One Hundred Explicit Definitions of Potentially Inappropriate Prescriptions of Antibiotics in Hospitalized Older Patients: The Results of an Expert Consensus Study
Abstract
1. Introduction
2. Results
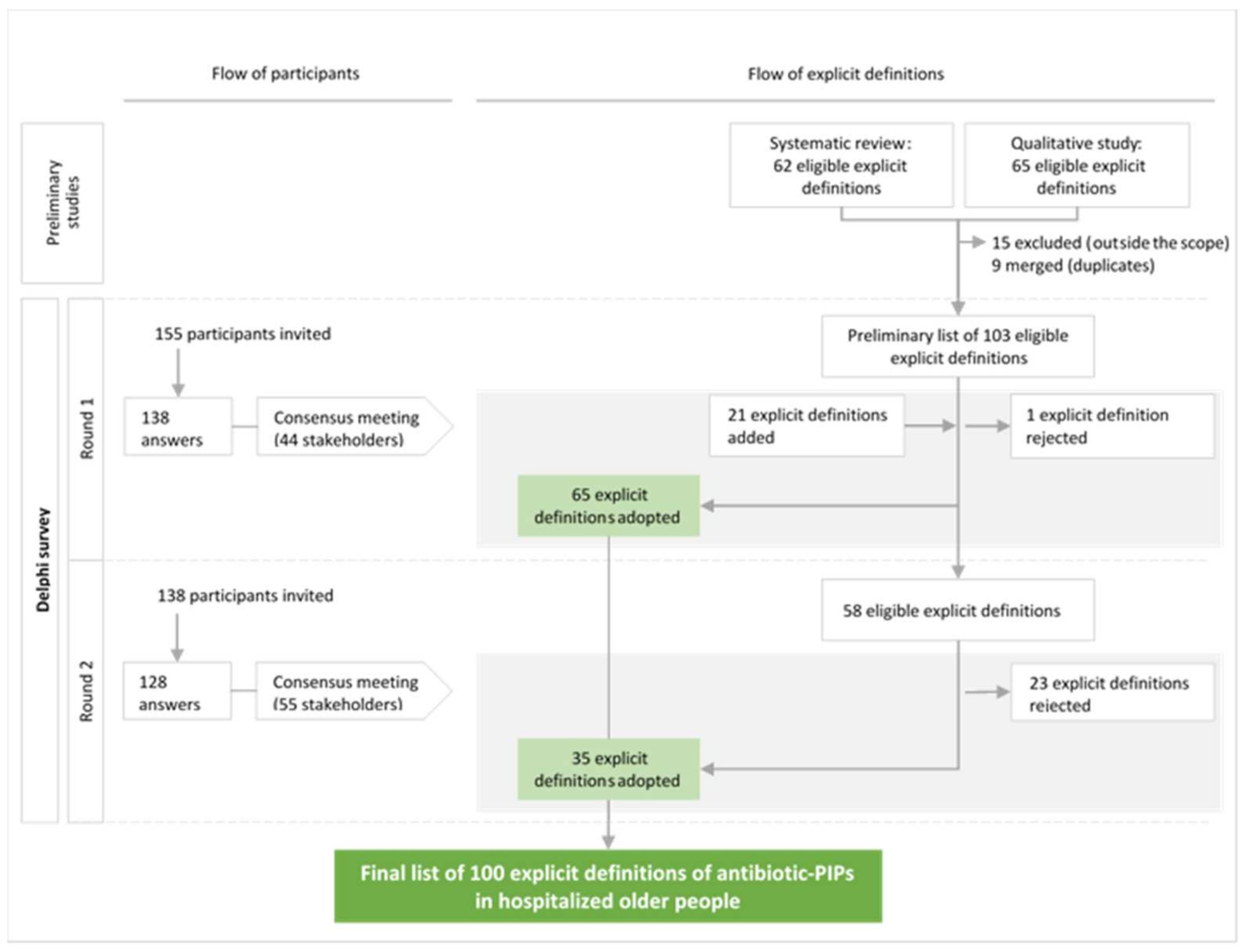
2.1. Participants
2.2. The Delphi Survey
2.3. The Consensus List of Explicit Definitions of Antibiotic-PIPs
3. Material and Methods
3.1. Study Design
3.2. Scope of the Study
3.3. Steering Committee
3.4. Ethical Approval
3.5. Preparation of the List of Eligible Explicit Definitions of Antibiotic-PIPs
3.6. The Panel of Experts for the Delphi Study
3.6.1. Inclusion Criteria for Participants
3.6.2. Number of Participants
3.6.3. Recruitment of Participants
3.7. Preparation of the Delphi Survey
3.7.1. The Online Platform
3.7.2. Briefing of the Participants
3.8. Definition of the Consensus Criteria
3.9. The Delphi Process
3.9.1. The Rounds
3.9.2. Analysis of Rounds and Preparation of the Consensus Meetings
3.9.3. Consensus Meetings
4. Discussion
4.1. A New Approach to Antimicrobial Stewardship
4.2. Explicit Definitions of Relevance to the Fight against Antibiotic Resistance
4.3. Perspectives for the Use of Explicit Definitions of Antibiotic-PIPs
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2016–2017; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151344-9. [Google Scholar]
- European Centre for Disease Prevention and Control; World Health Organization. Antimicrobial Resistance Surveillance in Europe: 2022: 2020 Data; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Willemsen, I.; Groenhuijzen, A.; Bogaers, D.; Stuurman, A.; van Keulen, P.; Kluytmans, J. Appropriateness of Antimicrobial Therapy Measured by Repeated Prevalence Surveys. Antimicrob. Agents Chemother. 2007, 51, 864–867. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Strategic Priorities on Antimicrobial Resistance: Preserving Antimicrobials for Today and Tomorrow; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-004138-7. [Google Scholar]
- European Centre for Disease Prevention and Control; European Medicines Agency. The Bacterial Challenge: Time to React: A Call to Narrow the Gap between Multidrug-Resistant Bacteria in the EU and the Development of New Antibacterial Agents; Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- Antimicrobial Stewardship Interventions: A Practical Guide; WHO Regional Office for Europe: Copenhagen, Denmark, 2021.
- American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694. [Google Scholar] [CrossRef]
- Laroche, M.-L.; Charmes, J.-P.; Merle, L. Potentially inappropriate medications in the elderly: A French consensus panel list. Eur. J. Clin. Pharmacol. 2007, 63, 725–731. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, D.; O’Sullivan, D.; Byrne, S.; O’Connor, M.N.; Ryan, C.; Gallagher, P. STOPP/START criteria for potentially inappropriate prescribing in older people: Version 2. Age Ageing 2015, 44, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Guaraldo, L.; Cano, F.G.; Damasceno, G.S.; Rozenfeld, S. Inappropriate medication use among the elderly: A systematic review of administrative databases. BMC Geriatr. 2011, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Beuscart, J.-B.; Genin, M.; Dupont, C.; Verloop, D.; Duhamel, A.; Defebvre, M.-M.; Puisieux, F. Potentially inappropriate medication prescribing is associated with socioeconomic factors: A spatial analysis in the French Nord-Pas-de-Calais Region. Age Ageing 2017, 46, 607–613. [Google Scholar] [CrossRef]
- Mor, A.; Frøslev, T.; Thomsen, R.W.; Oteri, A.; Rijnbeek, P.; Schink, T.; Garbe, E.; Pecchioli, S.; Innocenti, F.; Bezemer, I.; et al. Antibiotic use varies substantially among adults: A cross-national study from five European Countries in the ARITMO project. Infection 2015, 43, 453–472. [Google Scholar] [CrossRef]
- Scott, M.M.; Liang, S.Y. Infections in Older Adults. Emerg. Med. Clin. N. Am. 2021, 39, 379–394. [Google Scholar] [CrossRef]
- Cavalié, P.; Hider-Mlynarz, K. L’évolution des Consommations D’antibiotiques en France Entre 2000 et 2015; ANSM: Saint-Denis, France, 2017. [Google Scholar]
- Gavazzi, G.; Krause, K.-H. Ageing and infection. Lancet Infect. Dis. 2002, 2, 659–666. [Google Scholar] [CrossRef]
- Baclet, N.; Ficheur, G.; Alfandari, S.; Ferret, L.; Senneville, E.; Chazard, E.; Beuscart, J.-B. Explicit definitions of potentially inappropriate prescriptions of antibiotics in older patients: A compilation derived from a systematic review. Int. J. Antimicrob. Agents 2017, 50, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Motter, F.R.; Fritzen, J.S.; Hilmer, S.N.; Paniz, É.V.; Paniz, V.M.V. Potentially inappropriate medication in the elderly: A systematic review of validated explicit criteria. Eur. J. Clin. Pharmacol. 2018, 74, 679–700. [Google Scholar] [CrossRef] [PubMed]
- Dalkey, N.C. The Delphi Method. Available online: https://www.rand.org/pubs/research_memoranda/RM5888.html (accessed on 15 May 2019).
- Diamond, I.R.; Grant, R.C.; Feldman, B.M.; Pencharz, P.B.; Ling, S.C.; Moore, A.M.; Wales, P.W. Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J. Clin. Epidemiol. 2014, 67, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Standardised method for reporting exercise programmes: Protocol for a modified Delphi study. BMJ Open 2014, 4, e006682. [Google Scholar] [CrossRef] [PubMed]
- Boulkedid, R.; Abdoul, H.; Loustau, M.; Sibony, O.; Alberti, C. Using and Reporting the Delphi Method for Selecting Healthcare Quality Indicators: A Systematic Review. PLoS ONE 2011, 6, e20476. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.P.; Smyth, R.L.; Williamson, P.R. Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies. PLoS Med. 2011, 8, e1000393. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.H. Quality—Can We Measure It? N. Engl. J. Med. 1977, 296, 170–172. [Google Scholar] [CrossRef]
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007, 370, 173–184. [Google Scholar] [CrossRef]
- Baclet, N.; Calafiore, M.; Fregnac, C.; Gavazzi, G.; Forestier, E.; Roubaud-Baudron, C.; Fraisse, T.; Alfandari, S.; Senneville, E.; Beuscart, J.-B. Explicit definitions of potentially inappropriate prescriptions of antibiotics in hospitalized older patients. Infect. Dis. Now 2022, 52, 214–222. [Google Scholar] [CrossRef]
- Géodes—Santé Publique France—Indicateurs: Cartes, Données et Graphiques. Available online: https://geodes.santepubliquefrance.fr/#c=home (accessed on 12 May 2022).
- Likert, R. A Technique for the Measurement of Attitudes; Archives of Psychology: New York, NY, USA, 1932; Volume 22. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 12 March 2024).
- Earl, T.R.; Katapodis, N.D.; Schneiderman, S.R.; Shoemaker-Hunt, S.J. Using Deprescribing Practices and the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions Criteria to Reduce Harm and Preventable Adverse Drug Events in Older Adults. J. Patient Saf. 2020, 16, S23–S35. [Google Scholar] [CrossRef]
- Dalton, K.; O’Brien, G.; O’Mahony, D.; Byrne, S. Computerised interventions designed to reduce potentially inappropriate prescribing in hospitalised older adults: A systematic review and meta-analysis. Age Ageing 2018, 47, 670–678. [Google Scholar] [CrossRef]
- Hill-Taylor, B.; Walsh, K.A.; Stewart, S.; Hayden, J.; Byrne, S.; Sketris, I.S. Effectiveness of the STOPP/START (Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: Systematic review and meta-analysis of randomized controlled studies. J. Clin. Pharm. Ther. 2016, 41, 158–169. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151552-8. [Google Scholar]
- Gauzit, R.; Castan, B.; Bonnet, E.; Bru, J.P.; Cohen, R.; Diamantis, S.; Faye, A.; Hitoto, H.; Issa, N.; Lebeaux, D.; et al. Anti-infectious treatment duration: The SPILF and GPIP French guidelines and recommendations. Infect. Dis. Now 2021, 51, 114–139. [Google Scholar] [CrossRef] [PubMed]
- Wintenberger, C.; Guery, B.; Bonnet, E.; Castan, B.; Cohen, R.; Diamantis, S.; Lesprit, P.; Maulin, L.; Péan, Y.; Peju, E.; et al. Proposal for shorter antibiotic therapies. Médecine Mal. Infect. 2017, 47, 92–141. [Google Scholar] [CrossRef] [PubMed]
- Hanretty, A.M.; Gallagher, J.C. Shortened Courses of Antibiotics for Bacterial Infections: A Systematic Review of Randomized Controlled Trials. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, M.; Morris, A.M.; Thursky, K.; Pulcini, C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 2020, 26, 447–453. [Google Scholar] [CrossRef] [PubMed]
- CDC. The Core Elements of Hospital Antibiotic Stewardship Programs; US Department of Health and Human Services: Atlanta, GA, USA, 2019.
- Scott, I.A.; Pillans, P.I.; Barras, M.; Morris, C. Using EMR-enabled computerized decision support systems to reduce prescribing of potentially inappropriate medications: A narrative review. Ther. Adv. Drug Saf. 2018, 9, 559–573. [Google Scholar] [CrossRef]
- Cuvelier, E.; Robert, L.; Musy, E.; Rousselière, C.; Marcilly, R.; Gautier, S.; Odou, P.; Beuscart, J.-B.; Décaudin, B. The clinical pharmacist’s role in enhancing the relevance of a clinical decision support system. Int. J. Med. Inf. 2021, 155, 104568. [Google Scholar] [CrossRef]
- Robert, L.; Quindroit, P.; Henry, H.; Perez, M.; Rousselière, C.; Lemaitre, M.; Vambergue, A.; Décaudin, B.; Beuscart, J.-B. Implementation of a clinical decision support system for the optimization of antidiabetic drug orders by pharmacists. Br. J. Clin. Pharmacol. 2024, 90, 239–246. [Google Scholar] [CrossRef]
- Robert, L.; Cuvelier, E.; Rousselière, C.; Gautier, S.; Odou, P.; Beuscart, J.-B.; Décaudin, B. Detection of Drug-Related Problems through a Clinical Decision Support System Used by a Clinical Pharmacy Team. Healthcare 2023, 11, 827. [Google Scholar] [CrossRef]
| Class/Domain | Number of Definitions (n = 100) |
|---|---|
| Infection site | 52 |
| Upper respiratory tract | 12 |
| Urinary tract | 11 |
| Lower respiratory tract | 10 |
| Skin and soft tissues | 7 |
| Gastrointestinal tract | 5 |
| Bones/joints | 4 |
| Dental care | 2 |
| Bloodstream | 1 |
| Use | 18 |
| Administration route | 5 |
| Dose level | 4 |
| Antibiotic combination | 4 |
| Treatment time | 4 |
| Laboratory assays | 1 |
| General principles of antibiotic use | 16 |
| All infection sites | 7 |
| Undocumented infections | 5 |
| Community-acquired infections | 4 |
| Organisms | 14 |
| Clostridioides difficile | 4 |
| Viruses | 3 |
| Treponema pallidum | 2 |
| Neisseria gonorrhoeae | 2 |
| Pseudomonas aeruginosa | 1 |
| Helicobacter pylori | 1 |
| Salmonella spp. | 1 |
| Area | Domain | Sub-Domain | Explicit Definition: “It Is Potentially Inappropriate To …” |
|---|---|---|---|
| Site of infection | Urinary tract | General | 1. Prescribe nitrofurantoin for a urinary tract infection (apart from cystitis) |
| 2. Prescribe norfloxacin in a urinary tract infection (apart from cystitis) | |||
| 3. Prescribe amoxicillin–clavulanic acid for the empirical therapy of a urinary tract infection | |||
| Urinary tract colonization | 4. Prescribe antibiotics for urinary tract colonization (in the absence of urinary tract surgery, and regardless of the pathogen identified [ESBL, etc.]) | ||
| Cystitis | 5. Prescribe aminoglycosides in a case of cystitis | ||
| 6. Prescribe the following antibiotics for the empirical therapy of cystitis: amoxicillin, amoxicillin–clavulanic acid, azithromycin, cefadroxil, cefuroxime, and doxycycline | |||
| 7. Prescribe a 3GC in a case of cystitis | |||
| 8. Prescribe a 4GC in a case of cystitis | |||
| 9. Prescribe fluoroquinolones for the first-line treatment of cystitis | |||
| Urinary tract infections in men | 10. Prescribe amoxicillin for urinary tract infections in men (apart from Enterococci) | ||
| 11. Prescribe amoxicillin–clavulanic acid for urinary tract infections in men | |||
| Lower respiratory tract | Bronchitis | 12. Prescribe antibiotics for acute bronchitis | |
| AECOPD | 13. Prescribe antibiotics in the prophylaxis of AECOPD | ||
| Pneumonia | 14. Prescribe antibiotics in a case of viral pneumonia or pleurisy | ||
| 15. Prescribe amoxicillin–clavulanic acid for documented acute community-acquired pneumococcal pneumonia | |||
| 16. Prescribe ceftriaxone for documented acute community-acquired pneumococcal pneumonia | |||
| 17. Prescribe an injectable 3GC for community-acquired pneumonia without comorbidities | |||
| 18. Prescribe a 3GC–fluoroquinolone combination for the empirical therapy of pneumonia | |||
| 19. Prescribe fluoroquinolones for the first-line treatment of pneumonia | |||
| 20. Prescribe a macrolide for community-acquired pneumonia (apart from legionellosis) | |||
| 21. Prescribe antibiotics for an infiltrate on a chest X-ray in the absence of clinically significant symptoms of pneumonia | |||
| Upper respiratory tract | Non-specific URTI | 22. Prescribe antibiotics for nasopharyngitis (a common cold), acute laryngitis, and tracheitis | |
| 23. Prescribe doxycycline in acute pharyngitis | |||
| 24. Prescribe a 3GC for an URTI | |||
| 25. Prescribe a fluoroquinolone for the first-line treatment of an URTI | |||
| Sinusitis | 26. Prescribe antibiotics in a case of acute sinusitis, with symptoms for less than 5 days and/or no fever | ||
| 27. Prescribe doxycycline in acute sinusitis | |||
| Tonsillitis | 28. Prescribe other treatments than amoxicillin and/or penicillin V for acute pharyngotonsillitis | ||
| 29. Prescribe doxycycline for acute tonsillitis | |||
| 30. Prescribe antibiotics for viral tonsillitis | |||
| Otitis | 31. Prescribe erythromycin as an empirical therapy in acute otitis media | ||
| 32. Prescribe trimethoprim/sulfamethoxazole as an empirical therapy in acute otitis media | |||
| 33. Prescribe antibiotics for uncomplicated acute otitis externa unless there is extension beyond the ear canal or the presence of specific host factors that indicate a need for systemic treatment | |||
| Skin and soft tissues | 34. Prescribe any molecule other than amoxicillin for non-necrotizing cellulitis of the lower limb (uncomplicated erysipelas) | ||
| 35. Prescribe fluoroquinolones for skin and soft tissue infections | |||
| 36. Prescribe an anti-MRSA antibiotic for community-acquired non-necrotizing cellulitis | |||
| 37. Prescribe an antibiotic for the treatment of a wound in the absence of cellulitis (apart from a bite) | |||
| 38. Prescribe topical antibiotics (apart from Staphylococcus aureus decontamination) | |||
| 39. Prescribe antibiotics for a decubitus ulcer in an individual at the end of life | |||
| 40. Prescribe ceftriaxone for the empirical therapy of skin and soft tissue infections in immunocompetent hosts | |||
| Gastrointestinal tract | 41. Prescribe antibiotics for the empirical therapy of diarrhea | ||
| 42. Prescribe a fluoroquinolone for the empirical therapy of a gastro-intestinal infection | |||
| 43. Prescribe amoxicillin–clavulanic acid for nosocomial gastrointestinal infections | |||
| 44. Prescribe antibiotics for the empirical therapy of acute vomiting or diarrhea in the absence of a positive stool culture or a positive toxin assay for Clostridioides difficile | |||
| 45. Prescribe antibiotics that do not cover the following bacteria for secondary intra-abdominal infections: aerobic, anaerobic, or beta-lactamase-producing Gram-negative bacilli | |||
| Bones/joints | 46. Prescribe ceftriaxone for the empirical therapy of a bone or joint infection in immunocompetent hosts | ||
| 47. Prescribe fluoroquinolones for the empirical therapy of a bone or joint infection | |||
| 48. Prescribe rifampicin for the empirical therapy of a bone or joint infection | |||
| 49. Prescribe antibiotics for the empirical treatment of a bone or joint infection before reliable microbiological samples have been collected | |||
| Bloodstream | 50. Initiate antibiotic therapy more than 24 h after a positive blood culture (unless the sample is contaminated) | ||
| Dental care | 51. Prescribe antibiotics for the first-line therapy of pulpitis | ||
| 52. Prescribe antibiotics for acute dental pain unless patient has facial swelling, adenopathy, difficulty opening the mouth, fever, difficulty swallowing or ulcerative gingivitis | |||
| General principles of antibiotic use | All sites of infection | 53. Prescribe nitrofurantoin in men | |
| 54. Prescribe ertapenem as a first-line treatment | |||
| 55. Prescribe aminoglycosides when the severity criteria are not met | |||
| 56. Prescribe fluoroquinolones as a first-line treatment (apart from urinary tract infections in men or acute pyelonephritis) | |||
| 57. Prescribe antibiotics for an isolated elevation of CRP | |||
| 58. Prescribe oral 3GCs (except for in a documented case of acute pyelonephritis in a woman) | |||
| 59. Prescribe fluoroquinolones for empirical treatment in patients treated with fluoroquinolones in the previous 6 months | |||
| Undocumented infections | 60. Prescribe carbapenems as an empirical therapy | ||
| 61. Prescribe ertapenem as an empirical therapy | |||
| 62. Prescribe fluoroquinolones as an empirical therapy | |||
| 63. Prescribe rifampicin as an empirical therapy | |||
| 64. Prescribe cotrimoxazole as an empirical therapy (except when pneumocystosis is suspected) | |||
| Community-acquired infections | 65. Prescribe antibiotics that are effective against methicillin-resistant Staphylococci (vancomycin, teicoplanin, daptomycin, linezolide, and dalbavancin) as an empirical therapy for community-acquired infections | ||
| 66. Prescribe carbapenems for a community-acquired infection | |||
| 67. Prescribe piperacillin–tazobactam for a community-acquired infection | |||
| 68. Prescribe a 4GC for a community-acquired infection | |||
| Use | Dosing | 69. Reduce the dose level of aminoglycosides in the event of kidney failure | |
| 70. Underdose gentamicin (at least 10% below the recommended dose) | |||
| 71. Fail to re-evaluate the dose level as a function of changes in renal function changes | |||
| 72. Prescribe a continuous infusion of vancomycin in the absence of a loading dose | |||
| Duration of treatment | 73. Prescribe antibiotics for more than 5 days for AECOPD | ||
| 74. Prescribe antibiotics for more than 7 days for pneumonia | |||
| 75. Prescribe antibiotics for more than 7 days for non-necrotizing cellulitis | |||
| 76. Prescribe aminoglycosides for more than 3 days | |||
| Combination of antibiotics | 77. Combine amoxicillin–clavulanic acid with fluoroquinolones | ||
| 78. Combine amoxicillin–clavulanic acid with metronidazole | |||
| 79. Combine two aminoglycosides | |||
| 80. Prescribe systemic rifampicin alone (i.e., as a monotherapy) | |||
| Laboratory assays | 81. Prescribe a glycopeptide without assaying plasma concentrations (apart from orally administered glycopeptides) | ||
| Administration route | 82. Prescribe oral penicillin M | ||
| 83. Prescribe subcutaneous ceftriaxone if intravenous administration is possible | |||
84. Prescribe intravenous (i.v.) antibiotics when the patient meets the criteria for use of the per os (p.o.) formulation according to the i.v.–p.o. antibiotic switch protocol:
| |||
85. Intravenous (i.v.) fluoroquinolones after 48 h when the patient meets the criteria for use of the per os (p.o.) formulation:
| |||
| 86. Prescribe a subcutaneously administered aminoglycoside | |||
| Organisms | Viruses | 87. Prescribe antibiotics for a SARS-CoV-2 infection | |
| 88. Prescribe antibiotics for probable viral infections | |||
| 89. Prescribe antibiotics for influenza | |||
| Clostridioides difficile | 90. Prescribe metronidazole for a CDI | ||
| 91. Prescribe intravenous vancomycin for the treatment of a CDI | |||
| 92. Prescribe metronidazole rather than vancomycin for a severe CDI | |||
| 93. Prescribe antibiotics for empirical therapy for a mild-to-moderate CDI (i.e., not meeting the criteria for severe CDI), unless the recurrence of a recent CDI is suspected | |||
| Pseudomonas aeruginosa | 94. Prescribe a fluoroquinolone alone for the first-line treatment of Pseudomonas aeruginosa infections | ||
| Helicobacter pylori | 95. Prescribe an amoxicillin–clavulanic acid/tetracycline combination for the eradication of Helicobacter pylori | ||
| Salmonella | 96. Prescribe fluoroquinolones for the first-line treatment of salmonellosis | ||
| Neisseria gonorrhoeae | 97. Prescribe ciprofloxacin for uncomplicated gonococcal urethritis in men | ||
| 98. Prescribe amoxicillin for uncomplicated gonococcal urethritis in men | |||
| Treponema pallidum | 99. Prescribe ciprofloxacin for late syphilis | ||
| 100. Prescribe azithromycin for late syphilis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baclet, N.; Forestier, E.; Gavazzi, G.; Roubaud-Baudron, C.; Hiernard, V.; Hequette-Ruz, R.; Alfandari, S.; Aumaître, H.; Botelho-Nevers, E.; Caraux-Paz, P.; et al. One Hundred Explicit Definitions of Potentially Inappropriate Prescriptions of Antibiotics in Hospitalized Older Patients: The Results of an Expert Consensus Study. Antibiotics 2024, 13, 283. https://doi.org/10.3390/antibiotics13030283
Baclet N, Forestier E, Gavazzi G, Roubaud-Baudron C, Hiernard V, Hequette-Ruz R, Alfandari S, Aumaître H, Botelho-Nevers E, Caraux-Paz P, et al. One Hundred Explicit Definitions of Potentially Inappropriate Prescriptions of Antibiotics in Hospitalized Older Patients: The Results of an Expert Consensus Study. Antibiotics. 2024; 13(3):283. https://doi.org/10.3390/antibiotics13030283
Chicago/Turabian StyleBaclet, Nicolas, Emmanuel Forestier, Gaëtan Gavazzi, Claire Roubaud-Baudron, Vincent Hiernard, Rozenn Hequette-Ruz, Serge Alfandari, Hugues Aumaître, Elisabeth Botelho-Nevers, Pauline Caraux-Paz, and et al. 2024. "One Hundred Explicit Definitions of Potentially Inappropriate Prescriptions of Antibiotics in Hospitalized Older Patients: The Results of an Expert Consensus Study" Antibiotics 13, no. 3: 283. https://doi.org/10.3390/antibiotics13030283
APA StyleBaclet, N., Forestier, E., Gavazzi, G., Roubaud-Baudron, C., Hiernard, V., Hequette-Ruz, R., Alfandari, S., Aumaître, H., Botelho-Nevers, E., Caraux-Paz, P., Charmillon, A., Diamantis, S., Fraisse, T., Gazeau, P., Hentzien, M., Lanoix, J.-P., Paccalin, M., Putot, A., Ruch, Y., ... Beuscart, J.-B., on behalf of the GInGer (SPILF–SFGG Study Group). (2024). One Hundred Explicit Definitions of Potentially Inappropriate Prescriptions of Antibiotics in Hospitalized Older Patients: The Results of an Expert Consensus Study. Antibiotics, 13(3), 283. https://doi.org/10.3390/antibiotics13030283







