Influence of Interfacial Stress on the Structural Characteristics and Hydrogen Sensing Performance of WO3 Films
Abstract
1. Introduction
2. Experimental Section
2.1. Selection of Substrate
2.2. Sample Preparation and Characterization
2.3. Hydrogen Sensing Measurement
3. Results and Discussion
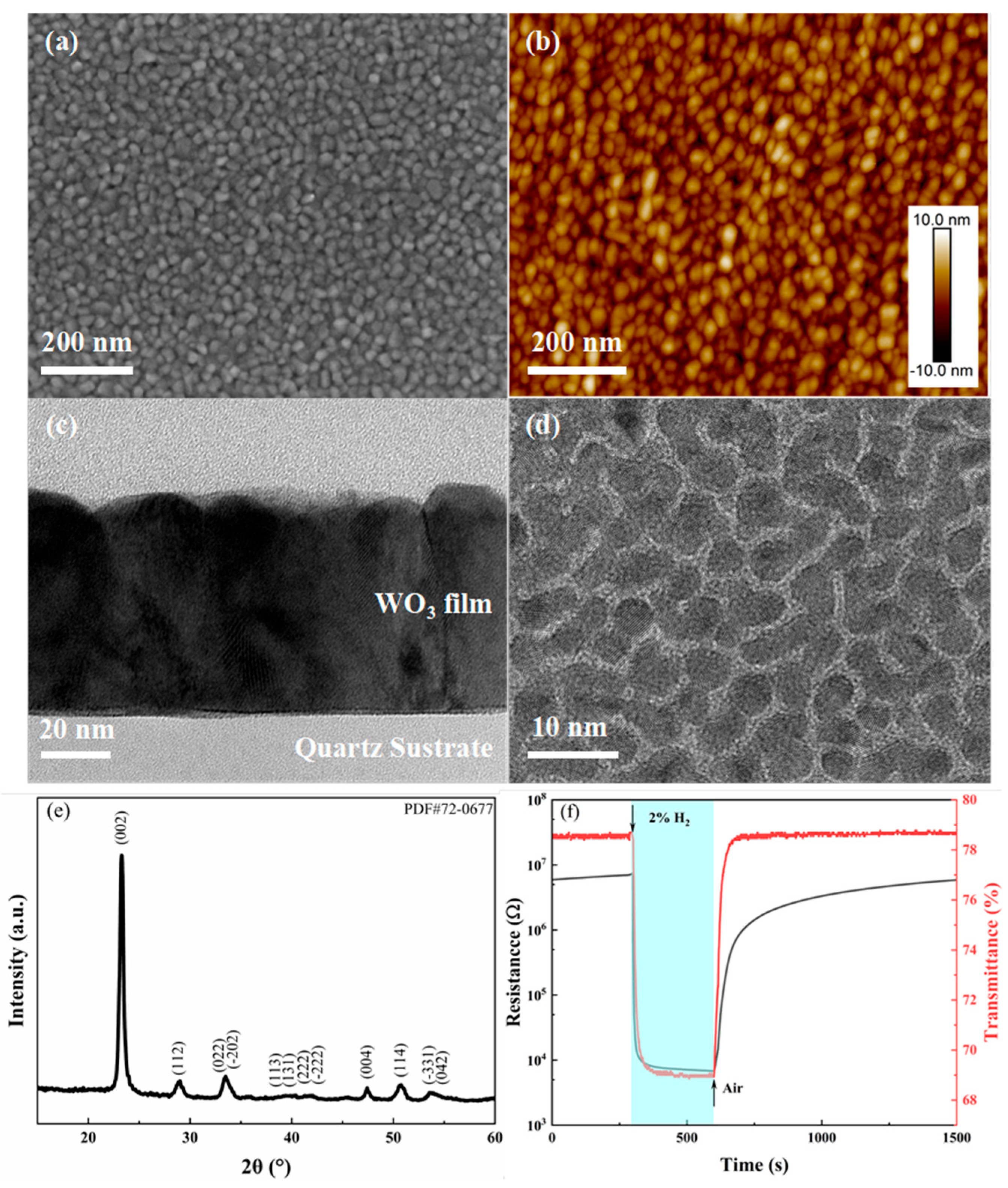
3.1. WO3 Film Deposited on Amorphous Quartz Substrate
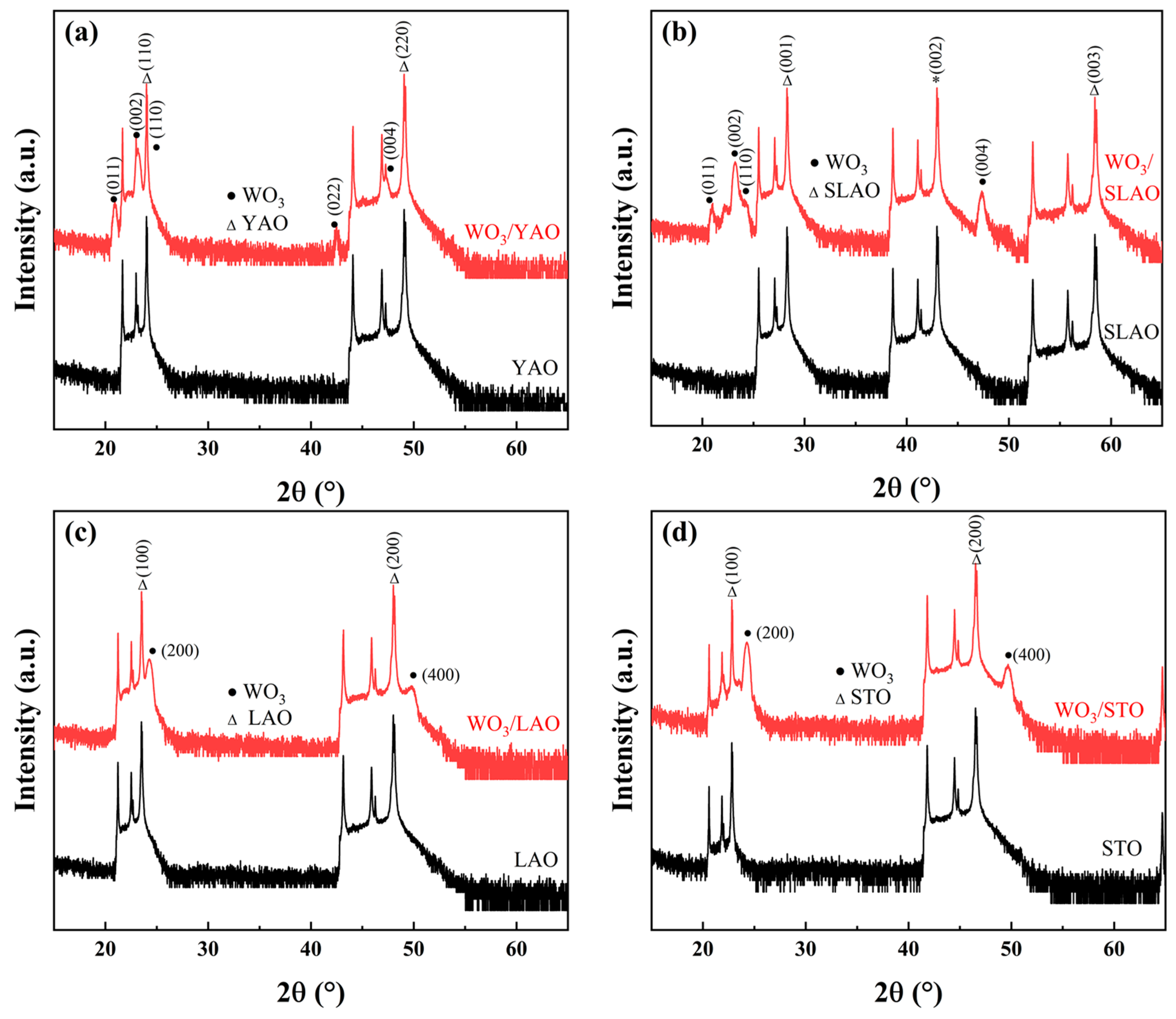
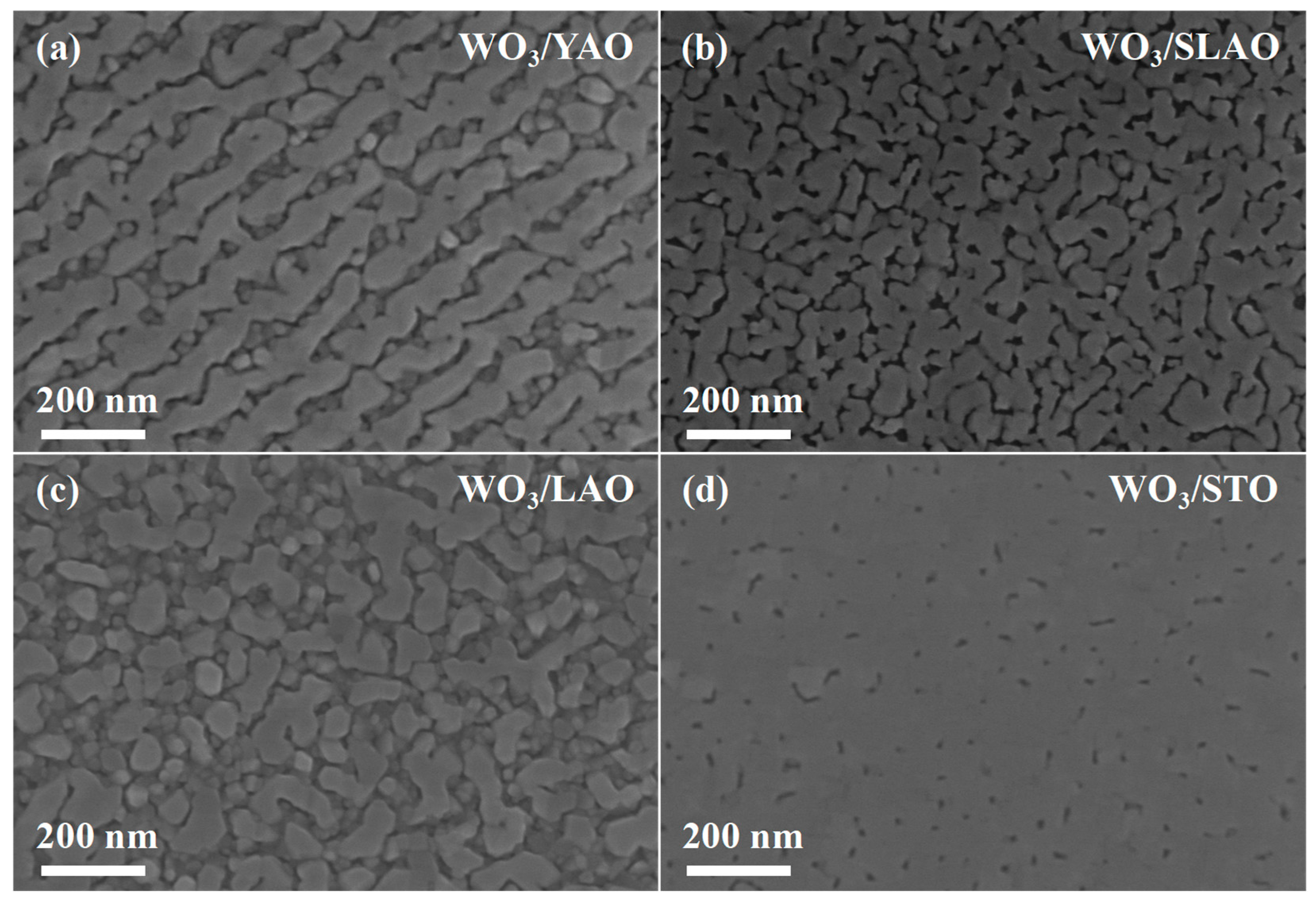
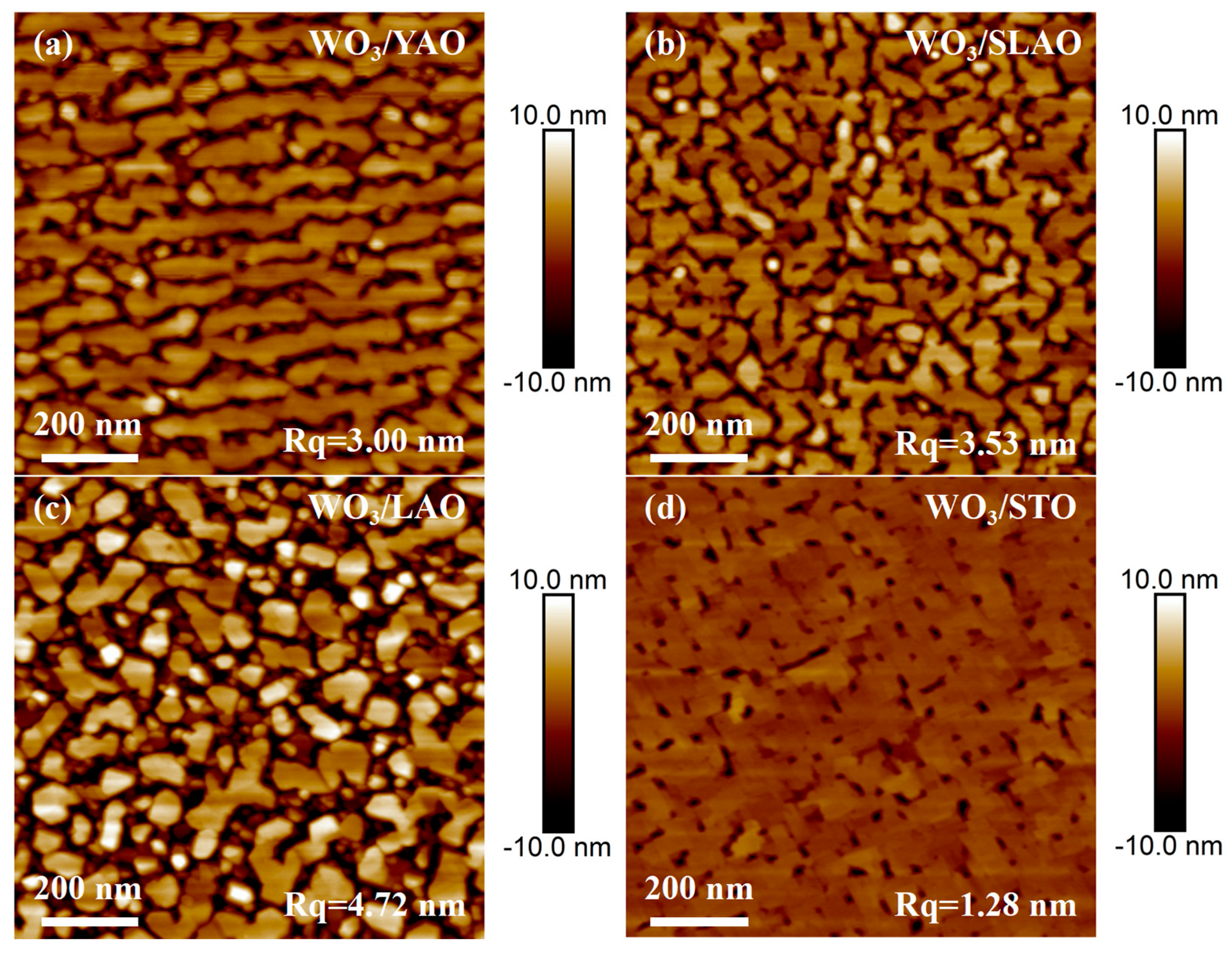
3.2. WO3 Film Deposited on Single Crystal Substrates
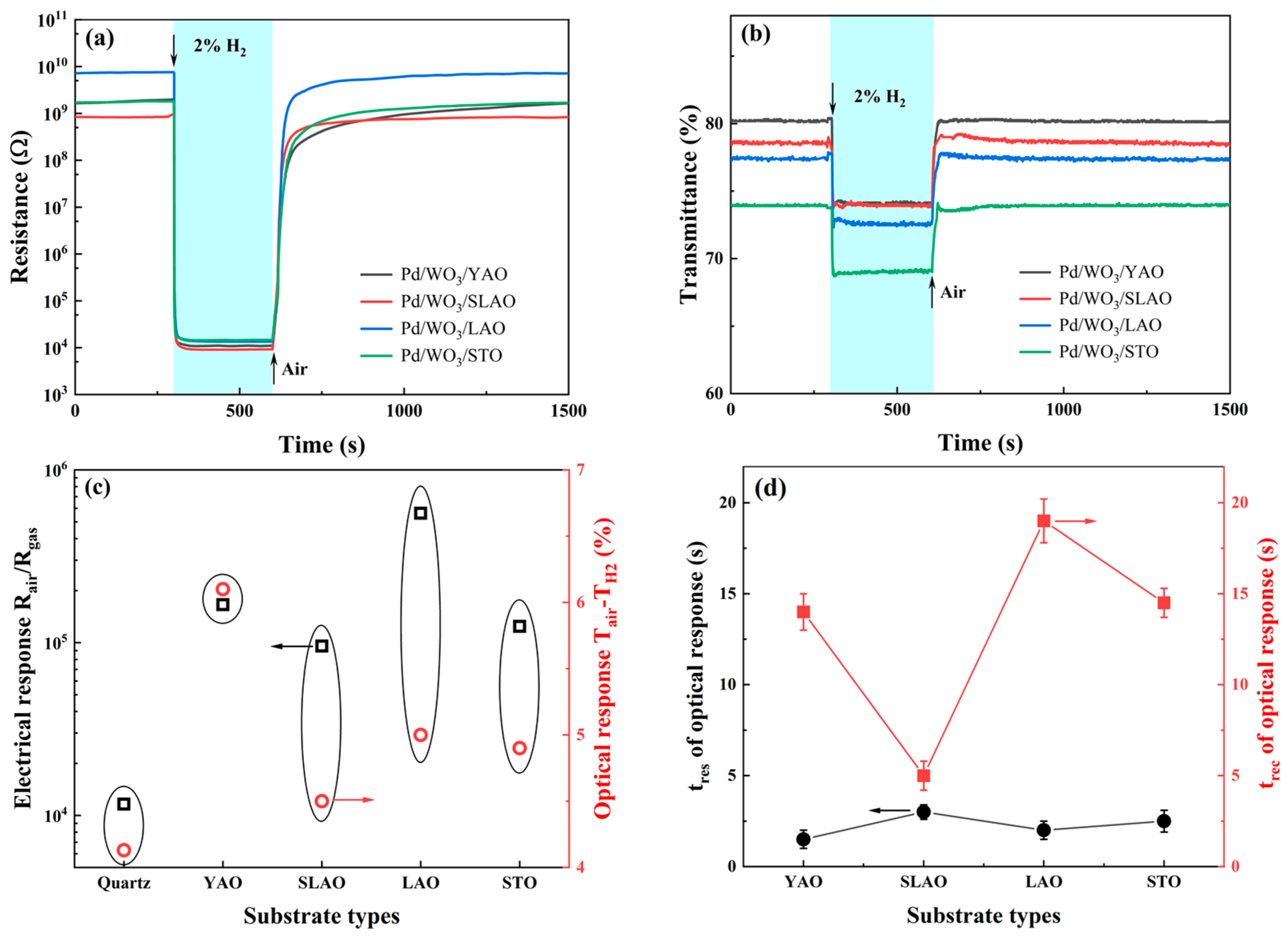
3.3. Hydrogen Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, L.; Fang, C.; Pan, T.; Zhu, Q.; Geng, T.; Li, G.; Li, X.; Yu, J. Hydrogen Production via Electrolysis of Wastewater. Nanomaterials 2024, 14, 567. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Zhang, L.; Lang, J.; Yuan, W.; An, G.; Lei, T.; Yan, J. A comprehensive review of advances and challenges of hydrogen production, purification, compression, transportation, storage and utilization technology. Renew. Sustain. Energy Rev. 2026, 226, 116196. [Google Scholar] [CrossRef]
- Boon-Brett, L.; Bousek, J.; Black, G.; Moretto, P.; Castello, P.; Hubert, T.; Banach, U. Identifying performance gaps in hydrogen safety sensor technology for automotive and stationary applications. Int. J. Hydrogen Energy 2010, 35, 373–384. [Google Scholar] [CrossRef]
- Ndaya, C.C.; Javahiraly, N.; Brioude, A. Recent Advances in Palladium Nanoparticles-Based Hydrogen Sensors for Leak Detection. Sensors 2019, 19, 4478. [Google Scholar] [CrossRef]
- Hubert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Davies, E.; Ehrmann, A.; Schwenzfeier-Hellkamp, E. Safety of Hydrogen Storage Technologies. Processes 2024, 12, 2182. [Google Scholar] [CrossRef]
- Qanbar, M.W.; Hong, Z. A Review of Hydrogen Leak Detection Regulations and Technologies. Energies 2024, 17, 4059. [Google Scholar] [CrossRef]
- Ma, Y.; Qiu, X.; Duan, Z.; Liu, L.; Li, J.; Wu, Y.; Yuan, Z.; Jiang, Y.; Tai, H. A Novel Calibration Scheme of Gas Sensor Array for a More Accurate Measurement Model of Mixed Gases. ACS Sens. 2024, 9, 6022–6031. [Google Scholar] [CrossRef]
- Suchikova, Y.; Nazarovets, S.; Konuhova, M.; Popov, A.I. Binary Oxide Ceramics (TiO2, ZnO, Al2O3, SiO2, CeO2, Fe2O3, and WO3) for Solar Cell Applications: A Comparative and Bibliometric Analysis. Ceramics 2025, 8, 119. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, D.; Zou, L.; Wang, Q.; Liu, C. A Review on the Properties and Applications of WO3 Nanostructure-Based Optical and Electronic Devices. Nanomaterials 2021, 11, 2136. [Google Scholar] [CrossRef]
- Wu, X.; Guo, X.; Gao, C.; Luo, J.; Nie, L.; Chen, J.; Peng, L. An innovative method for enhancing the hydrogen gasochromic performance of mesoporous Pt/WO3 films. Int. J. Hydrogen Energy 2024, 83, 1405–1414. [Google Scholar] [CrossRef]
- Gao, C.; Guo, X.; Nie, L.; Wu, X.; Peng, L.; Chen, J. A review on WO3 gasochromic film: Mechanism, preparation and properties. Int. J. Hydrogen Energy 2023, 48, 2442–2465. [Google Scholar] [CrossRef]
- Matsukawa, T.; Ishigaki, T. Effect of isothermal holding time on hydrogen-induced structural transitions of WO3. Dalton Trans. 2021, 50, 7590–7596. [Google Scholar] [CrossRef]
- An, B.X.; Yang, Y.F.; Wang, Y.R.; Li, R.X.; Wu, Z.K.; Wang, P.Z.; Zhang, T.Y.; Han, R.Q.; Xie, E.R. Observation on Switching Properties of WO3-Based H2 Sensor Regulated by Temperature and Gas Concentration. ACS Sens. 2024, 9, 5179–5187. [Google Scholar] [CrossRef]
- Bai, Y.; Ran, X.L.; Yang, X.H.; Xiong, S.X.; Gu, F.; Wang, S.F.; Li, J.C.; Fu, H.T.; An, X.Z. Room-Temperature Selective Detection of H2 by Pd Nanoparticle-Decorated SnO2@WO3 Core-Shell Hollow Structures. Anal. Chem. 2025, 97, 2819–2827. [Google Scholar] [CrossRef]
- Yang, X.Y.; Chen, H.N.; Yue, L.J.; Gong, F.L.; Xie, K.F.; Wei, S.Z.; Zhang, Y.H. Surface engineering of 1D Na-doped Pd/WO3 nanorods for chemiresistive H2 sensing. Sens. Actuators B Chem. 2025, 423, 136825. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Chan, C.-C.; Peng, C.-H.; Chang, C.-C. Hydrogen sensing characteristics of an electrodeposited WO3 thin film gasochromic sensor activated by Pt catalyst. Thin Solid Film. 2007, 516, 407–411. [Google Scholar] [CrossRef]
- Chan, C.-C.; Hsu, W.-C.; Chang, C.-C.; Hsu, C.-S. Hydrogen incorporation in gasochromic coloration of sol-gel WO3 thin films. Sens. Actuators B Chem. 2011, 157, 504–509. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.-H.; Lee, Y.-A.; Seo, H. Green deposition of Pd nanoparticles on WO3 for optical, electronic and gasochromic hydrogen sensing applications. Sens. Actuators B Chem. 2015, 221, 411–417. [Google Scholar] [CrossRef]
- Takahashi, H.; Okazaki, S.; Nishijima, Y.; Arakawa, T. Optimization of Hydrogen Sensing Performance of Pt/WO3 Gasochromic Film Fabricated by Sol-Gel Method. Sens. Mater. 2017, 29, 1259–1268. [Google Scholar] [CrossRef]
- Chen, M.; Zou, L.; Zhang, Z.; Shen, J.; Li, D.; Zong, Q.; Gao, G.; Wu, G.; Shen, J.; Zhang, Z. Tandem gasochromic-Pd-WO3/graphene/Si device for room-emperature high-performance optoelectronic hydrogen sensors. Carbon 2018, 130, 281–287. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T. Recent Progress of Nanostructured Sensing Materials from 0D to 3D: Overview of Structure–Property-Application Relationship for Gas Sensors. Small Methods 2021, 5, 2100515. [Google Scholar] [CrossRef]
- Panigrahi, P.K.; Chandu, B.; Puvvada, N. Recent Advances in Nanostructured Materials for Application as Gas Sensors. ACS Omega 2024, 9, 3092–3122. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Current state of knowledge on the metal oxide based gas sensing mechanism. Sens. Actuators B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Liu, Y.; Wang, S.; Li, T.; Feng, S.; Qin, S.; Zhang, T. Heteronanostructural metal oxide-based gas microsensors. Microsyst. Nanoeng. 2022, 8, 85. [Google Scholar] [CrossRef]
- Zhu, L.-Y.; Ou, L.-X.; Mao, L.-W.; Wu, X.-Y.; Liu, Y.-P.; Lu, H.-L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nano-Micro Lett. 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, M.; Park, S.; Ahn, J.; Kim, I.-D. Materials Engineering for Light-Activated Gas Sensors: Insights, Advances, and Future Perspectives. Adv. Mater. 2025, 37, e08204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, T.; Zhang, G.; Gao, R.; Gao, C.; Wang, Z.; Xuan, F. Rational Design and Fabrication of MEMS Gas Sensors with Long-Term Stability: A Comprehensive Review. Adv. Sci. 2025, 12, e11555. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-T.; Ma, C.; Ge, C.; Zhang, Q.-H.; Du, J.-Y.; Li, J.-K.; Huang, H.-Y.; He, M.; Wang, C.; Meng, S.; et al. Effects of line defects on the electronic and optical properties of strain-engineered WO3 thin films. J. Mater. Chem. C 2017, 5, 11694–11699. [Google Scholar] [CrossRef]
- Du, Y.G.; Gu, M.; Varga, T.; Wang, C.M.; Bowden, M.E.; Chambers, S.A. Strain Accommodation by Facile WO6 Octahedral Distortion and Tilting during WO3 Heteroepitaxy on SrTiO3(001). ACS Appl. Mater. Interfaces 2014, 6, 14253–14258. [Google Scholar] [CrossRef]
- Moulzolf, S.C.; Ding, S.A.; Lad, R.J. Stoichiometry and microstructure effects on tungsten oxide chemiresistive films. Sens. Actuators B Chem. 2001, 77, 375–382. [Google Scholar] [CrossRef]
- Moulzolf, S.C.; Legore, L.J.; Lad, R.J. Heteroepitaxial growth of tungsten oxide films on sapphire for chemical gas sensors. Thin Solid Film. 2001, 400, 56–63. [Google Scholar] [CrossRef]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Perri, J.A.; Banks, E.; Post, B. Study of Phase Transitions in WO3 with a High-Temperature X-Ray Diffractometer. J. Appl. Phys. 1957, 28, 1272–1275. [Google Scholar] [CrossRef]
- Mattoni, G.; de Jong, B.; Manca, N.; Tomellini, M.; Caviglia, A.D. Single-Crystal Pt-Decorated WO3 Ultrathin Films: A Platform for Sub-ppm Hydrogen Sensing at Room Temperature. ACS Appl. Nano Mater. 2018, 1, 3446–3452. [Google Scholar] [CrossRef]
- Deb, S.K. Opportunities and challenges in science and technology of WO3 for electrochromic and related applications. Sol. Energy Mater. Sol. Cells 2008, 92, 245–258. [Google Scholar] [CrossRef]
- Geller, S.; Wood, E.A. Crystallographic studies of perovskite-like compounds. I. Rare earth orthoferrites and YFeO3, YCrO3, YAlO3. Acta Crystallogr. 1956, 9, 563–568. [Google Scholar] [CrossRef]
- Ichinose, T.; Naganuma, H.; Mukaiyama, K.; Oogane, M.; Ando, Y. Preparation of monoclinic 0.9(BiFeO3)–0.1(BiCoO3) epitaxial films on orthorhombic YAlO3 (100) substrates by r.f. magnetron sputtering. J. Cryst. Growth 2015, 409, 18–22. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, M.; Wang, C.; Wang, J.; Ye, J.-M.; Wei, Y.-C.; Li, Z.-Y.; Zhao, R.; Liu, G.-Z.; Geng, Y.-H.; et al. Vacuum Based Gas Sensing Material Characterization System for Precise and Simultaneous Measurement of Optical and Electrical Responses. Sensors 2022, 22, 1014. [Google Scholar] [CrossRef]
- Zhao, M.; Ong, C.W. Improved H2-sensing performance of nanocluster-based highly porous tungsten oxide films operating at moderate temperature. Sens. Actuators B Chem. 2012, 174, 65–73. [Google Scholar] [CrossRef]
- Wu, C.-H.; Zhu, Z.; Huang, S.Y.; Wu, R.-J. Preparation of palladium-doped mesoporous WO3 for hydrogen gas sensors. J. Alloys Compd. 2019, 776, 965–973. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, B.; Zhou, R.; Liu, Z.; Li, Q.; Wang, T. Room-temperature H2 sensing interfered by CO based on interfacial effects in palladium-tungsten oxide nanoparticles. Sens. Actuators B Chem. 2018, 254, 966–972. [Google Scholar] [CrossRef]
- Amrehn, S.; Wu, X.; Wagner, T. Tungsten Oxide Photonic Crystals as Optical Transducer for Gas Sensing. ACS Sens. 2018, 3, 191–199. [Google Scholar] [CrossRef]
- Foroushani, F.T.; Tavanai, H.; Ranjbar, M.; Bahrami, H. Fabrication of tungsten oxide nanofibers via electrospinning for gasochromic hydrogen detection. Sens. Actuators B Chem. 2018, 268, 319–327. [Google Scholar] [CrossRef]
- Mazur, M.; Kapuscik, P.; Weichbrodt, W.; Domaradzki, J.; Mazur, P.; Kot, M.; Flege, J.I. WO3 Thin-Film Optical Gas Sensors Based on Gasochromic Effect towards Low Hydrogen Concentrations. Materials 2023, 16, 3831. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Wang, J.; Wang, Y.; Sun, G.; Cao, J.; Bala, H.; Zhang, Z. Enhanced Methane Sensing Properties of WO3 Nanosheets with Dominant Exposed (200) Facet via Loading of SnO2 Nanoparticles. Nanomaterials 2019, 9, 351. [Google Scholar] [CrossRef] [PubMed]

| Material | Crystalline Phase | Lattice Parameter | ICSD PDF No. | |||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | |||
| WO3 | Triclinic | 7.309 | 7.522 | 7.678 | 88.81 | 90.92 | 90.93 | 71-0305 |
| WO3 | Monoclinic | 5.277 | 5.156 | 7.663 | 90 | 91.76 | 90 | 87-2380 |
| WO3 | Monoclinic | 7.306 | 7.540 | 7.692 | 90 | 90.88 | 90 | 72-0677 |
| WO3 | Orthorhombic | 7.333 | 7.573 | 7.740 | 90 | 90 | 90 | 89-4477 |
| WO3 | Orthorhombic | 7.341 | 7.570 | 7.754 | 90 | 90 | 90 | 71-0131 |
| WO3 | Tetragonal | 5.250 | 5.250 | 3.915 | 90 | 90 | 90 | 85-0808 |
| WO3 | Hexagonal | 7.324 | 7.324 | 7.662 | 90 | 90 | 120 | 85-2460 |
| Substrate | Crystalline Phase | ICSD PDF No. | Lattice Constant | Crystal Plane Used | Diagonal (Å) | “2 × 2” Superlattice (Å) | ||
|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | ||||||
| YAlO3 | Orthorhombic | 89-7947 | 5.179 | 5.327 | 7.37 | (110) | 5.254 | 7.430 |
| SrLaAlO4 | Tetragonal | 81-0744 | 3.756 | 3.756 | 12.636 | (001) | 5.311 | 7.512 |
| LaAlO3 | Cubic | 70-4125 | 3.82 | 3.82 | 3.82 | (100) | 5.401 | 7.640 |
| SrTiO3 | Cubic | 79-0175 | 3.905 | 3.905 | 3.905 | (100) | 5.522 | 7.810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Z.; Ye, J.; Ye, W.; Wei, J.; Li, Y.; Lv, Z.; Zhao, M. Influence of Interfacial Stress on the Structural Characteristics and Hydrogen Sensing Performance of WO3 Films. Nanomaterials 2025, 15, 1785. https://doi.org/10.3390/nano15231785
Qiao Z, Ye J, Ye W, Wei J, Li Y, Lv Z, Zhao M. Influence of Interfacial Stress on the Structural Characteristics and Hydrogen Sensing Performance of WO3 Films. Nanomaterials. 2025; 15(23):1785. https://doi.org/10.3390/nano15231785
Chicago/Turabian StyleQiao, Zhihong, Jianmin Ye, Wen Ye, Jie Wei, Ying Li, Zhe Lv, and Meng Zhao. 2025. "Influence of Interfacial Stress on the Structural Characteristics and Hydrogen Sensing Performance of WO3 Films" Nanomaterials 15, no. 23: 1785. https://doi.org/10.3390/nano15231785
APA StyleQiao, Z., Ye, J., Ye, W., Wei, J., Li, Y., Lv, Z., & Zhao, M. (2025). Influence of Interfacial Stress on the Structural Characteristics and Hydrogen Sensing Performance of WO3 Films. Nanomaterials, 15(23), 1785. https://doi.org/10.3390/nano15231785







