Antimicrobial Treatment Challenges in the Management of Infective Spondylodiscitis Associated with Hemodialysis: A Comprehensive Review of Literature and Case Series Analysis
Abstract
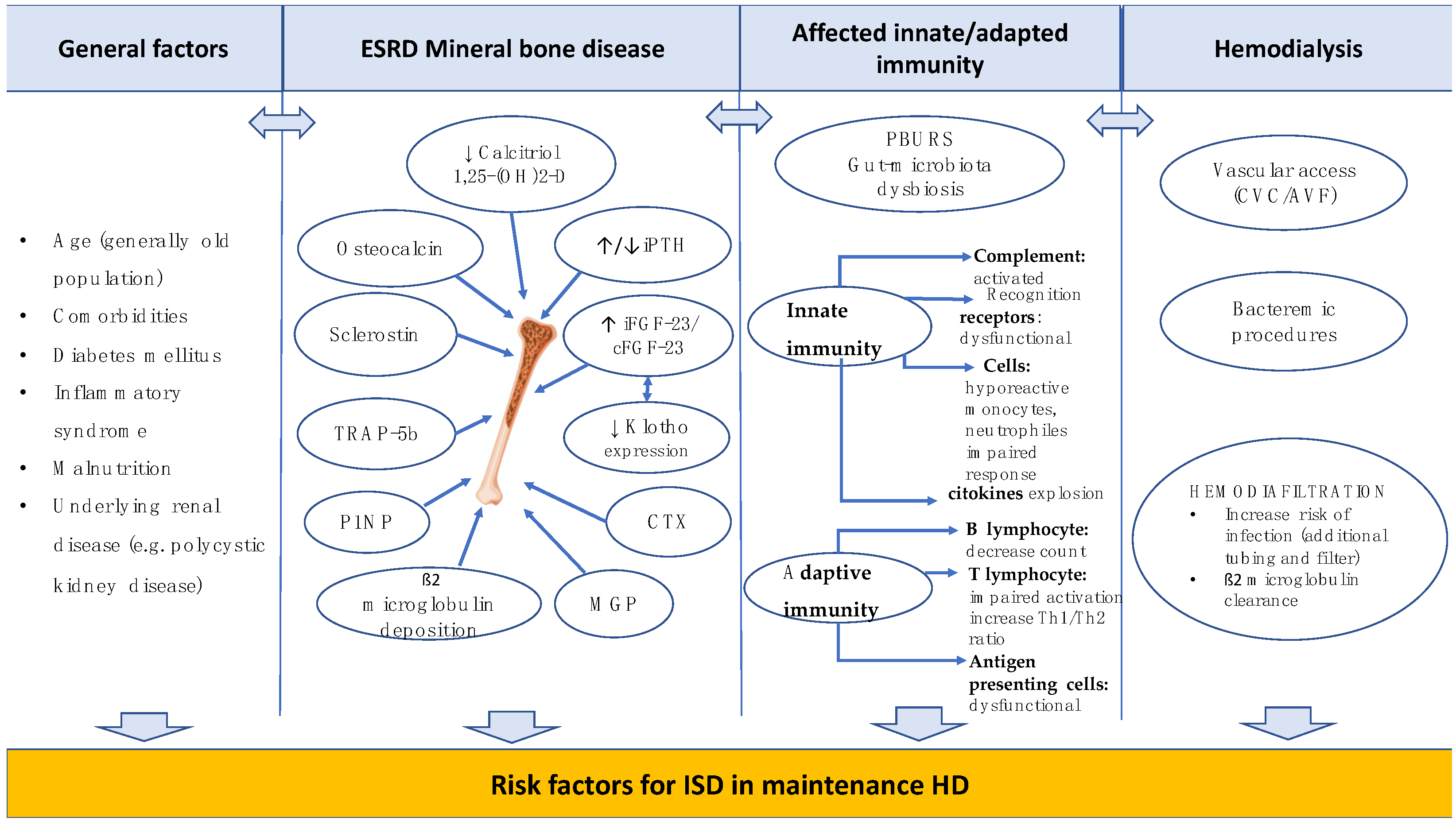
1. Introduction
2. Part I—Clinical Research
2.1. Materials and Method
2.2. Diagnosis Criteria
2.3. Hemodialysis Treatment Characteristics
2.4. Vascular Access Management in Hemodialysis Patients
2.5. Collected Data for the Two Groups of Patients
2.6. Statistical Analysis
3. Results
4. Part II—Review of the Literature
4.1. Materials and Methods
4.2. Research Results
5. Discussions
5.1. General/Demographic Considerations
5.2. Associated Diseases
5.3. Microbiological Findings. Vascular Access
5.4. Clinical and Biological ISD Features
5.5. Therapeutic Strategies
5.6. The Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Waheed, G.; Soliman, M.A.R.; Ali, A.M.; Aly, M.H. Spontaneous spondylodiscitis: Review, incidence, management, and clinical outcome in 44 patients. Neurosurg. Focus 2019, 46, E10. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Betjes, M.G.H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Koppe, L.; Fouque, D.; Thaunat, O. Chronic Kidney Disease-Associated Immune Dysfunctions: Impact of Protein-Bound Uremic Retention Solutes on Immune Cells. Toxins 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Heidari, B. C-reactive protein and other markers of inflammation in hemodialysis patients. Casp. J. Intern. Med. 2013, 4, 611–616. [Google Scholar]
- Docci, D.; Bilancioni, R.; Buscaroli, A.; Baldrati, L.; Capponcini, C.; Mengozzi, S.; Turci, F.; Feletti, C. Elevated Serum Levels of C-Reactive Protein in Hemodialysis Patients. Nephron 1990, 56, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.F. C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int. 1998, 54, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Yeun, J.Y.; Levine, R.A.; Mantadilok, V.; Kaysen, G.A. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. 2000, 35, 469–476. [Google Scholar] [CrossRef]
- Fusaro, M.; Barbuto, S.; Gallieni, M.; Cossettini, A.; Sartò, G.V.R.; Cosmai, L.; Cianciolo, G.; La Manna, G.; Nickolas, T.; Ferrari, S.; et al. Real-world usage of Chronic Kidney Disease—Mineral Bone Disorder (CKD–MBD) biomarkers in nephrology practices. Clin. Kidney J. 2024, 17, sfad290. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Kaliya-Perumal, A.-K.; Jenq, C.-C.; Niu, C.-C.; Ho, N.Y.-J.; Lee, T.-Y.; Lai, P.-L. The unresolved problem of beta-2 microglobulin amyloid deposits in the intervertebral discs of long-term dialysis patients. J. Orthop. Surg. Res. 2017, 12, 194. [Google Scholar] [CrossRef]
- Kanda, E.; Muenz, D.; Bieber, B.; Cases, A.; Locatelli, F.; Port, F.K.; Pecoits-Filho, R.; Robinson, B.M.; Perl, J. Beta-2 microglobulin and all-cause mortality in the era of high-flux hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study. Clin. Kidney J. 2021, 14, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Yang, C.-W.; Chiang, C.-C.; Chang, C.-T.; Huang, C.-C. Long-term on-line hemodiafiltration reduces predialysis beta-2-microglobulin levels in chronic hemodialysis patients. Blood Purif. 2001, 19, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Lornoy, W.; Because, I.; Billiouw, J.M.; Sierens, L.; Van Malderen, P.; D’Haenens, P. On-line haemodiafiltration. Remarkable removal of beta2-microglobulin. Long-term clinical observations. Nephrol. Dial. Transplant. 2000, 15, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Roumelioti, M.E.; Trietley, G.; Nolin, T.D.; Ng, Y.-H.; Zhi, X.; Alaini, A.; Figueroa, R.; Unruh, M.L.; Argyropoulos, C.P. Beta-2 microglobulin clearance in high-flux dialysis and convective dialysis modalities: A meta-analysis of published studies. Nephrol. Dial. Transplant. 2018, 33, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Greene, T.; Hartmann, B.; Samtleben, W. Resistance to intercompartmental mass transfer limits β2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int. 2006, 69, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-A.; Sun, W.-C.; Kuo, G.; Chen, C.-Y.; Kao, H.-K.; Lin, Y.; Lee, C.-H.; Hung, C.-C.; Tian, Y.-C.; Ko, Y.-S.; et al. Epidemiology and Outcomes of Infectious Spondylodiscitis in Hemodialysis Patients. Spine 2018, 43, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; De Silva, S.; Warwicker, P.; Farrington, K. Infective spondylodiscitis in patients on high-flux hemodialysis and on-line hemodiafiltration. Hemodial. Int. 2008, 12, 463–470. [Google Scholar] [CrossRef]
- Gouliouris, T.; Aliyu, S.H.; Brown, N.M. Spondylodiscitis: Update on diagnosis and management. J. Antimicrob. Chemother. 2010, 65, 311–324. [Google Scholar] [CrossRef]
- Madhavan, K.; Chieng, L.O.; Armstrong, V.L.; Wang, M.Y. Spondylodiscitis in end-stage renal disease: A systematic review. J. Neurosurg. Spine 2019, 30, 674–682. [Google Scholar] [CrossRef]
- Hopkinson, N.; Patel, K. Clinical features of septic discitis in the UK: A retrospective case ascertainment study and review of management recommendations. Rheumatol. Int. 2016, 36, 1319–1326. [Google Scholar] [CrossRef]
- Berbari, E.F.; Kanj, S.S.; Kowalski, T.J.; Darouiche, R.O.; Widmer, A.F.; Schmitt, S.K.; Hendershot, E.F.; Holtom, P.D.; Huddleston, P.M., 3rd; Petermann, G.W.; et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin. Infect. Dis. 2015, 61, e26–e46. [Google Scholar] [CrossRef] [PubMed]
- Micle, O.; Vicas, I.S.; Ratiu, A.I.; Muresan, M. Cystatin C, a low molecular weight protein in chronic renal failure. Farmacia 2015, 63, 872–876. [Google Scholar]
- Antonescu, A.; Vicaș, S.; Teușdea, A.C.; Rațiu, I.A.; Antonescu, I.A.; Micle, O.; Vicaș, L.; Mureșan, M.; Gligor, F. The levels of serum biomarkers of inflammation in hemodialysis patient. Farmacia 2014, 62, 950–960. [Google Scholar]
- Cheung, W.Y.; Luk, K.D.K. Pyogenic spondylitis. Int. Orthop. 2012, 36, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Jean, M.; Irisson, J.-O.; Gras, G.; Bouchand, F.; Simo, D.; Duran, C.; Perronne, C.; Mulleman, D.; Bernard, L.; Dinh, A. Diagnostic delay of pyogenic vertebral osteomyelitis and its associated factors. Scand. J. Rheumatol. 2017, 46, 64–68. [Google Scholar] [CrossRef]
- Yoon, S.H.; Chung, S.K.; Kim, K.J.; Kim, H.J.; Jin, Y.J.; Kim, H.B. Pyogenic vertebral osteomyelitis: Identification of microorganism and laboratory markers used to predict clinical outcome. Eur. Spine J. 2010, 19, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Kim, D.; van der Vlugt, T.; Vittum, D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine (Phila Pa 1976) 1997, 22, 2089–2093. [Google Scholar] [CrossRef]
- Maus, U.; Andereya, S.; Gravius, S.; Ohnsorge, J.A.K.; Miltner, O.; Niedhart, C. Procalcitonin (PCT) als Verlaufsparameter der Spondylodiszitis [Procalcitonin (PCT) as diagnostic tool for the monitoring of spondylodiscitis]. Z. Für Orthopädie Unfallchirurgie 2009, 147, 59–64. [Google Scholar] [CrossRef]
- Fleege, C.; Wichelhaus, T.; Rauschmann, M. Systemische und lokale Antibiotikatherapie bei konservativ und operativ behandelten Spondylodiszitiden [Systemic and local antibiotic therapy of conservative and operative treatment of spondylodiscitis]. Orthopade 2012, 41, 727–735. [Google Scholar] [CrossRef]
- Choi, S.-H.; Sung, H.; Kim, S.-H.; Lee, S.H.; Kim, Y.S.; Woo, J.H.; Kim, M.-N. Usefulness of a direct 16S rRNA gene PCR assay of percutaneous biopsies or aspirates for etiological diagnosis of vertebral osteomyelitis. Diagn. Microbiol. Infect. Dis. 2014, 78, 75–78. [Google Scholar] [CrossRef]
- Salaffi, F.; Ceccarelli, L.; Carotti, M.; Di Carlo, M.; Polonara, G.; Facchini, G.; Golfieri, R.; Giovagnoni, A. Differentiation between infectious spondylodiscitis versus inflammatory or degenerative spinal changes: How can magnetic resonance imaging help the clinician? La Radiol. Med. 2021, 126, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Ramadani, N.; Dedushi, K.; Kabashi, S.; Mucaj, S. Radiologic Diagnosis of Spondylodiscitis, Role of Magnetic Resonance. Acta Inform. Med. 2017, 25, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Homagk, L.; Marmelstein, D.; Homagk, N.; Hofmann, G.O. SponDT (Spondylodiscitis Diagnosis and Treatment): Spondylodiscitis scoring system. J. Orthop. Surg. Res. 2019, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Rose, L.; Iossifidis, A. Diagnosis and Treatment of Spondylodiscitis: Current Concepts. Int. J. Orthop. 2022, 9, 1670–1675. Available online: http://www.ghrnet.org/index.php/ijo/article/view/3334 (accessed on 22 January 2024).
- Lang, S.; Walter, N.; Neumann, C.; Bärtl, S.; Simon, M.; Ehrenschwender, M.; Hitzenbichler, F.; Alt, V.; Rupp, M. Aktuelle Praxis der empirischen Antibiotikatherapie bei Spondylodiszitis [Current practice of empiric antibiotic treatment for spondylodiscitis]. Orthopadie 2022, 51, 540–546. [Google Scholar] [CrossRef]
- Prost, M.; Röckner, M.E.; Vasconcelos, M.K.; Windolf, J.; Konieczny, M.R. Outcome of Targeted vs Empiric Antibiotic Therapy in the Treatment of Spondylodiscitis: A Retrospective Analysis of 201 Patients. Int. J. Spine Surg. 2023, 17, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Sobottke, R.; Seifert, H.; Fätkenheuer, G.; Schmidt, M.; Goßmann, A.; Eysel, P. Current Diagnosis and Treatment of Spondylodiscitis. Dtsch. Aerzteblatt Online 2008, 105, 181–187. [Google Scholar] [CrossRef]
- Cervan, A.M.; Colmenero, J.d.D.; Del Arco, A.; Villanueva, F.; Guerado, E. Spondylodiscitis in patients under haemodyalisis. Int. Orthop. 2012, 36, 421–426. [Google Scholar] [CrossRef]
- Lu, Y.-A.; Hsu, H.-H.; Kao, H.-K.; Lee, C.-H.; Lee, S.-Y.; Chen, G.-H.; Hung, C.-C.; Tian, Y.-C. Infective spondylodiscitis in patients on maintenance hemodialysis: A case series. Ren. Fail. 2017, 39, 179–186. [Google Scholar] [CrossRef]
- Kuo, G.; Sun, W.-C.; Lu, Y.-A.; Chen, C.-Y.; Kao, H.-K.; Lin, Y.; Chen, Y.-C.; Hung, C.-C.; Tian, Y.-C.; Hsu, H.-H. Chronic dialysis patients with infectious spondylodiscitis have poorer outcomes than non-dialysis populations. Ther. Clin. Risk Manag. 2018, 14, 257–263. [Google Scholar] [CrossRef]
- Originali, A.; Traversi, L.; Nava, E.; Xhaferi, B.; Bigatti, G.G.O.; Tedoldi, S.; Mazzullo, T.; Botticelli, I.M.C.; Martino, S.; Villa, M.N.; et al. Spondylodiscitis in hemodialysis patients: A new emerging disease? Data from an Italian Center. Giornale Italiano di Nefrologia. Organo Uff. Della Soc. Ital. Nefrol. 2020, 37, 15. [Google Scholar]
- Ramírez-Huaranga, M.A.; Sánchez de la Nieta-García, M.D.; Anaya-Fernández, S.; Arambarri-Segura, M.; Caparrós-Tortosa, G.; RiveraHernández, F.; Romera-Segorbe, A.; Vozmediano-Poyatos, M.C.; Ferreras-García, I. Spondylodiscitis, nephrology department’s experience. Nefrologia 2013, 33, 250–255. [Google Scholar] [PubMed]
- Ünal, A.; Arıkan, T.; Sipahioğlu, M.H.; Koçyiğit, I.; Tokgöz, B.; Kahriman, G.; Oymak, O. Metastatic Infections in Hemodialysis: An Analysis of 19 Cases. Turk. Nephrol. Dial. Transplant. 2017, 26, 317–322. [Google Scholar] [CrossRef]
- Cassó-Troche, L.R.; Echavarría-Uceta, J.A.; Quiñones-Robles, J.; Haché-Pagan, C.; Herrera, I.; Encarnación, J.; De la Rosa, S.; De la Cruz, D.M.; Rojas, L.; Vásquez, P.P.D. Infective spondylodiscitis in hemodialysis patients. Surg. Neurol. Int. 2022, 13, 549. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Fu, T.-S.; Kao, Y.-H.; Tsai, T.-T.; Lai, P.-L.; Niu, C.-C.; Chen, W.-J. Surgical treatment of infectious spondylitis in patients undergoing hemodialysis therapy. Eur. Spine J. 2010, 19, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Daka, S.; Pastewski, A.; Kyaw, W.; Chapnick, E.; Sepkowitz, D. Spinal Epidural Abscess in Hemodialysis Patients: A Case Series and Review. Clin. J. Am. Soc. Nephrol. 2011, 6, 1495–1500. [Google Scholar] [CrossRef]
- Kovalik, E.C.; Raymond, J.R.; Albers, F.J.; Berkoben, M.; Butterly, D.W.; Montella, B.; Conlon, P.J. A clustering of epidural abscesses in chronic hemodialysis patients: Risks of salvaging access catheters in cases of infection. J. Am. Soc. Nephrol. JASN 1996, 7, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.V.; Ravikumar, T.V. Surgical Management of Thoracolumbar Spondylodiscitis in End-Stage Renal Disease. Indian J. Orthop. 2020, 55, 176–181. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Fu, T.-S.; Chang, C.-H.; Hsu, H.-H.; Chang, M.-Y.; Tian, Y.-C.; Hung, C.-C.; Fang, J.-T.; Chen, L.-H.; Chen, Y.-C. Aggressive Surgical Intervention in End-Stage Renal Disease Patients with Spinal Epidural Abscess. Ren. Fail. 2011, 33, 582–586. [Google Scholar] [CrossRef]
- Kilic, G.; Onec, K.; Polat, O. Spondilodyscitis in Patients under Haemodyalisis. Online Turk. J. Health Sci. 2022, 7, 74–79. [Google Scholar]
- Yildirim, S.; Yilmaz, B.; Yilmaz, E.; Derici, U.B. Metastatic spondylodiscitis in central venous catheter related bloodstream infections in hemodialysis patients: Risk factors and prognosis. J. Vasc. Access 2022, 25, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Yamaoka, K.; Tanaka, K.; Sasaki, T. Bacterial spondylodiscitis in the patients with hemodialysis. Spine 2004, 29, 2533–2537. [Google Scholar] [CrossRef] [PubMed]
- Faria, B.; Canto; Moreira, N.; Sousa, T.C.; Pêgo, C.; Vidinha, J.; Garrido, J.; Lemos, S.; Soares, C.; Lima, C.; et al. Spondylodiscitis in hemodialysis patients: A case series. Clin. Nephrol. 2011, 76, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.; Benazzo, F.; De Rosa, F.; Boriani, S.; Dallagiacoma, G.; Franceschetti, G.; Cuzzocrea, F. A systematic review: Characteristics, complications and treatment of spondylodiscitis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 117–128. [Google Scholar] [PubMed]
- Türkmen, E. Metastatic infectious complications in tunneled dialysis catheter-associated infections: A single-center experience. J. Health Sci. Med. 2022, 5, 178–183. [Google Scholar] [CrossRef]
- Helewa, R.M.; Boughen, C.G.; Cheang, M.S.; Embil, J.M.; Goytan, M.; Zacharias, J.M. Infectious pondylodiscitis in patients receiving hemodialysis. Orthop. Procs. 2009, 91, 251. [Google Scholar]
- Dalrymple, L.S.; Mu, Y.; Romano, P.S.; Nguyen, D.V.; Chertow, G.M.; Delgado, C.; Grimes, B.; Kaysen, G.A.; Johansen, K.L. Outcomes of Infection-Related Hospitalization in Medicare Beneficiaries Receiving In-Center Hemodialysis. Am. J. Kidney Dis. 2015, 65, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Kesikburun, B.; Ekşioğlu, E.; Akdağ, İ.; Çakçı, A. Low back pain in hemodialysis patients: Risk factors and its impact on health-related quality of life. Turk. J. Phys. Med. Rehabil. 2017, 64, 66–71. [Google Scholar] [CrossRef]
- Ulrich Josef Albert, S.; Kilper, A.; Glasmacher, S.; Heyde, C.-E.; Josten, C. Welche Parameter haben Einfluss auf den stationären Verlauf bei Patienten mit Spondylodiszitis?Which factors influence the inpatient course for patients with spondylodiscitis? Der Unfallchirurg. 2020, 123, 724–730. [Google Scholar] [CrossRef]
- Lener, S.; Wipplinger, C.; Hartmann, S.; Rietzler, A.; Thomé, C.; Tschugg, A. Gender-Specific Differences in Presentation and Management of Spinal Infection: A Single-Center Retrospective Study of 159 Cases. Glob. Spine J. 2021, 11, 430–436. [Google Scholar] [CrossRef]
- Lang, S.; Walter, N.; Schindler, M.; Baertl, S.; Szymski, D.; Loibl, M.; Alt, V.; Rupp, M. The Epidemiology of Spondylodiscitis in Germany: A Descriptive Report of Incidence Rates, Pathogens, In-Hospital Mortality, and Hospital Stays between 2010 and 2020. J. Clin. Med. 2023, 12, 3373. [Google Scholar] [CrossRef] [PubMed]
- Şahintürk, F.; Sönmez, E.; Ayhan, S.; Ustaoğlu, D.; Gülşen, S.; Yılmaz, C. The effect of chronic kidney disease in patients with spontaneous spondylodiscitis. J. Turk. Spinal Surg. 2023, 34, 45–48. [Google Scholar] [CrossRef]
- Coelho, S. What is the Role of HbA1c in Diabetic Hemodialysis Patients? Semin. Dial. 2016, 29, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Bomholt, T.; Adrian, T.; Nørgaard, K.; Ranjan, A.G.; Almdal, T.; Larsson, A.; Vadstrup, M.; Rix, M.; Feldt-Rasmussen, B.; Hornum, M. The Use of HbA1c, Glycated Albumin and Continuous Glucose Monitoring to Assess Glucose Control in the Chronic Kidney Disease Population Including Dialysis. Nephron 2021, 145, 14–19. [Google Scholar] [CrossRef]
- Bomholt, T.; Kofod, D.; Nørgaard, K.; Rossing, P.; Feldt-Rasmussen, B.; Hornum, M. Can the Use of Continuous Glucose Monitoring Improve Glycemic Control in Patients with Type 1 and 2 Diabetes Receiving Dialysis? Nephron 2023, 147, 91–96. [Google Scholar] [CrossRef]
- Kim, D.K.; Ko, G.J.; Choi, Y.J.; Jeong, K.H.; Moon, J.Y.; Lee, S.H.; Hwang, H.S. Glycated hemoglobin levels and risk of all-cause and cause-specific mortality in hemodialysis patients with diabetes. Diabetes Res. Clin. Pract. 2022, 190, 110016. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, B. Pathogenesis of chronic viral hepatitis: Differential roles of T cells and NK cells. Nat. Med. 2013, 19, 859–868. [Google Scholar] [CrossRef]
- Sánchez, J.L.C.; Revuelta, M.G.; Mantecón, M.E.C.; Alonso, R.A.S.; Cano, M.S.S. Infectious spondylodiscitis in patients with central venous catheters for haemodialysis: A retrospective study. J. Ren. Care 2012, 38, 147–150. [Google Scholar] [CrossRef]
- Rocha, A.; Castro, R.; Santos, J. Endocarditis and Spondylodiscitis Associated with Tunneled Cuffed Hemodialysis Catheters: Hospitalizations with Poor Outcomes. Int. J. Artif. Organs 2018, 38, 173–177. [Google Scholar] [CrossRef]
- Topan, R.; Ambrose, T.; Small, M.; Lightman, E.; Nightingale, J.; Gabe, S.M. Spinal Infections Among Patients with Long-Term Central Venous Catheters for Home Parenteral Nutrition. Nutr. Clin. Pract. 2016, 32, 133–138. [Google Scholar] [CrossRef]
- Sharif, M.R.; Chitsazian, Z.; Moosavian, M.; Raygan, F.; Nikoueinejad, H.; Sharif, A.R.; Einollahi, B. Immune disorders in hemodialysis patients. Iran. J. Kidney Dis. 2015, 9, 84–96. [Google Scholar] [PubMed]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33 (Suppl. 3), 35–40. [Google Scholar] [CrossRef] [PubMed]
- Raţiu, I.A.; Raţiu, C.A.; Miclăuş, V.; Boşca, A.B.; Kazancioğlu, R.T.; Constantin, A.M.; Bako, G.C.; Şovrea, A.S. The pioneer use of a modified PRGF–Endoret® technique for wound healing in a hemodialyzed diabetic patient in a terminal stage of renal disease. Rom. J. Morphol. Embryol. 2021, 62, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.P. The Use of Carbapenems in the Treatment of Serious Infections. J. Intensiv. Care Med. 2009, 24, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Masterton, R.G. Carbapenems in serious infections: Maximizing the power, minimizing resistance. Clin. Microbiol. Infect. 1999, 5, 12–14. [Google Scholar] [CrossRef]
- Watkins, R.R.; Yendewa, G.; Burdette, S.D.; Horattas, S.; Haller, N.A.; Mangira, C.; Salata, R.A.; Bonomo, R.A. DISC: Describing Infections of the Spine treated with Ceftaroline. J. Glob. Antimicrob. Resist. 2018, 13, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.B.; Bistas, K.G.; Le, J.K. Clindamycin. [Updated 2023 May 23]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519574/ (accessed on 22 January 2024).
- Nagarkoti, D.; Prajapati, K.; Sharma, A.N.; Gyawali, A.; Manandhar, S. Distribution of Macrolide-Lincosamide-Streptogramin B Antibiotics Resistance Genes in Clinical Isolates of Staphylococci. J. Nepal Health Res. Counc. 2021, 18, 734–740. [Google Scholar] [CrossRef]
- Gemmell, C.G.; Edwards, D.I.; Fraise, A.P.; Gould, F.K.; Ridgway, G.L.; Warren, R.E. Behalf of the Joint Working Party of the British Society for Antimicrobial Chemotherapy, Hospital Infection Society and Infection Control Nurses Association, Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J. Antimicrob. Chemother. 2006, 57, 589–608. [Google Scholar] [CrossRef]
- Boeckh, M.; Lode, H.; Deppermann, K.M.; Grineisen, S.; Shokry, F.; Held, R.; Wernicke, K.; Koeppe, P.; Wagner, J.; Krasemann, C. Pharmacokinetics and serum bactericidal activities of quinolones in combination with clindamycin, metronidazole, and ornidazole. Antimicrob. Agents Chemother. 1990, 34, 2407–2414. [Google Scholar] [CrossRef]

| Patients’ Characteristics | Chronic HD (N = 13) | Non-HD Patients (N = 19) | p-Value, χ2 |
|---|---|---|---|
| Age (years), median (IQR) | 63 (8) | 66 (18) | 0.252 |
| Male gender number (%) | 10 (76.92) | 16 (84.2) | 0.355 |
| Recurrent infections (%) | 8 (61.53) | 11 (57.89) | 0.836 |
| Diabetes mellitus (%) | 1 (7.69) | 7 (36.84) | 0.077 |
| Chronic hepatitis C/B (%) | 5 (38.46) | 2 (15.38) | 0.060 χ2 = 3.524. |
| Positive blood culture (%) | 4 (30.78) | 3 (15.78) | 0.149 χ2 = 2.076 |
| Previous documented discopathy (%) | 4 (30.78) | 7 (36.84) | 0.355 |
Recent bacteremic procedures (3 months before ISD)
| 2 7 - | - 9 3 | |
Location ISD (MRI) (%)
| 4 (30.78) 9 (69.23) | 14 (73.68) (3 paraparesis) 5 (26.31) | 0.016 0.016 |
| Neurosurgical procedures (%) | 1(7.69%) | 4(21.05%) | 0.306 |
| Hemodialysis Patient Characteristics | Number (%) | p Value | |
|---|---|---|---|
| 1. Primary kidney disease | Chronic glomerulonephritis Tubulointerstitial diseases Polycystic kidney disease Vascular nephropathy Diabetic nephropathy | 4 (30.7) 3 (23) 2 (15.38) 3 (23) 1 (7.69) | |
| 2. Hemodialysis type: High-flux hemodialysis | 13 (100) | ||
| 3. Vascular access | Long term catheter Arteriovenous fistula | 4 (30.7) 9 (69.23) | 0.049 |
| 4. Duration of hemodialysis, mean (years) Long-term catheter Arteriovenous fistula | 6 3.25 7.22 | ||
| 5. Hemodialysis efficiency (kT/V) | 1.38 | ||
| 6. PTH (pg/mL) | 332.3 | ||
| 7. Staphyloccocus nasal carriage | 1 (7.69) | ||
| Symptoms | Diagnostic Tools | CRP | WBC | Neutrophils | Hb | RWD | Ferritin | iPTH | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dgn | 3M | dgn | 3M | dgn | 3M | dgn | 3M | dgn | dgn | dgn | |||
| 1 | pain fever | MRI T10-11 | 148 | 42 | 14,000 | 7800 | 12,000 | 6000 | 10.5 | 9.3 | 16 | 440 | 200 |
| 2 | pain, fever limb weakness | MRI L2-L3 | 150 | 39 | 9700 | 6200 | 7300 | 4900 | 9.5 | 9.4 | 17 | 720 | 320 |
| 3 | pain | MRI T12-L1 | 35 | 12 | 5500 | 5100 | 3800 | 3200 | 12.2 | 12.4 | 18 | 410 | 120 |
| 4 | fever limb weakness | MRI L1-L3 | 350 | 218 | 28,000 | 24,000 | 20,000 | 19,500 | 9.5 | 7.9 | 16.5 | 730 | 210 |
| 5 | pain limb weakness | MRI T10-T11 | 81 | 228 | 8200 | 6600 | 5700 | 4500 | 10.2 | 11.3 | 16.9 | 530 | 419 |
| 6 | pain, fever | MRI L1-L2 | 210 | 42 | 8500 | 8500 | 6200 | 6300 | 11.2 | 9 | 13.8 | 630 | 110 |
| 7 | pain fever limb weakness | MRICT L2-3 | 227 | 65 | 18,500 | 8900 | 15,600 | 7000 | 6.6 | 9 | 20.4 | 1089 | 93 |
| 8 | pain limb weakness | MRI L4-l5 | 290 | 11.5 | 12,000 | 6800 | 9900 | 4800 | 9.6 | 12.5 | 17.2 | 620 | 511 |
| 9 | pain fever | MRI L1-L2, L4-L5 | 62 | 23 | 4100 | 4200 | 2600 | 2400 | 8 | 9.6 | 16.1 | 419 | 802 |
| 10 | pain fever | MRI D11-D12 | 88 | 17 | 11,300 | 7300 | 7700 | 5200 | 10.5 | 11 | 15.5 | 520 | 340 |
| 11 | pain fever | MRI L2-L3 | 161 | 11.6 | 19,300 | 12,100 | 15,700 | 8200 | 9,6 | 11.7 | 16.9 | 530 | 570 |
| 12 | pain fever | MRI T10-T11 | 120 | NP | 12,000 | NP | 9800 | NP | 8.9 | NP | 15.3 | 470 | 265 |
| 13 | pain fever | MRI T7-T8 | 150 | 25 | 18,000 | 6500 | 15,300 | 4200 | 10.2 | 11.5 | 14 | 520 | 360 |
| Symptoms | Diagnostic Tools | CRP | WBC | Neutrophils | Hb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| dgn | 3M | dgn | 3M | dgn | 3M | dgn | 3M | |||
| 1. | pain | MRI | 56 | 4.5 | 9700 | 5200 | 7300 | 4200 | 13.6 | 14.5 |
| 2. | pain/limb weakness | MRI | 130 | NP | 11,600 | NP | 10,000 | NP | 12.4 | NP |
| 3. | pain | MRI | 48 | 3 | 7500 | 5300 | 6000 | 4900 | 14.5 | 14.7 |
| 4. | fever/limb weakness | MRI | 56 | 17 | 7900 | 5300 | 6300 | 4200 | 13.6 | 14.2 |
| 5. | pain/limb weakness | MRI | 120 | 37 | 9800 | 6500 | N/A | N/A | 12 | 11.9 |
| 6. | pain/hemiplegia | MRI T11/12 | 10.19 | 4.16 | 6100 | 7000 | 4000 | 5000 | 14.1 | 16 |
| 7. | pain/limb weakness | MRI L2-l3 | 161 | 6.4 | 16,000 | 9900 | 14,000 | 7100 | 12.3 | 12.9 |
| 8. | pain | MRI L4-l5 | 38 | 1.4 | 5700 | N/A | 2000 | N/A | 13.6 | N/A |
| 9. | pain | MRI T10-11 | 46 | NP | 7200 | NP | 4900 | NP | 13.3 | NP |
| 10. | pain | MRI L5-S1 | 1.7 | 12 | 10,200 | 8000 | 4600 | N/A | 14 | 15 |
| 11. | monoparesis | MRI L2-S2 (abscess) | 22.7 | 3.5 | 8700 | 8300 | 4700 | 3400 | 12.2 | 12 |
| 12. | pain /paraparesis | MRI T10-T11 | 85 | N/A | 12,000 | N/A | 9800 | N/A | 12 | 13.5 |
| 13. | pain | MRI T12 | 95 | N/A | 18,000 | N/A | 16,000 | N/A | 12.3 | N/A |
| 14. | pain | N/A | 113 | 61 | 7600 | 8400 | 4300 | 4900 | 10.2 | 10 |
| 15. | pain | MRI L4-L5 (abscess) | 42 | 31 | 7500 | 10,200 | 5100 | 6300 | 11 | 12.7 |
| 16. | pain | MRI L1-L2 | 11 | N/A | 7100 | N/A | 4400 | N/A | 12.5 | N/A |
| 17. | pain | N/A | 67 | N/A | 9700 | N/A | 6700 | N/A | 11 | N/A |
| 18. | pain | N/A | 129 | 5.4 | 9200 | N/A | 6800 | N/A | 13 | N/A |
| 19. | paraplegia | MRI T8-T9 | 108 | 8 | 7400 | 4200 | N/A | N/A | 9.7 | 10.2 |
| Laboratory Biomarkers | CRP (Mean) | WBC | Neutrophils | Hb Level | ||||
|---|---|---|---|---|---|---|---|---|
| dgn | 3M | dgn | 3M | dgn | 3M | dgn | 3M | |
| Chronic hemodialysis (mean) | 159.38 | 53.69 | 13,007 | 8578 | 10,123 | 6338 | 9.42 | 10.31 |
| Non-HD | 70.5 | 16.5 | 9415 | 7253 | 6876 | 5110 | 12.49 | 13.04 |
| p-value ES (effect size) | 0.0005 0.001 | 0.03 0.01 | 0.02 0.0001 | 0.19 - | 0.028 - | 0.1 - | 0.00001 0.09275 | 0.0001 0.08 |
| Blood Culture | Culture from Biopsy | Other Cultures | Antimicrobial Therapy | AB Therapy Length | Outcome | |
|---|---|---|---|---|---|---|
| 1. | Staphylococcus epidermidis | (NP) | E. coli (uro culture) | Vancomycin + Ceftazidime, then Vancomycin + Meropenem then Ciprofloxacin + Clindamycin | 2 w 4 w 6 w | recovered died 1 year later |
| 2. | Staphylococcus aureus | (-) | NP | Vancomycin + Meropenem Clindamycin | 3 w 2 w | died |
| 3. | (-) | (NP) | NP | Meropenem | 2 w | recovered |
| 4. | (-) | (NP) | (-) | Meropenem | 3 w | recovered |
| 5. | (-) | (NP) | (-) | Vancomycin + Meropenem | 3 w | died |
| 6. | (-) | (NP) | NP | Meropenem | 2 w | recovered |
| 7. | (-) | (NP) | NP | Vancomycin + Cefoperazon | 3 w | recovered |
| 8. | Staphylococcus aureus | (NP) | NP | Cefrtiaxon + Amikacin Vancomycin | 1 w 4 w | recovered |
| 9. | (-) | (-) | NP | Vancomycin + Meropenem Clindamycin | 4 w 4 w | recovered |
| 10. | (-) | (NP) | NP | Clindamycin Cefixime | 3 w 3 w | died |
| 11. | Providencia stuartii | Providencia stuartii | NP | Ceftazidime/avibactam | 8 w | died |
| 12. | (-) | (NP) | NP | Cefoperazone/sulbactam Vancomycin + Ciprofloxacin Ciprofloxacin + Cefuroxime | 2 w 2 w 2 w | died |
| 13. | (-) | (NP) | NP | Vancomycin + Cefoperazone + Ciprofloxacin Clindamycin | 2 w 2 w | recovered |
| Case No | Blood Culture | Other Cultures | Antimicrobial Therapy | AB Therapy Length | Outcome |
|---|---|---|---|---|---|
| 1. | (-) | Uro Culture (-) | Clindamycin Ciprofloxacin | 3 w 3 w | recovered |
| 2. | Staphylococcus aureus | (NA) | Clindamycin + Amikacin | 8 d | died |
| 3. | (-) | (-) | Clindamycin + Ciprofloxacin | 8 w | recovered |
| 4. | (-) | (-) | Clindamycin + Ciprofloxacin | recovered | |
| 5. | (-) | Staphylococcus Epidermidis | Vancomycin + Amikacin Amoxicillin/clavulanate + Ciprofloxacin | 2 w 3 w | recovered |
| 6. | (-) | (-) | Clindamycin + Ciprofloxacin | 6 w | recovered |
| 7. | E. coli | Coproculture (Candida Albicans) | Clindamycin + Ciprofloxacin Vancomycin + Imipenem Cefoperazone/sulbactam | 10 d 10 d 4 w | recovered |
| 8. | Staphylococcus epidermidis | Candida albicans | Vancomycin + Ceftriaxone Ceftriaxone + Clindamycin | 2 w 2 w | recovered |
| 9. | (-) | (-) | Vancomycin + Ceftriaxone Ciprofloxacin + Clindamycin then Teicoplanin | 10 d 10 d 2 w | died |
| 10. | (-) | (-) | Vancomycin + Clindamycin Amoxicillin/clavulanate + Ciprofloxacin | 6 w 2 w | recovered |
| 11. | NP | Candida albicans | Ciprofloxacin + Amikacin Clindamycin + Ciprofloxacin | 4 w 4 w | recovered |
| 12. | NP | (-) | Clindamycin + Ciprofloxacin Amoxicillin/clavulanat Doxycycline | 3 w 2 w 4 w | recovered |
| 13. | (-) | (-) | Vancomycin + Ceftriaxone | 2 d | died |
| 14. | (-) | (-) | Clindamycin+Ciprofloxacin + Gentamicin Clindamycin | 2 w 4 w | recovered |
| 15. | (-) | (-) | Meropenem + Gentamicin Clindamycin | 1 w 4 w | recovered |
| 16. | (-) | (-) | Clindamycine + Gentamicin Clindamycin | 3 w 3 w | recovered |
| 17. | (-) | (-) | Amikacin + Clindamycin Clindamycin | 2 w 4 w | recovered |
| 18. | (-) | (-) | Clindamycin + Ciprofloxacin Clindamycin | 3 w 3 w | recovered |
| 19. | (-) | NP | Vancomycin | 2 we | recovered |
| HD Patients | Meropenem | Vancomycin | p Value |
|---|---|---|---|
| Number of patients (%) | 5 (55.5%) | 5 (55.5%) | - |
| Length of treatment (days) | 19.6 | 19.6 | - |
| Recovered patients no (%) | 4 (80%) | 3 (60%) | 0.0032 |
| Monotherapy/ Recovered patients | 3 (60%)/ 3 (100%) | - | 0.00001 |
| Combined therapy/results | 2 (40%) Vancomycin + Meropenem, (died) Vancomycin + Meropenem, (recovered) | 5 (100%) Vancomycin + Meropenem (died) Vancomycin + Cefoperazon (recovered) Vancomycin + Meropenem (recovered) Vancomycin + Ciprofloxacin (died) Vancomycin + Cefoperazone + Ciprofloxaci (recovered) | 0.00001 |
| Nr. | Article | Number of Patients | Follow-Up Period |
|---|---|---|---|
| 1 | Cervan A.M., 2012 [38] | 23 | 1996–2010 (14 years) |
| 2 | Lu Y.A., 2017 [39] | 18 | 2005–2015 (10 years) |
| 3 | Kuo G., 2018 [40] | 105 | 2002–2015 (13 years) |
| 4 | Karthik Madhavan, 2019 [19] | 4 | N/A |
| 5 | Traversi L, 2020 [41] | 9 | 2005–2019 (14 years) |
| 6 | Ramírez-Huaranga, M.A., 2013 [42] | 5 | 2008–2012 (4 years) |
| 7 | Aydın üNAL, 2017 [43] | 9 | 2010–2016 (6 years) |
| 8 | Cassó-Troche L.R., 2022 [44] | 11 | 2011–2012 (1 year) |
| 9 | Abid S., 2008 [17] | 13 | 1997–2006 (9 years) |
| 10 | Chen, LH., 2010 [45] | 16 | 1997–2006 (9 years) |
| 11 | Wong, S.S.; 2011 [46] | 6 | 2000–2005 (5 years) |
| 12 | Kovalik, E C., 1996 [47] | 10 | 1991–1996 (5 years) |
| 13 | Vinay Jain K., 2020 [48] | 34 | 2014–2019 (5 years) |
| 14 | Mei-Yi Wu, [49] | 12 | 2003–2006 |
| 15 | Lu, Yueh-An, 2018. [50] | 102 | 13 years |
| 16 | Yildirim S., 2022; [51] | 15 | N/A |
| 17 | Tsuchiya K., 2004 [52] | 9 | N/A |
| 18 | Faria B., 2011 [53] | 11 | 5 years |
| Article | Etiological Agent | Antibiotic | No. of Patients (%) | Treatment Length | Outcome |
|---|---|---|---|---|---|
| Lu YA (2017) [39] | Enterococcus faecalis | Ampicillin | 1 | 35 d | Relapse |
| Enterobacter cloacae | Vancomycin + Piperacillin/Tazobactam | 1 | 42 d | Relapse | |
| MSSA | Cefazolin + Gentamicin | 1 | 30 d | Recovered | |
| Coagulase Negative Staphylococci | Teicoplanin | 1 | 25d | Death | |
| MRSA | Teicoplanin, Daptomycin, Teicoplanin + Rifampicin | 1 | 115 d | Recovered | |
| S. Epidermidis | Vancomycin, Teicoplanin | 1 | 53 d | Recovered | |
| Kuo G. (2018) [16] | MRSA | N/A | 30 (28.6) | N/A | N/A |
| MSSA | N/A | 9 (8.6) | N/A | N/A | |
| Coagulase Negative Staphylococci | N/A | 14 (13.3) | N/A | N/A | |
| Enterococcus | N/A | 7 (6.6) | N/A | N/A | |
| Streptococcus spp. | N/A | 2 (1.9) | N/A | N/A | |
| Klebsiella pneumoniae | N/A | 1 (0.95) | N/A | N/A | |
| Candida parapsilosis | N/A | 1 (0.95) | N/A | N/A | |
| Mycobacterium tuberculosis | N/A | 1 (0.95) | N/A | N/A | |
| Mycobacterium chelonae | N/A | 1 (0.95) | N/A | N/A | |
| Traversi L (2020) [41] | Staphylococcus aureus | Vancomycin + Gentamicin | 1 | 4 w | Relapse |
| Vancomycin + Ciprofloxacin + Ceftazidime | 1 | 8 w | Recovered | ||
| Vancomycin + Ciprofloxacin | 1 | 8 w | Paraplegia | ||
| Teicoplanin, then Linezolid | 1 | 8 w | Recovered | ||
| Streptococcus agalactiae | Vancomycin + Levofloxacin | 1 | 4 w | Recovered | |
| Marco A. (2013) [42] | MSSA | Vancomycin + Ceftazidime | 1 | N/A | Death |
| Ceftazidime, Levofloxacin IV, then Levofloxacin and Rifampicin PO | 1 | 1 m 6 w | Recovered | ||
| S. Epidermidis | Vancomycin then Daptomycin | 1 | 3 m + 3 m | Recovered | |
| Enterococcus faecalis | Vancomycin and Cefotaxime | 1 | N/A | Recovered | |
| Aydın Ünal (2017) [43] | S. Epidermidis | Ampicillin + Sulbactam | 1 | N/A | Recovered |
| Ampicillin + Sulbactam + Rifampicin | 1 | N/A | Recovered | ||
| Teicoplanin | 1 | N/A | N/A | ||
| Vancomycin + Meropenem | 1 | N/A | N/A | ||
| MSSA | Vancomycin + Piperacillin/ Tazobactam | 1 | N/A | Death | |
| Kovalik (1996) [47] | MRSA+MSSA | Vancomycin | 10 | 4–6w | |
| Length of treatment (days): mean/median/IQR: 54.53/42/26 Length of treatment (days) for recovered patients 56/50/68.5 | |||||
| Nr. | Article | Empiric Treatment Scheme | Duration | No. of Patients | Outcome |
|---|---|---|---|---|---|
| 1. | Cervan AM (2012) [38] | Mono or combined antibiotic therapy | iv (4 w), then oral (6w) after the clinical symptoms settled down | 11 | Death of 3 patients |
| 2. | Lu YA (2017) [39] | Teicoplanin + Ceftriaxone | 25 d | 1 | Death |
| Teicoplanin + Ceftazidime, Teicoplanin + Imipenem/Cilastatin, Vancomycin + Flomoxef | 38 d | 1 | Relapse | ||
| Vancomycin | 40 d | 1 | Recovered | ||
| Vancomycin + Ceftriaxone | 42 d | 1 | Recovered | ||
| Vancomycin +Ceftriaxone | 23 d | 1 | Recovered | ||
| Vancomycin + Meropenem | 55 d | 1 | Recovered | ||
| Ceftriaxone, Vancomycin + Piperacillin/Tazobactam | 18 d | 1 | Death | ||
| Cefazolin, Oxacillin | 24 d | 1 | Recovered | ||
| 3. | Traversi L. Nava E. (2020) [41] | Vancomycin + Ciprofloxacin; then Teicoplanin + Ceftazidime | 8 w | 1 | Recovered (2 m) |
| Teicoplanin + Ciprofloxacin | 4 w | 1 | Recovered (3 m) | ||
| Levofloxacin + Rifampicin | 4 w | 1 | Recovered (8 m) | ||
| Ciprofloxacin | 8 w | 1 | Recovered (3 m) | ||
| 4. | Marco A. Ramirez Huaranga (2013) [42] | Vancomycin + Ceftazidime | 4 w | 1 | Death |
| Vancomycin + Ceftazidime | 1 w | 1 | Treatment change (Enterococcus identified) | ||
| 5. | Aydın Ünal (2017) [43] | Teicoplanin | N/A | 1 | Recovered |
| Ampicillin + Sulbactam | N/A | 1 | Recovered | ||
| Teicoplanin | N/A | N/A | Recovered | ||
| Amoxicillin/clavulanic acid + Ciprofloxacin | N/A | 1 | Recovered | ||
| 6. | Chen, LH. (2010) [45] | Cefazoline + Gentamicin | Over 6 w | 5 | N/A |
| Vancomycin | N/A | 2 | N/A | ||
| Length of treatment (days): mean/median/IQR: 35,62/28/27.5 Length of treatment (days) for recovered patients 39,16/39/30.75 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratiu, I.A.; Moisa, C.F.; Țiburcă, L.; Hagi-Islai, E.; Ratiu, A.; Bako, G.C.; Ratiu, C.A.; Stefan, L. Antimicrobial Treatment Challenges in the Management of Infective Spondylodiscitis Associated with Hemodialysis: A Comprehensive Review of Literature and Case Series Analysis. Antibiotics 2024, 13, 284. https://doi.org/10.3390/antibiotics13030284
Ratiu IA, Moisa CF, Țiburcă L, Hagi-Islai E, Ratiu A, Bako GC, Ratiu CA, Stefan L. Antimicrobial Treatment Challenges in the Management of Infective Spondylodiscitis Associated with Hemodialysis: A Comprehensive Review of Literature and Case Series Analysis. Antibiotics. 2024; 13(3):284. https://doi.org/10.3390/antibiotics13030284
Chicago/Turabian StyleRatiu, Ioana A., Corina F. Moisa, Laura Țiburcă, Edy Hagi-Islai, Anamaria Ratiu, Gabriel Cristian Bako, Cristian Adrian Ratiu, and Liana Stefan. 2024. "Antimicrobial Treatment Challenges in the Management of Infective Spondylodiscitis Associated with Hemodialysis: A Comprehensive Review of Literature and Case Series Analysis" Antibiotics 13, no. 3: 284. https://doi.org/10.3390/antibiotics13030284
APA StyleRatiu, I. A., Moisa, C. F., Țiburcă, L., Hagi-Islai, E., Ratiu, A., Bako, G. C., Ratiu, C. A., & Stefan, L. (2024). Antimicrobial Treatment Challenges in the Management of Infective Spondylodiscitis Associated with Hemodialysis: A Comprehensive Review of Literature and Case Series Analysis. Antibiotics, 13(3), 284. https://doi.org/10.3390/antibiotics13030284







