Abstract
Microbial biofilms pose significant medical and industrial challenges due to their resistance to conventional antimicrobials, accounting for 40–80% of bacteria in various environments. This resistance primarily results from the extracellular polymeric matrix, a protective network of sugars, proteins, and other molecules produced by bacteria. The matrix restricts antibiotic penetration, facilitates microbial communication, and retains nutrients. Consequently, novel strategies to counteract biofilms are under investigation. Fatty acids have emerged as promising prebiotic agents, defined as substances that stimulate the growth of beneficial bacteria. These compounds can disrupt biofilm structure and increase microbial susceptibility to treatment. Short- and medium-chain fatty acids demonstrate direct antimicrobial activity and can alter microbial community composition, thereby inhibiting biofilm formation in several pathogens, including oral species. For instance, omega-3 fatty acids effectively inhibit Staphylococcus aureus and Pseudomonas aeruginosa biofilms through membrane disruption and quorum sensing (QS) inhibition. Additionally, long-chain fatty acids, particularly omega-3 and omega-6 polyunsaturated fatty acids, exhibit anti-inflammatory and antibacterial properties. This review synthesises current evidence on fatty acids as prebiotics, emphasising their mechanisms of action and therapeutic potential against drug-resistant biofilm-associated infections. Given the increasing prevalence of antimicrobial resistance, unsaturated and essential fatty acids rep-resent promising candidates for innovative biofilm-control strategies.
1. Introduction
The resilience of biofilms against antimicrobial agents and their persistent adherence to both biological and non-biological surfaces present significant challenges in healthcare and food processing settings. Microorganisms embedded within biofilm matrices exhibit altered physiological states, reduced metabolic activity, and heightened stress responses, which together enhance resistance to conventional antibiotics and disinfectants. Consequently, biofilm-associated infections often become chronic, recurrent, and difficult to eradicate, thereby significantly contributing to the global burden of antimicrobial resistance (AMR) [1,2,3].
The reduced effectiveness of conventional antimicrobials has intensified interest in alternative or complementary strategies to prevent biofilm formation or disrupt established biofilms without fostering resistance. In this context, naturally occurring compounds with multifunctional biological activities have garnered significant attention [4]. Fatty acids are particularly promising due to their structural diversity, broad-spectrum antimicrobial properties, and capacity to interfere with key biofilm-related processes [5,6]. Prebiotic short-chain fatty acids function as potent quorum-sensing (QS) inhibitors, suppressing signals that regulate biofilm formation and maintenance. Medium-chain and long-chain fatty acids, primarily derived from dietary sources, have also demonstrated antimicrobial and antibiofilm activities, often attributed to their ability to disrupt microbial membranes and interfere with essential signalling pathways [5,6,7,8,9,10,11]. Comparative analyses of these fatty acids and established quorum inhibitors elucidate the relationship between chain length and antibiofilm efficacy, suggesting a unifying mechanism underlying their effectiveness.
Despite the growing body of research on fatty acids as antimicrobial and antibiofilm agents, the literature remains fragmented and conceptually ambiguous. Distinctions among chain-length-dependent mechanisms, prebiotic versus bioactive effects, and indirect versus direct antibiofilm actions are frequently inadequately addressed. Furthermore, translational challenges related to formulation, delivery, and in vivo efficacy remain underexplored.
This review offers a critical and integrative synthesis of the role of fatty acids in biofilm control, with particular emphasis on chain-length-dependent mechanisms, microbiota-related effects, and translational limitations. The objective is to clarify current knowledge gaps and propose future research directions for the strategic application of fatty acids as antibiofilm agents.
The narrative review was conducted through systematic searches of PubMed, Scopus, and Web of Science for peer-reviewed articles published up to 2025. Search terms included combinations of “fatty acids”, “short-chain fatty acids”, “medium-chain fatty acids”, “long-chain fatty acids”, “polyunsaturated fatty acids”, “prebiotic”, “biofilm”, “antibiofilm activity”, “quorum sensing”, and “microbiota”. Additional relevant studies were identified by manually screening the reference lists of key articles.
2. Fatty Acids as Prebiotics: Conceptual Boundaries and Critical Perspectives
The International Scientific Association for Probiotics and Prebiotics (ISAPP) has updated the consensus definition of a prebiotic to “a substrate that is selectively utilised by host microorganisms to confer a health benefit.” This revision broadens the concept to potentially include non-carbohydrate compounds [12,13]. The updated definition emphasises the functionality of selectively fermented ingredients that induce specific changes in gastrointestinal microbiota composition or activity, thereby conferring benefits to the host [14,15,16,17,18,19]. This expanded perspective allows for the inclusion of compounds with diverse chemical structures as prebiotics, provided they are selectively utilised by the microbiota and confer a health benefit [2,20]. A substance is classified as a prebiotic if it possesses a defined structure and composition, is selectively utilised by the host microbiota, results in measurable modulation of the microbiome, and demonstrates a health benefit. These criteria distinguish prebiotics from other compounds that may influence the microbiota but lack selectivity or specific health benefits. Furthermore, a prebiotic must be resistant to gastric acidity, mammalian digestive enzymes, and gastrointestinal absorption, ensuring it remains intact until reaching the colon for fermentation by the intestinal microflora. Selective fermentation alters the composition or activity of the intestinal microbiota, thereby conferring health benefits to the host [21]. These benefits include supporting probiotics, which are essential for establishing and maintaining a favourable environment for beneficial gut microbiota. Distinguishing the functional differences among short-, medium-, and long-chain fatty acids is essential for accurately delineating their prebiotic potential, as these molecules exert distinct effects on the gut microbiota and host physiology. Clarifying these distinctions prevents conceptual ambiguity and semantic inflation of the term “prebiotic.” A more rigorous and biologically consistent framework should classify SCFAs as microbiota-derived signalling metabolites, while MCFAs and LCFAs should be considered bioactive dietary lipids with secondary microbiota-modulating properties. Recognising these boundaries is critical for accurate interpretation of experimental data and for guiding future mechanistic and translational research in this field [22]. SCFAs, which contain fewer than six carbon atoms and are primary metabolites of microbial fermentation of indigestible dietary fibre, play a critical role in modulating both intestinal and systemic health. Acetate, propionate, and butyrate exert prebiotic effects by selectively stimulating the growth of beneficial bacteria and inhibiting pathogenic species, a process also mediated by the reduction in caecal pH resulting from their production [23]. These fatty acids are key regulators of the host immune system, influencing processes such as phagocytosis, chemokine production, and cell signalling pathways [24]. Notably, butyrate is essential for intestinal health and immune regulation, serving as both an energy source for colonocytes and a signalling molecule with anti-inflammatory properties [25,26]. In contrast, MCFAs and LCFAs, including PUFAs, which are primarily obtained from the diet, do not meet the strict ISAPP criteria for prebiotics. However, increasing evidence suggests that these fatty acids can indirectly modulate gut microbial ecosystems through mechanisms such as antimicrobial pressure, competitive exclusion, and host-mediated effects, thereby shifting microbial composition and metabolic activity. These effects represent ecological restructuring rather than selective microbial utilisation and therefore do not align with the classical definition of prebiotic activity. Dietary fatty acids play significant roles in energy metabolism and influence the microbiota and intestinal physiology by modulating intestinal permeability and exerting anti-inflammatory activity, rather than through direct fermentation [27]. Although MCFAs and LCFAs are not conventionally classified as prebiotics, they exert indirect effects on gut microbiota composition and activity. MCFAs can modulate intestinal permeability and inflammation, thereby altering the environment for resident microorganisms and exhibiting direct antimicrobial properties that influence gut microbial composition [28]. LCFAs primarily support nutrient absorption and host energy metabolism and may also indirectly affect the microbiota by altering substrate availability and intercellular signalling. While these effects do not directly stimulate beneficial microbial species, the promotion of beneficial gut bacteria and the inhibition of harmful species [29] foster a healthier gut environment and enhance the production of beneficial metabolites, such as SCFAs [30]. These metabolites are vital for maintaining colonic epithelial health, strengthening the gut barrier, modulating immune response, and regulating systemic metabolism [31]. By being selectively utilised by beneficial microorganisms, these compounds contribute to bacteriocin production and inhibit pathogenic bacteria, thereby supporting a healthier gut microbiome [32]. Certain PUFAs, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exhibit anti-inflammatory and anti-diabetic properties, effects that are partly mediated by interactions with the gut microbiome [16,20,33]. These interactions demonstrate that fatty acids can function both as direct modulators of microbial communities and as indirect contributors to host systemic health through microbiome-mediated pathways [2]. Additionally, these fatty acids can alter the gut environment by modifying pH and oxygen levels, creating conditions less favourable for dysbiotic bacteria [34,35,36]. Notably, n-3 PUFAs restore eubiosis after dysbiosis and enhance SCFA production by acting as prebiotics for specific bacterial families, such as Bacteroidetes and Lachnospiraceae [23,37,38]. In individuals with inflammatory bowel disease (IBD), n-3 PUFA supplementation has been observed to restore a healthier microbiota composition, characterised by decreases in Faecalibacterium and increases in Lachnospiraceae and Bacteroidetes [23,38,39,40]. The effectiveness of omega-3 PUFAs appears to be dose-dependent, with studies indicating that higher doses (e.g., 60–90 mg in animal models) may be required to fully restore certain microbial taxa [41]. Human trials have employed various dosages, including 5 g/day of fish oil providing 1.9–2.2 g EPA and 1.1 g DHA [42], or DHA-enriched canola oil [37]. Although the sample sizes of studies demonstrating these direct changes in IBD patients vary, systematic reviews synthesising these findings suggest general trends [39]. However, some comprehensive meta-analyses of randomised controlled trials (RCTs), including studies with up to 69 RCTs, have reported little or no significant impact of omega-3 supplementation on IBD treatment or inflammatory status. This highlights potential study heterogeneity and underscores the need for further targeted research [43]. Table 1 summarises the definition of prebiotics and the mechanisms of action of fatty acids as prebiotics.

Table 1.
Definition of prebiotics and mechanisms of action of fatty acids as prebiotics.
2.1. Short-Chain Fatty Acids (SCFAs) as Prebiotics
Alterations in microbial composition, especially the proliferation of beneficial taxa and modifications in their metabolic activities, illustrate the intricate relationship between dietary fatty acids and the gut microbiome in both health and disease.
Among lipids, n-3 PUFAs, particularly those derived from marine sources, significantly modulate the gut microbiota. They increase beneficial bacteria, such as Lactobacillus, Lachnospiraceae, Bacteroidetes, and Roseburia, while reducing Faecalibacterium and Barnesiella. These changes promote butyrate production and gut homeostasis and attenuate inflammatory responses [23,38,42,43,44,45]. The evidence indicates that n-3 PUFAs possess substantial prebiotic potential, particularly in enhancing beneficial microbial populations and maintaining a balanced gut environment [46]. In animal models, marine lipids and n-3 PUFAs also promote the growth of Bifidobacteria and commensal lactic acid bacteria, thereby supporting gastrointestinal stability even under high-fat dietary conditions [47]. Alterations in gut microbiota composition are linked to increased production of short-chain fatty acids, especially butyrate, and reduced levels of pro-inflammatory mediators, which contribute to systemic health benefits [47,48,49]. These findings underscore the integral role of the gut microbiota in mediating the systemic effects of n-3 PUFAs, particularly in metabolic regulation and inflammation [50]. For instance, omega-3 PUFA supplementation increases the abundance of Bacteroides and Coprococcus species while reducing Collinsella species, which are linked to fatty liver, thereby demonstrating a direct effect on gut microbial populations [51]. The method of n-3 PUFA administration can also significantly affect the microbiota, as certain functional beverages exert a greater influence on butyrate-producing bacterial genera [52]. Furthermore, conjugated linoleic acid (CLA) and monounsaturated fatty acids exhibit notable prebiotic potential by selectively promoting beneficial gut bacteria and inhibiting pathogenic strains [53]. Fatty acids also support the repair of compromised intestinal mucosa [54]. Recent in vitro models, such as the Mucosal SHIME (Simulator of the Human Intestinal Microbial Ecosystem), indicate that ω-3 PUFAs primarily modulate mucolytic species, such as Akkermansia muciniphila, increasing their abundance and enhancing their metabolic activities [55]. Collectively, these findings demonstrate the substantial impact of n-3 PUFAs on the gut microbiome, particularly their capacity to enhance beneficial microbial populations and metabolic outputs, which is critical for maintaining intestinal and systemic health [56,57,58].
2.2. Medium- and Long-Chain Fatty Acids with Prebiotic Potential
Medium-chain fatty acids, such as those found in coconut oil, inhibit harmful bacteria and promote beneficial species. Long-chain fatty acids also counteract pathogens, modulate immune responses, and influence the gut microbiome, thereby helping to prevent inflammation [23]. Their interactions with gut bacteria involve alterations in fat-derived signalling molecules, such as oxylipins, which can either induce or reduce inflammation [59]. Conjugated linoleic acids further exemplify this complexity: cis-9, trans-11 CLA reduces inflammation by modulating immune cells, whereas trans-10, cis-12 CLA may exacerbate inflammatory responses [60].
Oxylipins serve as key modulators of the gut microbiome. Omega-6-derived oxylipins, including those from linoleic, linolenic, arachidonic, 5-hydroxyeicosatetraenoic acid (5-HETE), and adrenic acids, as well as plasma omega-3-derived oxylipins, are negatively associated with Sutterella. Oxylipins derived from arachidonic acid also show a negative correlation with Proteobacteria. Both omega-3 and omega-6 oxylipins regulate intestinal alkaline phosphatase, an enzyme that degrades bacterial toxins [59]. The ratio of omega-6 to omega-3 oxylipins is inversely associated with Clostridium cluster IV and Butyricimonas [59]. These findings indicate that dietary fats indirectly support gut health and modulate inflammation through selective and complex interactions with gut microbes, suggesting that dietary oxylipins may facilitate targeted interventions. The distinct effects of omega-3- and omega-6-derived oxylipins further underscore their opposing roles in gut ecology and disease risk [59,61]. Diets high in omega-3 fatty acids promote the growth of beneficial bacteria, such as Bacteroidetes and Firmicutes, while reducing populations of pro-inflammatory species. Conversely, omega-6-derived oxylipins from linoleic and dihomo-γ-linolenic acids are negatively associated with Acidaminococcus and Phascolarctobacterium. Increasing omega-3 intake and reducing omega-6 consumption may beneficially modulate the gut microbiota, metabolism, and immune function [59]. Elucidating the effects of lipid mediators on bacterial growth and activity is essential for developing precision nutrition strategies to address dysbiosis and inflammation [62,63,64]. Table 2 provides a summary of the effects of fatty acids, particularly n-3 polyunsaturated fatty acids (PUFAs), on gut microbiota and metabolic outcomes.

Table 2.
Effects of fatty acids on gut microbiota and metabolic outcomes.
3. Biofilm
A biofilm is a structured microbial community embedded within a self-produced matrix that adheres to biotic or abiotic surfaces [65]. This three-dimensional network, which may be homogeneous or heterogeneous [66], consists of extracellular polymeric substances, including exopolysaccharides, proteins, lipids, and nucleic acids, collectively referred to as the matrixome [67]. The matrix imparts mechanical stability, facilitates adhesion, and protects against host defences and antimicrobials [68,69], resulting in emergent properties such as enhanced surface attachment, structural heterogeneity, and antimicrobial tolerance [67,70]. The structural and biochemical characteristics of the matrixome confer additional emergent properties, including increased surface adhesion, spatial and chemical heterogeneity, and pronounced antimicrobial recalcitrance [67]. Consequently, biofilm-associated microbes are distinguished from their planktonic counterparts by their ability to acquire resources and persist in hostile environments [70,71].
The typical process of bacterial biofilm development is illustrated in Figure 1.
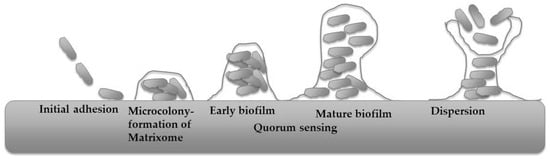
Figure 1.
Typical process of bacterial biofilm development (modified from [71]).
The process begins with the reversible attachment of planktonic cells, which is influenced by substrate characteristics such as roughness and hydrophobicity, as well as by hydrophobic, electrostatic, and Lifshitz–van der Waals forces. These factors complicate catheter sterilisation and present challenges for pipeline maintenance. Cellular appendages, including flagella, pili, fimbriae, and curli, also contribute to this initial attachment [72,73,74]. As cells synthesise extracellular polymeric substances (EPS), attachment transitions to an irreversible state, facilitated by adhesins such as collagen-binding proteins, lipopolysaccharides, and type IV pili [75,76]. Once stable attachment is established, cells proliferate, migrate across the surface, and form microcolonies, particularly under elevated cyclic di-GMP concentrations [77]. After microcolony formation, the maturation stage ensues. During this phase, microcolonies expand into macrocolonies, accompanied by increased production of EPS. This expansion produces a complex architecture with nutrient and waste channels that support metabolic activity [78,79]. Matrixome components, including polysaccharides, proteins, extracellular DNA, and lipids, stabilise the structure and enhance resistance to environmental stressors [80,81]. The viscoelastic properties of amyloids and cellulose contribute to both structural rigidity and adaptability [82]. Quorum-sensing mechanisms enable coordinated behaviour and phenotypic heterogeneity, allowing subpopulations to adapt to nutrient and oxygen gradients [83,84]. Ultimately, dispersal of cells from the mature biofilm enables recolonisation of new niches, thereby completing the biofilm life cycle [85,86].
3.1. Fatty Acids as Antibiofilm Agents
Fatty acids represent a promising strategy for antibiofilm therapy, as they can enhance the efficacy of existing antibiotic treatments and reduce bacterial resistance. These compounds function as multifunctional biofilm disruptors, effectively dismantling both bacterial and fungal biofilms. Unsaturated fatty acids, such as oleic acid and linoleic acid, demonstrate efficacy against diverse bacterial biofilms by disrupting initial cellular attachment or promoting biofilm dispersal [87,88]. Their broad-spectrum antibiofilm activity against various microbial pathogens supports their potential application in clinical infection management [6]. Certain fatty acids, including cis-2-decenoic acid (C2DA), serve as signalling molecules that induce biofilm dispersal and sensitise persister cells to antimicrobial agents, thereby expanding their functional scope beyond direct eradication [89]. This dispersal often occurs at sub-inhibitory concentrations, where fatty acids modulate cellular communication pathways or directly affect the biofilm matrix without inhibiting bacterial proliferation [89]. Omega-3 PUFAs have attracted considerable attention due to their diverse biological activities, particularly their potential as antibiofilm agents and disruptors of the extracellular matrixome [90,91]. Their amphipathic structure facilitates membrane disruption [92]. Although the development of conventional antibiotics initially overshadowed research into the antimicrobial properties of fatty acids, the rise in antibiotic resistance has renewed interest in unsaturated fatty acids as alternative or adjunct antimicrobial agents [93,94,95]. This renewed focus is supported by evidence that fatty acids can penetrate biofilm protective barriers, which often contribute to the reduced efficacy of standard antibiotics [22]. Monounsaturated and polyunsaturated fatty acids interact directly with bacterial membranes, compromising membrane integrity and inducing cell lysis, a process essential for accessing biofilm-embedded cells [95]. In addition to direct lysis, certain fatty acids act as diffusible signal factors in bacterial communication, promoting biofilm dispersal and inhibiting biofilm formation across diverse species [89]. These properties establish fatty acids as promising candidates for innovative antibiofilm therapies, particularly due to their ability to act synergistically with conventional antibiotics. This synergy arises from increased membrane permeability and alters efflux activity, which enhance intracellular antibiotic uptake. For example, myristoleic acid increases the antibiofilm efficacy of aminoglycoside antibiotics by promoting their penetration into the biofilm matrix, likely due to its surfactant-like properties [96]. Recent studies indicate that combining unsaturated fatty acids, such as palmitoleic acid and linoleic acid, with vancomycin leads to significant reductions in both methicillin-sensitive and methicillin-resistant Staphylococcus aureus (MRSA) populations, including antibiotic-tolerant cells [97]. Certain fatty acids can overcome multidrug resistance in pathogens such as Pseudomonas aeruginosa by targeting key virulence factors and biofilm-forming pathways, which is particularly valuable for addressing persistent infections [22]. Research also demonstrates that alpha-linolenic acid, a precursor to longer-chain omega-3 fatty acids, disrupts P. aeruginosa biofilm formation and reduces virulence factor production [22]. Synthetic unsaturated fatty acids have also shown selective antibacterial activity against MRSA [98]. The potential of fatty acids as antibiotic adjuvants extends beyond their direct antimicrobial effects, as they can enhance the efficacy of existing antibiotics and provide a strategic advantage in combating antimicrobial resistance [6]. Their biocompatibility is essential for clinical application, whether used as standalone agents or as enhancers of current antimicrobials, particularly given the significantly reduced susceptibility of biofilms to antibiotics compared to planktonic cells. Investigating fatty acids as antibiotic adjuvants is therefore a pivotal strategy for improving the effectiveness of conventional treatments against resistant pathogens and persistent biofilms. Recent research on omega-3 PUFAs, such as DHA, has confirmed their safety and efficacy as antibiofilm agents against pathogens, including S. aureus and MRSA, without inducing ica-ADBC-dependent biofilm formation or stress responses that could increase antibiotic resistance [91,99].
3.1.1. Inhibition of Initial Adhesion and Early Biofilm Development
Initial adhesion represents a critical stage targeted by fatty acids during early biofilm formation. Fatty acids influence bacterial surface charge, hydrophobicity, flexibility, and adhesin expression, which reduces attachment to both biotic and abiotic surfaces [22]. The primary mechanism is the incorporation of fatty acids into the bacterial outer membrane, altering membrane permeability and fluidity and impeding stable surface colonisation. Essential and unsaturated fatty acids, including EPA, DHA, and gamma-linolenic acid (GLA), demonstrate strong inhibitory effects on adhesion and early biofilm development, particularly in vancomycin-resistant Enterococcus faecium [6]. Oleic, palmitic, and linoleic acids, which are prevalent in various plant oils, modulate pathogen–probiotic ratios and suppress early biofilm formation [88]. Omega-3 fatty acids, such as EPA and DHA, further reduce bacterial adhesion by modifying cell-surface properties, thereby making attachment and community formation less favourable. These effects are especially notable in Streptococcus mutans, where EPA and DHA significantly decrease biofilm thickness [95].
3.1.2. Membrane Disruption and Detergent-like Activity
Many fatty acids, particularly unsaturated fatty acids, exert direct bactericidal and antibiofilm effects through detergent-like interactions with bacterial membranes. This mechanism is widely regarded as a primary target of fatty acid action [93,94]. The amphipathic nature of these molecules enables them to destabilise membrane lipids by inserting into the lipid bilayer [92], which increases membrane permeability and fluidity [100,101,102]. As a result, vital intracellular contents, such as ions, adenosine triphosphate (ATP), proteins, and nucleic acids, leak from the cell [93,94], ultimately leading to cell lysis [92,94]. Disruption of membrane integrity alters bacterial morphology and prevents cells from sustaining metabolic processes. The resulting loss of electron transport chain function and uncoupling of oxidative phosphorylation further impairs cellular energy production [93,94]. This process is essential for accessing and eliminating biofilm-embedded cells, which are typically protected by the EPS matrix. For example, the bactericidal effect of fatty acids derived from Hermetia illucens larvae fat against Klebsiella pneumoniae has been associated with increased bacterial outer membrane permeability [92]. Oleic, linoleic, and palmitoleic acids significantly inhibit biofilm growth in S. epidermidis, S. aureus, and S. mutans by compromising membrane integrity and disrupting cellular homeostasis [95]. Unsaturated fatty acids, including linoleic and arachidonic acids, generally demonstrate greater efficacy than their saturated counterparts by interfering with peptidoglycan and fatty acid synthesis pathways.
3.1.3. Disruption of the EPS Matrix
Fatty acids compromise mature biofilms by directly targeting and disrupting the extracellular polymeric substance (EPS) matrix, also known as the matrixome. The EPS matrix is a complex mixture of polysaccharides, proteins, extracellular DNA (eDNA), and lipids that encases bacterial cells and confers structural stability, protection against environmental stressors, and resistance to antibiotics [103,104]. Fatty acids disrupt the integrity of this matrix through several mechanisms:
Modulation of EPS Biosynthesis: Ginkgolic acid may inhibit and disrupt biofilms by affecting the expression of genes involved in exopolysaccharide biosynthesis. Although the precise molecular mechanisms remain incompletely characterised, existing studies indicate that fatty acids can modulate gene expression [105].
Weakening of Matrix Structure: Oleic and linoleic acids reduce cell-surface hydrophobicity, thereby weakening nascent biofilms and decreasing bacterial attachment to host tissues and abiotic surfaces [6,96]. This change in surface properties impedes the formation and maintenance of the EPS network.
Direct Degradation of Matrix Components: Both omega-3 and omega-6 polyunsaturated fatty acids are believed to impair EPS integrity and promote matrix degradation [87]. Specifically, EPA and DHA degrade essential matrix components, such as polysaccharides, proteins, and extracellular DNA, in biofilms of S. mutans, Porphyromonas gingivalis, and Fusobacterium nucleatum [95]. This degradation reduces the biofilm’s biomass and the viability of embedded cells.
Disruption of Structural organisation: Gamma-linolenic acid downregulates key genes in mature vancomycin-resistant E. faecium biofilms, disrupting their structural organisation [6]. This results in a less cohesive and more vulnerable biofilm structure.
Consequently, omega-3 PUFAs demonstrate broad-spectrum antibiofilm activity in oral, staphylococcal, and mixed-species biofilms [90,99].
3.1.4. Modulation of Quorum Sensing and Biofilm Dispersal
Several fatty acids disrupt quorum-sensing (QS) networks that control biofilm maturation and virulence. QS is a density-dependent mechanism in which bacteria communicate through small, diffusible signal molecules, known as autoinducers, to regulate gene expression for processes such as biofilm formation, virulence factor production, and stress tolerance [106,107,108,109]. Fatty acids function as quorum quenching agents [110], directly interfering with these signalling systems to inhibit biofilm establishment or facilitate its breakdown.
Suppression of QS-Regulated Genes: Linolenic acid suppresses QS-regulated biofilm formation in P. aeruginosa and reduces the expression of virulence genes [22].
Induction of Biofilm Dispersal: Cis-2-decenoic acid (C2DA), a diffusible signal factor produced by bacteria, induces biofilm dispersal at nanomolar concentrations without inhibiting bacterial growth. This process promotes the transition to planktonic cells and increases antibiotic susceptibility [89,93]. Such interference may involve inhibition of specific enzymes in the QS pathway or disruption of autoinducer production or detection, including N-acyl homoserine lactones (AHLs), which are prevalent in Proteobacteria [109,111].
Interference with Specific QS Systems: In Acinetobacter baumannii, unsaturated fatty acids may inhibit biofilm formation by blocking specific QS systems, thereby reducing virulence. Although the precise mechanisms of systems such as AbaIR are not fully described in the current literature, fatty acids are recognised to modulate virulence repression by inhibiting transcriptional activators [105].
Inhibition of QS by fatty acids disrupts the coordinated behaviours required for mature biofilm development, thereby increasing bacterial susceptibility to host defences and antimicrobial agents.
3.1.5. Modulation of Gene Expression and Virulence Factors
Beyond structural disruption, fatty acids modulate bacterial transcriptional programmes linked to virulence and biofilm maintenance. GLA significantly downregulates biofilm-associated genes in vancomycin-resistant E. faecium [6]. Fatty acids inhibit transcriptional regulators, thereby preventing DNA binding and the activation of virulence genes [105]. LCFAs act as allosteric inhibitors, reducing the DNA-binding affinity of transcriptional activators of virulence genes and modulating the activation of histidine kinase receptors. This modulation alters downstream intracellular signalling networks, resulting in the repression of virulence [105]. Fatty acids also influence bacterial metabolism; for example, propionate alters toxin production through metabolic reprogramming. Furthermore, alterations in fatty acid synthesis are associated with decreased expression of major virulence factors [112]. Figure 2 provides an overview of the mechanisms by which fatty acids function as antibiofilm agents.
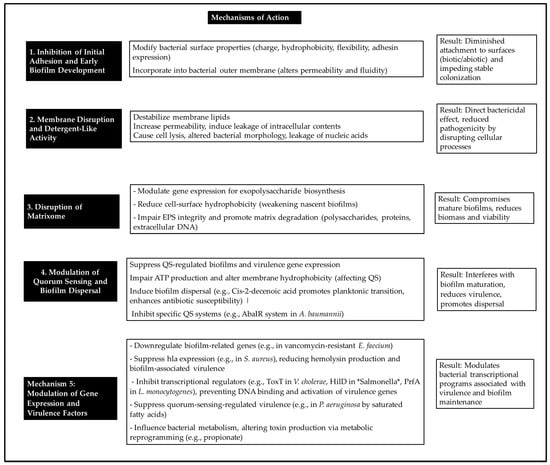
Figure 2.
Main mechanisms of action of fatty acids against bacterial biofilms.
3.1.6. Spectrum of Activity and Therapeutic Relevance
Oils containing high levels of omega-3 fatty acids demonstrate significant efficacy against specific pathogens. For instance, herring oil and its omega fatty acids at 100 µg/mL reduce S. aureus MSSA 6538 biofilms by 75%, while 20 µg/mL achieves a 65% reduction in S. aureus MSSA33591 [113]. Saw palmetto oil exhibits activity against S. aureus biofilm at 20 mg/mL [96]. Multiple seed oils, particularly those rich in omega-3 fatty acids, inhibit biofilm formation by various pathogens. Calophyllum seed oil at 20 µL/mL inhibits biofilm formation by A. baumannii and S. aureus by 50.34% and 56.09%, respectively. Prickly pear seed oil inhibits S. aureus and E. coli biofilm formation by 63% and 80%, respectively [114]. Fratianni et al. reported that borage seed oil inhibited the metabolism of sessile cells, notably L. monocytogenes (54.4% inhibition) and P. aeruginosa (50.83%) [114].
Additional oils, such as coffee, pumpkin, and watermelon oils at 20 µL/mL, inhibit mature S. aureus biofilms by 59.22%, 57.39%, and 66.92%, respectively. At this concentration, broccoli seed oil and green coffee seed oil inhibit mature L. monocytogenes biofilms by 53.41% and 55.99%, respectively. Green coffee seed oil also inhibits mature A. baumannii biofilms by 53.72% [115].
Individual fatty acids exhibit potent antibiofilm activities at relatively low concentrations. For example, DHA at 0.612 and 1.25 mg/L modulates gene expression associated with biofilm production in S. aureus [91]. Both DHA and EPA completely inhibit biofilm formation and P. gingivalis growth at 12.5 μM and significantly reduce F. nucleatum biofilm formation at 100 μM [116]. At 100 μM, DHA and EPA also inhibit S. mutans biofilm growth [116]. Additionally, 125 µg/mL cis-2-decenoic acid (C2DA) disperses 40% of P. aeruginosa biofilm [89]. N-acylethanolamines, including oleoylethanolamide and anandamide at 64 mg/mL, inhibit S. aureus biofilms by reducing surface motility, aggregation, and the expression of biofilm-associated genes [116,117,118]. Certain fatty acids at 1.17 mg/mL can overcome multidrug resistance in pathogens such as P. aeruginosa by targeting key virulence factors and biofilm-forming pathways [119].
The low toxicity of certain fatty acids, such as cis-2-decenoic acid, at antibiofilm concentrations up to 620 nM, suggests a reduced risk of resistance development [120]. Synthetic unsaturated fatty acids with selective activity against MRSA further underscore the potential of this compound class as leads for next-generation antibiofilm and antibacterial agents [98]. The multimodal activities of fatty acids, including membrane disruption, matrix degradation, quorum-sensing inhibition, virulence repression, and synergy with existing antibiotics, support their promise as candidates for innovative antibiofilm therapies, particularly against multidrug-resistant infections [6,7,105,119,120,121,122]. Table 3 summarises mechanistic roles of fatty acids in biofilm control.

Table 3.
Mechanistic Roles of Fatty Acids in Biofilm Control.
4. Specific Fatty Acids and Their Antibiofilm Effects
The structural diversity of fatty acids significantly influences their antimicrobial and antibiofilm activities. Key factors such as chain length, degree of saturation, and stereochemistry determine their capacity to disrupt bacterial membranes or interfere with quorum-sensing pathways [123]. Long-chain fatty acids (LCFAs) not only modulate bacterial functions but also serve as nutrient sources, underscoring their dual metabolic and therapeutic significance [105]. Several LCFAs inhibit hilA promoter activity by prevention of HilD binding to DNA, which reduces Salmonella virulence [105]. For instance, cis-2-hexadecenoic acid acts as a diffusible signal factor that binds to and inhibits HilD and modulates virulence gene expression in S. enterica [124]. Long-chain unsaturated fatty acids, such as cis-palmitoleate, also decrease HilD’s DNA-binding affinity and occupy a hydrophobic pocket in the N-terminal domain of ToxT, which alters its dimerization and DNA interactions [105,125]. Specific residues, including R267 and N44 in HilD, interact with fatty acid head groups and induce conformational changes that affect DNA binding [126]. The presence of a cis-2 unsaturation, characteristic of diffusible signal factors, is essential for regulatory activity and influences bacterial virulence, motility, and biofilm formation through quorum sensing [126]. Mono-PUFAs, such as palmitoleic and myristoleic acids, inhibit tcp gene expression in Vibrio cholerae by prevention of ToxT dimerization and promoter binding [109,127]. The targeted interactions demonstrate that LCFAs disrupt regulatory proteins to suppress virulence rather than bacterial growth, thereby limiting the development of resistance [124,126].
4.1. Butyrate and Its Role in Biofilm Inhibition
Butyrate (C4:0), a short-chain fatty acid, has garnered significant attention for its multifaceted roles in host physiology and potent antimicrobial and antibiofilm properties, primarily through modulation of the gut microbiota. Butyrate modulates host immunity and metabolism, directly influencing pathogen virulence and contributing to intestinal homeostasis [122]. Its antibiofilm activity arises from the regulation of bacterial gene expression and disruption of quorum-sensing pathways, which impedes biofilm formation and maturation. Additionally, butyrate acetylates lysine residues on transcriptional regulators, such as HilA in Salmonella SPI-1, leading to downregulation of virulence genes [128]. While this acetylation reduces virulence, it concurrently promotes biofilm formation in S. enterica, illustrating a complex interaction in which butyrate differentially modulates bacterial phenotypes based on gene targets and environmental context [128]. This paradox highlights an adaptive bacterial strategy within the host, in which diminished invasion may be compensated for by enhanced biofilm formation [122].
4.2. Propionate and Acetate in Biofilm Modulation
Beyond butyrate, the other two major SCFAs produced by the gut microbiota—propionate and acetate—also strongly influence bacterial biofilm formation and virulence. Propionate represses several Salmonella SPI-1 genes, including hilA and hilD, which reduces invasion by destabilisation of HilD and disruption of intracellular pH homeostasis [122]. Propionate and butyrate both inhibit S. enterica serovar Typhimurium biofilm formation in laboratory and food models, showing broad antibiofilm activity [129]. Acetate inhibits extracellular polysaccharide production and has anti-quorum-sensing effects in E. coli, reducing biofilm formation [122,130]. Although the molecular targets of acetate remain unclear, it is likely to interfere with autoinducer signalling [128]. Propionate also targets HilD directly, decreasing SPI-1 expression and limiting S. enteritidis invasion [131]. Notably, propionate and butyrate generally suppress Salmonella virulence, whereas acetate can enhance invasion gene expression in the distal small intestine at physiological concentrations [132].
4.3. Unsaturated Fatty Acids (UFAs) and Antibiofilm Activity
UFAs with defined chain lengths and structural features inhibit biofilm formation through disruption of signalling pathways, compromise of cell wall integrity, attenuation of efflux systems, and interference with quorum sensing. LCUFAs, such as oleic and linoleic acids, downregulate virulence genes including hilA and hilD in S. Typhimurium, which reduces bacterial invasion and dissemination [126,133,134,135]. Similar inhibitory effects are observed in other bacterial species. For instance, SCFAs produced by Cutibacterium acnes, such as propionic, isobutyric, and isovaleric acids, inhibit S. epidermidis biofilms through decreased exopolysaccharide production and increased antibiotic susceptibility via concentration-dependent mechanisms that modulate immune responses and reduce bacterial viability. Furthermore, short-chain fatty acid production by commensal C. acnes restricts the growth and biofilm formation of S. aureus [1,136]. SCFAs, including caproic and caprylic acids, also downregulate virulence genes such as fimA and hilA in S. Typhimurium, which diminishes its capacity to invade porcine intestinal epithelial cells [133].
4.4. Conjugated Linoleic Acid (CLA) and Biofilm Disruption
CLA and other SCFAs function as potent antibiofilm agents through disruption of bacterial membrane integrity and inhibition of EPS synthesis. Lipid-producing L. casei strains that enhance short-chain fatty acid (SCFA) output significantly modify the surface properties of Salmonella and E. coli, resulting in reduced hydrophobicity, decreased auto-aggregation, and diminished biofilm formation through interactions with the cytoplasmic membrane [137]. These changes lead to increased membrane permeability, leakage of intracellular contents, and impaired early adhesion, all of which are critical for biofilm development. SCFAs also modulate quorum-sensing–related gene expression, further inhibiting biofilm maturation [1]. At physiological concentrations, SCFAs suppress biofilm formation in several pathogens, including S. epidermidis, and frequently demonstrate greater efficacy than traditional antibiotics against established biofilms [1,137]. SCFA-overproducing L. casei strains markedly inhibit biofilm formation and reduce adhesion and invasion of S. Typhimurium and enterohaemorrhagic E. coli on INT-407 cells [137]. Genetically engineered L. casei strains with enhanced SCFA production confer even greater protection against enterohaemorrhagic E. coli (EHEC) growth and infection in both in vitro and in vivo models [138]. Table 4 summarises the effects of these fatty acids on specific bacterial biofilms.

Table 4.
Specific Fatty Acids and Their Antibiofilm/Antivirulence Effects.
5. Dosage and Toxicity Considerations
Fatty acids are generally regarded as safe dietary components; however, their antimicrobial and antibiofilm activities exhibit strong dose-dependency [23,153]. Concentrations required for effective biofilm inhibition frequently surpass physiological levels, especially for medium-chain and long-chain fatty acids (MCFAs and LCFAs) [38,44]. At higher doses, certain fatty acids may induce cytotoxicity, disrupt epithelial integrity, or trigger pro-inflammatory responses [45,46,47]. The efficacy and safety of fatty acids are influenced by microbial species, biofilm maturity, and environmental factors, emphasising the necessity of thorough evaluation of dosage, formulation, and delivery methods prior to translating in vitro results to clinical or industrial contexts [48,64].
5.1. Dose-Dependency of Antimicrobial and Antibiofilm Activity
Fatty acids such as stearic acid and oleic acid disrupt cell membranes in a concentration-dependent manner at levels above 1100 µM [155]. Saturated fatty acids, including myristic and palmitic acid, have been shown to reduce cellular growth rates in a dose-dependent fashion up to 250 µM and can induce apoptosis [156]. The minimum inhibitory concentration (MIC) is essential for determining the lowest effective concentration of MCFAs required to inhibit bacterial growth, demonstrating their dose-dependent antimicrobial activity against bacteria such as E. coli, S. Typhimurium, and Campylobacter coli [157]. Therefore, MIC determination is a prerequisite for subsequent assays, such as crystal violet and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) or 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (imethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) or 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), to assess antibiofilm activity. Long-chain PUFAs also inhibit bacterial growth in a dose-dependent manner, with MICs against pathogens including C. acnes and S. aureus ranging from 32 to 1024 mg/L [93].
5.2. Concentrations Exceeding Physiological Levels and Cytotoxicity
At higher concentrations, certain fatty acids exhibit lipotoxic effects. For instance, palmitic acid at micromolar concentrations induces apoptosis in pancreatic cells, while some monounsaturated fatty acids reduce membrane fluidity and contribute to lipotoxicity [156,158]. Excessive dietary intake of lipids, including oleic and palmitic acids, can negatively impact the structure and mechanotransduction of intestinal cells in vitro. Bergen et al. reported that exposing HCT116 cells to oleic and palmitic acid at concentrations above 25 µM significantly decreased cell membrane fluidity, with marked actin cytoskeleton rearrangement observed at 25 mM and 100 mM, respectively [159]. These results suggest that concentrations above physiological levels may compromise epithelial integrity.
5.3. Dependence on Microbial Species, Biofilm Maturity, and Environmental Conditions
The antimicrobial efficacy of fatty acids varies according to bacterial species, as shown in MIC studies with multiple pathogens [115,157]. Environmental factors, especially pH, significantly affect the antimicrobial and antibiofilm activities of short-chain carboxylic acids. For example, acidic conditions at pH 4.5 enhance efficacy against S. enterica [160].
5.4. Importance of Evaluating Dosage, Formulation, and Delivery Strategies
Developing innovative drug-delivery strategies is crucial for improvement of biofilm penetration, promotion of dispersal, and achievement of synergistic bactericidal effects of antibiofilm agents, including fatty acids, in clinical applications [161]. The therapeutic potential of specific fatty acids, such as gamma-linolenic acid, for treating biofilm-associated infections highlights the necessity for comprehensive evaluation prior to clinical use [16]. Furthermore, thorough safety assessments are essential when considering fatty acids for therapeutic purposes.
6. Clinical and Therapeutic Implications
The antibiofilm and immunomodulatory properties of fatty acids offer promising avenues for the development of novel therapeutics that target persistent bacterial infections. Linoleic acid-overproducing L. casei alters key physicochemical properties, such as hydrophobicity and auto-aggregation, in pathogens including Salmonella and E. coli, which highlights its potential for probiotic use. These engineered strains significantly reduce bacterial surface hydrophobicity and auto-aggregation, thereby interfering with adhesion mechanisms critical for biofilm formation [137]. Increased linoleic acid synthesis by L. casei induces both structural and functional damage to bacterial cell membranes, which leads to inhibited growth and reduced virulence of enteric pathogens. Furthermore, linoleic acid-overproducing L. casei decreases the attachment of Salmonella and enterohaemorrhagic E. coli to host cells, which demonstrates a direct impact on pathogen-host interactions [137].
Although CLA is often associated with beneficial effects on metabolism and inflammation, and its interactions with the gut microbiome are under investigation, direct evidence from human clinical trials that supports CLA as a prebiotic remains limited. Some studies suggest an indirect association; for example, a prebiotic dietary fibre intervention in obese patients increased faecal rumenic acid (a form of CLA), which correlated with the presence of Bifidobacterium [162]. This finding indicates that other prebiotics can influence CLA levels, which, in turn, are associated with beneficial bacteria. Another study assessed the impact of consumption of 1 L/day of cows’ milk containing 5 mg/g fat cis-9, trans-11 CLA, as well as higher concentrations of cis-9, trans-11 CLA and trans-10, cis-12 CLA for 8 weeks, on the human faecal microbiological profile. The results showed that CLA intake altered faecal microbiota composition [163]. However, these findings do not conclusively establish CLA as a direct prebiotic substrate for specific beneficial bacteria. The ability of the human gut microbiota to synthesise CLA from linoleic acid is still under investigation, with current evidence suggesting that this process is less well-characterised in humans than in vitro or animal models [164]. Several human trials involving CLA focus on its anti-obesity, metabolic, and anti-inflammatory properties, with changes in gut microbiota considered secondary outcomes rather than evidence of CLA functioning as a prebiotic [165,166]. For instance, Smedman and Vessby studied 53 healthy adults whose diets were randomly supplemented with CLA (4.2 g/day) or an equivalent amount of olive oil for 12 weeks in a double-blind design. The CLA-treated group experienced a 3.8% reduction in body fat that represented a significant difference compared to the control group [165]. In contrast, other fatty acids, particularly omega-3 PUFAs such as DHA and EPA, have shown promising in vitro antimicrobial and antibiofilm activities against various pathogens [6,91]. However, these findings are primarily based on laboratory or animal studies. To date, no human clinical trials have evaluated CLA as an antibiofilm agent for the treatment or prevention of biofilm-associated infections.
6.1. Fatty Acids in Gut Health Management
Targeted modulation of the gut microbiota using specific fatty acids constitutes an effective approach to enhance host defences and reduce gastrointestinal pathogen colonisation. This strategy is exemplified by probiotics such as L. casei, which can be engineered to overproduce beneficial fatty acids, including conjugated linoleic acid, thereby increasing their protective efficacy against enteric pathogens [137]. The cell-free supernatant from linoleic acid-overproducing L. casei significantly inhibits biofilm formation by enterohaemorrhagic E. coli and downregulates virulence genes, particularly those associated with type III secretion systems in S. Typhimurium and enterohaemorrhagic E. coli [137,138]. Suppression of these virulence factors restricts pathogen adhesion and invasion, which highlights the therapeutic potential of fatty acid-enriched probiotics [137].
6.2. Potential for Treating Biofilm-Related Infections
Fatty acids with demonstrated antibiofilm properties, such as CLA, offer promising alternatives or adjuncts to antibiotics in the context of rising multidrug resistance. Their capacity to disrupt established biofilms and inhibit initial microbial adhesion makes them strong candidates for antimicrobial strategies that bypass conventional resistance mechanisms. Probiotic strains, such as L. casei, can endogenously synthesise these bioactive fatty acids, reducing dependence on external antibiotics and lowering selective pressures that contribute to resistance. This endogenous production limits pathogenic biofilm formation, reinforcement of gut barrier integrity, and enhancement of host resilience [137]. Fatty acid metabolites further modulate inflammation by downregulating pro-inflammatory pathways and promoting anti-inflammatory mediators, thereby supporting gut and immune homeostasis. The combined immunomodulatory, antimicrobial, and antibiofilm effects of these metabolites position them as key regulators of host–pathogen interactions [137,152]. SCFAs also influence microbial growth, motility, biofilm development, and QS [153], thereby shaping microbial community composition and pathogenicity [154]. Additionally, CLA isomers can modulate immune responses and exhibit either pro-inflammatory or anti-inflammatory effects depending on their specific structure and biological context [150]. Clinical and therapeutic implications of fatty acids are summarised in Table 5.

Table 5.
Clinical and Therapeutic Implications of Fatty Acids.
7. Challenges and Opportunities in Therapeutic Applications
Despite their promising therapeutic potential, the clinical use of fatty acids as antibiofilm agents is limited by challenges in delivery, stability, and dose optimisation in complex physiological environments. Further research is required to clarify their mechanisms of action, including interactions with bacterial membranes and signalling pathways [167], thereby supporting the development of targeted strategies to disrupt or prevent biofilms. Exploring synergistic combinations of fatty acids with conventional antimicrobials may further enhance antibiofilm efficacy. Genetically engineering probiotic strains to boost fatty acid production and deliver them to specific host sites represents another promising avenue for personalised antibiofilm therapies. This aligns with the broader concept that free fatty acids can inhibit pathogens through multiple pathways without harming the gut microbiota [54]. Some fatty acids also help maintain skin microbial balance through their antimicrobial activity. For example, cis-2-decenoic acid induces biofilm dispersal at nanomolar levels and inhibits growth at higher concentrations [7]. When combined with conventional antibiotics, it shows enhanced antibiofilm effects [120], illustrating how coupling dispersal with growth inhibition offers a powerful strategy against persistent, biofilm-associated infections [7,120].
8. Future Directions and Research Gaps
Although numerous SCFAs demonstrate potent antibiofilm effects in vitro and in preclinical models, substantial gaps remain before translation of these findings into clinical practice. Comprehensive knowledge of their pharmacokinetics, bioavailability, and potential adverse effects in humans is required. Further research is also necessary to determine optimal dosing, delivery methods, and interactions with the host microbiome. Advanced encapsulation or targeted delivery approaches may enhance SCFA stability and bioavailability at infection sites. Additionally, the establishment of standardised protocols to evaluate antibiofilm activity across various bacterial species and biofilm architectures is critical [77]. Among SCFAs, C2DA is particularly promising and exhibits broad-spectrum activity against microorganisms such as P. aeruginosa and methicillin-resistant S. aureus [77,80]. This unsaturated fatty acid, produced by multiple bacterial species, primarily functions as a potent biofilm-dispersal signal and induces a transition from sessile to planktonic states without bactericidal effects at low concentrations [116,140]. At elevated concentrations, C2DA inhibits bacterial growth and prevents biofilm formation and demonstrates efficacy against S. aureus at 500 µg/mL and 125 µg/mL, respectively [77,80]. By dispersion of established biofilms, C2DA increases bacterial susceptibility to antimicrobials and facilitates synergistic effects with antibiotics or disinfectants that markedly reduce biofilm biomass [7,141]. Its capacity to function at nanomolar and environmentally safe concentrations, coupled with a low propensity for resistance development, underscores its potential for biofilm control in both industrial and clinical contexts [7]. This diminished risk of resistance further augments the effectiveness of prophylactic antibiotic regimens [77,140].
9. Comparison of Antibiofilm Strategies: Fatty Acids, Nanoparticles, Polyphenols, and Peptides
Several classes of molecules offer promising strategies for combating bacterial biofilms, each with distinct mechanisms and specific advantages (Table 5). Naturally occurring fatty acids disrupt bacterial membranes [92,93,94,95,102,155,168], inhibit bacterial adhesion, and modulate QS [89]. These compounds often act at subinhibitory concentrations and can enhance antibiotic efficacy [87,89,169].
Nanoparticles, engineered and biofabricated using micro- and nanotechnologies, are also used to combat biofilms. They can encapsulate and deliver antimicrobial agents [169,170,171,172], including natural compounds like carvacrol [170] or even fatty acids themselves (e.g., fatty acid-capped silver nanoparticles) [171], and provide an engineered approach for targeted and controlled release of antibiofilm agents, which improves biofilm penetration and reduces resistance development [172,173,174,175].
Polyphenols, natural plant-derived compounds, exert antibiofilm effects through multitarget mechanisms, including interference with QS and modulation of virulence factors, and show low potential to induce resistance [176,177,178,179,180].
Peptides, including antimicrobial and host defence peptides, permeabilise bacterial membranes, inhibit adhesion, and disaggregate the biofilm matrix. These molecules display broad-spectrum activity and a low propensity to induce resistance [181,182,183,184,185]. Together, these molecular classes offer complementary and potentially synergistic strategies to address the challenges posed by biofilm-associated infections.
Table 6 provides a comparative overview of these antibiofilm agents.

Table 6.
Comparative overview of fatty acids, nanoparticles, polyphenols and peptides as antibiofilm agents.
10. Limitations
Despite the considerable promise of fatty acids as antibiofilm agents, several limitations must be recognised when interpretation of the current evidence and evaluation of their potential for clinical application are considered.
10.1. Methodological Heterogeneity
Substantial methodological heterogeneity characterises the literature on fatty acid antibiofilm activity, which complicates direct comparisons across studies. Experimental designs vary across bacterial strains, biofilm cultivation methods (static versus flow systems), incubation durations, growth media composition, and biofilm maturity at the time of treatment. Additionally, diverse assessment methodologies—including crystal violet staining, metabolic assays (MTT, XTT), viable cell counts, and microscopic techniques—measure different aspects of biofilm integrity. This variability hinders the establishment of standardised efficacy thresholds and complicates meta-analytical synthesis. Adoption of standardised protocols, such as those recommended by international microbiology consortia, would facilitate more robust cross-study comparisons in future research.
10.2. In Vitro vs. In Vivo Discrepancies
Most of the evidence for fatty acid antibiofilm activity originates from in vitro studies conducted under controlled laboratory conditions. Whilst these models provide valuable mechanistic insights, they do not replicate the complexity of in vivo environments, where host immune responses, tissue architecture, blood flow, protein binding, and microbial community interactions significantly influence biofilm dynamics and antimicrobial efficacy. The limited in vivo studies, primarily in animal models, have produced variable results, making direct extrapolation to human clinical scenarios uncertain. Furthermore, the bioavailability, pharmacokinetics, and tissue distribution of fatty acids in vivo differ markedly from those in vitro, potentially impacting their antibiofilm efficacy at infection sites. The absence of clinical trials evaluating fatty acids as antibiofilm agents in humans represents a critical gap in translational research.
10.3. Dose Comparability and Concentration Issues
A major limitation involves comparability of effective concentrations reported across studies and their relevance to physiologically or clinically achievable levels. Many in vitro studies demonstrate antibiofilm activity at fatty acid concentrations exceeding those naturally present in biological fluids or attainable through dietary supplementation or pharmacological administration without adverse effects. For example, certain fatty acids exhibit potent antibiofilm effects at millimolar concentrations in vitro. However, achievement and maintenance of such levels systemically or at infection sites in vivo may be impractical or cytotoxic, as discussed in Section 5. Additionally, dose–response relationships are inconsistently reported, and minimum effective concentrations vary widely by bacterial species, biofilm age, and environmental conditions. This variability complicates the development of therapeutic dosing regimens and highlights the need for comprehensive pharmacokinetic and pharmacodynamic studies in relevant clinical settings.
10.4. Translational Constraints
Additional constraints hinder the immediate clinical translation of fatty acids as antibiofilm therapies. The stability and targeted delivery of fatty acids to infection sites are challenging due to their susceptibility to oxidation, enzymatic degradation, and rapid metabolism. Advanced formulation strategies, such as encapsulation, nanoparticle delivery systems, and prodrug approaches, are required but remain largely unexplored for antibiofilm applications. The potential for off-target effects, including changes in host lipid metabolism, modulation of inflammatory pathways, and impacts on commensal microbiota, necessitates thorough evaluation in long-term safety studies. Although fatty acids show synergistic effects with conventional antibiotics in vitro, optimal combinations, dosing regimens, and the risk of antagonistic interactions have not been systematically studied in clinical contexts. Furthermore, regulatory pathways for the approval of fatty acids as therapeutic agents, whether as standalone antimicrobials, adjuvants, or medical food ingredients, remain undefined and may differ across jurisdictions.
In summary, although current evidence supports the antibiofilm potential of fatty acids, substantial methodological, translational, and clinical gaps must be addressed before these compounds can be reliably incorporated into antimicrobial stewardship strategies. Future research should prioritise standardised methodologies, rigorous in vivo validation, pharmacokinetic optimisation, and well-designed clinical trials to facilitate the transition from promising preclinical findings to effective clinical applications.
11. Conclusions
Fatty acids represent a structurally diverse class of bioactive molecules with multifaceted roles extending from microbiota modulation to biofilm disruption. Their biological effects are highly dependent on chain length, degree of saturation, and concentration, resulting in distinct prebiotic-like, antimicrobial, and antibiofilm activities. While short-chain fatty acids primarily act as key mediators of host–microbiota interactions and demonstrate potent effects on quorum sensing and virulence gene expression, medium- and long-chain fatty acids exhibit more direct antibiofilm properties through membrane disruption, extracellular polymeric substance degradation, and interference with bacterial signalling pathways. The therapeutic potential of fatty acids is further enhanced by their synergistic interactions with conventional antibiotics, offering promising strategies to combat multidrug-resistant pathogens and persistent biofilm-associated infections. Their multimodal mechanisms of action—including modulation of bacterial adhesion, disruption of mature biofilms, and suppression of virulence factors—position them as valuable alternatives or adjuncts to traditional antimicrobials. The capacity of engineered probiotic strains to overproduce specific fatty acids adds another dimension to their clinical applicability, particularly in the management of gut health. However, as discussed in the preceding limitations section, significant knowledge gaps and translational challenges must be addressed before fatty acids can be reliably integrated into clinical practice. Methodological heterogeneity across studies, discrepancies between in vitro efficacy and in vivo performance, issues with dose comparability, and concerns about bioavailability and stability represent critical barriers to clinical translation. Future research should prioritise the development of standardised experimental protocols to enable robust cross-study comparisons and meta-analyses. Well-designed clinical trials are essential to establish optimal dosing regimens, evaluation of safety profiles, assessment of long-term effects, and validation of the efficacy of fatty acids in human biofilm-associated infections. Additionally, advancing delivery technologies—such as encapsulation systems, nanoparticle formulations, and targeted release mechanisms—will be crucial to enhance biofilm penetration and maintain therapeutic concentrations at infection sites while minimising systemic toxicity. Exploring synergistic combinations with other antibiofilm agents, including conventional antibiotics, antimicrobial peptides, and phytochemicals, may further amplify therapeutic efficacy and reduce the risk of resistance development. Elucidation of the precise molecular mechanisms underlying fatty acid antibiofilm activity, particularly their interactions with bacterial signalling networks and host immune responses, will enable rational design of next generation antibiofilm therapies. Ultimately, the successful translation of fatty acids from promising preclinical candidates to effective clinical interventions will require multidisciplinary collaboration among microbiologists, pharmacologists, clinicians, and pharmaceutical scientists. With continued rigorous investigation addressing the identified limitations, fatty acids hold substantial promise as complementary strategies against biofilm-associated infections in both clinical and food-related environments, contributing to the global effort to combat antimicrobial resistance.
Author Contributions
Conceptualization, F.N., F.C., F.F. and R.C.; writing—review and editing, F.N., F.C., F.F. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the European Commission—Next Generation EU, Project SUS-MIRRI.IT “Strengthening the MIRRI Italian Research Infrastructure for Sustainable Bioscience and Bioeconomy”, code n. IR0000005, and by the project “DBA.AD005.310—NATVALUE Valorizzazione biochimica e biologica di risorse vegetali e prodotti naturali per l’alimentazione, la salute e il benessere”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nakamura, K.; O’Neill, A.M.; Williams, M.R.; Cau, L.; Nakatsuji, T.; Horswill, A.R.; Gallo, R.L. Short chain fatty acids produced by Cutibacterium acnes inhibit biofilm formation by Staphylococcus epidermidis. Sci. Rep. 2020, 10, 21237. [Google Scholar] [CrossRef]
- Quigley, E.M.M. Prebiotics and probiotics. Nutr. Clin. Pract. 2012, 27, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Karley, D.; Nayak, K.K.; Kolla, V. Prebiotics and probiotics 2024. In Food Fortification; CRC Press eBooks: Boca Raton, FL, USA, 2024; p. 303. [Google Scholar]
- El-Mowafy, M.; Elgaml, A.; Shaaban, M.I. New approaches for competing microbial resistance and virulence. In Microorganisms; IntechOpen Limited: London, UK, 2019. [Google Scholar]
- Huang, C.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Wei, M.; Wang, P.; Li, T.; Wang, Q.; Su, M.; Gu, L.; Wang, S. Antimicrobial and antibiofilm effects of essential fatty acids against clinically isolated vancomycin-resistant Enterococcus faecium. Front. Cell. Infect. Microbiol. 2023, 13, 1266674. [Google Scholar] [CrossRef] [PubMed]
- Sepehr, S.; Rahmani-Badi, A.; Babaie-Naiej, H.; Soudi, M.R. Unsaturated fatty acid, cis-2-decenoic acid, in combination with disinfectants or antibiotics removes pre-established biofilms formed by food-related bacteria. PLoS ONE 2014, 9, e101677. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial biofilm eradication agents: A current review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.H.; Kim, Y.; Hu, L.; Lee, J. Fatty acids as aminoglycoside antibiotic adjuvants against Staphylococcus aureus. Front. Microbiol. 2022, 13, 876932. [Google Scholar] [CrossRef]
- Sidders, A.E.; Kedziora, K.M.; Arts, M.; Daniel, J.M.; de Benedetti, S.; Beam, J.E.; Bui, D.T.; Parsons, J.B.; Schneider, T.; Rowe, S.E.; et al. Antibiotic-induced accumulation of lipid II synergizes with antimicrobial fatty acids to eradicate bacterial populations. eLife 2023, 12, e80246. [Google Scholar] [CrossRef]
- Lazăr, V.; Holban, A.M.; Curuțiu, C.; Chifiriuc, M.C. Modulation of quorum sensing and biofilms in less investigated Gram-negative ESKAPE pathogens. Front. Microbiol. 2021, 12, 676510. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.W.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. ISAPP consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Hutkins, R.; Walter, J.; Gibson, G.R.; Bedu-Ferrari, C.; Scott, K.; Tancredi, D.J.; Wijeyesekera, A.; Sanders, M.E. Classifying compounds as prebiotics—Scientific perspectives and recommendations. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 54–70. [Google Scholar] [CrossRef]
- Swann, J.R.; Rajilić-Stojanović, M.; Salonen, A.; Sakwińska, O.; Gill, C.M.; Meynier, A.; Fança-Berthon, P.; Schelkle, B.; Segata, N.; Shortt, C.; et al. Considerations for the design and conduct of human gut microbiota intervention studies relating to foods. Eur. J. Nutr. 2020, 59, 3347–3368. [Google Scholar] [CrossRef]
- Rinninella, E.; Costantini, L. Editorial: Polyunsaturated fatty acids and gut microbiota. Front. Nutr. 2023, 10, 1256817. [Google Scholar] [CrossRef]
- Cullen, C.M.; Aneja, K.K.; Beyhan, S.; Cho, C.E.; Woloszynek, S.; Convertino, M.; McCoy, S.J.; Zhang, Y.; Anderson, M.Z.; Alvarez-Ponce, D.; et al. Emerging priorities for microbiome research. Front. Microbiol. 2020, 11, 136. [Google Scholar] [CrossRef]
- Bačić, A.; Gavrilović, J.; Rajilić-Stojanović, M. Polyphenols as a new class of prebiotics for gut microbiota manipulation. Arh. Farm. 2023, 73, 535–551. [Google Scholar] [CrossRef]
- Cherry, P.; Yadav, S.; Strain, C.; Allsopp, P.J.; McSorley, E.M.; Ross, R.P.; Stanton, C. Prebiotics from seaweeds: An ocean of opportunity? Mar. Drugs 2019, 17, 327. [Google Scholar] [CrossRef]
- Balasubramaniam, A.; Adi, P.; My, D.T.T.; Keshari, S.; Sankar, R.; Chen, C.L.; Huang, C.M. Repurposing INCI-registered compounds as skin prebiotics for probiotic Staphylococcus epidermidis against UV-B. Sci. Rep. 2020, 10, 78132. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Ng’Onga, L.; Amoah, K.; Chen, H.; Huang, Y.; Wang, B.; Shija, V.; Mpwaga, A.Y.; Fachri, M.; Cai, J.; Adjei-Boateng, D. The metabolism and antioxidant properties of probiotics and prebiotics in fish: A review. Front. Mar. Sci. 2025, 12, 1622474. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The power of microbiome studies: Some considerations on which alpha and beta metrics to use and how to report results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef]
- Waisundara, V.Y. Biochemistry and health benefits of fatty acids. In IntechOpen eBooks; IntechOpen: London, UK, 2018. [Google Scholar]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Albaladejo-Riad, N.; El Qendouci, M.; Cuesta, A.; Esteban, M.Á. Ability of short-chain fatty acids to reduce inflammation and attract leucocytes to the inflamed skin of gilthead seabream (Sparus aurata L.). Sci. Rep. 2024, 14, 31404. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Louis, P. Gut microbiota-derived short-chain fatty acids and their role in human health and disease. Nat. Rev. Microbiol. 2025, 23, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.I.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Santos, J.M.D.; Bellucci, E.R.B.; Carvalho, L.T.; Bis-Souza, C.V.; Lorenzo, J.M.; Barretto, A.C.S. Reduced fat–reduced sodium fermented meat products: A review of reformulation strategies. Food Sci. Technol. 2023, 43, e108322. [Google Scholar] [CrossRef]
- Zepeda-Hernández, A.; Garcia-Amezquita, L.E.; Requena, T.; García-Cayuela, T. Probiotics, prebiotics, and synbiotics added to dairy products: Uses and applications to manage type 2 diabetes. Food Res. Int. 2021, 142, 110208. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Costa, C.; Lopes, T.B.; Silva, S.; Veiga, M.; Monforte, A.R.; Nunes, J.; Vicente, A.A.; Pintado, M. Prebiotic effects of olive pomace powders in the gut: In vitro evaluation of the inhibition of adhesion of pathogens, prebiotic and antioxidant effects. Food Hydrocoll. 2021, 112, 106312. [Google Scholar] [CrossRef]
- Yao, N.; Yang, Y.; Li, X.; Wang, Y.; Guo, R.; Wang, X.; Li, J.; Xie, Z.; Li, B.; Cui, W. Effects of dietary nutrients on MAFLD: Based on the intestinal–hepatic axis. Front. Nutr. 2022, 9, 906511. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Neyrinck, A.M.; Cani, P.D. Modulation of the gut microbiota by nutrients with prebiotic properties: Consequences for host health in the context of obesity and metabolic syndrome. Microb. Cell Factories 2011, 10, S10. [Google Scholar] [CrossRef]
- Amedei, A.; Lamminpää, I.; Parolini, C. Potential and Future Therapeutic Applications of Eicosapentaenoic/Docosahexaenoic Acid and Probiotics in Chronic Low-Grade Inflammation. Biomedicines 2025, 13, 2428. [Google Scholar] [CrossRef]
- Lammi, C.; Ottaviano, E.; Fiore, G.; Bollati, C.; d’Adduzio, L.; Fanzaga, M.; Ceccarani, C.; Vizzuso, S.; Zuccotti, G.; Borghi, E.; et al. Effect of docosahexaenoic acid as an anti-inflammatory for ca-co-2 cells and modulating agent for gut microbiota in children with obesity (the DAMOCLE study). J. Endocrinol. Investig. 2025, 48, 465–481. [Google Scholar] [CrossRef]
- Ziółkowska, E.; Bogucka, J.; Rawski, M.; Mazurkiewicz, J.; Maiorano, G.; Stanek, M. First insights on trans-galactooligosaccharide effects on fatty acids profile of carp muscle. Ann. Anim. Sci. 2022, 22, 305–318. [Google Scholar] [CrossRef]
- Montanari, C.; Parolisi, S.; Borghi, E.; Putignani, L.; Bassanini, G.; Zuvadelli, J.; Bonfanti, C.; Tummolo, A.; Vici, C.D.; Biasucci, G.; et al. Dysbiosis, host metabolism, and non-communicable diseases: Trialogue in the inborn errors of metabolism. Front. Physiol. 2021, 12, 716520. [Google Scholar] [CrossRef]
- De Velasco, P.C.; Ferreira, A.R.C.; Crovesy, L.; Marine, T.; Carmo, M.G.T. Fatty acids, gut microbiota, and the genesis of obesity. In IntechOpen eBooks; IntechOpen: London, UK, 2018. [Google Scholar]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef] [PubMed]
- Origüela, V.; Gázquez, A.; López-Andreo, M.J.; Bueno-Vargas, P.; Vurma, M.; López-Pedrosa, J.M.; Leyshon, B.J.; Kuchan, M.; Chan, J.P.; Larqué, E. Effects of new lipid ingredients during pregnancy and lactation on rat offspring brain gene expression. Food Funct. 2025, 16, 1720–1730. [Google Scholar] [CrossRef]
- Khatun, P.; Li, X.; Xiaoxi, Z.; Zhai, J.; Ullah, A.; Bo, Y.; Lyu, Q. Effect of Omega-3 Polyunsaturated Fatty Acid Supplements on Cognitive Performance in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Nutr. Rev. 2025, nuaf167. [Google Scholar] [CrossRef]
- Reyes-Pérez, S.D.; González-Becerra, K.; Barrón-Cabrera, E.; Muñoz-Valle, J.F.; Armendáriz-Borunda, J.; Martínez-López, E. FADS1 genetic variant and omega-3 supplementation are associated with changes in fatty acid composition in red blood cells of subjects with obesity. Nutrients 2024, 16, 3522. [Google Scholar] [CrossRef]
- Ajabnoor, S.M.; Thorpe, G.; Abdelhamid, A.; Hooper, L. Long-term effects of increasing omega-3, omega-6 and total polyunsaturated fats on inflammatory bowel disease and markers of inflammation: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2021, 60, 2293–2316. [Google Scholar] [CrossRef] [PubMed]
- Forgie, A.J.; Drall, K.M.; Bourque, S.L.; Field, C.J.; Kozyrskyj, A.L.; Willing, B.P. The impact of maternal and early life malnutrition on health: A diet–microbe perspective. BMC Med. 2020, 18, 135. [Google Scholar] [CrossRef]
- Yang, Y.; Bi, Z.; Zhang, S.; Si, L.; Liu, X.; Li, F. Prebiotics for depression: How does the gut microbiota play a role? Front. Nutr. 2023, 10, 1206468. [Google Scholar] [CrossRef]
- Koçyiğit, E.; Gövez, N.E.; Arslan, S.; Ağagündüz, D. Dietary components and patterns and age-related macular degeneration: A narrative review. Nutr. Res. Rev. 2024, 37, 1–18. [Google Scholar] [CrossRef]
- Abril, A.G.; Carrera, M.; Pazos, M. Immunomodulatory effect of marine lipids on food allergy. Front. Nutr. 2023, 10, 1254681. [Google Scholar] [CrossRef]
- Lombardi, V.C.; De Meirleir, K.; Subramanian, K.; Nourani, S.; Dagda, R.K.; Delaney, S.L.; Palotás, A. Nutritional modulation of the intestinal microbiota and neuroimmune disease. J. Nutr. Biochem. 2018, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.; Ting, S.Y.; Sam, K.; Janaranjani, M.; Wong, S.C.; Wu, X.; Waiho, K.; Fazhan, H.; Shu-Chien, A.C. Comparative analyses of Scylla olivacea gut microbiota composition and function suggest the capacity for polyunsaturated fatty acid biosynthesis. Microb. Ecol. 2022, 86, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, F.; Ren, J.; Zhang, H.; Jing, C.; Wei, M.; Jiang, Y.; Xie, H. Dietary intervention improves metabolic levels in patients with type 2 diabetes through the gut microbiota: A systematic review and meta-analysis. Front. Nutr. 2024, 10, 1243095. [Google Scholar] [CrossRef] [PubMed]
- Djuričić, I.; Calder, P.C. Polyunsaturated fatty acids and metabolic health: Novel insights. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 436–442. [Google Scholar] [CrossRef]
- Laing, B.; Barnett, M.P.G.; Marlow, G.; Nasef, N.A.; Ferguson, L.R. An update on the role of gut microbiota in chronic inflammatory diseases, and potential therapeutic targets. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 969–983. [Google Scholar] [CrossRef]
- Mujico, J.R.; Baccan, G.C.; Gheorghe, A.; Díaz, L.E.; Marcos, A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br. J. Nutr. 2013, 110, 711–720. [Google Scholar] [CrossRef]
- Li, P.G.; Huang, Z. Editorial: Rising stars in nutrition and microbes: Nutrients and diets that shape gut microbiota. Front. Nutr. 2023, 10, 1328660. [Google Scholar] [CrossRef]
- Roussel, C.; Guebara, S.A.B.; Plante, P.L.; Desjardins, Y.; Marzo, V.D.; Silvestri, C. Short-term supplementation with ω-3 polyunsaturated fatty acids modulates mucolytic species from the gut mucin niche. Gut Microbes 2022, 14, 2120344. [Google Scholar] [CrossRef]
- Huyben, D.; Roehe, B.K.; Bekaert, M.; Ruyter, B.; Glencross, B. Dietary lipid: Protein ratio and n-3 long-chain polyunsaturated fatty acids alters the gut microbiome of Atlantic salmon under hypoxic and normoxic conditions. Front. Microbiol. 2020, 11, 589898. [Google Scholar] [CrossRef]
- Ağagündüz, D.; İçer, M.A.; Yeşildemir, Ö.; Koçak, T.; Koçyiğit, E.; Capasso, R. The roles of dietary lipids and lipidomics in gut–brain axis in type 2 diabetes mellitus. J. Transl. Med. 2023, 21, 4088. [Google Scholar] [CrossRef]
- Son, K.H.; Lee, J.; Yang, H.Y.; Heo, Y.; Lee, J.S.; Lee, D.-S.; Shin, K.-J.; Kwon, T.J. Combined effect of probiotics and omega-3 fatty acids on blood and liver function biomarkers and gut microbiota diversity in male mice with high fat diet-induced obesity. PLoS ONE 2025, 20, e0331981. [Google Scholar] [CrossRef]
- Xu, H.; Jurado-Fasoli, L.; Ortiz-Alvarez, L.; Osuna-Prieto, F.J.; Köhler, I.; Di, X.; Vilchez-Vargas, R.; Link, A.; Plaza-Díaz, J.; Gil, A.; et al. Plasma levels of omega-3 and omega-6 derived oxylipins are associated with fecal microbiota composition in young adults. Nutrients 2022, 14, 4991. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Qian, Y.-W.; Cui, X.; Zhao, J.; Ning, Z.; Cha, J.; Wang, K.; Ge, C.; Jia, J.; Dou, T.; et al. Immunomodulatory role of gut microbial metabolites: Mechanistic insights and therapeutic frontiers. Front. Microbiol. 2025, 16, 1675065. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A.; Hanyang, S. Omega-3 polyunsaturated fatty acids and gut microbiota. Curr. Opin. Clin. Nutr. Metab. Care 2025, 10, 1097. [Google Scholar] [CrossRef]
- Bourdeau-Julien, I.; Castonguay-Paradis, S.; Rochefort, G.; Perron, J.; Lamarche, B.; Flamand, N.; Marzo, V.D.; Veilleux, A.; Raymond, F. The diet rapidly and differentially affects the gut microbiota and host lipid mediators in a healthy population. Microbiome 2023, 11, 26. [Google Scholar] [CrossRef]
- Mohammadi, F.; Rudkowska, I. Dietary lipids, gut microbiota, and their metabolites: Insights from recent studies. Nutrients 2025, 17, 639. [Google Scholar] [CrossRef]
- Spisni, E.; Turroni, S.; Shahaj, S.; Spigarelli, R.; Ayala, D.; Valerii, M.C. Natural compounds in the modulation of the intestinal microbiota. In IntechOpen eBooks; IntechOpen: London, UK, 2020. [Google Scholar]
- El-Baky, R.M.A. Application of scanning electron microscopy for the morphological study of biofilm in medical devices. In InTech eBooks; InTechOpen: London, UK, 2012. [Google Scholar]
- Santosh, V.; Vadakkan, K.; Mani, B. A narrative review on bacterial biofilm: Its formation, clinical aspects and inhibition strategies. Futur. J. Pharm. Sci. 2023, 9, 1–15. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Wi, Y.M.; Patel, R. Understanding biofilms and novel approaches to the diagnosis, prevention, and treatment of medical device-associated infections. Infect. Dis. Clin. N. Am. 2018, 32, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.D.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; Coppola, R.; Ayala-Zavala, F.J.; da Cruz, A.G.; De Feo, V. Essential oils and microbial communication. In Essential Oils-Oils of Nature; IntechOpen: London, UK, 2019; pp. 1–26. [Google Scholar]
- Das, M.; Khamaru, D.; Santra, S.; Gupta, R.; Das, S.; Banerjee, R. The enigmatic world of biofilm. In Biofilm Applications to Revolutionize Food Technology; Springer: Cham, Switzerland, 2025; pp. 1–26. [Google Scholar]
- Reddy, T.S.; Hadi, M.A.; Navanita, S.K.; Karri, V.N.R.; Pagar, K.P.; Shanker, K. Targeting microbial biofilms altering chronic wound healing: New breakthrough in drug development using silver. J. Pharm. Negat. Results 2022, 13, 2024–2032. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Petrova, O.; Sauer, K. Escaping the biofilm in more than one way: Desorption, detachment or dispersion. Curr. Opin. Microbiol. 2016, 30, 67–73. [Google Scholar] [CrossRef]
- Ramanathan, S.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Lin, X. Bacterial biofilm inhibition: A focused review on recent therapeutic strategies for combating the biofilm mediated infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Jiang, Z.; Nero, T.; Mukherjee, S.; Olson, R.; Yan, J. Searching for the secret of stickiness: How biofilms adhere to surfaces. Front. Microbiol. 2021, 12, 686793. [Google Scholar] [CrossRef]
- Li, C.H.; Chao, W.H.; Wu, P.C.; Fan, C.H. Remote biofilm dislodgment using focused acoustic vortex. Ultr. Sonochem. 2025, 121, 107423. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Håkansson, A.; Darrieux, M.; Converso, T.R. Klebsiella pneumoniae biofilms and their role in disease pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef]
- Zafer, M.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Ghosh, S.; Bornman, C.; Elfaky, M.A. Biofilm-mediated infections by multidrug-resistant microbes: A comprehensive exploration and forward perspectives. Arch. Microbiol. 2024, 206, 101. [Google Scholar] [CrossRef] [PubMed]
- McConoughey, S.J.; Howlin, R.P.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.E.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Joshi, Y.; Rawat, K.; Bisht, R. Biofilms: Architecture, resistance, quorum sensing and control mechanisms. Indian J. Microbiol. 2018, 59, 3–15. [Google Scholar] [CrossRef]
- Shahid, A.; Rasool, M.; Akhter, N.; Aslam, B.; Hassan, A.N.; Sana, S.; Rasool, M.H.; Khurshid, M. Innovative strategies for the control of biofilm formation in clinical settings. In IntechOpen eBooks; IntechOpen: London, UK, 2019. [Google Scholar]
- Natanzi, A.S.; Poudineh, M.; Karimi, E.; Khaledi, A.; Kashani, H.H. Innovative approaches to combat antibiotic resistance: Integrating CRISPR/Cas9 and nanoparticles against biofilm-driven infections. BMC Med. 2025, 23, 486. [Google Scholar]
- Schulze, A.; Mitterer, F.; Pombo, J.P.N.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—Development, composition and regulation—Therapeutical strategies. Microb. Cell 2021, 8, 28–40. [Google Scholar] [CrossRef]
- Meroni, G.; Panelli, S.; Zuccotti, G.; Bandi, C.; Drago, L.; Pistone, D. Probiotics as therapeutic tools against pathogenic biofilms: Have we found the perfect weapon? Microbiol. Res. 2021, 12, 916–937. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Rohde, M.; Molinari, G.; Stadler, M.; Abraham, W.R. Unsaturated fatty acids control biofilm formation of Staphylococcus aureus and other gram-positive bacteria. Antibiotics 2020, 9, 788. [Google Scholar] [CrossRef]
- Lee, C.; Tribble, G.D. Roles of specialized pro-resolving mediators and omega-3 polyunsaturated fatty acids in periodontal inflammation and impact on oral microbiota. Front. Oral Health 2023, 4, 1217088. [Google Scholar] [CrossRef]
- Harrison, Z.L.; Awais, R.; Harris, M.A.; Raji, B.; Hoffman, B.C.; Baker, D.L.; Jennings, J.A. 2-Heptylcyclopropane-1-carboxylic acid disperses and inhibits bacterial biofilms. Front. Microbiol. 2021, 12, 645180. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Steixner, S.; Wurm, A.; Nogler, M. Antibacterial and anti-biofilm activity of omega-3 polyunsaturated fatty acids against periprosthetic joint infections-isolated multi-drug-resistant strains. Biomedicines 2021, 9, 334. [Google Scholar] [CrossRef]
- Spiegel, C.; Steixner, S.; Coraça-Huber, D.C. Antibiofilm activity of omega-3 fatty acids and its influence on the expression of biofilm formation genes on Staphylococcus aureus. Antibiotics 2022, 11, 932. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Tae, H.; Park, S.; Cho, N.J. Lipid membrane remodeling by the micellar aggregation of long-chain unsaturated fatty acids for sustainable antimicrobial strategies. Int. J. Mol. Sci. 2023, 24, 9639. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Obukhova, E.S.; Murzina, S.A. Mechanisms of the antimicrobial action of fatty acids: A review. Appl. Microbiol. Biotechnol. 2024, 60, 1035–1043. [Google Scholar] [CrossRef]
- Eder, A.E.; Munir, S.A.; Hobby, C.R.; Anderson, D.M.; Herndon, J.L.; Siv, A.W.; Symes, S.J.K.; Giles, D.K. Exogenous polyunsaturated fatty acids (PUFAs) alter phospholipid composition, membrane permeability, biofilm formation and motility in Acinetobacter baumannii. Microbiology 2017, 163, 1626–1636. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Lee, J. Antibiofilm activities of fatty acids including myristoleic acid against Cutibacterium acnes via reduced cell hydrophobicity. Phytomedicine 2021, 91, 153710. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Y.; Zhang, B.; Zhang, Y.H.P.J. Spatially structured exchange of metabolites enhances bacterial survival and resilience in biofilms. Nat. Commun. 2024, 15, 7575. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Progr. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Jackman, J.A.; Boyd, R.D.; Elrod, C.C. Medium-chain fatty acids and monoglycerides as feed additives for pig production: Towards gut health improvement and feed pathogen mitigation. J. Anim. Sci. Biotechnol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Demirbolat, G.M.; Coskun, G.P.; Erdogan, O.; Cevik, O. Long chain fatty acids can form aggregates and affect the membrane integrity. Colloids Surf. B Biointerfaces 2021, 204, 111795. [Google Scholar] [CrossRef]
- Herndon, J.L.; Peters, R.E.; Hofer, R.N.; Simmons, T.B.; Symes, S.J.; Giles, D.K. Exogenous polyunsaturated fatty acids (PUFAs) promote changes in growth, phospholipid composition, membrane permeability and virulence phenotypes in Escherichia coli. BMC Microbiol. 2020, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Lee, S.F.; Langelaan, D.N.; Lefsay, A.; Rupasinghe, H.V. Carvacrol alters the membrane phospholipids in erythromycin-resistant Streptococcus pyogenes. Phytomed. Plus 2024, 4, 100614. [Google Scholar] [CrossRef]
- Nakamura, Y.K.; Omaye, S.T. Metabolic diseases and pro- and prebiotics: Mechanistic insights. Nutr. Metab. 2012, 9, 60. [Google Scholar] [CrossRef]
- Pinto, R.M.; Lopes-de-Campos, D.; Martins, M.C.L.; Van Dijck, P.; Nunes, C.; Reis, S. Impact of nanosystems in Staphylococcus aureus biofilms treatment. FEMS Microbiol. Rev. 2019, 43, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.K.; Ellermann, M. Long chain fatty acids and virulence repression in intestinal bacterial pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 928503. [Google Scholar] [CrossRef]
- Mitra, S.; Sana, B.; Mukherjee, J. Ecological Roles and Biotechnological Applications of Marine and Intertidal Microbial Biofilms. In Productive Biofilms. Advances in Biochemical Engineering/Biotechnology; Muffler, K., Ulber, R., Eds.; Springe: Cham, Switzerland, 2014; pp. 163–205. [Google Scholar] [CrossRef]
- Juszczuk-Kubiak, E. Molecular aspects of the functioning of pathogenic bacteria biofilm based on quorum sensing (QS) signal-response system and innovative non-antibiotic strategies for their elimination. Int. J. Mol. Sci. 2024, 25, 2655. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Aranda-Pérez, J.; Sánchez-Aguilar, M.D.C.; Cutiño-Gobea, A.M.; Pérez-Montaño, F.; Medina, C. Cyclic di-GMP Modulation of Quorum Sensing and Its Impact on Type VI Secretion System Function in Sinorhizobium fredii. Microorganisms 2025, 13, 2232. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K.J.A.M. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef]
- Behzadnia, A.; Moosavi-Nasab, M.; Oliyaei, N. Anti-biofilm activity of marine algae-derived bioactive compounds. Front. Microbiol. 2024, 15, 1270174. [Google Scholar] [CrossRef]
- Wongdontree, P.; Millan-Oropeza, A.; Upfold, J.; Lavergne, J.P.; Halpern, D.; Lambert, C.; Page, A.; Kénanian, G.; Grangeasse, C.; Henry, C.; et al. Oxidative stress is intrinsic to staphylococcal adaptation to fatty acid synthesis antibiotics. iScience 2024, 27, 109505. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Raorane, C.J.; Oh, S.T.; Park, J.G.; Lee, J. Herring oil and omega fatty acids inhibit Staphylococcus aureus biofilm formation and virulence. Front. Microbiol. 2018, 9, 1241. [Google Scholar] [CrossRef]
- Fratianni, F.; Coppola, F.; Tavaniello, S.; Ombra, M.N.; De Giulio, B.; D’Agostino, N.; Zengin, G.; Coppola, R.; Nazzaro, F. Fatty Acid Profile and Some Useful Biological Aspects of Borage, Calophyllum, and Prickly Pear Seed Oils: Implications for Health and Dietary Use. Antioxidants 2025, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Amato, G.; De Feo, V.; d’Acierno, A.; Coppola, R.; Nazzaro, F. Potential therapeutic benefits of unconventional oils: Assessment of the potential in vitro biological properties of some Rubiaceae, Cucurbitaceae, and Brassicaceae seed oils. Front. Nutr. 2023, 10, 1171766. [Google Scholar] [CrossRef]
- Sun, X.; Ni, Z.; Tang, J.; Ding, Y.; Wang, X.; Li, F. The abaI/abaR quorum sensing system effects on pathogenicity in Acinetobacter baumannii. Front. Microbiol. 2021, 12, 679241. [Google Scholar] [CrossRef]
- Ellermann, M. Emerging mechanisms by which endocannabinoids and their derivatives modulate bacterial populations within the gut microbiome. Adv. Drug Alcohol Res. 2023, 3, 11359. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2018, 8, 17696. [Google Scholar] [CrossRef]
- Chanda, W.; Joseph, T.P.; Padhiar, A.A.; Guo, X.; Min, L.; Wang, W.; Lolokote, S.; Ning, A.; Cao, J.; Huang, M.; et al. Combined effect of linolenic acid and tobramycin on Pseudomonas aeruginosa biofilm formation and quorum sensing. Exp. Ther. Med. 2017, 14, 193–204. [Google Scholar] [CrossRef]
- Jennings, J.A.; Courtney, H.S.; Haggard, W.O. Cis-2-decenoic acid inhibits Staphylococcus aureus growth and biofilm in vitro: A pilot study. Clin. Orthop. Relat. Res. 2012, 470, 2663–2670. [Google Scholar] [CrossRef]
- Kumar, V.; Yasmeen, N.; Pandey, A.; Chaudhary, A.A.; Alawam, A.S.; Rudayni, H.A.; Islam, A.; Lakhawat, S.S.; Sharma, P.K.; Shahid, M. Antibiotic adjuvants: Synergistic tool to combat multi-drug resistant pathogens. Front. Cell. Infect. Microbiol. 2023, 13, 1293633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Pedicord, V.A.; Peng, T.; Hang, H.C. Site-specific acylation of a bacterial virulence regulator attenuates infection. Nat. Chem. Biol. 2019, 16, 95–102. [Google Scholar] [CrossRef]
- Han, L.; Ren, J.; Xue, Y.; Gao, J.; Fu, Q.; Shao, P.; Zhu, H.; Zhang, M.; Ding, F. Fatty acid synthesis promoted by PA1895–1897 operon delays quorum sensing activation in Pseudomonas aeruginosa. AMB Express 2024, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Trirocco, R.; Pasqua, M.; Tramonti, A.; Colonna, B.; Paiardini, A.; Prosseda, G. Diffusible signal factors (DSFs) bind and repress VirF, the leading virulence activator of Shigella flexneri. Sci. Rep. 2023, 13, 13170. [Google Scholar] [CrossRef] [PubMed]
- Woodbrey, A.K.; Onyango, E.O.; Pellegrini, M.; Kovacikova, G.; Taylor, R.K.; Gribble, G.W.; Kull, F.J. A new class of inhibitors of the AraC family virulence regulator Vibrio cholerae ToxT. Sci. Rep. 2017, 7, 45011. [Google Scholar] [CrossRef]
- Chowdhury, R.; Bitar, P.D.P.; Keresztes, I.; Condo, A.M.; Altier, C. A diffusible signal factor of the intestine dictates Salmonella invasion through its direct control of the virulence activator HilD. PLoS Pathog. 2021, 17, e1009357. [Google Scholar] [CrossRef]
- Luizet, J. Host Cell Modulation by Brucella Effectors. Ph.D. Thesis, University of Lyon, Lyon, France, 2019. [Google Scholar]
- Zhan, Z.; Tang, H.; Zhang, Y.; Huang, X.; Xu, M. Potential of gut-derived short-chain fatty acids to control enteric pathogens. Front. Microbiol. 2022, 13, 976406. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, W.; Qin, N.; Ren, X.; Xia, X. Propionate and butyrate inhibit biofilm formation of Salmonella Typhimurium grown in laboratory media and food models. Foods 2022, 11, 3493. [Google Scholar] [CrossRef]
- Deng, Z.; Luo, X.; Liu, J.; Wang, H. Quorum sensing, biofilm, and intestinal mucosal barrier: Involvement the role of probiotic. Front. Cell. Infect. Microbiol. 2020, 10, 538077. [Google Scholar] [CrossRef]
- Gart, E.V.; Suchodolski, J.S.; Welsh, T.H.; Alaniz, R.C.; Randel, R.D.; Lawhon, S.D. Salmonella Typhimurium and multidirectional communication in the gut. Front. Microbiol. 2016, 7, 1827. [Google Scholar] [CrossRef]
- Rangan, K.J.; Hang, H.C. Biochemical mechanisms of pathogen restriction by intestinal bacteria. Trends Biochem. Sci. 2017, 42, 887–898. [Google Scholar] [CrossRef]
- Boyen, F.; Haesebrouck, F.; Vanparys, A.; Volf, J.; Mahu, M.; Van Immerseel, F.; Rychlík, I.; Dewulf, J.; Ducatelle, R.; Pasmans, F. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet. Microbiol. 2008, 132, 319–327. [Google Scholar] [CrossRef]
- Van Immerseel, F.; De Buck, J.; Boyen, F.; Bohez, L.; Pasmans, F.; Volf, J.; Ducatelle, R. Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 2004, 70, 3582–3587. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Russell, J.B.; Flythe, M.D.; Gantois, I.; Timbermont, L.; Pasmans, F.; Ducatelle, R. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathol. 2006, 35, 182–188. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, H.; Kim, A.-R.; Yun, C.; Han, S.H. Propionate ameliorates Staphylococcus aureus skin infection by attenuating bacterial growth. Front. Microbiol. 2019, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Tabashsum, Z.; Patel, P.; Bernhardt, C.; Biswas, D. Linoleic acids overproducing Lactobacillus casei limits growth, survival, and virulence of Salmonella Typhimurium and enterohaemorrhagic Escherichia coli. Front. Microbiol. 2018, 9, 2663. [Google Scholar] [CrossRef] [PubMed]
- Tabashsum, Z.; Peng, M.; Salaheen, S.; Comis, C.; Biswas, D. Competitive elimination and virulence property alteration of Campylobacter jejuni by genetically engineered Lactobacillus casei. Food Control 2018, 85, 283–291. [Google Scholar] [CrossRef]
- Worthington, R.J.; Richards, J.J.; Melander, C. Small molecule control of bacterial biofilms. Org. Biomol. Chem. 2012, 10, 7457–7466. [Google Scholar] [CrossRef]
- Rahmani-Badi, A.; Sepehr, S.; Fallahi, H.; Heidari-Keshel, S. Dissection of the cis-2-decenoic acid signaling network in Pseudomonas aeruginosa using microarray technique. Front. Microbiol. 2015, 6, 383. [Google Scholar]
- Pierro, F.D.; Sagheddu, V.; Galletti, S.; Forti, M.; Elli, M.; Bertuccioli, A.; Gaeta, S. Antibacterial activity of a fractionated Pistacia lentiscus oil against pharyngeal and ear pathogens, alone or in combination with antibiotics. Front. Microbiol. 2021, 12, 686942. [Google Scholar] [CrossRef]
- Mansara, P.; Deshpande, R.; Vaidya, M.M.; Kaul-Ghanekar, R. Differential ratios of omega fatty acids (AA/EPA+DHA) modulate growth, lipid peroxidation and expression of tumor regulatory MARBPs in breast cancer cell lines MCF7 and MDA-MB-231. PLoS ONE 2015, 10, e0136542. [Google Scholar] [CrossRef]
- Dragičević Tomičić, D.; Lešić, N.; Škrlec, I.; Steigmann, L.; Tseneva, K.; Čalušić Šarac, M.; Crnić, T.; Tomičić, I.; Perić Kačarević, Ž.; Čandrlić, M. Effects of Vitamin D, Melatonin, and Omega-3 Fatty Acids on Periodontal Health: A Narrative Review. Dent. J. 2025, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Maddela, N.R. Is “quorum quenching” a promising tool to combat antibiotic-resistant bacteria? Universa Med. 2023, 42, 249–250. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and antivirulence efficacy of myrtenol enhances the antibiotic susceptibility of Acinetobacter baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.; Alexandre, S.; Luizet, J.B.; Skogman, M.; Jouenne, T.; Salcedo, S.P.; Dé, E. Unsaturated fatty acids affect quorum sensing communication system and inhibit motility and biofilm formation of Acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 214. [Google Scholar] [CrossRef]
- Azuama, O.C. Searching for New Bioactive Compounds to Tackle the Opportunistic Human Pathogen Pseudomonas aeruginosa. Ph.D. Thesis, Universitéde Rouen Normandie, Normandie Université, Évreux, France, 2020. [Google Scholar]
- Zhe-bin, H.; Wu, C.; Fan, G.; Tang, Z.; Cao, F. The antibacterial activity and mechanism of ginkgolic acid C15:1. BMC Biotechnol. 2017, 17, 5. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Ryu, S.Y.; Cho, M.H.; Lee, J. Ginkgolic acids and Ginkgo biloba extract inhibit Escherichia coli O157: H7 and Staphylococcus aureus biofilm formation. Int. J. Food Microbiol. 2014, 174, 47–55. [Google Scholar] [CrossRef]
- Ohshima, T.; Kawai, T.; Maeda, N. Bacterial cell-free probiotics using effective substances produced by probiotic bacteria, for application in the oral cavity. In IntechOpen eBooks; IntechOpen: London, UK, 2019. [Google Scholar]
- Juárez-Rodríguez, M.M.; Cortés-López, H.; García-Contreras, R.; González-Pedrajo, B.; Díaz-Guerrero, M.; Martínez-Vázquez, M.; Rivera-Chávez, J.; Soto-Hernández, M.; Castillo-Juárez, I. Tetradecanoic acids with anti-virulence properties increase the pathogenicity of Pseudomonas aeruginosa in a murine cutaneous infection model. Front. Cell. Infect. Microbiol. 2021, 10, 597517. [Google Scholar] [CrossRef]
- Aditya, A.; Peng, M.; Young, A.; Biswas, D. Antagonistic mechanism of metabolites produced by Lactobacillus casei on lysis of enterohemorrhagic Escherichia coli. Front. Microbiol. 2020, 11, 574422. [Google Scholar] [CrossRef]
- Baldewijns, S.; Sillen, M.; Palmans, I.; Vandecruys, P.; Van Dijck, P.; Demuyser, L. The role of fatty acid metabolites in vaginal health and disease: Application to candidiasis. Front. Microbiol. 2021, 12, 705779. [Google Scholar] [CrossRef]
- Basson, A.; Trotter, A.; Rodriguez-Palacios, A.; Cominelli, F. Mucosal interactions between genetics, diet, and microbiome in inflammatory bowel disease. Front. Immunol. 2016, 7, 290. [Google Scholar] [CrossRef]
- Jiang, D.; Lin, Y.C.; Shin, S.; Choe, Y.; Cho, N.J. Elucidating the pH Effects on Oleic Acid and Interactions with Lipid Membranes. J. Phys. Chem. B 2025, 129, 5976–5988. [Google Scholar] [CrossRef]
- Gindlhuber, J.; Schinagl, M.; Liesinger, L.; Darnhofer, B.; Tomin, T.; Schittmayer, M.; Birner-Gruenberger, R. Hepatocyte proteome alterations induced by individual and combinations of common free fatty acids. Int. J. Mol. Sci. 2022, 23, 3356. [Google Scholar] [CrossRef]
- Cochrane, A.; Remfry, L.; Nagaraja, W.; Dritz, T.; Niederwerder, J. Determining the Minimum Inhibitory Concentration of Medium Chain Fatty Acids for Generic Escherichia coli, Enterotoxigenic Escherichia coli, Salmonella Typhimurium, and Campylobacter coli. Kansas Agric. Exp. Station Res. Rep. 2018, 4, 10. [Google Scholar] [CrossRef]
- Wieder, N.; Fried, J.C.; Kim, C.; Sidhom, E.H.; Brown, M.R.; Marshall, J.L.; Arevalo, C.; Dvela-Levitt, M.; Kost-Alimova, M.; Sieber, J.; et al. FALCON systematically interrogates free fatty acid biology and identifies a novel mediator of lipotoxicity. Cell Metab. 2023, 35, 887–905. [Google Scholar] [CrossRef] [PubMed]
- Bergen, J.; Karasova, M.; Bileck, A.; Pignitter, M.; Marko, D.; Gerner, C.; Del Favero, G. Exposure to dietary fatty acids oleic and palmitic acid alters structure and mechanotransduction of intestinal cells in vitro. Arch. Toxicol. 2023, 97, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.S.; Bambace, M.F.; Andersen, E.B.; Meyer, R.L.; Schwab, C. Environmental pH and compound structure affect the activity of short-chain carboxylic acids against planktonic growth, biofilm formation, and eradication of the food pathogen Salmonella enterica. Microbiol. Spectr. 2024, 12, e01658-24. [Google Scholar] [CrossRef]
- Choi, E.; Murray, B.; Choi, S. Biofilm and cancer: Interactions and future directions for cancer therapy. Int. J. Mol. Sci. 2023, 24, 12836. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Rodriguez, J.; Zhang, Z.; Seethaler, B.; Sánchez, C.R.; Roumain, M.; Hiel, S.; Bindels, L.B.; Cani, P.D.; Paquot, N.; et al. Prebiotic dietary fibre intervention improves fecal markers related to inflammation in obese patients: Results from the Food4Gut randomized placebo-controlled trial. Eur. J. Nutr. 2021, 60, 3159–3170. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R.; Chouinard, Y.P.; Jacques, H.; Venkatramanan, S.; Maf, A.A.; Defnoun, S.; Jones, P.J. The effect of drinking milk containing conjugated linoleic acid on fecal microbiological profile, enzymatic activity, and fecal characteristics in humans. Nutr. J. 2007, 6, 15. [Google Scholar] [CrossRef]
- Carafa, I.; Masuero, D.; Vrhovsek, U.; Bittante, G.; Franciosi, E.; Tuohy, K.M. Production of conjugated linoleic acid (CLA): Effect of inulin on microbial composition and CLA concentration in a human intestinal model. Proceed. Nutr. Soc. 2020, 79, E628. [Google Scholar] [CrossRef]
- Smedman, A.; Vessby, B. Conjugated linoleic acid supplementation in humans—Metabolic effects. Lipids 2001, 36, 773–781. [Google Scholar] [CrossRef]
- Dilzer, A.; Park, Y. Implication of conjugated linoleic acid (CLA) in human health. Crit. Rev. Food Sci. Nutr. 2012, 52, 488–513. [Google Scholar] [CrossRef]
- Yazici, A. The strain-dependent antimicrobial and antibiofilm effect of cis- and trans-vaccenic acid against Pseudomonas aeruginosa. Cumhuriyet Sci. J. 2024, 45, 1–10. [Google Scholar] [CrossRef]
- Hassanein, A.; Marusich, E.; Pustovalova, M.; Leonov, S. Mechanism of bactericidal efficacy against nosocomial pathogenic Staphylococcus aureus strain caused by fatty acids from Hermetia illucens larvae fat. Sci. Rep. 2025, 15, 30305. [Google Scholar] [CrossRef]
- Hawas, U.W.; Shaher, F.; Ghandourah, M.; Abou El-Kassem, L.T.; Satheesh, S.; Al-Sofyani, A.M.A. Lipids and free fatty acids of Red Sea Avrainvillea amadelpha, Holothuria atra, and Sarcocornia fruticosa inhibit marine bacterial biofilms. Lett. Org. Chem. 2020, 17, 466–471. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Juarez, J.; Valdez, M.A.; Ayala-Zavala, J.F.; Del-Toro-Sánchez, C.L.; Encinas-Basurto, D. Hydrophobic chitosan nanoparticles loaded with carvacrol against Pseudomonas aeruginosa biofilms. Molecules 2022, 27, 699. [Google Scholar] [CrossRef] [PubMed]
- Asiri, M.A.; Alzohairy, S.M.M.; Alomary, M.A.; Almatroudi, M.N.; Khan, F.A. Biofabricated fatty acids-capped silver nanoparticles as potential antibacterial, antifungal, antibiofilm and anticancer agents. Pharmaceuticals 2021, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Ivanova, K.; Fiandra, L.; Mantecca, P.; Catelani, T.; Natan, M.; Banin, E.; Jacobi, G.; Tzanov, T. Antibacterial, antibiofilm, and antiviral farnesol-containing nanoparticles prevent Staphylococcus aureus from drug resistance development. Int. J. Mol. Sci. 2022, 23, 7527. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Fazly Bazzaz, B.S.; Mirhadi, E.; Tajani, A.S.; Khameneh, B. The role of nanotechnology in combating biofilm-based antibiotic resistance. J. Drug Deliv. Sci. Technol. 2020, 60, 101880. [Google Scholar] [CrossRef]
- Thambirajoo, M.; Maarof, M.; Lokanathan, Y.; Katas, H.; Ghazalli, N.F.; Tabata, Y.; Fauzi, M.B. Potential of nanoparticles integrated with antibacterial properties in preventing biofilm and antibiotic resistance. Antibiotics 2021, 10, 1338. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, H.; Teng, Z.; Ma, J.; Liu, G. Nanomaterial-enabled anti-biofilm strategies: New opportunities for treatment of bacterial infections. Nanoscale 2025, 17, 5605–5628. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling antibiotic resistance with compounds of natural origin: A comprehensive review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; d’Acierno, A.; De Feo, V.; Ayala-Zavala, F.J.; Gomes-Cruz, A.; Granato, D.; Coppola, R. Effect of polyphenols on microbial cell-cell communications. In Quorum Sensing; Tommonaro, G., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 195–223. [Google Scholar]
- Nazzaro, F.; Coppola, F.; Fratianni, F.; Abdalrazeq, M.; Ombra, M.N.; De Giulio, B.; Coppola, R.; Zengin, G. Polyphenols Bioactive Metabolites, and Their Anti-Biofilm and Neuroprotective Potential. Foods 2025, 14, 3976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Shi, M.; Chen, R.; Zhang, Y.; Sheng, Y.; Tong, C.; Cao, G.; Shou, D. Natural phytochemical-based strategies for antibiofilm applications. Chin. Med. 2025, 20, 96. [Google Scholar] [CrossRef]
- Dostert, M.; Trimble, M.J.; Hancock, R.E. Antibiofilm peptides: Overcoming biofilm-related treatment failure. RSC Adv. 2021, 11, 2718–2728. [Google Scholar] [CrossRef]
- Fontanot, A.; Ellinger, I.; Unger, W.W.; Hays, J.P. A comprehensive review of recent research into the effects of antimicrobial peptides on biofilms—January 2020 to September 2023. Antibiotics 2024, 13, 343. [Google Scholar] [CrossRef]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and antibiofilm peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef]
- Wongchai, M.; Wongkaewkhiaw, S.; Kanthawong, S.; Roytrakul, S.; Aunpad, R. Dual-function antimicrobial-antibiofilm peptide hybrid to tackle biofilm-forming Staphylococcus epidermidis. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 44. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of antimicrobial peptides against bacterial biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.