Influence of Bioinspired Lithium-Doped Titanium Implants on Gingival Fibroblast Bioactivity and Biofilm Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Surface Modification
2.2. Culture of Human Gingival Fibroblasts
2.3. Cell Attachment and Spread Morphology
2.4. Cell Count
2.5. Gene Expression by Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Extracellular Matrix Expression by Luminex
2.7. Ethics Approval and Saliva Collection
2.8. Biofilm Development
2.8.1. Single Species Biofilms
2.8.2. Multispecies Biofilms
2.8.3. Biofilm Culture and Development
2.9. Bacterial Metabolic Activity
2.10. Biofilm Viability Staining
2.11. Statistical Analysis
3. Results
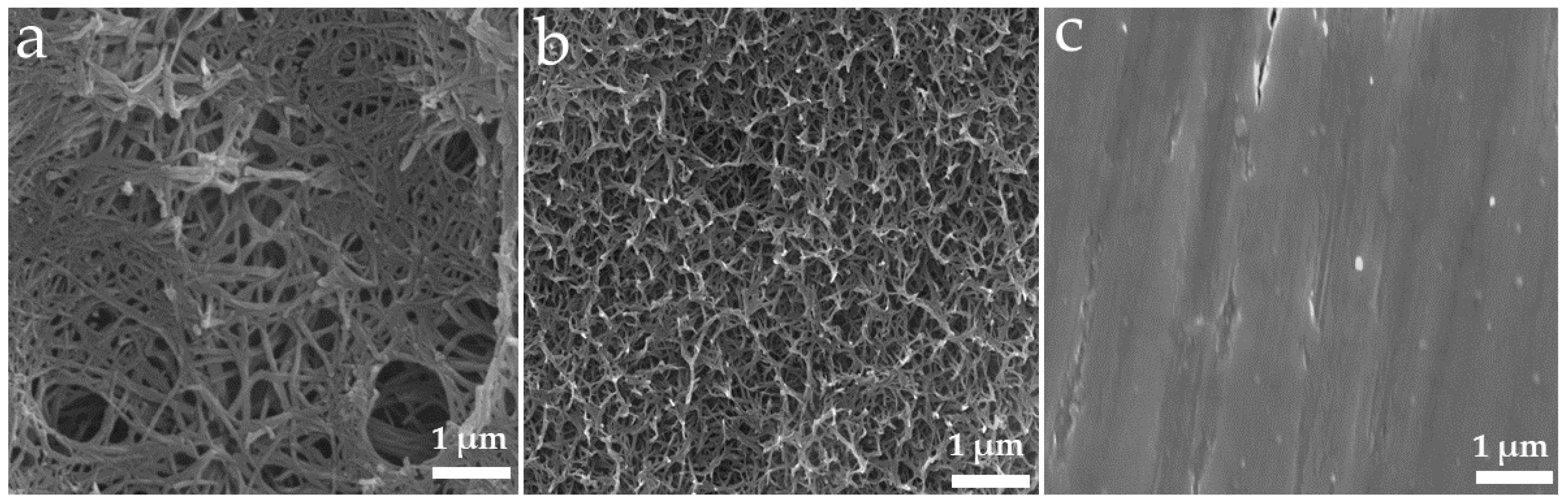
3.1. Surface Characterisation of Ti Substrates
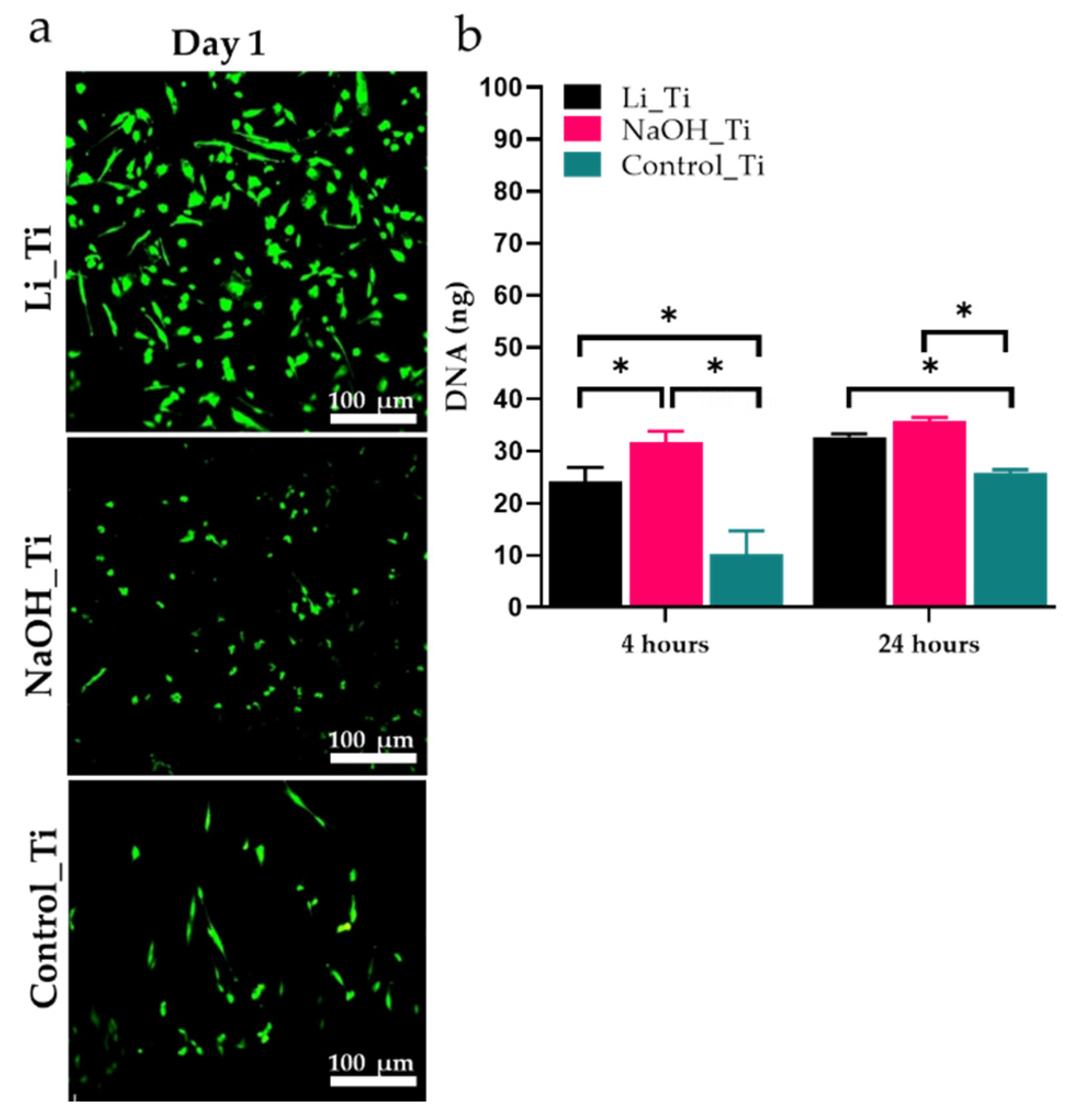
3.2. HGF Viability and Early Proliferation
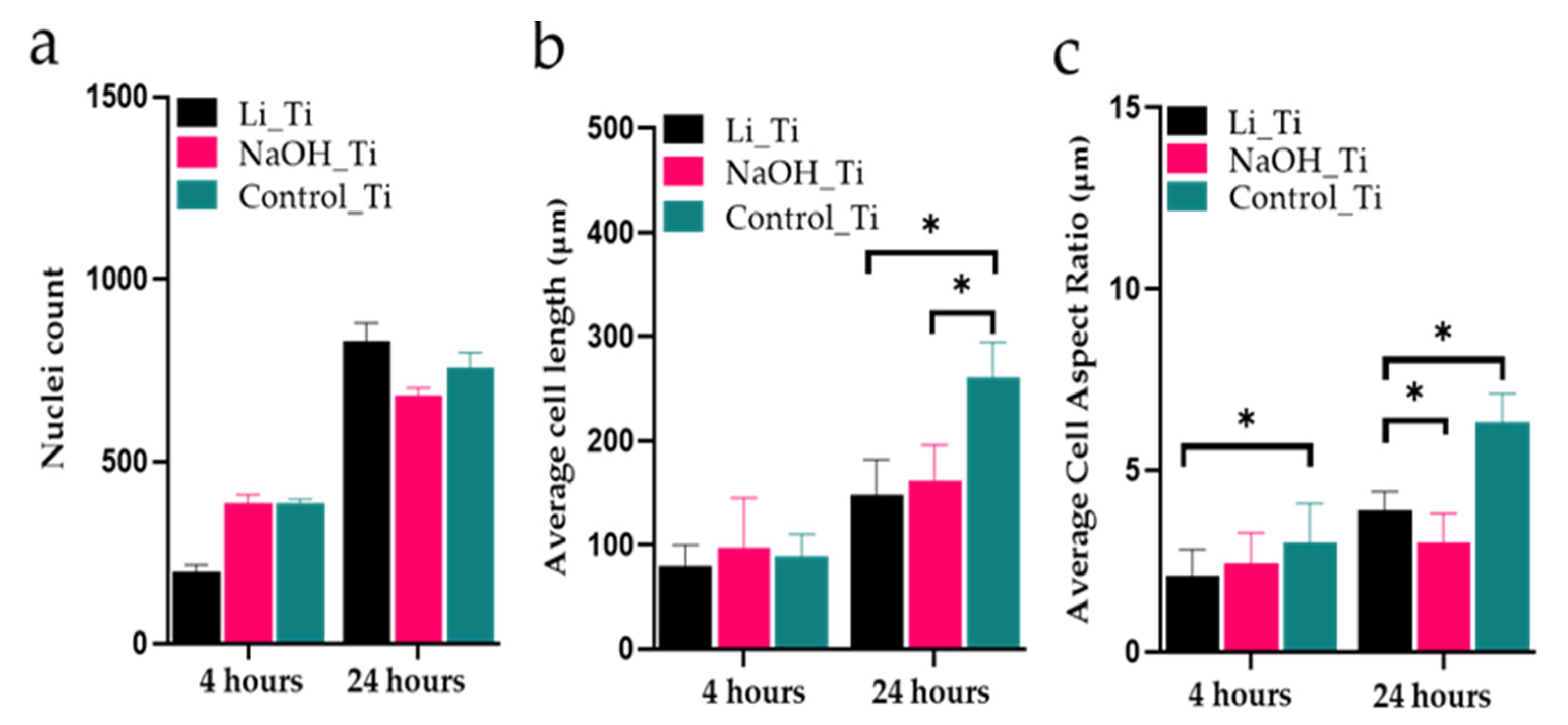
3.3. HGF Attachment and Morphology
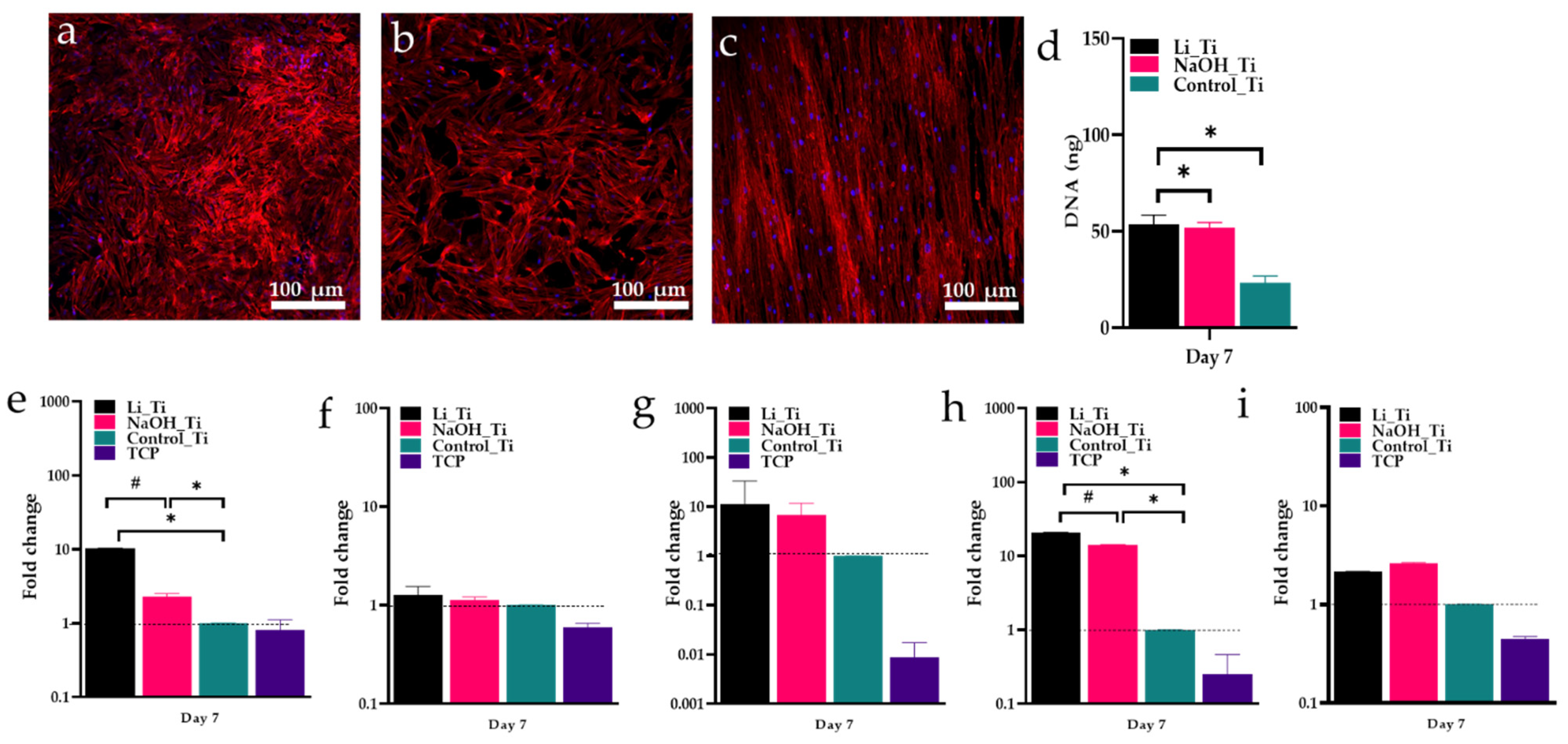
3.4. HGF Proliferation and Gene Expression of after 7 Days of Culture
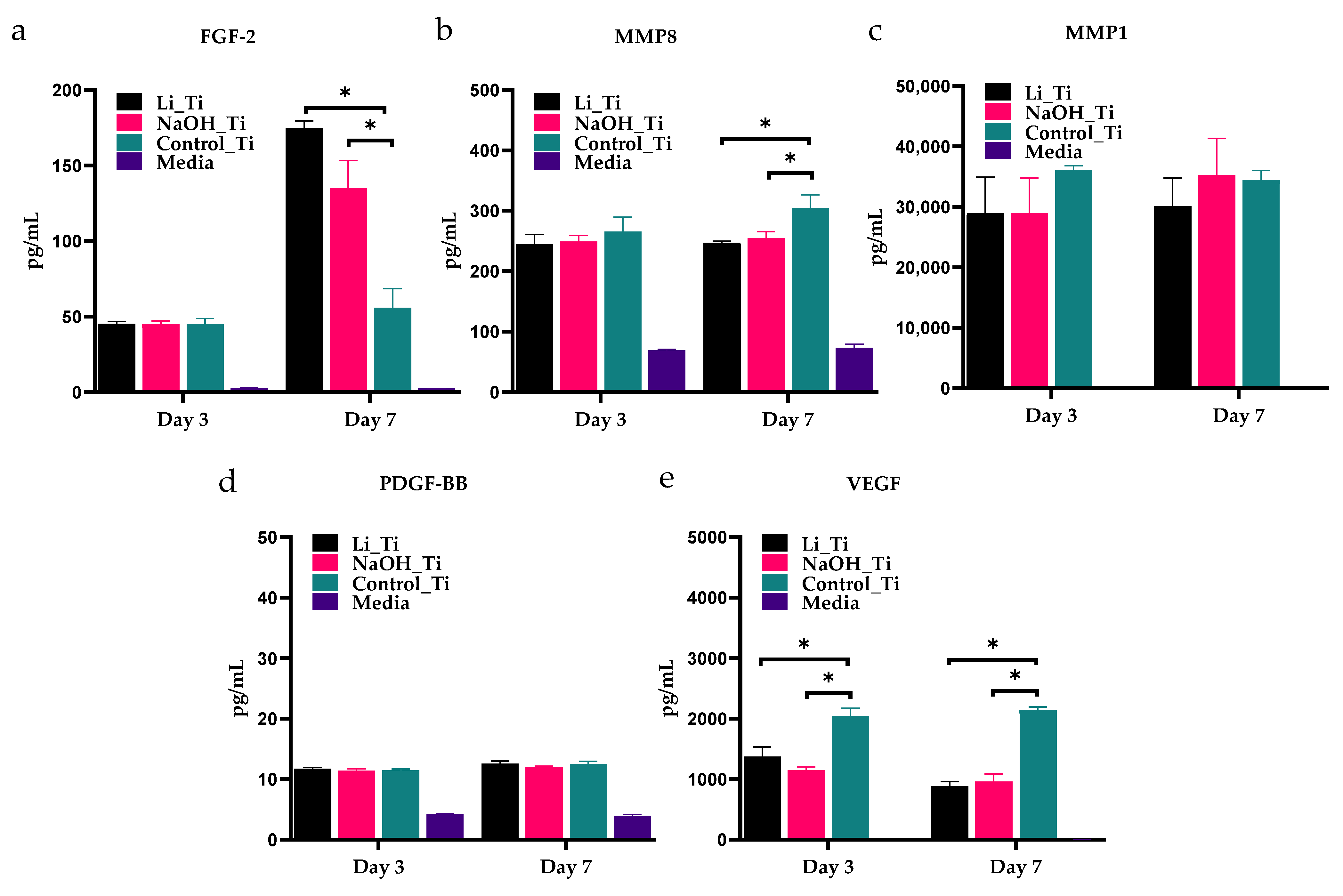
3.5. Analysis of Selected HGF-Secreted Proteins
3.6. Analysis of Bacterial Metabolic Activity
3.7. Biofilm Viability
4. Discussion
4.1. Gingival Fibroblasts Response to the Surface
4.2. Bacterial Activity over the Surface
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roos, J.; Sennerby, L.; Lekholm, U.L.F.; Jemt, T.; Gröndahl, K.; Albrektsson, T. A qualitative and quantitative method for evaluating implant success: A 5-year retrospective analysis of the Branemark implant. Int. J. Oral Maxillofac. Implants 1997, 12, 1–20. [Google Scholar]
- Becker, W.; Becker, B.E.; Newman, M.G.; Nyman, S. Clinical and microbiologic findings that may contribute to dental implant failure. Int. J. Oral Maxillofac. Implant. 1990, 5, 1–17. [Google Scholar]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontology 2000 2018, 76, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, M.A. Manipulation of the peri-implant tissue for better maintenance: A periodontal perspective. J. Oral Implant. 2003, 29, 120–123. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Race to invade: Understanding soft tissue integration at the transmucosal region of titanium dental implants. Dent. Mater. 2021, 37, 816–831. [Google Scholar] [CrossRef]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Orchestrating soft tissue integration at the transmucosal region of titanium implants. Acta Biomater. 2021, 124, 33–49. [Google Scholar] [CrossRef]
- Kim, H.; Murakami, H.; Chehroudi, B.; Textor, M.; Brunette, D.M. Effects of surface topography on the connective tissue attachment to subcutaneous implants. Int. J. Oral Maxillofac. Implant. 2006, 21, 354–365. [Google Scholar]
- Hao, J.; Li, Y.; Li, B.; Wang, X.; Li, H.; Liu, S.; Liang, C.; Wang, H. Biological and mechanical effects of micro-nanostructured titanium surface on an osteoblastic cell line In Vitro and osteointegration In Vivo. Appl. Biochem. Biotechnol. 2017, 183, 280–292. [Google Scholar] [CrossRef]
- Gui, N.; Xu, W.; Myers, D.; Shukla, R.; Tang, H.; Qian, M. The effect of ordered and partially ordered surface topography on bone cell responses: A review. Biomater. Sci. 2017, 6, 250–264. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of dental implant surface modifications on osseointegration. Biol. Med. Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, J.C.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Jain, S.; Chatterjee, K. Nanoscale topography on black titanium imparts multi-biofunctional properties for orthopedic applications. Sci. Rep. 2017, 7, 41118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadra, C.M.; Truong, V.K.; Pham, V.T.H.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.; Juodkazis, S.; Crawford, R.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modaresifar, K.; Azizian, S.; Ganjian, M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Bactericidal effects of nanopatterns: A systematic review. Acta Biomater. 2019, 83, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandara, C.D.; Singh, S.; Afara, I.O.; Wolff, A.; Tesfamichael, T.; Ostrikov, K.; Oloyede, A. Bactericidal effects of natural nanotopography of dragonfly wing on Escherichia coli. ACS Appl. Mater. Interfaces 2017, 9, 6746–6760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaggessar, A.; Mathew, A.; Wang, H.; Tesfamichael, T.; Yan, C.; Yarlagadda, P.K. Mechanical, bactericidal and osteogenic behaviours of hydrothermally synthesised TiO2 nanowire arrays. J. Mech. Behav. Biomed. Mater. 2018, 80, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanova, E.P.; Hasan, J.; Webb, H.; Truong, V.K.; Watson, G.; Watson, J.; Baulin, V.; Pogodin, S.; Wang, J.; Tobin, M.; et al. Natural bactericidal surfaces: Mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Gulati, K.; Ivanovski, S. Understanding and optimising the antibacterial functions of anodized nano-engineered titanium implants. Acta Biomater. 2021, 127, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Ferrà-Cañellas, M.D.M.; Llopis-Grimalt, M.A.; Monjo, M.; Ramis, J.M. Tuning nanopore diameter of titanium surfaces to improve human gingival fibroblast response. Int. J. Mol. Sci. 2018, 19, 2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, K.; Moon, H.J.; Kumar, P.S.; Han, P.; Ivanovski, S. Anodized anisotropic titanium surfaces for enhanced guidance of gingival fibroblasts. Mater. Sci. Eng. C. 2020, 112, 110860. [Google Scholar] [CrossRef]
- Guida, L.; Oliva, A.; Basile, M.A.; Giordano, M.; Nastri, L.; Annunziata, M. Human gingival fibroblast functions are stimulated by oxidized nano-structured titanium surfaces. J. Dent. 2013, 41, 900–907. [Google Scholar] [CrossRef]
- Gulati, K.; Moon, H.-J.; Li, T.; Kumar, P.S.; Ivanovski, S. Titania nanopores with dual micro-/nano-topography for selective cellular bioactivity. Mater. Sci. Eng. C. 2018, 91, 624–630. [Google Scholar] [CrossRef]
- Ziegler, N.; Sengstock, C.; Mai, V.; Schildhauer, T.A.; Köller, M.; Ludwig, A. Glancing-angle deposition of nanostructures on an implant material surface. Nanomaterials 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Jiang, L. Bio-inspired design of multiscale structures for function integration. Nano Today 2011, 6, 155–175. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; García-Martín, J.M.; Alvarez, R.; Palmero, A.; Esteban, J.; Pérez-Jorge, C.; Arcos, D.; Vallet-Regí, M. Nanocolumnar coatings with selective behavior towards osteoblast and Staphylococcus aureus proliferation. Acta Biomater. 2015, 15, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Zang, R.; Yang, K.K.; Liu, N.; Yang, S.-T. Three-dimensional fibrous scaffolds with microstructures and nanotextures for tissue engineering. RSC Adv. 2012, 2, 10110–10124. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.Y.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Dalby, M.J.; Riehle, M.O.; Johnstone, H.; Affrossman, S.; Curtis, A.S.G. Investigating the limits of filopodial sensing: A brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol. Int. 2004, 28, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, P.; Bathala, L.R.; Sangur, R. Techniques for dental implant nanosurface modifications. J. Adv. Prosthodont. 2014, 6, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, D.L. Alkaline Hydrothermal Treatment of Titanate Nanostructures. Ph.D. ThesIs, Queensland University of Technology, Brisbane City, QLD, Australia, 2010. [Google Scholar]
- Dorkhan, M.; Yucel-Lindberg, T.; Hall, J.; Svensäter, G.; Davies, J.R. Adherence of human oral keratinocytes and gingival fibroblasts to nano-structured titanium surfaces. BMC Oral Health 2014, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- Miao, X.; Wang, D.; Xu, L.; Wang, J.; Zeng, D.; Lin, S.; Huang, C.; Liu, X.; Jiang, X. The response of human osteoblasts, epithelial cells, fibroblasts, macrophages and oral bacteria to nanostructured titanium surfaces: A systematic study. Int. J. Nanomed. 2017, 12, 1415–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Guo, J.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases 2012, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Chen, D.; Jiang, G.; Li, Q.; Wang, Q.; Cheng, M.; He, G.; Zhang, X. A lithium-containing nanoporous coating on entangled titanium scaffold can enhance osseointegration through Wnt/β-catenin pathway. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 153–164. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Q.; Zhang, X.; Qiu, J.; Qian, S.; Liu, X. Co-implantation of magnesium and zinc ions into titanium regulates the behaviors of human gingival fibroblasts. Bioact. Mater. 2021, 6, 64–74. [Google Scholar] [CrossRef]
- Galli, C.; Piemontese, M.; Lumetti, S.; Manfredi, E.; Macaluso, G.M.; Passeri, G. GSK3b-inhibitor lithium chloride enhances activation of Wnt canonical signaling and osteoblast differentiation on hydrophilic titanium surfaces. Clin. Oral Implant. Res. 2013, 24, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Abdal-Hay, A.; Gulati, K.; Fernandez-Medina, T.; Qian, M.; Ivanovski, S. In Situ hydrothermal transformation of titanium surface into lithium-doped continuous nanowire network towards augmented bioactivity. Appl. Surf. Sci. 2020, 505, 144604. [Google Scholar] [CrossRef]
- Tang, G.H.; Xu, J.; Chen, R.J.; Qian, Y.F.; Shen, G. Lithium delivery enhances bone growth during midpalatal expansion. J. Dent. Res. 2011, 90, 336–340. [Google Scholar] [CrossRef]
- Han, P.; Wu, C.; Chang, J.; Xiao, Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/β-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials 2012, 33, 6370–6379. [Google Scholar] [CrossRef]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z.; Ivanovski, S. Bridging the gap: Optimized fabrication of robust titania nanostructures on complex implant geometries towards clinical translation. J. Colloid Interface Sci. 2018, 529, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Ivanovski, S. Effect of saliva collection methods on the detection of periodontium-related genetic and epigenetic biomarkers-a pilot study. Int. J. Mol. Sci. 2019, 20, 4729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, N.; Bandara, H.; Leishman, S.J.; Walsh, L.J. Inhibitory effects of fruit berry extracts on Streptococcus mutans biofilms. Eur. J. Oral Sci. 2019, 127, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Koban, I.; Matthes, R.; Hübner, N.O.; Welk, A.; Sietmann, R.; Lademann, J.; Kramer, A.; Kocher, T. XTT assay of Ex Vivo saliva biofilms to test antimicrobial influences. GMS Krankenhaushygiene Interdisziplinär 2012, 7, Doc06. [Google Scholar]
- Kim, H.-J.; Cho, M.-Y.; Lee, E.-S.; Jung, H.I.; Kim, B.-I. Effects of short-time exposure of surface pre-reacted glass-ionomer eluate on dental microcosm biofilm. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.W.; Blankenship, J.A. Migration of gingival fibroblasts on fibronectin and laminin. J. Periodontol. 1997, 68, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Isoshima, K.; Ueno, T.; Arai, Y.; Saito, H.; Chen, P.; Tsutsumi, Y.; Hanawa, T.; Wakabayashi, N. The change of surface charge by lithium ion coating enhances protein adsorption on titanium. J. Mech. Behav. Biomed. Mater. 2019, 100, 103393. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Takei, H.H.; Carranza, F.A. Carranza’s Clinical Periodontology; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Roman-Malo, L.; Bullon, B.; De Miguel, M.; Bullon, P. Fibroblasts collagen production and histological alterations in hereditary gingival fibromatosis. Diseases 2019, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Meyle, J. Cell adhesion and spreading on different implant surfaces. In Proceedings of the 3rd European Workshop on Periodontology, Waldenburg, Switzerland, 1999. [Google Scholar]
- Knowles, G.C.; McKeown, M.; Sodek, J.; McCulloch, C.A. Mechanism of collagen phagocytosis by human gingival fibroblasts: Importance of collagen structure in cell recognition and internalization. J. Cell Sci. 1991, 98, 551–558. [Google Scholar] [CrossRef]
- Wang, X.; Lu, T.; Wen, J.; Xu, L.; Zeng, D.; Wu, Q.; Cao, L.; Lin, S.; Liu, X.; Jiang, X. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyetheretherketone with multilevel nanostructured TiO2. Biomaterials 2016, 83, 207–218. [Google Scholar] [CrossRef]
- Zhang, P.; Katz, J.; Michalek, S.M. Glycogen synthase kinase-3beta (GSK3beta) inhibition suppresses the inflammatory response to Francisella infection and protects against tularemia in mice. Mol. Immunol. 2009, 46, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Hormia, M.; Könönen, M. Immunolocalization of fibronectin and vitronectin receptors in human gingival fibroblasts spreading on titanium surfaces. J. Periodontal Res. 1994, 29, 146–152. [Google Scholar] [CrossRef]
- Zhou, Z.; Dai, Y.; Liu, B.-B.; Xia, L.-L.; Liu, H.-B.; Vadgama, P.; Liu, H.-R. Surface modification of titanium plate enhanced fibronectin-mediated adhesion and proliferation of MG-63 cells. Trans. Nonferrous Met. Soc. China 2014, 24, 1065–1071. [Google Scholar] [CrossRef]
- Qi, H.; Shi, M.; Ni, Y.; Mo, W.; Zhang, P.; Jiang, S.; Zhang, Y.; Deng, X. Size-confined effects of nanostructures on fibronectin-induced macrophage inflammation on titanium implants. Adv. Health Mater. 2021, e2100994. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.S.; Schreiner, C.L.; Giancotti, F.G.; Ruoslahti, E.; Juliano, R.L. Motility of fibronectin receptor-deficient cells on fibronectin and vitronectin: Collaborative interactions among integrins. J. Cell Biol. 1992, 118, 217. [Google Scholar] [CrossRef] [Green Version]
- Ohshima, M.; Yamaguchi, Y.; Ambe, K.; Horie, M.; Saito, A.; Nagase, T.; Nakashima, K.; Ohki, H.; Kawai, T.; Abiko, Y.; et al. Fibroblast VEGF-receptor 1 expression as molecular target in periodontitis. J. Clin. Periodontol. 2015, 43, 128–137. [Google Scholar] [CrossRef]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The role of matrix metalloproteinases in periodontal disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef] [PubMed]
- Maddaluno, L.; Urwyler, C.; Werner, S. Fibroblast growth factors: Key players in regeneration and tissue repair. Development 2017, 144, 4047–4060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.; Huang, X.; Gao, Y.; Ling, J.; Huang, Y.; Xiao, Y. FGF-2 induces the proliferation of human periodontal ligament cells and modulates their osteoblastic phenotype by affecting Runx2 expression in the presence and absence of osteogenic inducers. Int. J. Mol. Med. 2015, 36, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Takayama, S.I.; Yoshida, J.; Hirano, H.; Okada, H.; Murakami, S. Effects of basic fibroblast growth factor on human gingival epithelial cells. J. Periodontol. 2002, 73, 1467–1473. [Google Scholar] [CrossRef]
- Wang, L.; Xie, X.; Li, C.; Liu, H.; Zhang, K.; Zhou, Y.; Chang, X.; Xu, H.H. Novel bioactive root canal sealer to inhibit endodontic multispecies biofilms with remineralising calcium phosphate ions. J. Dent. 2017, 60, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Pourhashemi, S.J.; Sadegh, M.; Salehi, Y.; Fard, M.J.K. Antibiofilm efficacy of silver nanoparticles as a vehicle for calcium hydroxide medicament against Enterococcus faecalis. J. Dent. 2015, 43, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Garbacz, K.; Jarzembowski, T.; Kwapisz, E.; Daca, A.; Witkowski, J. Do the oral Staphylococcus aureus strains from denture wearers have a greater pathogenicity potential? J. Oral. Microbiol. 2018, 11, 1536193. [Google Scholar] [CrossRef]
- Willems, H.M.; Xu, Z.; Peters, B.M. Polymicrobial biofilm studies: From basic science to biofilm control. Curr. Oral Health Rep. 2016, 3, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M.; Sedghi, A. A comparative study on the In Vitro formation of hydroxyapatite, cytotoxicity and antibacterial activity of 58S bioactive glass substituted by Li and Sr. Mater. Sci. Eng. C. 2018, 91, 349–360. [Google Scholar] [CrossRef]


| Gene Symbol | Direction | Primer Sequence | Length (bp) |
|---|---|---|---|
| hCOL1A1 | Forward | CCTGCGTGTACCCCACTCA | 115 |
| Reverse | ACCAGACATGCCTCTTGTCCTT | 115 | |
| hCOL3A1 | Fwd | CCGTTCTCTGCGATGACATAA | 142 |
| Rev | CCTTGAGGTCCTTGACCATTAG | 142 | |
| hGAPDH | Fwd | TCAGCAATGCATCCTGCAC | 117 |
| Rev | TCTGGGTGGCAGTGATGGC | 117 | |
| h18S | Fwd | CAGACATTGACCTCACCAAGAG | 99 |
| Rev | GAATCTTCTTCAGTCGCTCCAG | 99 | |
| hIL_1B | Fwd | GGTGTTCTCCATGTCCTTTGTA | 125 |
| Rev | GCTGTAGAGTGGGCTTATCATC | 125 | |
| hCXCL8 | Fwd | GAGAGTGATTGAGAGTGGACCAC | 112 |
| Rev | CACAACCCTCTGCACCCAGTTT | 112 | |
| hFN1 | Fwd | CACAGTCAGTGTGGTTGCCT | 68 |
| Rev | CTGTGGACTGGGTTCCAATCA | 68 |
| Figure | Test/Assay | Time Points | Description | Inference |
|---|---|---|---|---|
| Figure 2a | Livedead staining | D1 | Viability of cells | Live cells were observed in all groups ( no signs of cytotoxicity) |
| Figure 2b | Picogreen | D1 | Early cell proliferation measured by DNA content | Some significance in DNA content was observed |
| Figure 3a–f | Immunofluorescence staining (DAPI, phalloidin) | 4 h, D1 | Early visualization of nuclei and actin filaments | Generated images (at least 3 samples per group) were used for the analysis showed in Figure 4 |
| Figure 3g–i | Scanning electron microscopy | D1 | Detailed information of the surface and attached cells | Closer visualization of cellular morphology |
| Figure 5a–c | Immunofluorescence staining (DAPI, Phalloidin) | D7 | 1 week old visualization of nuclei and actin filaments | Some difference of the filaments density was observed |
| Figure 5d | Picogreen | D7 | 1 week old cell proliferation measured by DNA content | Significance of DNA content between groups |
| Figure 5e–i | Real time PCR | D7 | Quantification of mRNA levels of selected primers | Significant increase in the expression of COL 1 and Fibronectin between Li_Ti and NaOH_Ti |
| Figure 6 | Multiplex-ELISA | D7 | Quantification of protein concentrations in the culture media | Significant difference in protein concentration between treated titanium groups vs. control titanium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alali, A.Q.; Abdal-hay, A.; Gulati, K.; Ivanovski, S.; Fournier, B.P.J.; Lee, R.S.B. Influence of Bioinspired Lithium-Doped Titanium Implants on Gingival Fibroblast Bioactivity and Biofilm Adhesion. Nanomaterials 2021, 11, 2799. https://doi.org/10.3390/nano11112799
Alali AQ, Abdal-hay A, Gulati K, Ivanovski S, Fournier BPJ, Lee RSB. Influence of Bioinspired Lithium-Doped Titanium Implants on Gingival Fibroblast Bioactivity and Biofilm Adhesion. Nanomaterials. 2021; 11(11):2799. https://doi.org/10.3390/nano11112799
Chicago/Turabian StyleAlali, Aya Q., Abdalla Abdal-hay, Karan Gulati, Sašo Ivanovski, Benjamin P. J. Fournier, and Ryan S. B. Lee. 2021. "Influence of Bioinspired Lithium-Doped Titanium Implants on Gingival Fibroblast Bioactivity and Biofilm Adhesion" Nanomaterials 11, no. 11: 2799. https://doi.org/10.3390/nano11112799
APA StyleAlali, A. Q., Abdal-hay, A., Gulati, K., Ivanovski, S., Fournier, B. P. J., & Lee, R. S. B. (2021). Influence of Bioinspired Lithium-Doped Titanium Implants on Gingival Fibroblast Bioactivity and Biofilm Adhesion. Nanomaterials, 11(11), 2799. https://doi.org/10.3390/nano11112799









