Abstract
Designing metal–semiconductor-based core–shell nanostructures with strong interactions is emerging as a unique component for chemiresistor applications. Here, we have developed an effective hydrogen (H2) gas sensor based on Au-In2O3 core–shell nanostructures, which were synthesized through a short-time microwave hydrothermal process. At an optimal temperature of 375 °C, the device based on Au-In2O3 displays a high sensitivity of ~42, which is five times greater than that of the In2O3 toward 100 ppm of H2 gas. Moreover, the Au-In2O3 sensor showed higher selectivity toward H2 gas and stability over a long period. The excellent H2 gas-sensing performance of Au-In2O3 core–shell nanoparticles can be credited to the sensitization and Au catalytic effect core, and their strong interaction with the In2O3 component. Our work not only accounts for a facile synthesis approach for Au-In2O3 core–shell nanoparticles by synergistic properties of Schottky heterojunctions, but also offers a new insight into how strong metal–semiconductor interactions (SMSIs) play a dynamic role in developing high-performance gas-sensing devices.
1. Introduction
Hydrogen (H2) energy has recently been considered a promising alternative to solve environmental problems caused by fossil fuel energies. As an energy source, hydrogen has several benefits, such as it does not produce any pollution byproducts, is easily stored and delivered, prepared from water, and can be used in several areas, including domestic, industrial, and automotive [,]. However, hydrogen gas is highly flammable and explosive if the concentration is higher than 4% in air. In addition, hydrogen is tasteless, odorless, and colorless, hence it cannot be noticed by human senses [,]. Therefore, it is highly essential to develop techniques that make selective and accurate detection of hydrogen gas for safe purposes, especially in production areas, storage, and conveyance, which are all related to the hydrogen economy. For the quick detection of H2 gas, chemiresistors are among the simplest chemical sensor architectures due to their simple operation, low power consumption, portability, excellent miniaturization, quick measurement capability, and cost-effectiveness []. In 1992, the first chemiresistor was designed by Hughes and Schubert for H2 gas detection. After that, several researchers designed various chemiresistors based on metal oxide semiconductors (MOSs), which have possessed good sensing activity, but their higher operating temperature and durability are major drawbacks [,,,,,]. Therefore, the exploration of consistent, stable, and repeatable gas-sensing elements is a persistent endeavor with considerable promise from MOSs.
Indium oxide (In2O3) is an n-type semiconducting metal oxide with a direct bandgap of 3.5–3.7 eV, widely useful in chemiresistor applications because of its high conductivity, surface defects, good catalytic activity, low toxicity, and stability [,,,]. The gas-sensing performance of In2O3 depends on the electrical resistance changes due to the interaction of test gases and the In2O3 surface. Since the interaction of gas molecules mostly occurs at the surface, the gas response of In2O3 is meticulously connected to their morphology, grain size, and surface-to-volume ratios []. But pristine In2O3 nanostructures display poor sensitivity and selectivity, which restricts further practical applications.
To enrich the gas-sensing activity of In2O3 nanomaterials, fabrication of heterostructures with other semiconducting materials is a promising strategy. For example, In2O3-SnO2 [], In2O3-WOx [], In2O3-ZnO [], In2O3-rGO [], In2O3-Co3O4 [], and In2O3-NiO [], etc., were prepared by several researchers, where heterostructures offered high gas response due to the synergic effects at the heterojunctions. In addition, the heterointerfaces could increase charge transduction, work function, and potential barrier modulation at the interfaces and surface properties such as defects and active sites, which are beneficial for sensing devices []. Another important and effective strategy to boost the sensing activity of In2O3 is a surface decoration with noble metal nanoparticles (NPs) such as Au [], Ag [], Pd [], and Pt []. These noble metals improve the sensor activity toward the target gas by promotional effects, namely chemical sensitization and/or electronic sensitization processes. Here, the chemical sensitization occurs via the spill-over effect, which means the noble metal increases the sensor activity by increasing the reaction rate of chemical processes on the In2O3 surface. Next, electronic sensitization arises from the electronic interaction between two components at the interfaces of metal and In2O3 []. However, when the metal NPs are decorated on the In2O3 surface, they will suffer from undesired aggregation at moderate and higher operating temperatures, thereby losing their catalytic activity. In addition, the loaded metal NPs can be oxidized and adulterated by the gas byproducts, leading to the detachment from the In2O3 component [,,]. Hence, to avoid the drawbacks of traditional approaches, metal–semiconductor core–shell heteronanostructures (CSHNSs) have gained more attention due to their exceptional properties in gas-sensing applications. These CSHNSs could offer improved specific surface area, synergistic properties, and stability. In addition, the metal NPs are protected by the semiconductor shell, which can prevent aggregation and delamination during the sensing activity [,,,]. Further, the CSHNSs also increase the electron transfer between the two components and thereby improve the gas-sensing properties. Therefore, it is important to fabricate a high-performance H2 gas-sensing device based on the CSHNSs.
In this regard, we prepared a novel H2 gas-sensing element, Au-In2O3 core–shell heteronanostructures. Different from conventionally tested hydrogen gas-sensing systems, the hybrid of metal Au NPs and semiconductor In2O3 nanostructures is designed as a core–shell system in two steps. Primarily, Au metal NPs were obtained by a citrate reduction method, and then an In2O3 shell was grown on the surface of Au NPs via a microwave hydrothermal process and followed by the calcination process. The suggested approach has many exceptional properties. First, Au NPs can be protected against aggregation due to the creation of a thick In2O3 shell on the surface of Au metal. Second, among precious metals, Au is promising for improving the gas-sensing properties since it exhibits excellent catalytic activity for oxidizing gases. Besides this, when Au is in contact with In2O3, a Schottky junction could be created, and an efficient electron transfer takes place. Third, the small-sized Au metallic NPs offer ideal adsorption sites for the test gas, from which activated species are spilled over to the In2O3 surface to react with the adsorbed oxygen ions. Lastly, the proposed microwave hydrothermal approach enables the Au-In2O3 CSHNSs with strong metal–semiconductor interactions, which not only avoids the aggregation of Au NPs, but also improves the electron transfer between Au and In2O3 components. With all these benefits, the Au-In2O3 CSHNSs show an excellent H2 gas-sensing response of 42, with good response and recovery times of 10 sec and 7 min, respectively, and also a high stability. At the end of this article, the reason for the improved H2 gas response of the Au-In2O3 sensor device compared to bare In2O3 and hydrothermally prepared Au-In2O3 CSHNSs is also discussed.
2. Materials and Methods
2.1. Chemicals and Reagents
All the chemicals used in the present work are of analytical grade. Indium tri-chloride (InCl3, 99.99%, Aldrich, Burlington, MA, USA), trisodium citrate dihydrate (C6H5Na3O7·2H2O, 98%, Showa Chemicals, Tokyo, Japan), HAuCl4·4H2O, 99%, Showa Chemicals, Tokyo, Japan, and urea (NH2CONH2, 98% Aldrich) were procured for the synthesis procedures. For all solution preparations, double-distilled water from a Milli-Q water purification system was utilized.
2.2. Preparation of Au-In2O3 Core–Shell HNSs
Au metal NPs with a 15 nm size were synthesized by using the trisodium citrate reduction method [], and the detailed synthesis procedure is provided in the supporting information. To make Au-In2O3 Core–Shell samples, take 5 mL of prepared Au NPs in a separate vial and keep on stirring for 5 min. A solution containing 2.4 mM trisodium citrate and 2.0 mM InCl3 was gently added and kept on stirring for 0.5 hr. After stirring, 2 mM of urea was added and stirred for another 0.5 hr. Finally, the mixed solution was transferred to a 100 mL Teflon liner and heated in the microwave oven (CEM, MARS 6) at 180 °C for one hour. The ramping time to reach 180 °C from 20 °C is 10 min. After completing the reaction, the product was washed with distilled water five times and then dried at 60 °C overnight and finally calcined at 400 °C for two hours (ramping rate of 2 degrees per minute) in an air atmosphere.
The physical characterization of samples and the gas sensor device fabrication details are provided in the supporting information file.
3. Results
3.1. Formation Mechanism of Au-In2O3 CSHNSs
The fabrication of Au-In2O3 CSHNSs was attained by a two-step method in which metal Au NPs were first prepared by a sodium citrate reduction method, and then the microwave hydrothermal process was carried out to produce the In(OH)3 shell, resulting in the Au@In(OH)3 core-shell nanoparticles (CSNPs). Next, the calcination of Au@In(OH)3 results in the formation of Au-In2O3. The detailed approach for the synthesis of Au@In2O3 core–shell HNSs is shown in Scheme 1. Citrate-stabilized Au NPs (~15 nm) were synthesized through the reduction of chloroauric acid by trisodium citrate at a temperature of ~95 °C for 15 min. The formation of the In(OH)3 shell is accomplished by a microwave hydrothermal reaction (at 180 °C) with the precursor solution. When Indium chloride is dissolved in the deionized water, it produces In3+ ions, and these are easily attached to citrate ions. During the microwave hydrothermal reaction, urea worked as a pH buffer by gradually releasing the OH− ions through the thermal decomposition, and then reacted with pre-formed In3+ ions to form In(OH)3 nanoparticles. In(OH)3 nanoparticles were slowly formed and stabilized in the solution due to the adsorption of citrate ions on the positively charged In3+ ions. This reaction simultaneously produces a number of In(OH)3 nanoparticles; these nanoparticles specially aggregated and self-assembled onto the Au NPs surface for the minimization of the total energy of the system [,,]. After calcination at 400 °C, the formed Au@In(OH)3 core–shell HNSs will be transformed to the Au-In2O3 core–shell HNSs without changing the shape of core–shell particles.
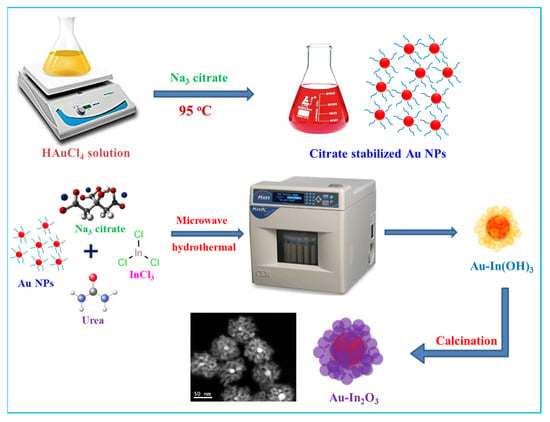
Scheme 1.
Detailed synthesis protocol for the preparation of Au NPs and Au-In2O3 core–shell NPs.
3.2. Morphological and Structural Studies
The surface morphology of Au-In2O3 CSHNSs was studied by taking FE-SEM images. As shown in Figure 1a,b, the synthesized Au-In2O3 possessed spherical-shaped nanostructures in which In2O3 NPs were aggregated onto the surface of Au NPs. The diameter of the as-prepared CSNPs was around 100 nm. Additionally, the energy dispersive X-ray spectroscopy (EDS) and mapping profiles were recorded to detect the elemental distribution of Au-In2O3 CSNPs, and the results are shown in Figure 1c and Figure S1, respectively. These EDS and mapping profiles revealed that the Au, In, and O elements co-existed in the prepared sample, and no other impurity peaks were detected. Further, the morphologies of the as-synthesized CSHNSs were also examined by TEM images. Figure 2a shows the TEM image of Au NPs having a diameter of below 15 nm.
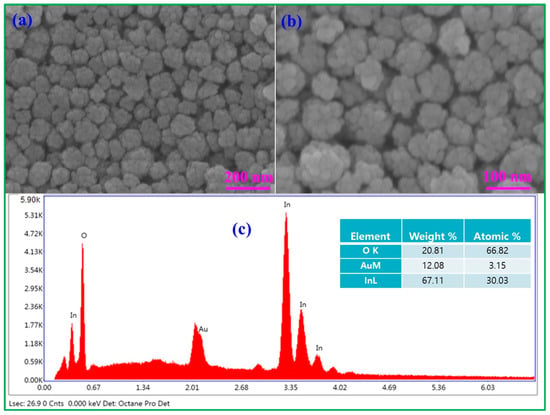
Figure 1.
FE-SEM images (a,b) and EDAX profile (c) of Au@In2O3 CSHNSs.
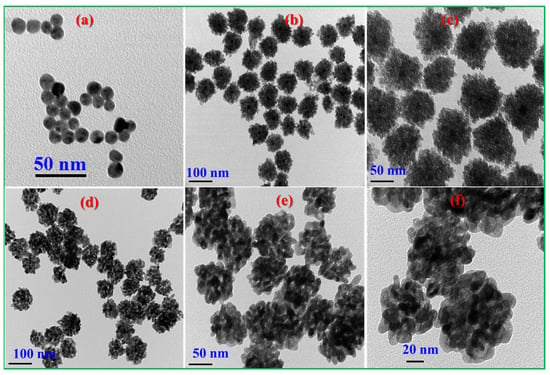
Figure 2.
TEM images of (a) citrate-stabilized Au NPs, (b,c) Au@In(OH)3, and (d–f) Au@In2O3 core–shell NPs.
In Figure 2b,c, the morphology of Au@In(OH)3 nanostructures was characterized by core–shell structures, in which Au NPs were observed as the core and an In(OH)3 shell was grown on the surface of Au NPs. After calcination, the Au@In(OH)3 CSNPs were converted into Au@In2O3 CSNPs, which are displayed in Figure 2d–f. A close observation of Figure 2f suggests that the shell comprises numerous In2O3 nanoparticles, and the size of the final Au@In2O3 CSNP was around 100 nm.
To distinguish the Au core and In2O3 shell, the STEM-HAADF image is presented in Figure 3a. As seen from Figure 3a, the bright metal cores were Au NPs, whereas the surrounding shell was In2O3. Figure 3b shows the SAED patterns of Au@In2O3 CSNPs, in which the bright circular fringes are characteristic crystalline planes of In2O3. The heterojunctions between Au and In2O3 components were studied by HR-TEM image (Figure 3c), in which the lattice spacings for Au (111) and In2O3 (104) planes were determined as 0.23 and 0.27 nm, respectively. Next, Figure 3d–g present the STEM-EDS line scanning profiles, and these results confirmed the formation of Au@In2O3 CSNPs. In addition, STEM-EDS elemental mapping profiles (Figure 3h–k) also revealed that the core is made of Au NPs and the shell is made of In2O3 NPs. All these studies validate the formation of Au@In2O3 CSHNSs.
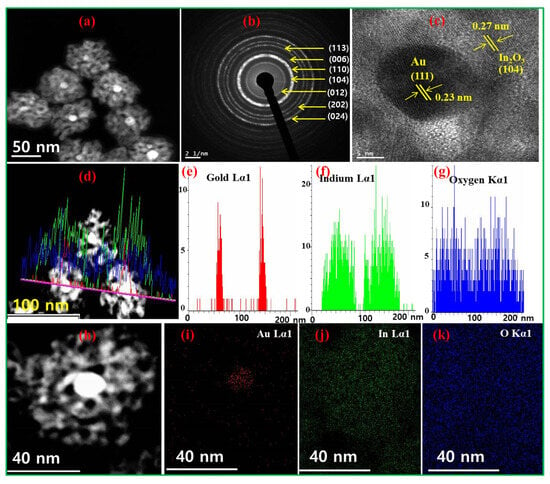
Figure 3.
(a) STEM-HAADF, (b) SAED, (c) HR-TEM image of Au@In2O3 CSNP, which depicts the lattice spacings of both Au and In2O3 components, (d–g) line scanning profiles, and (h–k) STEM-EDS elemental mapping details of Au@In2O3 CSNPs.
Figure 4a shows the powder XRD patterns of as-synthesized Au-In2O3 CSHNSs. From Figure 4a, it is observed that Au metal NPs exhibited diffraction peaks at 38.22°, 44.43°, 64.58°, and 77.55°, corresponding to (111), (200), (220), and (311), respectively, of diffraction planes of Au with a cubic structure (JCPDS No: 00-004-0784). Meanwhile, strong diffraction peaks located at 22.32°, 30.96°, 32.59°, 37.14°, 39.95°, 45.64°, 50.24°, 54.12°, 57.20°, 58.21°, 67.43°, 68.32° and 71.02° corresponded to the planes of (012), (104), (110), (006), (202), (024), (116), (018), (214), (300), (1010), (220), (306) and (128), respectively, of In2O3 with the Rhombohedral structure (JCPDS No: 00-021-0406). No other peaks were identified in the diffraction patterns of Au-In2O3, suggesting the purity of the prepared sample.
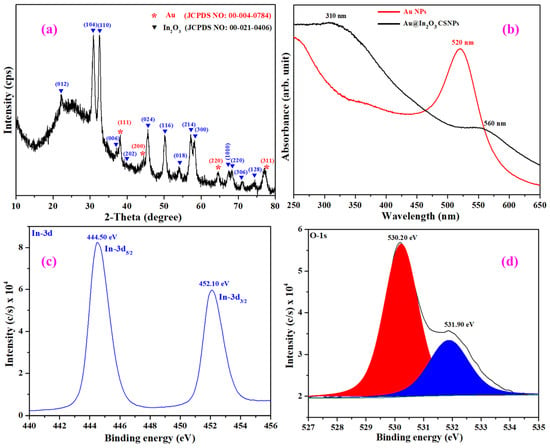
Figure 4.
Powder X-ray diffraction patterns (a), UV-vis absorbance spectra of Au NPs and Au@In2O3 CSNPs (b); high-resolution XPS spectrum of (c) In-3d, and (d) O-1s core-levels.
The formation of Au-In2O3 CSHNSs was further established by studying the UV-vis absorption spectra, as shown in Figure 4b. As seen from Figure 4b, the Au NPs exhibited a strong absorption peak at 520 nm, which is according to the surface plasmon resonance (SPR) peak of Au metal NPs. Figure 4b also shows the absorption spectrum of Au-In2O3 CSHNSs, which displayed two absorption bands around 310 nm and 560 nm; the first absorption band is attributed to the intrinsic absorption band of In2O3. The other absorption band around 560 nm arises from the SPR feature of the Au NP in the core–shell nanoparticles. After making the core–shell nanoparticles, it should be noted that the SPR peak is redshifted (~40 nm) due to the increase in the refractive index of the surrounding medium, and a similar phenomenon was observed in several core–shell nanoparticles [,,].
Further, X-ray photoelectron spectroscopy analysis verifies the chemical states of constituent elements in the prepared core–shell nanostructures. The XPS survey spectrum (Figure S2) confirms the presence of In and O elements; however, the peaks related to the Au element were not well resolved, which might be due to the In2O3 shielding effect, and the same behavior was also supported by the reduced SPR peak intensity in Figure 4b. The relative atomic% of the In, O, and Au elements are calculated as 33.85, 66.01, and 0.14%, respectively. The high-resolution XPS of In-3d and O-1s core level spectra are provided in Figure 4. The binding energy values for the In-3d region (Figure 4c) were observed at 444.50 and 452.10 eV, which were assigned to In-3d5/2 and In-3d3/2, respectively. It is also observed that the In-3d region is characterized by well-separated spin–orbit components (Δ = 7.6 eV), which confirms that the oxidation state of the indium element in the prepared core–shell nanostructures is +3 [,]. Next, Figure 4d displays the high-resolution XPS spectrum of O-1s, which is deconvoluted into three peaks at 530.0, 530.50, and 531.90 eV, and are noted as OL (lattice oxygen), OV (oxygen vacancies), and OC (chemisorbed oxygen), respectively. It should be noted that the Au 4f XPS signal was not detected for the Au- In2O3 core–shell nanoparticles. This absence might be attributed to the thickness of the In2O3 shell or lower content of the Au core, which signals fall below the detection limit of the instrument.
3.3. Hydrogen Gas Sensing Properties of Microwave Hydrothermal Synthesized Au-In2O3 Core–Shell NPs
To explore the advantages of Au-In2O3, we implemented the gas-sensing tests toward acetaldehyde, hydrogen, ethanol, nitrogen dioxide and carbon monoxide gases. The operating temperature is a substantial factor for metal oxide-based gas-sensing devices because it intensely affects the reaction to a testing gas. Therefore, the effect of temperature on the gas-sensing response of the Au-In2O3 device was tested at 100 ppm of H2 gas concentration, and the temperature was optimized. Figure 5a presents the corresponding curve of relative responses of the Au@In2O3 gas-sensing device at a temperature range of 50–400 °C. As shown in Figure 5a, as the temperature increases, the response also increases, and the highest response is achieved at 375 °C; hence, we chose 375 °C as the operating temperature for the successive gas-sensing measurements. The low responses below 375 °C are due to the lower surface activity, but as the temperature increases beyond a certain value, the response will diminish due to the accelerated desorption of the primarily adsorbed oxygen [,,,]. Figure 5a also reveals that the sensor device exhibited a response of ~42 at a temperature of 375 °C toward 100 ppm of H2 gas. Figure 5b shows the responses of the Au@In2O3 gas-sensing device at various temperatures ranging from 50 to 400 °C towards 1–100 ppm of H2 gas concentrations. It can be observed that the responses increase with the H2 gas concentration at all temperatures. Among them, the sensor device exhibited higher responses at 375 °C toward all gas concentrations. The responses of the Au-In2O3 gas sensor are 42.1, 28.0, 20.5, 7.1, 4.4, 2.4, 1.5, and 1.2 to 100, 80, 50, 20, 10, 5, 2, and 1 ppm, respectively, of H2 gas.
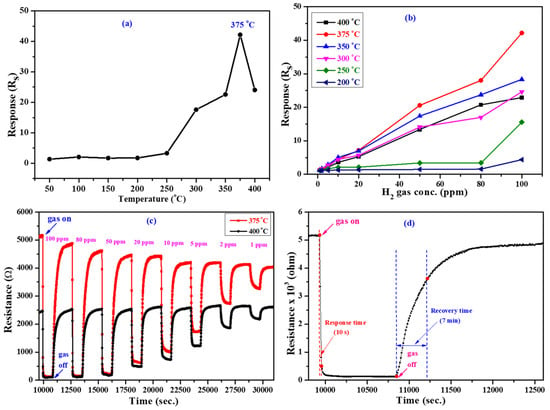
Figure 5.
(a) H2 gas-sensing performance of Au@In2O3 gas sensor device (a) at different temperatures toward 100 ppm, (b) response at various temperatures toward 1–100 ppm H2 gas concentrations, (c) dynamic H2 gas-sensing response curves at 375 and 400 °C, and (d) response and recovery times.
Therefore, the sensor device exhibits a good performance in terms of higher response at an operating temperature of 375 °C; hence, other gas-sensing measurements are mainly focused on the temperature mentioned above. From Figure 5b, it is also concluded that the Au-In2O3 core–shell NPs sensor also responded to lower temperatures, typically at 50 °C. Figure 5c shows the dynamic response curves of the as-prepared Au@In2O3 gas-sensing device for different concentrations (100–1 ppm) of H2 gas at 375 °C and 400 °C. When the sensor device is exposed to air, the resistance increases sharply and instantly. After the sensor contacts the H2 gas, the resistance decreases, and this specifies that the sensor exhibits n-type gas-sensing behavior. As seen from Figure 5c, the sensor device tested at 375 °C displayed a higher baseline resistance when compared to that at 400 °C, confirming that the device showed higher responses at 375 °C at all concentrations of H2 gas. It can be seen that the sensor displays a very fast response and recovery speed with gas on and off states. The sensor response also increases with increasing H2 gas concentration, demonstrating the outstanding detection ability for different gas concentrations. Next, Figure 5d presents the response and recovery times of the Au@In2O3 gas sensor. As mentioned in Figure 5d, the response and recovery times to 100 ppm H2 gas at 375 °C were determined as 10 s and 7 min, respectively.
Of particular importance, the designed Au@In2O3 gas sensor device also displayed a significant, rapid response to low temperatures in the range of 50–150 degrees. During these measurements, with the increase of H2 gas concentration from 1 to 100 ppm, the sensor response also slightly increased from 1 to 1.37 at 50 °C and 1–2 at 100, and 150 °C. This response at lower temperatures is attributed to the formation of heterointerfaces between Au and In2O3 components. The continuous dynamic response and recovery curves of the H2 gas sensor based on Au@In2O3 were tested at 50, 100, and 150 °C (Figure 6a) upon exposure to hydrogen vapor at different concentrations from 1 to 100 ppm. As the hydrogen gas with a higher concentration was introduced into the testing chamber, the response of the sensor rapidly increased. As the gas supply is switched off, the responses almost recover to their initial value. In addition, the comprehensive responses of the Au@In2O3 sensor to H2 gas of different concentrations are summarized in Figure 6b.
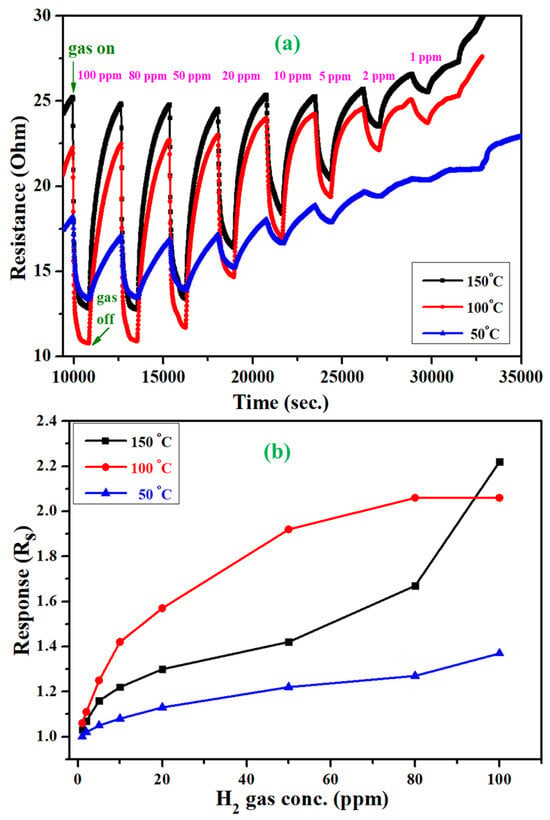
Figure 6.
Dynamic gas-sensing response curves (a,b) response values of Au/In2O3 core–shell NPs tested at temeperatures of 150, 100 and 50 °C.
Repeatability and stability are two significant parameters in evaluating the practical application of gas sensors in harmful and explosive gas environments. Therefore, the Au-In2O3 sensor device was tested at 100 ppm of H2 gas for nine successive cycles. As shown in Figure 7a, the sensor device shows response and recovery curves at 375 °C toward 100 ppm H2 gas. There were no significant changes in the sensing performance during the continuous cycles of the adsorption–desorption of H2 gas, confirming the excellent stability and repeatability of the Au-In2O3 sensor device. Further, the long-term stability of the Au/In2O3 sensor device was assessed by measuring the gas response separately after one week, one month, and three months in a dry atmosphere. As displayed in Figure 7b, the response sustained the initial value, suggesting that the Au-In2O3 core–shell NPs have sufficient stability, which extends the applications of Au-In2O3 core–shell NPs in industries. Table S1 summarizes the previous studies focused on metal–metal oxide core–shell nanostructured layers for sensing applications. Evidently, Au-In2O3 exhibits a superior and comparable H2 sensing performance to that of various core–shell structures.
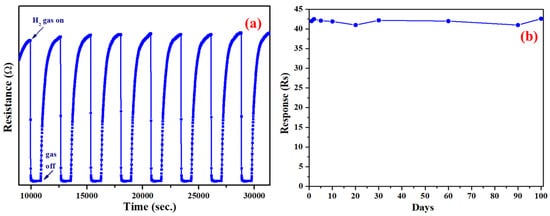
Figure 7.
(a) Short-term and (b) long-term stability tests of Au-In2O3 CSHNSs toward 100 ppm of H2 gas.
3.4. H2 Gas-Sensing Mechanism over Au-In2O3 Core–Shell NP Surfaces
The sensing mechanism on the surface of metal oxides can be defined as the redox reaction between the surface-adsorbed oxygen molecules and exposed gas molecules, resulting in a change in the concentration of charge carriers. Due to the higher electron affinity of oxygen molecules, the oxygen molecule will capture electrons from the conduction band of indium oxide and form different oxygen species (O2−, O−, and O2−). The activated H2 molecules react with the oxygen species that are adsorbed on metal oxides, producing H2O, and releasing the electrons. In an air atmosphere, the oxygen molecules adsorb on the surface of the In2O3 sensor film and capture electrons, forming the oxygen ionic species O2−, O−, and O2−. The relevant reactions are noted as
O2 (gas) ↔ O2 (ads)
O2 (ads) + e− → O2−
O2 (ads) + 2e− → 2O−
O2 (ads) + 4e− → 2O2−
Hence, an electron depletion layer is generated on the In2O3 surface, and its electrical resistance increases. When the In2O3 sensor device is exposed to H2 gas, the chemisorbed oxygen species interact with H2 gas to generate water and release free electrons. These free electrons then flow back to In2O3; as a result, the electrical resistance of In2O3 decreases. These reactions are written as follows:
H2 (gas) ↔ H2 (ads)
H2 (ads) + O− (ads) → H2O(gas) + e−
H2 (ads) +O2− → H2O(gas) + 2e−
Based on the experimental results and discussions put together, overall, the improved gas-sensing response of Au-In2O3 core–shell nanostructures is credited to the following aspects. The Au NPs play a significant role in improving the response and selectivity of the Au-In2O3 core–shell HNSs. Due to the good catalytic nature, the electron-rich Au could enrich the process of catalytic oxidation and chemical sensitization by changing the surface energy via the spill-over effect []. As a result, the concentration of active oxygen species on the surface of the Au-In2O3 sensor device. Hence, the overall rate of adsorption–desorption of oxygen molecules on the sensors’ surface is improved. The electrons in the conduction band of In2O3 convert oxygen molecules into oxygen ions, which are exposed to the target gas (H2), release the electrons back to the Au-In2O3 sensor film via redox reaction, and improve the conductivity of the sensor. To start with the electronic sensitization effects, the formation of the Schottky junction between the Au metal and In2O3 components results in the transfer of electrons from In2O3 to Au metal, which acts as an electron sink []. Secondly, these metal Au NPs are good catalysts for oxygen dissociation and have high accessibility for catalyzing molecular oxygen dissociation []. The dissociated oxygen molecules could be absorbed on Au NP in the air to form the chemisorbed oxygen ions. Meanwhile, the chemisorbed oxygen ions produced on the Au could easily overflow to the In2O3 surface, resulting in more chemisorbed oxygen ions on the core–shell nanostructure surface. The detailed gas-sensing mechanism is presented in Figure 8.
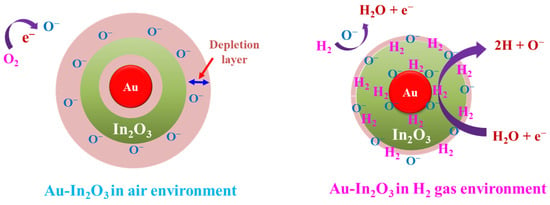
Figure 8.
Behavior of depletion layer and gas-sensing mechanism of Au-In2O3 core–shell NPs in air and H2 gas exposures.
3.5. Comparison of H2 Gas Sensing Properties of In2O3 and Au-In2O3 Core–Shell Nanostructures Synthesized via Hydrothermal and Microwave Methods
To understand the advantages of the microwave synthesis method and core–shell heteronanostructures, here we have attempted to compare the gas responses of other sensor devices based on bare In2O3 and Au-In2O3 core–shell samples, which were synthesized via the hydrothermal method []. For convenience, all the prepared samples are denoted as IO-H, AIO-H, IO-MH, and AIO-MH, where H refers to hydrothermal and MH refers to microwave hydrothermal methods. Figure 9 shows the gas-sensing performances and their selectivities of different sensor devices. It can be seen that gas-sensing performance was greatly improved between bare In2O3 and Au-In2O3 core–shell nanostructures, further Au metal NP significantly changes the selectivity of gases that were exposed, which makes the fabrication of core-shell heterostrucres is an attractive toward their implementation into gas sensing elements for industrial applications.
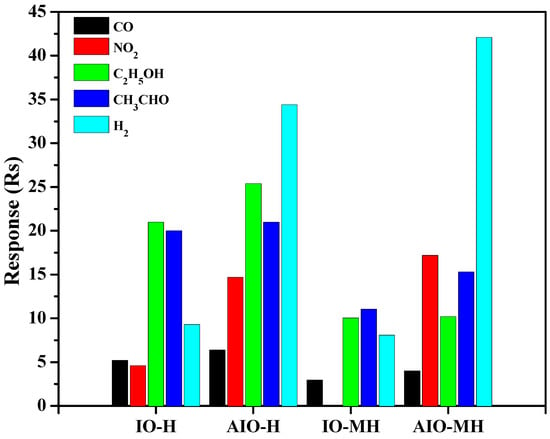
Figure 9.
The investigation of responses and selectivities of the IO and AIO gas sensor devices prepared via hydrothermal and microwave hydrothermal methods. All sensing measurements were performed at 375 °C toward 100 ppm of test gases.
According to Figure 9, the sensor device based on the AIO-MH showed the highest gas response of 42.1, which is 4.5, 5.2, and 1.2 times greater than that of the IO-H, IO-MH, and AIO-H sensor devices toward 100 ppm of H2 gas. Selectivity is an essential factor in sensor devices and defines whether the device can work efficiently in a complex environment with multicomponent gases. Therefore, the sensor device was tested to 100 ppm of several gases such as acetaldehyde, ethanol, carbon monoxide, and nitrogen dioxide for selectivity performance. The selectivity toward H2 gas was achieved via the core–shell morphology of Au-In2O3 nanostructures. As given in Figure 9, all these gases show lower responses, suggesting that our device has great potential in discriminating against the H2 gas. Hence, the obtained results suggest that Au/In2O3 is an effective and stable active component for H2 gas sensors. Further, AIO-MH displayed an excellent and greater H2 gas-sensing response over the AIO-H, and the significant parameters that affected the gas response of AIO-MH over AIO-H are outlined as follows:
Firstly, the metal–semiconductor interactions were extensively studied to understand the charge transfer abilities between the two components. For this, the XPS characterization has been undertaken to examine the changes in the electron density on the surfaces of the Au metal core and In2O3 shell by investigating the shift in the binding energy values. If the electron transfers from one component’s surface to another, it can cause a change in its binding energy. Specifically, a decrease in the electron density results in a positive shift in binding energy, whereas a negative shift in binding energy values specifies an increase in the electron density []. According to Figure S3, the XPS of In-3d core level in AIO-MH sample displayed a negative shift in binding energy values when compared to IO-MH and AIO-H samples, depicting the electron-rich In2O3 surface, which in turn is accessible to the H2 sensing reactions. As a result, the AIO-MH sample exhibited a greater response toward H2 gas.
Next, oxygen vacancies and adsorbed oxygen species on metal oxide surfaces play important roles in gas-sensing applications [,]. Further oxygen vacancies are confirmed by X-ray photoelectron spectra, and could behave as additional electron donors, resulting in the enhanced H2 gas-sensing activity of AIO-MH samples. The high-resolution deconvoluted O-1s XPS spectra confirm the presence of a higher number of O-vacancies (Ov) on the surface of AIO-MH (35.35%). In contrast, AIO-H core–shell nanostructures possess only 20.2% (Figure S4). In addition, the XPS spectra of O-1s reveal that the total concentration of O-vacancies and chemisorbed oxygen (Ov + Oc) is around 70.06% in AIO-MH, whereas AIO-H only accounts for 65.15%, indicating that the higher number of O-species displayed, the greater response in the AIO-MH sensor device. Finally, oxygen vacancies are termed as active sites for oxygen gas molecule adsorption, and these O-vacancies expedite the creation of numerous chemisorbed oxygen species on the indium oxide surface []. The reaction between O2- and H occurs effectively on the In2O3 surface, and as a result, a higher response was observed in AIO-MH core–shell nanostructures [,]. Additionally, the AIO-MH sensor device responds even at low temperature regions at 50–150 °C, signifying the importance of microwave-synthesized Au-In2O3 core–shell nanostructures for domestic and industrial applications. This low-temperature sensing activity is mainly due to the chemisorbed O2- species present on the surface of In2O3. When a sensor device is exposed to H2 gas, the gas molecules react with O2− species, releasing electrons to In2O3; consequently, the depletion layer is reduced, and the resistance decreases (O2− + H2 → H2O + 2e). All these results indicate that the promotional (electronic and chemical sensitization) effects of Au NP and Schottky junction with In2O3 semiconductor; O-vacancies and strong interactions between Au and In2O3 enabled the efficient electron transportation and favored achieving the efficient H2 gas-sensing elements.
4. Conclusions
In this study, metal–semiconductor-based core–shell heterostructures composed of Au-In2O3 were synthesized to achieve selectivity and enhance the sensitivity toward H2 gas. The results show that the sample Au-In2O3 obtained from microwave hydrothermal reaction displayed the highest H2 gas response of ~42, which is 4.5, 5.2, and 1.2 times greater when compared to the bare In2O3, the hydrothermally prepared In2O3, and Au-In2O3-based sensors. The synergetic effect of the strong metal–semiconductor interactions between Au and In2O3, and oxygen vacancies led to significantly enhanced H2 gas sensitivity, and even enabled the sensor to work at a lower temperature of 50 °C. Thus, microwave hydrothermal synthesized Au-In2O3 core–shell nanostructures can be widely applied as efficient sensing layers in the H2 monitoring industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15231786/s1. This file contains the synthesis of Au NPs, physical characterization of samples, gas-sensing device fabrication details, FE-SEM EDAX mapping profiles, and XPS spectra of In-3d and O-1s elements. Refs. [,,,,,,,,,,] are cited in the supplementary material file.
Author Contributions
Conceptualization, methodology, investigation, formal analysis, data curation, writing—original draft preparation, R.K.C.; investigation, validation, writing—review and editing. R.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting the findings of this study have been included as part of the supporting information and are also available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wadell, C.; Syrenova, S.; Langhammer, C. Plasmonic Hydrogen Sensing with Nanostructured Metal Hydrides. ACS Nano 2014, 8, 11925–11940. [Google Scholar] [CrossRef]
- Gao, Z.M.; Wang, T.Q.; Li, X.F.; Li, Q.A.; Zhang, X.M.; Cao, T.L.; Li, Y.N.; Zhang, L.Y.; Guo, L.; Fu, Y. Pd-Decorated PdO Hollow Shells: A H2-Sensing System in Which Catalyst Nanoparticle and Semiconductor Support are Interconvertible. ACS Appl. Mater. Interfaces 2020, 12, 42971–42981. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Chen, H.-I.; Chou, P.-C.; Liou, J.-K.; Chen, C.-C.; Chang, C.-F.; Liu, W.-C. Hydrogen-Sensing Properties of a Pd/AlGaN/GaN-Based Field-Effect Transistor under a Nitrogen Ambience. IEEE Sens. J. 2013, 13, 1787–1793. [Google Scholar] [CrossRef]
- Hubert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen Sensors-A Review. Sens. Actuators B 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Jha, R.K.; Bhat, N. Recent Progress in Chemiresistive Gas Sensing Technology Based on Molybdenum and Tungsten Chalcogenide Nanostructures. Adv. Mater. Interfaces 2020, 7, 1901992. [Google Scholar] [CrossRef]
- Kumar, M.; Bhatt, V.; Kim, J.D.; Abhyankar, A.C.; Chung, H.-J.; Singh, K.; Cho, Y.B.; Yun, Y.J.; Lim, K.S.; Yun, J.-H. Holey engineered 2D ZnO-nanosheets architecture for supersensitive ppm level H2 gas detection at room temperature. Sens. Actuators B 2021, 326, 128839. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, X.P.; Xue, D.Y.; Zong, F.Y.; Zhang, J.M.; Duan, X.C.; Li, Q.H.; Wang, T.H. Enhanced H2 gas sensing properties by Pd-loaded urchin-like W18O49 hierarchical nanostructures. Sens. Actuators B 2018, 260, 900–907. [Google Scholar] [CrossRef]
- Mai, H.D.; Jeong, S.M.; Nguyen, T.K.; Youn, J.-S.; Ahn, S.B.; Park, C.-M.; Jeon, K.-J. Pd Nanocluster/Monolayer MoS2 Heterojunctions for Light-Induced Room-Temperature Hydrogen Sensing. ACS Appl. Mater. Interfaces 2021, 13, 14644–14652. [Google Scholar] [CrossRef] [PubMed]
- Badie, C.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Sayegh, S.; Bechelany, M. Lionel Santinacci and Sang Sub Kim, Enhanced sensitivity towards hydrogen by a TiN interlayer in Pd-decorated SnO2 nanowires. J. Mater. Chem. A 2023, 11, 12202–12213. [Google Scholar] [CrossRef]
- Shi, Y.S.; Xu, H.Y.; Liu, T.Y.; Zeb, S.; Nie, Y.; Zhao, Y.M.; Qin, C.Y.; Jiang, X.C. Advanced development of metal oxide nanomaterials for H2 gas sensing applications. Mater. Adv. 2021, 2, 1530–1569. [Google Scholar] [CrossRef]
- Luo, Y.B.; An, B.X.; Bai, J.L.; Wang, Y.R.; Cheng, X.; Wang, Q.A.; Li, J.P.; Yang, Y.F.; Wu, Z.K.; Xie, E.Q. Ultrahigh-response hydrogen sensor based on PdO/NiO co-doped In2O3 nanotubes. J. Colloid Interface Sci. 2021, 599, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, Y.; Li, Y.N.; Cao, L.; Nan, N.; Li, C.; Yu, L.M. Tunable NH4F-Assisted Synthesis of 3D Porous In2O3 Microcubes for Outstanding NO2 Gas-Sensing Performance: Fast Equilibrium at High Temperature and Resistant to Humidity at Room Temperature. ACS Appl. Mater. Interfaces 2021, 13, 14355–14364. [Google Scholar] [CrossRef]
- Hana, B.Q.; Wang, J.H.; Yang, W.Y.; Chen, X.W.; Wang, H.R.; Chen, J.L.; Zhang, C.; Sund, J.H.; Wei, X.Y. Hydrothermal synthesis of flower-like In2O3 as a chemiresistive isoprene sensor for breath analysis. Sens. Actuators B 2020, 309, 127788. [Google Scholar] [CrossRef]
- Liu, W.; Sun, J.A.; Xu, L.; Zhu, S.D.; Zhou, X.G.; Yang, S.; Dong, B.; Bai, X.; Lu, G.Y.; Song, H.W. Understanding the noble metal modifying effect on In2O3 nanowires: Highly sensitive and selective gas sensors for potential early screening of multiple diseases. Nanoscale Horiz. 2019, 4, 1361–1371. [Google Scholar] [CrossRef]
- Xu, H.Y.; Akbari, M.K.; Wei, Z.H.; Hu, J.; Verpoort, F.; Zhuiykov, S. Plasma-induced sub-10 nm Au-SnO2-In2O3 heterostructures fabricated by atomic layer deposition for highly sensitive ethanol detection on ppm level. Appl. Surf. Sci. 2021, 563, 150400. [Google Scholar] [CrossRef]
- He, P.; Fu, H.T.; Yang, X.H.; Xiong, S.X.; Han, D.Z.; An, X.Z. Variable gas sensing performance towards different volatile organic compounds caused by integration types of ZnS on In2O3 hollow spheres. Sens. Actuators B 2021, 345, 130316. [Google Scholar] [CrossRef]
- Wan, K.C.; Wang, D.; Wang, F.; Li, H.J.; Xu, J.C.; Wang, X.Y.; Yang, J. Hierarchical In2O3@SnO2 Core−Shell Nanofiber for High Efficiency Formaldehyde Detection. ACS Appl. Mater. Interfaces 2019, 11, 45214–45225. [Google Scholar] [CrossRef]
- Cao, Y.Y.; He, Y.; Zou, X.X.; Li, G.-D. Tungsten oxide clusters decorated ultrathin In2O3 nanosheets for selective detecting formaldehyde. Sens. Actuators B 2017, 252, 232–238. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, S.W.; Tang, P.G.; Feng, Y.J.; Li, D.Q. Ultra-sensitive ethanol gas sensors based on nanosheet-assembled hierarchical ZnO-In2O3 heterostructures. J. Hazard. Mater. 2020, 391, 122191. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Gundimeda, V.; Hou, T.F.; Lee, D.W. Realizing Synergy between In2O3 Nanocubes and Nitrogen-Doped Reduced Graphene Oxide: An Excellent Nanocomposite for the Selective and Sensitive Detection of CO at Ambient Temperatures. ACS Appl. Mater. Interfaces 2017, 9, 31728–31740. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, N.G.; Wang, S.M.; Zhang, H.M. Electronic structure-dependent formaldehyde gas sensing performance of the In2O3/Co3O4 core/shell hierarchical heterostructure sensors. J. Colloid Interface Sci. 2020, 577, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Xu, Y.J.; He, W.M. High acetone sensing properties of In2O3–NiO one-dimensional heterogeneous nanofibers based on electrospinning. RSC Adv. 2021, 11, 11215–11223. [Google Scholar] [CrossRef]
- Jian, Y.Y.; Hu, W.W.; Zhao, Z.H.; Cheng, P.F.; Haick, H.; Yao, M.S. Gas Sensors Based on Chemiresistive Hybrid Functional Nanomaterials. Nano-Micro Lett. 2020, 12, 71. [Google Scholar] [CrossRef]
- Gu, F.B.; Di, M.Y.; Han, D.M.; Hong, S.; Wang, Z.H. Atomically Dispersed Au on In2O3 Nanosheets for Highly Sensitive and Selective Detection of Formaldehyde. ACS Sens. 2020, 5, 2611–2619. [Google Scholar] [CrossRef]
- Wang, S.M.; Xiao, B.X.; Yang, T.Y.; Wang, P.; Xiao, C.H.; Li, Z.F.; Zhao, R.; Zhang, M.Z. Enhanced HCHO gas sensing properties by Ag-loaded sunflower-like In2O3 hierarchical nanostructures. J. Mater. Chem. A 2014, 2, 6598–6604. [Google Scholar] [CrossRef]
- Wang, Z.H.; Men, G.L.; Zhang, R.X.; Gu, F.B.; Han, D.M. Pd loading induced excellent NO2 gas sensing of 3DOM In2O3 at room temperature. Sens. Actuators B 2018, 263, 218–228. [Google Scholar] [CrossRef]
- Ma, R.-J.; Zhao, X.; Zou, X.X.; Li, G.-D. Enhanced formaldehyde sensing performance at ppb level with Pt-doped nanosheet-assembled In2O3 hollow microspheres. J. Alloys Compd. 2018, 732, 863–870. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.H.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Rai, P.; Yoon, J.-W.; Jeong, M.; Hwang, S.-J.; Kwak, C.-H.; Lee, J.-H. Design of highly sensitive and selective Au@NiO yolk–shell nanoreactors for gas sensor applications. Nanoscale 2014, 6, 8292–8299. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Yoon, W.; Kwak, H.; Lee, H. Role of Pd nanoparticles on gas sensing behaviour of Pd@In2O3 yolkshell nanoreactors. J. Mater. Chem. A 2016, 4, 264–269. [Google Scholar] [CrossRef]
- Nguyen, T.T.D.; Dao, D.V.; Kim, D.-S.; Lee, H.-J.; Oh, S.-Y.; Lee, I.-H.; Yu, Y.-T. Effect of core and surface area toward hydrogen gas sensing performance using Pd@ZnO core-shell nanoparticles. J. Colloid Interface Sci. 2021, 587, 252–259. [Google Scholar] [CrossRef]
- Lee, H.-J.; Dao, D.V.; Yu, Y.-T. Superfast and efficient hydrogen gas sensor using PdAu alloy@ZnO core–shell nanoparticles. J. Mater. Chem. A 2020, 8, 12968–12974. [Google Scholar] [CrossRef]
- Enüstün, B.V.; Turkevich, J. Coagulation of Colloidal Gold. J. Am. Chem. Soc. 1963, 85, 3317–3328. [Google Scholar] [CrossRef]
- Zhu, S.B.; Tian, X.; Chen, J.J.; Shan, L.M.; Xu, X.L.; Zhou, Z.W. A Facile Approach to Construct Multiple Structured ZnO Crystals by Trisodium Citrate-Assisted Hydrothermal Growth Toward Performance Enhancement of Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 16401–16407. [Google Scholar] [CrossRef]
- Zai, J.T.; Zhu, J.; Qi, R.R.; Qian, X.F. Nearly monodispersed In(OH)3 hierarchical nanospheres and nanocubes: Tunable ligand-assisted synthesis and their conversion into hierarchical In2O3 for gas sensing. J. Mater. Chem. A 2013, 1, 735–745. [Google Scholar] [CrossRef]
- Parkinson, J.A.; Sun, H. New Approach to the Solution Chemistry of Bismuth Citrate Antiulcer Complexes. Chem. Commun. 1998, 8, 881–882. [Google Scholar] [CrossRef]
- Li, B.X.; Gu, T.; Ming, T.; Wang, J.X.; Wang, P.; Wang, J.F.; Yu, J.C. (Gold Core)@(Ceria Shell) Nanostructures for Plasmon-Enhanced Catalytic Reactions under Visible Light. ACS Nano 2014, 8, 8152–8162. [Google Scholar] [CrossRef]
- Ngaw, C.K.; Xu, Q.C.; Tan, T.T.Y.; Hu, P.; Cao, S.W.; Loo, J.C.S.C. A strategy for in-situ synthesis of well-defined core–shell Au@TiO2 hollow spheres for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2014, 257, 112–121. [Google Scholar] [CrossRef]
- Majhi, S.M.; Rai, P.; Yu, Y.-T. Facile Approach to Synthesize Au@ZnO Core−Shell Nanoparticles and Their Application for Highly Sensitive and Selective Gas Sensors. ACS Appl. Mater. Interfaces 2015, 7, 9462–9468. [Google Scholar] [CrossRef]
- Tao, K.; Han, X.; Yin, Q.; Wang, D.; Han, L.; Chen, L.A. Metal-Organic Frameworks-Derived Porous In2O3 Hollow Nanorod for High-Performance Ethanol Gas Sensor. ChemistrySelect 2017, 2, 10918–10925. [Google Scholar] [CrossRef]
- Xu, Y.S.; Zheng, L.L.; Yang, C.; Zheng, W.; Liu, X.H.; Zhang, J. Oxygen Vacancies Enabled Porous SnO2 Thin Films for Highly Sensitive Detection of Triethylamine at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 20704–20713. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ma, T.; Zheng, L.; Sun, L.; Liu, X.; Zhao, Y.; Zhang, J. Rational design of Au/Co3O4-functionalized W18O49 hollow heterostructures with high sensitivity and ultralow limit for trimethylamine detection. Sens. Actuators B 2019, 284, 202–212. [Google Scholar] [CrossRef]
- Rao, A.; Long, H.; Harley-Trochimczyk, A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. In Situ Localized Growth of Ordered Metal Oxide Hollow Sphere Array on Microheater Platform for Sensitive, Ultra-Fast Gas Sensing. ACS Appl. Mater. Interfaces 2017, 9, 2634–2641. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, X.; Wang, C.; Shimanoe, K.; Lu, G.; Yamazoe, N. Hollow SnO2/alpha-Fe2O3 spheres with a double-shell structure for gas sensors. J. Mater. Chem. A 2014, 2, 1302–1308. [Google Scholar] [CrossRef]
- Pak, Y.; Kim, S.-M.; Jeong, H.; Kang, C.G.; Park, J.S.; Song, H.; Lee, R.; Myoung, N.S.; Lee, B.H.; Seo, S.; et al. Palladium-Decorated Hydrogen-Gas Sensors Using Periodically Aligned Graphene Nanoribbons. ACS Appl. Mater. Interfaces 2014, 6, 13293–13298. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, A.; Barman, P.B.; Hazra, S.K. Role of catalyst oxidation states in the selective detection of reducing gases: A comparative study of nanocomposites based on Au and chemically/thermally reduced graphene oxide. Mater. Sci. Eng. B 2024, 308, 117605. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Zhang, X.; Liu, M.; Yu, D.; Yuan, Y.L. Aldous and X. Jiang Experimental and theoretical studies of gold nanoparticle decorated zinc oxide nanoflakes with exposed {1 0 0} facets for butylamine sensing. Sens. Actuators B 2016, 230, 581–591. [Google Scholar] [CrossRef]
- Chava, R.K.; Oh, S.-Y.; Yu, Y.-T. Enhanced H2 gas sensing properties of Au@In2O3 core-shell hybrid metal-semiconductor heteronanostructures. CrystEngComm 2016, 18, 3655–3666. [Google Scholar] [CrossRef]
- Low, J.X.; Jiang, C.J.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J.G. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Jung, G.W.; Ju, S.Y.; Choi, K.W.; Kim, J.H.; Hong, S.B.; Park, J.W.; Shin, W.J.; Jeong, Y.J.; Han, S.W.; Choi, W.Y.; et al. Reconfigurable Manipulation of Oxygen Content on Metal Oxide Surfaces and Applications to Gas Sensing. ACS Nano 2023, 17, 17790–17798. [Google Scholar] [CrossRef]
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of Oxygen Vacancies in Nanostructured Metal-Oxide Gas Sensors: A Review. Sens. Actuators B 2019, 301, 126845. [Google Scholar] [CrossRef]
- Zhao, N.; Chang, X.; Liu, X.; Li, J.; Zheng, W.; Zhang, J. Probing the Structural Dynamics of In2O3 Using in Situ Raman Spectroscopy: Bridging Material Dynamics and Sensor Functionality. Angew. Chem. Int. Ed. 2025, 64, e202512808. [Google Scholar] [CrossRef]
- Zhu, H.L.; Guo, Y.; Zheng, F.S.; Li, C.; Wu, J.B.; Wang, T.Q.; Fu, Y.; Zhang, X.M. Introducing oxygen vacancies on sodium titanate via NaBH4 treatment for conductometric hydrogen gas sensors. Sens. Actuators B 2023, 375, 132916. [Google Scholar] [CrossRef]
- Ivanovskaya, M.; Ovodok, E.; Kotsikau, D.; Azarko, I.; Micusik, M. Gas-sensing properties of In2O3-Au sensors as a function of gold particle size and method of their synthesis. Microchem. J. 2025, 209, 112676. [Google Scholar] [CrossRef]
- Li, X.; Zhou, X.; Guo, H.; Wang, C.; Liu, J.Y.; Sun, P.; Liu, F.; Lu, G. Design of Au@ZnO Yolk–Shell Nanospheres with Enhanced Gas Sensing Properties. ACS Appl. Mater. Interfaces 2014, 6, 18661–18667. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Rai, P.; Yu, Y.-T. Microwave assisted hydrothermal synthesis of Au@ TiO2 core–shell nanoparticles for high temperature CO sensing applications. Sens. Actuators B 2013, 186, 633–639. [Google Scholar] [CrossRef]
- Liu, X.J.; Sun, X.L.; Duan, X.P.; Zhang, C.; Zhao, K.R.; Xu, X.J. Core-shell Ag@In2O3 hollow hetero-nanostructures for selective ethanol detection in air Sens. Actuators B Chem. 2020, 305, 127450. [Google Scholar] [CrossRef]
- Majhi, S.M.; Naik, G.K.; Lee, H.J.; Song, H.G.; Lee, C.R.; Lee, I.H.; Yu, Y.T. Au@NiO core-shell nanoparticles as a p-type gas sensor: Novel synthesis, characterization, and their gas sensing properties with sensing mechanism. Sens. Actuators B 2018, 268, 223–231. [Google Scholar] [CrossRef]
- Lee, H.Y.; Bang, J.H.; Majhi, S.M.; Mirzaei, A.; Shin, K.Y.; Yu, D.J.; Oum, W.; Kang, S.; Lee, M.L.; Kim, S.S.; et al. Conductometric ppb-level acetone gas sensor based on one-pot synthesized Au@Co3O4 core-shell nanoparticles. Sens. Actuators B 2022, 359, 131550. [Google Scholar] [CrossRef]
- Wu, C.H.; Zhu, Z.; Chang, H.M.; Jiang, Z.X.; Hsieh, C.Y.; Wu, R.J. Pt@NiO core-shell nanostructure for a hydrogen gas sensor. J. Alloy. Compd. 2020, 814, 151815. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Du, L.L.; Xing, X.X.; Wang, C.; Feng, D.L.; Li, Z.X.; Yang, D.C. Surface-broken palladium and palladium oxide core/shell nanowires for stable hydrogen sensing at room temperature. Sens. Actuators B 2023, 375, 132914. [Google Scholar] [CrossRef]
- Zhao, S.K.; Shen, Y.B.; Zhou, P.F.; Zhong, X.X.; Han, C.; Zhao, Q.; Wei, D.Z. Design of Au@WO3 core-shell structured nanospheres for ppb-level NO2 sensing. Sens. Actuators B 2019, 282, 917–926. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).