Recent Advances in Porphyrin-Based COFs Boosting CO2 Photocatalytic and Electrocatalytic Conversion
Abstract
1. Introduction
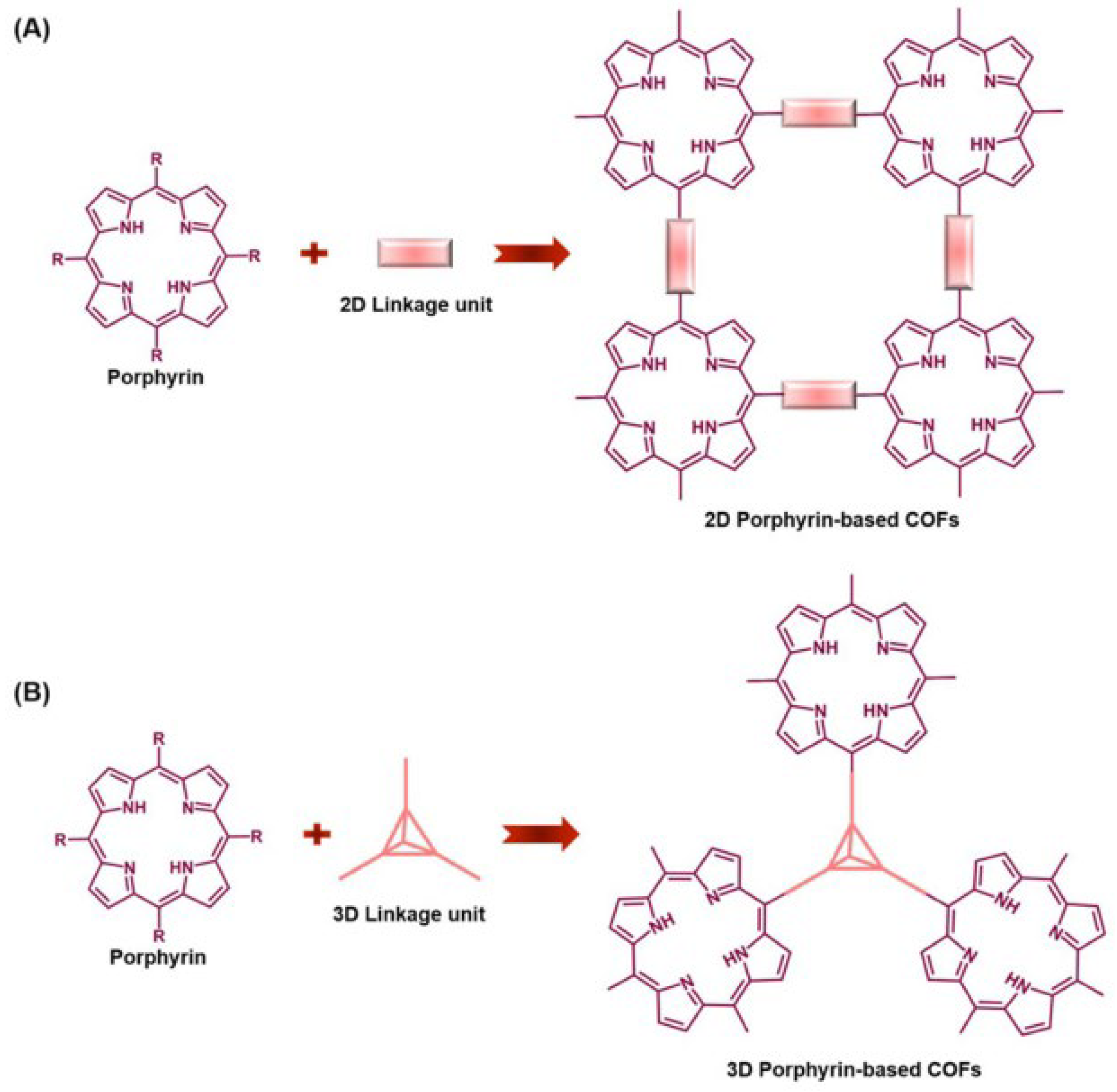
2. Structure and Properties of Porphyrin-Based COFs
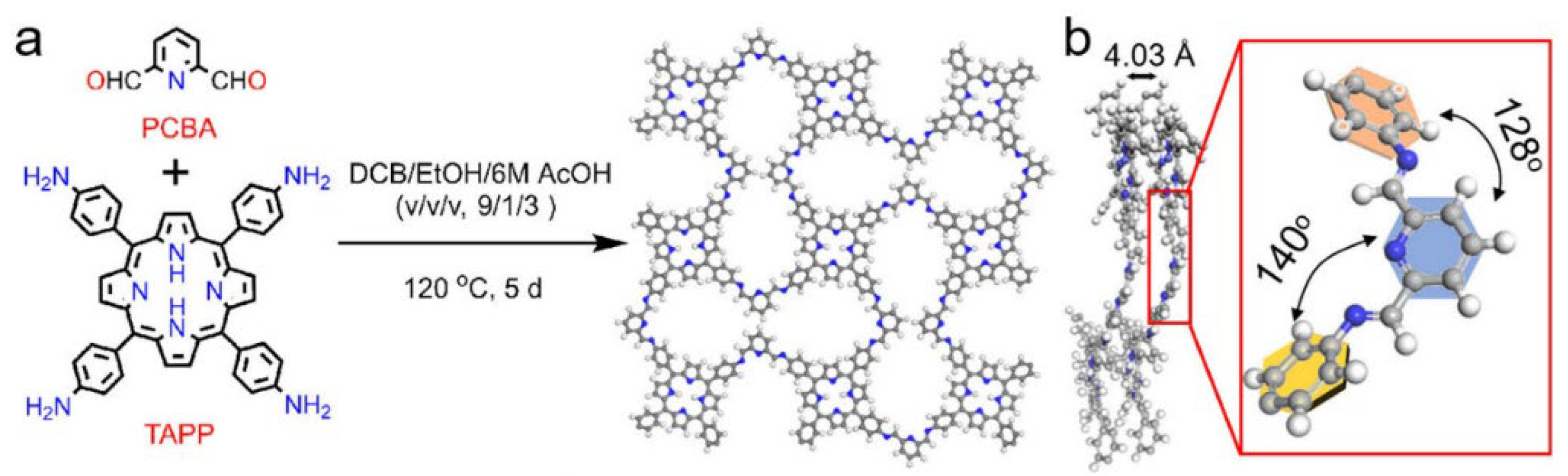
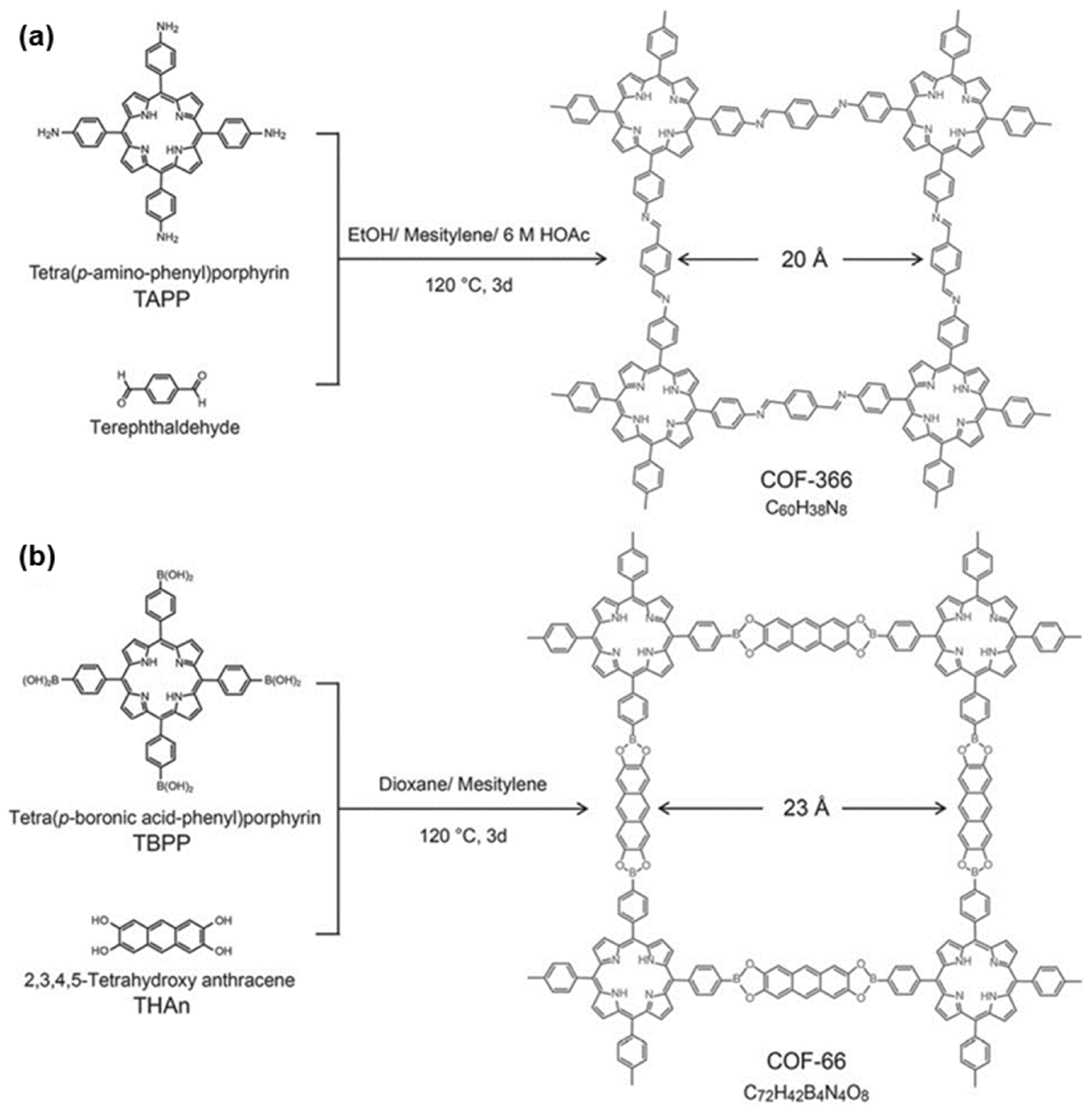
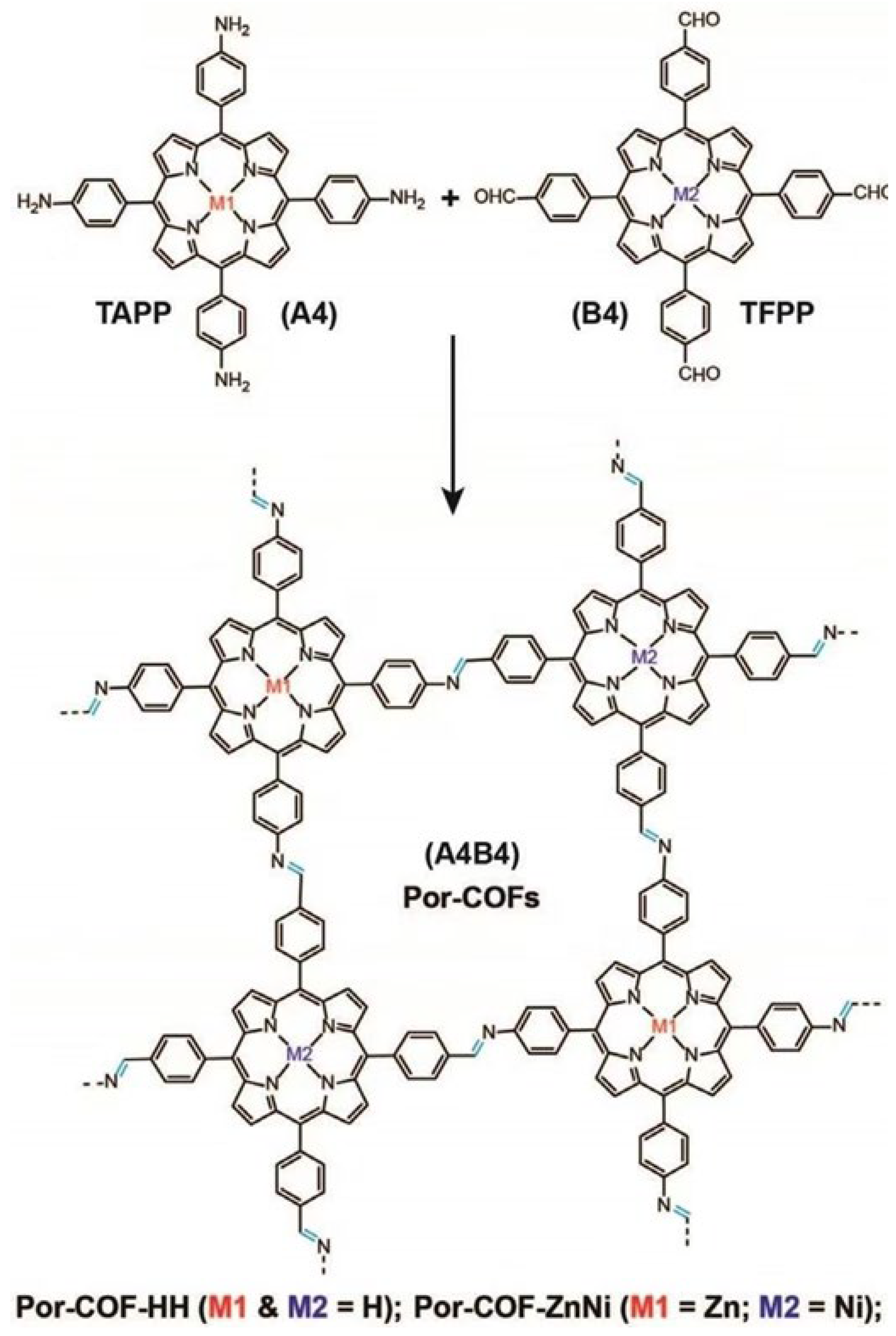
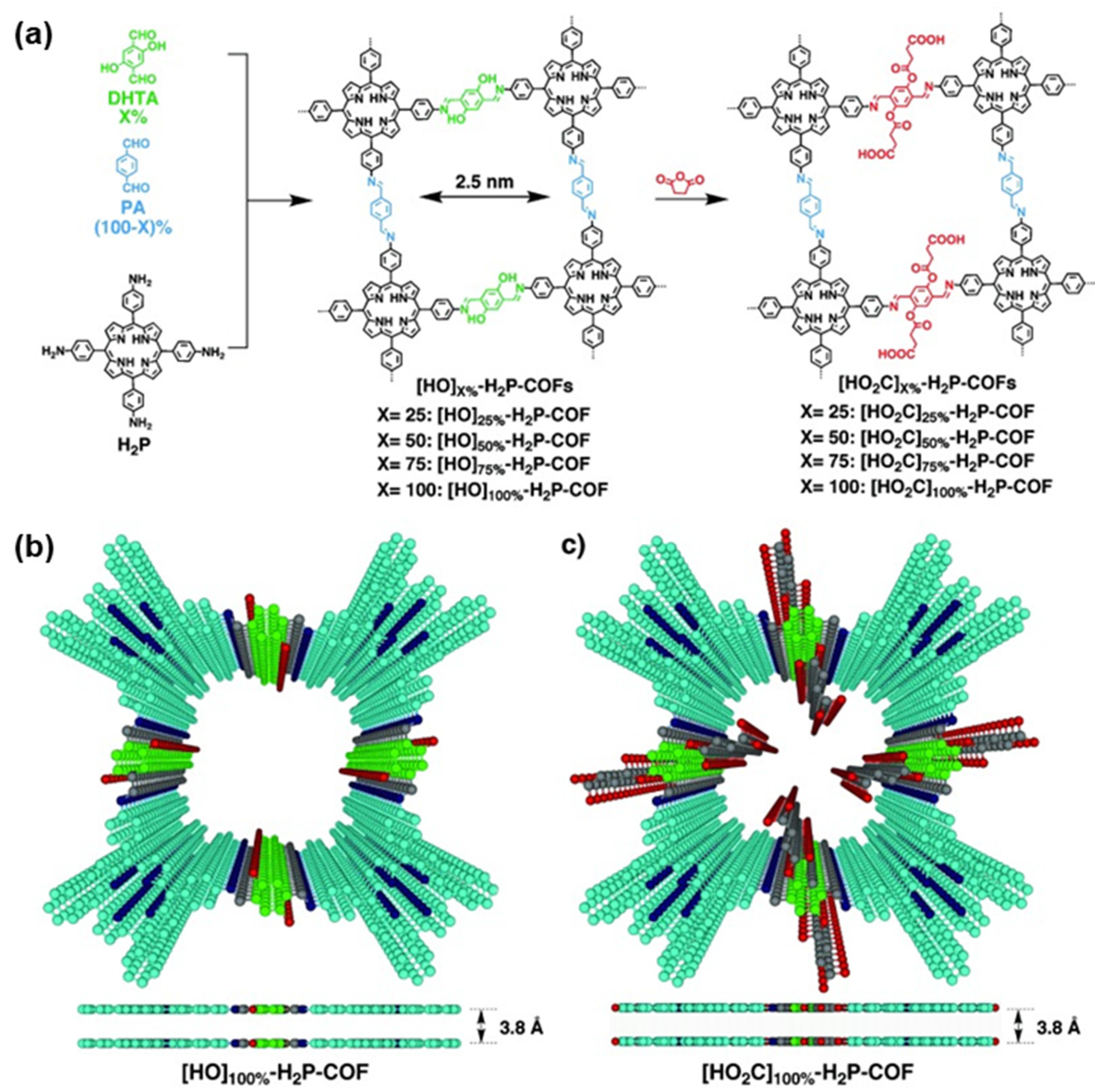
2.1. Two-Dimensional Porphyrin COFs
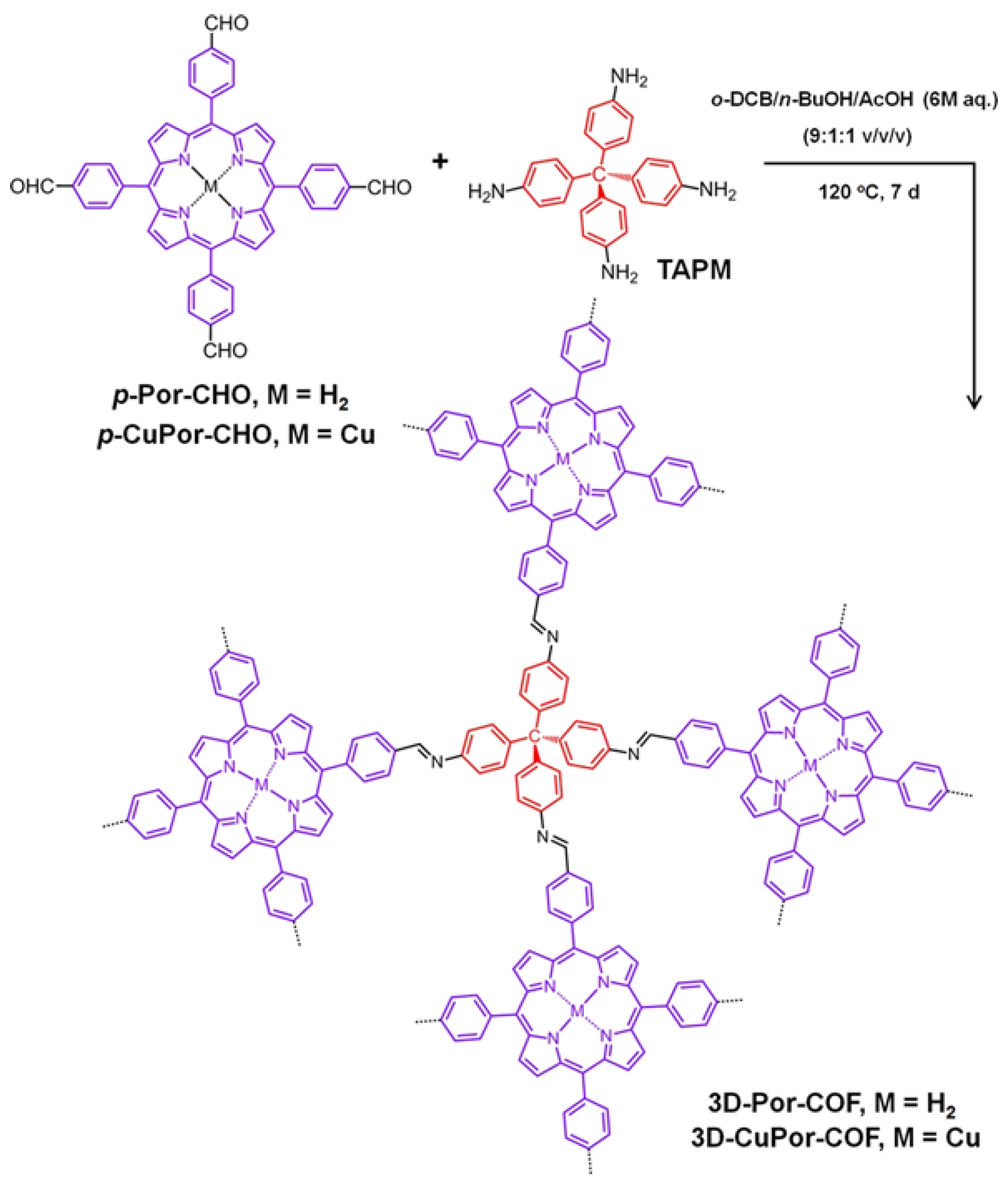
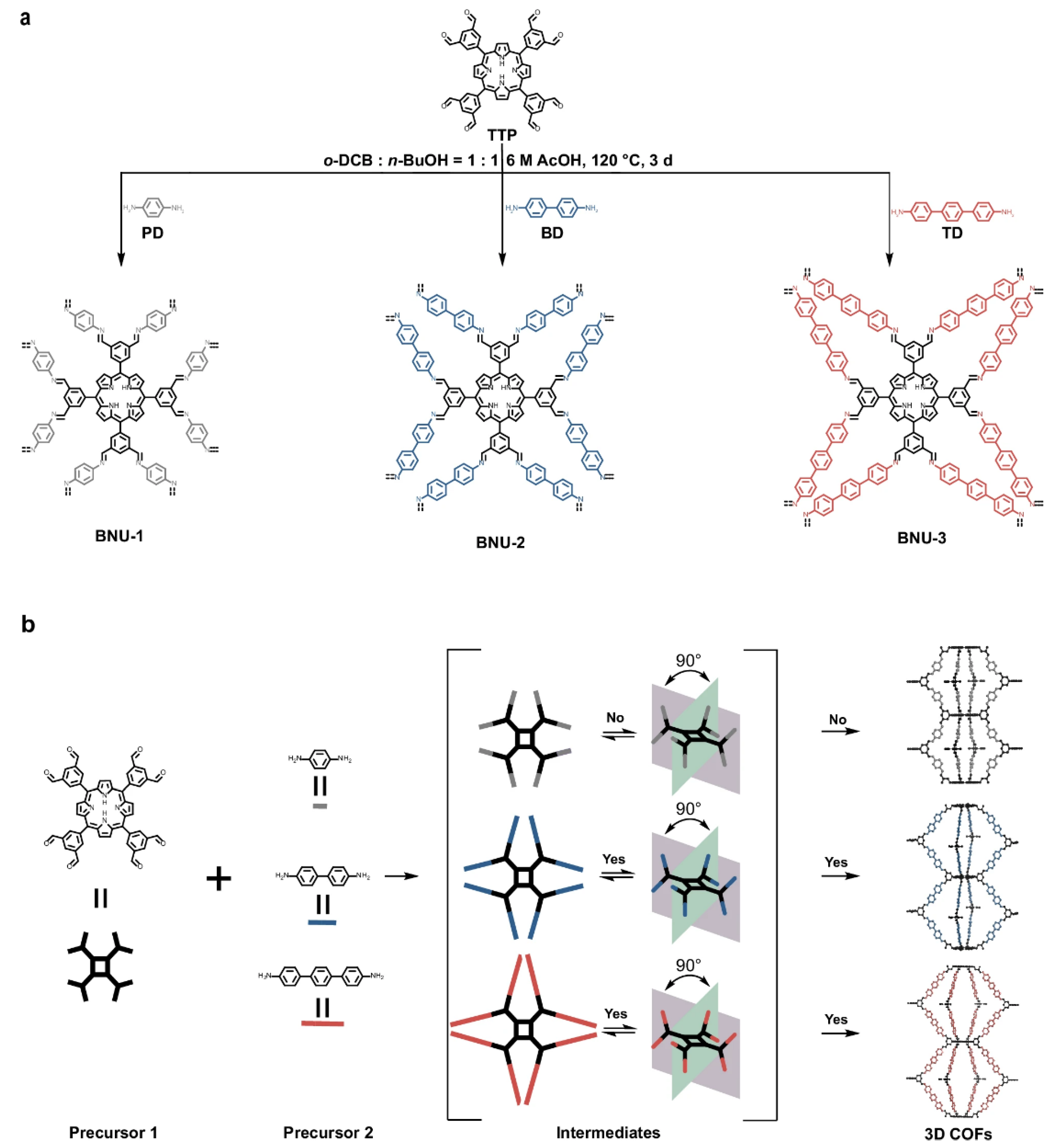
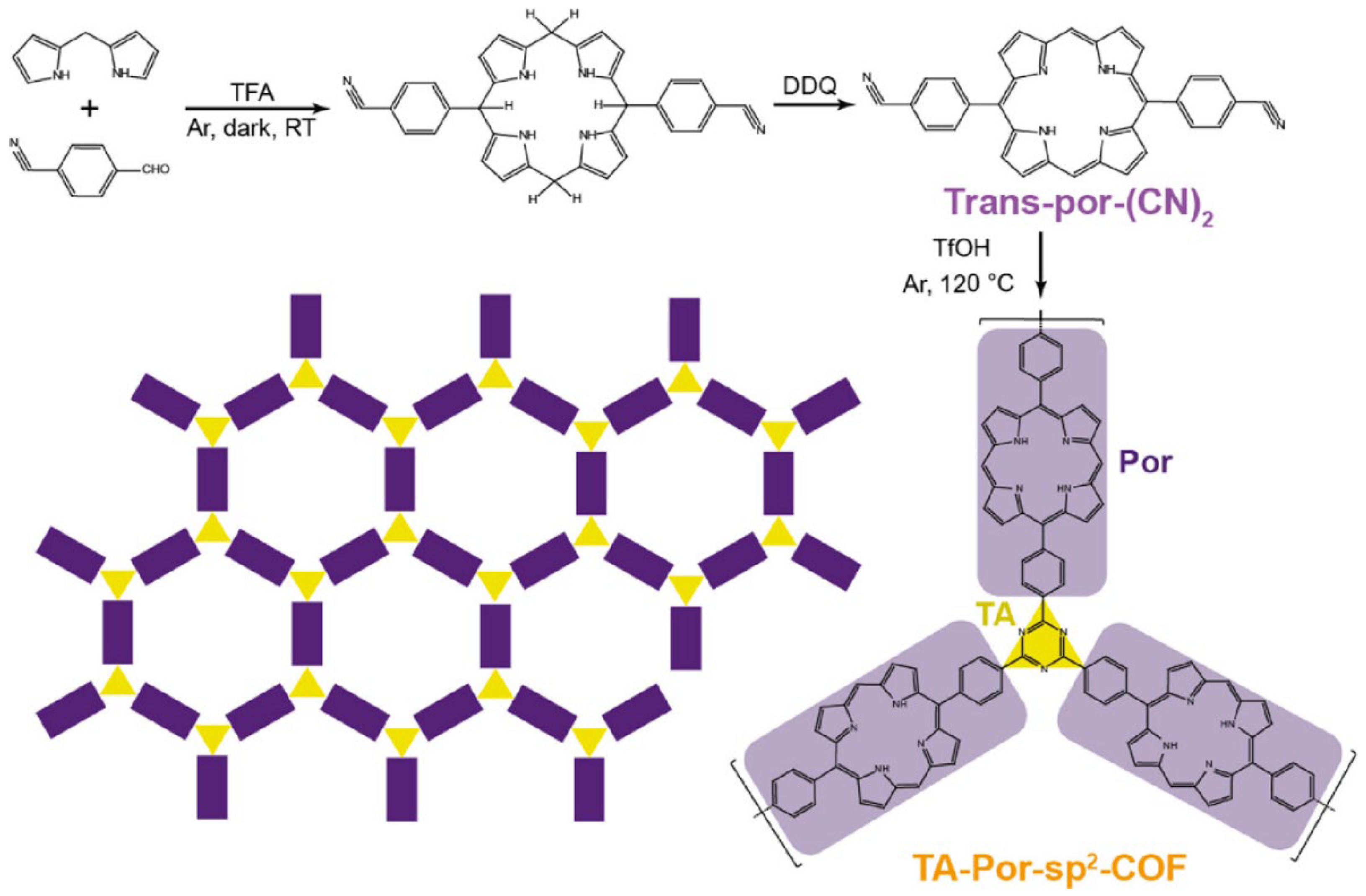
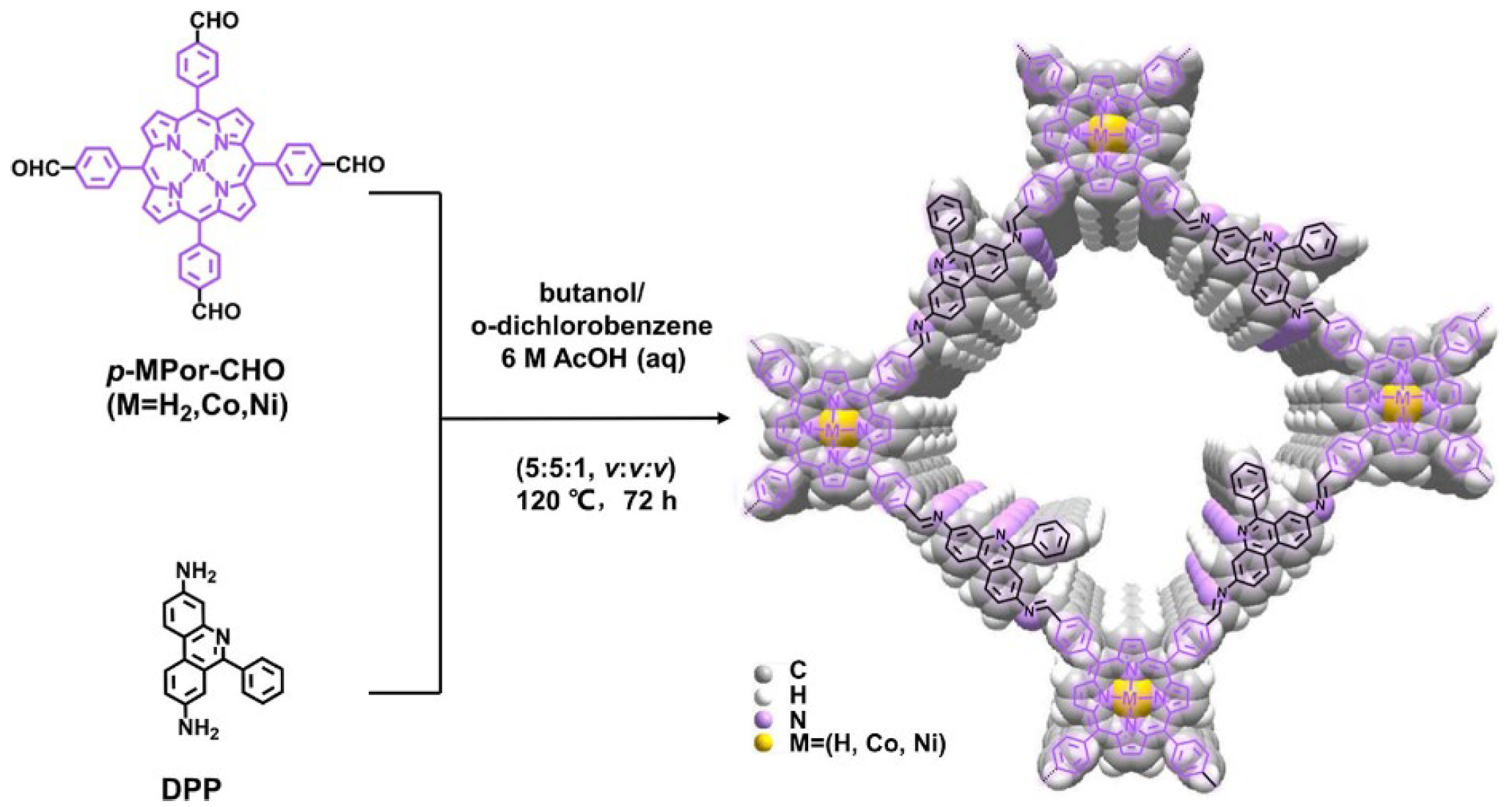
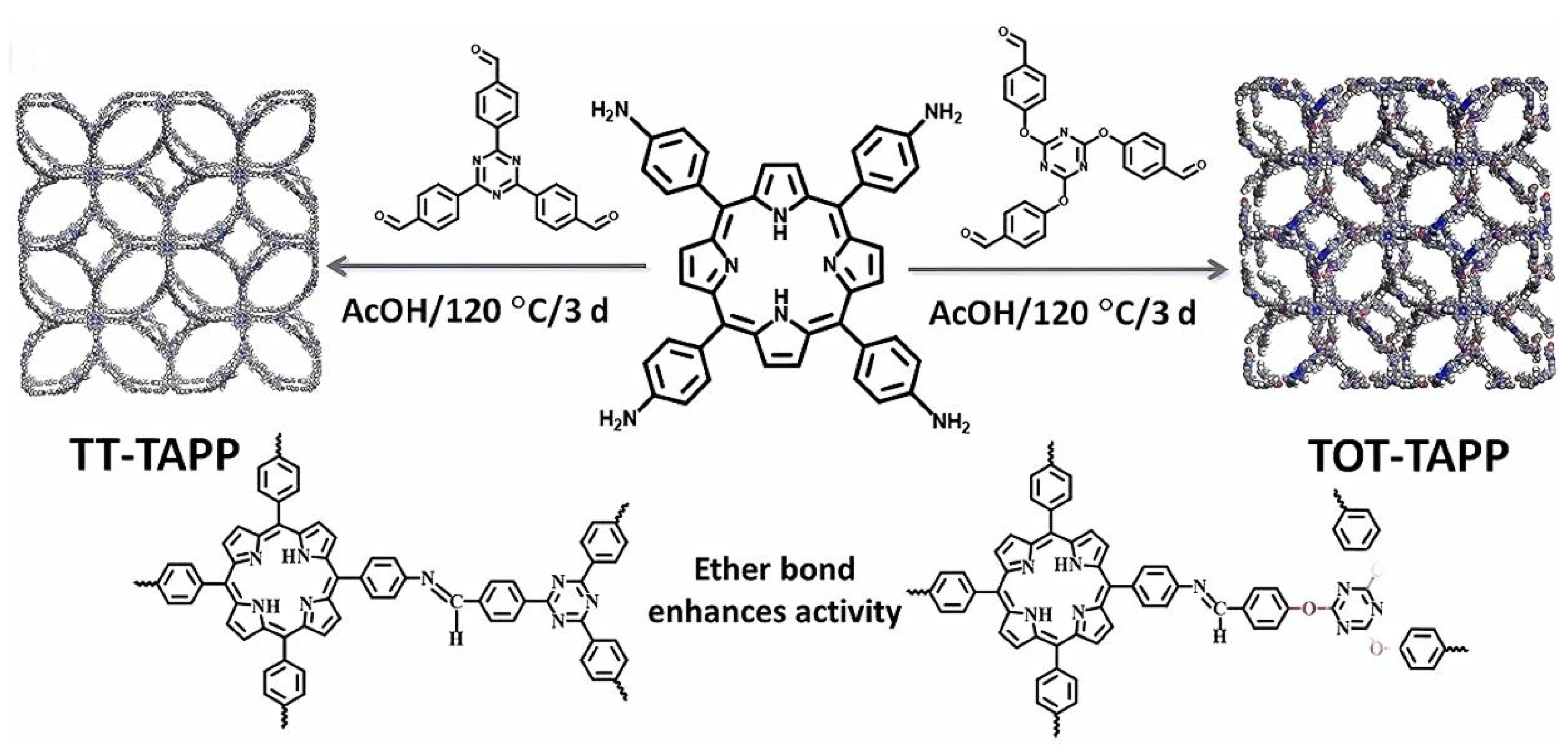
2.2. Three-Dimensional Porphyrin COFs
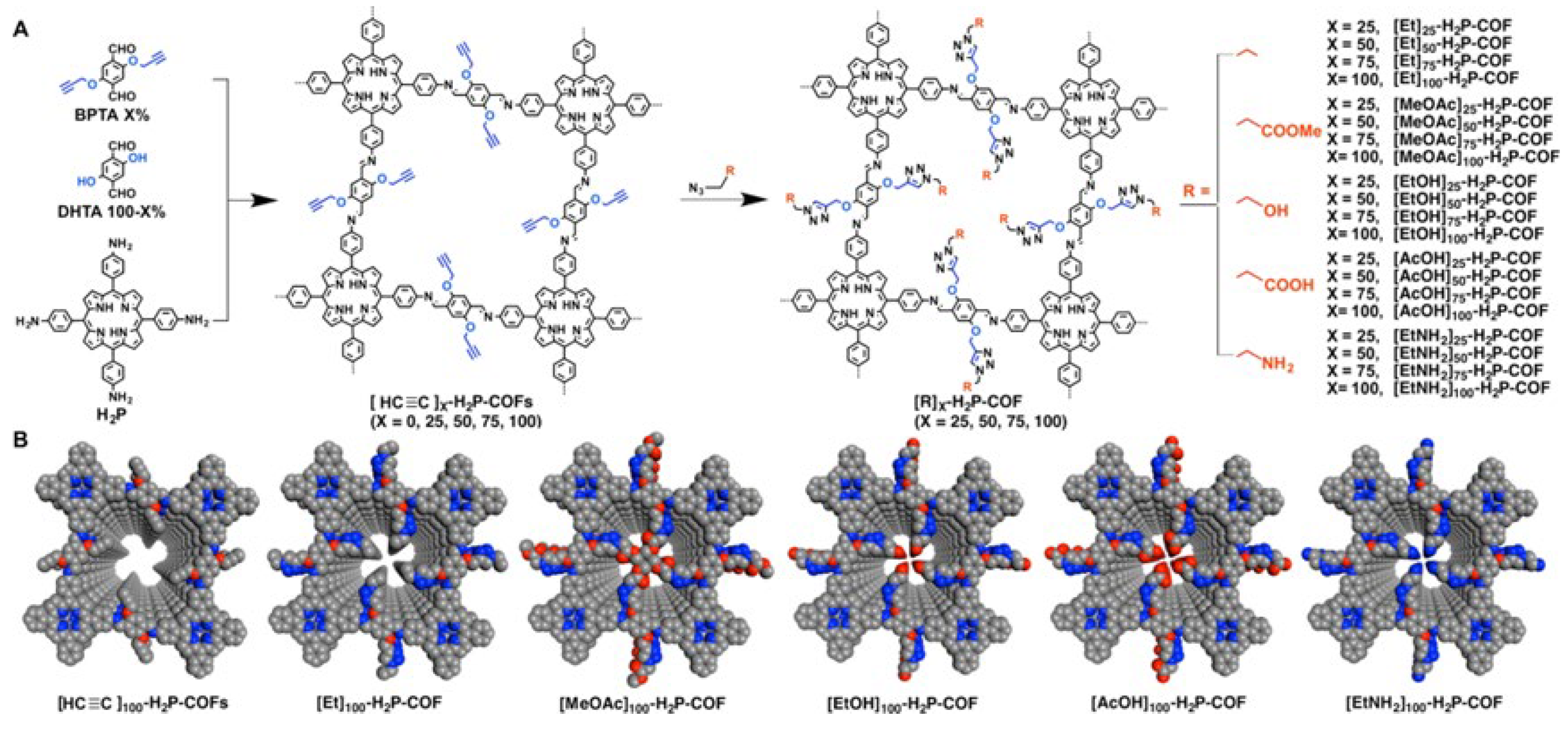
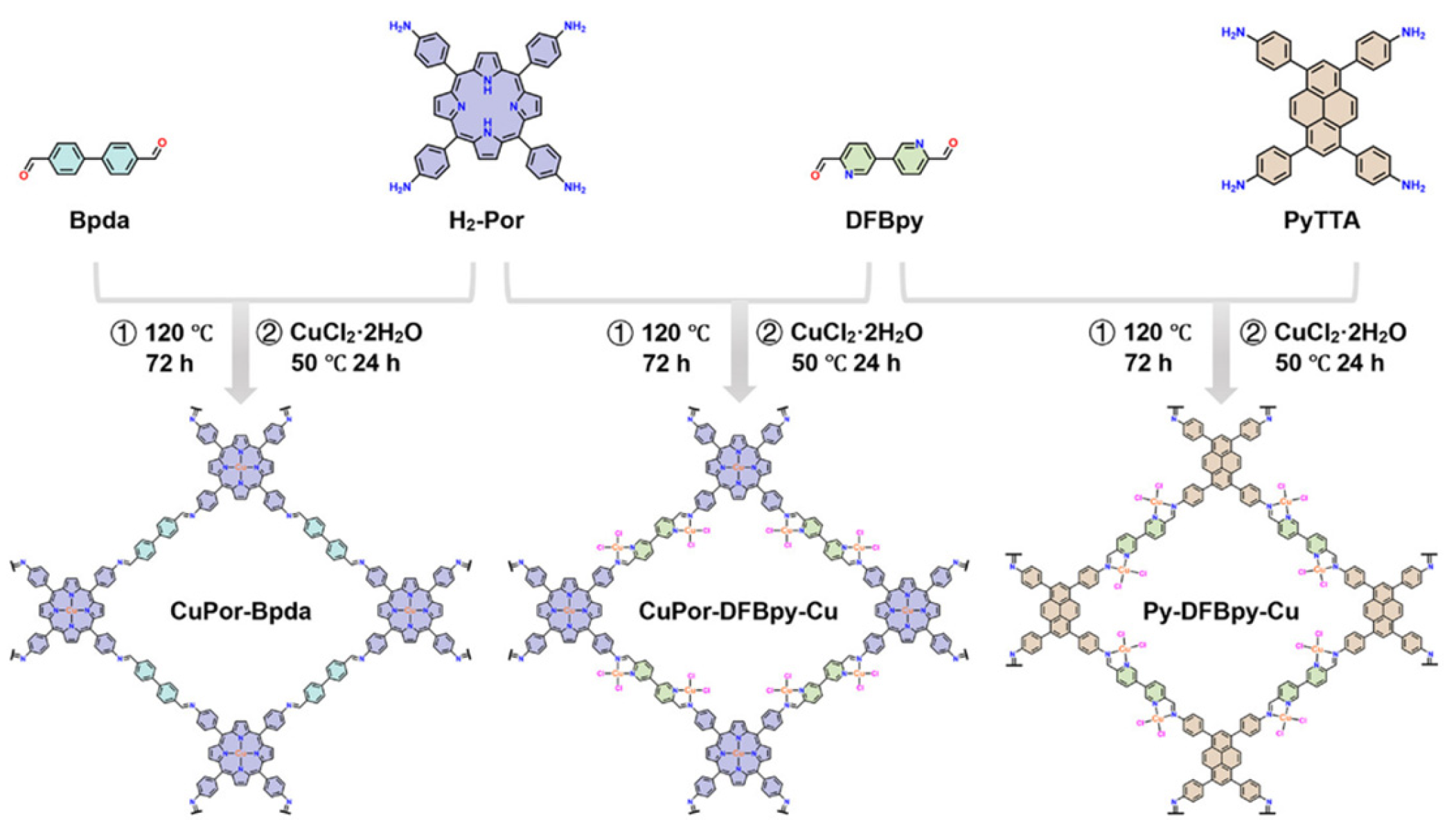
3. Synthesis of Porphyrin COFs
4. Applications
4.1. CO2 Capture and Storage
4.2. Catalytic Reduction Reaction of CO2 (CO2RR)
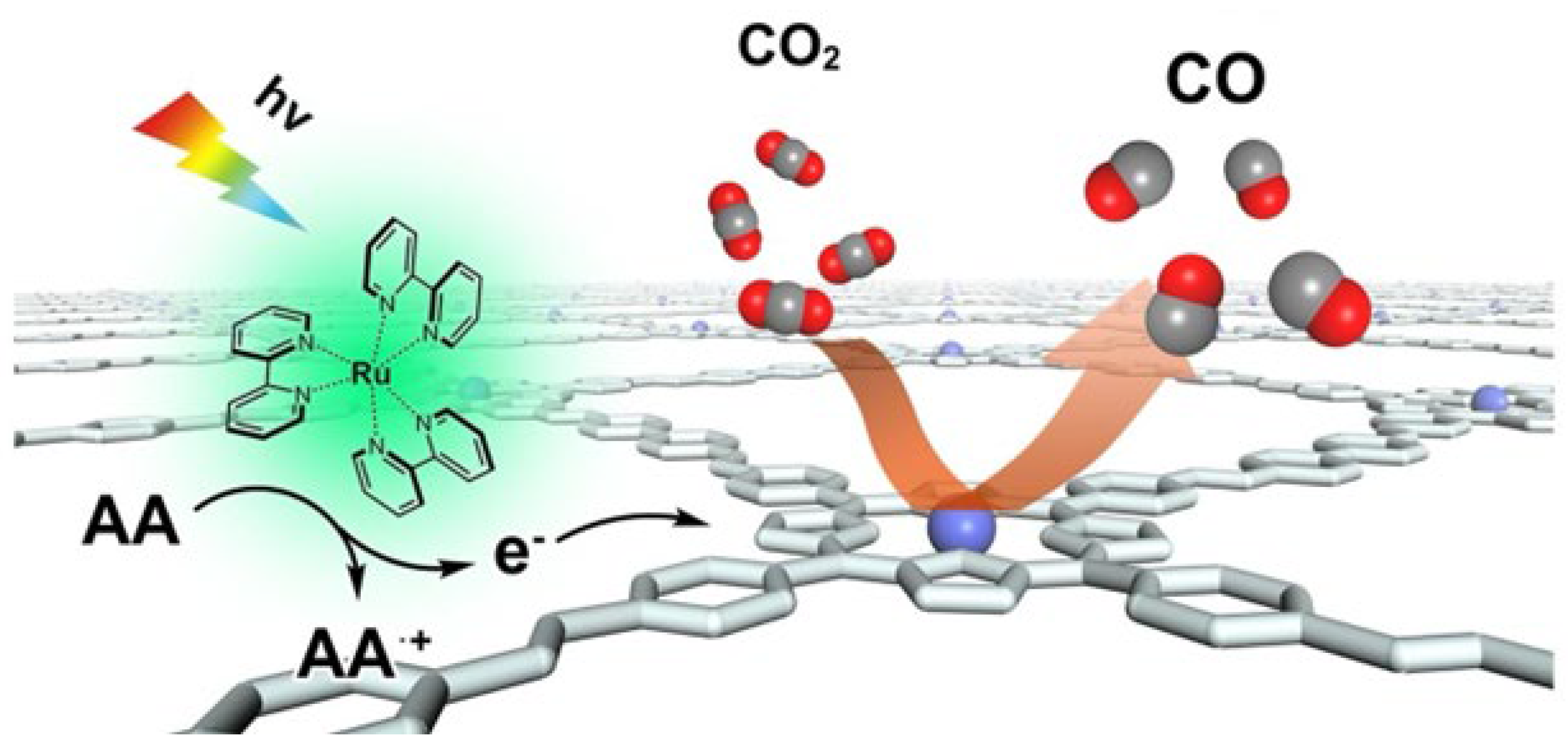
4.2.1. Photocatalysis
4.2.2. Electrocatalysis
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| COF | covalent organic framework |
| CO2RR | CO2 reduction reaction |
| fs-TAS | femtosecond transient absorption spectroscopy |
| PL | photoluminescence |
| TRPL | time-resolved photoluminescence |
| DRIFTS | diffuse reflectance infrared Fourier transform spectroscopy |
| DFT | density functional theory |
| PXRD | powder X-ray diffraction |
| AQE/AQY | apparent quantum efficiency/apparent quantum yield |
References
- Toganoh, M.; Furuta, H. Creation from Confusion and Fusion in the Porphyrin World—The Last Three Decades of N-Confused Porphyrinoid Chemistry. Chem. Rev. 2022, 122, 8313–8437. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Shi, J.; Zou, X.; Huang, X.; Tahir, H.E. Preparation of Conducting Polyaniline/Protoporphyrin Composites and Their Application for Sensing VOCs. Food Chem. 2019, 276, 291–297. [Google Scholar] [CrossRef]
- Gu, H.; Huang, X.; Chen, Q.; Sun, Y. Rapid Assessment of Total Polar Material in Used Frying Oils Using Manganese Tetraphenylporphyrin Fluorescent Sensor with Enhanced Sensitivity. Food Anal. Methods 2020, 13, 2080–2086. [Google Scholar] [CrossRef]
- Lin, H.; Lin, J.; Man, Z.; Jin, H.; Kutsanedzie, F.Y.H.; Chen, Q. Development of Colorimetric Detection of 2,4,5-Trimethyloxazole in Volatile Organic Compounds Based on Porphyrin Complexes for Vinegar Storage Time Discrimination. Food Anal. Methods 2020, 13, 2192–2203. [Google Scholar] [CrossRef]
- Bin, Z.; Feng, L.; Yan, Y. Biomimetic Metalloporphyrin Oxidase Modified Carbon Nanotubes for Highly Sensitive and Stable Quantification of Anti-Oxidants Tert-Butylhydroquinone in Plant Oil. Food Chem. 2022, 388, 132898. [Google Scholar] [CrossRef]
- Gu, H.; Dong, Y.; Lv, R.; Huang, X.; Chen, Q. Rapid Quantification of Acid Value in Frying Oil Using Iron Tetraphenylporphyrin Fluorescent Sensor Coupled with Density Functional Theory and Multivariate Analysis. Food Qual. Saf. 2022, 6, fyac046. [Google Scholar] [CrossRef]
- Gu, H.; Dong, Y.; Zhu, S.; Huang, X.; Sun, Y.; Chen, Q. Development of a Sensor-Based Fluorescent Method for Quality Evaluation of Used Frying Oils. J. Food Compos. Anal. 2022, 112, 104640. [Google Scholar] [CrossRef]
- Gu, H.; Huang, X.; Sun, Y.; Lv, R.; Chen, Q. Qualitative and Quantitative Analysis of Oxidative Degradation Products in Frying Oil by Three-Dimensional Fluorescence Spectroscopy with Metalloporphyrin-Based Sensor. Food Anal. Methods 2022, 15, 1143–1153. [Google Scholar] [CrossRef]
- Jiaojiao, X.; Feng, L.; Lishi, Y.; Hongbo, S.; Jingya, Q.; Bin, Z. Simultaneous Determination of Tert-Butylhydroquinone, Butylated Hydroxyanisole and Phenol in Plant Oil by Metalloporphyrin-Based Covalent Organic Framework Electrochemical Sensor. J. Food Compos. Anal. 2023, 122, 105486. [Google Scholar] [CrossRef]
- Wang, S.; Liang, N.; Hu, X.; Li, W.; Guo, Z.; Zhang, X.; Huang, X.; Li, Z.; Zou, X.; Shi, J. Carbon Dots and Covalent Organic Frameworks Based FRET Immunosensor for Sensitive Detection of Escherichia Coli O157:H7. Food Chem. 2024, 447, 138663. [Google Scholar] [CrossRef]
- Ji, Z.; Zhu, J.; Deng, J.; Meng, F.; Jiang, H.; Chen, Q. High-Precision Identification of Zearalenone Contamination in Wheat Based on Olfactory Sensor Combined with near-Infrared Spectroscopy. J. Food Compos. Anal. 2025, 145, 107805. [Google Scholar] [CrossRef]
- Liu, X.; Qi, R.; Li, S.; Liu, W.; Yu, Y.; Wang, J.; Wu, S.; Ding, K.; Yu, Y. Triazine–Porphyrin-Based Hyperconjugated Covalent Organic Framework for High-Performance Photocatalysis. J. Am. Chem. Soc. 2022, 144, 23396–23404. [Google Scholar] [CrossRef]
- Tavakoli, E.; Kakekhani, A.; Kaviani, S.; Tan, P.; Ghaleni, M.M.; Zaeem, M.A.; Rappe, A.M.; Nejati, S. In Situ Bottom-up Synthesis of Porphyrin-Based Covalent Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 19560–19564. [Google Scholar] [CrossRef]
- Noreen, J.; Amin, M.A.; Rani, S.; Ullah, H.; Zaki, Z.I.; Khalifa, M.E.; Nadeem, M.; Sohail, M.; Khan, S.; Ahmad, Z.; et al. High Performance in Water Splitting(OER) Mediated by Porphyrin Coupled with Polyoxometalates in COF: A Breakthrough in Electrocatalysis. J. Electroanal. Chem. 2025, 990, 119172. [Google Scholar] [CrossRef]
- Wu, J.; Meng, T.; Zhang, X.; Tang, S.; Liu, L.; Xue, J.; Liu, X.; Wang, J.; Wen, J.; Hu, D.; et al. Glucose-Responsive Zn(II)-Porphyrin COF Adhesive Hydrogels With Dual-Active Sites and GOX-Like Activity for Accelerated Wound Healing. Adv. Healthc. Mater. 2025, 14, 2404076. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, Z.; Wang, Y.; Xu, Q.; Wang, K.; Jiang, J. Porphyrin-Based Covalent Organic Frameworks for CO2 Photo/Electro-Reduction. Angew. Chem. Int. Ed. 2025, 64, e202422814. [Google Scholar] [CrossRef]
- Yin, Y.; Fang, L.; Wu, G.; Xu, H.; Li, L. Enhanced Electrocatalytic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid via N-Hydroxyphthalimide-Integrated Porphyrin(Co) Covalent Organic Frameworks. ChemCatChem 2025, 17, e202401674. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Ding, X.; Li, X.; Zhao, S.; Feng, Z.; Ke, W.; Xiao, S.; Zhao, J.; Ma, K. 3D Porphyrin-Based Covalent Organic Frameworks for Efficient Thorium Adsorption: Structural Engineering and Mechanism Insights. Sep. Purif. Technol. 2025, 382, 135731. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Z.; Gong, L.; Wang, K.; Wu, B.; Jiang, J. Integrating Urea-Based Units Into Porphyrinic Covalent Organic Framework for Efficient Artemisinin Photosynthesis. Angew. Chem. Int. Ed. 2025, 64, e202506462. [Google Scholar] [CrossRef]
- Guo, J.; Guo, Y.; Zhang, M.; Yu, H.; Bi, H.; Feng, J.; Wang, J.; Geng, K.; Heine, T.; Sun, J.; et al. Exploring Three-Dimensional Porphyrin-Based Covalent Organic Frameworks with Outstanding Solar Energy Conversion. J. Am. Chem. Soc. 2025, 147, 30369–30379. [Google Scholar] [CrossRef]
- Ma, X.; Hu, J.; Li, S.; Zheng, T.; Gao, Y.; Han, Y.; Pan, H.; Bian, Y.; Jiang, J. Porphyrin-Based Covalent Organic Frameworks with Undulated Layers for Efficient Photocatalytic CO2 Reduction. Sci. Bull. 2025, 70, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Gao, Y.; Li, J.; Cai, Z.; Liu, N.; Jiang, J. Porphyrin-based Vinylene-linked 3D Covalent Organic Framework with Unprecedented Cya Topology for Photocatalytic H2O2 Production. Angew. Chem. Int. Ed. 2025, 64, e202423205. [Google Scholar] [CrossRef]
- Zhong, H.; Sa, R.; Lv, H.; Yang, S.; Yuan, D.; Wang, X.; Wang, R. Covalent Organic Framework Hosting Metalloporphyrin-Based Carbon Dots for Visible-Light-Driven Selective CO2 Reduction. Adv. Funct. Mater. 2020, 30, 2002654. [Google Scholar] [CrossRef]
- Li, X.-G.; Li, J.; Chen, J.; Rao, L.; Zheng, L.; Yu, F.; Tang, Y.; Zheng, J.; Ma, J. Porphyrin-Based Covalent Organic Frameworks from Design, Synthesis to Biological Applications. Biomater. Sci. 2024, 12, 2766–2785. [Google Scholar] [CrossRef]
- Chen, R.; Wang, Y.; Ma, Y.; Mal, A.; Gao, X.-Y.; Gao, L.; Qiao, L.; Li, X.-B.; Wu, L.-Z.; Wang, C. Rational Design of Isostructural 2D Porphyrin-Based Covalent Organic Frameworks for Tunable Photocatalytic Hydrogen Evolution. Nat. Commun. 2021, 12, 1354. [Google Scholar] [CrossRef]
- Lin, G.; Ding, H.; Chen, R.; Peng, Z.; Wang, B.; Wang, C. 3D Porphyrin-Based Covalent Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 8705–8709. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Huang, X.; Dong, L.; Lu, M.; Guo, C.; Yuan, D.; Chen, Y.; Xu, G.; Li, S.; et al. Porphyrin-Based COF 2D Materials: Variable Modification of Sensing Performances by Post-Metallization. Angew. Chem. 2022, 134, e202115308. [Google Scholar] [CrossRef]
- Wonanke, A.D.D.; Addicoat, M.A. Effect of Unwanted Guest Molecules on the Stacking Configuration of Covalent Organic Frameworks: A Periodic Energy Decomposition Analysis. Phys. Chem. Chem. Phys. 2022, 24, 15494–15501. [Google Scholar] [CrossRef] [PubMed]
- Pelkowski, C.E.; Natraj, A.; Malliakas, C.D.; Burke, D.W.; Bardot, M.I.; Wang, Z.; Li, H.; Dichtel, W.R. Tuning Crystallinity and Stacking of Two-Dimensional Covalent Organic Frameworks through Side-Chain Interactions. J. Am. Chem. Soc. 2023, 145, 21798–21806. [Google Scholar] [CrossRef]
- Kharel, P.; Carmichael, P.T.; Natraj, A.; Pelkowski, C.E.; Bae, S.H.; Dichtel, W.R.; Huang, P.Y. 3D Imaging Reveals Widespread Stacking Disorder in Single Crystal 2D Covalent Organic Frameworks. J. Am. Chem. Soc. 2025, 147, 11821–11828. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Deng, W.; Lu, X. Metallosalen Covalent Organic Frameworks for Heterogeneous Catalysis. Interdiscip. Mater. 2024, 3, 87–112. [Google Scholar] [CrossRef]
- Kohn, J.T.; Li, H.; Evans, A.M.; Brédas, J.-L.; Grimme, S. Quantum Chemistry Insight into the Multifaceted Structural Properties of Two-Dimensional Covalent Organic Frameworks. Chem. Mater. 2023, 35, 2820–2826. [Google Scholar] [CrossRef]
- Jin, F.; Lin, E.; Wang, T.; Yan, D.; Yang, Y.; Chen, Y.; Cheng, P.; Zhang, Z. Rationally Fabricating 3D Porphyrinic Covalent Organic Frameworks with Scu Topology as Highly Efficient Photocatalysts. Chem 2022, 8, 3064–3080. [Google Scholar] [CrossRef]
- Lai, J.; Liu, S.; Ma, W.; Wu, F.; Yu, P.; Mao, L. Steric Effect of Intermediates Induces Formation of Three-Dimensional Covalent Organic Frameworks. Nat. Commun. 2025, 16, 6071. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.-Y.; Joseph, E.; Zhou, H.-C. Catalytic Porphyrin Framework Compounds. Trends Chem. 2020, 2, 555–568. [Google Scholar] [CrossRef]
- Liao, H.; Wang, H.; Ding, H.; Meng, X.; Xu, H.; Wang, B.; Ai, X.; Wang, C. A 2D Porous Porphyrin-Based Covalent Organic Framework for Sulfur Storage in Lithium–Sulfur Batteries. J. Mater. Chem. A 2016, 4, 7416–7421. [Google Scholar] [CrossRef]
- Calik, M.; Auras, F.; Salonen, L.M.; Bader, K.; Grill, I.; Handloser, M.; Medina, D.D.; Dogru, M.; Löbermann, F.; Trauner, D.; et al. Extraction of Photogenerated Electrons and Holes from a Covalent Organic Framework Integrated Heterojunction. J. Am. Chem. Soc. 2014, 136, 17802–17807. [Google Scholar] [CrossRef] [PubMed]
- Neti, V.S.P.K.; Wu, X.; Hosseini, M.; Bernal, R.A.; Deng, S.; Echegoyen, L. Synthesis of a Phthalocyanine 2D Covalent Organic Framework. CrystEngComm 2013, 15, 7157. [Google Scholar] [CrossRef]
- Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S.K.; Liao, L.; Ambrogio, M.W.; Botros, Y.Y.; Duan, X.; et al. Covalent Organic Frameworks with High Charge Carrier Mobility. Chem. Mater. 2011, 23, 4094–4097. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent Organic Frameworks Comprising Cobalt Porphyrins for Catalytic CO2 Reduction in Water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef]
- Biswal, B.P.; Valligatla, S.; Wang, M.; Banerjee, T.; Saad, N.A.; Mariserla, B.M.K.; Chandrasekhar, N.; Becker, D.; Addicoat, M.; Senkovska, I.; et al. Nonlinear Optical Switching in Regioregular Porphyrin Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2019, 58, 6896–6900. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, J.-P.; Li, R.-H.; Sun, S.-N.; Lu, M.; Dong, L.-Z.; Huang, P.; Li, S.-L.; Cai, Y.-P.; Lan, Y.-Q. Green Synthesis of Bifunctional Phthalocyanine-Porphyrin COFs in Water for Efficient Electrocatalytic CO2 Reduction Coupled with Methanol Oxidation. Natl. Sci. Rev. 2023, 10, nwad226. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Wang, H.; Yi, H.; Li, B.; Liu, S.; Zhang, M.; et al. Recent Advances in Covalent Organic Frameworks (COFs) as a Smart Sensing Material. Chem. Soc. Rev. 2019, 48, 5266–5302. [Google Scholar] [CrossRef]
- Li, L.-H.; Feng, X.-L.; Cui, X.-H.; Ma, Y.-X.; Ding, S.-Y.; Wang, W. Salen-Based Covalent Organic Framework. J. Am. Chem. Soc. 2017, 139, 6042–6045. [Google Scholar] [CrossRef]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A.; Jiang, D. An Azine-Linked Covalent Organic Framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, L.; Honsho, Y.; Saeki, A.; Seki, S.; Irle, S.; Dong, Y.; Nagai, A.; Jiang, D. High-Rate Charge-Carrier Transport in Porphyrin Covalent Organic Frameworks: Switching from Hole to Electron to Ambipolar Conduction. Angew. Chem. Int. Ed. 2012, 51, 2618–2622. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Wang, T.; Li, H.; Wang, X.; Ma, L.; Zhang, N.; Yue, P.; Li, Y. Design and Synthesis of Dual Functional Porphyrin-Based COFs as Highly Selective Adsorbent and Photocatalyst. Chem. Eng. J. 2023, 470, 144135. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Liu, J.; Parvez, K.; Liang, H.; Shu, J.; Sachdev, H.; Graf, R.; Feng, X.; Müllen, K. High-Performance Electrocatalysts for Oxygen Reduction Derived from Cobalt Porphyrin-Based Conjugated Mesoporous Polymers. Adv. Mater. 2014, 26, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, L.; Dai, S.; Zhao, C.; Ma, C.; Wei, L.; Zhu, M.; Chong, S.Y.; Yang, H.; Liu, L.; et al. Reconstructed Covalent Organic Frameworks. Nature 2022, 604, 72–79. [Google Scholar] [CrossRef]
- Tohidi, M.E.; Amiri, A. Literature on the Synthesis of Modulator-Assisted Covalent–Organic Frameworks (MA-COFs): A Review. Adv. Mater. 2025, e11083. [Google Scholar] [CrossRef]
- Xiang, Z.; Xue, Y.; Cao, D.; Huang, L.; Chen, J.; Dai, L. Highly Efficient Electrocatalysts for Oxygen Reduction Based on 2D Covalent Organic Polymers Complexed with Non-precious Metals. Angew. Chem. Int. Ed. 2014, 53, 2433–2437. [Google Scholar] [CrossRef]
- Huang, N.; Chen, X.; Krishna, R.; Jiang, D. Two-Dimensional Covalent Organic Frameworks for Carbon Dioxide Capture through Channel-Wall Functionalization. Angew. Chem. Int. Ed. 2015, 54, 2986–2990. [Google Scholar] [CrossRef]
- Chen, X.; Addicoat, M.; Jin, E.; Zhai, L.; Xu, H.; Huang, N.; Guo, Z.; Liu, L.; Irle, S.; Jiang, D. Locking Covalent Organic Frameworks with Hydrogen Bonds: General and Remarkable Effects on Crystalline Structure, Physical Properties, and Photochemical Activity. J. Am. Chem. Soc. 2015, 137, 3241–3247. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Synfacts 2006, 2006, 231. [Google Scholar] [CrossRef]
- Huang, N.; Krishna, R.; Jiang, D. Tailor-Made Pore Surface Engineering in Covalent Organic Frameworks: Systematic Functionalization for Performance Screening. J. Am. Chem. Soc. 2015, 137, 7079–7082. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cai, K.; Tao, D.-J.; Zhao, T. The Mega-Merger Strategy: M@COF Core-Shell Hybrid Materials for Facilitating CO2 Capture and Conversion to Monocyclic and Polycyclic Carbonates. Appl. Catal. B Environ. 2024, 341, 123317. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Chong, S.Y.; Little, M.A.; Wu, Y.; Zhu, W.-H.; Clowes, R.; Yan, Y.; Zwijnenburg, M.A.; Sprick, R.S.; et al. Sulfone-Containing Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution from Water. Nat. Chem. 2018, 10, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Kapustin, E.A.; Yin, S.X.; Liang, L.; Zhou, Z.; Niu, J.; Li, L.-H.; Wang, Y.; Su, J.; Li, J.; et al. Single-Crystal x-Ray Diffraction Structures of Covalent Organic Frameworks. Science 2018, 361, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.D.; Petrus, M.L.; Jumabekov, A.N.; Margraf, J.T.; Weinberger, S.; Rotter, J.M.; Clark, T.; Bein, T. Directional Charge-Carrier Transport in Oriented Benzodithiophene Covalent Organic Framework Thin Films. ACS Nano 2017, 11, 2706–2713. [Google Scholar] [CrossRef]
- Babarao, R.; Jiang, J. Exceptionally High CO2 Storage in Covalent-Organic Frameworks: Atomistic Simulation Study. Energy Environ. Sci. 2008, 1, 139. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Z.; Zhang, L.; Zhang, Q. Covalent Organic Frameworks for Photocatalytic Reduction of Carbon Dioxide: A Review. ACS Nano 2024, 18, 21804–21835. [Google Scholar] [CrossRef]
- Che, W.; Zhao, S.; Byun, W.J.; Tao, T.; Jeon, J.; Zhao, Q.; Shao, Y.; Li, J.; Kim, J.; Lee, J.S.; et al. From Carbon Nitrides to COFs: Opportunities and Prospects in Photocatalytic CO2 Reduction. Adv. Mater. 2025, 37, e06961. [Google Scholar] [CrossRef]
- Zhong, W.; Sa, R.; Li, L.; He, Y.; Li, L.; Bi, J.; Zhuang, Z.; Yu, Y.; Zou, Z. A Covalent Organic Framework Bearing Single Ni Sites as a Synergistic Photocatalyst for Selective Photoreduction of CO2 to CO. J. Am. Chem. Soc. 2019, 141, 7615–7621. [Google Scholar] [CrossRef]
- Lu, M.; Liu, J.; Li, Q.; Zhang, M.; Liu, M.; Wang, J.; Yuan, D.; Lan, Y. Rational Design of Crystalline Covalent Organic Frameworks for Efficient CO2 Photoreduction with H2O. Angew. Chem. 2019, 131, 12522–12527. [Google Scholar] [CrossRef]
- Lu, M.; Li, Q.; Liu, J.; Zhang, F.-M.; Zhang, L.; Wang, J.-L.; Kang, Z.-H.; Lan, Y.-Q. Installing Earth-Abundant Metal Active Centers to Covalent Organic Frameworks for Efficient Heterogeneous Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2019, 254, 624–633. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Wang, C.; Pan, H.; Liu, W.; Wang, K.; Zeng, Q.; Wang, R.; Jiang, J. A Scalable General Synthetic Approach toward Ultrathin Imine-Linked Two-Dimensional Covalent Organic Framework Nanosheets for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 17431–17440. [Google Scholar] [CrossRef]
- Li, S.-Y.; Meng, S.; Zou, X.; El-Roz, M.; Telegeev, I.; Thili, O.; Liu, T.X.; Zhu, G. Rhenium-Functionalized Covalent Organic Framework Photocatalyst for Efficient CO2 Reduction under Visible Light. Microporous Mesoporous Mater. 2019, 285, 195–201. [Google Scholar] [CrossRef]
- Bi, J.; Xu, B.; Sun, L.; Huang, H.; Fang, S.; Li, L.; Wu, L. A Cobalt-Modified Covalent Triazine-Based Framework as an Efficient Cocatalyst for Visible-Light-Driven Photocatalytic CO2 Reduction. ChemPlusChem 2019, 84, 1149–1154. [Google Scholar] [CrossRef]
- Yang, S.; Hu, W.; Zhang, X.; He, P.; Pattengale, B.; Liu, C.; Cendejas, M.; Hermans, I.; Zhang, X.; Zhang, J.; et al. 2D Covalent Organic Frameworks as Intrinsic Photocatalysts for Visible Light-Driven CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 14614–14618. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Fujisawa, Y.; Satake, A. Photocatalytic CO2 Reduction Mediated by Electron Transfer via the Excited Triplet State of Zn(II) Porphyrin. J. Am. Chem. Soc. 2020, 142, 705–709. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and Perspectives of CO2 Conversion into Fuels and Chemicals by Catalytic, Photocatalytic and Electrocatalytic Processes. Energy Environ. Sci. 2013, 6, 3112. [Google Scholar] [CrossRef]
- Kojima, T.; Honda, T.; Ohkubo, K.; Shiro, M.; Kusukawa, T.; Fukuda, T.; Kobayashi, N.; Fukuzumi, S. A Discrete Supramolecular Conglomerate Composed of Two Saddle-Distorted Zinc(II)-Phthalocyanine Complexes and a Doubly Protonated Porphyrin with Saddle Distortion Undergoing Efficient Photoinduced Electron Transfer. Angew. Chem. Int. Ed. 2008, 47, 6712–6716. [Google Scholar] [CrossRef]
- Jana, A.; Bähring, S.; Ishida, M.; Goeb, S.; Canevet, D.; Sallé, M.; Jeppesen, J.O.; Sessler, J.L. Functionalised Tetrathiafulvalene- (TTF-) Macrocycles: Recent Trends in Applied Supramolecular Chemistry. Chem. Soc. Rev. 2018, 47, 5614–5645. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.M.; Kawashima, Y.; Ohkubo, K.; Lim, J.M.; Kim, D.; Fukuzumi, S.; Sessler, J.L. Photoinduced Electron Transfer from a Tetrathiafulvalene-Calix [4]Pyrrole to a Porphyrin Carboxylate within a Supramolecular Ensemble. J. Phys. Chem. C 2014, 118, 13503–13513. [Google Scholar] [CrossRef]
- Graetzel, C.K.; Graetzel, M. Light-Driven Electron Transfer from Tetrathiafulvalene to Porphyrins and Ru(Bipy)32+. Charge Separation by Organized Assemblies. J. Phys. Chem. 1982, 86, 2710–2714. [Google Scholar] [CrossRef]
- Wang, X.; Ding, X.; Wang, T.; Wang, K.; Jin, Y.; Han, Y.; Zhang, P.; Li, N.; Wang, H.; Jiang, J. Two-Dimensional Porphyrin-Based Covalent Organic Framework with Enlarged Inter-Layer Spacing for Tunable Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2022, 14, 41122–41130. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Gong, L.; Li, J.; Qi, D.; Liu, N.; Bian, Y.; Jiang, J. Unprecedented POSS-Linked 3D Covalent Organic Frameworks with 2-Fold Interpenetrated Scu or Sqc Topology Regulated by Porphyrin Center for Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2024, 63, e202404156. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Q.; Zhang, J.; Wang, T.; Liu, Z.-Q. Ether-Embedded Covalent Organic Frameworks Enable Efficient Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2025, 64, e202500329. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, C.; Dong, T.; Song, Y.; Wang, T.; Che, H.; Sheng, H.; Wu, B.; Wang, C. Fullerene C70-Encapsulated Tetrathiafulvalene-Co Porphyrin Covalent Organic Framework: Driving Multistep Charge Transfer to Boost CO2 Photoreduction. Adv. Sci. 2025, 12, 2505161. [Google Scholar] [CrossRef]
- Cheng, D.; Ding, L.; Gong, C.; Zhang, L.; Ma, L.; Peng, Y.; Yuan, G. Electron Cloud Density Modulation in Three-Dimensional Porphyrin-Based Covalent Organic Frameworks for Enhanced Photocatalytic CO2 Reduction. ACS Mater. Lett. 2025, 7, 1235–1241. [Google Scholar] [CrossRef]
- Dong, H.; Lu, M.; Wang, Y.; Tang, H.-L.; Wu, D.; Sun, X.; Zhang, F.-M. Covalently Anchoring Covalent Organic Framework on Carbon Nanotubes for Highly Efficient Electrocatalytic CO2 Reduction. Appl. Catal. B Environ. 2022, 303, 120897. [Google Scholar] [CrossRef]
- Lu, C.; Yang, J.; Wei, S.; Bi, S.; Xia, Y.; Chen, M.; Hou, Y.; Qiu, M.; Yuan, C.; Su, Y.; et al. Atomic Ni Anchored Covalent Triazine Framework as High Efficient Electrocatalyst for Carbon Dioxide Conversion. Adv. Funct. Mater. 2019, 29, 1806884. [Google Scholar] [CrossRef]
- Li, J.; Tan, Y.-X.; Lin, J.; Feng, Y.; Zhang, X.; Zhou, E.; Yuan, D.; Wang, Y. Coupling Electrocatalytic Redox-Active Sites in a Three-Dimensional Bimetalloporphyrin-Based Covalent Organic Framework for Enhancing Carbon Dioxide Reduction and Oxygen Evolution. J. Mater. Chem. A 2024, 12, 9478–9485. [Google Scholar] [CrossRef]
- Veldhuizen, H.; Abdinejad, M.; Gilissen, P.J.; Albertsma, J.; Burdyny, T.; Tichelaar, F.D.; Van Der Zwaag, S.; Van Der Veen, M.A. Combining Nickel- and Zinc-Porphyrin Sites via Covalent Organic Frameworks for Electrochemical CO2 Reduction. ACS Appl. Mater. Interfaces 2024, 16, 34010–34019. [Google Scholar] [CrossRef]
- Endo, K.; Raza, A.; Yao, L.; Van Gele, S.; Rodríguez-Camargo, A.; Vignolo-González, H.A.; Grunenberg, L.; Lotsch, B.V. Downsizing Porphyrin Covalent Organic Framework Particles Using Protected Precursors for Electrocatalytic CO2 Reduction. Adv. Mater. 2024, 36, 2313197. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhong, S.; Jing, Z.; Fan, Y. Efficient CO2 Electroreduction to Methane on Covalent Organic Frameworks with CuN4 and CuN2 Cl2 Dual Sites. Adv. Funct. Mater. 2025, 35, 2509578. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Sang, L.; Wang, Y. Recent Advances in Porphyrin-Based COFs Boosting CO2 Photocatalytic and Electrocatalytic Conversion. Nanomaterials 2025, 15, 1787. https://doi.org/10.3390/nano15231787
Yin J, Sang L, Wang Y. Recent Advances in Porphyrin-Based COFs Boosting CO2 Photocatalytic and Electrocatalytic Conversion. Nanomaterials. 2025; 15(23):1787. https://doi.org/10.3390/nano15231787
Chicago/Turabian StyleYin, Jiatong, Linxue Sang, and Yue Wang. 2025. "Recent Advances in Porphyrin-Based COFs Boosting CO2 Photocatalytic and Electrocatalytic Conversion" Nanomaterials 15, no. 23: 1787. https://doi.org/10.3390/nano15231787
APA StyleYin, J., Sang, L., & Wang, Y. (2025). Recent Advances in Porphyrin-Based COFs Boosting CO2 Photocatalytic and Electrocatalytic Conversion. Nanomaterials, 15(23), 1787. https://doi.org/10.3390/nano15231787






