Abstract
Fresh sausages are highly perishable, and the preservatives allowed in these types of meat preparations are limited. Balkan-style fresh sausages were prepared in triplicate without antimicrobials (Control), with an aqueous hops extract (30 mL/kg), with Zataria multiflora Boiss essential oil (1 mL/kg), or a combination of both (15 and 0.5 mL/kg, respectively), and refrigerator-stored under a 20% CO2 and 80% N2 atmosphere. The spoilage microbial growth, i.e., lactic acid bacteria (LAB), Brochothrix thermosphacta, Enterobacteriaceae, Micrococcaceae, molds and yeasts, the pH value, and the production of biogenic amines in the sausages were monitored weekly and compared with a control sausage during a 35-day storage period. Furthermore, 349 colonies of presumptive LAB (isolated from the De Mann, Rogose-Sharpe agar plates) were identified using a MALDI-TOF-based method. Growth levels to ≈ 9 Log colony forming units (CFU) per g were reached by LAB, with a predominance of Lactobacillus sakei. Enterobacteriaceae and B. thermosphacta also showed significant growth (up to 6 Log CFU/g). Biogenic amine levels increased, and tyramine values overcame 250 mg/kg. The study could not demonstrate a significant effect of antimicrobial source treatments in any of the characteristics studied, and thus, the shelf-life of sausages.
1. Introduction
Fresh sausages are produced with comminuted meat, salt, species, and condiments and a limited number of allowed additives. Their formulation, preparation, and dimensions strongly depend on local preparation. Fresh sausages must be refrigerator-stored and cooked before consumption. They are considered to be highly perishable, with pH values >5.5 and water activity (aw) ≥0.97 [1]. To retard microbial growth, fresh sausages are commonly stored at low temperatures under anaerobic CO2-containing modified atmosphere packaging (MAP). The spoilage microbiota of fresh sausages on these conditions consists of facultative anaerobic microorganisms such as lactic acid bacteria (LAB), Brochothrix thermosphacta, and Enterobacteriaceae, with LAB being observed as the predominant group [2,3]. The shelf-life of fresh sausages refrigerator-stored under anaerobiosis depends on the hygienic quality of raw materials, pH, aw, storage temperature, atmosphere, etc. [4]. Some authors, based on the appearance of off-odors and discoloration, have found a shelf-life for these sausages slightly longer than 10 days [3] and others of more than 20 days [5,6,7].
During refrigerated storage of fresh sausages under vacuum or anaerobic MAP, and most probably due to the growth of LAB and Enterobacteriaceae, a significant production of biogenic amines (BA) such as tyramine, putrescine, and cadaverine occurs [4,8]. In a previous study [2], levels of tyramine higher than 100 mg/kg were found in a Mexican fresh sausage stored in anaerobic MAP for more than two weeks, which represents a health risk and corroborates the need to control the production of BA in fresh sausages.
A current approach to extend the shelf-life of fresh sausages is the use of natural antimicrobials [9]. Hops, the strobiles (female flowers) of the Humulus lupulus L. plant, which are commonly used in brewery and have found application in other foods [10], appear to be a potentially suitable ingredient for this purpose. Hops contains antimicrobial compounds, such as prenylated acylphloroglucinols, bitter acids or xanthohumol, among others, which have been probed to inhibit Gram-positive bacteria [11,12]. The Food Safety and Inspection Service from the USA has approved the use of hops α-acids as antimicrobials for cooked meat and casings [13]. Moreover, Kramer et al. [11] found hops extract to inhibit total aerobic microbial growth in marinated pork. However, the effect of hops in fresh sausages packaged under anaerobic conditions seems to have been rarely studied. Hops could interfere in the growth of Gram-positive spoilage microorganisms such as LAB or Brochothrix thermosphacta, thus extending the sausage shelf-life.
Plant-derived essential oils (EO) obtained from aromatic and medical plant materials have proved wide antimicrobial spectra against bacteria, yeasts, and molds [14]. Nonetheless, among bacteria, the Gram-positive are more susceptible than the Gram-negative [15]. The effectiveness of EO at levels up to 2% in extending the lag phase or reducing the final population of spoilage microbiota in minced meat and meat products during refrigerated storage has been reported [16,17]. EO has been claimed to be one of the best alternatives to synthetic preservatives in meat and meat products [17]. However, the use of EO in meat as well as in other foods as natural preservatives present relevant limitations regarding deleterious effects in sensory quality due to their strong flavor, loss of antimicrobial activity due to interactions with food components, and regulatory or safety issues [18]. In this context, the use of EO combined with other synergistic or complementary natural antimicrobials has been suggested as a viable approach to using lower amounts of EO, thus not affecting the sensory acceptation, while achieving a significant antimicrobial effect.
Among the EO, that obtained from Zataria multiflora Boiss (ZM), which contains carvacrol and thymol as its main components, has shown a significant antimicrobial effect, this effect being greater on Gram-negative bacteria [19,20,21]. Zataria multiflora Boiss, belonging to the Laminaceae family, is cultivated in warm parts of the Middle East, where it is popularly used in traditional medicine and as food flavoring and preservative [22]. Regarding processed meat, it has been reported that the addition of ZM’s EO at levels up to 0.1% reduced the growth of total viable microbiota, Pseudomonas spp., and LAB in buffalo burgers during aerobic storage [23,24]. Moreover, a chitosan film containing this EO (5–10 g/kg) also reduced the counts of total viable microbiota at the surface of mortadella-type slices packaged in oxygen permeable polyethylene bags during refrigerated storage [25]. The above-mentioned studies were carried out using aerobic storage; however, no study has been found in the literature addressing the antimicrobial effect of ZM’s EO on meat or meat products stored under anaerobic MAP.
This study has aimed to evaluate the growth of spoilage microorganisms and BA production in a typical fresh sausage during refrigerated storage under CO2 plus N2 MAP, and to assess the effect of two natural antimicrobials: hops and ZM’s EO, alone or combined. The study focused on LAB population considering them as the predominant spoilage microorganisms in fresh sausages packaged under this type of atmosphere and responsible for the BA formation.
2. Results and Discussion
2.1. Water Activity, pH, Microbial Contents, and Biogenic Amine Production
The mean (standard deviation) aw and pH values of the sausages from the three batches at day 0 were 0.987 (±0.004) and 6.01 (±0.02), respectively. During storage, the pH values decreased steadily (P < 0.05) from day 7 to day 28 for all the treatments, with the effect of either treatment or treatment x storage time interaction being non-significant. The mean values of pH in sausages (the four treatments) at days 7, 14, 28, and 35 were 6.05 (±0.01), 5.89 (±0.01), 5.66 (±0.02) and 5.61 (±0.03), respectively (data not shown in tables for brevity).
As is shown in Figure 1, the counts of LAB, B. thermosphacta, Enterobacteriaceae, and Micrococcaceae were not significantly affected by antimicrobial treatment. The mean counts of the Gram-positive LAB and B. thermosphacta tended to be higher in control (C) sausages, although the P values from the analysis of variance (ANOVA) were not significant, i.e., 0.263 and 0.397, respectively (not shown in figures). ZM’s EO contains high amounts of antimicrobial molecules, i.e., thymol, carvacrol, α-terpinene, and a contrasted antimicrobial effect in in vitro experiments [19,21]. In this study, the lack of effect of ZM’s EO on microbial growth in the fresh sausages could be explained by a loss of inhibitory efficacy due to interactions between the antimicrobials and sausage matrix compounds such as fat or specific proteins, which could be influenced by the sausage pH and aw [15,18].
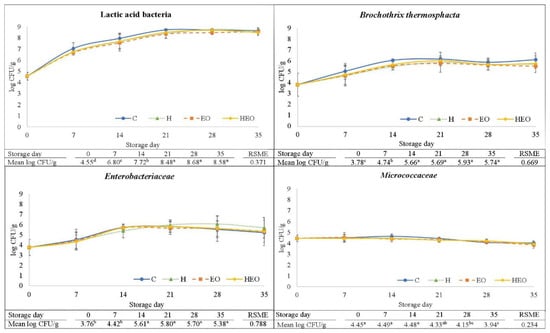
Figure 1.
Effect of the addition of different natural antimicrobial sources on lactic acid bacteria: Brochothrix thermosphacta, Enterobacteriaceae, and Micrococcaceae counts (mean values, n = 3, and standard deviation, vertical bars) in fresh lamb sausages packaged under modified atmosphere (80% N2, 20% CO2) during refrigerated storage (4 °C). CFU: Colony forming units. RSME: Root square mean error. C: Control sausages; H: hops; EO essential oil (Zataria multiflora Boiss); HEO: hops and essential oil. abcd: Total means (n = 12) with different superscripts time-related indicate statistical differences (Tukey test, P < 0.05).
In contrast with our results, the addition of different EO, i.e., bay leaf, cassia, clove, holy basil, lemon, thyme, or sage, to fresh sausages at levels between 0.01% and 0.25% has shown significant reducing effects on the growth of spoilage microflora during refrigerated storage of fresh sausages packaged under aerobic atmosphere [26,27,28,29]. Moreover, the ZM’s EO at levels up to 0.1% also significantly decreased the growth of total viable microbiota, Pseudomonas spp. and LAB in burgers during aerobic refrigerated storage [23,24]. Nevertheless, a clear difference between those studies and this one is that in the formers, the atmosphere was aerobic and in this study it was anaerobic. This suggests that the antimicrobial effect of EO in fresh comminuted meat products might be higher on the microbiota growing in fresh minced meat products with O2 than in that growing when the presence of O2 is restricted.
Hops extracts have been demonstrated to be useful as antimicrobials in casings, cooked ready-to-eat meat, and marinated meat products [11,13]. However, no study has been found investigating their antimicrobial effect in fresh comminuted meat products. In this study, hops extract given alone or combined with ZM’s EO did not reduce the growth of spoilage bacteria. Again, chemical interactions between the hops antimicrobials and the food matrix would be the reasons for the lack of significant antimicrobial activity. In order to achieve a positive antimicrobial effect due to the use of hops extracts in fresh sausages stored under anaerobiosis, it is suggested to use a higher amount of hops antimicrobials or reduce the sausage pH, due to the reported higher effect of hops in food matrix with pH close to 5 [11].
Regarding the changes on microbial growth (Figure 1), LAB became the dominant microbial group from day 7 onwards, reaching final values slightly higher than 8 Log colony forming units (CFU) per g at day 21, which can be considered as the onset of the stationary growth phase. Psychrotrophic LAB have been found to become the major microorganisms in fresh sausages refrigerator-stored under anaerobic CO2-containing MAP over the third week of storage [3,30], with the maximum LAB levels being comparable to those from this study. Lactic acid bacteria are considered as the principal spoilage-specific microorganisms in meat and fresh sausages stored under vacuum or CO2-containing anaerobic MAP [31]. Thus, the appearance of off-flavors, i.e., sour or putrid, in fresh pork sausage has been related to LAB counts over 7–8 Log CFU/g [5,32].
Both B. thermosphacta and Enterobacteriaceae showed similar growth patterns between them, i.e., starting with counts near to 4 Log CFU/g at day 0 and reaching the stationary phase at day 14 with counts of 5–6 Log CFU/g (Figure 1). In both cases, the growth phase was slower and shorter than that for LAB, which suggests a competitive effect of LAB, probably due to a higher ability of LAB for the consumption of limiting nutrients under the anaerobic MAP fresh sausage conditions [33].
Other studies also described how B. thermosphacta steadily increases its levels in fresh meats during refrigerated storage under vacuum and anaerobic MAP, becoming one of the dominant spoilage species and originating cheesy, buttery, or sour odors [34]. According to Samelis [35], the levels of B. thermosphacta associated to fresh meat spoilage are around 7 Log CFU/g.
The control of Enterobacteriaceae in fresh sausages seems to be desirable since levels of 4–5 Log CFU/g [36] have been associated with meat spoilage—counts higher than this level were overcome in this study at day 14. The growth pattern of Enterobacteriaceae in fresh sausages stored under CO2- and N2-containing MAP has shown variability among studies. In agreement with our results, Benson et al. [32] reported an exponential growth of Enterobacteriaceae during the first two weeks of storage of a fresh pork sausage, reaching counts around 6 Log CFU/g; however, Ruíz-Capillas and Jiménez Colmenero [3] found the levels of Enterobacteriaceae in fresh pork sausages to decrease after 10 days of refrigerated storage. These differences might be explained by variations among studies in spice mixtures, sausage pH, or bacterial communities and their competence.
Micrococcaceae counts were stable up to day 21 of storage and then decreased slightly until day 35 (Figure 1). A decrease after some weeks of storage has been described in other studies on fresh sausages during anaerobic refrigerated storage [37,38], and attributed to both pH decrease and low O2 and nutrients availability. No differences were found due either to treatment nor storage time in the molds and yeast counts (the mean values considering all the treatments and days were 2.99 ± 0.17 Log CFU/g; n = 24; data not shown in tables for brevity), which is probably due to their low growing ability under diminishing O2 levels [38].
Changes in BA production in sausages are shown in Table 1. The levels of BA were not affected by antimicrobial treatment except for spermine (P = 0.037), with slightly higher amounts in the hops extract and essential oil (HEO) sausages than in the C sausages. Mono and diamines in fresh sausages are presumably produced from microbial enzymatic decarboxylation of free amino acids. In ripened sausages, this is mainly carried out by LAB and Enterococci, this ability being strain-dependent [39]. Enterobacteriaceae and B. thermosphacta can also contribute to the production of BA in LAB-fermented meats, with the first being more active in cadaverine and putrescine formation and the latter in histamine and tyramine [40,41,42]. The lack of effect of treatment on BA in the fresh sausage is thus coherent with the absence of a significant effect on microbial growth. However, the levels of tryptamine, putrescine, and histamine were significantly different between the experimental batches (data not shown in tables). Thus, the levels of putrescine and histamine in the second batch were respectively more than 5 times and 20 times higher than in the other two batches, which would corroborate the dependence of BA production on microbial strains.

Table 1.
Biogenic amine contents (mg/kg) in fresh sausages stored at 2 °C under anaerobic modified atmosphere storage as a function of antimicrobial treatment (Treat) and storage day (Time).
In contrast with our results, Lu et al. [43] reported a reduction in biogenic mono and diamine production in Chinese smoked sausages as a result of the addition of a mixture of essential oils (from cinnamon, cloves, ginger, and anise; 0.12% in total) and tea polyphenols (0.19%) to the sausage mix. The discrepancy between both studies could be attributed to differences in the antimicrobial source used, the making process, and storage conditions (i.e., 50 °C smoking, 20–22 °C fermentation and 10–12 °C ripening-drying steps versus continuous refrigerated storage under CO2-containing MAP).
Storage time affected the amounts of all mono and diamines in the fresh sausage, which increased steadily, indicating a continuous formation of those BA by the active microbiota during storage. However, time did not affect the content of polyamines originated from de novo synthesis in animal tissues [44]. Overall, the time-related changes in the content of BA in this study have been observed in other studies on fresh sausages stored under anaerobic MAP [2,8]. Biogenic monoamines can produce toxic effects on the consumers resulting in migraine, hypertensive crisis, or allergy [45]. Among them, histamine and tyramine present the highest health concern [46]. The maximum recommended levels for both amines in fermented sausages are over 100 mg/kg—although their toxicity depends not only on their levels in food but also on dietary factors and consumers’ susceptibility [47]. Tyramine content in the fresh sausages from this study exceeded that limit (100 mg/kg) at day 14. On the other hand, although the diamines putrescine and cadaverine are not considered toxic per se, they can enhance the toxic effect of histamine and tyramine [48].
2.2. Identification of Lactic Acid Bacteria
Only eight out of the 346 isolates from the DeMan-Rogosa-Sharpe (MRS) agar plates were not positively identified. Among the identified isolates, 90% corresponded to LAB (70% of isolates were identified as LAB at day 0 and ≥90% at the other sampling days). Among the non-LAB bacteria, the genus identified in order of abundance were Staphylococcus spp., Enterobacter spp., Serratia spp., Filifactor spp., Escherichia spp., and Macrococcus spp. (not shown in tables). Table 2 shows the frequency (%) of the LAB species identified at different storage days considering the isolates in sausages from the four antimicrobial treatments. The genus Lactobacillus was the most abundant (84% of the LAB isolates) regardless of the storage time. Lactobacillus sakei was the predominant LAB, with its frequency overcoming 50% from day 7 onwards—when LAB counts showed significant growth (counts higher than 7 Log CFU/g; Figure 1). Among the isolates identified as Lb. sakei, 59% were identified as Lb. sakei subsp. carnosus (not shown in tables) and the others were identified as Lb. sakei (only species level). On the other hand, approximately 40% of the isolates ascribed to the Lactobacillus genus were not positively identified at species level (provided as Lactobacillus spp. in Table 2). Comparing between the first weeks (especially day 0) and the last weeks of storage, the diversity (number) of LAB species showed a tendency to decrease. On days 28 and 35, there was a clear dominance of Lactobacillus, and among them, Lb. sakei (<90% and ≤70% of total LAB, respectively).

Table 2.
Lactic acid bacteria (LAB) species in the sausages # at the different storage days (expressed in % of total isolates identified as LAB).
Figure 2 depicts the frequency of LAB species or genus, as identified by the matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) analysis, obtained for each of the experimental treatments from day 7 to 35. Lb. sakei was predominant for each of the treatment-day combinations except for H-day 14 (ranging from 40% to 85%). The contingency chi-square test showed no significant effect of treatment either on the frequency of Lb. sakei or on Lactobacillus spp. (P = 0.419 and P = 0.729, respectively).
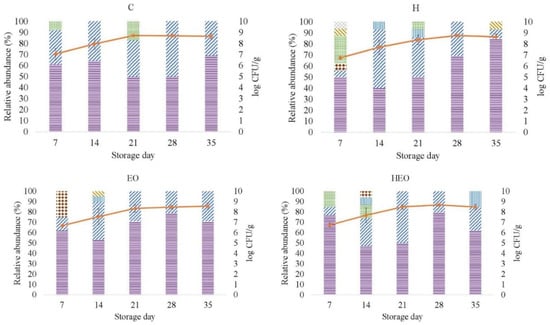
Figure 2.
Relative abundance (%) and growth curve (mean and standard deviation, vertical bars; n = 3) of the isolates identified as lactic acid bacteria in fresh lamb sausages packaged under modified atmosphere (80% N2, 20 %CO2) during refrigerated storage (4 °C). CFU: Colony forming unit. C: Control sausages; H: hops; EO essential oil (Zataria multiflora Boiss); HEO: hops and essential oil. Lactobacillus sakei ( ), Lactobacillus spp. (
), Lactobacillus spp. ( ), Leuconostoc mesenteroides (
), Leuconostoc mesenteroides ( ), Enterococcus faecalis (
), Enterococcus faecalis ( ), Carnobacterium maltromaticum (
), Carnobacterium maltromaticum ( ), Lactobacillus curvatus (
), Lactobacillus curvatus ( ), Lactococcus lactis (
), Lactococcus lactis ( ).
).
Most of the studies on the succession of microbial population in fresh sausages during refrigerated storage have been carried out with the sausages stored under aerobic normal or modified atmosphere [1,4,6,32]. In these studies, LAB species have been one of the major microorganisms detected together with species belonging to Enterobacteriaceae, Micrococcaceae, Pseudomonas spp., and B. thermosphacta. However, LAB (Lb. sakei, Lb. curvatus/graminis) and B. thermosphacta tended to be the most predominant groups at the end of storage. The degree of abundance of LAB would directly depend not only on storage time but also on the reduction degree of redox potential during storage [49]. On the other hand, in the study by Fougy et al. [7], the sausages were packaged under vacuum and anaerobic 50% CO2-containing atmosphere. The authors, in agreement with the results of the present study, found, using metagenetic 16S rRNA pyrosequencing, Lb. sakei to be the most abundant species in spoiled sausages (after 21 days of storage). Moreover, they also reported the presence of Lactococcus piscium, Carnobacterium divergens, Carnobacterium maltaromaticum, Serratia proteamaculans, and B. thermosphacta.
Lb. sakei together with Lb. curvatus appears to be the dominant LAB species in fermented sausages produced by spontaneous fermentation, and Lb. sakei is the most used LAB species as a starter culture for these sausages [50]. This species has been demonstrated to have an effective metabolic adaptation to the meat environment, and to cold temperature and high NaCl concentration. These abilities explain its growth in refrigerator-stored fresh sausages. This LAB have been demonstrated to be resistant to the presence of the antimicrobials tested in the present study at the levels used.
In contrast with traditional fermented sausages, fresh sausages became spoiled after fermentation, which would be due to differences in the LAB metabolism in a meat environment with higher aw and the concomitant activity of other spoilage microorganisms, i.e., B. thermosphacta and Enterobacteriaceae.
3. Materials and Methods
3.1. Experimental Plan
Three batches of ćevapi, a Bosnian-style fresh sausage, were prepared using lamb lean meat at the pilot plant at the Faculty of Veterinary Medicine, University of León (Spain). Each batch was composed of four treatments: control (C), with no antimicrobial additives in the formulation; hops extract (H), including an aqueous hops extract; essential oil (EO), with ZM’s EO, and with both hops extract and essential oil (HEO). Sausages were packaged in bags under a modified atmosphere (20% of CO2 and 80% N2) and then stored under refrigeration at 2 °C for 35 days. Water activity (aw) was determined at the day of packaging (day 0), and pH, the presence of relevant microbial groups, i.e., lactic acid bacteria (LAB), Enterobacteriaceae, Brochothrix thermosphacta, Micrococcaceae and yeast and mold, and the concentration BA were analyzed weekly (storage days 0, 7, 14, 21, 28, and 35). Moreover, 346 colonies (4–5 per batch, day, and treatment) were picked from De Man Rogosa agar plates used for LAB counts and identified by MALDI-TOF mass spectrometry.
3.2. Lamb Meat, Hops Extract, and Essential Oil
Lamb meat came from the legs of six male Assaf lambs reared at the Instituto de Ganadería de Montaña (CSIC; Grulleros, León, Spain). The lambs were weaned with 14 ± 2 kg of weight and fattened to 50 ± 4 kg of weight ad libitum on a pelleted complete diet based on straw (150 g/kg), cereals (barley, corn and soybean meal; 810 g/kg), molasses (10 g/kg), a mineral-vitamin premix (25 g/kg), and sodium bicarbonate (5 g/kg). The animals were then slaughtered in a local abattoir, and their legs were separated from the right-hand carcasses after 24 h post-mortem and then deboned. The meat was then cut into approximately 3 cm cubes, which were trimmed of visible fat. The lean meat from each leg was packaged under vacuum and frozen (−20 °C) until being used (up to 3 months).
The aqueous hops extract used in the study was obtained from recently cropped Nugget variety hop, with α-acid, β-acid, and co-humulone composition of 4.8–5.3%, 12–16%, and 22–28%, respectively. The hops was provided by a local producer (Orbigo Valley S.L., Madrid, Spain). An amount of 50 g of hops was boiled into 1 L of water for 30 min and the final volume was filled up to 1 L, which was filtered through a Whatman number 1 filter paper (GE Healthcare Europe, Barcelona, Spain) and frozen at −18 °C until further use. ZM’s EO was obtained from the Faculty of Agriculture, University of Tehran, Iran. Crushed dried leaves of ZM plant were transferred to an all-glass Clevenger-type apparatus and steam distilled for 2.5 h. The essential oil was then dried over anhydrous Na2SO4 and stored in opaque glass bottles until further use.
3.3. Sausage Manufacture
Three batches of sausages were produced on different days using the meat from the legs of 2 among the 6 lambs for each of the batches. The sausage-making process was based on a Balkans-style ćevapi recipe. Lamb meat was thawed at 5 °C for 24 h and minced using a butcher’s mincer equipped with a 5 mm diameter sieve. A total of 3.8 kg of minced meat was mixed with salt (80 g) for 10 min and placed into a bowl covered with cling film and stored at 4 °C until the next day (24 h). A mixture of finely cut fresh garlic and pepper was boiled in water for 2 min. The mixture (spices solution) was cooled, filtered, and then stored (4 °C) until the next day. The salted minced meat was divided into four parts of 950 g each, one for each of the four above-mentioned treatments (C, H, EO, and HEO) using the ingredients provided in Table 3. All the portions were mixed (for 5 min) with the spice solution (20 mL/kg) and 3 g/kg of sodium bicarbonate. C, EO, and HEO were also mixed with an amount of water, H and HEO treatment with hops extract, and EO and HEO with essential oil (see Table 3). The amount of hops extract added to the H sausage was equivalent to 1.5 g of hops per kg of sausage (i.e., 30 mL of the solution obtained from boiling 50 g of hops per L), which is that commonly used in brewery. The amount of ZM’s EO used (1 mL/kg) was within the concentration ranges reported for antimicrobial activity of EOs in food [16], i.e., around 0.5–20 mL of EO per kg. When both antimicrobial sources were added, their amounts were halved.

Table 3.
Ingredients and amounts (expressed in g or mL, solid or liquids, respectively) used in the sausage preparation for the experimental treatments.
The sausage mixtures were stuffed into lamb casings (20/22 cm diameter) and drained for 3 h at 12 °C. The sausages were then cut into 100 g portions, which were individually packaged in bags (150 μm plastic film, oxygen permeability of 30 cm3/(m2 × bar × 24 h) at 23 °C and 0% relative humidity) under a 20% CO2 and 80% N2 atmosphere at 750 mbars, and refrigerator-stored (2 °C). One C portion was used for analysis at day 0 and one packaged portion for each of the treatments was sampled after 7, 14, 21, 28, and 35 days of storage for subsequent analysis.
3.4. Analysis of Water Activity, pH, Microbial Content, and Biogenic Amine Production
Water activity (aw) was determined in duplicate at 25 °C using a CX-2 hygrometer (Decagon Devices Inc., Pullman, WA, USA) following the manufacturer’s instructions, and pH using a pHmeter (Model 507; Crison, Barcelona, Spain) according to the International Organization for Standardization (ISO) guideline 2917 [51]. For microbiological analysis, samples of 25 ± 0.1 g of sausages were homogenized with 225 mL of peptone water (0.1% peptone) for 2 min in sterile bags using a Stomacher-400 circulator (Seward, West Sussex, UK). Serial decimal dilutions were prepared, and aliquots of the appropriate dilutions were cultured in duplicate on the corresponding media and incubated, according to the procedure described by the culture media manufacturer, as follows: 1 mL on the De Man-Rogosa-Sharpe agar (Oxoid) with double agar layer at 30 °C for 72 h for LAB; 1 mL in Mannitol Salt Agar (Oxoid) at 35 °C for 48 h for Micrococcaceae; 1 mL in Violet Red Bile Glucose Agar (VRBGA; Oxoid) with double agar layer at 35 °C for 48 h for Enterobacteriaceae; 1 mL in Oxytetracycline Glucose Yeast extract agar (Oxoid) at 22 °C for 5 days for molds and yeast, and 0.1 mL onto the surface of STAA Agar Base (CM 0881; Oxoid) plates containing STA Selective Supplement (0.4 mL/100 mL) and sterilized glycerol (1.5 g/100 mL), at 22 °C for 48 h (only the straw colored oxidase-negative colonies were considered).
Biogenic amine contents were analyzed following the Eerola, Hinkkanen, Lindfors, and Hirvi [52] procedure using a high performance liquid chromatograph (HPLC) Alliance (Waters 2695) equipped with a double wavelength detector (Waters 2996, Waters Corporation, Milford, MA, USA) and a Spherisorb ODS2 column (125 × 4 mm ID; 5 μm; Waters). The standards used for detection and quantification were tryptamine cadaverine dihydrochloride, histamine dihydrochloride, putrescine dihydrochloride, spermidine, spermine, tryptamine hydrochloride, and tyramine hydrochloride (Sigma-Aldrich Química, Madrid, Spain).
3.5. Identification of Lactic Acid Bacteria
From the growth in the MRS plates, 4–5 colonies were picked for each experimental treatment (4), sampling day (7) and batch (3), giving 346 colonies in total. These isolates were then grown in Tryptone Soy Broth (TSB; Bacto, Mt Printchard, Australia) with 0.5% (w/v) of yeast extract (YE; Difco, Leeuwarden, The Netherlands) (TSB-YE) at 37 °C for 24 h. One mL aliquot was centrifuged (12,000 rpm, 3 min) in Eppendorf tubes (Eppendorf Ibérica, San Sebastián de los Reyes, Madrid). The supernatants were discarded, and the pellets were suspended in 1 mL of MRS broth with 50% (v/v) of glycerol. The isolates were maintained at −40 °C for storage purposes. Isolates were recovered for their identification as follows: they were grown at 30 °C on MRS broth (Oxoid) with 0.5% (w/v) of YE (Difco) at 37 °C for 24 h, and then a loopful of bacteria was sub-cultured in MRS agar (Oxoid).
The analysis was carried out at the Laboratory for Instrumental Analysis, University of Valladolid (Valladolid, Spain). For the analysis, one colony from the MRS plate was picked using a sterilized toothpick and smeared gently onto a MALDI-TOF target plate (Bruker Daltonik GmbH, Leipzig, Germany). After air-drying, 1 µL of formic acid was added. The dried sample was overlaid with 1 μL matrix solution containing 10 mg/mL α-cyano-4-hydroxycinnamic acid (HCCA) in a mixture of acetonitrile, deionized water, and trifluoracetic acid (50/47.5/2.5, v/v/v). The target plate with samples were introduce in the MALDI-TOF equipment for analysis. Not all the bacteria were amenable to analysis: approximately 30% of the isolates were not accurately identified, i.e., identification score at genus level <1.7. For these isolates, the analysis was repeated including an ethanol extraction tube protocol before analysis to extract ribosomal proteins according to the manufacturer’s instructions (Bruker Daltonik). Briefly, one colony from MRS plates was sub-cultured in TSB + 0.5% (w/v) of yeast extract at 35 °C overnight. One mL aliquot of the isolate was transferred into an Eppendorf tube and centrifuged at 12,000 rpm for 2 min. The supernatant was discarded, and the pellet was mixed thoroughly with 1 mL of deionized water and centrifuged at the same speed and time. This stage was performed twice. Afterward, 900 µL of absolute ethanol and 300 µL deionized water were added, mixed for 2 min, and the tube was centrifuged at 15,000 rpm for 5 min. The supernatant was discarded, and the pellet was air-dried for a minimum of 30 min until dryness. The pellet was re-suspended with 15 µL of formic acid (70%) and mixed thoroughly. Moreover, the mix was kept for 5 min at room temperature and then 15 µL of acetonitrile was added and mixed. The mixture was centrifuged at 15,000 rpm for 3 min and subsequently, 1 mL of the supernatant was spotted onto a MALDI-TOF target plate. After being air-dried, the sample was overlaid with 1 μL of matrix solution (HCCA).
For identification, each series of measurements was preceded by a calibration step with a bacterial test standard (BTS 155 255343; Bruker Daltonik) to validate the run. Mass spectra were generated by a Flex Analysis MALDI-TOF mass spectrometer (Bruker Daltonik) equipped with a nitrogen laser (l1⁄4337 nm) operating in linear positive ion detection mode under the Bruker Flex Control software (Bruker Daltonik). The Autoflex LT Speed was periodically calibrated by using the Bruker Daltonik Escherichia coli bacterial test standard DH5. Automated analysis of the raw spectral data was performed by the MALDI BioTyper automation (version 3.1) software (Bruker Daltonik) using a library of 5627 main spectra (MSPs; database update of 7/15/2015). Identifications at species or genus level were considered if scores were above 2.0 and 1.7 respectively, according to the report generated by Bruker Compass [53,54].
3.6. Statistical Analysis
Data on microbial counts and BA levels were analyzed by two-way analysis of variance (ANOVA) with treatment and storage day as fixed factors. When the fixed factors or their interaction showed significant differences (P < 0.05), the ANOVA was followed by the Tukey’s post-hoc test. For the results of the LAB identification, a contingency table (4 by 2; treatment by positive or negative) chi-square analysis was used to test the eventual dependence between treatment and the frequency of the presence in the sausages of the main genus or species identified, considering the entire storage period. The statistical analysis was performed using the SPSS Statistics software (version 24; IBM, Somers, NY, USA).
4. Conclusions
The results from this study demonstrate that Balkan-style fresh sausages stored under anaerobic atmosphere are already fermented in the first week of storage and the predominant responsible species are Lactobacillus spp., specifically Lb. sakei. The fermentation was compatible with a controlled growth of B. thermosphacta and Enterobacteriaceae and resulted in BA production to a concerning level, thus suggesting that the contents of BA in anaerobic MAP fresh sausages should be controlled. The use of Zataria multiflora Boiss EO, hops extract, or the combination of both at the levels used did not significantly affect the microbial development in the sausages. More studies using higher amounts of these antimicrobial sources, different combinations with other antimicrobials, extracts with higher concentrations of active compounds, or previous encapsulation, would be needed to achieve their effectiveness in fresh sausage preservation.
Author Contributions
F.J.G. and S.A., growth lambs and meat preparation; D.E.C., J.M., S.O., sausage preparation; D.E.C., I.C., J.M., E.J.Q., A.K., microbial analysis; D.E.C., J.M. and I.C. biogenic amines; all the authors have contributed in planning the experiment and writing, reviewing, and editing the manuscript.
Funding
This research was funded by Junta de Castilla y León, project CSI042 P17. D.E. Carballo is grateful for a doctoral grant from CONACYT (MEX/Ref. 288189).
Acknowledgments
The authors would like to thank Mr. José Manuel Martínez Fernández from Orbigo Valley SL for providing the hops and to Javier Gutiérrez Reguera from the LTI, University of Valladolid for the technical support on the MALDI-TOF analysis.
Conflicts of Interest
None of the authors has a financial or personal relationship with other people/organizations that could inappropriately influence or bias the paper or present other types of conflict interest.
References
- Cocolin, L.; Rantsiou, K.; Iacumin, L.; Urso, R.; Cantoni, C.; Comi, G. Study of the ecology of fresh sausages and characterization of populations of lactic acid bacteria by molecular methods. Appl. Environ. Microbiol. 2004, 70, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- González-Tenorio, R.; Fonseca, B.; Caro, I.; Fernández-Diez, A.; Kuri, V.; Soto, S.; Mateo, J. Changes in biogenic amine levels during storage of Mexican-style soft and Spanish-style dry-ripened sausages with different aw values under modified atmosphere. Meat Sci. 2013, 94, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Effect of an argon-containing packaging atmosphere on the quality of fresh pork sausages during refrigerated storage. Food Control 2010, 21, 1331–1337. [Google Scholar]
- Raimondi, S.; Nappi, M.R.; Sirangelo, T.M.; Leonardi, A.; Amaretti, A.; Ulrici, A.; Magnani, R.; Montanari, C.; Tabanelli, G.; Gardini, F.; et al. Bacterial community of industrial raw sausage packaged in modified atmosphere throughout the shelf life. Int. J. Food Microbiol. 2018, 280, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Djenane, D.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Effect of different concentrations of carbon dioxide and low concentration of carbon monoxide on the shelf-life of fresh pork sausages packaged in modified atmosphere. Meat Sci. 2005, 71, 563–570. [Google Scholar] [CrossRef]
- Dias, F.S.; Ramos, C.L.; Schwan, R.F. Characterization of spoilage bacteria in pork sausage by PCR-DGGE analysis. Food Sci. Technol. 2013, 33, 468–474. [Google Scholar] [CrossRef]
- Fougy, L.; Desmonts, M.-H.; Coeuret, G.; Fassel, C.; Hamon, E.; Hézard, B.; Champomier-Vergès, M.-C.; Chaillou, S. Reducing salt in raw pork sausages increases spoilage and correlates with reduced bacterial diversity. Appl. Environ. Microbiol. 2016, 82, 3928–3939. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Pintado, T.; Jiménez-Colmenero, F. Biogenic amine formation in refrigerated fresh sausage “chorizo” keeps in modified atmosphere. J. Food Biochem. 2012, 36, 449–457. [Google Scholar] [CrossRef]
- Hugo, C.J.; Hugo, A. Current trends in natural preservatives for fresh sausage products. Trends Food Sci. Technol. 2015, 45, 12–23. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Španinger, E.; Košir, I.; Knez, Ž.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef]
- Kramer, B.; Thielmann, J.; Hickisch, A.; Muranyi, P.; Wunderlich, J.; Hauser, C. Antimicrobial activity of hop extracts against foodborne pathogens for meat applications. J. Appl. Microbiol. 2015, 118, 648–657. [Google Scholar] [CrossRef]
- Bogdanova, K.; Kolar, M.; Langova, K.; Dusek, M.; Mikyska, A.; Bostikova, V.; Bostik, P.; Olsovska, J. Inhibitory effect of hop fractions against Gram-positive multi-resistant bacteria. A pilot study. Biomed. Pap. 2018, 162, 276–283. [Google Scholar] [CrossRef]
- Singh, M.; Smith, J.; Bailey, M. Using natural antimicrobials to enhance the safety and quality of poultry. In Handbook of Natural Antimicrobials for Food Safety and Quality; Taylor, T.M., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 375–401. [Google Scholar]
- Tiwari, B.K.; Valdramidis, V.P.; O’ Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of natural antimicrobials for food preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems–A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jo, C. Essential oils as potential antimicrobial agents in meat and meat products: A review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Nevárez-Moorillon, G.V.; Caro, I.; Quinto, E.J.; Mateo, J. Natural antimicrobial agents to improve foods shelf life. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Nederland, 2019; pp. 125–157. [Google Scholar]
- Saei-Dehkordi, S.S.; Tajik, H.; Moradi, M.; Khalighi-Sigaroodi, F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol. 2010, 48, 1562–1567. [Google Scholar] [CrossRef]
- Shakeri, M.-S.; Shahidi, F.; Beiraghi-Toosi, S.; Bahrami, A. Antimicrobial activity of Zataria multiflora Boiss. essential oil incorporated with whey protein based films on pathogenic and probiotic bacteria. Int. J. Food Sci. Technol. 2011, 46, 549–554. [Google Scholar] [CrossRef]
- Rezaeigolestani, M.; Misaghi, A.; Khanjari, A.; Basti, A.A.; Abdulkhani, A.; Fayazfar, S. Antimicrobial evaluation of novel poly-lactic acid based nanocomposites incorporated with bioactive compounds in-vitro and in refrigerated vacuum-packed cooked sausages. Int. J. Food Microbiol. 2017, 260, 1–10. [Google Scholar] [CrossRef]
- Basti, A.A.; Gandomi, H.; Noori, N.; Khanjari, A. Shirazi thyme (Zataria multiflora Boiss) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Nederland, 2016; pp. 731–736. [Google Scholar]
- Tajik, H.; Aminzare, M.; Mounesi Raad, T.; Hashemi, M.; Hassanzad Azar, H.; Raeisi, M.; Naghili, H. Effect of Zataria multiflora Boiss essential oil and grape seed extract on the shelf life of raw buffalo patty and fate of onoculated Listeria monocytogenes. J. Food Process. Preserv. 2015, 39, 3005–3013. [Google Scholar] [CrossRef]
- Torab, M.; Basti, A.A.; Khanjari, A. Effect of free and nanoencapsulated forms of Zataria multiflora boiss. Essential oil on some microbial and chemical properties of beef burger. Carpathian J. Food Sci. Technol. 2017, 9, 93–102. [Google Scholar]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R. Effectiveness of Zataria multiflora Boiss essential oil and grape seed extract impregnated chitosan film on ready-to-eat mortadella-type sausages during refrigerated storage. J. Sci. Food Agric. 2011, 91, 2850–2857. [Google Scholar] [CrossRef]
- da Silveira, S.M.; Luciano, F.B.; Fronza, N.; Cunha, A.; Scheuermann, G.N.; Vieira, C.R.W. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT Food Sci. Technol. 2014, 59, 86–93. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Incoronato, A.L.; Conte, A.; Del Nobile, M.A. Shelf life of reduced pork back-fat content sausages as affected by antimicrobial compounds and modified atmosphere packaging. Int. J. Food Microbiol. 2011, 150, 1–7. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agrawal, R.K.; Gurunathan, K.; Kumar, S.; Singh, T.P. Use of various essential oils as bio preservatives and their effect on the quality of vacuum packaged fresh chicken sausages under frozen conditions. LWT Food Sci. Technol. 2017, 81, 118–127. [Google Scholar] [CrossRef]
- Šojić, B.; Pavlić, B.; Zeković, Z.; Tomović, V.; Ikonić, P.; Kocić-Tanackov, S.; Džinić, N. The effect of essential oil and extract from sage (Salvia officinalis L.) herbal dust (food industry by-product) on the oxidative and microbiological stability of fresh pork sausages. LWT 2018, 89, 749–755. [Google Scholar] [CrossRef]
- Lerasle, M.; Federighi, M.; Simonin, H.; Anthoine, V.; Rezé, S.; Chéret, R.; Guillou, S. Combined use of modified atmosphere packaging and high pressure to extend the shelf-life of raw poultry sausage. Innov. Food Sci. Emerg. Technol. 2014, 23, 54–60. [Google Scholar] [CrossRef]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef]
- Benson, A.K.; David, J.R.D.; Gilbreth, S.E.; Smith, G.; Nietfeldt, J.; Legge, R.; Kim, J.; Sinha, R.; Duncan, C.E.; Ma, J.; et al. Microbial successions are associated with changes in chemical profiles of a model refrigerated fresh pork sausage during an 80-day shelf life study. Appl. Environ. Microbiol. 2014, 80, 5178–5194. [Google Scholar] [CrossRef]
- Narasimha Rao, D.; Sachindra, N.M. Modified atmosphere and vacuum packaging of meat and poultry products. Food Rev. Int. 2002, 18, 263–293. [Google Scholar] [CrossRef]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Tamplin, M.; Chandry, P.S. Insight into the genome of Brochothrix thermosphacta, a problematic meat spoilage bacterium. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J. Food Spoilage Microorganisms; Blackburn, C.D.W., Ed.; Woodhead Publishing: Cambridge, UK, 2006. [Google Scholar]
- Gribble, A.; Brightwell, G. Spoilage characteristics of Brochothrix thermosphacta and campestris in chilled vacuum packaged lamb, and their detection and identification by real time PCR. Meat Sci. 2013, 94, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Reale, A.; Maiorano, G.; Coppola, R. Shelf life of fresh sausages stored under modified atmospheres. J. Food Prot. 2005, 68, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Chiavaro, E.; Zanardi, E.; Bottari, B.; Ianieri, A. Efficacy of different storage practices in maintaining the physicochemical and microbiological properties of fresh pork sausage. J. Muscle Foods 2008, 19, 157–174. [Google Scholar] [CrossRef]
- Vignolo, G.; Castellano, P.; Fontana, C.; Cocconcelli, P.S.; Fadda, S. Lactic acid bacteria in meat fermentations: Role of autochthonous starter. In Lactic Acid Bacteria: Microbiological and Functional Aspects; Vinderola, G., Ouwehand, A., Salminen, S., von Wright, A., Eds.; CRC Press: Boca Ratón, FL, USA, 2019; p. 764. [Google Scholar]
- Durlu-Özkaya, F.; Ayhan, K.; Vural, N. Biogenic amines produced by Enterobacteriaceae isolated from meat products. Meat Sci. 2001, 58, 163–166. [Google Scholar] [CrossRef]
- Suzzi, G.; Gardini, F. Biogenic amines in dry fermented sausages: A review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef]
- Nowak, A.; Czyzowska, A. In vitro synthesis of biogenic amines by Brochothrix thermosphacta isolates from meat and meat products and the influence of other microorganisms. Meat Sci. 2011, 88, 571–574. [Google Scholar] [CrossRef]
- Lu, S.; Ji, H.; Wang, Q.; Li, B.; Li, K.; Xu, C.; Jiang, C. The effects of starter cultures and plant extracts on the biogenic amine accumulation in traditional Chinese smoked horsemeat sausages. Food Control 2015, 50, 869–875. [Google Scholar] [CrossRef]
- Santos, M.H.S. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 2005, 44, 489–599. [Google Scholar] [CrossRef]
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic amines in cheese and other fermented foods: A Review. J. Food Prot. 1991, 54, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Halász, A.; Baráth, Á.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Sattler, J.; Häfner, D.; Klotter, H.J.; Lorenz, W.; Wagner, P.K. Food-induced histaminosis as an epidemiological problem: Plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO). Agents Actions 1988, 23, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Del Blanco, A.; Caro, I.; Quinto, E.J.; Mateo, J. Quality changes in refrigerated stored minced pork wrapped with plastic cling film and the effect of glucose supplementation. Meat Sci. 2017, 126, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.A.; Fadda, S.; Cocconcelli, P.S.; Vignolo, G. Lactic acid bacteria in meat fermentations. In Lactic acid Bacteria: Microbiological and Functional Aspects; Lahtinen, S., Ouwehand, A.C., Salminen, S., von Wright, A., Eds.; CRC Press: Boca Ratón, FL, USA, 2011; pp. 247–259. [Google Scholar]
- Meat and Meat Products: Measurment of pH; ISO: 2917; International Organization for Standardization: Geneva, Switzerland, 1999.
- Eerola, S.; Hinkkanen, R.; Lindfors, E.; Hirvi, T. Liquid chromatographic determination of biogenic amines in dry sausages. J. AOAC Int. 1993, 76, 575–577. [Google Scholar]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix-assisted laser desorption ionization-time of flight mass spectrometry: A fundamental shift in the routine practice of clinical microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef]
- Patel, R. Matrix-assisted laser desorption ionization-time of flight mass spectrometry in clinical microbiology. Clin. Infect. Dis. 2013, 57, 564–572. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).