Abstract
Background: In geriatrics, explicit criteria for potentially inappropriate prescriptions (PIPs) are useful for optimizing drug use. Objective: To produce an expert consensus on explicit definitions of antibiotic-PIPs for hospitalized older patients. Methods: We conducted a Delphi survey involving French experts on antibiotic stewardship in hospital settings. During the survey’s rounds, the experts gave their opinion on each explicit definition, and could suggest new definitions. Definitions with a 1-to-9 Likert score of between 7 and 9 from at least 75% of the participants were adopted. The results were discussed during consensus meetings after each round. Results: Of the 155 invited experts, 128 (82.6%) participated in the whole survey: 59 (46%) infectious diseases specialists, 45 (35%) geriatricians, and 24 (19%) other specialists. In Round 1, 65 explicit definitions were adopted and 21 new definitions were suggested. In Round 2, 35 other explicit definitions were adopted. The results were validated during consensus meetings (with 44 participants after Round 1, and 54 after Round 2). Conclusions: The present study is the first to have provided a list of explicit definitions of potentially inappropriate antibiotic prescriptions for hospitalized older patients. It might help to disseminate key messages to prescribers and reduce inappropriate prescriptions of antibiotics.
1. Introduction
The development of antimicrobial resistance remains a major public health issue [1] and is promoted by the inappropriate use of antibiotics [2,3] (defined as under-use, over-use, incorrect choice, or incorrect use with regard to the dose level, administration route, duration of treatment, etc.) [4]. Many national and international action plans have been developed to reduce inappropriate antibiotic prescribing and to combat antibiotic resistance [5,6,7]. The appropriateness or inappropriateness of antibiotic prescriptions is usually assessed by an expert with regard to clinical practice guidelines and the individual patient’s situation; this is a so-called implicit approach. The problem of inappropriate prescriptions of antibiotics remains significant, despite effective interventions. This usual approach is time-consuming and resource-consuming for antibiotic stewardship teams to deal with a large number of prescriptions. The development of new tools remains useful for improving the use of antibiotics and consequently limiting the increase in antibiotic resistance. Another approach (already used in the field of geriatrics) is based on explicit criteria for potentially inappropriate prescriptions (PIPs) [8,9,10]. These explicit criteria (i) provide training tools for prescribers; (ii) allow the development of computerized tools for the automatic detection of PIPs; and (iii) might provide epidemiological data on these prescriptions [11,12]. The explicit approach could be an additional support for expert teams, for example by helping to disseminate messages to prescribers about prescriptions to be avoided, or by helping to better identify patients requiring optimization of the antibiotics prescribed. Older patients are particularly susceptible to bacterial infections and are frequently prescribed antibiotics [13,14,15]. In this population, possible atypical clinical presentations can affect the diagnosis, which contributes to inappropriate prescriptions of antibiotics [16].
The explicit approach has not yet been applied in antibiotic stewardship programs, and there are no validated criteria for PIPs of antibiotics (henceforth referred to as “antibiotic-PIPs”) [17]. The objective of the present study was to develop a list of explicit definitions of antibiotic-PIPs for hospitalized patients aged 75 or over.
2. Results
2.1. Participants
A total of 155 people were invited to participate in the study. Of these, 128 completed the entire survey (i.e., Rounds 1 and 2), giving a full participation rate of 82.6%. A total of 59 participants (46.1%) were ID specialists, 45 (35.2%) were geriatricians, and 24 (18.8%) were other specialists (18 hospital pharmacists, 3 microbiologists, 1 infection control practitioner, 1 general practitioner, and 1 neurologist). The characteristics of the study participants are summarized in Supplementary Data S3 and their geographical distribution is shown in Supplementary Data S4.
2.2. The Delphi Survey
The study flow chart for participants and explicit definitions is shown in Figure 1. The consensus meetings after Round 1 and Round 2 were attended by 44 and 55 stakeholders, respectively. At the end of Round 1, 65 of the 103 explicit definitions in the eligible list were adopted and 1 definition was rejected. The participants suggested a total of 113 new, explicit definitions of antibiotic-PIPs and several reformulations of definitions. The first consensus meeting validated the reformulation of 16 definitions and the introduction of 21 new definitions. The remaining 37 explicit definitions lacking a consensus and the 21 new definitions were submitted to Round 2 of the survey. After Round 2, 35 explicit definitions were adopted. Of the 21 new, explicit definitions suggested by the participants, 8 did not have a consensus. During the consensus meeting following Round 2, the 55 stakeholders evaluated these eight definitions and decided: (i) not to submit them to a third round, considering that they were not derived from the same standardized process as the definitions in the eligible list; (ii) to reject the five definitions if 60% or less of the experts rated them with a score of 7–9; (iii) to discuss and then vote for the three remaining definitions rated by >60% of the experts with a score of 7–9. Finally, the three remaining definitions were included (>90% of the participants voted “Yes”).
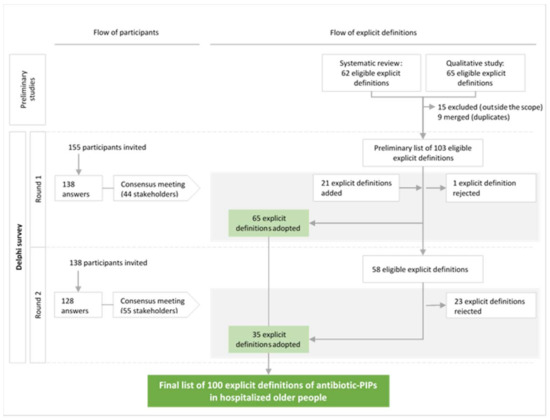
Figure 1.
Diagram showing the flow of participants and explicit definitions of potentially inappropriate prescriptions of antibiotics (antibiotic-PIPs) during the Delphi survey and the consensus meetings.
2.3. The Consensus List of Explicit Definitions of Antibiotic-PIPs
The Delphi survey resulted in a consensus list of 100 explicit definitions of antibiotic-PIPs for hospitalized older patients, covering 23 areas of antibiotic prescribing (Table 1). The experts specified that these explicit definitions should be applied only if the clinical presentation was non-severe and in the absence of a known drug allergy. The final list of explicit definitions of antibiotic-PIPs is given in Table 2.

Table 1.
Numbers of explicit definitions of antibiotic-PIPs in hospitalized older patients, by domain or usage.

Table 2.
Classification of explicit definitions of potentially inappropriate prescriptions of antibiotics in hospitalized older patients (caution: the definitions should not be applied to severe cases or patients with known drug allergies).
3. Material and Methods
3.1. Study Design
We conducted a Delphi survey, i.e., a method frequently used to design and validate explicit criteria [18]. This sequential process collects the opinion of a panel of experts on a preliminary list of items. Each stage is called a round, in which participants fill out a questionnaire. Anonymity is a fundamental principle of the Delphi survey. Participants interact indirectly, in order to avoid the dominant influence of certain participants [19]. Our work followed expert recommendations on the reporting of Delphi studies [20,21,22,23].
3.2. Scope of the Study
In clinical pharmacology, PIPs can be assessed via an implicit judgment or via explicit criteria [24,25]. Explicit definitions state situations that are usually considered to be inappropriate, according to the literature or an expert consensus. In the present explicit approach, we sought to develop a consensus list of explicit definitions of antibiotic-PIPs in older patients (aged 75 or over) hospitalized in acute care units. Explicit definitions of PIPs are usually intended to limit adverse events at the individual patient level [8,9,10]. In the present work, we conceived explicit definitions as a means of combatting antimicrobial resistance on both the individual and collective scales, as presented in two preliminary studies [17,26].
3.3. Steering Committee
A steering committee (NB, ES, and JBB) was set up to validate the study’s methodological principles and to validate the results at each stage. The work was carried out in partnership with the GInGer (a joint study group for infections in the elderly, created by the French Infectious Diseases Society (Société de pathologie infectieuse de langue française, SPILF) and the French Gerontology and Geriatrics Society (Société Française de Gériatrie et Gérontologie, SFGG)).
3.4. Ethical Approval
We confirmed that the experts had agreed to participate in the study and had consented to the collection of personal data, in accordance with the European Union’s General Data Protection Regulation.
3.5. Preparation of the List of Eligible Explicit Definitions of Antibiotic-PIPs
The list of eligible explicit definitions of antibiotic-PIPs to be submitted to the Delphi group was prepared in two preliminary studies. Firstly, a list of 62 explicit definitions of antibiotic-PIPs was identified through a systematic review of the literature [17]. Secondly, a list of 65 explicit definitions was established in a qualitative survey [26]. The sets of explicit definitions from the two studies overlapped to some extent. We, therefore, sought to identify and merge similar definitions and to independently validate the choices made. The key steps in the preparation of this list were (i) translation of the list of definitions from the systematic literature review into French; (ii) the grouping together, merger, or reformulation of explicit definitions if necessary (performed by two researchers: NB and RHR); and (iii) validation of the list by the steering committee and external experts comprising infectious disease (ID) specialists and geriatricians. The methodology used to prepare the list of eligible explicit definitions is detailed in Supplementary Data S1. Supplementary Data S2 shows the list of eligible explicit definitions of antibiotic-PIPs.
3.6. The Panel of Experts for the Delphi Study
We sought a variety of opinions from ID specialists, geriatricians, and other experts in the use of antibiotics in older patients in hospitals from throughout metropolitan France, in order to take account of possible local and regional disparities in practice [27].
3.6.1. Inclusion Criteria for Participants
Three groups of participants were identified, with the following target distribution: ID specialists (40%), geriatricians (40%), and other specialists involved in antibiotic stewardship in hospitals (20%). Participants had to meet at least one of the following criteria: prescriber of antibiotics, an advisory role on antibiotic therapy, membership of a hospital antibiotic committee, active membership of a learned society’s antibiotic working group, or membership of public health authority dealing with antibiotic use.
3.6.2. Number of Participants
With a view to being representative and to take account of differences in expert opinion, we sought to recruit between 140 and 160 participants. We expected at least 75% of the recruited experts to participate throughout the duration of the study.
3.6.3. Recruitment of Participants
The steering committee and GInGer identified 16 nationally known experts as local coordinators (EF, CBR, SA, HA, EBN, PCP, AC, SD, TF, PG, MH, JPL, MP, AP, YR, and ES). These experts were asked to (i) recruit at least three geriatricians, three ID specialists, and two other specialists meeting the inclusion criteria from within their local network; (ii) participate in the Delphi survey; (iii) participate in the consensus meetings; and (iv) help the steering committee to remind participants to fill out the questionnaire, if necessary.
3.7. Preparation of the Delphi Survey
3.7.1. The Online Platform
The online survey was prepared on the SmartSurveyTM platform (https://www.smartsurvey.com/, accessed on 12 March 2024). An introductory page presented the concepts needed to understand the study’s scope and objectives, with a synopsis, an explanatory video, and the results of the preliminary studies (i.e., an information kit). The participants were informed that their answers would be anonymous and were given information about the regulatory framework that covered the data collected. After confirming that they did not object to these conditions, the participants filled out a questionnaire collecting data on their age, sex, year of their MD/PharmD thesis, city of practice, type of hospital (general or university), specialty, antimicrobial stewardship activity, membership of an antibiotic committee, membership of a learned society’s antibiotic working group, and membership of a public health authority dealing with antibiotic use. Next, the main questionnaire presented all the explicit definitions of an antibiotic-PIP, which were scored on a Likert scale ranging from 1 (strongly disagree) to 9 (strongly agree) [28]. To avoid exhaustion bias, the various definitions were presented in random order.
3.7.2. Briefing of the Participants
An individual meeting (1 h) with each local coordinator was used to present the study and the recruitment method and to explain the coordinator’s role. Next, the steering committee and the local coordinator met each participant and presented the study process (1 h). Lastly, each participant received an e-mail message containing the information kit (the one available online) and a personal link to the online survey.
3.8. Definition of the Consensus Criteria
Participants were asked to express their level of agreement with each explicit definition of antibiotic-PIP by rating it on the above-mentioned 1-to-9 Likert scale [28]. The objective was to adopt explicit definitions with a high level of consensus. The numerical criteria for consensus were as follows. If at least 75% of the participants gave a Likert score of between 7 and 9, the definition was adopted. If at least 75% of the participants gave a Likert score of between 1 and 3, the definition was rejected. In all other cases, no consensus was formed.
The Delphi survey method encourages discussion of the numerical results by the participants. Consensus meetings were organized at the end of each round and were attended by the local coordinators and available experts from the Delphi panel. These meetings were intended to present the results of each round, summarize the comments, and discuss contentious cases. Each proposed change in the wording of an explicit definition was discussed and voted on during the meetings, using the Wooclap application (https://www.wooclap.com/, accessed on 12 March 2024).
3.9. The Delphi Process
3.9.1. The Rounds
In Round 1 of the survey, each participant expressed his/her level of agreement with each explicit definition on a scale of 1 to 9 and could also provide a free text comment if so wished. Participants were also allowed to suggest new, explicit definitions in this round. In Round 2, each participant again expressed his/her opinion on explicit definitions on the 1-to-9 scale. The explicit definitions of antibiotic-PIPs considered in Round 2 included (i) the explicit definitions from the eligible list (Supplementary Data S2) that did not achieve a consensus in Round 1, and (ii) the new, explicit definitions suggested in Round 1. If a participant did not reply, he/she was sent a reminder email by the investigators. Another reminder was sent by the regional coordinator, if needed.
3.9.2. Analysis of Rounds and Preparation of the Consensus Meetings
The data were analyzed statistically using R software (version 4.1.2) [29]. With regard to the participants’ characteristics, qualitative variables were quoted as the frequency (percentage) per category, and quantitative variables were described as the median (range). Explicit definitions were classified as having been adopted, rejected, or lacking a consensus. Each free text comment was analyzed independently and in a standardized manner by two researchers (NB and VH), in order to identify critical comments that might prompt the reformulation of explicit definitions. Disagreements were resolved by the two researchers and then by the steering committee, if necessary. The new definitions of antibiotic-PIPs suggested by the participants in Round 1 were checked for explicitness by two researchers (NB and VH). The steering committee validated the results of the quantitative analysis and the text content analysis for submission to the consensus meeting.
3.9.3. Consensus Meetings
Consensus meetings (videoconferences) were organized by the investigators at the end of each round. The investigators first chose the date with the regional coordinators (so that all of the latter could attend the meeting) and then invited all Delphi participants. The results obtained by the research team were presented and discussed after each round. The objectives of the meeting after Round 1 were to (i) adopt the explicit definitions of antibiotic-PIPs that meet the consensus criteria; (ii) validate the reformulation of certain explicit definitions; and (iii) validate the new, explicit definitions proposed in Round 1, in order to submit them to Round 2.
The consensus meeting after Round 2 notably concerned the explicit definitions from the eligible list (Supplementary Data S2); the objective was to adopt those that met the consensus criteria. Definitions that did not have a consensus were definitively rejected. This second meeting also concerned new, explicit definitions of antibiotic-PIPs suggested by the participants in Round 1. The objectives were to (i) adopt those that met the consensus criteria; and (ii) discuss those classified as lacking a consensus for protocol adaptation. These new, explicit definitions did not result from the same preparation process as the list of eligible explicit definitions (Supplementary Data S1). We used the results for the definitions from the eligible list in Rounds 1 and 2: none of the definitions with a favorable score (7–9) from 60% or less of the experts in Round 1 were adopted in Round 2. We, therefore, decided that new, explicit definitions with a favorable score (7–9) from 60% or less of the experts could be rejected directly and that definitions with a favorable score of between 60 and 75% of the experts were contentious and had to be discussed and then voted on (yes/no) for definitive adoption or exclusion.
4. Discussion
The present study is the first to have provided a list (n = 100) of explicit definitions of antibiotic-PIPs for hospitalized older patients. This list provides key messages for prescribers and could be used in specific computer-based tools for the detection of inappropriate situations. Application of this list might help to reduce antibiotic-PIPs and, thus, contribute to the fight against antimicrobial resistance.
4.1. A New Approach to Antimicrobial Stewardship
The explicit approach is already used in the field of geriatrics, where several lists of explicit criteria for inappropriate drug prescriptions have been validated [8,9,10]. The explicit criteria developed in geriatrics are intended to limit individual adverse events for older patients and have proven value in reducing inappropriate prescriptions [30] and related clinical events, such as falls, confusion, and hospital readmission [31,32].
Our work was inspired by this approach, with application in the use of antibiotics. Explicit definitions were developed with a view to limiting the development of bacterial resistance on individual and collective levels (e.g., by using broad-spectrum compounds sparingly and by limiting treatment times). These explicit definitions were designed in a very general way so that a wide range of PIPs could be detected. It should be kept in mind that explicit definitions of antibiotic-PIPs can flag up situations considered to be potentially inappropriate but cannot determine with certainty the inappropriateness of a given prescription. There is a degree of overlap between explicit approaches, which can provide general information, and implicit approaches, which must subsequently be validated by an expert in antibiotic prescribing. For example, the explicit definitions of antibiotic-PIPs might help to flag up situations that are potentially inappropriate and can then be reassessed by an expert. This approach might improve the detection of inappropriate prescribing situations, enable actions to be targeted more effectively, and foster the efforts of antibiotic stewardship teams.
4.2. Explicit Definitions of Relevance to the Fight against Antibiotic Resistance
The explicit definitions in our list corresponded to ongoing issues in antibiotic stewardship. For example, 40% of the definitions were concerned with urinary tract, respiratory tract, or skin infections—the main infections encountered in hospitalized older patients. The most commonly cited antibiotics in the definitions were those qualified as critical: fluoroquinolones, amoxicillin–clavulanic acid, cephalosporins, aminoglycosides, and carbapenems [33]. Many of the definitions were intended to limit the use of these antibiotics when the infection was documented (i.e., the infection site and/or the microorganism) and to facilitate the application of general principles when the infection was not documented, with the aim of limiting the use of broad-spectrum antibiotics. Other explicit definitions specified treatment times that should not be exceeded (e.g., for respiratory or skin infections), in accordance with recent guidelines [34,35,36]. In our systematic review [17], we showed that the published explicit definitions did not adequately cover some issues in antibiotic use. Our list provides new, explicit definitions that address the domains most commonly encountered in practice by multidisciplinary antibiotic therapy teams and the core elements of hospital antibiotic stewardship programs [37,38], such as the general principles of antibiotic use (e.g., definitions #54: It is potentially inappropriate to prescribe ertapenem as a first-line treatment; and #57: It is potentially inappropriate to prescribe antibiotics for an isolated elevation of C-reactive protein).
4.3. Perspectives for the Use of Explicit Definitions of Antibiotic-PIPs
The list of explicit definitions of antibiotic-PIPs could be used to provide training messages to prescribers on situations considered to be inappropriate and that should be avoided. These explicit definitions could also be integrated into computer-based decision support systems for the detection of antibiotic-PIPs [31,39]. This type of detection would provide epidemiological data on antibiotic-PIPs on different scales (e.g., a department, a hospital, etc.). This information would also facilitate audits and assessments of professional practice, in order to provide personalized messages on improving antibiotic use. Lastly, real-time detection could help multidisciplinary antibiotic stewardship teams to re-evaluate potentially inappropriate treatments in a more targeted way to increase the efficiency of interventions. Clinical validation of the definitions provided in this study could, therefore, be based on studies evaluating the validation rate between automatic detection based on explicit definitions, using a digital tool, and the opinion of an expert in infectiology. Implementation in clinical decision support systems will require a set of procedures for their use in everyday practice: translating the explicit definitions into semi-natural language, then translating them into computer language, testing the rules (technical errors, clinical relevance, effective changes to prescriptions), deploying them within a hospital structure, and measuring an outcome [40,41,42]. Studies using this explicit approach would be able to assess the effect of antibiotic therapy re-evaluation interventions, based on the detection of antibiotic-PIPs, on patient outcomes and, in the longer term, the effects on the development of bacterial resistance.
The list developed here was designed for use with older inpatients. However, many of the definitions do not appear to be limited to older adults and might be applicable to hospital patients in general (e.g., definitions #60: “It is potentially inappropriate to prescribe carbapenems for empirical treatment definitions” and #74: “It is potentially inappropriate to prescribe antibiotics for more than 7 days for pneumonia”). Some of the definitions of antibiotic-PIPs that are applicable to adults in general are necessarily applicable to older adults. Although this was not one of the present study’s objectives, it might be possible to use a subset of the definitions in younger adults—subject to expert validation.
4.4. Strengths and Limitations
All the steps in this work followed a robust methodology. The list of eligible explicit definitions was derived from two published preliminary studies [17,26]. Furthermore, all the steps followed a dual analysis, with validation by the steering committee and then by the participants. A large number of experts participated in the Delphi survey, with a high full-study participation rate. A large number of experts also participated in the consensus meetings after each round.
The study had some limitations. Firstly, it was carried out in France, where the epidemiology of antimicrobial resistance and challenges in antibiotic use might differ from those in other countries in Europe or worldwide. However, several aspects of our list cover universal issues such as reducing treatment times, using broad-spectrum antibiotics sparingly, and avoiding unnecessary courses of antibiotics [5,6,7]. Secondly, the Delphi survey (carried out in 2021–2022) came several years after the qualitative study (2019) and the systematic review (2017). This long time interval was due mainly to the COVID-19 pandemic, during which infectious disease experts and geriatricians were not available for participation in the study. Hence, some new data in the literature might have been overlooked, although giving the experts an opportunity to suggest new, explicit definitions and holding discussions at the consensus meetings enabled us to include some recent scientific developments in this field. The consensus criteria were modified at the second consensus meeting with regard to eight definitions from those suggested by participants (not for the definitions of the eligible list). These modifications are allowed by the Delphi method [22]. They were discussed and validated by the 55 participants at the consensus meeting, in order to take into account the fact that the new definitions suggested in Round 1 were not derived from the same standardized process as the definitions in the eligible list. Therefore, eight explicit definitions that lacked a consensus after Round 2 were not submitted to a third round. Some participants may have had conflicts of interest that could have influenced their responses. However, the potential impact on the results was mitigated by the large number of participants and the variety of specialties.
5. Conclusions
We produced the first ever consensus list of explicit definitions (n = 100) of antibiotic-PIPs for hospitalized older patients, by using a structured Delphi method with a large number of French experts and a high participation rate. The list provides key messages to prescribers and can be used in specific computer-based tools for detecting PIPs and for improving antibiotic stewardship. Application of this list might help to reduce antibiotic-PIPs and, thus, contribute to the fight against antimicrobial resistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13030283/s1, Supplementary Data S1: Preparation of the preliminary list; Supplementary Data S2: Eligible list of definitions; Supplementary Data S3: Geographical distribution of participants; Supplementary Data S4: Characteristics of participants.
Author Contributions
Conceptualization, N.B., E.S. and J.-B.B.; data curation, N.B.; formal analysis, N.B., V.H. and R.H.-R.; funding acquisition, N.B.; investigation, N.B., E.F., C.R.-B., R.H.-R., S.A., H.A., E.B.-N., P.C.-P., A.C., S.D., T.F., P.G., M.H., J.-P.L., M.P., A.P. and Y.R.; methodology, N.B., E.F., G.G., C.R.-B., V.H., E.S. and J.-B.B.; project administration, J.-B.B.; software, N.B. and V.H.; supervision, E.S. and J.-B.B.; validation, E.F., G.G., C.R.-B., R.H.-R., S.A., H.A., E.B.-N., P.C.-P., A.C., S.D., T.F., P.G., M.H., J.-P.L., M.P., A.P., Y.R. and J.-B.B.; visualization, N.B. and V.H.; writing—original draft, N.B.; writing—review and editing, N.B., E.F., G.G., C.R.-B., V.H., R.H.-R., S.A., H.A., E.B.-N., P.C.-P., A.C., S.D., T.F., P.G., M.H., J.-P.L., M.P., A.P., Y.R., E.S. and J.-B.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded partially by a grant from the Stop Sida association. The funding source had no involvement in the study design, data collection, analysis, or interpretation; the writing of the report; or the decision to submit the report for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Study data are available on request.
Acknowledgments
The authors thank Serge Alfandari (Tourcoing), Anne Charpentier (Lille), Fabien Visade (Lille), and Pierre Weyrich (Lille) for their help in preparing the eligible list of explicit definitions of antibiotic-PIPs. The authors thank D. Satour for her help in preparing the content on the online platform used for the survey. The authors thank the contribution of the Delphi survey participants: Marion Andro (Brest), Cyprien Arlaud (Villeneuve-de-Berg), Julien Azzi (Nancy), Jeremy Barben (Dijon), Elisabeth Baux (Nancy), Anna Belkacem (Villeneuve-Saint-Georges), Nysrine Bennouna (Amiens), Guillaume Beraud (Poitiers), Julien Berthaud (Provins), Naïma Berthol (Fontainebleau), Mathieu Blot (Dijon), Caroline Bonhoure (Perpignan), Morgane Bonnet (Reims), Benjamin Bord (Perpignan), Emmanuelle Boschetti (Nancy), Kevin Bouiller (Besançon), Elsa Bourcier (Villeneuve-Saint-Georges), Emma Breugnon (Saint-Étienne), Thomas Brunet (Poitiers), Philippe Cabaret (Lille), Bertrand Cappeliez (Rouen), Karine Castro-Lionard (Saint-Étienne), Melanie Catroux (Poitiers), Leslie Cavee (Strasbourg), Thomas Celarier (Saint-Étienne), Adjodah Chandra (Rang-du-Fliers), Gilles Chapelle (Poitiers), Florence Chaumel (Villeneuve-Saint-Georges), Cedric Chol (Saint-Étienne), Marie Dafour (Brest), Benoit De Waziere (Nîmes), Astrid Depontfarcy (Melun), Amandine Devun (Saint-Étienne), Youcef Douadi (Saint-Quentin), Maria Dubos (Pessac), Florence Dupont (Amiens), Antoine Dupuis (Poitiers), Clementine Esteve (Dijon), Sandrine Estivin (Brest), Nicolas Eychenne (Villeneuve-Saint-Georges), Arthur Fourmy (Poitiers), Jean-Marc Galempoix (Charleville-Mézières), Olivier Gallon (Charleville-Mézières), Remy Gauzit (Paris), Paul Gerard (Nancy), Marine Gilis (Besançon), Carine Greib (Bordeaux), Marine Grosset (Angoulème), Yves Hansmann (Strasbourg), Caroline Hyernard (Bruges), Delphine Imperiale (Strasbourg), Danielle Jaafar (Villeneuve-Saint-Georges), Valérie Jacob Corazza (Alès), Cédric Joseph (Amiens), Claudie Lamoureux (Brest), Sarah Laurent-Badr (Reims), Hervé Le Bars (Brest), Georges Le Falher (Béziers), Vincent Le Moing (Montpellier), Nicolas Lefebvre (Strasbourg), Antoine Lefebvre (Lille), Benjamin Lefevre (Nancy), Philippe Lesprit (Grenoble), Thierry Levent (Maubeuge), Florence Lieutier-Colas (Strasbourg), Antoine Louvel (Annecy), Marie-France Lutz (Saint-Étienne), Camille Mabille (Amiens), Abd-El-Rachid Mahmoudi (Reims), Delphine Matelot (Alès), Hugues Melliez (Helfaut), Florence Meyer (Vandoeuvre-lès-Nancy), Marine Micolau (Chambéry), Chantal Miquel (Perpignan), Marine Moliere (Perpignan), Julien Moyet (Amiens), Maroua Mrouki (Perpignan), Jacques Naturel (Villeneuve-Saint-Georges), Sophie Nguyen (Béthune), Yasmine Nivoix (Strasbourg), Clément Ourghanlian (Créteil), Virginie Paul (Amiens), Marie Pichenot (Roubaix), Emilie Piet (Annecy), Delphine Poitrenaud (Ajaccio), Hélène Poujol (Nîmes), Florence Poupet (Saint-Estève), Genevieve Prevot (Chambéry), Mathieu Prinet (Poitiers), Mathilde Puges (Bordeaux), Sophie Putot (Dijon), Luc Quaesaet (Brest), Alexis Redor (Perpignan), Chrystelle Rey (Saint-Étienne), Schéhérazade Rezig (Brest), Juliette Romaru (Reims), Jean-Luc Schmit (Amiens), Elise Schmitt (Strasbourg), Benoit Siaud (Provins), Serge Sirvain (Alès), Didier Tande (Brest), Thomas Tannou (Besançon), Nabil Tayech (Roubaix), Gianpierro Tebano (Forlì), Alina Tone (Valenciennes), David Tran (Reims), Franck Trinchero (Chambéry), Axel Ursenbach (Strasbourg), Marie-Neige Videau (Bordeaux), Fabien Visade (Lille), and Pierre Weyrich (Lille). The following authors are members of GInGer (a joint study group for infections in the elderly, created by the French Infectious Diseases Society (Société de pathologie infectieuse de langue française, SPILF) and the French Gerontology and Geriatrics Society (Société Française de Gériatrie et Gérontologie, SFGG): Nicolas Baclet (Lille), Emmanuel Forestier (Chambéry), Gaëtan Gavazzi (Grenoble), Claire Roubaud-Baudron (Bordeaux), Rozenn Hequette-Ruz (Roubaix), Elisabeth Botelho-Nevers (Saint-Etienne), Pauline Caraux-Paz (Villeneuve St George), Sylvain Diamantis (Melun), Thibaut Fraisse (Alès), Maxime Hentzien (Reims), Jean-Philippe Lanoix (Amiens), Marc Paccalin (Poitiers), and Alain Putot (Dijon).
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years. Other relationships or activities, not related to the present study: CRB has received grants or contracts from ANSM—French drug administration grants not related to the present study (to her institution), she states leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, and to be member of the executive committee of a study group (ESCMID—ESGIE). RHR has received support for attending meetings and/or travel by PFIZER. TF has received honoraria from Expression sante for manuscript writing and editorial board of the review “Repères en Geriatrie”. JPL has received grants from PARATEK for Mycobacterium avium experiments (to his institution), from GIRCI–NO for clinical trial for appropriate use of antibiotic in pneumonia in elderly (to his institution); consulting fees from ViiV healthcare, MSD; honoraria for lectures, presentations, manuscript writing, or educational events from ViiV healthcare, MSD, and Sanofi; support for attending meetings and/or travel from MSD, ViiV, and Gilead.
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–2017; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151344-9. [Google Scholar]
- European Centre for Disease Prevention and Control; World Health Organization. Antimicrobial Resistance Surveillance in Europe: 2022: 2020 Data; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Willemsen, I.; Groenhuijzen, A.; Bogaers, D.; Stuurman, A.; van Keulen, P.; Kluytmans, J. Appropriateness of Antimicrobial Therapy Measured by Repeated Prevalence Surveys. Antimicrob. Agents Chemother. 2007, 51, 864–867. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Strategic Priorities on Antimicrobial Resistance: Preserving Antimicrobials for Today and Tomorrow; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-004138-7. [Google Scholar]
- European Centre for Disease Prevention and Control; European Medicines Agency. The Bacterial Challenge: Time to React: A Call to Narrow the Gap between Multidrug-Resistant Bacteria in the EU and the Development of New Antibacterial Agents; Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- Antimicrobial Stewardship Interventions: A Practical Guide; WHO Regional Office for Europe: Copenhagen, Denmark, 2021.
- American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Laroche, M.-L.; Charmes, J.-P.; Merle, L. Potentially inappropriate medications in the elderly: A French consensus panel list. Eur. J. Clin. Pharmacol. 2007, 63, 725–731. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Guaraldo, L.; Cano, F.G.; Damasceno, G.S.; Rozenfeld, S. Inappropriate medication use among the elderly: A systematic review of administrative databases. BMC Geriatr. 2011, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Beuscart, J.-B.; Genin, M.; Dupont, C.; Verloop, D.; Duhamel, A.; Defebvre, M.-M.; Puisieux, F. Potentially inappropriate medication prescribing is associated with socioeconomic factors: A spatial analysis in the French Nord-Pas-de-Calais Region. Age Ageing 2017, 46, 607–613. [Google Scholar] [CrossRef]
- Mor, A.; Frøslev, T.; Thomsen, R.W.; Oteri, A.; Rijnbeek, P.; Schink, T.; Garbe, E.; Pecchioli, S.; Innocenti, F.; Bezemer, I.; et al. Antibiotic use varies substantially among adults: A cross-national study from five European Countries in the ARITMO project. Infection 2015, 43, 453–472. [Google Scholar] [CrossRef]
- Scott, M.M.; Liang, S.Y. Infections in Older Adults. Emerg. Med. Clin. N. Am. 2021, 39, 379–394. [Google Scholar] [CrossRef]
- Cavalié, P.; Hider-Mlynarz, K. L’évolution des Consommations D’antibiotiques en France Entre 2000 et 2015; ANSM: Saint-Denis, France, 2017. [Google Scholar]
- Gavazzi, G.; Krause, K.-H. Ageing and infection. Lancet Infect. Dis. 2002, 2, 659–666. [Google Scholar] [CrossRef]
- Baclet, N.; Ficheur, G.; Alfandari, S.; Ferret, L.; Senneville, E.; Chazard, E.; Beuscart, J.-B. Explicit definitions of potentially inappropriate prescriptions of antibiotics in older patients: A compilation derived from a systematic review. Int. J. Antimicrob. Agents 2017, 50, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Motter, F.R.; Fritzen, J.S.; Hilmer, S.N.; Paniz, É.V.; Paniz, V.M.V. Potentially inappropriate medication in the elderly: A systematic review of validated explicit criteria. Eur. J. Clin. Pharmacol. 2018, 74, 679–700. [Google Scholar] [CrossRef] [PubMed]
- Dalkey, N.C. The Delphi Method. Available online: https://www.rand.org/pubs/research_memoranda/RM5888.html (accessed on 15 May 2019).
- Diamond, I.R.; Grant, R.C.; Feldman, B.M.; Pencharz, P.B.; Ling, S.C.; Moore, A.M.; Wales, P.W. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J. Clin. Epidemiol. 2014, 67, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Standardised method for reporting exercise programmes: Protocol for a modified Delphi study. BMJ Open 2014, 4, e006682. [Google Scholar] [CrossRef] [PubMed]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and Reporting the Delphi Method for Selecting Healthcare Quality Indicators: A Systematic Review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.P.; Smyth, R.L.; Williamson, P.R. Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies. PLoS Med. 2011, 8, e1000393. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.H. Quality—Can We Measure It? N. Engl. J. Med. 1977, 296, 170–172. [Google Scholar] [CrossRef]
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007, 370, 173–184. [Google Scholar] [CrossRef]
- Baclet, N.; Calafiore, M.; Fregnac, C.; Gavazzi, G.; Forestier, E.; Roubaud-Baudron, C.; Fraisse, T.; Alfandari, S.; Senneville, E.; Beuscart, J.-B. Explicit definitions of potentially inappropriate prescriptions of antibiotics in hospitalized older patients. Infect. Dis. Now 2022, 52, 214–222. [Google Scholar] [CrossRef]
- Géodes—Santé Publique France—Indicateurs: Cartes, Données et Graphiques. Available online: https://geodes.santepubliquefrance.fr/#c=home (accessed on 12 May 2022).
- Likert, R. A Technique for the Measurement of Attitudes; Archives of Psychology: New York, NY, USA, 1932; Volume 22. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 12 March 2024).
- Earl, T.R.; Katapodis, N.D.; Schneiderman, S.R.; Shoemaker-Hunt, S.J. Using Deprescribing Practices and the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions Criteria to Reduce Harm and Preventable Adverse Drug Events in Older Adults. J. Patient Saf. 2020, 16, S23–S35. [Google Scholar] [CrossRef]
- Dalton, K.; O’Brien, G.; O’Mahony, D.; Byrne, S. Computerised interventions designed to reduce potentially inappropriate prescribing in hospitalised older adults: A systematic review and meta-analysis. Age Ageing 2018, 47, 670–678. [Google Scholar] [CrossRef]
- Hill-Taylor, B.; Walsh, K.A.; Stewart, S.; Hayden, J.; Byrne, S.; Sketris, I.S. Effectiveness of the STOPP/START (Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: Systematic review and meta-analysis of randomized controlled studies. J. Clin. Pharm. Ther. 2016, 41, 158–169. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151552-8. [Google Scholar]
- Gauzit, R.; Castan, B.; Bonnet, E.; Bru, J.P.; Cohen, R.; Diamantis, S.; Faye, A.; Hitoto, H.; Issa, N.; Lebeaux, D.; et al. Anti-infectious treatment duration: The SPILF and GPIP French guidelines and recommendations. Infect. Dis. Now 2021, 51, 114–139. [Google Scholar] [CrossRef] [PubMed]
- Wintenberger, C.; Guery, B.; Bonnet, E.; Castan, B.; Cohen, R.; Diamantis, S.; Lesprit, P.; Maulin, L.; Péan, Y.; Peju, E.; et al. Proposal for shorter antibiotic therapies. Médecine Mal. Infect. 2017, 47, 92–141. [Google Scholar] [CrossRef] [PubMed]
- Hanretty, A.M.; Gallagher, J.C. Shortened Courses of Antibiotics for Bacterial Infections: A Systematic Review of Randomized Controlled Trials. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, M.; Morris, A.M.; Thursky, K.; Pulcini, C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 2020, 26, 447–453. [Google Scholar] [CrossRef] [PubMed]
- CDC. The Core Elements of Hospital Antibiotic Stewardship Programs; US Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Scott, I.A.; Pillans, P.I.; Barras, M.; Morris, C. Using EMR-enabled computerized decision support systems to reduce prescribing of potentially inappropriate medications: A narrative review. Ther. Adv. Drug Saf. 2018, 9, 559–573. [Google Scholar] [CrossRef]
- Cuvelier, E.; Robert, L.; Musy, E.; Rousselière, C.; Marcilly, R.; Gautier, S.; Odou, P.; Beuscart, J.-B.; Décaudin, B. The clinical pharmacist’s role in enhancing the relevance of a clinical decision support system. Int. J. Med. Inf. 2021, 155, 104568. [Google Scholar] [CrossRef]
- Robert, L.; Quindroit, P.; Henry, H.; Perez, M.; Rousselière, C.; Lemaitre, M.; Vambergue, A.; Décaudin, B.; Beuscart, J.-B. Implementation of a clinical decision support system for the optimization of antidiabetic drug orders by pharmacists. Br. J. Clin. Pharmacol. 2024, 90, 239–246. [Google Scholar] [CrossRef]
- Robert, L.; Cuvelier, E.; Rousselière, C.; Gautier, S.; Odou, P.; Beuscart, J.-B.; Décaudin, B. Detection of Drug-Related Problems through a Clinical Decision Support System Used by a Clinical Pharmacy Team. Healthcare 2023, 11, 827. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).