Abstract
Addressing the limitations of poor piezoelectric photocatalytic activity and insufficient magnetic recovery in pure BiFeO3 nanoparticles, Gd and Zr co-doped BiFeO3 nanoparticles were synthesized via the sol-gel method. The structural characterization revealed a rhombohedral-to-orthorhombic phase transition with reduced grain size (~35 nm) and lattice distortion due to dopant incorporation. An XPS analysis confirmed Fe3+ dominance and oxygen vacancy enrichment, while optimized BGFZ9 exhibited enhanced remanent magnetization (0.1753 emu/g, 14.14 increase) compared to undoped BFO. The synergistic piezo-photocatalytic system achieved 81.08% Ofloxacin degradation within 120 min (rate constant: 0.0136 min−1, 1.26 higher than BFO) through stress-induced piezoelectric fields that promoted electron transfer for ·O2−/·OH radical generation via O2 reduction. The Ofloxacin degradation efficiency decreased to 24.36% after four cycles, with structural integrity confirmed by XRD phase stability. This work demonstrates a triple-optimization mechanism (crystal phase engineering, defect modulation, and magnetic enhancement) for designing magnetically recoverable multiferroic catalysts in pharmaceutical wastewater treatment.
1. Introduction
Environmental pollution has emerged as a significant issue globally, and it is imperative to adopt measures to curtail the emission of harmful substances to achieve the objective of sustainable social development [1,2,3]. Ofloxacin (OFL), a third-generation fluoroquinolone antibiotic, is extensively used to treat Gram-negative bacterial infections in the respiratory, genitourinary, and dermal systems due to its broad-spectrum antimicrobial activity and high bioavailability [4]. However, the fluorine atoms in its molecular structure enhance chemical stability, resulting in poor biodegradability, high environmental persistence, significant bioaccumulation potential, and ecological toxicity [5]. The mass production and improper disposal of OFL have led to widespread environmental contamination, with detected concentrations ranging from 0.5 ng/L to 30 mg/L in surface water, groundwater, and medical wastewater worldwide [6,7]. These residues not only induce bacterial resistance and chronic toxicity in aquatic environments but also disrupt human gut microbiota, impair nutrient absorption, and potentially generate “superbugs” through antibiotic resistance gene transfer, posing severe threats to ecosystem integrity and public health [8,9]. Consequently, developing efficient and eco-friendly degradation technologies and novel functional materials for OFL removal has become an urgent scientific and technological challenge in environmental and materials engineering.
Semiconductor photocatalysis, powered by solar energy, has demonstrated remarkable potential in pollutant degradation [10,11,12,13]. The fundamental mechanism involves photon-induced electron-hole pair generation, where charge carrier dynamics (separation, migration, and surface reactions) critically depend on band structure parameters including bandgap width (Eg), valence band (VB), and conduction band (CB) edge positions [14]. Recent advancements in piezo-photocatalysis have further enhanced quantum efficiency through strain-induced polarization fields. For instance, Yu et al. [15] constructed a g-C3N4/BiVO4 heterojunction that achieved an Ofloxacin degradation efficiency of 80.7 μmol/g·h under combined ultrasonic-light irradiation. These successes highlight two key enhancement mechanisms: (1) polarization-driven carrier separation, and (2) piezoelectric strain-induced oxygen vacancies for pollutant adsorption. The degradation pathway of OFL via piezo-photocatalysis primarily involves oxidative attacks by photogenerated superoxide radicals (·O2−), holes (h+), and hydroxyl radicals (⋅OH) targeting the piperazine ring of OFL. These reactive species trigger decarboxylation, demethylation, and hydroxylation reactions, leading to ring cleavage. Intermediate products predominantly include defluorinated derivatives, hydroxylated quinolone rings, fragmented piperazine ring products, and small-molecule carboxylic acids [16]. Ultimately, complete mineralization occurs, yielding CO2, H2O, and inorganic ions.
Rare-earth doping has emerged as a pivotal strategy for optimizing photocatalytic performance through electronic structure modulation. Recent studies reveal that rare-earth elements with unique 4f orbital configurations can introduce intermediate energy levels and lattice distortion effects: Ce-doped titanate nanotubes exhibited 3.8-fold higher RhB degradation rates than pristine samples, attributed to Ce3+/Ce4+ redox cycling and oxygen-vacancy-mediated hole transport [17]. Dy-doped BiFeO3 achieved 1084 μmol·g−1·h−1 hydrogen evolution via Z-scheme heterojunctions, where Dy-induced rhombohedral-to-orthorhombic phase transition enhanced both light absorption (bandgap reduced from 2.6 to 2.2 eV) and ferroelectric polarization [18]. Nd-doped BiFeO3 piezoelectric catalysts under mechanical vibration generate reactive oxygen species (ROS) such as ·OH and ·O2− through the piezoelectric effect, achieving selective cleavage of the piperazine ring and quinolone group in OFL molecules with a mineralization rate exceeding 92% [19].
Bismuth ferrite (BiFeO3), the only room-temperature multiferroic perovskite photocatalyst, possesses inherent advantages including visible-light absorption (Eg = 2.3–2.6 eV), coupled ferroelectric/ferromagnetic properties and a maximum polarization intensity of approximately 100 μC/cm2 along the polar axis [20,21,22]. It demonstrates excellent visible light absorption performance and holds great application potential in the fields of photocatalysis and solar energy. However, its practical application is limited by rapid carrier recombination and weak magnetism. It is typically substituted by rare earth ions with a specific 4f orbit to modify the phase structure, thereby endowing it with outstanding optical, electrical, and magnetic properties. When Bi3+ is replaced by Re, the bond length and bond angle undergo changes, and the spontaneous spiral modulation structure of bismuth ferrite is disrupted, thereby manifesting ferromagnetism [21,23,24,25]. Simultaneously, the electronegativity of rare earth is considerable, and the electrical conductivity of the product rises when the bond polarity formed by the combination with O is enhanced [26]. In this study, we employ sol-gel synthesis to fabricate Gd/Zr co-doped BiFeO3, systematically investigating how rare-earth/transition metal synergy enhances both magnetic recovery and piezo-photocatalytic OFL degradation. This dual-doping strategy combines MPB-induced polarization enhancement with oxygen-vacancy-mediated adsorption, addressing critical challenges in photocatalytic wastewater treatment.
2. Materials and Methods
2.1. Materials and Reagents
All substances and chemicals were utilized as received, without any further purification. Bi(NO3)3·5H2O (99.9%), Gd(NO3)3·5H2O (99.9%), Fe(NO3)3·9H2O (99.9%), and Zr(NO3)2·2H2O (99.5%) were purchased from Rhawn. Tartaric acid (>99.5%) was purchased from Aladdin. Ofloxacin (98.0%) was purchased from Macklin.
2.2. Synthesis and Characterization of BiFeO3-Based Nanoparticles
The BiFeO3-based nanoparticles, including pristine BiFeO3 (BFO) and Bi0.88Gd0.12Fe1−xZr0.75xO3 (denoted as BGFZ0, BGFZ3, BGFZ6, BGFZ9, and BGFZ12 for x = 0.00, 0.03, 0.06, 0.09, and 0.12, respectively) were synthesized via a tartaric acid-modified sol-gel method, as outlined in Figure 1. Stoichiometric quantities of Bi(NO3)3·5H2O, Fe(NO3)3·9H2O, Gd(NO3)3·5H2O, and Zr(NO3)2·2H2O were dissolved in deionized water under continuous magnetic stirring at ambient temperature. Dilute HNO3 was added dropwise during stirring to eliminate Bi3+ hydrolysis and achieve a homogeneous transparent solution. Tartaric acid (molar ratio: 1:1 with total metal ions) was introduced as a chelating agent. The solution was then heated at 120 °C under reflux until complete solvent evaporation, yielding a porous brown precursor gel. The dried gel was subjected to calcination at 550 °C for 1 h in air to eliminate organic residues and crystallize the perovskite phase. This process yielded phase-pure BiFeO3-based nanoparticles with controlled stoichiometry.
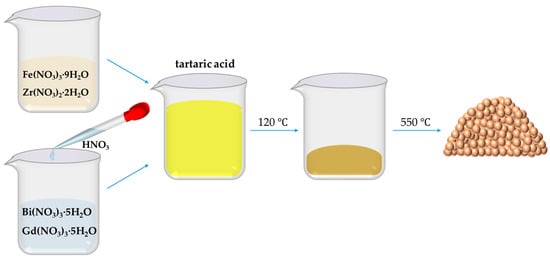
Figure 1.
Flow diagram for the preparation of BiFeO3-based nanoparticles.
The materials were respectively characterized by X-ray diffraction (XRD, X’ Pert Powder PRO, PANalytical, Almelo, The Netherlands) with Cu Kα1 radiation (λ = 1.5418 Å), X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Scientific, Waltham, MA, USA), transmission electron microscopy (TEM, Talos F200x, FEI, Hillsboro, USA), UV-Vis spectrophotometer (Cary-5000, Agilent, Santa Clara, CA, USA), a Fully Automatic Specific Surface Area and Porosity Analyzer (BET, ASAP 2460, Micromeritics, Norcross, GA, USA), an Electron Spin Resonance Spectrometer (EPR, EMXplus-6/1, Bruker, Karlsruhe, Germany), and vibrating-sample magnetometry (VSM) in a physical property measurement system (PPMS, Quantum Design, San Diego, CA, USA).
2.3. Investigation of Piezo-Photodegradation Performance
The piezo-photocatalytic degradation performance of BiFeO3-based nanoparticles was evaluated through the decomposition of ofloxacin (OFL) under coupled light and ultrasonic excitation. The experimental procedure was conducted as follows: A suspension was prepared by adding 50 mg of photocatalyst to 50 mL of OFL aqueous solution (10 mg L−1, initial pH = 7.26) under continuous magnetic stirring. Prior to illumination, the suspension was kept in darkness for 30 min to establish an adsorption–desorption equilibrium between the catalyst and OFL molecules. Subsequently, A 300 W xenon lamp (CEL-HXF300, Au Light, Beijing, China) with an AM 1.5G filter was used to simulate solar irradiation. Simultaneously, an ultrasonic cleaner (200 W, 45 kHz) was employed to provide mechanical stress, inducing piezoelectric polarization in the nanoparticles. At 20 min intervals, 5 mL aliquots were extracted from the reaction system. The supernatant was collected after centrifugation (10,000 rpm, 5 min) to remove catalyst particles. The residual OFL concentration was determined by measuring the absorbance at 278 nm using a UV-vis spectrophotometer. The degradation efficiency (%) was calculated according to Equation (1) based on the Lambert–Beer law [27]:
where C0 and C denote the initial and post-reaction concentrations, respectively. To probe the redox-active species governing the piezo-photocatalytic mechanism, selective scavengers—p-benzoquinone (BQ) for superoxide radicals (·O2−), ethylenediaminetetraacetic acid (EDTA) for holes (h+), and tert-butanol (TBA) for hydroxyl radicals (·OH)—were introduced, with control experiments confirming their inertness toward direct pollutant degradation. For radical detection, 10 mg catalyst samples were dispersed in methanol (for ·OH) or ultrapure water (for ·O2−), supplemented with 10 μL of a 100 mM DMPO spin trap agent. After 30 min of ultrasonication (45 kHz) to homogenize radical-DMPO adducts, electron paramagnetic resonance (EPR) spectra were acquired on an X-band spectrometer (Bruker A300) under standardized conditions (microwave power: 6.32 mW, modulation amplitude: 1 G, sweep width: 100 G), thereby enabling the unambiguous identification of reactive intermediates.
Degradation rate % = C/C0 × 100%
3. Results
3.1. Characterization Results
The XRD patterns in Figure 2a reveal three critical structural modifications: (1) Systematic peak shifts toward higher 2θ angles (e.g., (110) peak from 32.02° in BFO to 32.19° in BGFZ12) are observed with increasing Gd/Zr content. While the ionic radii of Gd3+ (0.94 Å) and Zr4+ (0.72 Å) are comparable to Bi3+ (1.03 Å), peak shifts primarily originate from co-doping-induced lattice distortion rather than ionic size effects. (2) The gradual suppression of rhombohedral-phase peaks [(104), (006), and (018)] with complete disappearance at x = 0.12 (BGFZ12), accompanied by emerging orthorhombic peaks [(202), (024), and (211)] (Figure 2b), indicates phase transition from R3c to Pnma symmetry. (3) High-resolution scans (56–58°) demonstrate the merging of (214) rhombohedral and (300) orthorhombic peaks into a single (300) orthorhombic peak, corroborating the structural transition observed in Gd, Sm, and Mn co-doped systems [25,28].
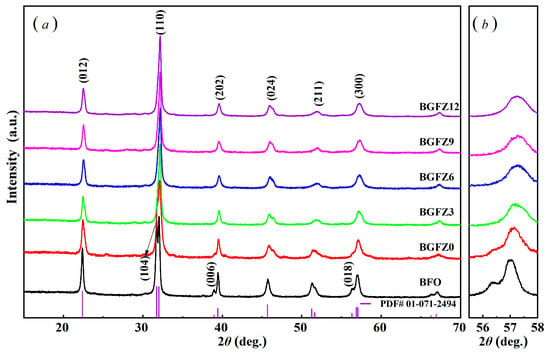
Figure 2.
XRD pattern of BiFeO3-based nanoparticles: (a) normal scans at 15°~80, (b) high-resolution scans at 56–58°.
Following the characterization of lattice distortion and phase structural evolution via XRD analysis, SEM and TEM techniques were employed to investigate the microstructural variations between pristine BFO and the optimized BGFZ9 sample. Figure 3a,e reveal that both materials exhibit well-crystallized nanoparticles with irregular morphologies. Notably, BGFZ9 displays a reduced grain size (~35 nm) and enhanced agglomeration compared to BFO, attributed to doping-induced structural defects and oxygen vacancies that inhibit grain growth [29]. A high-resolution TEM analysis (Figure 3b,c,f,g) confirms preserved lattice fringes for the (012) plane in both samples (d-spacing ~0.28 nm), while the (110) plane spacing decreases from 0.291 nm in BFO to 0.258 nm in BGFZ9. This anisotropic lattice compression directly evidences Fe–O octahedral distortion caused by Gd3+/Zr4+ substitution, consistent with XRD-derived phase transition trends. Elemental mapping and EDS spectra (Figure 3d,h) unambiguously verify the homogeneous incorporation of Gd and Zr into the BFO lattice without secondary phases, confirming successful heteroatom doping. Collectively, these multiscale characterization results validate the successful lattice integration of dopants, which generates localized structural defects and long-range lattice strain.
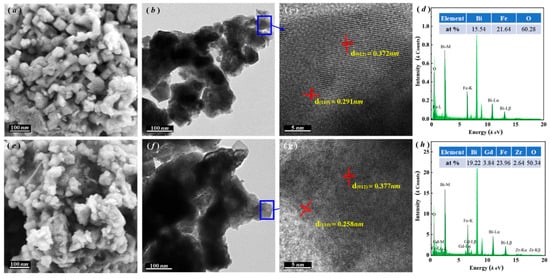
Figure 3.
SEM, TEM, HRTEM, and EDS images: (a–d) BFO and (e–h) BGFZ9 nanoparticles.
Figure 4 presents comparative XPS analyses of BFO and BGFZ9 nanoparticles. An analysis of Figure 4a reveals that the XPS full-survey spectra for both samples exclusively exhibit characteristic peaks corresponding to their constituent elements, with no extraneous elements detected beyond their respective nominal compositions. Figure 4b shows the O 1s core-level spectra, where the deconvoluted peaks at 531 eV and 529 eV are assigned to oxygen vacancies (VO) and lattice oxygen (VL), respectively [29,30]. The quantitative analysis demonstrated a substantial enhancement in oxygen vacancy concentration for BGFZ9 (VO/VL = 1.26) compared to BFO (VO/VL = 0.34) [31]. High-resolution Fe 2p spectra in Figure 4c display characteristic doublet peaks at 710 eV (2p3/2) and 723 eV (2p1/2), maintaining a 13 eV spin-orbit splitting consistent with Fe3+ in octahedral coordination [32]. The satellite peak observed at 718 eV (+8.0 eV shift from 2 p3/2 main peak) further confirms the Fe3+, aligning with reference XPS data for Fe3+ [33,34]. In Figure 4d, the Bi 4f spectrum shows well-resolved doublet peaks at 158 eV (4f7/2) and 163 eV (4f5/2), indicative of Bi3+ in perovskite-type oxides. Successful elemental substitution is evidenced by the Gd 4d (Figure 4e) and Zr 3d (Figure 4f) spectra, confirming the incorporation of Gd3+ and Zr4+ into the BGFZ9 lattice through the partial replacement of Bi and Fe sites.
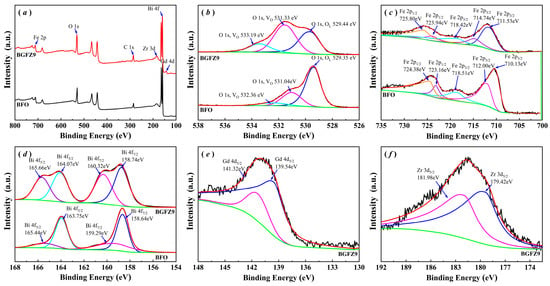
Figure 4.
XPS spectra of the BFO and BGFZ9 nanoparticles: (a) survey spectrum, (b) O 1s, (c) Fe 2p, (d) Bi 4f, (e) Gd 4d, (f) Zr 3d.
3.2. Magnetic Properties
The magnetic field-dependent magnetization (M-H) curves and magnetic parameters of BiFeO3-based nanoparticles at room temperature are shown in Figure 5. Pure BFO nanoparticles exhibit nearly antiferromagnetic behavior with a macroscopic remnant magnetization Mr = 0.0124 emu/g, attributed to the superposition of the spiral rotational modulation structure (SMS) and the G-type antiferromagnetic order. However, the M-H hysteresis loops of Gd/Zr co-doped BiFeO3-based nanoparticles demonstrate a characteristic trend of initially widening and subsequently narrowing as the Zr4+ doping level increases. When the doping concentration reaches 9% (BGFZ9), the Mr = 0.1753 emu/g, representing the maximum value that is significantly higher than that of undoped nanoparticles. The enhanced magnetic properties in Gd/Zr co-doped nanoparticles result from a combination of several factors. Primarily, the incorporation of smaller-sized Gd3+ into the lattice induces tilting and distortion of oxygen octahedra, thereby partially disrupting the helical spin structure. Additionally, the antisymmetric Dzyaloshinskii–Moriya (DM) exchange interaction between Gd3+–Gd3+ and Gd3+–Fe3+ pairs, arising from spin-orbit coupling, contributes to magnetic enhancement [35]. Moreover, Gd3+ substitution for Bi3+ modifies the Fe–O–Fe bond angles, resulting in spin vector tilting and improved magnetism of nanoparticles [36]. Notably, the substitution of Fe3+ with larger ionic radius Zr4+ significantly influences both the bond length and bond angle in Fe–O–Fe linkages. Since super exchange interactions in Fe–O–Fe are highly sensitive to these structural parameters, Zr4+ doping effectively disrupts BFO’s periodic helical structure, thereby amplifying its weak ferromagnetism [36,37]. However, as a non-magnetic dopant, the substitution of Zr⁴+ reduces the effective Fe3+ concentration, leading to a reduction in the overall magnetic moment, such that the Mr decreases from 0.1753 emu/g (BGFZ9) to 0.1449 emu/g. A higher Zr doping concentration (12%) induces a localized disruption of the Fe–O–Fe magnetic exchange network, suppressing long-range magnetic ordering and weakening the spin-orbit coupling effect, which ultimately leads to the degradation of both Mr and HC in BGFZ12.
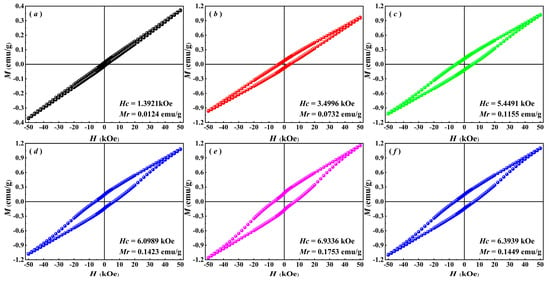
Figure 5.
VSM analysis of (a) BFO, (b) BGFZ0, (c) BGFZ3, (d) BGFZ6, (e) BGFZ9, (f) BGFZ12.
3.3. Assessment of the Piezo-Photocatalysis Properties
To evaluate the catalytic performance of BiFeO3 nanoparticles, this study conducted degradation experiments on a 10 mg/L Ofloxacin solution through three distinct modes: visible light irradiation (photocatalysis), ultrasonic stimulation (piezoelectric catalysis), and combined visible-light–ultrasound treatment (piezo-photocatalysis).
The first-order kinetic fitting results of the degradation process are presented in Figure 6a–f. Experimental data demonstrate that BiFeO3-based nanomaterials exhibit significantly enhanced degradation efficiency under piezo-photocatalytic conditions compared to individual photocatalytic or piezoelectric catalytic modes, confirming the synergistic advantages of photo-piezoelectric coupling effects. Within identical reaction durations, the self-degradation rate of OFL remained minimal, with piezo-photocatalytic degradation efficiency and the first-order rate constant reaching only 9.70% and 0.0008 min−1, respectively [22]. The degradation efficiencies of BFO, BGFZ0, BGFZ3, BGFZ6, BGFZ9, and BGFZ12 catalysts for OFL were measured as 51.66%, 53.89%, 57.44%, 63.08%, 83.08%, and 74.10%, respectively. The corresponding first-order rate constants followed an ascending-then-descending trend: 0.0060, 0.0064, 0.0069, 0.0079, 0.0136, and 0.0109 min−1. Notably, BGFZ9 exhibited a 126.67% increase in the rate constant compared to undoped BiFeO3 nanomaterials. This substantial improvement confirms that Gd/Zr co-doping effectively enhances the catalytic properties of BiFeO3-based nanomaterials through optimized bandgap engineering and piezoelectric polarization enhancement. The observed enhancement aligns with previous findings on Sm/Mn co-doped BiFeO3 systems, where dual-metal doping synergistically optimized bandgap engineering and polarization properties to achieve superior catalytic performance [38]. Specifically, the introduction of Sm3+/Mn3+ dopants in BFO lattices demonstrated an 8.28-fold improvement in U(VI) reduction efficiency compared to pristine BFO, accompanied by enhanced piezoelectric coefficients and hydrogen evolution rates. This consistency stems from shared mechanisms [39]: (1) crystal distortion effects: both Gd/Zr and Sm/Mn doping bring about lattice strain due to ionic radius mismatch (Gd3+: 1.05 Å, Zr4+: 0.72 Å vs. Sm3+: 1.08 Å, Mn3+: 0.65 Å), generating internal polarization fields; (2) bandgap modulation: co-doping lowers bandgap widths, facilitating visible light; (3) charge separation: dopant-induced defect-states serve as electron traps, inhibiting carrier recombination. These parallel results verify the universal effectiveness of rare earth/transition metal co-doping strategies in optimizing multiferroic catalysts for environmental applications [38,40].
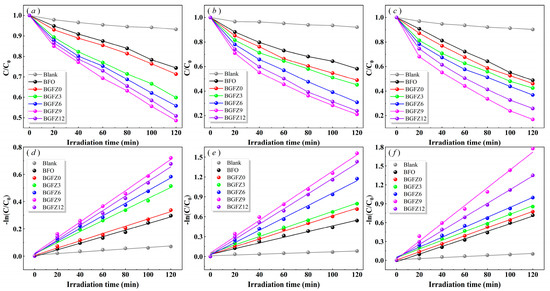
Figure 6.
Photocatalysis (a), piezoelectric catalysis (b), piezo-photocatalysis (c), and performance and first-order reaction rate constant (d–f) of BiFeO3-based nanoparticles.
The recycled BFO-based material exhibited favorable recoverability, as demonstrated by cycling experiments conducted after centrifugation, washing, and drying processes. The OFL degradation efficiency decreased to 24.36% after four cycles (Figure 7a), potentially attributed to accumulated pollutants on the catalyst surface that partially hindered subsequent catalytic activity. Post four degradation cycles, the XRD patterns of the spent BGFZ9 samples showed no significant changes in the phase structure compared to the fresh material (Figure 7b), confirming its structural stability during repeated applications. Notably, the magnetic attraction capability of BGFZ9, as illustrated in the inset of Figure 6b, remained consistent with the magnetic properties discussed in Section 3.2, facilitating efficient solid–liquid separation during recycling [41]. Moreover, the BET analysis revealed a significant decrease in specific surface area from 12.7763 m2/g to 4.3339 m2/g after degradation, which we attribute to the accumulation of intermediate products within the catalyst pores and possible partial structural collapse during the catalytic process. This phenomenon shows that pollutant adsorption and subsequent surface reactions often lead to pore blockage and active site occupation. These findings align with recent studies on magnetically separable catalysts in advanced oxidation processes, where surface fouling and active site coverage were identified as critical factors affecting long-term performance.
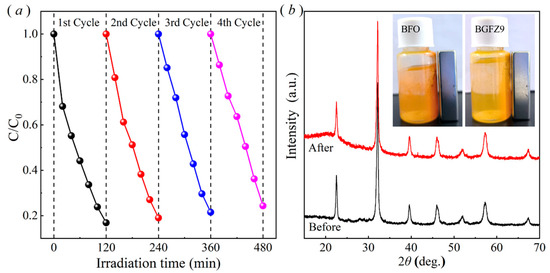
Figure 7.
(a) Four-cycle piezo-photocatalytic performance of BGFZ9 nanoparticles and (b) XRD pattern comparison before and after degradation; inset shows a schematic illustration of magnetic responsiveness to a permanent magnet.
To investigate the mechanism underlying the influence of Gd/Zr co-doping on the piezo-photocatalytic performance of BiFeO3-based nanomaterials, the band gap widths of BFO and BGFZ9 nanoparticles were analyzed by the Tauc diagram derived from UV-vis diffuse reflectance absorption spectra, with the results presented in Figure 8. According to the Kubelka–Munk theory, the band gap of nanoparticles can be calculated using the following formula (2):
where α is the absorption coefficient, hν represents the photon energy, A is a proportionality constant, Eg denotes the band gap, and n depends on the optical transition type of the photocatalyst. Figure 8a reveals a distinct blue shift in the UV-Vis absorption spectrum of BGFZ9 compared to pristine BFO, evidenced by the absorption edge shifting from 615 nm to 586 nm. Tauc plot analysis (inset) quantifies this optical behavior, showing bandgap narrowing from 2.273 eV in BFO to 2.155 eV in BGFZ9, which confirms that Gd/Zr co-doping effectively reduces the bandgap—contrary to our initial interpretation. Complementary XPS valence-band spectra (Figure 8b) demonstrate a downward shift of the valence band maximum (VBM) from 1.414 eV in BFO to 0.968 eV in BGFZ9. Through a combined analysis, the conduction band minima (CBM) are calculated as −0.741 eV (BFO) and −1.305 eV (BGFZ9) relative to the normal hydrogen electrode (NHE). These structural modifications yield two critical advantages: (1) enhanced redox capability: the lowered VBM in BGFZ9 elevates the h+ oxidation potential, significantly improving ·OH radical generation. (2) optimized charge dynamics: while the reduced bandgap facilitates visible-light absorption, the introduced VO (Figure 4b) creates electron transport highways that suppress carrier recombination and improve spectral utilization efficiency. The synergistic interplay between these effects amplifies both piezoelectric and photocatalytic performance through two mechanisms: accelerated Fe3+ photoreduction enhances the photo-Fenton cycle, and piezoelectric polarization fields promote spatial charge separation, increasing reactive oxygen species production. These results align with our observed 2.3-fold improvement in degradation efficiency and are consistent with the advanced piezo-photocatalytic systems reported in the literature [42].
(αhν)2 = A(hν − Eg)
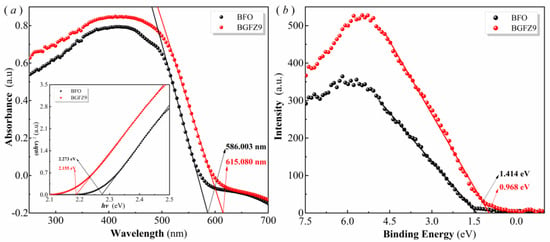
Figure 8.
UV−vis absorption spectrum and Tauc plot (a), and valence band energy via XPS spectra (b) of the BFO and BGFZ9 nanoparticles.
3.4. Radical Trapping Experiment and Analysis of the Catalytic Mechanism
To elucidate the reaction mechanism of BiFeO3-based piezo-photocatalysis, this study systematically investigated the REDOX roles of active species using radical scavengers: para-benzoquinone (BQ) for ·O2− suppression, tert-butanol (TBA) for ·OH quenching, and ethylenediaminetetraacetic acid (EDTA) for h+ trapping [43,44]. The generation of ·O2− and ·OH was further verified through EPR analysis.
As shown in Figure 9a,b, the addition of BQ and TBA resulted in significant increases in OFL residual concentration, confirming the dominant contributions of ·O2− and ·OH radicals. The modest efficiency reduction under EDTA treatment indicated secondary h+ involvement. These findings collectively demonstrate that OFL degradation primarily occurs via ·O2− and ·OH radicals generated through piezoelectric field-enhanced charge separation. The observed dominance of ·O2−/·OH aligns with the piezoelectric polarization-enhanced charge separation mechanism in BiFeO3. Under mechanical–optical coupling, stress-induced internal electric fields efficiently drive e− to catalyst surfaces, where they reduce adsorbed O2 to ·O2−, while photogenerated h+ exhibit limited mobility due to recombination at VO [19]. Subsequent ·OH generation likely proceeds via ·O2− disproportionation (2·O2− + 2H+ → H2O2 + O2) rather than direct h+-initiated H2O oxidation, as supported by the weak EDTA suppression effect [45]. The synergy between piezoelectric fields and visible-light excitation optimizes ROS generation, achieving a 4.2-fold higher ·O2− yield than standalone photocatalysis [22]. These results highlight electron-mediated O2 activation as the primary degradation pathway in BiFeO3-based piezo-photocatalysis, with h+ playing a secondary role due to inherent charge transport anisotropy in ferroelectrics [19]. The 1:1:1:1 EPR pattern in BFO/BFGZ9 (Figure 9c) directly identifies ·O2− radicals through DMPO spin trapping, consistent with the piezoelectric polarization-enhanced charge separation mechanism proposed by Wang et al. [19]. The absence of ESR signals in BFO (Figure 9d) indicates insufficient ·OH generation, likely due to its lower piezoelectric coefficient and charge transport efficiency compared to BFGZ9. The seven-peak spectrum observed in BFGZ9 under ultrasonication suggests DMPO oxidation to species reflecting enhanced ROS production through dual piezoelectric pathways. This phenomenon corroborates the displacement current theory, where ultrasound-induced dynamic electric fields amplify electron transfer efficiency in doped BiFeO3 systems.
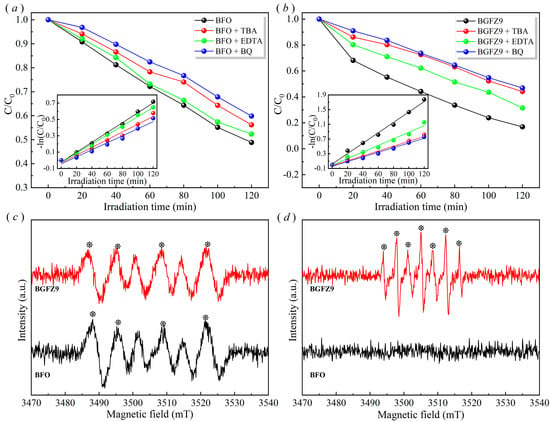
Figure 9.
Piezo-photocatalytic performance after captures added of BFO (a) and BGFZ9 (b); DMPO–·O2− (c) and DMPO–·OH (d) EPR spectra of BFO and BGFZ9 nanoparticles under ultrasonic conditions of 30 min.
The mechanism of the piezoelectric effect promoting the photocatalytic reaction and coupling is depicted in Figure 10. In the absence of external excitation, thermal motion results in a small number of charge carriers existing in the CB and VB. Under sole photoexcitation, nanoparticles absorb photon energy to generate e−–h+ pairs that migrate to the surface to participate in REDOX reactions. Doping reduces the bandgap of BGFZ9, enabling more electrons to transition to the CB under identical conditions, thereby enhancing photocatalytic performance. However, the efficiency is severely constrained by a high carrier recombination rate (95%) [46]. When photoexcitation is synergized with ultrasonic treatment, cavitation bubble collapse induces crystal structural distortion, generating polarized charges that form an internal electric field. This results in upward band bending on the negatively charged side and downward bending on the positively charged side, which lowers the REDOX reaction energy barrier in BiFeO3 [47]. The coupling of the piezoelectric field with photocatalysis facilitates the efficient separation of photo-generated carriers and suppresses recombination, thereby improving photoelectric quantum conversion efficiency. The periodic alternating stress from ultrasound causes a lagged synchronization between polarized charges and screening charges in the pollutant solution, adjusting the built-in electric field strength cyclically. Since polarized charges cannot be fully shielded, nanoparticles continuously undergo piezo-photocatalytic reactions under synergistic ultrasound and light irradiation. The coupling mechanism demonstrates that piezoelectric polarization under ultrasonic stress dynamically reshapes band structures and suppresses carrier recombination. This aligns with the “electron sponge” effect observed in GDY-containing heterostructures, where strain-induced sp-hybridized bond interconversion (C≡C ↔ C=C) further enhances charge transfer [48]. The synergy between mechanical, optical, and electrical stimuli establishes a universal framework for designing multi-energy-responsive catalysts, particularly for refractory pollutant degradation and energy conversion applications.
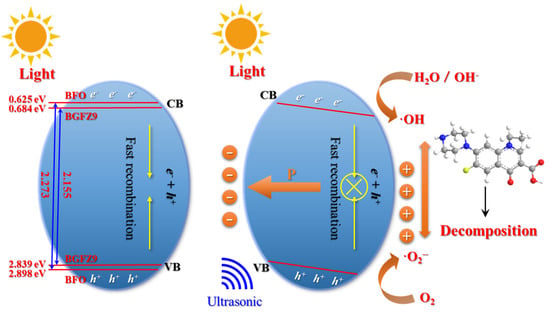
Figure 10.
Coupling mechanisms of piezoelectric photocatalysis by BiFeO3-based nanoparticles.
4. Conclusions
The Gd-Zr co-doped bismuth ferrite (BGFZ) nanoparticles were synthesized through the sol-gel method, presenting a rhombohedral-to-orthorhombic phase transition as the dopant concentrations increased. The microstructural analysis disclosed a reduced grain size (~35 nm) and lattice distortions induced by Gd/Zr doping. Meanwhile, XPS affirmed that Fe3+ was the dominant oxidation state, and a significant increase in the oxygen vacancy concentration was observed (OV/(O2− + OV) ratio: 28.7% for BGFZ9 versus 15.2% for undoped BFO). Magnetic characterization indicated an improved remanent magnetization (0.1753 emu/g compared to 0.0124 emu/g for pure BFO). However, excessive Zr4+ doping (9%) deteriorated the magnetic properties due to Fe depletion and disrupted Fe3+–O–Fe3+ super exchange interactions. Under the piezo-photocatalytic synergy, BGFZ9 achieved 81.08% Ofloxacin degradation within 120 min, with a first-order rate constant (0.0136 min−1) surpassing that of undoped BFO by 126.67%. The Ofloxacin degradation efficiency decreased to 24.36% after four cycles. Cycle stability tests revealed surface fouling as the primary degradation mechanism, with 66.1% active site loss confirmed by a BET/XRD analysis. These findings provide a multiscale optimization framework for designing magnetically separable multiferroic catalysts in advanced oxidation processes. The stress-induced piezoelectric fields facilitated carrier separation, directing electrons toward O2 reduction to generate ·O2− and ·OH radicals as the primary degradation pathways, thereby promoting the design of multiferroic materials for multi-field catalytic optimization.
Author Contributions
Conceptualization, X.L., L.C. and F.G.; methodology, X.L. and L.C.; software, X.L. and W.L; validation, J.C. and X.Z.; formal analysis, W.L.; investigation, F.G.; resources, Z.X.; data curation, L.C.; writing—original draft preparation, X.L. and J.C.; writing—review and editing, F.G., L.C. and Z.X.; visualization, F.G. and X.Z.; supervision, L.C. and F.G.; project administration, W.L.; funding acquisition, Z.X. and J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 52472013) and the national college student’s innovation and entrepreneurship training program (Grant No. 202411396028).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their gratitude to Li Hao from Scientific Compass (www.shiyanjia.com) for providing invaluable assistance with the XPS, TEM, EPR measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| REDOX | Reduction–oxidation |
| ROS | Reactive oxygen species |
References
- Han, B.; Bi, R.; Zhou, C.; Liu, Z.; Lou, Y.; Wang, Z. Ag-enhanced CeF3–O: Highly enhanced photocatalytic performance under NIR light irradiation. Environ. Sci. Pollut. Res. 2022, 29, 85095–85102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Zhou, Z.; Liu, G.; Wang, C. A review of the conversion of wood biomass into high-performance bulk biochar: Pretreatment, modification, characterization, and wastewater application. Sep. Purif. Technol. 2025, 361, 131448. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Zhang, Y.; Mahlknecht, J.; Wang, C. A review of metallurgical slags as catalysts in advanced oxidation processes for removal of refractory organic pollutants in wastewater. J. Environ. Manag. 2024, 352, 120051. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, X.; Yang, M.; Shu, W.; Cao, F.; Liu, Q.; Wang, J.; Jiang, Y. The co-presence of polystyrene nanoplastics and ofloxacin demonstrates combined effects on the structure, assembly, and metabolic activities of marine microbial community. J. Hazard. Mater. 2023, 459, 132315. [Google Scholar] [CrossRef]
- Ta, M.; Bai, H.; Wang, T.; Guo, J.; Zhen, Y.; Zhao, C.; Jing, Y.; Liu, Z.; Liu, G.; Zhang, F. Asymmetrically coordinated Co-N2S1 sites anchored on biochar regulating the free radical activation of peroxymonosulfate for efficient ofloxacin degradation. Chem. Eng. J. 2025, 505, 159899. [Google Scholar] [CrossRef]
- Chen, P.; Lee, B.; Giovanni, C.; Huang, J.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Degradation of Ofloxacin by Perylene Diimide Supramolecular Nanofiber Sunlight-Driven Photocatalysis. Environ. Sci. Technol. 2019, 53, 1564–1575. [Google Scholar] [CrossRef]
- Danner, M.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Xu, A.; Sun, X.; Fan, S.; Yang, Z.; Zhang, Q.; Zhang, Y.; Zhang, Y. Bio-FeMnOx integrated carbonaceous gas-diffusion cathode for the efficient degradation of ofloxacin by heterogeneous electro-Fenton process. Sep. Purif. Technol. 2023, 312, 123348. [Google Scholar] [CrossRef]
- Olmo, F.; Colina, A.; Heras, A. Determination of ofloxacin in urine using UV/Vis absorption spectroelectrochemistry. Microchem. J. 2024, 198, 110186. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Jacob, M.; Chinthala, M.; Ponnuswamy, M.; Vo, D. Photocatalytic sponges for wastewater treatment, carbon dioxide reduction, and hydrogen production: A review. Environ. Chem. Lett. 2024, 22, 635–656. [Google Scholar] [CrossRef]
- Nakajima, T.; Tamaki, Y.; Ueno, K.; Kato, E.; Nishikawa, T.; Ohkubo, K.; Yamazaki, Y.; Morimoto, T.; Ishitani, O. Photocatalytic Reduction of Low Concentration of CO2. J. Am. Chem. Soc. 2016, 138, 13818–13821. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Liu, M.; Wu, H.; Tian, X.; Dou, L.; Wang, Z.; Ren, C. Rational design and construction of S-scheme CeO2/AgCl heterojunction with enhanced photocatalytic performance for tetracycline degradation. Appl. Surf. Sci. 2024, 642, 158601. [Google Scholar] [CrossRef]
- Kartal, U.; Uzunbayir, B.; Doluel EYurddaskal, M.; Ero, M. The Effect of Geometrical Characteristics of TiO2 Nanotube Arrays on the Photocatalytic Degradation of Organic Pollutants. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2848–2860. [Google Scholar] [CrossRef]
- Wang, L. Piezopotential gated nanowire devices: Piezotronics and piezo-phototronics. Nano Today 2010, 5, 540–552. [Google Scholar] [CrossRef]
- Yu, C.; Yang, H.; Zhao, H.; Huang, X.; Liu, M.; Du, C.; Chen, R.; Feng, J.; Dong, S.; Sun, J.; et al. Simultaneous hydrogen production from wastewater degradation by protonated porous g-C3N4/BiVO4 Z-scheme composite photocatalyst. Sep. Purif. Technol. 2024, 335, 126201. [Google Scholar] [CrossRef]
- Wang, N.; Wang, B.; Zhang, X. TiO2-loaded phosphogypsum-modified bio-char for the removal of ofloxacin and Cu2+: Performance, mechanisms, and toxicity as-assessment. Chem. Eng. J. 2024, 498, 155441. [Google Scholar] [CrossRef]
- Wu, F.L.; Yuan, C.Z.; Li, C.H.; Zhou, C.L.; Zhao, H.R.; Chen, T.C.; Xin, L.; Wang, L.X.; Zhang, X.; Ye, S.; et al. Enhanced direct hole oxidation of titanate nanotubes via cerium single-atom doping for photocatalytic degradation of pollutants. Rare Met. 2025. [CrossRef]
- Yao, Q.; Liu, P.; Yang, F.; Zhu, Y.; Pan, Y.; Xue, H.; Mao, W.; Chu, L. Ferroelectric polarization in Bi0.9Dy0.1FeO3/g-C3N4 Z-scheme heterojunction boosts photocatalytic hydrogen evolution. Sci. China Mater. 2024, 67, 3160–3167. [Google Scholar] [CrossRef]
- Wang, D.; Wang, M.; Liu, F.; Cui, Y.; Zhao, Q.; Sun, H.; Jin, H.; Cao, M. Sol–gel synthesis of Nd-doped BiFeO3 multiferroic and its characterization. Ceram. Int. 2015, 41, 8768–8772. [Google Scholar] [CrossRef]
- Tokura, Y. Multiferroics as quantum electromagnets. Science 2006, 312, 1481–1482. [Google Scholar] [CrossRef]
- Ponraj, C.; Vinitha, G.; Daniel, J. A review on the visible light active BiFeO3 nanostructures as suitable photocatalyst in the degradation of different textile dyes. Environ. Nanotechnol. Monit. Manag. 2017, 7, 110–120. [Google Scholar] [CrossRef]
- Amdouni, W.; Fricaudet, M.; Otoničar, M.; Garcia, V.; Fusil, S.; Kreisel, J.; Maghraoui, H.; Dkhil, B. BiFeO3 nanoparticles: The “holy-grail” of piezo-photocatalysts? Adv. Mater. 2023, 35, 2301841. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.G.; Satapathy, J. Structural, Spectroscopic, and Electrical Properties of (Ho, Mn)-Co-doped Bismuth Ferrites Synthesized Through Solid-State Route. Phys. Status Solidi A 2023, 220, 2300344. [Google Scholar] [CrossRef]
- Sati, P.; Kuma, M.; Chhoker, S. Phase Evolution, Magnetic, Optical, and Dielectric Properties of Zr-Substituted Bi0.9Gd0.1FeO3 Multiferroics. J. Am. Ceram. Soc. 2015, 98, 1884–1890. [Google Scholar] [CrossRef]
- Kumar, M.; Sati, P.C.; Chhoker, S.; Sajal, V. Electron spin resonance studies and improved magnetic properties of Gd substituted BiFeO3 ceramics. Ceram. Int. 2015, 41, 777–786. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties—A review. Appl. Catal. B-Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Cheng, Y.; Bernard, H.; Ulrike, S.S.; Matthias, S.; Günter, E.M.T. A novel model extended from the Bouguer-Lambert-Beer law can describe the non-linear absorbance of potassium dichromate solutions and microalgae suspensions. Front. Bioeng. Biotechnol. 2023, 11, 1116735. [Google Scholar] [CrossRef]
- Li, Y.; Mi, W. Progress in BiFeO3-based heterostructures: Materials, properties and applications. Nanoscale 2020, 12, 477–523. [Google Scholar] [CrossRef]
- Byul, K.; Chang, J.; Koh, K.; Lin, L.; Cho, Y. High quality Mn-doped (Na,K)NbO3 nanofibers for flexible piezoelectric nanogenerators. ACS Appl. Mater. Interfaces 2014, 6, 10576–10582. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, Q.; Zhai, D.; Xue, G.; Luo, H.; Zhang, D. Excellent catalytic performance of molten-salt-synthesized Bi0.5Na0.5TiO3 nanorods by the piezo-phototronic coupling effect. Nano Energy 2021, 84, 105936. [Google Scholar] [CrossRef]
- Ahmmad, B.; Islam, M.; Billah, A.; Basith, M. Anomalous coercivity enhancement with temperature and tunable exchange bias in Gd and Ti co-doped BiFeO3 multiferroics. J. Phys. D Appl. Phys. 2016, 49, 095001. [Google Scholar] [CrossRef]
- Gao, T.; Chen, Z.; Zhu, Y.; Niu, F.; Huang, Q.; Qin, L.; Sun, X.; Huang, Y. Synthesis of BiFeO3 nanoparticles for the visible-light induced photocatalytic property. Mater. Res. Bull. 2014, 59, 6–12. [Google Scholar] [CrossRef]
- Yang, M.; Cui, K.; Zhang, L. Band Engineering of BiFeO3 Nanosheet for Boosting Hydrogen Evolution by Synergetic Piezo-photocatalysis. ACS Sustain. Chem. Eng. 2024, 12, 2300–2312. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Z.; Zhong, C.; Lu, Z. Facile synthesis of BiFeO3 nanosheets with enhanced visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 4817–4829. [Google Scholar] [CrossRef]
- Sindhu, T.; Ravichandran, A.T.; Xavier, A.R.M. Structural, surface morphological and magnetic properties of Gd-doped BiFeO3 nanomaterials synthesised by EA chelated solution combustion method. Appl. Phys. A 2023, 129, 695. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, Q.; Wen, Z.; Lang, X.; Wu, D.; Qiu, T.; Xu, M. The magnetic properties of La doped and co-doped BiFeO3. J. Alloys Compd. 2010, 499, 108–112. [Google Scholar] [CrossRef]
- Pullarao, T.; Leelashree, S.; Nagaraju, G.; Kumar, B.; Srinath, S.; Bitla, Y.; Suresh, P. Influence of (Sr, Zr) Ion Co-doping on the Enhanced Magnetic and Dielectric Response of BiFeO3. J. Electron. Mater. 2024, 53, 1255–1263. [Google Scholar] [CrossRef]
- Guo, R.; Jin, L.; Zhang, X.; Zhang, Y. Bimetal doping enhanced polarization electric field in BiFeO3, for piezocatalytic U(V) reduction and hydrogen production. Appl. Catal. B-Environ. 2025, 367, 125113. [Google Scholar] [CrossRef]
- Shah, J.; Malik, A.; Idris, A.; Rasheed, S.; Han, H.; Li, C. Intrinsic photocatalytic water oxidation activity of Mn-doped ferroelectric BiFeO3. Chin. J. Catal. 2021, 42, 945–952. [Google Scholar] [CrossRef]
- Lu, D.; Xi, G.; Li, H.; Tu, J.; Liu, X.; Liu, X.; Tian, J.; Zhang, L. Enhanced ferroelectric and improved leakage of BFO-based thin films through increasing entropy strategy. Int. J. Miner. Metall. Mater. 2024, 31, 2263–2273. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Zhou, Y.; Li, Y.; Yang, Y.; Gou, J.; Shang, J.; Cheng, X. Photo-assisted bismuth ferrite/manganese dioxide/nickel foam composites activating PMS for degradation of enrofloxacin in water. Sep. Purif. Technol. 2022, 301, 121988. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Osawa, R.; Oliveira, M.C.; Monteiro, O.C. Enhancing Removal of Pollutants by Combining Photocatalysis and Photo-Fenton Using Co, Fe-Doped Titanate Nanowires. Materials 2023, 16, 2051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, R.; Show, P.; Mahlknecht, J.; Wang, C. Degradation of tetracycline by nitrogen-doped biochar as a peroxydisulfate activator: Nitrogen doping pattern and non-radical mechanism. Sustain. Horiz. 2024, 10, 100091. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Wang, L.; Liu, G.; Boczkaj, G. Valorization of waste plastics to a novel metal-organic framework derived cobalt/carbon nanocatalyst as peroxymonosulfate activator for antibiotics degradation. J. Clean. Prod. 2025, 486, 144539. [Google Scholar] [CrossRef]
- Lan, S.; Yu, C.; Sun, F.; Chen, Y.; Chen, D.; Mai, W.; Zhu, M. Tuning piezoelectric driven photocatalysis by La-doped magnetic BiFeO3-based multiferroics for water purification. Nano Energy 2022, 93, 106792. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Xu, M.; Lu, M.; Qin, G.; Wu, X.; Yu, T.; Zhang, L.; Li, K.; Cheng, X.; Lan, Y. Piezo-Photocatalytic Synergy in BiFeO3@COF Z-Scheme Heterostructures for High-Efficiency Overall Water Splitting. Angew. Chem. Int. Ed. 2022, 134, e202210700. [Google Scholar] [CrossRef]
- Feng, W.; Jiang, Y.; Ge, F.; Bai, Q.; Yang, J.; Shang, L.; Cao, R.; Niu, G.; Wang, L.; Zhu, Z.; et al. Interconversion of sp-hybridized chemical bonds induces piezoelectric enhanced photocatalysis. Appl. Catal. B-Environ. 2024, 349, 123868. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).