Biomaterials in Traumatic Brain Injury: Perspectives and Challenges
Abstract
Simple Summary
Abstract
1. Introduction
2. Biomaterials in Neurological Disorders
3. Biomaterials and Their Mechanisms of Action in TBI
3.1. Biomaterials Utilized in TBI Therapy
3.1.1. Hydrogels
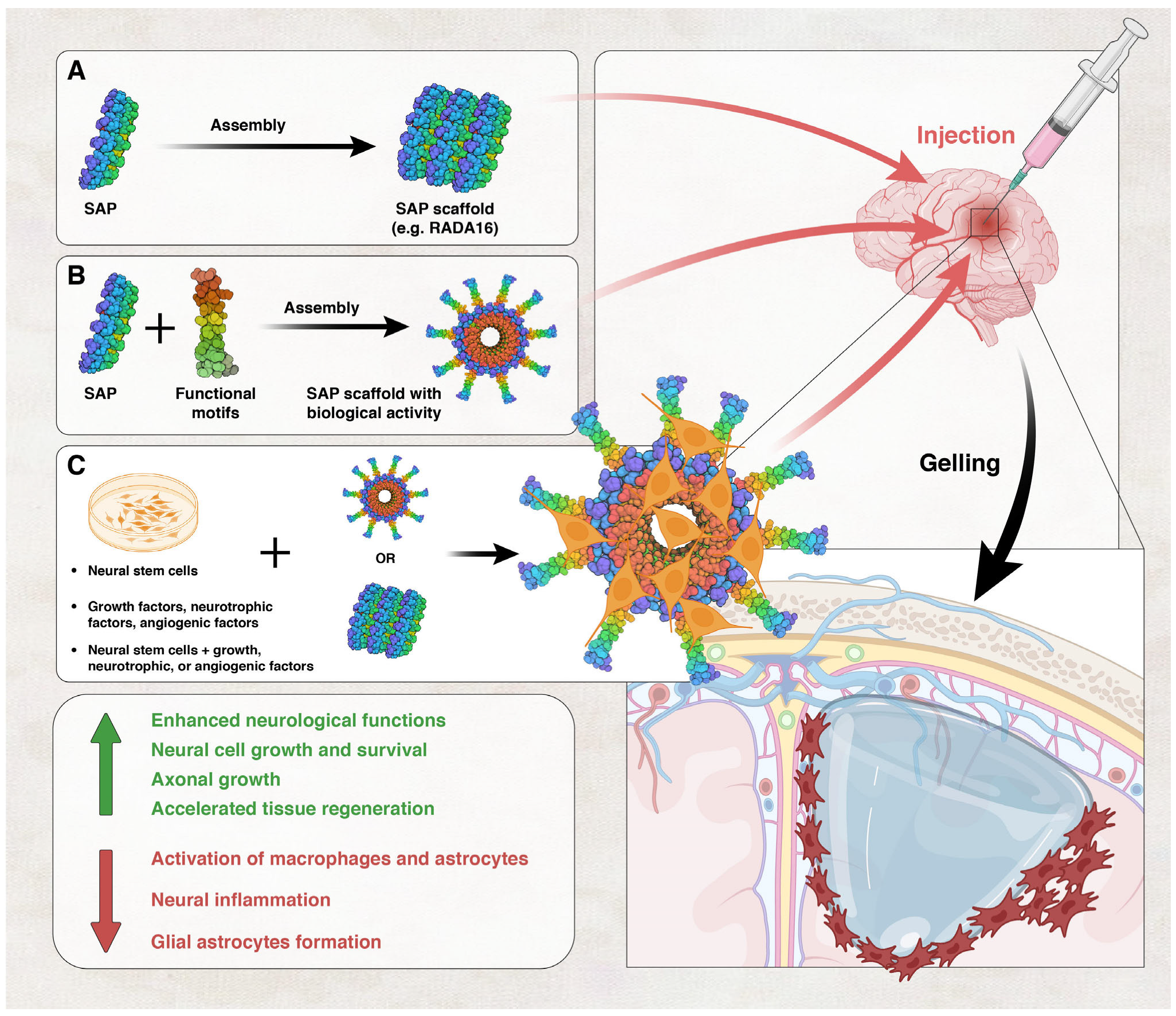
Self-Assembling Peptides
3.1.2. Electrospun Nanofibers
3.2. Mechanisms of Repair by Biomaterials in TBI
3.3. Complications, Limitations and Recommendations
| Biomaterial | Characteristic | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Natural hydrogels | Cross-linked macromolecular networks | -No mechanical/spatial restrictions compared to synthetic polymer scaffolds -Mesh size and porosity of hydrogels can be modified -Biocompatible -Injectable -Porous | -Heterogeneity between batches -May carry natural pathogens -Difficulty in precise modification of the material | [119,146] |
| Synthetic hydrogels | Can be modified according to need | -Biologically inert -Chemically stable -Easier to control important perimeters | -Premade, require invasive implantation surgery -Cause more inflammatory response than natural hydrogels | [223,224,225] |
| Self-assembling peptides SAPNs | Composed of repeating units of amino acids and characterized by the formation of double-β-sheet structures | -High porosity -Increased cell signaling from bioactive peptides that are present in high density at the damaged site -Highly biocompatible -Allow minimally invasive treatments | -Lack of understanding of their degradability -Lack of data on long-term electroactivity of the scaffold | [182,226,227] |
| Electrospun nanofibers | A nonwoven mat of micro- and nanofibers is created when fluid filament is stretched in a powerful electric field | -Aligned nanofibers can resemble the topographical characteristics of the extracellular matrix in the brain -Due to large surface-to-volume ratio, electrospun fibers improve cell adhesion, mass transfer characteristics, and drug loading | -pH difference, local enzymes may degrade the fibers | [150,228] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Peeters, W.; van den Brande, R.; Polinder, S.; Brazinova, A.; Steyerberg, E.W.; Lingsma, H.F.; Maas, A.I. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015, 157, 1683–1696. [Google Scholar] [CrossRef]
- Phillips, S.; Woessner, D. Sports-related traumatic brain injury. Prim. Care Clin. Off. Pract. 2015, 42, 243–248. [Google Scholar] [CrossRef]
- Wojcik, B.E.; Stein, C.R.; Bagg, K.; Humphrey, R.J.; Orosco, J. Traumatic brain injury hospitalizations of US army soldiers deployed to Afghanistan and Iraq. Am. J. Prev. Med. 2010, 38, S108–S116. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Mahon, S.; Hume, P.; Starkey, N.; Barker-Collo, S.; Jones, K.; Majdan, M.; Feigin, V.L. Incidence of sports-related traumatic brain injury of all severities: A systematic review. Neuroepidemiology 2020, 54, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.E.; Berry, J.G.; Jamieson, L.M. Head and traumatic brain injuries among Australian youth and young adults, July 2000–June 2006. Brain Inj. 2012, 26, 996–1004. [Google Scholar] [CrossRef]
- Peplow, P.V.; Martinez, B.; Gennarelli, T.A. Prevalence, needs, strategies, and risk factors for neurodegenerative diseases. Neurodegener. Dis. Biomark. Towards Transl. Res. Clin. Pract. 2022, 2022, 3–8. [Google Scholar]
- Norup, A.; Kruse, M.; Soendergaard, P.L.; Rasmussen, K.W.; Biering-Sørensen, F. Socioeconomic consequences of traumatic brain injury: A danish nationwide register-based study. J. Neurotrauma 2020, 37, 2694–2702. [Google Scholar] [CrossRef]
- Howlett, J.R.; Nelson, L.D.; Stein, M.B. Mental Health Consequences of Traumatic Brain Injury. Biol. Psychiatry 2022, 91, 413–420. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Blennow, K.; Ikonomovic, M.D.; Gandy, S. Acute and chronic traumatic encephalopathies: Pathogenesis and biomarkers. Nat. Rev. Neurol. 2013, 9, 192–200. [Google Scholar] [CrossRef]
- Crane, P.K.; Gibbons, L.E.; Dams-O’Connor, K.; Trittschuh, E.; Leverenz, J.B.; Keene, C.D.; Sonnen, J.; Montine, T.J.; Bennett, D.A.; Leurgans, S.; et al. Association of Traumatic Brain Injury with Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol. 2016, 73, 1062–1069. [Google Scholar] [CrossRef]
- Vespa, P.M. Hormonal dysfunction in neurocritical patients. Curr. Opin. Crit. Care 2013, 19, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Foreman, B.; Lee, H.; Mizrahi, M.A.; Hartings, J.A.; Ngwenya, L.B.; Privitera, M.; Tortella, F.C.; Zhang, N.; Kramer, J.H. Seizures and Cognitive Outcome After Traumatic Brain Injury: A Post Hoc Analysis. Neurocritical Care 2022, 36, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Monsour, M.; Ebedes, D.; Borlongan, C.V. A review of the pathology and treatment of TBI and PTSD. Exp. Neurol. 2022, 351, 114009. [Google Scholar] [CrossRef]
- Alouani, A.T.; Elfouly, T. Traumatic Brain Injury (TBI) Detection: Past, Present, and Future. Biomedicines 2022, 10, 2472. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Haidar, M.A.; Shakkour, Z.; Barsa, C.; Tabet, M.; Mekhjian, S.; Darwish, H.; Goli, M.; Shear, D.; Pandya, J.D.; Mechref, Y. Mitoquinone Helps Combat the Neurological, Cognitive, and Molecular Consequences of Open Head Traumatic Brain Injury at Chronic Time Point. Biomedicines 2022, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention; National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2003. [Google Scholar]
- Dikmen, S.; Machamer, J.; Fann, J.R.; Temkin, N.R. Rates of symptom reporting following traumatic brain injury. J. Int. Neuropsychol. Soc. JINS 2010, 16, 401–411. [Google Scholar] [CrossRef]
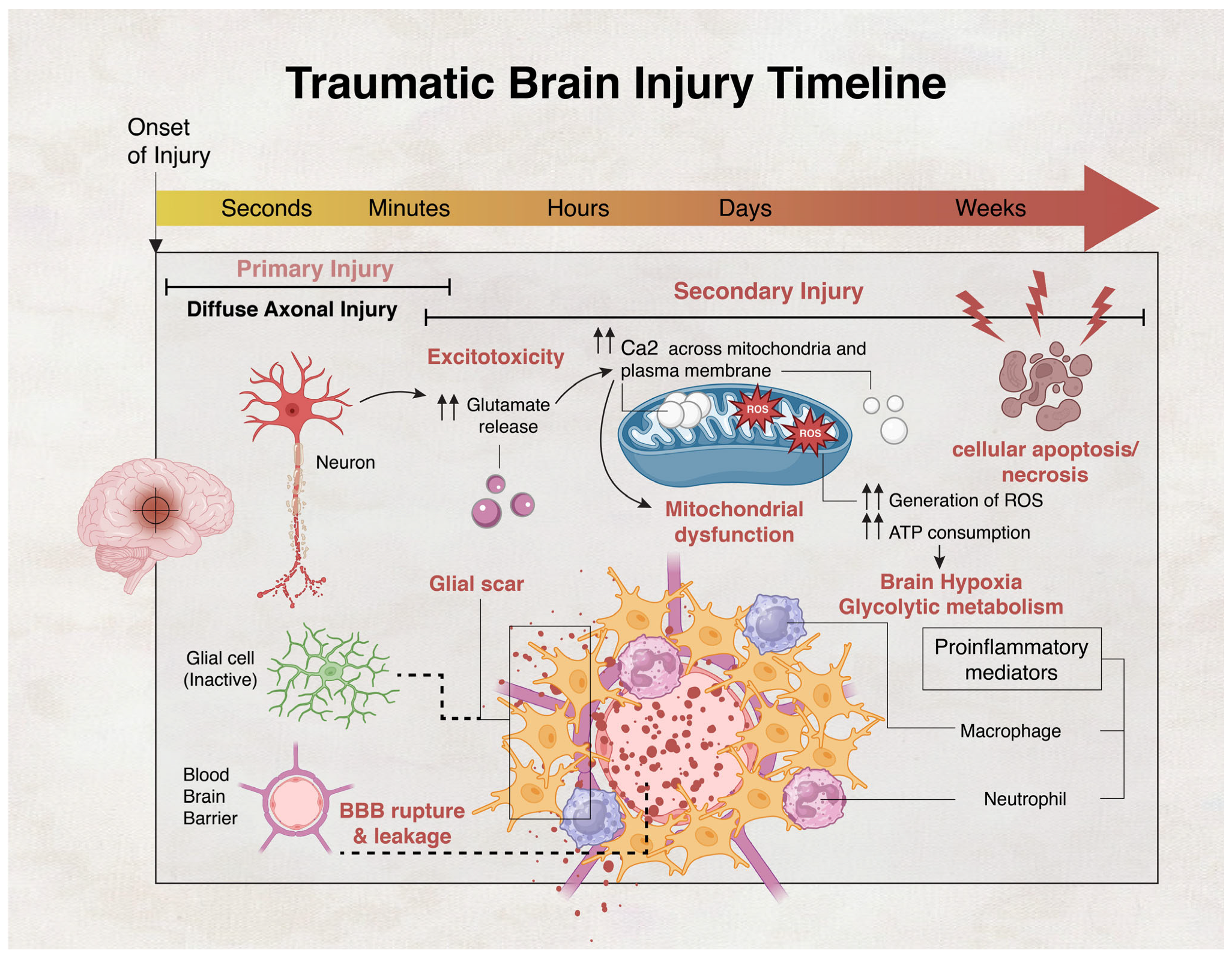
- Dixon, K.J. Pathophysiology of Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 215–225. [Google Scholar] [CrossRef]
- McKee, A.C.; Daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [PubMed]
- Kaur, P.; Sharma, S. Recent advances in pathophysiology of traumatic brain injury. Curr. Neuropharmacol. 2018, 16, 1224–1238. [Google Scholar] [CrossRef]
- Dong, G.-C.; Kuan, C.-Y.; Subramaniam, S.; Zhao, J.-Y.; Sivasubramaniam, S.; Chang, H.-Y.; Lin, F.-H. A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan based hydrogel. Mater. Sci. Eng. C 2015, 49, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.; El Tal, T.; Gul, R.; Mondello, S.; Zhang, Z.; Boustany, R.M.; Guingab, J.; Wang, K.K.; Kobeissy, F. Neuroproteomics approach and neurosystems biology analysis: ROCK inhibitors as promising therapeutic targets in neurodegeneration and neurotrauma. Electrophoresis 2012, 33, 3659–3668. [Google Scholar] [CrossRef] [PubMed]
- Kobeissy, F.H.; Guingab-Cagmat, J.D.; Zhang, Z.; Moghieb, A.; Glushakova, O.Y.; Mondello, S.; Boutté, A.M.; Anagli, J.; Rubenstein, R.; Bahmad, H.; et al. Neuroproteomics and Systems Biology Approach to Identify Temporal Biomarker Changes Post Experimental Traumatic Brain Injury in Rats. Front. Neurol. 2016, 7, 198. [Google Scholar] [CrossRef] [PubMed]
- Ottens, A.K.; Kobeissy, F.H.; Fuller, B.F.; Liu, M.C.; Oli, M.W.; Hayes, R.L.; Wang, K.K. Novel neuroproteomic approaches to studying traumatic brain injury. Prog. Brain Res. 2007, 161, 401–418. [Google Scholar] [CrossRef]
- Stirling, D.P.; Cummins, K.; Wayne Chen, S.; Stys, P. Axoplasmic reticulum Ca2+ release causes secondary degeneration of spinal axons. Ann. Neurol. 2014, 75, 220–229. [Google Scholar] [CrossRef]
- Mu, J.; Song, Y.; Zhang, J.; Lin, W.; Dong, H.; Pathology, E. Calcium signaling is implicated in the diffuse axonal injury of brain stem. Int. J. Clin. Exp. Pathol. 2015, 8, 4388. [Google Scholar]
- Arciniegas, D.B.; Silver, J.M. Pharmacotherapy of posttraumatic cognitive impairments. Behav. Neurol. 2006, 17, 25–42. [Google Scholar] [CrossRef]
- Weil, Z.M.; Gaier, K.R.; Karelina, K. Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 2014, 70, 108–116. [Google Scholar] [CrossRef]
- Shaito, A.; Hasan, H.; Habashy, K.J.; Fakih, W.; Abdelhady, S.; Ahmad, F.; Zibara, K.; Eid, A.H.; El-Yazbi, A.F.; Kobeissy, F.H. Western diet aggravates neuronal insult in post-traumatic brain injury: Proposed pathways for interplay. EBioMedicine 2020, 57, 102829. [Google Scholar] [CrossRef]
- Fehily, B.; Fitzgerald, M. Repeated mild traumatic brain injury: Potential mechanisms of damage. Cell Transplant. 2017, 26, 1131–1155. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.G.; Wang, J.A.; Carrico, K.M.; Hall, E.D. Pharmacological inhibition of lipid peroxidation attenuates calpain-mediated cytoskeletal degradation after traumatic brain injury. J. Neurochem. 2011, 117, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muneer, P.; Chandra, N.; Haorah, J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 2015, 51, 966–979. [Google Scholar] [CrossRef]
- Kobeissy, F.H.; Liu, M.C.; Yang, Z.; Zhang, Z.; Zheng, W.; Glushakova, O.; Mondello, S.; Anagli, J.; Hayes, R.L.; Wang, K.K. Degradation of βII-Spectrin Protein by Calpain-2 and Caspase-3 Under Neurotoxic and Traumatic Brain Injury Conditions. Mol Neurobiol 2015, 52, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.W.; Zheng, W.; Kobeissy, F.H.; Cheng Liu, M.; Hayes, R.L.; Gold, M.S.; Larner, S.F.; Wang, K.K. Calpain- and caspase-mediated alphaII-spectrin and tau proteolysis in rat cerebrocortical neuronal cultures after ecstasy or methamphetamine exposure. Int. J. Neuropsychopharmacol. 2007, 10, 479–489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alves, J.L. Blood–brain barrier and traumatic brain injury. J. Neurosci. Res. 2014, 92, 141–147. [Google Scholar] [CrossRef]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Chell, J.M.; Le Merre, P.; Wu, Y.; Zamboni, M.; Bergenstråhle, J.; Stenudd, M.; Sopova, E.; Lundeberg, J.; Shupliakov, O. A latent lineage potential in resident neural stem cells enables spinal cord repair. Science 2020, 370, eabb8795. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, L.; Yi, X.; Zhou, Z.; Liu, C.; Fu, R.; Dai, C.; Wang, Z.; Chen, X.; Yu, P. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano 2018, 12, 10957–10967. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Perez, E.J.; Tapanes, S.A.; Loris, Z.B.; Balu, D.T.; Sick, T.J.; Coyle, J.T.; Liebl, D.J. Enhanced astrocytic d-serine underlies synaptic damage after traumatic brain injury. J. Clin. Investig. 2017, 127, 3114–3125. [Google Scholar] [CrossRef]
- Chen, Y.; Swanson, R.A. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003, 23, 137–149. [Google Scholar] [CrossRef]
- Al-Haj, N.; Issa, H.; Zein, O.E.; Ibeh, S.; Reslan, M.A.; Yehya, Y.; Kobeissy, F.; Zibara, K.; Eid, A.H.; Shaito, A. Phytochemicals as Micronutrients: What Is their Therapeutic Promise in the Management of Traumatic Brain Injury? In Role of Micronutrients in Brain Health; Springer: Berlin/Heidelberg, Germany, 2022; pp. 245–276. [Google Scholar]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for structural modification of small molecules to improve blood–brain barrier penetration: A recent perspective. J. Med. Chem. 2021, 64, 13152–13173. [Google Scholar] [CrossRef]
- Silva, G.A. Nanotechnology approaches for the regeneration and neuroprotection of the central nervous system. Surg. Neurol. 2005, 63, 301–306. [Google Scholar] [CrossRef]
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sinica. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, P.M.; Bramlett, H.M.; Dixon, C.E.; Dietrich, W.D.; Mondello, S.; Wang, K.K.; Hayes, R.L.; Lafrenaye, A.; Povlishock, J.T.; Tortella, F.C. Operation brain trauma therapy: 2016 update. Mil. Med. 2018, 183, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.L.; Bennett, N.K.; Halikere, A.; Pang, Z.P.; Moghe, P.V. Self-assembling peptide nanofiber scaffolds for 3-D reprogramming and transplantation of human pluripotent stem cell-derived neurons. ACS Biomater. Sci. Eng. 2016, 2, 1030–1038. [Google Scholar] [CrossRef]
- Gonzalez-Perez, F.; Cobianchi, S.; Heimann, C.; Phillips, J.B.; Udina, E.; Navarro, X. Stabilization, rolling, and addition of other extracellular matrix proteins to collagen hydrogels improve regeneration in chitosan guides for long peripheral nerve gaps in rats. Neurosurgery 2017, 80, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, C.; Wang, T.; Wang, Y.; Wang, J.; Quan, D.; Deng, D.Y. PTMAc-PEG-PTMAc hydrogel modified by RGDC and hyaluronic acid promotes neural stem cells’ survival and differentiation in vitro. RSC Adv. 2017, 7, 41098–41104. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, A.; Huang, Z.; Yin, G.; Pu, X.; Jin, J. Enhancement of neurite adhesion, alignment and elongation on conductive polypyrrole-poly (lactide acid) fibers with cell-derived extracellular matrix. Colloids Surf. B Biointerfaces 2017, 149, 217–225. [Google Scholar] [CrossRef]
- Xue, C.; Zhu, H.; Tan, D.; Ren, H.; Gu, X.; Zhao, Y.; Zhang, P.; Sun, Z.; Yang, Y.; Gu, J. Electrospun silk fibroin-based neural scaffold for bridging a long sciatic nerve gap in dogs. J. Tissue Eng. Regen. Med. 2018, 12, e1143–e1153. [Google Scholar] [CrossRef]
- Al-Thani, N.; Haider, M.Z.; Al-Mansoob, M.; Patel, S.; Ahmad, S.M.S.; Kobeissy, F.; Shaito, A. Nano-Engineering in Traumatic Brain Injury. In Impact of Engineered Nanomaterials in Genomics and Epigenomics; John Wiley and Sons Ltd.: West Sussex, UK, 2023; pp. 217–228. [Google Scholar] [CrossRef]
- Khan, J.; Rudrapal, M.; Bhat, E.A.; Ali, A.; Alaidarous, M.; Alshehri, B.; Banwas, S.; Ismail, R.; Egbuna, C. Perspective Insights to Bio-Nanomaterials for the Treatment of Neurological Disorders. Front. Bioeng. Biotechnol. 2021, 9, 724158. [Google Scholar] [CrossRef]
- Hicks, A.U.; Lappalainen, R.S.; Narkilahti, S.; Suuronen, R.; Corbett, D.; Sivenius, J.; Hovatta, O.; Jolkkonen, J. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: Cell survival and functional recovery. Eur. J. Neurosci. 2009, 29, 562–574. [Google Scholar] [CrossRef]
- Tuladhar, A.; Payne, S.L.; Shoichet, M.S. Harnessing the potential of biomaterials for brain repair after stroke. Front. Mater. 2018, 5, 14. [Google Scholar] [CrossRef]
- Elkin, B.S.; Azeloglu, E.U.; Costa, K.D.; Morrison, B., 3rd. Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J. Neurotrauma 2007, 24, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Moshayedi, P.; Nih, L.R.; Llorente, I.L.; Berg, A.R.; Cinkornpumin, J.; Lowry, W.E.; Segura, T.; Carmichael, S.T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, S.; Liang, M.; Yang, H.; Chang, C. Biomaterials Based Growth Factor Delivery for Brain Regeneration after Injury. Smart Mater. Med. 2022, 3, 352–360. [Google Scholar] [CrossRef]
- Davim, J.P. Biomedical Composites: Materials, Manufacturing and Engineering; Walter de Gruyter: Boston, MA, USA, 2013; Volume 2. [Google Scholar]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hoffman, A.S. Hydrogels. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 153–166. [Google Scholar]
- Gradinaru, V.; Treweek, J.; Overton, K.; Deisseroth, K. Hydrogel-tissue chemistry: Principles and applications. Annu. Rev. Biophys. 2018, 47, 355–376. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Practice. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Youssef, G. Dynamic properties of hydrogels and fiber-reinforced hydrogels. J. Mech. Behav. Biomed. Mater. 2018, 85, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Aurand, E.R.; Lampe, K.J.; Bjugstad, K.B. Defining and designing polymers and hydrogels for neural tissue engineering. Neurosci. Res. 2012, 72, 199–213. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Yan, W. A Prosperous Application of Hydrogels with Extracellular Vesicles Release for Traumatic Brain Injury. Front. Neurol. 2022, 13, 908468. [Google Scholar] [CrossRef] [PubMed]
- Saracino, G.A.; Cigognini, D.; Silva, D.; Caprini, A.; Gelain, F. Nanomaterials design and tests for neural tissue engineering. Chem. Soc. Rev. 2013, 42, 225–262. [Google Scholar] [CrossRef]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, M.; Smeets, N.M.; Hoare, T. Designing injectable, covalently cross-linked hydrogels for biomedical applications. Macromol. Rapid Commun. 2014, 35, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Bakarich, S.E.; Pidcock, G.C.; Balding, P.; Stevens, L.; Calvert, P. Recovery from applied strain in interpenetrating polymer network hydrogels with ionic and covalent cross-links. Soft Matter. 2012, 8, 9985–9988. [Google Scholar] [CrossRef]
- Führmann, T.; Obermeyer, J.; Tator, C.H.; Shoichet, M.S. Click-crosslinked injectable hyaluronic acid hydrogel is safe and biocompatible in the intrathecal space for ultimate use in regenerative strategies of the injured spinal cord. Methods 2015, 84, 60–69. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Alvarez, G.S.; Hélary, C.; Mebert, A.M.; Wang, X.; Coradin, T.; Desimone, M.F. Antibiotic-loaded silica nanoparticle–collagen composite hydrogels with prolonged antimicrobial activity for wound infection prevention. J. Mater. Chem. B 2014, 2, 4660–4670. [Google Scholar] [CrossRef]
- Desai, R.M.; Koshy, S.T.; Hilderbrand, S.A.; Mooney, D.J.; Joshi, N.S. Versatile click alginate hydrogels crosslinked via tetrazine–norbornene chemistry. Biomaterials 2015, 50, 30–37. [Google Scholar] [CrossRef]
- Zamproni, L.N.; Mundim, M.T.; Porcionatto, M.A. Biology, d. Neurorepair and regeneration of the brain: A decade of bioscaffolds and engineered microtissue. Front. Cell Dev. Biol. 2021, 9, 649891. [Google Scholar] [CrossRef]
- Nih, L.R.; Gojgini, S.; Carmichael, S.T.; Segura, T.J. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 2018, 17, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.; Ma, Y.; Bruekers, S.M.; Ma, S.; Huck, W.T. 25th anniversary article: Designer hydrogels for cell cultures: A materials selection guide. Adv. Mater. 2014, 26, 125–147. [Google Scholar] [CrossRef]
- Namba, R.; Cole, A.; Bjugstad, K.; Mahoney, M. Development of porous PEG hydrogels that enable efficient, uniform cell-seeding and permit early neural process extension. Acta Biomater. 2009, 5, 1884–1897. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Nguyen, C.; Chun, H.N.; L Llorente, I.; Chiu, A.S.; Machnicki, M.; Zarembinski, T.I.; Carmichael, S.T. Metabolism. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 2017, 37, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.; Holloway, J.L.; Stabenfeldt, S.E. Hyaluronic Acid Biomaterials for Central Nervous System Regenerative Medicine. Cells 2020, 9, 2113. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Humpel, C. Collagen for brain repair: Therapeutic perspectives. Neural Regen. Res. 2018, 13, 595–598. [Google Scholar] [CrossRef]
- Peppas, N.A.; Moynihan, H.J.; Lucht, L.M. The structure of highly crosslinked poly(2-hydroxyethyl methacrylate) hydrogels. J. Biomed. Mater. Res. 1985, 19, 397–411. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Huglin, M.B. DSC studies on states of water in crosslinked poly (methyl methacrylate-co-n-vinyl-2-pyrrolidone) hydrogels. Polym. Int. 1994, 33, 273–277. [Google Scholar] [CrossRef]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef]
- Phipps, M.C.; Clem, W.C.; Grunda, J.M.; Clines, G.A.; Bellis, S.L. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials 2012, 33, 524–534. [Google Scholar] [CrossRef]
- Paşcu, E.I.; Stokes, J.; McGuinness, G.B. Electrospun composites of PHBV, silk fibroin and nano-hydroxyapatite for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4905–4916. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Farrugia, B.L.; Brown, T.D.; Upton, Z.; Hutmacher, D.W.; Dalton, P.D.; Dargaville, T.R. Dermal fibroblast infiltration of poly(ε-caprolactone) scaffolds fabricated by melt electrospinning in a direct writing mode. Biofabrication 2013, 5, 025001. [Google Scholar] [CrossRef]
- Izal, I.; Aranda, P.; Sanz-Ramos, P.; Ripalda, P.; Mora, G.; Granero-Moltó, F.; Deplaine, H.; Gómez-Ribelles, J.L.; Ferrer, G.G.; Acosta, V.; et al. Culture of human bone marrow-derived mesenchymal stem cells on of poly(L-lactic acid) scaffolds: Potential application for the tissue engineering of cartilage. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1737–1750. [Google Scholar] [CrossRef]
- Grad, S.; Kupcsik, L.; Gorna, K.; Gogolewski, S.; Alini, M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: Potential and limitations. Biomaterials 2003, 24, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
- Bini, T.; Gao, S.; Wang, S.; Lim, A.; Hai, L.B.; Ramakrishna, S. Electrospun poly (L-lactide-co-glycolide) biodegradable polymer nanofibre tubes for peripheral nerve regeneration. Nanotechnology 2004, 15, 1459. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef]
- Teo, W. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.; Bhaduri, S.J.P. Biomedical applications of electrospun nanofibers: Drug and nanoparticle delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef]
- Venugopal, J.; Low, S.; Choon, A.T.; Ramakrishna, S. Interaction of cells and nanofiber scaffolds in tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Ma, P. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2004, 39, 125–131. [Google Scholar] [CrossRef]
- Kumbar, S.; James, R.; Nukavarapu, S.; Laurencin, C. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 7, 25–34. [Google Scholar] [CrossRef]
- Nisbet, D.R.; Rodda, A.E.; Horne, M.K.; Forsythe, J.S.; Finkelstein, D.I. Neurite infiltration and cellular response to electrospun polycaprolactone scaffolds implanted into the brain. Biomaterials 2009, 30, 4573–4580. [Google Scholar] [CrossRef]
- Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2022, 15, 65. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; He, Y.; Ma, J.; Ni, G.; Zhou, S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018, 81, 80–113. [Google Scholar] [CrossRef]
- Zech, J.; Leisz, S.; Goettel, B.; Syrowatka, F.; Greiner, A.; Strauss, C.; Knolle, W.; Scheller, C.; Maeder, K. Biopharmaceutics. Electrospun Nimodipine-loaded fibers for nerve regeneration: Development and in vitro performance. Eur. J. Pharm. Biopharm. 2020, 151, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Gommans, H.; Cheyns, D.; Aernouts, T.; Girotto, C.; Poortmans, J.; Heremans, P. Electro-Optical Study of Subphthalocyanine in a Bilayer Organic Solar Cell. Adv. Funct. Mater. 2007, 17, 2653–2658. [Google Scholar] [CrossRef]
- Li, W.; Guo, Y.; Wang, H.; Shi, D.; Liang, C.; Ye, Z.; Qing, F.; Gong, J. Electrospun nanofibers immobilized with collagen for neural stem cells culture. J. Mater. Sci. Mater. Med. 2008, 19, 847–854. [Google Scholar] [CrossRef]
- Qian, J.; Lin, Z.; Liu, Y.; Wang, Z.; Lin, Y.; Gong, C.; Ruan, R.; Zhang, J.; Yang, H. Functionalization strategies of electrospun nanofibrous scaffolds for nerve tissue engineering. Smart Mater. Med. 2021, 2, 260–279. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, B.E.; Maas, A.I.; Narayan, R.K.; van der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N. A Clinical Trial of Progesterone for Severe Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, Y.; He, Y.; Chang, R.; Guo, S.; Ma, S.; Guan, F.; Yao, M. In situ forming and biocompatible hyaluronic acid hydrogel with reactive oxygen species-scavenging activity to improve traumatic brain injury repair by suppressing oxidative stress and neuroinflammation. Mater. Today Bio. 2022, 15, 100278. [Google Scholar] [CrossRef]
- Bender, E.C.; Kraynak, C.A.; Huang, W.; Suggs, L.J. Cell-Inspired Biomaterials for Modulating Inflammation. Tissue Eng. Part B Rev. 2022, 28, 279–294. [Google Scholar] [CrossRef]
- Babensee, J.E.; McIntire, L.V.; Mikos, A.G. Growth factor delivery for tissue engineering. Pharm. Res. 2000, 17, 497–504. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, X.-M.; Spector, M.; Cui, F.-Z. Scaffolds for central nervous system tissue engineering. Front. Mater. Sci. 2012, 6, 1–25. [Google Scholar] [CrossRef]
- Su, Z.; Niu, W.; Liu, M.-L.; Zou, Y.; Zhang, C.-L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 2014, 5, 3338. [Google Scholar] [CrossRef]
- Tamariz, E.; Varela-Echavarría, A. The discovery of the growth cone and its influence on the study of axon guidance. Front. Neuroanat. 2015, 9, 51. [Google Scholar] [CrossRef]
- Musah, S.; Morin, S.A.; Wrighton, P.J.; Zwick, D.B.; Jin, S.; Kiessling, L.L. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano 2012, 6, 10168–10177. [Google Scholar] [CrossRef]
- Li, X.; Duan, L.; Kong, M.; Wen, X.; Guan, F.; Ma, S. Applications and Mechanisms of Stimuli-Responsive Hydrogels in Traumatic Brain Injury. Gels 2022, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Jurga, M.; Dainiak, M.B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.N.; Strojek, L.; Jungvid, H.; Kumar, A. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Cholas, R.H.; Hsu, H.-P.; Spector, M. The reparative response to cross-linked collagen-based scaffolds in a rat spinal cord gap model. Biomaterials 2012, 33, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramos, C.; Gómez-Pinedo, U.; Soria, J.M.; Barcia, J.A.; Pradas, M.M. Neural tissue regeneration in experimental brain injury model with channeled scaffolds of acrylate copolymers. Neurosci. Lett. 2015, 598, 96–101. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Clarkson, E.D.; Zawada, W.M.; Adams, F.S.; Bell, K.P.; Freed, C.R. Strands of embryonic mesencephalic tissue show greater dopamine neuron survival and better behavioral improvement than cell suspensions after transplantation in parkinsonian rats. Brain Res. 1998, 806, 60–68. [Google Scholar] [CrossRef]
- Sautter, J.; Tseng, J.; Braguglia, D.; Aebischer, P.; Spenger, C.; Seiler, R.; Widmer, H.; Zurn, A. Implants of polymer-encapsulated genetically modified cells releasing glial cell line-derived neurotrophic factor improve survival, growth, and function of fetal dopaminergic grafts. Exp. Neurol. 1998, 149, 230–236. [Google Scholar] [CrossRef]
- Yan, F.; Li, M.; Zhang, H.-Q.; Li, G.-L.; Hua, Y.; Shen, Y.; Ji, X.-M.; Wu, C.-J.; An, H.; Ren, M. Collagen-chitosan scaffold impregnated with bone marrow mesenchymal stem cells for treatment of traumatic brain injury. Neural Regen. Res. 2019, 14, 1780. [Google Scholar]
- Latchoumane, C.V.; Betancur, M.I.; Simchick, G.A.; Sun, M.K.; Forghani, R.; Lenear, C.E.; Ahmed, A.; Mohankumar, R.; Balaji, N.; Mason, H.D.; et al. Engineered glycomaterial implants orchestrate large-scale functional repair of brain tissue chronically after severe traumatic brain injury. Sci. Adv. 2021, 7, eabe0207. [Google Scholar] [CrossRef]
- Donaghue, I.E.; Tam, R.; Sefton, M.V.; Shoichet, M.S. Cell and Biomolecule Delivery for Tissue Repair and Regeneration in the Central Nervous System. J. Control Release 2014, 190, 219–227. [Google Scholar] [CrossRef]
- Pettikiriarachchi, J.T.S.; Parish, C.L.; Shoichet, M.S.; Forsythe, J.S.; Nisbet, D.R. Biomaterials for Brain Tissue Engineering. Aust. J. Chem. 2010, 63, 1143–1154. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Q.; Fang, Z.; Gu, L.; Bian, L. Structurally dynamic hydrogels for biomedical applications: Pursuing a fine balance between macroscopic stability and microscopic dynamics. Chem. Rev. 2021, 121, 11149–11193. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, W.; Deng, C.; Li, G.; Chang, J.; Zhang, Z.; Jiang, X.; Wu, C. 3D Printing of Lotus Root-Like Biomimetic Materials for Cell Delivery and Tissue Regeneration. Adv. Sci. 2017, 4, 1700401. [Google Scholar] [CrossRef] [PubMed]
- Kyburz, K.A.; Anseth, K.S. Synthetic mimics of the extracellular matrix: How simple is complex enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef]
- Ventre, M.; Netti, P.A. Engineering Cell Instructive Materials to Control Cell Fate and Functions through Material Cues and Surface Patterning. ACS Appl. Mater. Interfaces 2016, 8, 14896–14908. [Google Scholar] [CrossRef]
- Wu, R.-X.; Xu, X.-Y.; Wang, J.; He, X.-T.; Sun, H.-H.; Chen, F.-M. Biomaterials for endogenous regenerative medicine: Coaxing stem cell homing and beyond. Appl. Mater. Today 2018, 11, 144–165. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Harn, H.-J.; Chiou, T.-W. The Role of Biomaterials in Implantation for Central Nervous System Injury. Cell Transpl. 2018, 27, 407–422. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Cheng, X.; Liu, P.; Feng, Q.; Wang, S.; Li, Y.; Gu, H.; Zhong, L.; Chen, M.; et al. Integrated printed BDNF-stimulated HUCMSCs-derived exosomes/collagen/chitosan biological scaffolds with 3D printing technology promoted the remodelling of neural networks after traumatic brain injury. Regen. Biomater. 2023, 10, rbac085. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Guo, S.; Zhao, C.; Wang, L.; Ma, S.; Guan, F.; Yao, M. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int. J. Biol. Macromol. 2021, 187, 200–213. [Google Scholar] [CrossRef]
- Tang, W.; Fang, F.; Liu, K.; Huang, Z.; Li, H.; Yin, Y.; Wang, J.; Wang, G.; Wei, L.; Ou, Y.; et al. Aligned Biofunctional Electrospun PLGA-LysoGM1 Scaffold for Traumatic Brain Injury Repair. ACS Biomater. Sci. Eng. 2020, 6, 2209–2218. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, G.; Chen, L.; Zhang, Y.; Luo, Y.; Zheng, Y.; Hu, F.; Forouzanfar, T.; Lin, H.; Liu, B. Neuro-regenerative imidazole-functionalized GelMA hydrogel loaded with hAMSC and SDF-1α promote stem cell differentiation and repair focal brain injury. Bioact. Mater. 2021, 6, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Mahumane, G.D.; Kumar, P.; Pillay, V.; Choonara, Y.E. Repositioning N-Acetylcysteine (NAC): NAC-Loaded Electrospun Drug Delivery Scaffolding for Potential Neural Tissue Engineering Application. Pharmaceutics 2020, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Tu, J.; Fang, G.; Deng, L.; Gao, X.; Guo, K.; Kong, J.; Lv, J.; Guan, W.; Yang, C. Combining PLGA Scaffold and MSCs for Brain Tissue Engineering: A Potential Tool for Treatment of Brain Injury. Stem Cells Int. 2018, 2018, 5024175. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, Z.; Castaño, O.; Castells, A.A.; Mateos-Timoneda, M.A.; Planell, J.A.; Engel, E.; Alcántara, S. Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials 2014, 35, 4769–4781. [Google Scholar] [CrossRef] [PubMed]
- Sulejczak, D.; Andrychowski, J.; Kowalczyk, T.; Nakielski, P.; Frontczak-Baniewicz, M.; Kowalewski, T. Electrospun nanofiber mat as a protector against the consequences of brain injury. Folia Neuropathol. 2014, 52, 56–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Emanuele, M.; Zhang, L.; Zhang, Z.G.; Lu, M.; Zhang, T.; Mahmood, A.; Xiong, Y. Treatment of Traumatic Brain Injury with Vepoloxamer (Purified Poloxamer 188). J. Neurotrauma 2018, 35, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Macks, C.; Jeong, D.; Bae, S.; Webb, K.; Lee, J.S. Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model. Int. J. Mol. Sci. 2022, 23, 11153. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chang, Z.H.; Chen, C.; Liang, J.; Shi, J.X.; Fan, X.; Shao, Q.; Meng, W.W.; Wang, J.J.; Li, X.H. 3D printing of injury-preconditioned secretome/collagen/heparan sulfate scaffolds for neurological recovery after traumatic brain injury in rats. Stem Cell Res. Ther. 2022, 13, 525. [Google Scholar] [CrossRef]
- Sahab Negah, S.; Oliazadeh, P.; Jahanbazi Jahan-Abad, A.; Eshaghabadi, A.; Samini, F.; Ghasemi, S.; Asghari, A.; Gorji, A. Transplantation of human meningioma stem cells loaded on a self-assembling peptide nanoscaffold containing IKVAV improves traumatic brain injury in rats. Acta Biomater. 2019, 92, 132–144. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Zhang, Y.; Chen, S.; Ding, J.; Chen, Z.; Wu, K.; Wu, X.; Zhou, T.; Zeng, M.; et al. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr. Polym. 2023, 306, 120578. [Google Scholar] [CrossRef]
- Tanikawa, S.; Ebisu, Y.; Sedlačík, T.; Semba, S.; Nonoyama, T.; Kurokawa, T.; Hirota, A.; Takahashi, T.; Yamaguchi, K.; Imajo, M.; et al. Engineering of an electrically charged hydrogel implanted into a traumatic brain injury model for stepwise neuronal tissue reconstruction. Sci. Rep. 2023, 13, 2233. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jia, Y.; Wang, S.; Ma, Y.; Huang, G.; Ding, T.; Feng, D.; Genin, G.M.; Wei, Z.; Xu, F. An ECM-Mimicking, Injectable, Viscoelastic Hydrogel for Treatment of Brain Lesions. Adv. Healthc. Mater. 2023, 12, e2201594. [Google Scholar] [CrossRef]
- Moisenovich, M.M.; Plotnikov, E.Y.; Moysenovich, A.M.; Silachev, D.N.; Danilina, T.I.; Savchenko, E.S.; Bobrova, M.M.; Safonova, L.A.; Tatarskiy, V.V.; Kotliarova, M.S.; et al. Effect of Silk Fibroin on Neuroregeneration After Traumatic Brain Injury. Neurochem. Res. 2019, 44, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, X.; Liu, C.; Li, S.; Yang, Z.; Zhang, F.; Chen, X.; Shan, H.; Tao, L.; Zhang, M. Surface-fill H2S-releasing silk fibroin hydrogel for brain repair through the repression of neuronal pyroptosis. Acta Biomater. 2022, 154, 259–274. [Google Scholar] [CrossRef]
- Jiang, J.; Dai, C.; Liu, X.; Dai, L.; Li, R.; Ma, K.; Xu, H.; Zhao, F.; Zhang, Z.; He, T.; et al. Implantation of regenerative complexes in traumatic brain injury canine models enhances the reconstruction of neural networks and motor function recovery. Theranostics 2021, 11, 768–788. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Han, Y.; Han, Z.; Zhang, D.; Zhang, L.; Zhao, G.; Li, S.; Jin, G.; Yu, R.; Liu, H. In Situ implantable, post-trauma microenvironment-responsive, ROS Depletion Hydrogels for the treatment of Traumatic brain injury. Biomaterials 2021, 270, 120675. [Google Scholar] [CrossRef]
- Chen, T.; Xia, Y.; Zhang, L.; Xu, T.; Yi, Y.; Chen, J.; Liu, Z.; Yang, L.; Chen, S.; Zhou, X.; et al. Loading neural stem cells on hydrogel scaffold improves cell retention rate and promotes functional recovery in traumatic brain injury. Mater. Today Bio. 2023, 19, 100606. [Google Scholar] [CrossRef]
- Ma, X.; Agas, A.; Siddiqui, Z.; Kim, K.; Iglesias-Montoro, P.; Kalluru, J.; Kumar, V.; Haorah, J. Angiogenic peptide hydrogels for treatment of traumatic brain injury. Bioact. Mater. 2020, 5, 124–132. [Google Scholar] [CrossRef]
- Che, L.; Lei, Z.; Wu, P.; Song, D. A 3D printable and bioactive hydrogel scaffold to treat traumatic brain injury. Adv. Funct. Mater. 2019, 29, 1904450. [Google Scholar] [CrossRef]
- Yang, B.; Liang, C.; Chen, D.; Cheng, F.; Zhang, Y.; Wang, S.; Shu, J.; Huang, X.; Wang, J.; Xia, K.; et al. A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact. Mater. 2022, 15, 103–119. [Google Scholar] [CrossRef]
- Woerly, S.; Fort, S.; Pignot-Paintrand, I.; Cottet, C.; Carcenac, C.; Savasta, M. Development of a sialic acid-containing hydrogel of poly [N-(2-hydroxypropyl) methacrylamide]: Characterization and implantation study. Biomacromolecules 2008, 9, 2329–2337. [Google Scholar] [CrossRef]
- Woerly, S.; Petrov, P.; Sykova, E.; Roitbak, T.; Simonova, Z.; Harvey, A. Neural tissue formation within porous hydrogels implanted in brain and spinal cord lesions: Ultrastructural, immunohistochemical, and diffusion studies. Tissue Eng. 1999, 5, 467–488. [Google Scholar] [CrossRef] [PubMed]
- Lesný, P.; De Croos, J.; Přádný, M.; Vacık, J.; Michalek, J.; Woerly, S.; Syková, E. Polymer hydrogels usable for nervous tissue repair. J. Chem. Neuroanat. 2002, 23, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Bellamkonda, R.V. Biomaterials for the central nervous system. J. R. Soc. Interface 2008, 5, 957–975. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Sun, M.; Wang, X.; Pei, D.; Lei, B.; Li, A. Engineering antioxidant poly (citrate-gallic acid)-Exosome hybrid hydrogel with microglia immunoregulation for Traumatic Brain Injury-post neuro-restoration. Compos. Part B Eng. 2022, 242, 110034. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, Y.H.; Huang, A.P.; Hsu, S.H. Semi-Interpenetrating Polymer Network of Hyaluronan and Chitosan Self-Healing Hydrogels for Central Nervous System Repair. ACS Appl. Mater. Interfaces 2020, 12, 40108–40120. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, D.; Ren, Y.; Guo, S.; Li, J.; Ma, S.; Yao, M.; Guan, F. Injectable hyaluronic acid hydrogel loaded with BMSC and NGF for traumatic brain injury treatment. Mater. Today Bio. 2022, 13, 100201. [Google Scholar] [CrossRef]
- Yao, M.; Chen, Y.; Zhang, J.; Gao, F.; Ma, S.; Guan, F. Chitosan-based thermosensitive composite hydrogel enhances the therapeutic efficacy of human umbilical cord MSC in TBI rat model. Mater. Today Chem. 2019, 14, 100192. [Google Scholar] [CrossRef]
- Chassenieux, C.; Tsitsilianis, C. Recent trends in pH/thermo-responsive self-assembling hydrogels: From polyions to peptide-based polymeric gelators. Soft Matter 2016, 12, 1344–1359. [Google Scholar] [CrossRef]
- Hernandez, A.; Hartgerink, J.D.; Young, S. Self-assembling peptides as immunomodulatory biomaterials. Front. Bioeng. Biotechnol. 2023, 11, 1139782. [Google Scholar] [CrossRef]
- Bakhtiary, N.; Ghalandari, B.; Ghorbani, F.; Varma, S.N.; Liu, C. Advances in Peptide-Based Hydrogel for Tissue Engineering. Polymers 2023, 15, 1608. [Google Scholar] [CrossRef]
- Kartha, K.K.; Babu, S.S.; Srinivasan, S.; Ajayaghosh, A. Attogram sensing of trinitrotoluene with a self-assembled molecular gelator. J. Am. Chem. Soc. 2012, 134, 4834–4841. [Google Scholar] [CrossRef]
- Hosseinkhani, H.; Hong, P.D.; Yu, D.S. Self-assembled proteins and peptides for regenerative medicine. Chem. Rev. 2013, 113, 4837–4861. [Google Scholar] [CrossRef]
- Cheng, T.-Y.; Chen, M.-H.; Chang, W.-H.; Huang, M.-Y.; Wang, T.-W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 2013, 34, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv. Sci. 2022, 9, e2104165. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.H. Modular self-assembling biomaterials for directing cellular responses. Soft Matter 2008, 4, 2310–2315. [Google Scholar] [CrossRef]
- Guo, J.; Leung, K.K.G.; Su, H.; Yuan, Q.; Wang, L.; Chu, T.-H.; Zhang, W.; Pu, J.K.S.; Ng, G.K.P.; Wong, W.M. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 345–351. [Google Scholar] [CrossRef]
- Ellis-Behnke, R.; So, K.-F.; Zhang, S. Molecular repair of the brain using self-assembling peptides. Chim. Oggi 2006, 24, 42–45. [Google Scholar]
- Ellis-Behnke, R.; Teather, L.; Schneider, G.; So, K.-F. Using nanotechnology to design potential therapies for CNS regeneration. Curr. Pharm. Des. 2007, 13, 2519–2528. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, Y.; He, L.; Zhou, L.; Wu, W. A self-assembly peptide nanofibrous scaffold reduces inflammatory response and promotes functional recovery in a mouse model of intracerebral hemorrhage. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Ma, X.; Agas, A.; Siddiqui, Z.; Iglesias-Montoro, P.; Nguyen, P.K.; Kim, K.K.; Haorah, J.; Kumar, V.A. In vivo neuroprotective effect of a self-assembled peptide hydrogel. Chem. Eng. J. 2021, 408, 127295. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, T.C.; DiPersio, C.M.; Hynes, R.O.; Su, X.; Rich, A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 1995, 16, 1385–1393. [Google Scholar] [CrossRef]
- Leung, G.K.K.; Wang, Y.C.; Wu, W. Peptide nanofiber scaffold for brain tissue reconstruction. Methods Enzymol. 2012, 508, 177–190. [Google Scholar]
- Ellis-Behnke, R.G.; Liang, Y.-X.; You, S.-W.; Tay, D.K.; Zhang, S.; So, K.-F.; Schneider, G.E. Nano neuro knitting: Peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc. Natl. Acad. Sci. USA 2006, 103, 5054–5059. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Nakajima, C.; Ajioka, I.; Muraoka, T.; Yaguchi, A.; Fujioka, T.; Akimoto, S.; Matsuo, M.; Lotfy, A.; Nakamura, S.; et al. Amphiphilic peptide-tagged N-cadherin forms radial glial-like fibers that enhance neuronal migration in injured brain and promote sensorimotor recovery. Biomaterials 2023, 294, 122003. [Google Scholar] [CrossRef] [PubMed]
- Maclean, F.L.; Ims, G.M.; Horne, M.K.; Williams, R.J.; Nisbet, D.R. A Programmed Anti-Inflammatory Nanoscaffold (PAIN) as a 3D Tool to Understand the Brain Injury Response. Adv. Mater. 2018, 30, e1805209. [Google Scholar] [CrossRef] [PubMed]
- Maclean, F.L.; Lau, C.L.; Ozergun, S.; O’Shea, R.D.; Cederfur, C.; Wang, J.; Healy, K.E.; Walker, F.R.; Tomas, D.; Horne, M.K.; et al. Galactose-functionalised PCL nanofibre scaffolds to attenuate inflammatory action of astrocytes in vitro and in vivo. J. Mater. Chem. B 2017, 5, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, M.; Guo, Q.; Liu, N.; Wang, Y.; Wu, T. Modulating axonal growth and neural stem cell migration with the use of uniaxially aligned nanofiber yarns welded with NGF-loaded microparticles. Mater. Today Adv. 2023, 17, 100343. [Google Scholar] [CrossRef]
- Yao, M.; Gao, F.; Xu, R.; Zhang, J.; Chen, Y.; Guan, F. A dual-enzymatically cross-linked injectable gelatin hydrogel loaded with BMSC improves neurological function recovery of traumatic brain injury in rats. Biomater. Sci. 2019, 7, 4088–4098. [Google Scholar] [CrossRef]
- Jeong, D.U.; Bae, S.; Macks, C.; Whitaker, J.; Lynn, M.; Webb, K.; Lee, J.S. Hydrogel-mediated local delivery of dexamethasone reduces neuroinflammation after traumatic brain injury. Biomed. Mater. 2021, 16, 035002. [Google Scholar] [CrossRef]
- Kuan, C.-Y.; Lin, Y.-Y.; Chen, C.-Y.; Yang, C.-C.; Chi, C.-Y.; Li, C.-H.; Dong, G.-C.; Lin, F.-H.J. The preparation of oxidized methylcellulose crosslinked by adipic acid dihydrazide loaded with vitamin C for traumatic brain injury. J. Mater. Chem. B 2019, 7, 4499–4508. [Google Scholar] [CrossRef]
- Lu, J.; Yan, X.; Sun, X.; Shen, X.; Yin, H.; Wang, C.; Liu, Y.; Lu, C.; Fu, H.; Yang, S. Synergistic effects of dual-presenting VEGF-and BDNF-mimetic peptide epitopes from self-assembling peptide hydrogels on peripheral nerve regeneration. Nanoscale 2019, 11, 19943–19958. [Google Scholar] [CrossRef]
- Zheng, R.Z.; Lee, K.Y.; Qi, Z.X.; Wang, Z.; Xu, Z.Y.; Wu, X.H.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; He, L.; Wu, W. Self-assembling peptide nanofibrous hydrogel as a promising strategy in nerve repair after traumatic injury in the nervous system. Neural Regen. Res. 2016, 11, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Cerpa, W. Regulation of Phosphorylated State of NMDA Receptor by STEP(61) Phosphatase after Mild-Traumatic Brain Injury: Role of Oxidative Stress. Antioxidants 2021, 10, 1575. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, J.; Huang, T.; Zhang, Z.; Xing, Q.; Zhou, X.; Zhang, K.; Yao, M.; Cheng, T.; Wang, X.; et al. Sodium alginate/collagen/stromal cell-derived factor-1 neural scaffold loaded with BMSCs promotes neurological function recovery after traumatic brain injury. Acta Biomater. 2021, 131, 185–197. [Google Scholar] [CrossRef]
- Shi, W.; Huang, C.J.; Xu, X.D.; Jin, G.H.; Huang, R.Q.; Huang, J.F.; Chen, Y.N.; Ju, S.Q.; Wang, Y.; Shi, Y.W.; et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016, 45, 247–261. [Google Scholar] [CrossRef]
- Wang, T.W.; Chang, K.C.; Chen, L.H.; Liao, S.Y.; Yeh, C.W.; Chuang, Y.J. Effects of an injectable functionalized self-assembling nanopeptide hydrogel on angiogenesis and neurogenesis for regeneration of the central nervous system. Nanoscale 2017, 9, 16281–16292. [Google Scholar] [CrossRef] [PubMed]
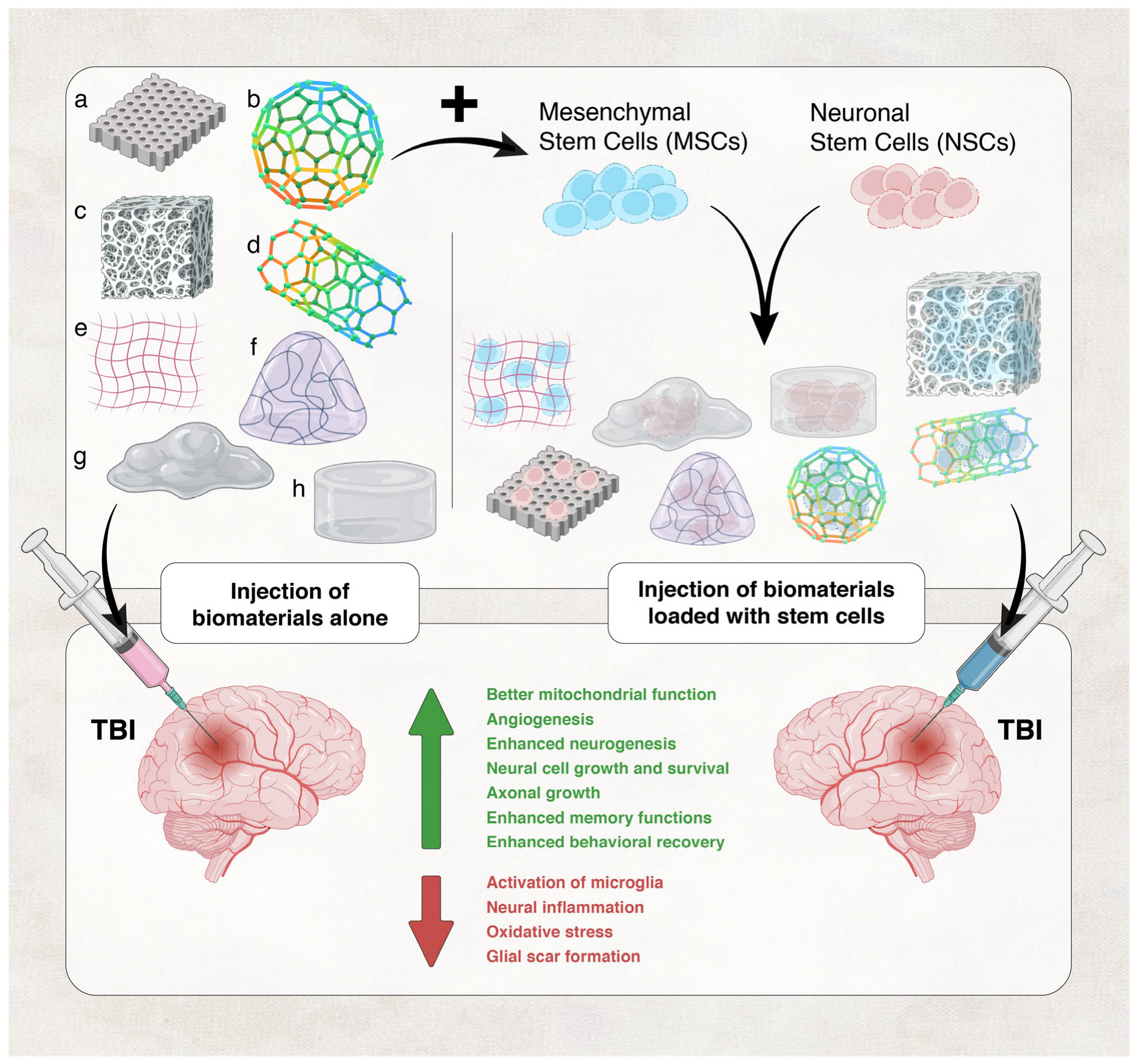
- Tabet, M.; Hasan, H.; Abdelhady, S.; Mahavadi, A.K.; Clervius, H.; Nasrallah, L.; Ahmad, F.; Shaito, N.; Ramakrawala, R.; Zibara, K.; et al. Evaluation of Evidence: Stem Cells as a Treatment Option for Traumatic Brain Injury. eLS 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Zoneff, E.; Thomas, J.; Hong, N.; Tan, L.; McGillivray, D.; Perriman, A.; Law, K.; Thompson, L.; Moriarty, N. Hydrogel oxygen reservoirs increase functional integration of neural stem cell grafts by meeting metabolic demands. Nat. Commun. 2023, 14, 457. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zimmermann, D.; Reuss, R.; Feilen, P.; Manz, B.; Katsen, A.; Weber, M.; Ihmig, F.; Ehrhart, F.; Gessner, P. Towards a medically approved technology for alginate-based microcapsules allowing long-term immunoisolated transplantation. J. Mater. Sci. Mater. Med. 2005, 16, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.M.; Oyen, M.L. Hydrogel composite materials for tissue engineering scaffolds. Jom 2013, 65, 505–516. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R.; Gadegaard, N. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, J.E.; Rozema, F.; Bos, R.; Boering, G.; De Bruijn, W.; Pennings, A. In vivo degradation and biocompatibility study of in vitro pre-degraded as-polymerized polylactide particles. Biomaterials 1995, 16, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.-J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef]
- Saha, S.K.; Tsuji, H. Effects of molecular weight and small amounts of d-lactide units on hydrolytic degradation of poly (l-lactic acid) s. Polym. Degrad. Stab. 2006, 91, 1665–1673. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and morphology of poly (L-lactide). III. Effects of initial crystallinity on long-term in vitro hydrolysis of high molecular weight poly (L-lactide) film in phosphate-buffered solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. [Google Scholar] [CrossRef]
- Suzui, M.; Futakuchi, M.; Fukamachi, K.; Numano, T.; Abdelgied, M.; Takahashi, S.; Ohnishi, M.; Omori, T.; Tsuruoka, S.; Hirose, A.; et al. Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors. Cancer Sci. 2016, 107, 924–935. [Google Scholar] [CrossRef]
- Akhtar, A.; Farzam Rad, V.; Moradi, A.-R.; Yar, M.; Bazzar, M. Emerging polymeric biomaterials and manufacturing-based tissue engineering approaches for neuro regeneration-A critical review on recent effective approaches. Smart Mater. Med. 2023, 4, 337–355. [Google Scholar] [CrossRef]
- Masaeli, R.; Zandsalimi, K.; Tayebi, L. Biomaterials Evaluation: Conceptual Refinements and Practical Reforms. Ther. Innov. Regul. Sci. 2019, 53, 120–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Li, X.; Zheng, D.; Gao, J.; Yang, Z. Supramolecular hydrogels of self-assembled zwitterionic-peptides. Chin. Chem. Lett. 2021, 32, 3636–3640. [Google Scholar] [CrossRef]
- Williams, D. Benefit and risk in tissue engineering. Mater. Today 2004, 7, 24–29. [Google Scholar] [CrossRef]
- Hu, H.; Chen, X.; Zhao, K.; Zheng, W.; Gao, C. Recent Advances in Biomaterials-Based Therapies for Alleviation and Regeneration of Traumatic Brain Injury. Macromol. Biosci. 2023, 23, e2200577. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Liu, Z.; Mao, S.; Han, L. Unraveling the Mechanobiology Underlying Traumatic Brain Injury with Advanced Technologies and Biomaterials. Adv. Healthc. Mater. 2022, 11, e2200760. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef] [PubMed]
- Keimpema, E.; Fokkens, M.R.; Nagy, Z.; Agoston, V.; Luiten, P.G.; Nyakas, C.; Boddeke, H.W.; Copray, J.C. Early transient presence of implanted bone marrow stem cells reduces lesion size after cerebral ischaemia in adult rats. Neuropathol. Appl. Neurobiol. 2009, 35, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Hackelbusch, S.; Rossow, T.; Steinhilber, D.; Weitz, D.A.; Seiffert, S. Hybrid microgels with thermo-tunable elasticity for controllable cell confinement. Adv. Healthc. Mater. 2015, 4, 1841–1848. [Google Scholar] [CrossRef]
- Bjugstad, K.; Lampe, K.; Kern, D.; Mahoney, M. Biocompatibility of poly (ethylene glycol)-based hydrogels in the brain: An analysis of the glial response across space and time. J. Biomed. Mater. Res. Part A 2010, 95, 79–91. [Google Scholar] [CrossRef]
- Tamariz, E.; Wan, A.C.; Pek, Y.S.; Giordano, M.; Hernández-Padrón, G.; Varela-Echavarría, A.; Velasco, I.; Castaño, V.M. Delivery of chemotropic proteins and improvement of dopaminergic neuron outgrowth through a thixotropic hybrid nano-gel. J. Mater. Sci. Mater. Med. 2011, 22, 2097–2109. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.; Bañobre-López, M.; Martins, J.A. Magnetic dehydrodipeptide-based self-assembled hydrogels for theragnostic applications. Nanomaterials 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Koss, K.; Unsworth, L. Neural tissue engineering: Bioresponsive nanoscaffolds using engineered self-assembling peptides. Acta Biomater. 2016, 44, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhou, Y.; Chang, J. Electrospun nanofibrous materials for tissue engineering and drug delivery. Sci. Technol. Adv. Mater. 2010, 11, 014108. [Google Scholar] [CrossRef] [PubMed]
| Property | Synthetic Biomaterial | Natural Biomaterial |
|---|---|---|
| Source | Artificially synthesized | Biological sources |
| Biodegradability | Variable, controllable | Naturally degradable |
| Immunogenicity | Generally low | Potential immune response |
| Mechanical properties | Customizable for specific needs | Variable |
| Biocompatibility | Reduced, can be optimized | Good biocompatibility |
| Growth factors | Controlled release | Potential endogenous release |
| Examples | Poly-anhydrides and poly-orthoesters. | Collagen, chitosan, hyaluronic acid |
| Study | Biomaterial | Species | Outcome | References |
|---|---|---|---|---|
| Liu et al. (2023) | Collagen/chitosan/BMExos scaffold | Rat |
| [143] |
| Li et al. (2021) | Gelatin hydrogel | In vitro & mice |
| [144] |
| Tang et al. (2020) | aPLGA-LysoGM1 scaffold | In vitro & rat |
| [145] |
| Zheng et al. (2020) | Gelatin methacrylate hydrogel with polydopamine nanoparticles and hAMSCs | Rat |
| [146] |
| Mahumane et al. (2020) | N-acetylcysteine (NAC)-loaded poly(lactic-co-glycolic acid) (PLGA) electrospun nanofiber | In vitro & ex vivo (Rat pheochromocytoma PC12 cells) and human glioblastoma multiform A172 cells) |
| [147] |
| Zhou et al. (2018) | poly(lactic-co-glycolic acid) (PLGA) scaffold | In vitro & in vivo Mesenchymal stem cells (MSCs) and neurons |
| [148] |
| Álvarez et al. (2014) | poly-L/DL lactic acid (PLA70/30) nanofibers | Mice |
| [149] |
| Sulejczak et al. (2014) | Electrospun nanofiber/L-lactide-caprolactone copolymer nanofiber net | Rat |
| [150] |
| Zhang et al. (2018) | Vepoloxamer | Rat |
| [151] |
| Macks et al. (2022) | poly(Ethylene) glycol-bis-(acryloyloxy acetate) (PEG-bis-AA) with dexamethasone (DX)-conjugated hyaluronic acid (HA-DXM) | Rat |
| [152] |
| Latchoumane et al. (2021) | Engineered Chondroitin sulfate (eCS) | Rat |
| [134] |
| Liu et al. (2022) | Secretome/collagen/heparan sulfate scaffold | Rat |
| [153] |
| Sahab Negah et al. (2019) | Self-assembling peptide hMgSCs + R-GSIK | Rat |
| [154] |
| Liu et al. (2023) | Bone marrow mesenchymal stem cell-derived exosomes (BME) + hyaluronan-collagen hydrogel (DHC-BME) | Rat |
| [155] |
| Tanikawa et al. (2023) | Electrically charged hydrogels (C1A1) + VEGF | Mice |
| [156] |
| Hu et al. (2023) | Self-healing hydrogel (HA-PBA/Gel-Dopa) | Mice |
| [157] |
| Moisenovich et al. (2019) | Silk fibroin scaffold | Rat |
| [158] |
| Chen et al. (2022) | Hydrogen sulfide(H2S)-releasing silk fibroin (SF) hydrogel (H2S@SF) | Mice |
| [159] |
| Jiang et al. (2021) | Collagen/Silk fibroin (SF) scaffold | Canine |
| [160] |
| Qian et al. (2021) | TM/PC hydrogel (tri-glycerol monostearate, propylene sulfide, and curcumin) | Mice |
| [161] |
| Zhang et al. (2022) | HT/HGA hydrogel (hyaluronic acid-tyramine + antioxidant gallic acid-grafted hyaluronic acid) | Mice |
| [119] |
| Chen et al. (2023) | Gelatin methacrylate and sodium alginate hydrogel (GelMA/Alg) | Rat |
| [162] |
| Ma et al. (2020) | Self-assembling peptide-based hydrogel | Rat |
| [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aqel, S.; Al-Thani, N.; Haider, M.Z.; Abdelhady, S.; Al Thani, A.A.; Kobeissy, F.; Shaito, A.A. Biomaterials in Traumatic Brain Injury: Perspectives and Challenges. Biology 2024, 13, 21. https://doi.org/10.3390/biology13010021
Aqel S, Al-Thani N, Haider MZ, Abdelhady S, Al Thani AA, Kobeissy F, Shaito AA. Biomaterials in Traumatic Brain Injury: Perspectives and Challenges. Biology. 2024; 13(1):21. https://doi.org/10.3390/biology13010021
Chicago/Turabian StyleAqel, Sarah, Najlaa Al-Thani, Mohammad Z. Haider, Samar Abdelhady, Asmaa A. Al Thani, Firas Kobeissy, and Abdullah A. Shaito. 2024. "Biomaterials in Traumatic Brain Injury: Perspectives and Challenges" Biology 13, no. 1: 21. https://doi.org/10.3390/biology13010021
APA StyleAqel, S., Al-Thani, N., Haider, M. Z., Abdelhady, S., Al Thani, A. A., Kobeissy, F., & Shaito, A. A. (2024). Biomaterials in Traumatic Brain Injury: Perspectives and Challenges. Biology, 13(1), 21. https://doi.org/10.3390/biology13010021








