Optimization of Nano-Tangeretin Recrystallization via Natural Surfactants in the Antisolvent Precipitation Process: Physicochemical Characterization and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
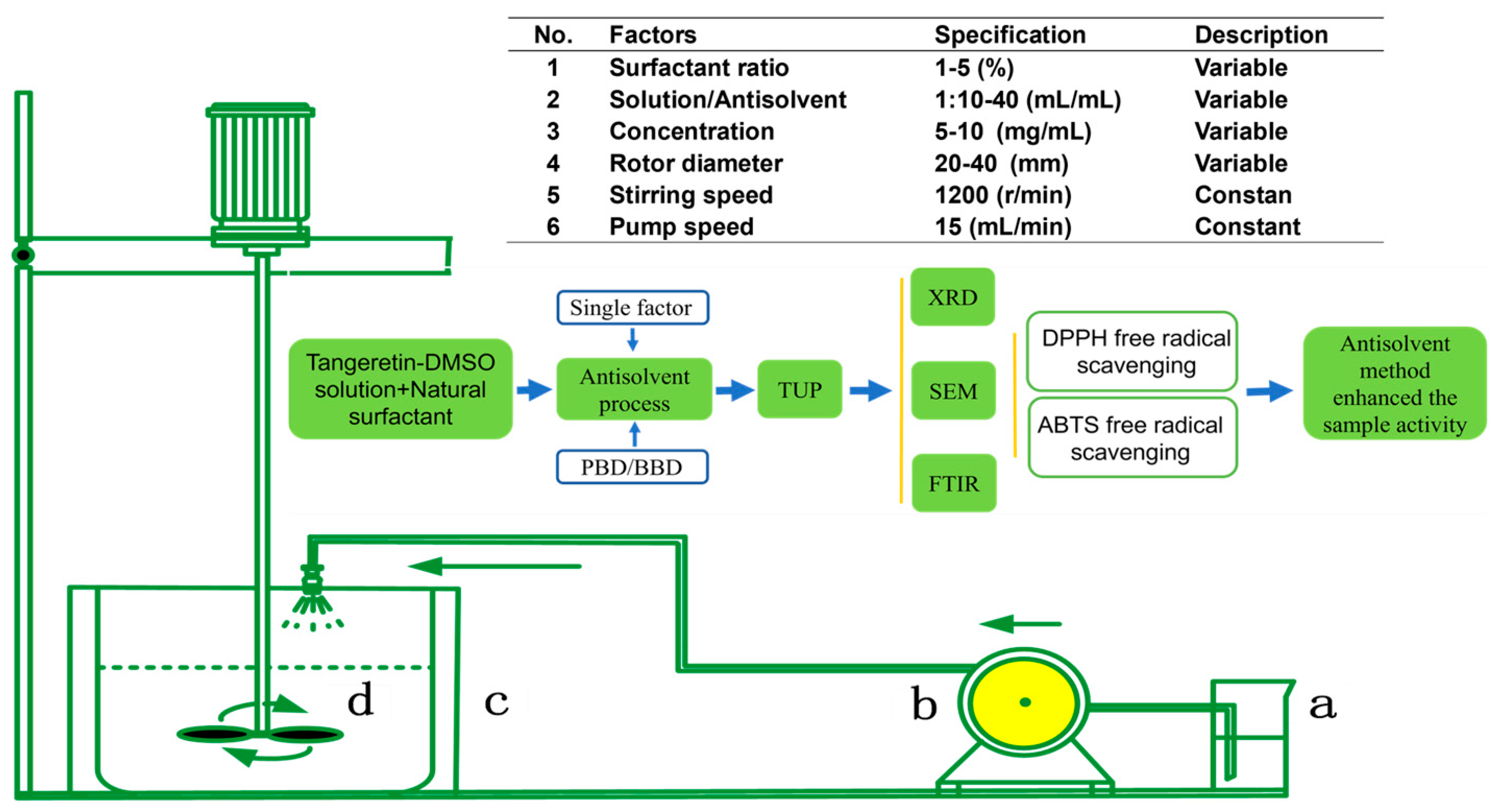
2.2. Preparation of Nano-Tangeretin Particles
2.3. Surfactant Screening
2.4. Response Surface Plackett–Burman Design Experiment
2.5. Response Surface Box–Behnken Design to Optimize Nano-Tangeretin Particles Size
2.6. Microstructure of Powders
2.7. The Method of Infrared Spectral Analysis
2.8. Crystal Properties of the Samples
2.9. Thermal Analysis of Tangeretin Samples
2.10. Determination of Antioxidant Capacity
2.10.1. Preparation of Sample Solution
2.10.2. DPPH/ABTS Free Radical Scavenging Experiment
3. Results and Discussion
3.1. Influence of the Type of Natural Surfactant on the Size of Samples
3.2. Plackett–Burman Design for Particle Size Optimization
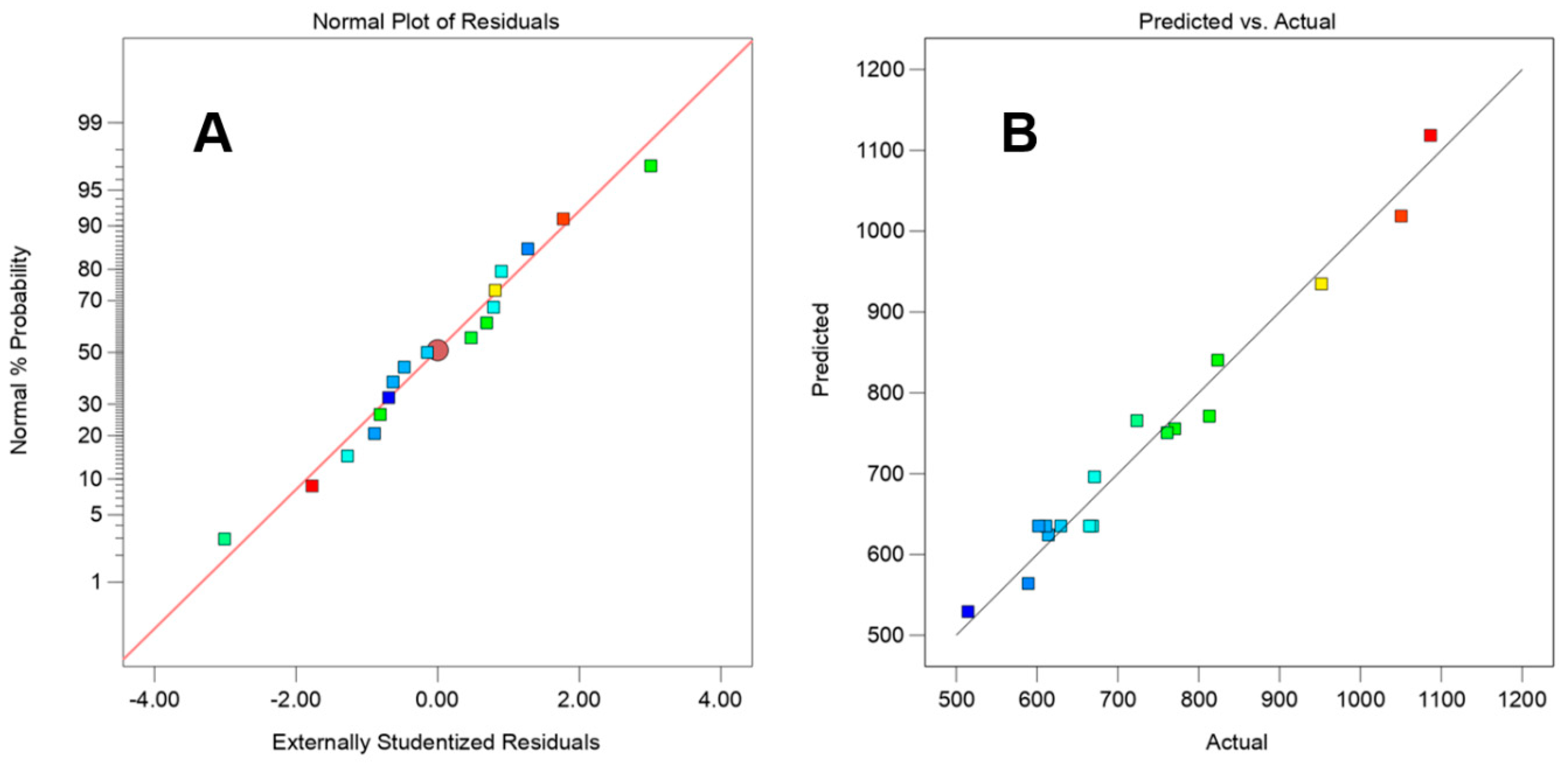
3.3. Particle Size Optimization by Response Surface
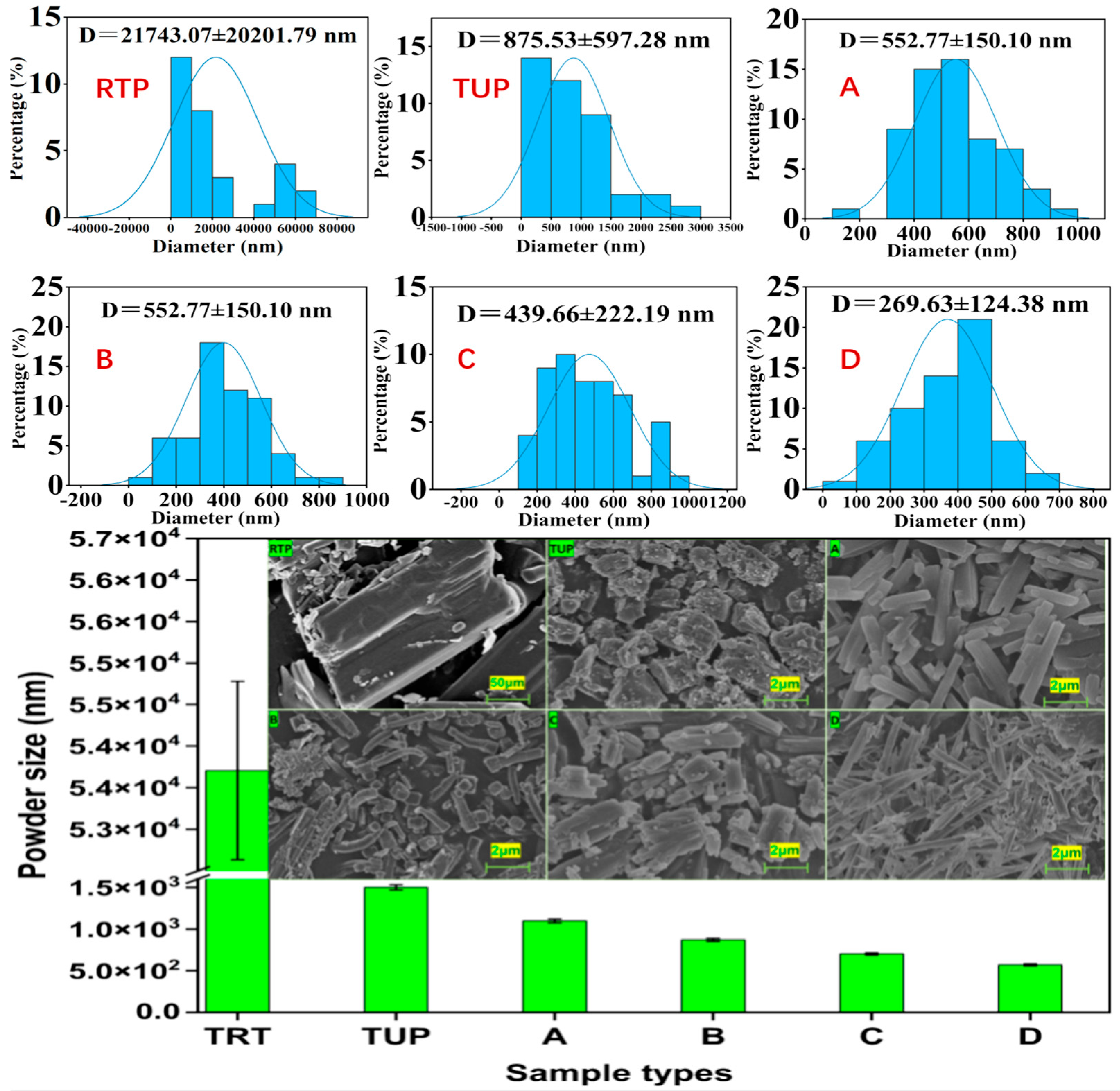
3.4. The Microscopic Morphology of the Samples
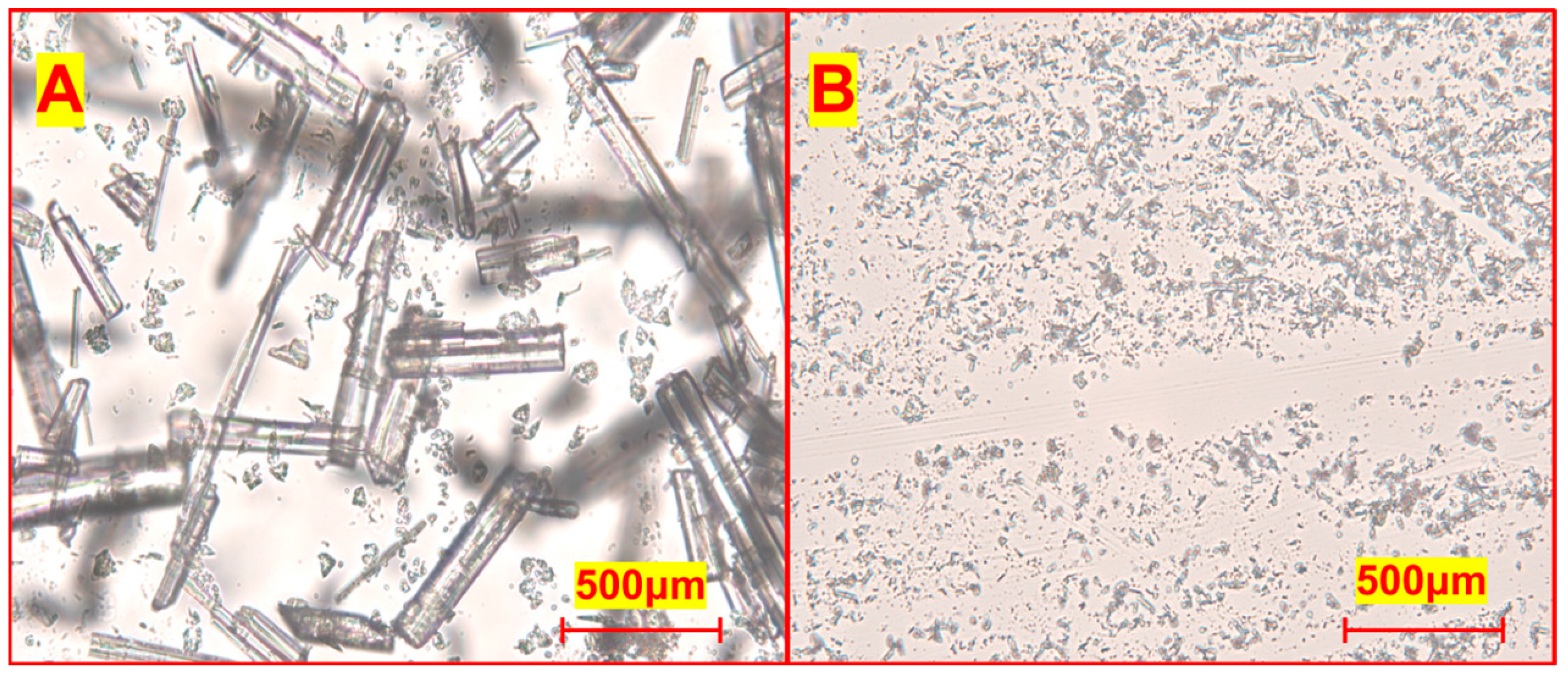
3.4.1. Morphological Observation of Raw and Nano-Tangeretin Powders
3.4.2. Influence of Different Natural Surfactants on Powder Morphology
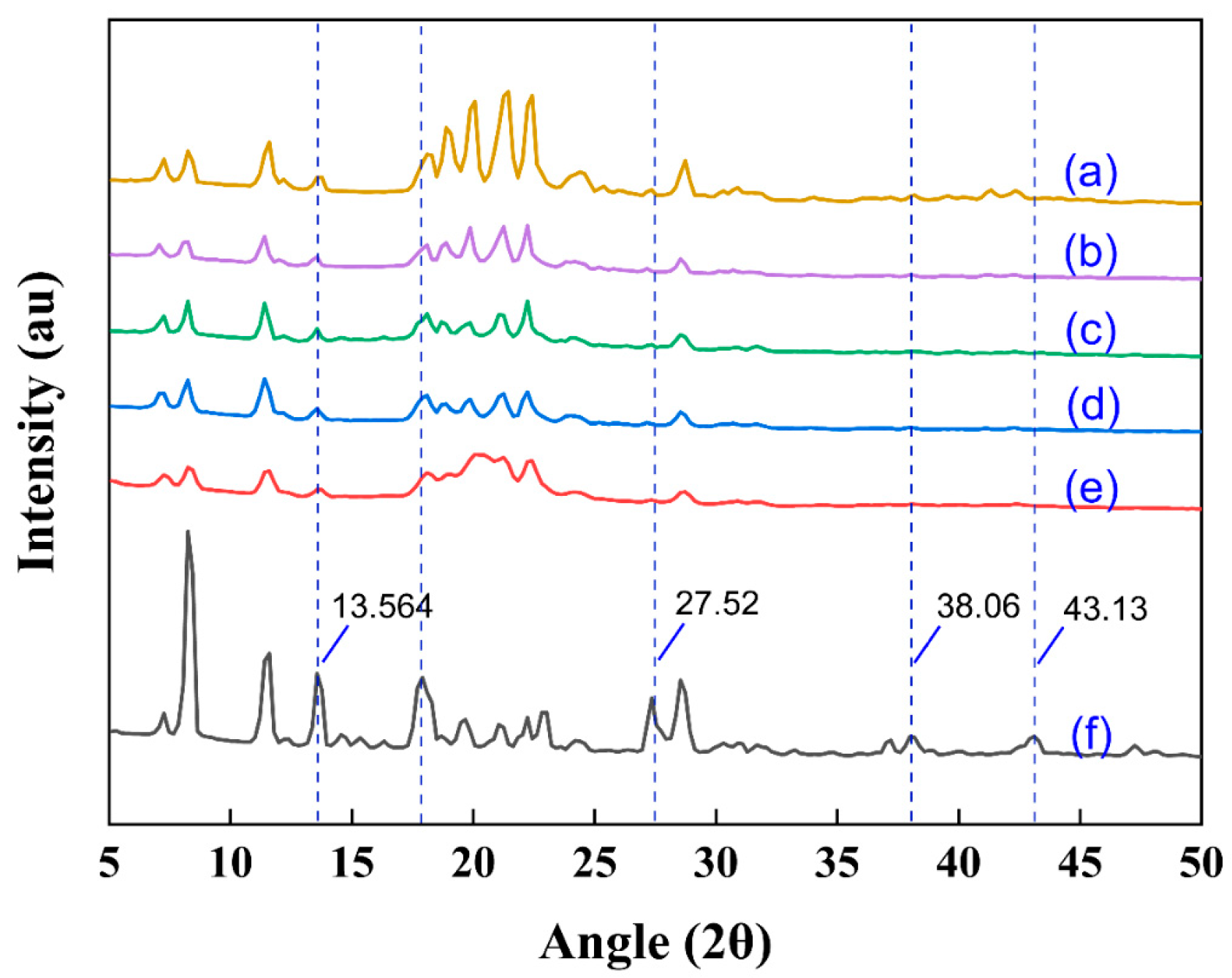
3.5. The Crystal Morphologies of Samples Were Analyzed by X-Ray Diffraction
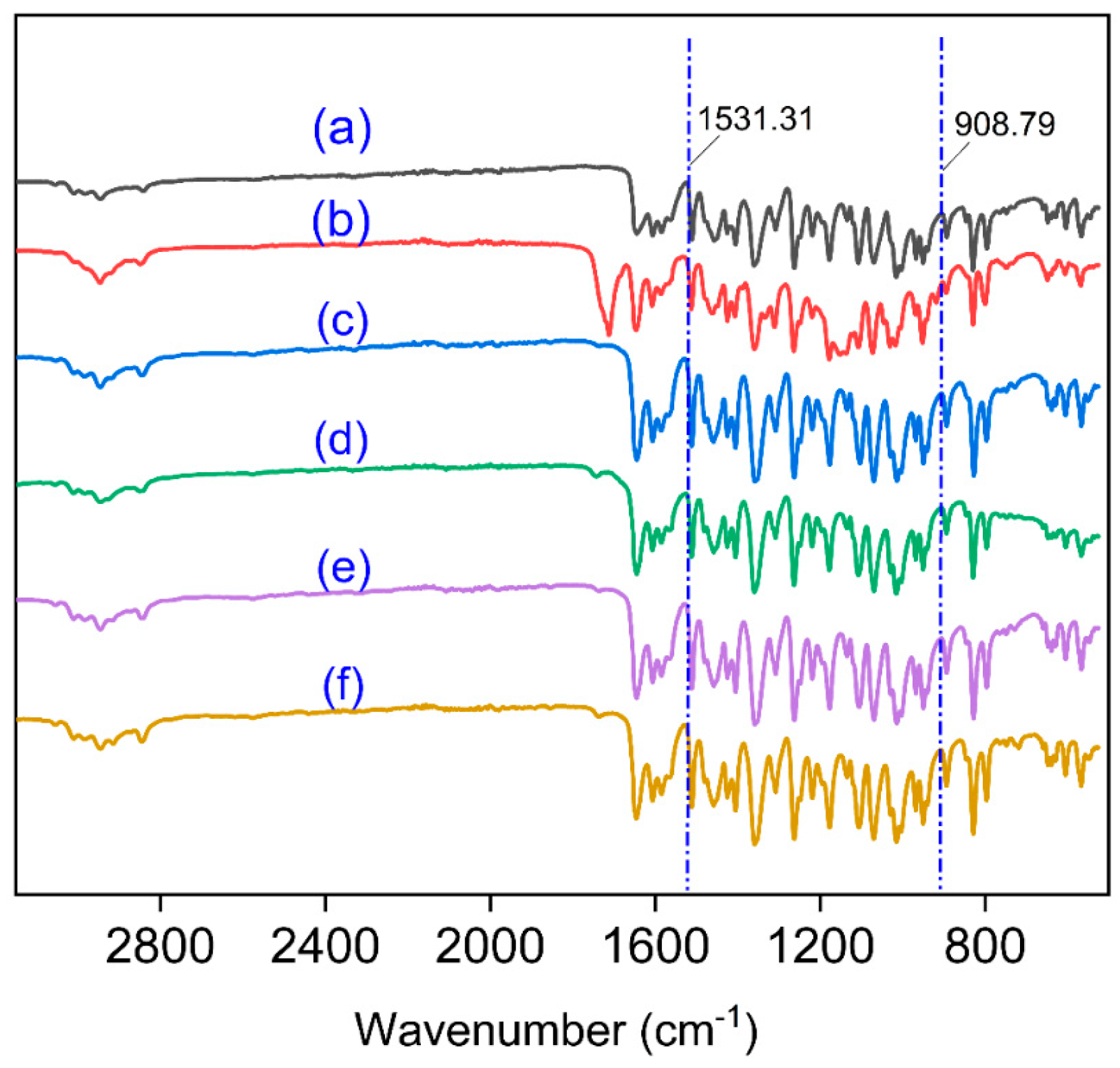
3.6. Infrared Spectrum Scanning of Samples Structure
3.7. Thermodynamic Analysis of Samples
3.8. Comparison of Antioxidant Properties of Samples Before and After Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RTP | Raw tangeretin powder |
| PBD | Plackett–Burman design |
| BBD | Box–Behnken design |
| DMSO | Dimethyl sulfoxide |
References
- Razzaghi-Koolaee, F.; Mehrabianfar, P.; Soltani Soulgani, B.; Esfandiarian, A. A Comprehensive Study on the Application of a Natural Plant-Based Surfactant as a Chemical Enhanced Oil Recovery (CEOR) Agent in the Presence of Different Ions in Carbonate Reservoirs. J. Environ. Chem. Eng. 2022, 10, 108572. [Google Scholar] [CrossRef]
- Khan, J.; Mariatti, M. Effect of Natural Surfactant on the Performance of Reduced Graphene Oxide Conductive Ink. J. Clean. Prod. 2022, 376, 134254. [Google Scholar] [CrossRef]
- Norouzpour, M.; Azdarpour, A.; Nabipour, M.; Santos, R.M.; Khaksar Manshad, A.; Iglauer, S.; Akhondzadeh, H.; Keshavarz, A. Red Beet Plant as a Novel Source of Natural Surfactant Combined with ‘Smart Water’ for EOR Purposes in Carbonate Reservoirs. J. Mol. Liq. 2023, 370, 121051. [Google Scholar] [CrossRef]
- Norouzpour, M.; Nabipour, M.; Azdarpour, A.; Akhondzadeh, H.; Santos, R.M.; Keshavarz, A. Experimental Investigation of the Effect of a Quinoa-Derived Saponin-Based Green Natural Surfactant on Enhanced Oil Recovery. Fuel 2022, 318, 123652. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, R.; Wang, Y.; Liu, T.; Tian, H.; Yin, X.; Yang, L.; Sui, X. Natural Surfactant Used as an Additive in the Homogenate-Ultrasound-Synergistic Extraction of Regaloside A and Polysaccharides from Lilium Lancifolium Bulbs Using Doehlert Matrix Design. Ind. Crops Prod. 2022, 188, 115689. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, H.; Xu, J.; Chang, Y.; Liu, C.; Zhang, Y.; Yang, H.; Duan, C.; Huang, J.; Fu, Y. A Sustainable and Integrated Natural Surfactant Mediated Microwave-Assisted Extraction Technique Enhances the Extraction of Phytochemicals from Plants. Ind. Crops Prod. 2022, 184, 115043. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Formation of PVP/Nimesulide Microspheres by Supercritical Antisolvent Coprecipitation. J. Supercrit. Fluids 2016, 118, 19–26. [Google Scholar] [CrossRef]
- Yu, G.; Zhu, H.; Huang, Y.; Zhang, X.; Sun, L.; Wang, Y.; Xia, X. Preparation of Daidzein Microparticles through Liquid Antisolvent Precipitation under Ultrasonication. Ultrason. Sonochem. 2021, 79, 1350–4177. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, L.; Mu, L.; Ma, J.; Wang, X.; Li, C.; Cui, Y.; Li, A. Antagonistic Effect of Zinc Oxide Nanoparticle and Surfactant on Anaerobic Digestion: Focusing on the Microbial Community Changes and Interactive Mechanism. Bioresour. Technol. 2020, 297, 122382. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Xia, X.; Pu, N.; Yu, Z.; Nabih, M.; Zhu, Y.; Zhang, S.; Jiang, L. Preparation and Physicochemical Characterization of Soy Isoflavone (SIF) Nanoparticles by a Liquid Antisolvent Precipitation Method. Adv. Powder Technol. 2019, 30, 1522–1530. [Google Scholar] [CrossRef]
- Guo, N.; Tong, T.; Ren, N.; Tu, Y.; Li, B. Saponins from Seeds of Genus Camellia: Phytochemistry and Bioactivity. Phytochemistry 2018, 149, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Su, J.; Shu, X.; Yuan, F.; Mao, L.; Gao, Y. Development of High Methoxyl Pectin-Surfactant-Pea Protein Isolate Ternary Complexes: Fabrication, Characterization and Delivery of Resveratrol. Food Chem. 2020, 321, 126706. [Google Scholar] [CrossRef]
- Krawczyk, J. Aggregation Properties of Sucrose Fatty Acid Esters and Some Other Sugar-Based Surfactants at Different Temperatures. J. Mol. Liq. 2018, 271, 610–620. [Google Scholar] [CrossRef]
- Li, G.; Chen, L.; Ruan, Y.; Guo, Q.; Liao, X.; Zhang, B. Alkyl Polyglycoside: A Green and Efficient Surfactant for Enhancing Heavy Oil Recovery at High-Temperature and High-Salinity Condition. J. Pet. Explor. Prod. Technol. 2019, 9, 2671–2680. [Google Scholar] [CrossRef]
- Mi, J.; Cheng, J.; Ng, K.H.; Yan, N. Biomass to Green Surfactants: Microwave-Assisted Transglycosylation of Wheat Bran for Alkyl Glycosides Production. Bioresour. Technol. 2024, 401, 130738. [Google Scholar] [CrossRef]
- Valenzuela, C.P.; Rodriguez-Llamazares, S. cryst: Calculate the Relative Crystallinity of Starch by XRD and FTIR. Available online: https://cran.r-project.org/web/packages/cryst/index.html (accessed on 20 April 2025).
- Zaheer, Z.; Kosa, S.A.; Akram, M. Solubilization of Congo Red into Non-Ionic Bolaform Sugar Based Surfactant: A Multi Spectroscopic Approach. J. Saudi Chem. Soc. 2021, 25, 101257. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, H.; Guo, Y.; Ma, L.; Wang, Q.; Hu, D. Stability and Rheological Properties of Foams Co-Stabilized by Hydrophilic Silica Nanoparticles and Amino Acid/Alkyl Glycoside Surfactants. J. Mol. Liq. 2023, 382, 122009. [Google Scholar] [CrossRef]
- Barón, M. Definitions of Basic Terms Relating to Low-Molar-Mass and Polymer Liquid Crystals (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 845–895. [Google Scholar] [CrossRef]
- Goodby, J.W.; Görtz, V.; Cowling, S.J.; Mackenzie, G.; Martin, P.; Plusquellec, D.; Benvegnu, T.; Boullanger, P.; Lafont, D.; Queneau, Y.; et al. Thermotropic Liquid Crystalline Glycolipids. Chem. Soc. Rev. 2007, 36, 1971–2032. [Google Scholar] [CrossRef]
- Ahmad, N.; Ramsch, R.; Llinàs, M.; Solans, C.; Hashim, R.; Tajuddin, H.A. Influence of Nonionic Branched-Chain Alkyl Glycosides on a Model Nano-Emulsion for Drug Delivery Systems. Colloids Surf. B Biointerfaces 2014, 115, 267–274. [Google Scholar] [CrossRef]
- Gao, P.; Guo, L.; Sun, J.; Wang, Y.; She, Z.; Gao, M.; Zhao, Y.; Jin, C. Effect of Alkyl Polyglycosides on the Performance of Thermophilic Bacteria Pretreatment for Saline Waste Sludge Hydrolysis. Bioresour. Technol. 2020, 296, 122307. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M. Process Optimization of Intermediate-Wave Infrared Drying: Screening by Plackett–Burman; Comparison of Box-Behnken and Central Composite Design and Evaluation: A Case Study. Ind. Crops Prod. 2021, 162, 113287. [Google Scholar] [CrossRef]
- Wei, C.; Xiao, K.; Li, H.; Qi, Y.; Zou, Z.; Liu, Z. Optimization of Ultrasound Assisted Aqueous Enzymatic Extraction of Oil from Cinnamomum Camphora Seeds. LWT 2022, 164, 113689. [Google Scholar] [CrossRef]
- Ardestani, N.S.; Amani, M. Production of Anthraquinone Violet 3RN Nanoparticles via the GAS Process: Optimization of the Process Parameters Using Box-Behnken Design. Dye. Pigment. 2021, 193, 109471. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, T.; Liu, X.; Kuang, W.; Zhong, Y.; Zhang, M.; Huang, Y.; Liu, Z. Anti-Solvent Precipitation for the Preparation of Nobiletin Nano-Particles under Ultrasonication-Cis/Reverse Homogenization. Ultrason. Sonochem. 2023, 96, 106433. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Nakamura, K. Effects of Solvent Composition and Temperature on Polymorphism and Crystallization Behavior of Thiazole-Derivative. J. Cryst. Growth 2002, 236, 676–686. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Scognamiglio, M.; Reverchon, E. Folic Acid–PVP Nanostructured Composite Microparticles by Supercritical Antisolvent Precipitation. Chem. Eng. J. 2015, 277, 286–294. [Google Scholar] [CrossRef]
- Liu, C.; Xu, B.; McClements, D.J.; Xu, X.; Cui, S.; Gao, L.; Zhou, L.; Xiong, L.; Sun, Q.; Dai, L. Properties of Curcumin-Loaded Zein-Tea Saponin Nanoparticles Prepared by Antisolvent Co-Precipitation and Precipitation. Food Chem. 2022, 391, 133224. [Google Scholar] [CrossRef]
- Xie, L.; Cai, C.; Cao, Y.; Li, X. Tea Saponins as Novel Stabilizers for the Development of Diosmin Nanosuspensions: Optimization and in Vitro Evaluation. J. Drug Deliv. Sci. Technol. 2023, 90, 105118. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Y.; Wang, X.; Zu, X.; Wang, H. Utilization of Peanut Protein Isolate Amyloid-like Fibers as Stabilizers for High Internal Phase Pickering Emulsions. Ind. Crops Prod. 2024, 213, 118414. [Google Scholar] [CrossRef]
- Gaudin, T.; Lu, H.; Fayet, G.; Berthauld-Drelich, A.; Rotureau, P.; Pourceau, G.; Wadouachi, A.; van Hecke, E.; Nesterenko, A.; Pezron, I. Impact of the Chemical Structure on Amphiphilic Properties of Sugar-Based Surfactants: A Literature Overview. Adv. Colloid Interface Sci. 2019, 270, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Sou, T.; Orlando, L.; McIntosh, M.P.; Kaminskas, L.M.; Morton, D.A. Investigating the Interactions of Amino Acid Components on a Mannitol-Based Spray-Dried Powder Formulation for Pulmonary Delivery: A Design of Experiment Approach. Int. J. Pharm. 2011, 421, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Sahoo, N.; Li, L. Precipitation of Poorly Water-Soluble Antioxidant Hesperetin for Improved Solubility and Dissolution. In Proceedings of the Chemeca 2011, Barton, Australia, 18–21 September 2011. [Google Scholar]
- Patel, M.K.; Tanna, B.; Mishra, A.; Jha, B. Physicochemical Characterization, Antioxidant and Anti-Proliferative Activities of a Polysaccharide Extracted from Psyllium (P. Ovata) Leaves. Int. J. Biol. Macromol. 2018, 118, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Baratto, M.C.; Tattini, M.; Galardi, C.; Pinelli, P.; Romani, A.; Visioli, F.; Basosi, R.; Pogni, R. Antioxidant Activity of Galloyl Quinic Derivatives Isolated from P. Lentiscus Leaves. Free Radic. Res. 2003, 37, 405–412. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, L. Antisolvent Precipitation for the Preparation of High Polymeric Procyanidin Nanoparticles under Ultrasonication and Evaluation of Their Antioxidant Activity in Vitro. Ultrason. Sonochem. 2018, 43, 208–218. [Google Scholar] [CrossRef]



| No. | A: Homogenate Speed (r/min) | B: Surfactant Ratio (%) | C: Rotor Diameter (mm) | D: Conentration (mg/mL) | E: Liquid–Liquid Ratio (mL/mL) | Particle Size (nm) |
|---|---|---|---|---|---|---|
| 1 | 600 | 1.00 | 20.00 | 5.00 | 10.00 | 1220 |
| 2 | 600 | 5.00 | 20.00 | 5.00 | 40.00 | 990 |
| 3 | 1200 | 5.00 | 40.00 | 5.00 | 40.00 | 412 |
| 4 | 600 | 1.00 | 40.00 | 10.00 | 10.00 | 1646 |
| 5 | 1200 | 1.00 | 40.00 | 10.00 | 40.00 | 1399 |
| 6 | 600 | 5.00 | 40.00 | 10.00 | 40.00 | 1481 |
| 7 | 1200 | 1.00 | 40.00 | 5.00 | 10.00 | 838 |
| 8 | 600 | 5.00 | 40.00 | 5.00 | 10.00 | 526 |
| 9 | 1200 | 5.00 | 20.00 | 10.00 | 10.00 | 1430 |
| 10 | 1200 | 1.00 | 20.00 | 5.00 | 40.00 | 1290 |
| 11 | 1200 | 5.00 | 20.00 | 10.00 | 10.00 | 1088 |
| 12 | 600 | 1.00 | 20.00 | 10.00 | 40.00 | 1880 |
| ANOVA | Sum of squares | Degree of freedom | Mean square | F-value | p-value | Significance level |
| Model | 1.959 × 106 | 5 | 3.918 × 105 | 16.57 | 0.0019 | ** |
| Residual | 1.418 × 105 | 6 | 23,638.67 | |||
| Cor Total | 2.101 × 106 | 11 | ||||
| Regression equation | Coefficient | Standard Error | F-Value | p-Value | Significance level | |
| A | −107.17 | 44.38 | 5.83 | 0.0522 | NS | |
| B | −195.50 | 44.38 | 19.40 | 0.0045 | ** | |
| C | −133.00 | 44.38 | 8.98 | 0.0241 | * | |
| D | 304.00 | 44.38 | 46.91 | 0.0005 | ** | |
| E | 58.67 | 44.38 | 1.75 | 0.2344 | NS |
| Run | BBD | ANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (mg/mL) | B (%) | C (mm) | Y (nm) | Source | SS | DF | Mean Square | F-Value | p-Value | ||||
| 1 | 10 | 5 | 30 | 813.21 | Model | 4.356 × 105 | 9 | 48,400.54 | 34.23 | <0.0001 ** | |||
| 2 | 10 | 3 | 20 | 1086.68 | A-Concentration | 32,951.30 | 1 | 32,951.30 | 23.31 | 0.0019 | |||
| 3 | 7.5 | 3 | 30 | 629.38 | B-Surfactant ratio | 24,355.35 | 1 | 24,355.35 | 17.23 | 0.0043 | |||
| 4 | 7.5 | 1 | 20 | 951.89 | C-Rotor diameter | 1629.06 | 1 | 1629.06 | 1.15 | 0.3187 | |||
| 5 | 7.5 | 3 | 30 | 668.65 | AB | 15,854.59 | 1 | 15,854.59 | 11.21 | 0.0123 | |||
| 6 | 7.5 | 5 | 40 | 823.45 | AC | 2.149 × 105 | 1 | 2.149 × 105 | 151.97 | <0.0001 ** | |||
| 7 | 5 | 5 | 30 | 514.59 | BC | 40,096.06 | 1 | 40,096.06 | 28.36 | 0.0011 | |||
| 8 | 10 | 1 | 30 | 770.32 | A2 | 15,035.74 | 1 | 15,035.74 | 10.63 | 0.0138 | |||
| 9 | 7.5 | 3 | 30 | 664.89 | B2 | 462.69 | 1 | 462.69 | 0.33 | 0.5852 | |||
| 10 | 7.5 | 5 | 20 | 613.97 | C2 | 84775.84 | 1 | 84775.84 | 59.96 | 0.0001 ** | |||
| 11 | 7.5 | 1 | 40 | 760.89 | Residual | 9897.38 | 7 | 1413.91 | |||||
| 12 | 10 | 3 | 40 | 670.98 | Lack of Fit | 6171.97 | 3 | 2057.32 | 2.21 | 0.2295 | |||
| 13 | 5 | 1 | 30 | 723.53 | Pure Error | 3725.41 | 4 | 931.35 | |||||
| 14 | 7.5 | 3 | 30 | 601.98 | Cor Total | 4.455 × 105 | 16 | ||||||
| 15 | 5 | 3 | 40 | 1050.51 | Credibility analysis of the regression equations | ||||||||
| 16 | 7.5 | 3 | 30 | 610.96 | Index mark | Standard deviation | Mean | CV% | Press | R2 | Adjust R2 | Predicted R2 | Adequacy precision |
| 17 | 5 | 3 | 20 | 539.13 | Y | 37.6 | 735 | 5.12 | 76.75 | 0.9778 | 0.9492 | 0.7653 | 20.645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Huang, W.; Zhang, X.; Liu, Z. Optimization of Nano-Tangeretin Recrystallization via Natural Surfactants in the Antisolvent Precipitation Process: Physicochemical Characterization and Antioxidant Activity. Nanomaterials 2025, 15, 791. https://doi.org/10.3390/nano15110791
Huang Y, Huang W, Zhang X, Liu Z. Optimization of Nano-Tangeretin Recrystallization via Natural Surfactants in the Antisolvent Precipitation Process: Physicochemical Characterization and Antioxidant Activity. Nanomaterials. 2025; 15(11):791. https://doi.org/10.3390/nano15110791
Chicago/Turabian StyleHuang, Yan, Wenxuan Huang, Xiaonan Zhang, and Zhiwei Liu. 2025. "Optimization of Nano-Tangeretin Recrystallization via Natural Surfactants in the Antisolvent Precipitation Process: Physicochemical Characterization and Antioxidant Activity" Nanomaterials 15, no. 11: 791. https://doi.org/10.3390/nano15110791
APA StyleHuang, Y., Huang, W., Zhang, X., & Liu, Z. (2025). Optimization of Nano-Tangeretin Recrystallization via Natural Surfactants in the Antisolvent Precipitation Process: Physicochemical Characterization and Antioxidant Activity. Nanomaterials, 15(11), 791. https://doi.org/10.3390/nano15110791






