Abstract
Aniba rosaeodora is one of the most widely used plants in the perfumery industry, being used as medicinal plant in the Brazilian Amazon. This work aimed to evaluate the chemical composition of A. rosaeodora essential oil and its biological activities. A. rosaeodora essential oil presented linalool (93.60%) as its major compound. The A. rosaeodora essential oil and linalool showed activity against all the bacteria strains tested, standard strains and marine environment bacteria, with the lower minimum inhibitory concentration being observed for S. aureus. An efficient antioxidant activity of A. rosaeodora essential oil and linalool (EC50: 15.46 and 6.78 µg/mL, respectively) was evidenced by the inhibition of the 2,2-azinobis- (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical. The antitrypanosomal activity of A. rosaeodora essential oil and linalool was observed at high concentrations against epimatigote forms (inhibitory concentration for 50% of parasites (IC50): 150.5 ± 1.08 and 198.6 ± 1.12 µg/mL, respectively), and even higher against intracellular amastigotes of T. cruzi (IC50: 911.6 ± 1.15 and 249.6 ± 1.18 µg/mL, respectively). Both A. rosaeodora essential oil and linalool did not exhibit a cytotoxic effect in BALB/c peritoneal macrophages, and both reduced nitrite levels in unstimulated cells revealing a potential effect in NO production. These data revealed the pharmacological potential of A. rosaeodora essential oil and linalool, encouraging further studies.
Keywords:
rosewood; linalool; marine bacteria; ABTS; Trypanosoma cruzi; cytotoxicity; nitrite; nitric oxide 1. Introduction
In recent decades, many studies have concentrated on the search for potential antimicrobials, with an increase in the worldwide spending on finding new antimicrobial agents. Faced with bacterial resistance to antibiotic treatment as well as the discovery of new pathogens, the need for new antimicrobials arises [1].
There are promising reports of different plant-derived natural phytochemicals, and a growing interest in exploring their potential [2]. Medicinal plants have been used around the world for various purposes, just as their active chemical compounds have been used to combat various diseases. Essential oils are well known for their pharmacological activities, including antibacterial [3] and trypanocidal activity [4], and may represent a promising source of new natural drugs.
Aniba rosaeodora Ducke, Lauraceae is a large tree that reaches 30 m in height, with yellow-brown bark (hence the name rosewood) and grows in the Amazon region (Figure 1A) [5]. Rosewood essential oil (Figure 1B) is widely used in the perfumery industry and its extraction for industrial purposes began in the interior of the state of Pará, Brazil, around 1930 [6]. All parts of the tree are fragrant, although only the trunk wood is harvested and hydrodistilled to obtain rosewood oil, a valuable product. The species has been used in the Amazon as a medicinal plant to control epileptic seizures, to compose regional fragrances and as an ornamental plant [7].

Figure 1.
Aniba rosaeodora tree (A) and essential oil (B).
Rosewood oil is a colorless to pale yellow liquid with a woody floral fragrance, containing monoterpenic alcohol linalool as the main constituent. It is of interest to the flavor and fragrance industries because it is transformed into several valuable derivatives [8]. There are few reports of pharmacological properties of Rosewood oil associated with its chemical profile. These properties are the result of the synergism of all the molecules present in the oil or reflect the activity of its major compound linalool, presenting antibacterial and antifungal [9,10], antioxidant [11] and antiprotozoan properties [12].
Available data on the biological activity of rosewood essential oil are quite limited, because most studies have focused on linalool, its main constituent [13]. Thus, this work aims to evaluate the chemical composition of Aniba rosaeodora essential oil extracted in the Amazon biome, as well as its antibacterial, antioxidant and trypanocidal activity.
2. Results
2.1. Physical and Chemical Characterization of A. rosaeodora Essential Oil
The yield of A. rosaeodora essential oil obtained from dried leaves thin branches was 2.8%. The oil showed a yellow color and a clean appearance (Figure 1B), and presented a density of 0.89 g/mL at 25 °C, and a refractive index (ND 25) of 1.459. It was soluble in ethanol 70% in a ratio of 1:2. Chemical compounds identified and quantified in A. rosaeodora essential oil are presented in the chromatogram (Figure 2) and Table 1. Three compounds were identified and enumerated accordingly with elution order and retention time. The major constituent of A. rosaeodora essential oil was linalool with 93.60%. In addition, α-terpinolene and linalool cis-oxide were identified and quantified at 3.37% and 3.03%, respectively.
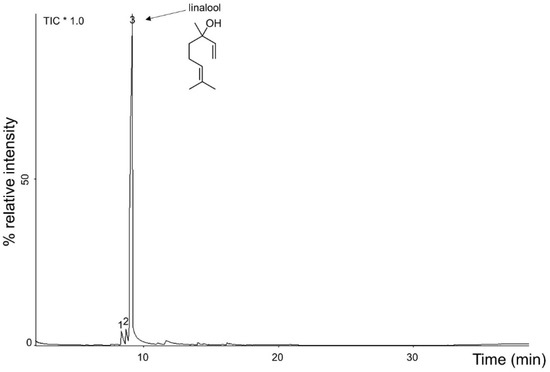
Figure 2.
Chromatogram of Aniba rosaeodora essential oil. * TIC: total ion chromatogram.

Table 1.
Chemical composition of Aniba rosaeodora essential oil.
2.2. Antimicrobial Activity of A. rosaeodora Essential Oil and Linalool
Bacteria from the marine environment were evaluated by the disc-diffusion method against A. rosaeodora, linalool and several reference antibiotics (Table 2). The bacteria culture displayed inhibition halos ranging from 7 to 25 mm, besides the non-inhibition presented in some cultures. Comparing linalool with A. rosaeodora essential oil, we found that A. rosaeodora was more efficient against Aeromonas caviae and Enterococcus faecalis than the standard linalool. Linalool exhibited greater activity against Klebsiella pneumonia and Providencia stuartii than A. rosaeodora essential oil, while both compounds presented the same activity against Aeromonas hydrophila. The susceptibility test performed with antibiotics showed that E. faecalis was the strain that presented sensibility for all the antibiotics analyzed, while the other four strains displayed mixed sensibility to the antibiotics.

Table 2.
Inhibitory zone diameters of Aniba rosaeodora essential oil, linalool and antibiotics on different bacteria isolated from marine environment after 24 h of treatment.
The preliminary antibacterial activity against standard strain bacteria evaluated by the disc-diffusion method showed a growth inhibitory halo on A. rosaeodora essential oil and linalool disks against Gram-positive (Staphylococcus aureus) and Gram-negative strains (Escherichia coli, Pseudomonas aeruginosa and Salmonella sp.) (Table 3). Gram-positive bacteria exhibited the highest inhibition halo from both essential oil and linalool. The minimum inhibitory concentration (MIC) of A. rosaeodora essential oil ranged from 250 to 450 μg/mL against the tested strains. As observed in the disc-diffusion method, S. aureus was the strain more sensible to A. rosaeodora essential oil and linalool activity by MIC analysis. Likewise, both methodologies showed that A. rosaeodora essential oil presented better antimicrobial activity than linalool to all strains analyzed.

Table 3.
Inhibitory zone diameters and minimum inhibitory concentration of Aniba rosaeodora essential oil on different bacterial cultures after 24 h of treatment.
2.3. Antioxidant Activity of A. rosaeodora Essential Oil and Linalol
Aniba roseadora essential oil and linalool presented antioxidant activity concentration-dependent, as observed in the graph that relates A. rosaeodora essential oil and linalool concentration versus the percentage of inhibition of the 2,2-azinobis- (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical (Figure 3). The calculated EC50 was 15.46 µg/mL for A. rosaeodora essential oil and 6.78 µg/mL for linalool.
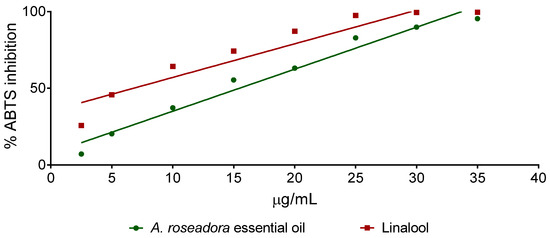
Figure 3.
Inhibition of the 2,2-azinobis- (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical by Aniba rosaeodora essential oil and linalool.
2.4. Cytotoxicity, Antitrypanosomal Activivy and Selectivity Index of A. rosaeodora Essential Oil and Linalool
The activity of A. rosaeodora essential oil and linalool was evaluated against the epimastigote and intracellular amastigote forms of Trypanosoma cruzi, as well as its cytotoxic effect against mammal cells. Both compounds presented concentration-dependent inhibitory activity against epimastigote and intracellular amastigote forms of T. cruzi (Figure 4). The inhibitory concentration for 50% of parasites (IC50) values for epimastigote forms was lower for A. rosaeodora essential oil than linalool. Analyzing the activity against different forms of the T. cruzi, A. rosaeodora essential oil exhibited IC50 value against epimastigote 6.0-fold higher in comparison to the IC50 against intracellular amastigotes forms. In contrast, linalool was 3.65-fold more effective against T. cruzi intracellular amastigote when compared to A. rosaeodora essential oil. Both compounds presented higher IC50 values when compared to benznidazole. Cytotoxicity assay revealed that A. roseadora essential oil and linalool not showed toxicity for BALB/c peritoneal macrophages even at the highest concentration analyzed (1000 μg/mL). Thus, linalool exhibited higher SI value than A. roseadora essential oil (Table 4).
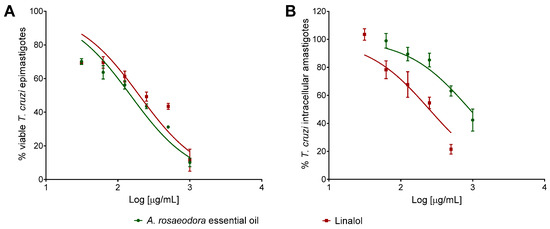
Figure 4.
Activity of Aniba rosaeodora essential oil and linalool against Trypanosoma cruzi epimastigote (A) and intracellular amastigote forms (B) after 24 h of treatment. Data represent media ± standard deviation of three independent experiment realized in triplicate.

Table 4.
BALB/c peritoneal macrophage cytotoxicity, trypanocidal activity and selectivity index of Aniba rosaeodora essential oil and linalool.
The parameters of infection analysis (Figure 5) showed that A. rosaeodora essential oil treatment displayed significant low number of amastigotes per 100 cells at 1000 μg/mL (p = 0.0001) and 500 μg/mL (p = 0.0011) (Figure 5A). Linalool showed a low number of amastigotes per 100 cells at 500 μg/mL (p = 0.0001), 250 μg/mL (p = 0.0014) and 125 μg/mL (p = 0.0290) (Figure 5B). On the other hand, the treatment with A. rosaeodora essential oil and linalool only presented a significant low mean number of amastigotes per infected cells at 1000 μg/mL (p = 0.0398, Figure 5C) and 500 μg/mL (p = 0.0229, Figure 5D), respectively. The alterations in intracellular amastigotes of T. cruzi after treatment with A. rosaeodora essential oil and linalool are represented in photomicrography images of Figure 5E.
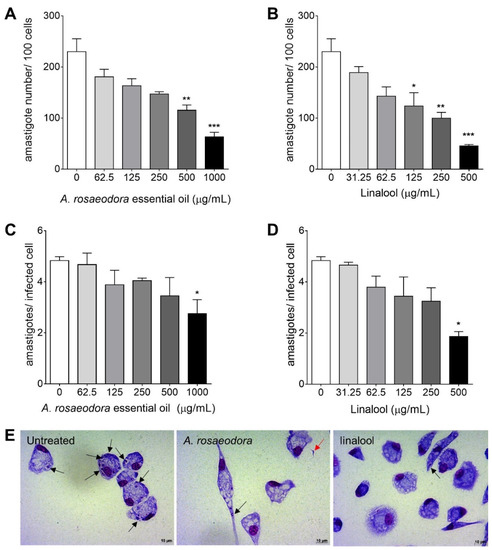
Figure 5.
BALB/c peritoneal macrophages infected with Trypanosoma cruzi and treated for 24 h with Aniba rosaeodora essential oil or linalool. (A–D) Parameters of infection and (E) light microscopy after A. rosaeodora or linalool treatment at 1000 or 500 μg/mL respectively. Intracellular amastigotes inside macrophages (black arrows) and non-internalized parasite (red arrows). The images and data (mean ± standard deviation) represent two independent experiments performed in quadruplicate. * p < 0.05, ** p < 0.01 and *** p < 0.001 when compared with untreated infected cells by Kruskal–Wallis and Dunn’s multiple comparison test. Giemsa, 40× objective.
2.5. Nitrite Quantification in T. Cruzi-Infected Peritoneal Macrophages Treated with A. rosaeodora Essential Oil and Linalool
The nitrite quantification in the supernatant of BALB/c peritoneal macrophages showed low nitrite levels in cells treated with A. rosaeodora essential oil (0.150 ± 0.220 μM NaNO2, p = 0.0259) and linalool (0.175 ± 0.146 μM NaNO2, p = 0.0490) when compared to untreated-unstimulated cells (1.129 ± 0.501 μM NaNO2). In T. cruzi-stimulated cells, although nitrite levels after treatment with A. rosaeodora essential oil (0.952 ± 0.779 μM NaNO2) and linalool (1.047 ± 0.702 μM NaNO2) were lower than stimulated-untreated cells (1.347 ± 0.416 μM NaNO2), the difference was not statistically significant for both compounds (p = 0.945 and p > 0.999, respectively) (Figure 6).
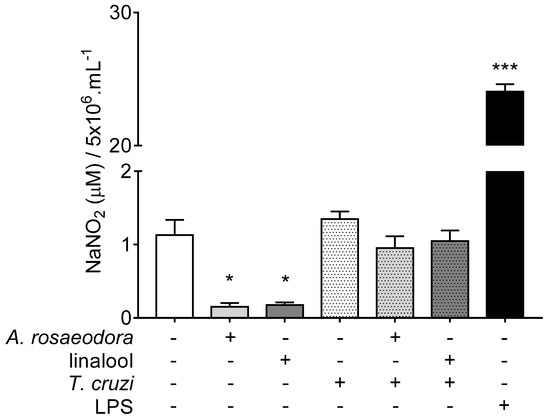
Figure 6.
Nitrite quantification in the supernatant of the BALB/c peritoneal macrophage treated with Aniba rosaeodora essential oil (500 µg/mL) or linalool (125 µg /mL), and stimulated or not with Trypanosoma cruzi. Data represents mean ± standard deviation of experiment realized in sextuplicate; * p < 0.05, *** p < 0.001 when compared with untreated and unstimulated macrophages by Kruskal–Wallis and Dunn’s multiple comparison test.
3. Discussion
Essential oil may change depending on the chemical nature of its constituents and can be modified by air, light, heat, water and various impurities of natural origin or from falsifications. The changes can be recognized both by changes in their organoleptic characteristics (aroma, color, taste, transparency, fluidity), as well as the values of their chemical and physical parameters. Thus the density, refractive index, solubility, color and appearance were analyzed and the physical characteristics of the essential oil were similar to the pattern described in previous studies of A. rosaeodora [14].
Studies has identified and quantified chemical compounds of A. rosaeodora essential oil, revealing that this species has chemotypes similar to essential oil extracted in Belém, state of Pará, Brazil, with linalool (84.8%) as the major compound, followed by α-terpineol (2.9%), geraniol (1.0%), benzyl benzoate (0.6%) and minimal amounts of monoterpene hydrocarbons and oxygenated sesquiterpenes (9.2%) [15]. The same was observed in the study of A. rosaeodora essential oil extracted in São Paulo, Brazil, where the presence of linalool (81.45%), trans-linalool oxide (1.19%), R-terpineol (1.09%) were observed [16]. Almeida et al. (2013) also reported that linalool is the main compound in essential oil obtained from wood, leaves and branches of the Brazilian rosewood [17].
The disk diffusion test carried out against standard strain bacteria and against bacteria isolated from a marine environment evidenced antibacterial activity, preliminarily. The mixed sensibility observed to the several antibiotics revealed the resistance pattern of marine environment bacteria. The sensibility observed to A. rosaeodora essential oil and linalool showed that both compounds have activity against marine environment bacteria used in this study. It is known that the bacterial cell wall influences in an important way on the action of certain antibiotics. The difference between Gram-positive and Gram-negative bacterial walls would be one of the responses to antibiotic resistance between two bacteria [14]. Bacterial resistance is best evidenced in environmental bacteria in the last few years. With the advent of modernization, an increasing amount of antibiotics was released into the environment along with the residues from domestic, industrial, agricultural and medical activities. This has ended up generating a selection of antibiotic-resistant bacteria or genes in the environment, which threatens the efficiency of antibiotics in fighting bacterial infections [18].
The MIC of A. rosaeodora essential oil resulted in concentrations lower than linalool. Holetz, et al. (2002) classifies samples that have MIC values below 100 μg/mL with good antibacterial activity; 100 to 500 μg/mL moderate; and 500 to 1000 μg/mL weak above 1000 μg/mL inactive [19]. Following this classification, A. rosaeodora essential oil showed moderate activity, while linalool displayed weak activity. The difference between the activity of both compounds may be related to the synergistic effect of the compounds present in the essential oil of A. rosaeodora. A synergistic interaction can be verified between the essential oils of A. rosaeodora and Pelargonium graveolens with gentamicin, and a very strong synergistic interaction against Acinetobacter baumannii ATCC 19606 (fractional inhibitory concentration/FIC index = 0.11) [20]. While research conducted with linalool showed low activity against Gram-positive and Gram-negative bacteria. Jabir et al. (2018) found that linalool loaded in gold nanoparticles modified with glutathione (LIN-GNPs) has effective antibacterial activity against Gram-positive bacteria, proving that LIN-GNPs acted on the bacterial cell membrane, in giving up and increasing cell wall permeability and stimulated reactive oxygen species (ROS) production that leads to bacterial nucleic acid damage [21].
Linalool is a compound widely used by the cosmetics industry [22] In the study by Herman et al. (2016) [10], a significant increase in antimicrobial efficacy was observed by the addition of linalool to essential oil, reducing its concentrations in products (cosmetics, medicine), making it possible to obtain its synergistic and additive effects. In addition, several studies have been reported on the commercial availability of oxidized linalool samples possibly causing allergic contact dermatitis [23,24,25]. Thus, essential oil from A. rosaeodora has potential applicability in edible and/or dermatological preparations.
Biological activity may be directly related to phenolic compounds as they are good electron donors and therefore have efficient antioxidant activity among secondary plant metabolites. These compounds are capable of control oxidative damage generated by reactive oxygen species or radicals [26]. We can also classify the antioxidant activity according to the excellent (IC50 < 15 µg/mL), good (15 µg/mL < IC50 < 50 µg/mL), medium (50 µg/mL < IC50 < 100 µg/mL), and weak activity (IC50 ≥ 100 µg/mL). A. rosaeodora essential oil antioxidant activity was considered good while the linalool was optimal, corroborating with previous studies that verified excellent antioxidant activity of Aniba species [26].
In traditional medicine, plant essential oils are known as a rich source of secondary metabolites with relevant biological activities, as an alternative in antiparasitic therapy [27,28]. The trypanocidal activity of essential oils of Aniba genus was described in the literature [14,29]. Currently, the drugs available for the treatment of Chagas disease are benznidazole and nifurtimox, which have limited efficacy, serious adverse effects and have been in use since the late 1960s [30]. Thus, in an attempt to search for new therapeutic alternatives for Chagas disease, we report the effect of A. rosaeodora essential oil and its main component linalool in the growth of epimastigote and intracellular amastigote forms of T. cruzi.
In the present study, A. rosaeodora essential oil showed activity against epimastigote forms. Literature data showed anti-T. cruzi activity in vitro in extracts and substances of different species of the Aniba genus of plants collected in the Amazon [29], with promising antileishmanial activity [14]. To understand whether linalool is responsible for the inhibitory activity, an analysis of linalool against epimastigote was performed. The results showed an inhibitory effect close to the values of A. rosaeodora essential oil. Therefore, it is worth inferring that the inhibitory effect of the essential oil occurs due to the high concentration of linalool, or due to a possible synergistic and/or additive effect of the constituents of the essential oil acting as trypanocidal agents [31].
Previous data demonstrated that the IC50/24 h for linalool was 162.5 μg/mL for epimastigotes and 264 μg/mL for T. cruzi trypomastigotes (Y strain) [32], corroborating with data presented in this study. However, linalool had a potent trypanocidal effect against the trypomastigote form of T. cruzi (clone Dm28c) derived from cells, with IC50/24 h of 306 ng/mL, indicating that different forms and/or origin and different strains may differ in their susceptibility to essential oil derivatives [33].
The search for new therapeutic drugs requires conditions that simulate the environment found by the parasite–cell interaction, therefore, the assay against intracellular amastigote forms of tripanosomatids may represent ideal conditions, with macrophages playing an important role in the evaluation of drug-mediated toxicity [34]. Thus, it was evaluated whether A. rosaeodora essential oil and linalool could inhibit T. cruzi intracellular amastigote. However, the inhibitory effect was observed only when infected cells were treated with linalool although in high concentration, while A. rosaeodora essential oil presented activity at an even higher concentration.
Piper aduncum essential oil (PaEO), with nerolidol (25.22%) and linalool (13.42%) as main constituents, effectively inhibits the intracellular survival/replication of T. cruzi amastigotes. PaEO at a concentration of 12.5 µg/mL decreased the rate of T. cruzi amastigote infection by 71.5%, with an IC50/24 h of 9 µg/mL. As linalool showed trypanocidal activity, with IC50/24 h of 306 ng/mL against trypomastigotes [33], it is possible to infer that activity against intracellular amastigote forms it is possibly due to linalool presence. In addition, previous data demonstrated that at low concentrations of purified linalool derived from the Croton cajucara essential oil, the number of parasites internalized in the macrophages decreased (treated before and after the interaction). On the other hand, no cytotoxic effects of essential oil and linalool were observed in peritoneal macrophages of Swiss mice and Vero cells [35]. As in our study, A. rosaeodora essential oil and linalool not exhibited cytotoxicity against peritoneal macrophages in the concentration range under analysis. As a result, linalool showed a select activity to the parasites when compared to mammalian cells [14].
Literature data with L. infantum chagasi determined that the post-interaction treatment with linalool has antiparasitic activity against intracellular amastigotes, inducing a decrease in the number of parasites within the macrophages [36]. In the same study, it was observed that linalool is capable of providing a drastic change in oxygen consumption, probably related to mitochondrial dysfunction. P. aduncum essential oil rich in linalool induced mitochondria dysfunction altering the mitochondrial membrane potential of the T. cruzi epimastigote [33]. Mitochondrial alterations as swelling and important changes in the organization of nuclear and kinetoplastic chromatins were observed by electron microscopy when L. amazonensis parasites were treated with C. cajucara essential oil [35]. Linalool may interfere with the integrity of protozoan mitochondria, however, further studies are needed to elucidate the mechanism involved in the trypanocidal activity observed in our study.
An indirect mechanism involved with antitrypanosomal activity is related with macrophage activation, particularly the nitric oxide (NO) induction. The NO-mediation directly kills T. cruzi in vitro [37]. Thus, we carried out an analysis of the nitrite quantification of T. cruzi-stimulated peritoneal macrophages treated with A. rosaeodora essential oil or linalool. However, a significant decrease in nitrite levels was observed in cells non-stimulated with T. cruzi and treated with A. rosaeodora essential oil or linalool. Reactive oxygen species decrease was also observed in cancer cells lines treated with A. rosaeodora essential oil, inhibiting apoptosis in these cells [13].
Otherwise, in T. cruzi-stimulated cells the treatment with A. rosaeodora essential oil or linalool did not significantly decrease nitrite levels. Linalool has a known anti-inflammatory activity [38] and inhibits NO formation in vitro [39], but interestingly, an in vitro experiment of macrophages treated with linalool (250 or 350 µg/mL) for 24 h before or after interactions with the Leishmania infantum was also not associated with any difference in NO production [36]. The inhibition of NO production observed in macrophages treated with A. rosaeodora essential oil and linalool, although it is not associated with antitrypanosomal activity, is an interesting finding that should be better elucidated in further studies.
4. Materials and Methods
4.1. Reagents
Anhydrous sodium sulfate, ethanol, ethyl acetate, dimethyl sulfoxide (DMSO), eugenol, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), penicillin, streptomycin, N-benzyl-2-nitro-1H-imidazole-1-acetamide (Benznidazole), Brewer thioglycolate medium, RPMI 1640 medium, 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), sulfanilamide, H3PO4, N-(1-naphthyl)ethylenediamine and sodium nitrite were purchased from Sigma, St Louis, MO, USA. Giemsa’s azur-eosin-methylene blue, Brain Heart Infusion broth, Mueller-Hinton agar and Mueller-Hinton broth were purchased from MERK, Darmstadt, Germany. The other bacteria culture medium were purchased from BD, Becton Dickinson, Franklin Lakes, NJ, USA. API® 20 E system was purchased from bioMérieux, Durham, NC, USA. Fetal bovine serum (FBS) was purchased from Gibco, Gaithersburg, MD, USA.
4.2. Plant Material
Authentic samples of the A. rosaeodora species were obtained from three trees cultivated at the Adolpho Ducke Forest Reserve, Highway AM-010, km 26 (latitude −2.908185, longitude −59.975457), Manaus, Brazil. Leaves and thin branches were harvested with a trimmer from the treetops in the dry season, March 2017. The taxonomic identification was undertaken by the Herbarium of the Department of Botany of the Universidade Federal do Amazonas, registry number 5982. The leaves were selected and dried in an oven at 37 °C for 48 h and sprayed in an electric knife mill at the Food and Water Quality Control Laboratory of the Federal University of Maranhão.
4.3. Essential Oil Extraction
The extraction of the essential oil of A. rosaeodora was carried out with 100 g of dried leaves from thin branches diluted in water in the proportion of 1:10 by hydrodistillation using the Clevenger system for 3 h at 100 °C. The essential oil collected were dried with anhydrous sodium sulfate (Na2SO4) and the final volume found was used to determine the yield through the mass/volume ratio by measuring the density. Mass/volume ratios were calculated from the mass (g) of the initial vegetal material and the volume (mL) of essential oil obtained after extraction. The essential oil samples were kept at 25 °C and then weighed. For the verification of biological activity in vitro, the essential oil and the reference drugs were diluted in DMSO and subsequently made serial dilutions in an appropriate culture medium until reaching a final concentration below 1% DMSO.
4.4. Physical-Chemical Analysis of Essential Oil
Physical-chemical analyzes performed on A. rosaeodora essential oil were: density, measured with a glass pycnometer; refractive index, calculated with an ABBE 2WAJ refractometer (PCE Instruments, Southampton, UK); the color and appearance, that were visually verified by three different people; and the determination of solubility, carried out through the ratio of 1:1 of oil and 70% ethanol until its complete solubilization.
4.5. Gas Chromatography–Mass Spectrometry (GC–MS)
The standard used in the development of the analytical methodology was linalool. Standard solutions of monoterpenes were prepared by dilution in absolute ethyl alcohol and chloroform at different concentrations. The essential oil of A. rosaeodora was solubilized in ethyl acetate and was analyzed by a gas chromatograph Shimadzu QP 5000 (Shimadzu Corp., Kyoto, Japan), a column used with a capillary ZB-5 ms (5% phenyl arylene 95% dimethylpolysiloxane) coupled to 70 eV (40–500 Da) HP 5MS electronic impact detector with a transfer temperature of 280 °C. In the analysis, 0.3 μL of ethyl acetate and helium gas (99.99%) were injected at a temperature of 280 °C, using a split mode (1:10) with an initial temperature gradient of 40 to 300 °C.min−1, with a chromatographic run that lasted 30 min.
4.6. Bacteria from Marine Enviroment
Bacteria strains isolated from the marine environment Aeromonas caviae, Aeromonas hydrophila, Enterococcus faecalis, Klebsiella pneumoniae and Providencia stuartii were gently provided by the Laboratory of Microbiology of the Water Quality Control Program at the Federal University of Maranhão. Water samples were aseptically collected from approximately 30 cm below the water surface of the Jansen lagoon, Maranhão Brazil (latitude −2.499629, longitude −44.301211). Then, the samples were transported to the Microbiology Laboratory of the Federal University of Maranhão in isothermal boxes containing ice to perform the identification. To Aeromonas isolation, successive decimal dilutions of water samples (10−1 to 10−7) were prepared in alkaline peptone water (APA), with subsequent distribution of 1 mL aliquots in five series of five tubes containing tryptic soy broth (TSB Broth) and 0.1 mL in plates containing the selective medium, agar gelatin phosphate salt (GSP Agar) (duplicates), both with 20 µg/mL of ampicillin, an antibiotic used as an inhibitor of the accompanying microbiota of Aeromonas. Colonies suspected of being Aeromonas were seeded in tilted BD trypticase soy agar (TSA agar) tubes, followed by incubation at 28 °C for 24 h. After, the cultures on TSA agar were subjected to biochemical tests of oxidase, catalase, gas production from glucose for species identification, indole production, O/129 resistance, amino acid decarboxylation (test on triple sugar agar and iron—TSI agar), motility: nitrate reduction, esculine hydrolysis, Voges–Proskauer (VP) assay, carbohydrate fermentation and growth at 3% and 6% sodium chloride. To Enterobacteriaceae isolation and identification of Klebsiella pneumoniae and Providencia stuartii in the water samples, initially, 25 mL of each sample were homogenized in 225 mL of brain and heart infusion broth (BHI broth) and incubated in a bacteriological oven at 37 °C for three hours. After the incubation period, the entire inoculum was transferred to 250 mL of broth for Escherichia coli and incubated at 37 °C for 24 h. Isolation was performed using selective and differential media, methylene blue eosin agar (EMB agar) and MacConkey sorbitol agar (MCS agar). For the identification of the species, initially five colonies were selected from the selective culture media, small colonies with metallic green or black without gloss in EMB agar and those of intense pink color (positive sorbitol) and yellow (negative sorbitol) in MCS agar. Then, the colonies were isolated in tubes containing TSA agar inclined with subsequent incubation at 37 °C for 24 h. Biochemical identification was performed using conventional tests: indole, simmons citrate, methyl red, VP, malonate, carbohydrate fermentation—sorbitol, rhamnose, mannitol, arabinose, inositol and raffinose, decarboxylation of amino acids lysine and ornithine, motility and H2S production in sulfide indole motility (SIM) agar [40] and by the API® 20 E system. For Enterococcus research, 9 mL of each sample were diluted in 90 mL of buffered peptide water and incubated for 24 h/35 °C. Subsequently dilutions (10−1 to 10−7) and the highest dilution were plated on M-Enterococcus agar and incubated at 35 °C for 48 h. Brick red colonies were inoculated on TSI agar. Tubes that showed suggestive characteristics were analyzed by acid ramp, acid-base, H2S (-), catalase, oxidase, 6% NaCl, glucose and esculin tests, and by the API® 20 E system.
4.7. Bacterial Strains and Culture Conditions
To perform the preliminary antimicrobial tests, the standard strains Escherichia coli (Migula) Castellani and Chalmers (ATCC® 25922™), Staphylococcus aureus subsp. aureus Rosenbach (ATCC® 12600™), Pseudomonas aeruginosa (Schroeter) Migula (ATCC® 27853™) and Salmonella enterica subsp. enterica (ex Kauffmann and Edwards) Le Minor and Popoff serovar Choleraesuis (ATCC® 12011™) were used. The tests were carried out at the Microbiology Laboratory of the Federal University of Maranhão. The strains were grown in BHI broth for 24 h at 37 °C and the inoculum was adjusted to a cell concentration of 108 colony forming unit (CFU)/mL following the MacFarland scale, recommended by the Clinical and Laboratory Standards Institute [41].
4.8. Antimicrobial Assays
During the preliminary test of diffusion in solid medium, 100 μL of inoculum of each bacterium sown on Mueller–Hinton agar plates were used, and on the agar surface, a paper disc impregnated with 50 μL of essential oil of A. rosaeodora, standard linalool or reference drugs were added; then the plates were incubated at 35 °C and after 24 h the inhibition zone was measured with a millimeter rule [42]. The MIC was also performed according to the broth dilution methodology performed in triplicate with the same bacteria used in the diffusion tests in solid medium [41]. Initially, serial dilutions were performed resulting in concentrations of 5–1000 μg/mL of A. rosaeodora essential oil, linalool or reference drugs and transferred to a test tube containing Mueller-Hinton broth. To each concentration, 100 μL of the microbial suspension containing 1.5 × 108 CFU/mL were added and subsequently incubated at 35 °C for 24 h. It was also reserved control of broth sterility and bacterial growth. After the incubation period, the MIC was determined, being defined as the lowest concentration that visibly inhibited bacterial growth (absence of visible turbidity). To confirm growth inhibition, the broth was subjected to the microbial seeding test of the inoculum on the surface of the plate-count agar.
4.9. Antioxidant Assay
Antioxidant activity was assessed using a reaction mixture of 2,2-azinobis- (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) at 3840 μg/mL with 88 μL of 37,840 μg/mL potassium persulfate solution left in the dark at room temperature for 16 h giving rise to the ABTS radical which was diluted in ethanol to obtain an absorbance of 0.7 to 734 nm. The results were obtained in a dark environment, in which 30 µL of each concentration of essential oil (200 to 15 μg/mL) and eugenol (90 to 5 μg/mL) was transferred in test tubes containing 3.0 mL of the cation radical ABTS and homogenized on a tube shaker, and after 6 min the absorbance of the reaction mixture was read on a spectrophotometer at a length of 734 nm [43]. The analyses were carried out in triplicates and the determination of the activity was demonstrated as percentage of inhibition (% I) of the ABTS radical cation according to the equation: % inhibition = (absorbance of the solution of the radical ABTS—absorbance of the sample)/(solution of ABTS absorbance radical) × 100 [44]. We also verified the efficient concentration or EC50% that represents the concentration necessary to sequester 50% of the ABTS root. The essential oil will be considered active when it has an EC50 < 500 μg/mL [45].
4.10. Parasites
Parasite cultures employed in this study were Trypanosoma cruzi (SC2005 strain). Trypomastigote forms were obtained from Vero cells infected and used to infect the macrophages. Epimatigote forms were originated from the suspension of cell culture trypomastigotes in 3 mL of liver infusion tryptose (LIT) medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin and 100 μg/mL of streptomycin), and incubated in an oven at 28 °C until complete differentiation of parasites.
4.11. Anti-Epimastigote Assay
Epimastigote forms of T. cruzi, from a 2- to 4-day-old culture were incubated for 24 h in the absence or in the presence of different concentrations (1000–15.625 µg/mL) of A. rosaeodora essential oil or linalool, obtained by serial dilutions (1:2), at a final volume of 100 µL per well. The controls were identified as blank (wells without parasites), untreated control (parasites and DMSO 1%) and reference drug (benznidazole). Incubation took place in a 96-wells plate, in a BOD incubator at 28 °C in LIT medium using a parasite concentration of 106 promastigotes/mL. After 24 h, with the aid of the Neubauer chamber and light microscopy [46], viability was evaluated by counting parasites and the results were used to calculate the IC50 (50% inhibition of parasite growth) following the formula: IC50 = (sample counting)/(control counting) ×100 [47].
4.12. Animals and Ethical Statements
BALB/c female mice from 4 to 6 weeks of age were purchased from the Institute of Science and Technology in Biomodels of the Institute of Science and Technology in Biomodels. All procedures were performed in accordance with the National Council for the Control of Animal Experimentation National Council for Animal Experimentation Control—CONCEA) and approved by the Ethics Committee on Animal Care and Utilization (CEUA/IOC—L018/2018).
4.13. Peritoneal Macrophage Collection and Culture
Peritoneal macrophages from BALB/c mice were collected after elicited with 3 mL 3% Brewer thioglycollate medium broth injection for 72 h, and maintained in RPMI 1640 medium supplemented with 10% FBS, 100 U/mL of penicillin and 100 µg/mL of streptomycin, overnight at 37 °C and 5% CO2.
4.14. Cytotoxicity Assay
Peritoneal macrophages (5 × 105 cells/mL) were cultured in 96-well plates with different concentrations, obtained by serial dilutions (1:2), of A. rosaeodora essential oil or linalool (1000–7.8 μg/mL) or benznidazole (200–0.78 μg/mL) up to a final volume of 100 μL per well. The controls were categorized as blanks (wells with culture medium without cells), untreated control (cells and DMSO 1%) and reference drug (benznidazole). After 72 h, the cell viability was analyzed by the MTT colorimetric method [48]. Absorbance was measured in a spectrophotometer at 540 nm wavelength. The concentration inhibiting 50% of cell growth (CC50) was calculated following the formula: CC50 = (sample absorbance-blank absorbance)/(control absorbance-blank absorbance) × 100 [49].
4.15. Activitiy Against Intracellular Amastigotes and Selectivity Index (SI)
BALB/c peritoneal macrophages cultured in 24-well plates (5 × 105 cells/well), with coverslips, were infected with trypomastigote forms of T. cruzi, obtained from cultured Vero cells, using the ratio of parasite/cell 10:1, at 37 °C and 5% CO2 for 6 h. After incubation, well plates were washed with phosphate-buffered saline (PBS, pH 7.2) to remove the non-internalized parasites. The infected cells were treated with different concentrations of A. rosaeodora essential oil or linalool (1000–31.25 µg/mL), or benzonidazole (100–6.25 µg/mL) for 24 h. The amastigotes couting by analysis of light microscopy were carried out to determine the IC50 calculation. Selectivity index were obtained from the relationship of macrophage cytotoxicity and antiamastigote activity. Parameters of infection analysis were performed according to Teles et al. [50].
4.16. Nitrite Quantification
BALB/c peritoneal macrophages (5 × 106 cells/mL) was treated with A. rosaeodora essential oil (500 µg/mL) or linalool (250 µg/mL), and either stimulated or not stimulated with T. cruzi trypomastigotes (5 × 107 parasites/mL) for 48 h. Nitrite quantification of the supernatant of the cells was performed with Griess reagent. Briefly, 50 µL of culture supernatant and 50 µL of Griess reagent (25 µL of sulfanilamide 1% in 2.5% H3PO4 solution and 25 µL of N-(1-naphthyl)-ethylenediamine 0.1% solution) were added in 96-well plates. After incubation in a dark environment for 10 min, absorbance was obtained at 570 nm on the spectrophotometer. The nitrite values were obtained from the standard curve of sodium nitrite (100–1.5 µM) [51].
4.17. Statistical Analysis
The numerical results from at least two independent assays were expressed as mean ± standard deviation and the IC50 and CC50 determination were performed with the GraphPad Prism 7.00 software package (GraphPad Software, San Diego, CA, USA). Kruskal-Wallis and Dunn’s multiple comparison test was used to analyze the data and the difference at p < 0.05 was considered significant.
5. Conclusions
The essential oil of A. rosaeodora showed activity against all the strains tested, with a lower minimum inhibitory concentration being observed for S. aureus. An efficient antioxidant activity of the essential oil was evidenced by the ABTS radical discoloration technique, fully inhibiting the radical in relatively low concentrations. These results point to an important potential for use as an antimicrobial and antioxidant agent. The antitrypanosomal activity of A. rosaeodora essential oil and linalool were observed at high concentrations against epimatigote forms, and even higher against intracellular amastigotes of T. cruzi. Both A. rosaeodora essential oil and linalool reduced nitrite levels in unstimulated cells revealing a potential effect in NO production. These data revealed the pharmacological potential of the A. rosaeodora essential oil and linalool, which encourage further studies.
Author Contributions
Conceptualization, A.M.T., J.V.S.-S., A.N.M. and F.A.-S.; methodology, A.M.T., J.V.S.-S., J.M.P.F. and S.C.M.; formal analysis, A.M.T., J.V.S.-S., V.E.M.F., S.C.M. and F.A.-S.; resources, K.d.S.C., A.L.A.-S., A.N.M., V.E.M.F. and F.A.-S.; data curation, A.M.T., J.V.S.-S., A.N.M., S.C.M., V.E.M.F. and F.A.-S.; writing—original draft preparation, A.M.T., J.V.S.-S. and F.A.-S.; writing—review and editing, A.M.T., J.V.S.-S., K.d.S.C., A.L.A.-S., A.N.M., V.E.M.F., S.C.M. and F.A.-S.; visualization, J.M.P.F., V.E.M.F. and F.A.-S.; supervision, A.N.M., V.E.M.F. and F.A.-S.; project administration, K.d.S.C., A.L.A.-S., A.N.M., V.E.M.F. and F.A.-S.; funding acquisition, K.d.S.C., A.L.A.-S., A.N.M., V.E.M.F. and F.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aper feiçoamento de Pessoal de Nível Superior do Brazil—CAPES) [grant number Finance Code 001]; and the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ) [grant number E-26/010.001759/2019]. The APC was funded by Oswaldo Cruz Institute (Instituto Oswaldo Cruz—IOC). Fernando Almeida-Souza is postdoctoral researcher fellow of CAPES [grant number 88887.363006/2019-00]. Dra. Ana Lucia Abreu-Silva is research productivity fellow of National Scientific and Technological Development Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq) [grant number 309885/2017-5].
Institutional Review Board Statement
The experiments with animals were conducted in accordance with the guidelines for experimental procedures of the Conselho Nacional de Controle de Experimentação Animal (the National Council for the Control of Animal Experimentation) (CONCEA) and approved by the Comissão de Ética no Uso de Animais (the Animal Research Ethics Committee) of the Instituto Oswaldo Cruz (CEUA-IOC/FIOCRUZ), License nº L018/2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets presented in this study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial Resistance and the Alternative Resources with Special Emphasis on Plant-Based Antimicrobials-A Review. Plants 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Kota, B.P.; Razmovski, V.; Roufogalis, B.D. Herbal or natural medicines as modulators of peroxisome proliferator-activated receptors and related nuclear receptors for therapy of metabolic syndrome. Basic Clin. Pharmacol. Toxicol. 2005, 96. [Google Scholar] [CrossRef] [PubMed]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid. Based Complementary Altern. Med. Ecam 2017, 2017. [Google Scholar] [CrossRef]
- Morais, M.C.; Souza, J.V.; da Silva Maia Bezerra Filho, C.; Dolabella, S.S.; Sousa, D.P. Trypanocidal Essential Oils: A Review. Molecules 2020, 25, 4568. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Couto, H.A.R.; Silva, A.C.M.; Marx, F.; Henke, C. Plant sources of amazon rosewood oil. Química Nova 2007, 30, 1906–1910. [Google Scholar] [CrossRef]
- May, P.H.; Barata, L.E.S. Rosewood exploitation in the Brazilian Amazon: Options for sustainable production. Econ. Bot. 2004, 58, 257–265. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Zoghbi, M.G.B.; Andrade, E.H.A. Essential Oils of Aeollanthus suaveolens Matt. ex Spreng. J. Essent. Oil Res. 2003, 15, 86–87. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A. Database of the Amazon aromatic plants and their essential oils. Química Nova 2009, 32, 595–622. [Google Scholar] [CrossRef]
- Pattnaik, S.; Subramanyam, V.R.; Bapaji, M.; Kole, C.R. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 1997, 89, 39. [Google Scholar]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef]
- Seol, G.H.; Kang, P.; Lee, H.S.; Seol, G.H. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurol. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Bero, J.; Kpoviessi, S.; Quetin-Leclercq, J. Anti-Parasitic Activity of Essential Oils and their Active Constituents against Plasmodium, Trypanosoma and Leishmania. Nov. Plant Bioresour. 2014, 455–469. [Google Scholar] [CrossRef]
- Soeur, J.; Marrot, L.; Perez, P.; Iraqui, I.; Kienda, G.; Dardalhon, M.; Meunier, J.R.; Averbeck, D.; Huang, M.E. Selective cytotoxicity of Aniba rosaeodora essential oil towards epidermoid cancer cells through induction of apoptosis. Mutat. Res. 2011, 718. [Google Scholar] [CrossRef]
- Da Silva, Y.C.; Silva, E.M.S.; Fernandes, N.S.; Lopes, N.L.; Orlandi, P.P.; Nakamura, C.V.; Costa, E.V.; da Veiga Junior, V.F. Antimicrobial substances from Amazonian Aniba (Lauraceae) species. Nat. Prod. Res. 2019, 1–4. [Google Scholar] [CrossRef]
- Sampaio Lde, F.; Maia, J.G.; de Parijos, A.M.; de Souza, R.Z.; Barata, L.E. Linalool from rosewood (Aniba rosaeodora Ducke) oil inhibits adenylate cyclase in the retina, contributing to understanding its biological activity. Phytother. Res. 2012, 26, 73–77. [Google Scholar] [CrossRef]
- D’ACAMPORA Zellner, B.; Lo Presti, M.; Barata, L.E.; Dugo, P.; Dugo, G.; Mondello, L. Evaluation of leaf-derived extracts as an environmentally sustainable source of essential oils by using gas chromatography-mass spectrometry and enantioselective gas chromatography-olfactometry. Anal. Chem. 2006, 78. [Google Scholar] [CrossRef]
- Almeida, M.R.; Fidelis, C.H.; Barata, L.E.; Poppi, R.J. Classification of Amazonian rosewood essential oil by Raman spectroscopy and PLS-DA with reliability estimation. Talanta 2013, 117, 305–311. [Google Scholar] [CrossRef]
- Ye, M.; Sun, M.; Huang, D.; Zhang, Z.; Zhang, H.; Zhang, S.; Hu, F.; Jiang, X.; Jiao, W. A review of bacteriophage therapy for pathogenic bacteria inactivation in the soil environment. Environ. Int. 2019, 129. [Google Scholar] [CrossRef]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.; Nakamura, C.V.; Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz. 2002, 97, 1027–1031. [Google Scholar] [CrossRef]
- Rosato, A.; Piarulli, M.; Corbo, F.; Muraglia, M.; Carone, A.; Vitali, M.E.; Vitali, C. In vitro synergistic antibacterial action of certain combinations of gentamicin and essential oils. Curr. Med. Chem. 2010, 17, 3289–3295. [Google Scholar] [CrossRef]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I. Linalool loaded on glutathione-modified gold nanoparticles: A drug delivery system for a successful antimicrobial therapy. Artif. Cells Nanomed Biotechnol. 2018, 46, 345–355. [Google Scholar] [CrossRef]
- Vainstein, A.; Lewinsohn, E.; Pichersky, E.; Weiss, D. Floral fragrance. New inroads into an old commodity. Plant Physiol. 2001, 127, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Christensson, J.B.; Matura, M.; Gruvberger, B.; Bruze, M.; Karlberg, A.T. Linalool—A significant contact sensitizer after air exposure. Contact Dermat. 2010, 62. [Google Scholar] [CrossRef] [PubMed]
- Bråred Christensson, J.; Andersen, K.E.; Bruze, M.; Johansen, J.D.; Garcia-Bravo, B.; Gimenez Arnau, A.; Goh, C.L.; Nixon, R.; White, I.R. Air-oxidized linalool: A frequent cause of fragrance contact allergy. Contact Dermat. 2012, 67. [Google Scholar] [CrossRef] [PubMed]
- Audrain, H.; Kenward, C.; Lovell, C.R.; Green, C.; Ormerod, A.D.; Sansom, J.; Chowdhury, M.M.; Cooper, S.M.; Johnston, G.A.; Wilkinson, M.; et al. Allergy to oxidized limonene and linalool is frequent in the U.K. Br. J. Dermatol. 2014, 171. [Google Scholar] [CrossRef]
- Martins, F.J.; Caneschi, C.A.; Vieira, J.L.; Barbosa, W.; Raposo, N.R. Antioxidant activity and potential photoprotective from amazon native flora extracts. J. Photochem. Photobiol. B Biol. 2016, 161. [Google Scholar] [CrossRef]
- Oliveira de Souza, L.I.; Bezzera-Silva, P.C.; do Amaral Ferraz Navarro, D.M.; da Silva, A.G.; Dos Santos Correia, M.T.; da Silva, M.V.; de Figueiredo, R.C.B.Q. The chemical composition and trypanocidal activity of volatile oils from Brazilian Caatinga plants. Biomed. Pharmacother. 2017, 96. [Google Scholar] [CrossRef]
- Dos Santos Sales, V.; Monteiro, Á.B.; Delmondes, G.A.; do Nascimento, E.P.; Sobreira Dantas Nóbrega de Figuêiredo, F.R.; de Souza Rodrigues, C.K.; Evangelista de Lacerda, J.F.; Fernandes, C.N.; Barbosa, M.O.; Brasil, A.X.; et al. Antiparasitic activity and essential oil chemical analysis of the piper tuberculatum jacq fruit. Iran. J. Pharm. Res. IJPR 2018, 17, 268. [Google Scholar]
- Giongo, J.L.; Vaucher, R.A.; Da Silva, A.S.; Oliveira, C.B.; de Mattos, C.B.; Baldissera, M.D.; Sagrillo, M.R.; Monteiro, S.G.; Custódio, D.L.; Souza de Matos, M.; et al. Trypanocidal activity of the compounds present in Aniba canelilla oil against Trypanosoma evansi and its effects on viability of lymphocytes. Microb. Pathog. 2017, 103. [Google Scholar] [CrossRef]
- Jackson, Y.; Wyssa, B.; Chappuis, F. Tolerance to Nifurtimox and Benznidazole in Adult Patients With Chronic Chagas’ Disease. J. Antimicrob. Chemother. 2020, 75. [Google Scholar] [CrossRef]
- Izumi, E.; Ueda-Nakamura, T.; Veiga, V.F.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. J. Med. Chem. 2012, 55. [Google Scholar] [CrossRef]
- Santoro, G.F.; Cardoso, M.G.; Guimarães, L.G.; Mendonça, L.Z.; Soares, M.J. Trypanosoma cruzi: Activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp. Parasitol. 2007, 116, 283–290. [Google Scholar] [CrossRef]
- Villamizar, L.H.; Cardoso, M.G.; Andrade, J.; Teixeira, M.L.; Soares, M.J. Linalool, a Piper aduncum essential oil component, has selective activity against Trypanosoma cruzi trypomastigote forms at 4 °C. Memórias Do Inst. Oswaldo Cruz. 2017, 112, 131–139. [Google Scholar] [CrossRef]
- Suman Gupta, N. Visceral leishmaniasis: Experimental models for drug discovery. Indian J. Med. Res. 2011, 133, 27–39. [Google Scholar]
- Do Socorro S Rosa, M.d.S.; Mendonça-Filho, R.R.; Bizzo, H.R.; de Almeida Rodrigues, I.; Soares, R.M.; Souto-Padrón, T.; Alviano, C.S.; Lopes, A.H. Antileishmanial activity of a linalool-rich essential oil from Croton cajucara. Antimicrob. Agents Chemother. 2003, 47. [Google Scholar] [CrossRef]
- Dutra, F.L.; Oliveira, M.M.; Santos, R.S.; Silva, W.S.; Alviano, D.S.; Vieira, D.P.; Lopes, A.H. Effects of linalool and eugenol on the survival of Leishmania (L.) infantum chagasi within macrophages. Acta Trop. 2016, 164, 69–76. [Google Scholar] [CrossRef]
- Vespa, G.N.; Cunha, F.Q.; Silva, J.S. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect. Immun. 1994, 62. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.M.; Min, J.H.; Kwon, O.K.; Park, M.H.; Park, J.W.; Ahn, H.I.; Hwang, J.Y.; Oh, S.R.; Lee, J.W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74. [Google Scholar] [CrossRef]
- Peana, A.T.; Marzocco, S.; Popolo, A.; Pinto, A. (-)-Linalool inhibits in vitro NO formation: Probable involvement in the antinociceptive activity of this monoterpene compound. Life Sci. 2006, 78. [Google Scholar] [CrossRef]
- Salfinger, Y.; Tortorello, M.L. Compendium of Methods for the Microbiological Examination of Foods; American Public Health Association (APHA): Washington, DC, USA, 1992. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 4th ed.; CLSI: Wayne, PA, USA, 2009; p. 99. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J. Food Sci. 2011, 76. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.; Webby, R.F.; Markham, K.R.; Mitchell, K.A.; Da Cunha, A.P. Age-induced diminution of free radical scav-enging capacity in bee pollens and the contribution of constituent flavonoids. J. Agric. Food. Chem. 2003, 51. [Google Scholar] [CrossRef] [PubMed]
- Rottini, M.M.; Amaral, A.C.F.; Ferreira, J.L.P.; Oliveira, E.S.C.; Silva, J.R.A.; Taniwaki, N.N.; Dos Santos, A.R.; Almeida-Souza, F.; de Souza, C.S.F.; Calabrese, K.S. Endlicheria bracteolata (Meisn.) essential oil as a weapon against Leishmania amazonensis: In vitro assay. Molecules 2019, 24, 2525. [Google Scholar] [CrossRef]
- Da Silva, V.D.; Almeida-Souza, F.; Teles, A.M.; Neto, P.A.; Mondego-Oliveira, R.; Mendes Filho, N.E.; Taniwaki, N.N.; Abreu-Silva, A.L.; Calabrese, K.d.S.; Mouchrek Filho, V.E. Chemical composition of Ocimum canum Sims. essential oil and the antimicrobial, antiprotozoal and ultrastructural alterations it induces in Leishmania amazonensis promastigotes. Ind. Crop. Prod. 2018, 119, 201–208. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65. [Google Scholar] [CrossRef]
- Oliveira, I.S.S.; Colares, A.V.; Cardoso, F.O.; Tellis, C.J.M.; Chagas, M.S.S.; Behrens, M.D.; Calabrese, K.S.; Almeida-Souza, F.; Abreu-Silva, A.L. Vernonia polysphaera baker: Anti-inflammatory activity in vivo and inhibitory effect in lps-stimulated RAW 264.7 cells. PLoS ONE 2019, 14, e0225275. [Google Scholar] [CrossRef]
- Teles, A.M.; Rosa, T.; Mouchrek, A.N.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Almeida-Souza, F. Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa essential oils: Chemical composition, antimicrobial and antileishmanial activity. Evid Based Complement Alternat. Med. 2019, 2019, 2421695. [Google Scholar] [CrossRef]
- Almeida-Souza, F.; Silva, V.D.D.; Silva, G.X.; Taniwaki, N.N.; Hardoim, D.J.; Buarque, C.D.; Abreu-Silva, A.L.; Calabrese, K.D.S. 1,4-Disubstituted-1,2,3-Triazole compounds induce ultrastructural alterations in leishmania amazonensis promastigote: An in vitro antileishmanial and in silico pharmacokinetic study. Int. J. Mol. Sci. 2020, 21, 6839. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).