adriatic Sea | F + M | 291 | 890 | 12 | sectioned | vbt | 986 | 0.18 | −0.95 | 5.24 | [60] | |||
| Mediterranean Sea | Northern Tyrrhenian Sea | F + M | 262 | 800 | sectioned | log.p | 709.2 | 0.55 | 190 | 5.44 | [61] | ||||
| Mediterranean Sea | Central Tyrrhenian Sea | F + M | 118 | 864 | vbt | 929.6 | 0.21 | −0.73 | 5.26 | ||||||
| Mediterranean Sea | Sardinian seas | F + M | 235 | 824 | vbt | 876.1 | 0.14 | −1.79 | 5.03 | ||||||
| Mediterranean Sea | Western Ionian Sea | F + M | 105 | 826 | vbt | 870.50 | 0.19 | −0.88 | 5.16 | ||||||
| Mediterranean Sea | Tunisian seas | F | 160 | 1040 | 15 | sectioned | vbt | 1146.00 | 0.11 | −1.23 | 5.16 | [62] | |||
| M | 125 | 850 | 12 | 1008.00 | 0.14 | −1.13 | 5.15 | ||||||||
| Mediterranean Sea | Strait of Sicily | F | 224 | sectioned | vbt | 1265.00 | 0.10 | −0.51 | 5.20 | [63] | |||||
| M | 200 | 1162.00 | 0.11 | −0.41 | 5.16 | ||||||||||
| Dipturus oxyrinchus | Mediterranean Sea | Sardinian seas | F + M | 61 | 1120 | 13 | sectioned | vbt0p | 1461.87 | 0.10 | −1.90 | 30 | 5.33 | this study | |
| Mediterranean Sea | Sardinian seas | F + M | 130 | 1155 | 17 | sectioned | vbL1p | 1275.5 | 0.14 | 5.36 | [49] | ||||
| Mediterranean Sea | Turkish seas | F + M | 169 | 885 | 9 | sectioned | vbt | 2564.6 | 0.04 | −1.17 | 5.42 | [51] | |||
| Mediterranean Sea | Tunisian seas | F | 175 | 1050 | 25 | sectioned | vbt | 1239 | 0.08 | −1.26 | 38 | 5.09 | [50] | ||
| M | 110 | 950 | 26 | 1021 | 0.12 | −1.18 | 26 | 5.10 | |||||||
| Torpedo marmorata | Mediterranean Sea | Sardinian seas | F + M | 60 | 557 | 10 | sectioned | vbL1p | 581.72 | 0.18 | - | 214.56 | 17 | 4.78 | this study |
| Mediterranean Sea | Sardinian seas | F | 65 | 560 | 17 | sectioned | vbL1p | 622.44 | 0.16 | 23 | 4.78 | [48] | |||
| M | 77 | 432 | 10 | vbt | 485.01 | 0.14 | 15 | 4.52 | |||||||
| Mediterranean Sea | Turkish Sea | F + M | 117 | 560 | 6 | sectioned | vbt | 573.17 | 0.19 | −0.39 | 4.79 | [47] | |||
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.K.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Cortés, E.; Brooks, E.; Gedamke, T. Population dynamics, demography, and stock assessment. In Biology of Sharks and Their Relatives; Carrier, J., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 453–485. [Google Scholar]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.N.K.; Krawchuk, M.A.; Dulvy, N.K. Why have global shark and ray landings declined: Improved management or overfishing? Fish Fish. 2015, 17, 438–458. [Google Scholar] [CrossRef]
- Stevens, J.; Walker, T.; Cook, S.; Fordham, S. Threats faced by chondrichthyan fishes. In Sharks, Rays, Chimaeras: The Status of the Chondrichthyan Fishes; Fowler, S., Cavanagh, R., Camhi, M., Eds.; IUCN Species Survival Commission Shark Specialist Group: Gland, Switzerland; Cambridge, UK, 2005; pp. 48–57. [Google Scholar]
- Clarke, S.C.; McAllister, M.K.; Milner-Gulland, E.J.; Kirkwood, G.P.; Michielsens, C.G.J.; Agnew, D.J.; Pikitch, E.K.; Nakano, H.; Shivji, M.S. Global estimates of shark catches using trade records from commercial markets. Ecol. Lett. 2006, 9, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- White, W.T.; Blaber, S.J.M.; Craig, J.F. The current status of elasmobranchs: Biology, fisheries and conservation. J. Fish Biol. 2012, 80, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Erikstein, K.; D’Offay, B.; Barone, M.; Guggisberg, S. Review of the Implementation of the International Plan of Action for the Conservation and Management of Sharks; FAO Fisheries and Aquaculture Circular No. 1076; FAO: Rome, Italy, 2012; 120p. [Google Scholar]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; 243p. [Google Scholar]
- Carbonara, P.; Follesa, M.C. (Eds.) Handbook on Fish Age Determination: A Mediterranean Experience; FAO Studies and Review N. 98; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/3/ca2745en/ca2745en.pdf?eloutlink=imf2fao (accessed on 20 September 2023).
- Campana, S.E. Age Determination of Elasmobranchs, with Special Reference to Mediterranean Species: A Technical Manual; FAO-GFCM Studies and Reviews No. 94; FAO: Rome, Italy, 2014; p. 38. [Google Scholar]
- Goldman, K.J.; Cailliet, G.M.; Andrews, A.H.; Natanson, L.J. Assessing the age and growth of Chondrichthyan species. In Biology of Sharks and Their Relatives; Carrier, J., Musick, J.A., Heithaus, M., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 423–453. [Google Scholar]
- Beamish, R.J.; McFarlane, G.A. A discussion of the importance of aging errors, and an application to walleye pollock: The world’s largest fishery. In Recent Developments in Fish Otolith Research; Secor, D.H., Dean, J.M., Campana, S.E., Eds.; University of South Carolina Press: Columbia, SC, USA, 1995; pp. 545–565. [Google Scholar]
- ICES. Report of the Workshop on Age Estimation of Atlantic Mackerel (Scomber scombrus) (WKARMAC2), 22–26 October 2018; ICES CM 2018/EOSG:32; ICES: San Sebastian, Spain, 2019; 96p. [Google Scholar] [CrossRef]
- Coggins, L.G.; Gwinn, D.C.; Allen, M.S. Evaluation of age–length key sample sizes required to estimate fish total mortality and growth. Trans. Am. Fish. Soc. 2013, 142, 832–840. [Google Scholar] [CrossRef]
- Smith, B.J.; Dembkowski, D.J.; James, D.A.; Wuellner, M.R. A simple method to reduce interpretation error of ages estimated from otoliths. Open Fish Sci. J. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Hüssy, K.; Radtke, K.; Plikshs, M.; Oeberst, R.; Baranova, T.; Krumme, U.; Sjöberg, R.; Walther, Y.; Mosegaard, H. Challenging ICES age estimation protocols: Lessons learned from the eastern Baltic cod stock. ICES Mar. Sci. 2016, 73, 2138–2149. [Google Scholar] [CrossRef]
- Schindler, D.E.; Geib, S.I.; Williams, M.R. Patterns of fish growth along a residential development gradient in north temperate lakes. Ecosystems 2000, 3, 229–237. [Google Scholar] [CrossRef]
- Carbonara, P.; Ciccolella, A.; De Franco, F.; Palmisano, M.; Bellodi, A.; Lembo, G.; Neglia, C.; Spedicato, M.T.; Zupa, W.; Guidetti, P. Does fish growth respond to fishing restrictions within Marine Protected Areas? A case study of the striped red mullet in the south-west Adriatic Sea (Central Mediterranean). Aquat. Conserv. 2022, 32, 417–429. [Google Scholar] [CrossRef]
- Carbonara, P.; Intini, S.; Kolitari, J.; Joksimović, A.; Milone, N.; Lembo, G.; Casciaro, L.; Bitetto, I.; Zupa, W.; Spedicato, M.T.; et al. A holistic approach to the age validation of Mullus barbatus L., 1758 in the Southern Adriatic Sea (Central Mediterranean). Sci. Rep. 2018, 8, 13219. [Google Scholar] [CrossRef] [PubMed]
- Isely, J.J.; Noble, R.L.; Koppelman, J.B.; Philipp, D.P. Spawning Period and First-Year Growth of Northern, Florida, and Intergrade Stocks of Largemouth Bass. Trans. Am. Fish. Soc. 1987, 116, 757–762. [Google Scholar] [CrossRef]
- Carbonara, P.; Zupa, W.; Anastasopoulou, A.; Bellodi, A.; Bitetto, I.; Charilaou, C.; Chatzispyrou, A.; Ellebode, R.; Esteban, A.; Follesa, M.C.; et al. Explorative analysis on red mullet (Mullus barbatus) ageing data variability in the Mediterranean. Sci. Mar. 2019, 83, 271–279. [Google Scholar] [CrossRef]
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Amelot, M.; Batsleer, J.; Foucher, E.; Girardin, R.; Marchal, P.; Poos, J.J.; Sys, K. Evidence of difference in landings and discards patterns in the English Channel and North Sea Rajidae complex fishery. Fish. Res. 2021, 242, 106028. [Google Scholar] [CrossRef]
- Notarbartolo di Sciara, G.; Serena, F.; Ungaro, N.; Ferretti, F.; Pheeha, S.; Human, B.; McCully, S.; Buscher, E. Torpedo marmorata (Europe assessment). IUCN Red List Threat. Species 2015, e.T161328A48956090. [Google Scholar]
- Ellis, J.; Abella, A.; Serena, F.; Stehmann, M.F.W.; Walls, R. Dipturus oxyrinchus. IUCN Red List Threat. Species 2015, e.T63100A48908629. [Google Scholar] [CrossRef]
- Neer, J.A.; Thompson, B.A. Life history of the Cownose ray, Rhinoptera bonasus, in the northern Gulf of Mexico, with comments on geographic variability in life history traits. Environ. Biol. Fish. 2005, 73, 321–331. [Google Scholar] [CrossRef]
- Thys, K.; Lemey, L.; Van Bogaert, N. Blondes do it better? A comparative study on the morphometry and life-history traits of commercially important skates blonde ray Raja brachyura, thornback ray Raja clavata, and spotted ray Raja montagui, with management implications. Fish. Res. 2023, 263, 106679. [Google Scholar] [CrossRef]
- Chilton, D.E.; Beamish, R.J. Age Determination Methods for Fishes Studied by the Groundfish Program at the Pacific Biological Station; Canadian Special Publication of Fisheries and Aquatic Sciences 60; Department of Fisheries and Oceans: Ottawa, ON, Canada, 1982; 102p. [Google Scholar]
- Panfili, J.; Troadec, H.; Pontual, H.D.; Wright, P.J. Manual of Fish Sclerochronology; Ifremer-IRD Coedition: Brest, France, 2002. [Google Scholar]
- Vitale, F.; Worsøe Clausen, L.; Ní Chonchúir, G. Handbook of Fish Age Estimation Protocols and Validation Methods; ICES Cooperative Research Reports (CRR) No. 346; ICES: Copenhagen, Denmark, 2019; 180p. [Google Scholar] [CrossRef]
- Beamish, R.J.; Fournier, D.A. A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 1981, 38, 982–983. [Google Scholar] [CrossRef]
- von Bertalanffy, L. A quantitative theory of organic growth (inquires of growth laws II). Hum. Biol. 1938, 10, 181–183. [Google Scholar]
- Winsor, C. The Gompertz curve as a growth equation. Proc. Natl. Acad. Sci. USA 1938, 18, 1–8. [Google Scholar] [CrossRef]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–301. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Sakamo, Y.; Ishiguro, M.; Kitagawa, G. Akaike Information Criterion Statistics; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Pauly, D. Gill Size and Temperature as Governing Factors in Fish Growth: A Generalization of von Bertalanffy’s Growth Formula; University of Kiel and Institut für Meereskunde: Kiel, Germany, 1979. [Google Scholar]
- Pauly, D.; Munro, J.L. Once more on the comparison of growth in fish and invertebrates. Fishbyte 1984, 2, 1–21. [Google Scholar]
- Sparre, P.; Ursin, E.; Venema, S.C. Introduction to Tropical Fish Stock Assessment. Part 1: Manual; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1987. [Google Scholar]
- Froese, R.; Binohlan, C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Chen, Y.; Jackson, D.A.; Harvey, H.H. A comparison of von bertalanffy and polynomial functions in modelling fish growth data. Can. J. Fish. Aquat. Sci. 1992, 49, 1228–1235. [Google Scholar] [CrossRef]
- Holden, M.J. The Growth Rates of Raja brachyura, R. clavata and R. montagui as determined from tagging data. ICES J. Mar. Sci. 1972, 34, 161–168. [Google Scholar] [CrossRef]
- Serra-Pereira, B.; Figueiredo, I.; Bordalo-Machado, P.; Farias, I.; Moura, T.; Gordo, L.S. Description of Portuguese Mixed-Fisheries with Positive Landings of Raja brachyura Lafont, 1873 and Raja montagui Fowler, 1910; ICES CM 2005/N:18—Elasmobranch Fisheries Science; ICES: Copenhagen, Denmark, 2005; Volume 18, pp. 1–10. [Google Scholar]
- Serra-Pereira, B.; Figueiredo, I.; Farias, I.; Moura, T.; Gordo, L.S. Description of dermal denticles from the caudal region of Raja clavata and their use for the estimation of age and growth. ICES J. Mar. Sci. 2008, 65, 1701–1709. [Google Scholar] [CrossRef]
- Duman, Ö.V.; Başusta, N. Age and growth characteristics of the Marbled Electric ray Torpedo marmorata (Risso, 1810) inhabiting Iskenderun Bay, North-eastern Mediterranean Sea. Turk. J. Fish. Aquat. Sci. 2013, 13, 541–549. [Google Scholar] [CrossRef]
- Bellodi, A.; Mulas, A.; Carbonara, P.; Cau, A.; Cuccu, D.; Marongiu, M.F.; Mura, V.; Pesci, P.; Zupa, W.; Porcu, C.; et al. New insights into life–history traits of Mediterranean Electric rays (Torpediniformes: Torpedinidae) as a contribution to their conservation. Zoology 2021, 146, 125922. [Google Scholar] [CrossRef] [PubMed]
- Bellodi, A.; Porcu, C.; Cannas, R.; Cau, A.; Marongiu, M.F.; Mulas, A.; Vittori, S.; Follesa, M.C. Life-history traits of the long-nosed skate Dipturus oxyrinchus. J. Fish Biol. 2017, 90, 867–888. [Google Scholar] [CrossRef] [PubMed]
- Kadri, H.; Marouani, S.; Bradai, M.N.; Bouaïn, A.; Morize, E. Age, growth, longevity, mortality and reproductive biology of Dipturus oxyrinchus, (Chondrichthyes: Rajidae) off the Gulf of Gabès (Southern Tunisia, central Mediterranean). J. Mar. Biol. Assoc. United Kingd. 2014, 95, 569–577. [Google Scholar] [CrossRef]
- Yigin, C.; Ismen, A. Age, growth, reproduction and feed of long-nosed skate, Dipturus oxyrinchus (Linnaeus 1758) in Saros Bay, the north Aegean Sea. J. Appl. Ichthyol. 2010, 26, 913–919. [Google Scholar] [CrossRef]
- Mulas, A.; Bellodi, A.; Cannas, R.; Carbonara, P.; Cau, A.; Marongiu, M.F.; Pesci, P.; Porcu, C.; Follesa, M.C. Resource partitioning among sympatric elasmobranchs in the central-western Mediterranean continental shelf. Mar. Biol. 2019, 166, 153. [Google Scholar] [CrossRef]
- Girard, M.; Du Buit, M.H. Reproductive biology of two deep-water sharks from the British Isles, Centroscymnus coelolepis and Centrophorus squamosus. J. Mar. Biol. Assoc. United Kingd. 1999, 79, 923–931. [Google Scholar] [CrossRef]
- Aranha, A.; Menezes, G.; Pinho, M.R. Biological aspects of the velvet belly, Etmopterus spinax (Linnaeus 1758) off the Azores, north East Atlantic. Mar. Biol. Res. 2009, 5, 257–267. [Google Scholar] [CrossRef]
- Chevolot, M.; Hoarau, G.; Rijnsdorp, A.D.; Stam, W.T.; Olsen, J.L. Phylogeography and population structure of thornback rays (Raja clavata L., Rajidae). Mol. Ecol. 2006, 15, 3693–3705. [Google Scholar] [CrossRef]
- Fahy, E. The south eastern ray Raja spp. fishery, with observations on the growth of rays in Irish waters and their commercial grading. In Irish Fisheries Investigation Series B: Marine; The Stationery Office: Dublin, Ireland, 1991; pp. 3–18. [Google Scholar]
- Gallagher, M.J.; Nolan, C.P.; Jeal, F. Age, growth and maturity of the commercial ray species from the Irish sea. J. Northwest Atl. Fish. Sci. 2005, 35, 47–66. [Google Scholar] [CrossRef]
- Porcu, C.; Bellodi, A.; Cannas, R.; Marongiu, M.F.; Mulas, A.; Follesa, M.C. Life-history traits of a commercial ray, Raja brachyura from the central western Mediterranean Sea. Medit. Mar. Sci. 2015, 16, 90–102. [Google Scholar] [CrossRef]
- Whittamore, J.; McCarthy, I. The population biology of the thornback ray, Raja clavata in caernarfon bay, north wales. J. Mar. Biol. Assoc. United Kingd. 2005, 85, 1089–1094. [Google Scholar] [CrossRef]
- Carbonara, P.; Bellodi, A.; Palmisano, M.; Mulas, A.; Porcu, C.; Zupa, W.; Donnaloia, M.; Carlucci, R.; Sion, L.; Follesa, M.C. Growth and Age Validation of the Thornback Ray (Raja clavata Linnaeus, 1758) in the South Adriatic Sea (Central Mediterranean). Front. Mar. Sci. 2020, 7, 586094. [Google Scholar] [CrossRef]
- Bellodi, A.; Massaro, A.; Zupa, W.; Donnaloia, M.; Follesa, M.C.; Ligas, A.; Mulas, A.; Palmisano, M.; Carbonara, P. Assessing thornback ray growth pattern in different areas of Western-Central Mediterranean Sea through a Multi-Model Inference analysis. J. Sea Res. 2022, 179, 102141. [Google Scholar] [CrossRef]
- Kadri, H.; Marouani, S.; Saïdi, B.; Bradai, M.N.; Bouaïn, A.; Morize, E. Age, growth, sexual maturity and reproduction of the thornback ray, Raja clavata (L.), of the Gulf of Gabès (south-central Mediterranean Sea). Mar. Biol. Res. 2014, 10, 416–425. [Google Scholar] [CrossRef]
- Cannizzaro, L.; Garofalo, G.; Levi, D.; Rizzo, P.; Gancitano, S. Raja clavata nel canale di sicilia: Crescita, distribuzione e abbondanza. Biol. Mar. Medit. 1995, 2, 257–262. [Google Scholar]


| Species | Sex | N | TL (mm) | Age (Year) | ||
|---|---|---|---|---|---|---|
| Mean ± SE | Range | Mean ± SE | Range | |||
| Dipturus oxyrinchus | F + M | 61 | 697.2 ± 33.5 | 220–1120 | 5 ± 1.28 | 0–13 |
| F | 41 | 714.6 ± 44.8 | 220–1120 | 5 ± 1.71 | 0–13 | |
| M | 20 | 661.5 ± 43.4 | 245–963 | 4 ± 1.34 | 0–9 | |
| Raja brachyura | F + M | 115 | 585.2 ± 22.6 | 70–990 | 3 ± 0.46 | 0–10 |
| F | 54 | 574.9 ± 21.8 | 175–990 | 3 ± 0.68 | 0–8 | |
| M | 61 | 594.3 ± 28.7 | 70–955 | 3 ± 0.64 | 0–10 | |
| R. clavata | F + M | 428 | 574.6 ± 7.18 | 131–955 | 4 ± 0.14 | 0–11 |
| F | 224 | 587.0 ± 10.7 | 131–955 | 4 ± 0.27 | 0–11 | |
| M | 204 | 561.0 ± 9.29 | 209–900 | 4 ± 0.21 | 0–9 | |
| Torpedo marmorata | F + M | 60 | 321.7 ± 14.9 | 127–557 | 4 ± 0.90 | 0–10 |
| F | 29 | 368.8 ± 23.8 | 138–557 | 5 ± 1.67 | 0–10 | |
| M | 31 | 277.7 ± 14.5 | 127–432 | 2 ± 0.54 | 0–6 | |
| Species | N Vertebrae | Preparation Method | N Readers | Ageing Precision Results | |||
|---|---|---|---|---|---|---|---|
| Whole | Sectioned | CV | PA | APE | |||
| Raja brachyura | 115 | 45 | 70 | 11 | 49% | 44% | 37% |
| R. clavata | 428 | 215 | 213 | 11 | 30% | 52% | 21% |
| Dipturus oxyrinchus | 61 | - | 61 | 9 | 34% | 45% | 26% |
| Torpedo marmorata | 60 | - | 60 | 9 | 47% | 49% | 33% |
| Species | Area | Sex | Sampling | Growth | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | TL Max | TL Min | Age Min | Age Max | Growth Model | TL∞ (mm) | k | t0 | TL1 | Lifespan (tmax) | φ | |||
| Raja brachyura | Atlantic Ocean | F + M | 45 | 990 | 191.25 | 0 | 8 | vbt0p | 1052.196 | 0.245 | −0.664 | 12 | 5.433 | |
| F | 24 | 910 | 220 | 1 | 7 | vbp | 911.278 | 0.397 | 0.000 | 8 | 5.519 | |||
| M | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Mediterranean Sea | F + M | 60 | 955 | 301 | 0 | 10 | vbt0p | 1166.318 | 0.168 | −1.331 | 18 | 5.360 | ||
| F | 37 | 955 | 347 | 0 | 10 | vbp | 885.650 | 0.429 | 0.000 | 7 | 5.527 | |||
| M | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Dipturus oxyrinchus | Mediterranean Sea | F + M | 61 | 1120 | 252.5 | 0 | 13 | vbt0p | 1461.872 | 0.101 | −1.904 | 30 | 5.335 | |
| R. clavata | Atlantic Ocean | F + M | 214 | 955 | 339.444 | 1 | 10 | log.p | 897.251 | 0.458 | 229.03 | 7 | 5.567 | |
| F | 126 | 955 | 341.25 | 1 | 10 | vbt0p | 1267.266 | 0.132 | −1.127 | 23 | 5.325 | |||
| M | 88 | 900 | 335.833 | 1 | 9 | vbt0p | 901.265 | 0.231 | −0.701 | 13 | 5.273 | |||
| Mediterranean Sea | F + M | 212 | 819 | 239.25 | 0 | 11 | log.p | 713.144 | 0.490 | 237.49 | 6 | 5.396 | ||
| F | 97 | 819 | 188 | 0 | 11 | vbt0p | 858.364 | 0.196 | −1.250 | 15 | 5.160 | |||
| M | 115 | 790 | 290.5 | 0 | 9 | log.p | 674.832 | 0.537 | 244.40 | 6 | 5.388 | |||
| Torpedo marmorata | Mediterranean Sea | F + M | 60 | 557 | 142.88 | 0 | 10 | vbL1p | 581.715 | 0.175 | 214.56 | 17 | 4.772 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellodi, A.; Carbonara, P.; MacKenzie, K.M.; Agus, B.; Bekaert, K.; Greenway, E.S.I.; Follesa, M.C.; Madia, M.; Massaro, A.; Palmisano, M.; et al. Measurement of the Growth of the Main Commercial Rays (Raja clavata, Raja brachyura, Torpedo marmorata, Dipturus oxyrinchus) in European Waters Using Intercalibration Methods. Biology 2024, 13, 20. https://doi.org/10.3390/biology13010020
Bellodi A, Carbonara P, MacKenzie KM, Agus B, Bekaert K, Greenway ESI, Follesa MC, Madia M, Massaro A, Palmisano M, et al. Measurement of the Growth of the Main Commercial Rays (Raja clavata, Raja brachyura, Torpedo marmorata, Dipturus oxyrinchus) in European Waters Using Intercalibration Methods. Biology. 2024; 13(1):20. https://doi.org/10.3390/biology13010020
Chicago/Turabian StyleBellodi, Andrea, Pierluigi Carbonara, Kirsteen M. MacKenzie, Blondine Agus, Karen Bekaert, Eleanor S. I. Greenway, Maria C. Follesa, Manfredi Madia, Andrea Massaro, Michele Palmisano, and et al. 2024. "Measurement of the Growth of the Main Commercial Rays (Raja clavata, Raja brachyura, Torpedo marmorata, Dipturus oxyrinchus) in European Waters Using Intercalibration Methods" Biology 13, no. 1: 20. https://doi.org/10.3390/biology13010020
APA StyleBellodi, A., Carbonara, P., MacKenzie, K. M., Agus, B., Bekaert, K., Greenway, E. S. I., Follesa, M. C., Madia, M., Massaro, A., Palmisano, M., Romano, C., Sinopoli, M., Ferragut-Perello, F., & Mahé, K. (2024). Measurement of the Growth of the Main Commercial Rays (Raja clavata, Raja brachyura, Torpedo marmorata, Dipturus oxyrinchus) in European Waters Using Intercalibration Methods. Biology, 13(1), 20. https://doi.org/10.3390/biology13010020