Functionalization of Gold Nanoparticles by Inorganic Entities
Abstract
1. Introduction
2. Notions on Stabilization of Gold and Silver Nanoparticles
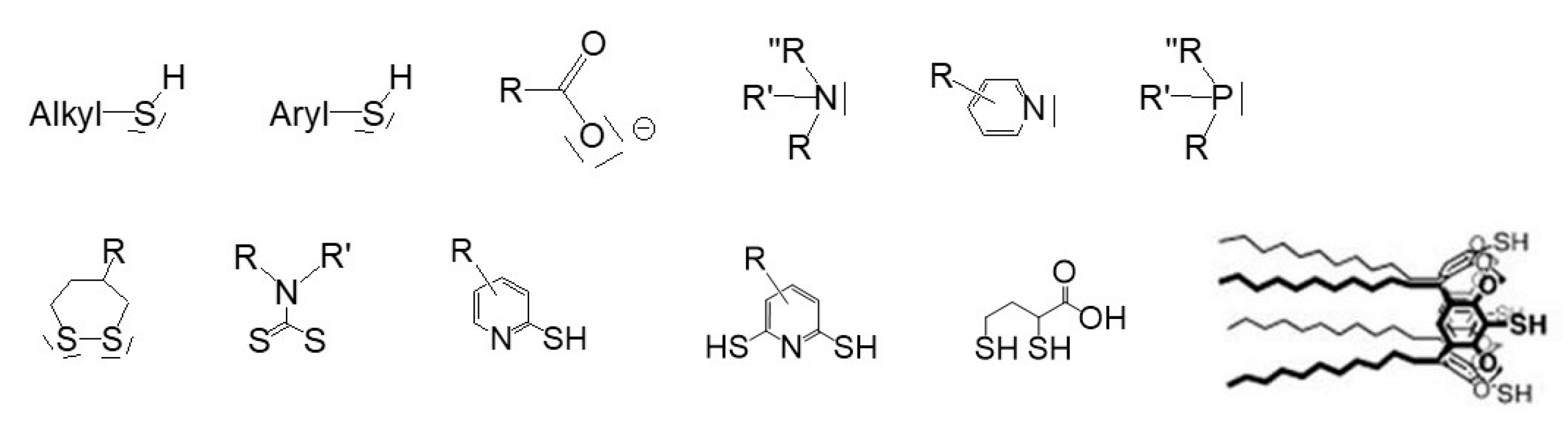
2.1. Role and Nature of the Stabilizing Agent
2.2. Synthetic Methods
3. Prerequisite for Functionalizing Agents
3.1. Definition
3.2. Method to Functionalize Au°-Nanoparticles
3.3. Technical Characterization of Functionalized Au-NPs
4. Functionalization by Inorganic Entities from P- Block
4.1. Molecular Clusters
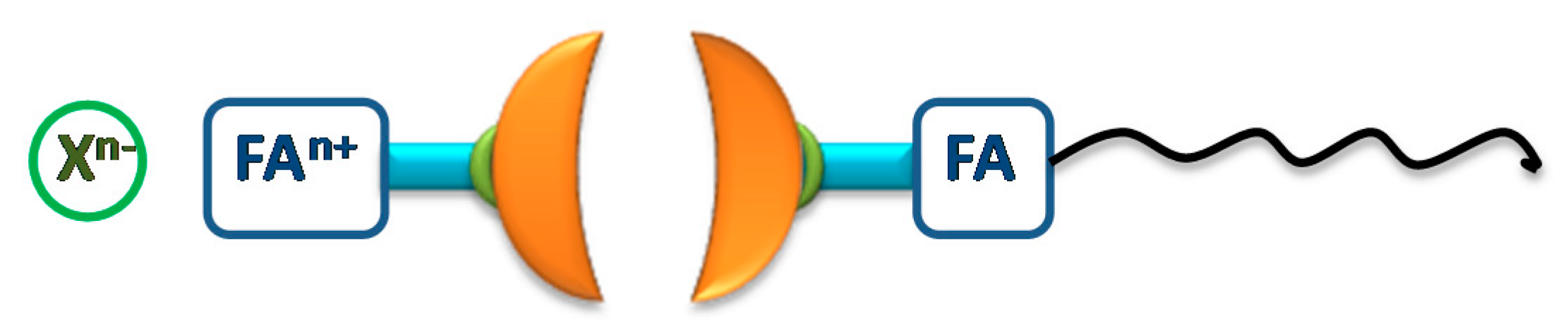
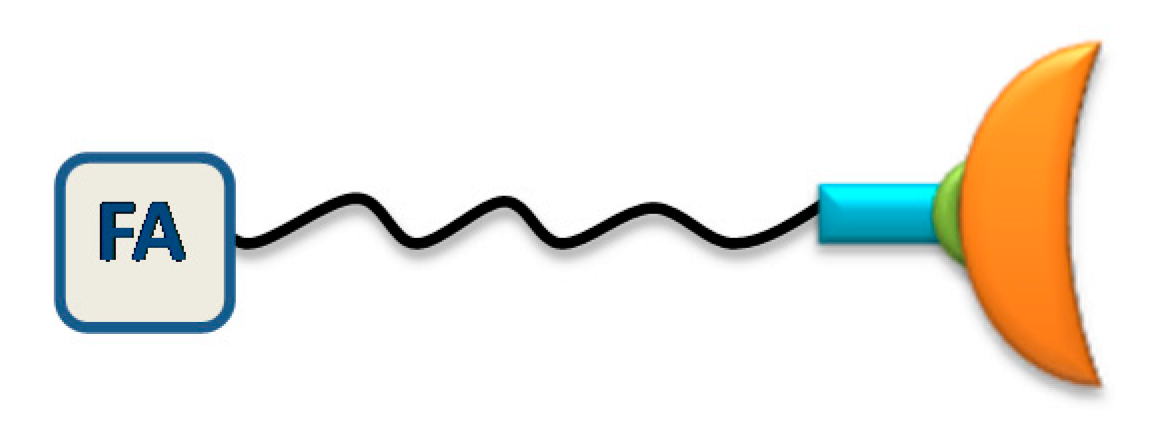
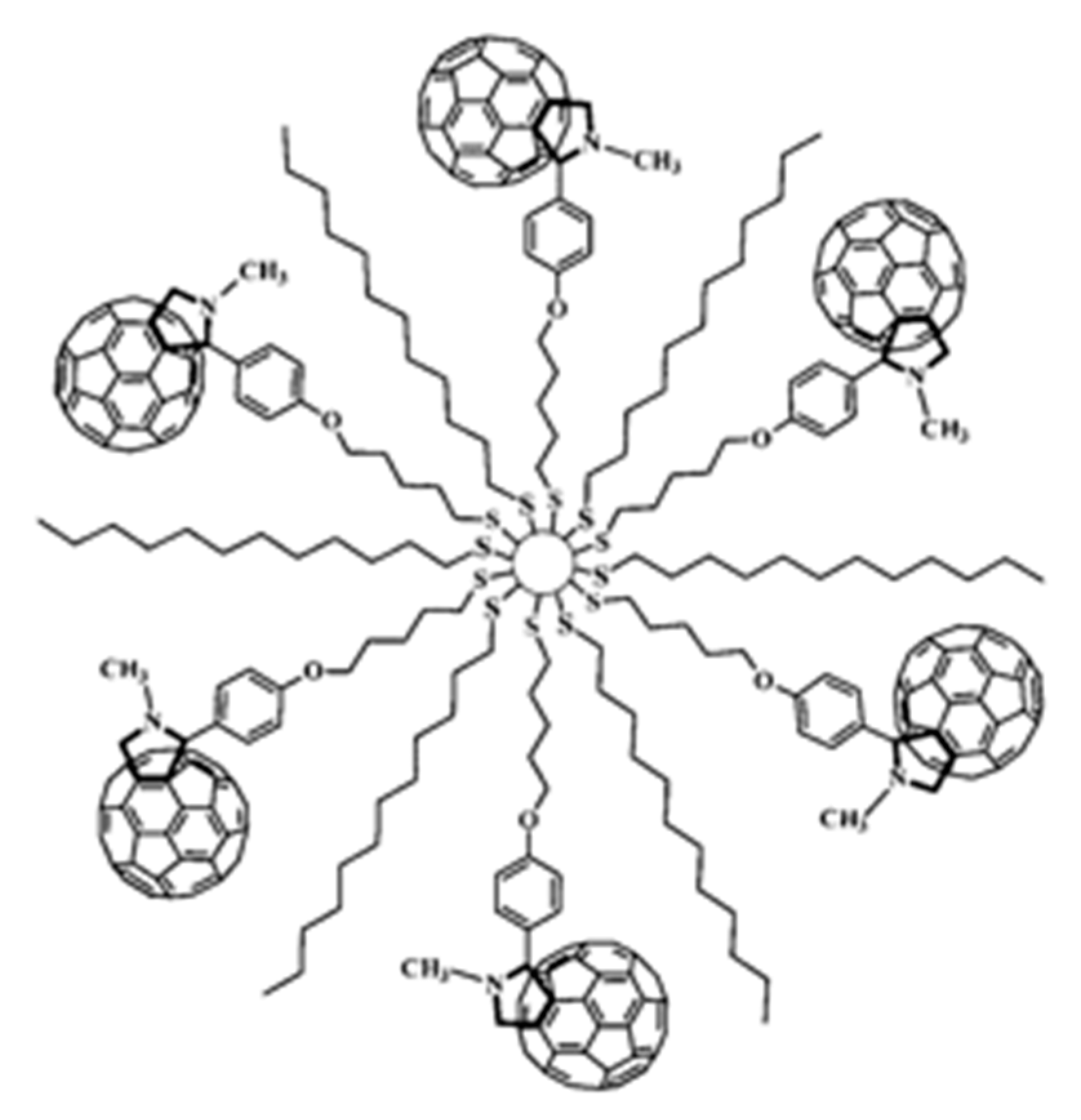
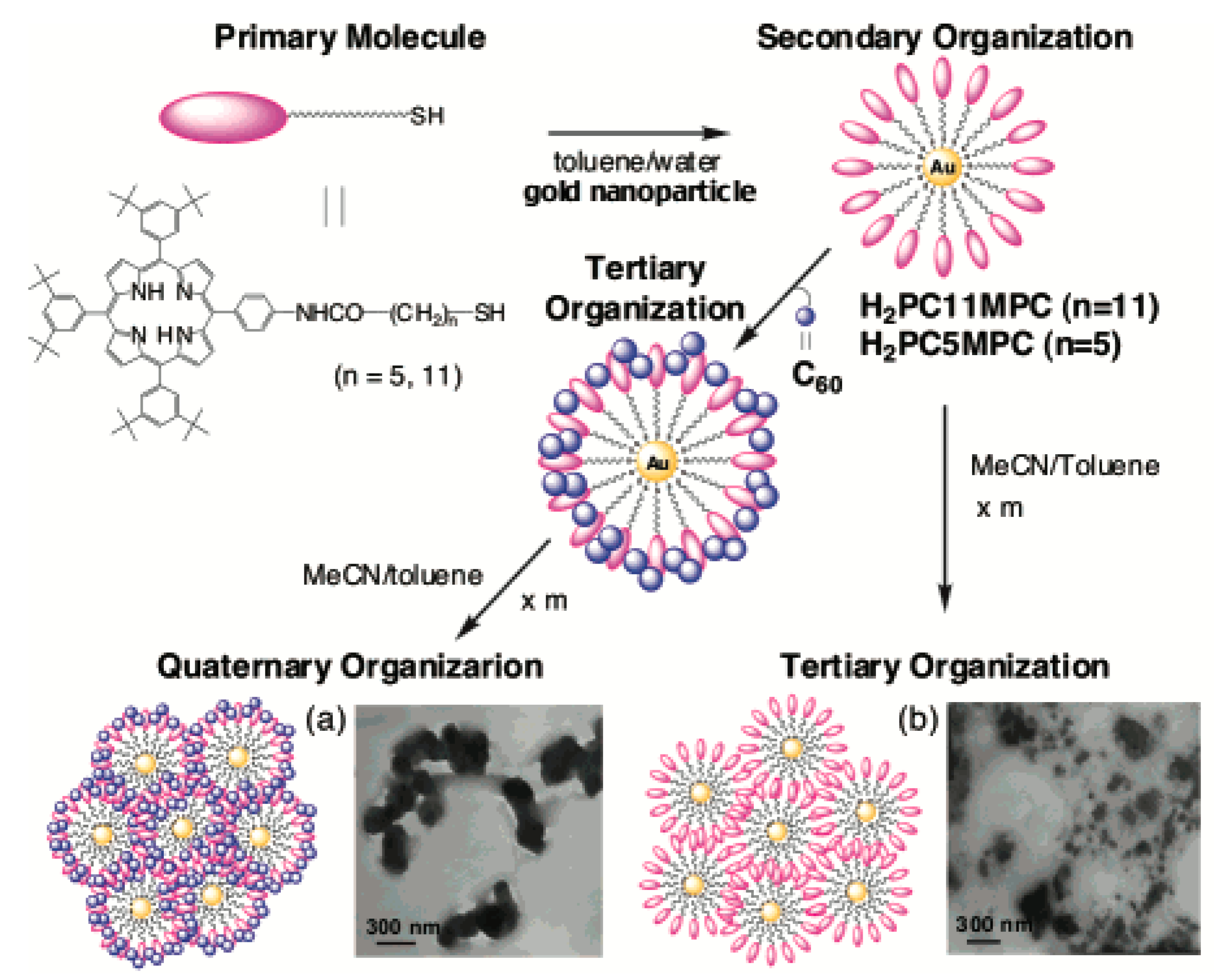
4.1.1. Fullerene (C60) Clusters
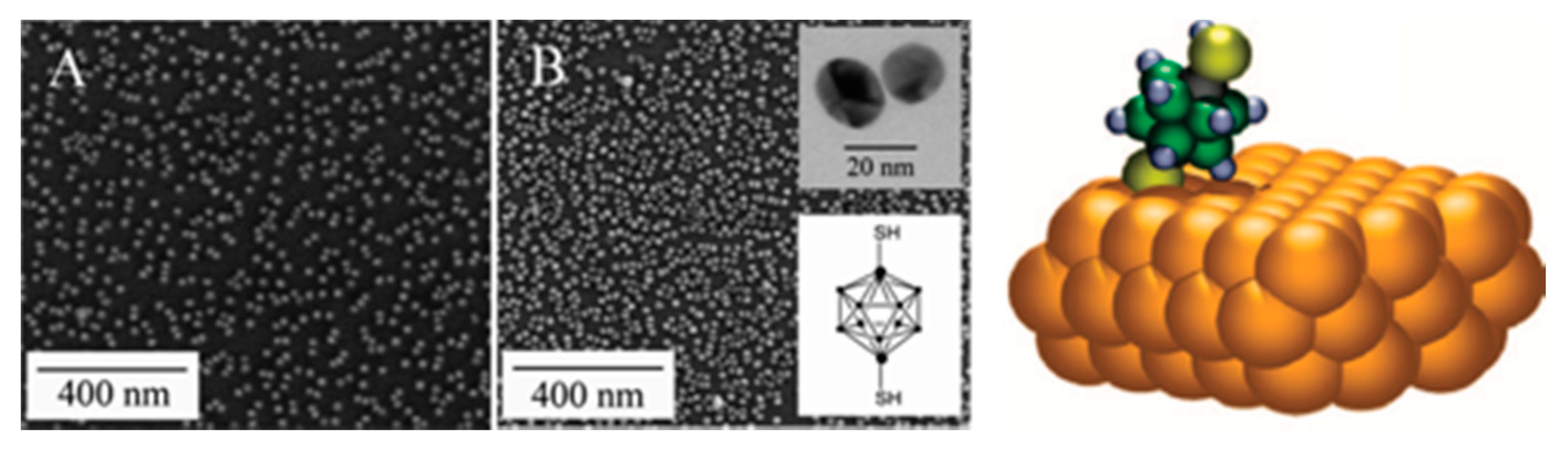
4.1.2. Carborane Clusters
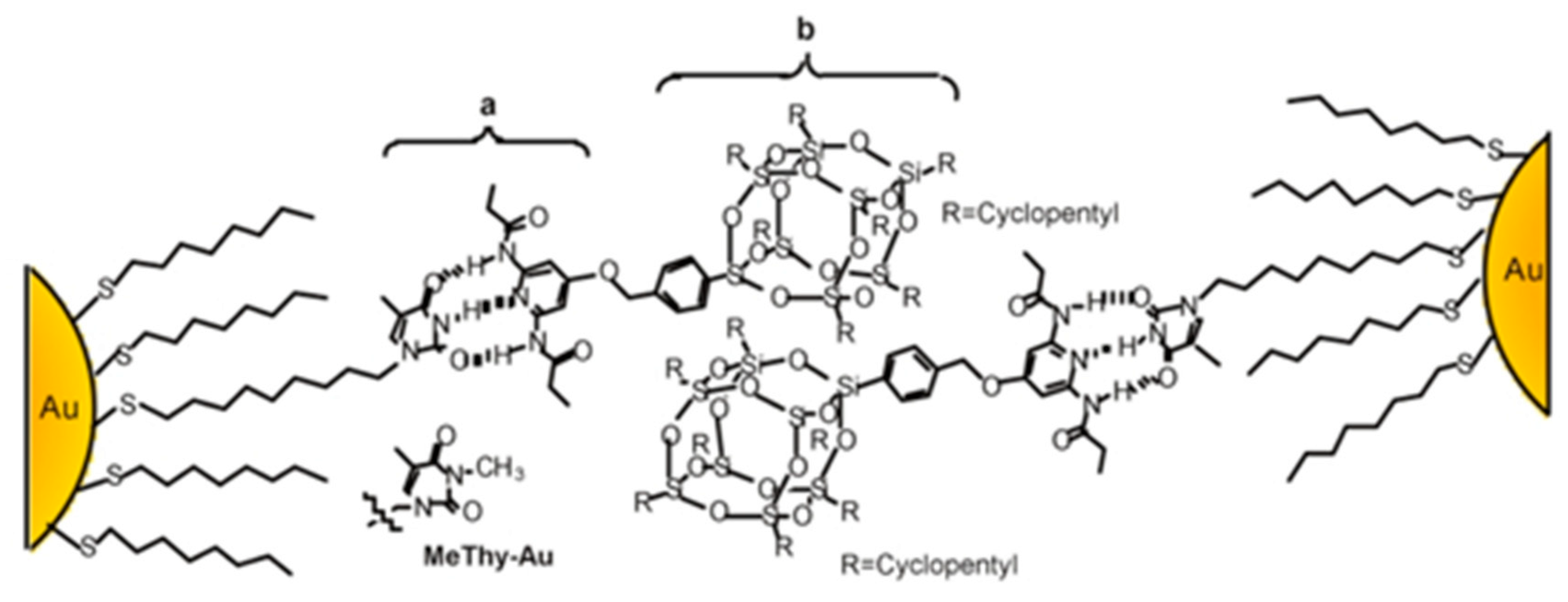
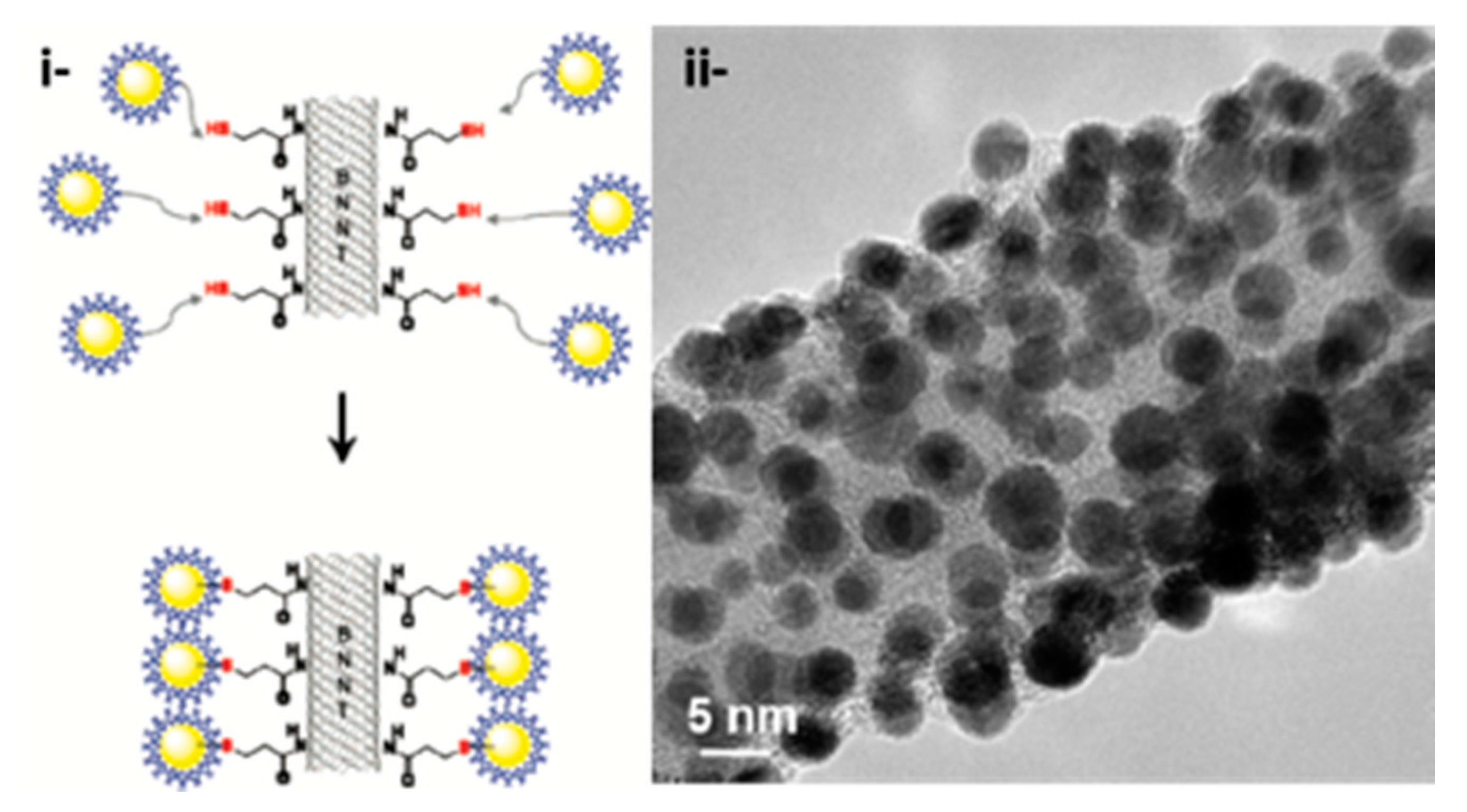
4.1.3. POSS Clusters
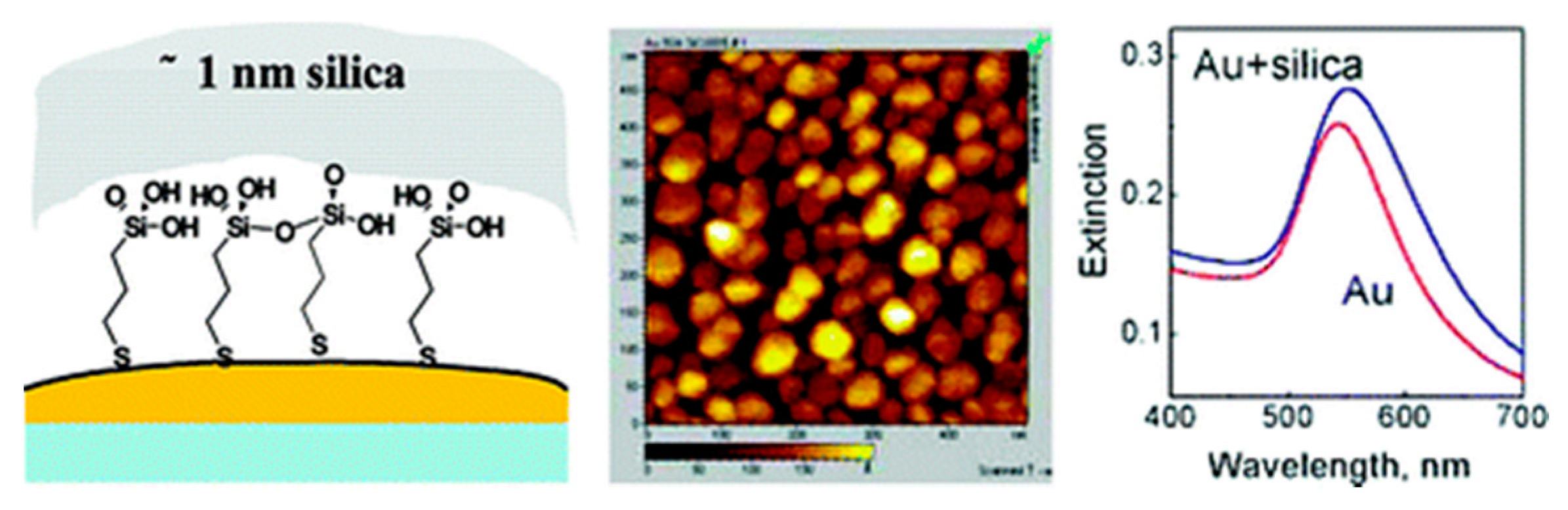
4.2. Silica Coating
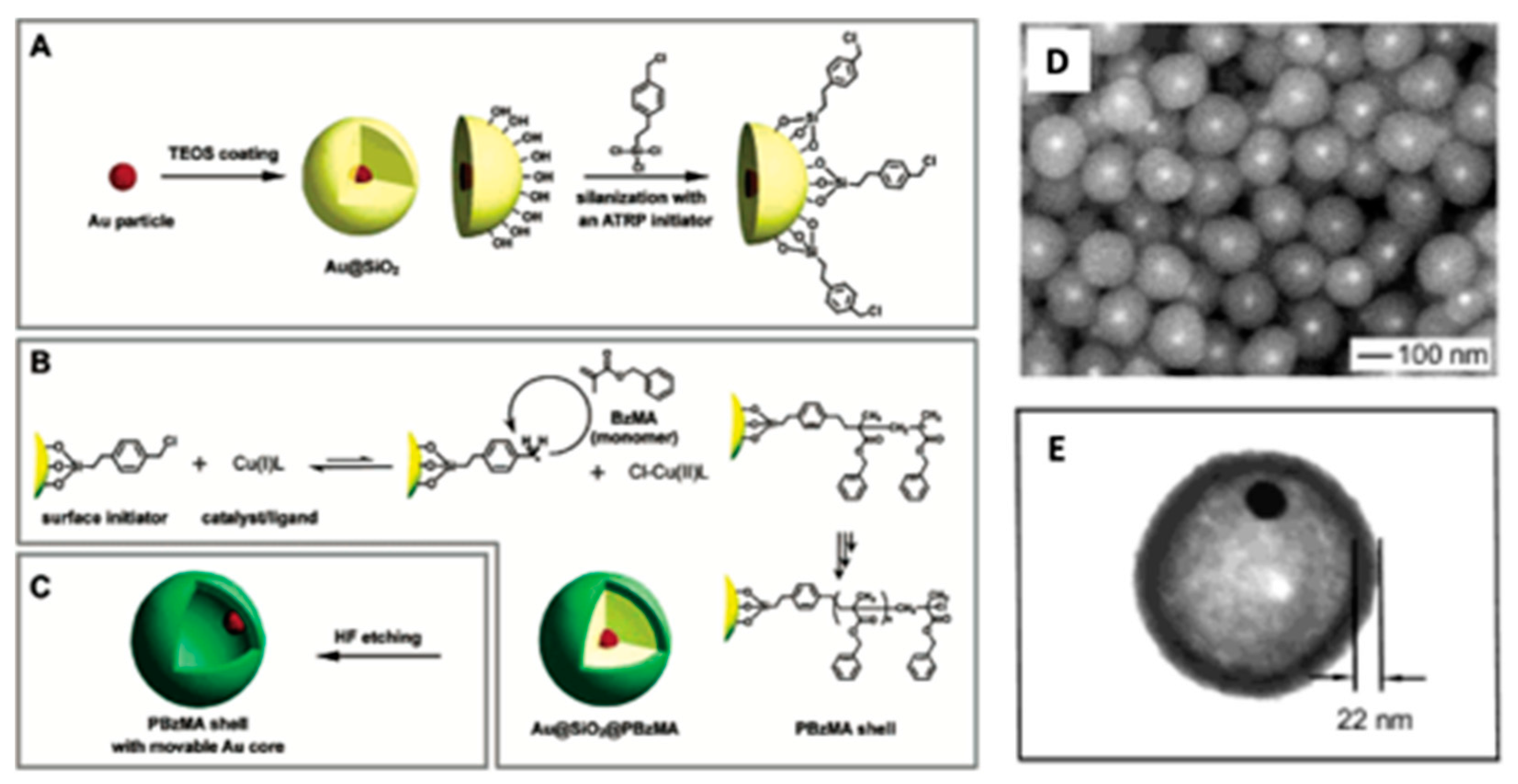
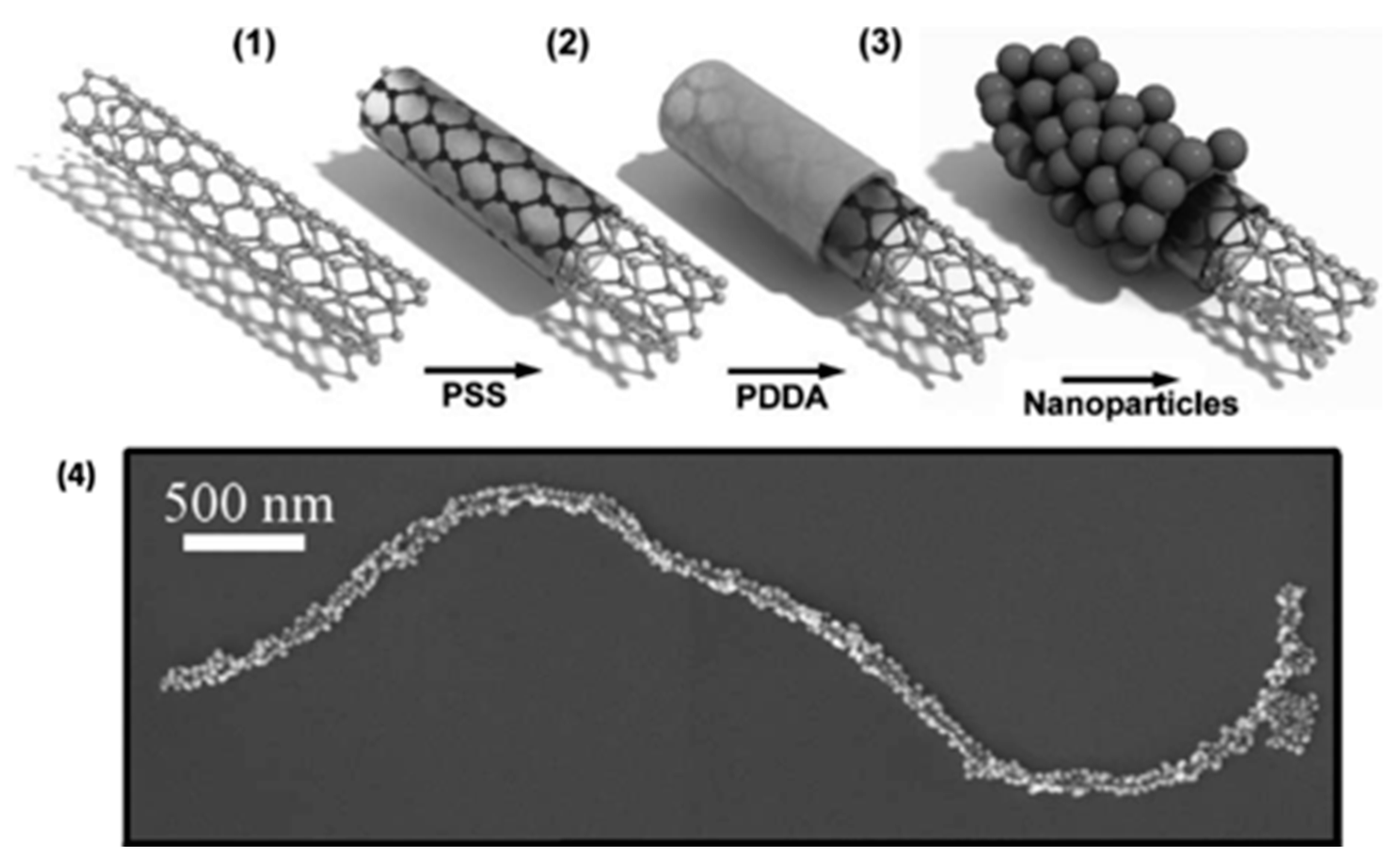
4.3. Combination Au-NPs and Carbon Nanotubes.
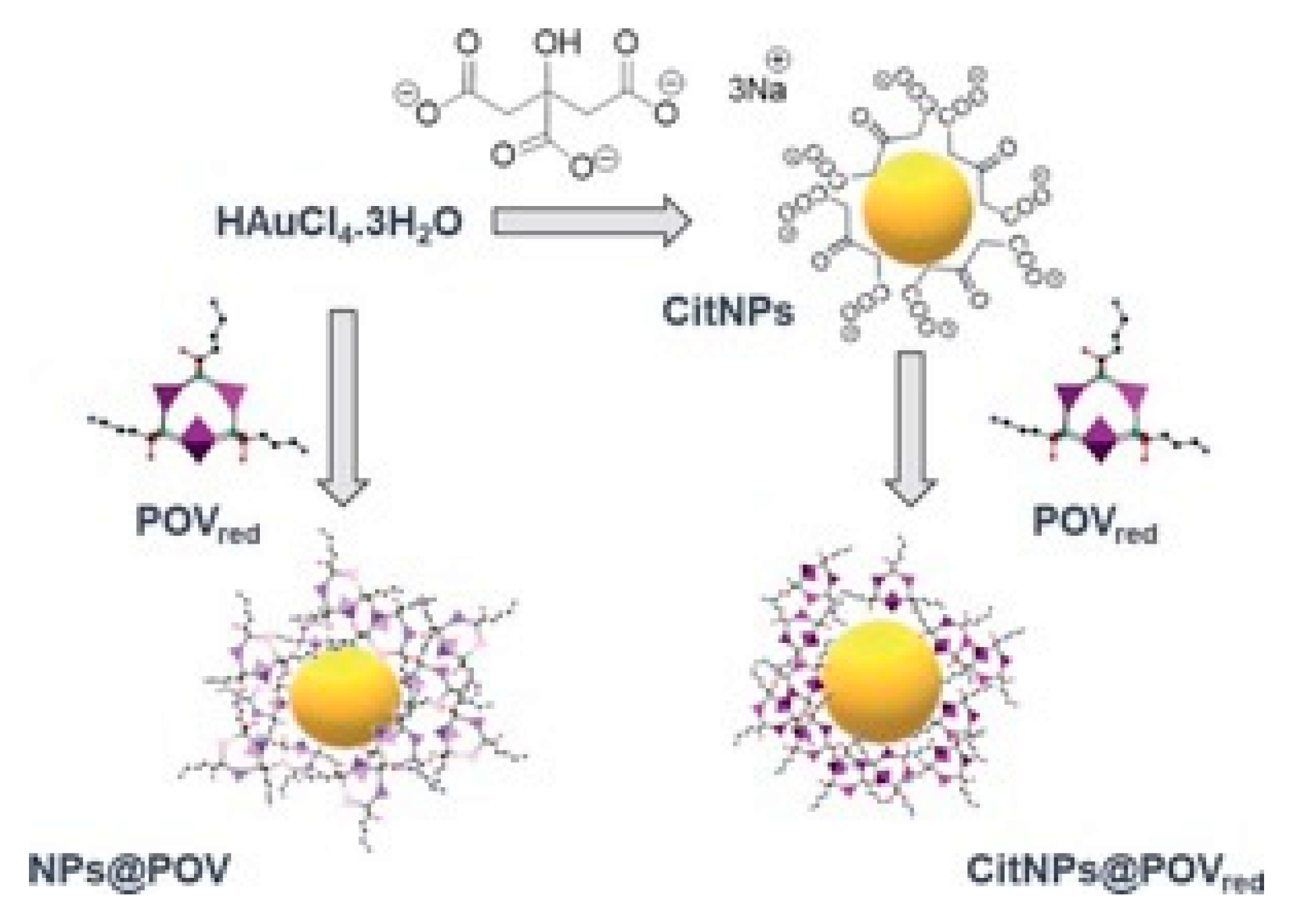
5. Functionalization by Polyoxometalate Compounds (POM)
6. Functionalization by Organometallic Complexes
6.1. Single Ferroncenyl Complexes
6.2. Variety of Organometallics-Au-NPs.
7. Functionalization by Coordination Complexes from D-Block Elements
7.1. Prussian Blue Derivatives
7.2. Terpyridinyl Metallic Complexes
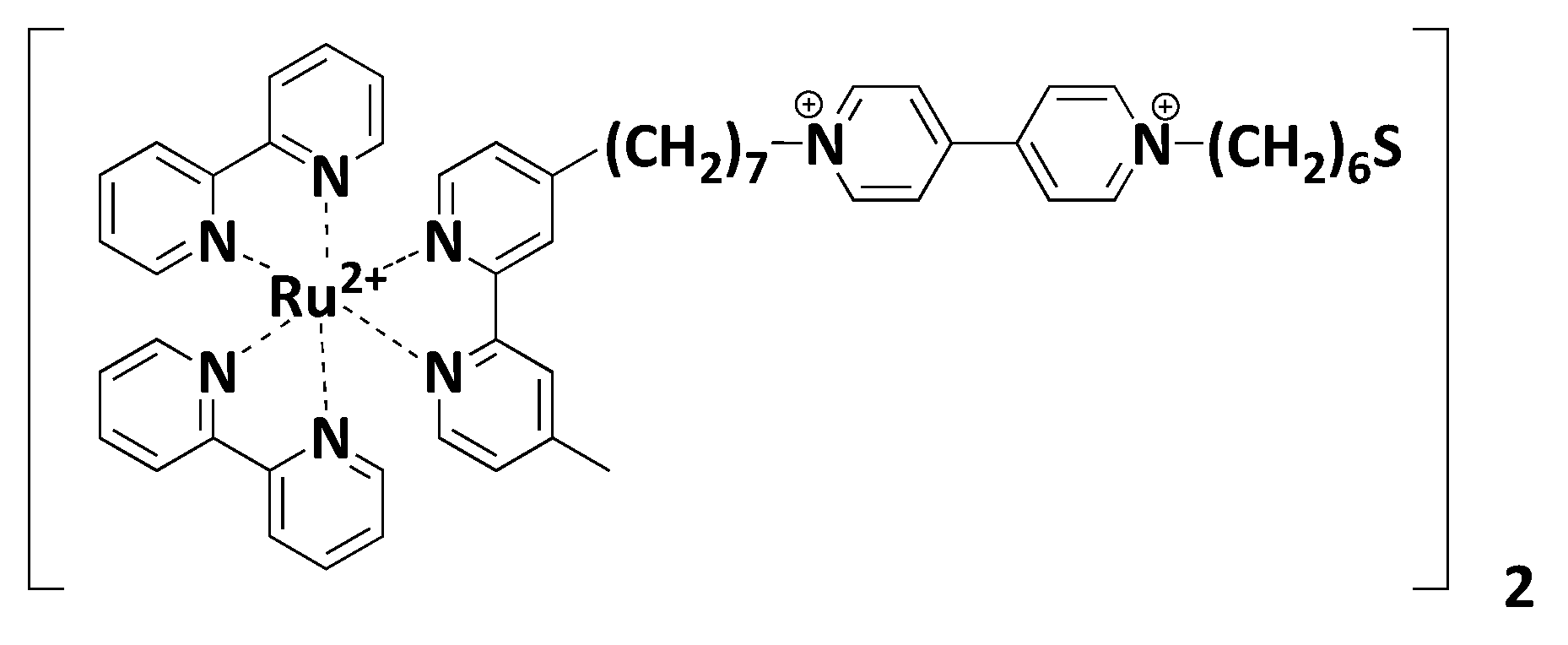
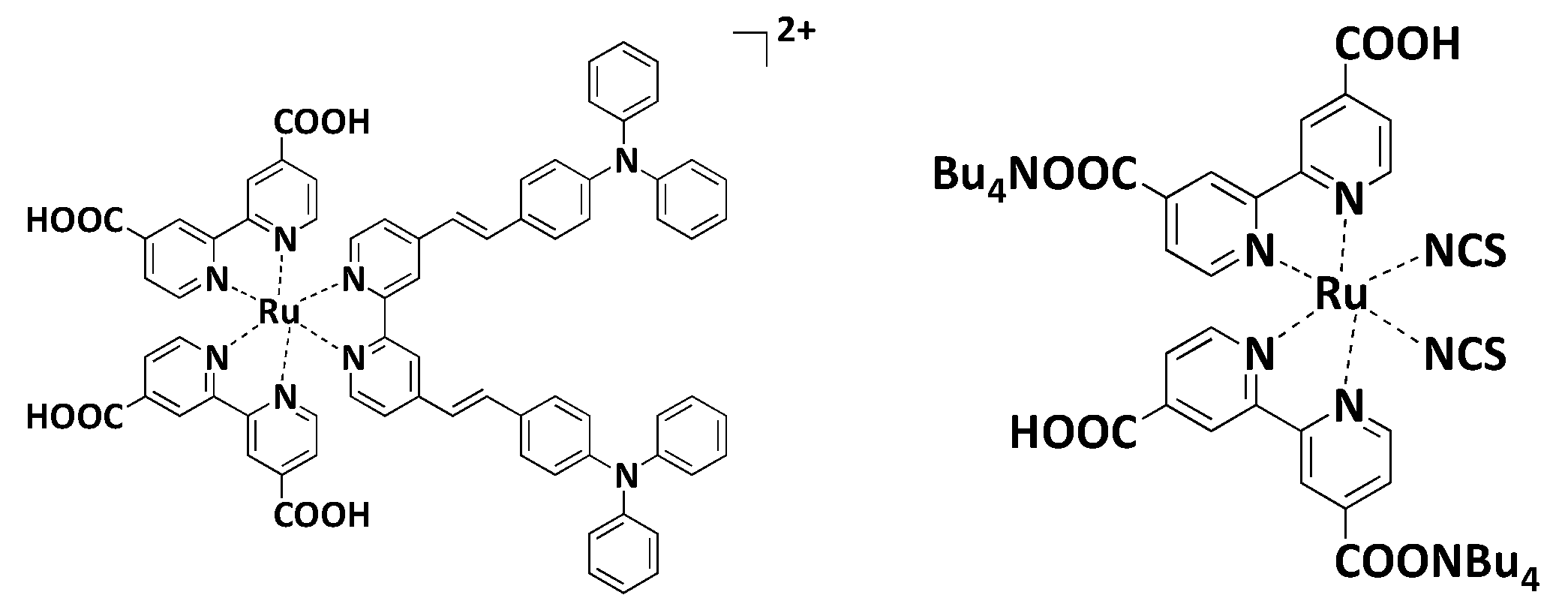
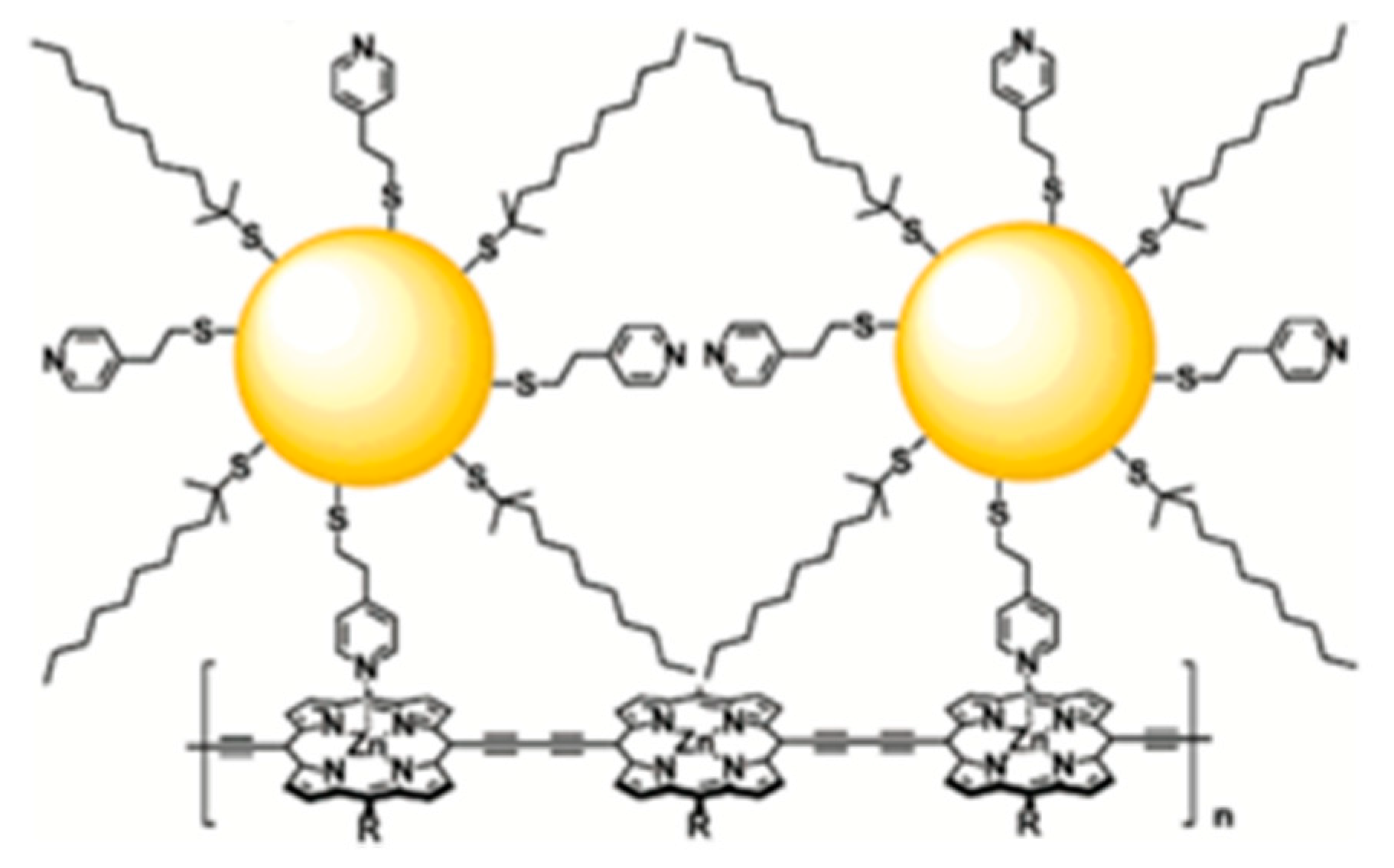
7.3. Polypyridyls Metallic Complexes
7.4. Coordination Complexes Grafted on Au-NPs for Multiple Applications
7.5. Carboxylates and Base Shiff Coordination Complexes
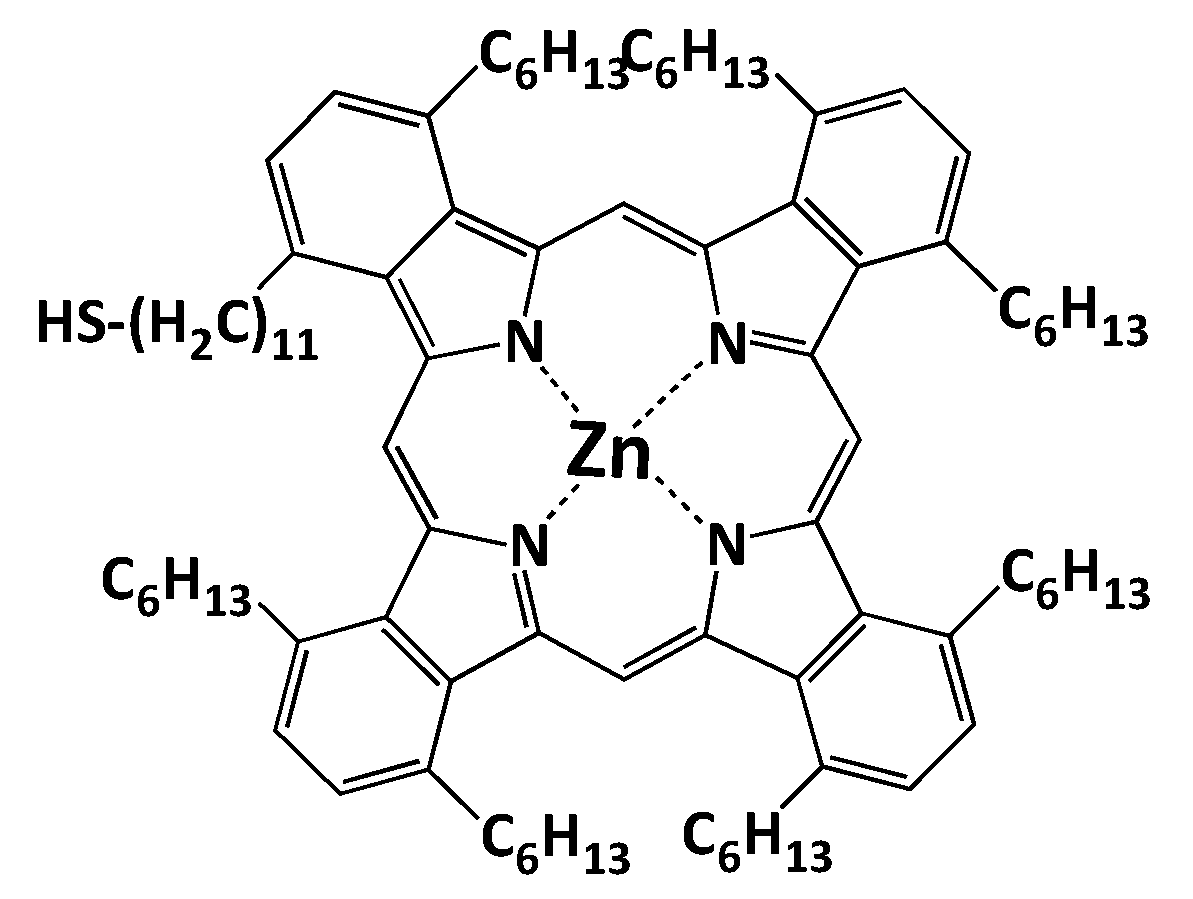
7.6. Bioinorganic Complexes
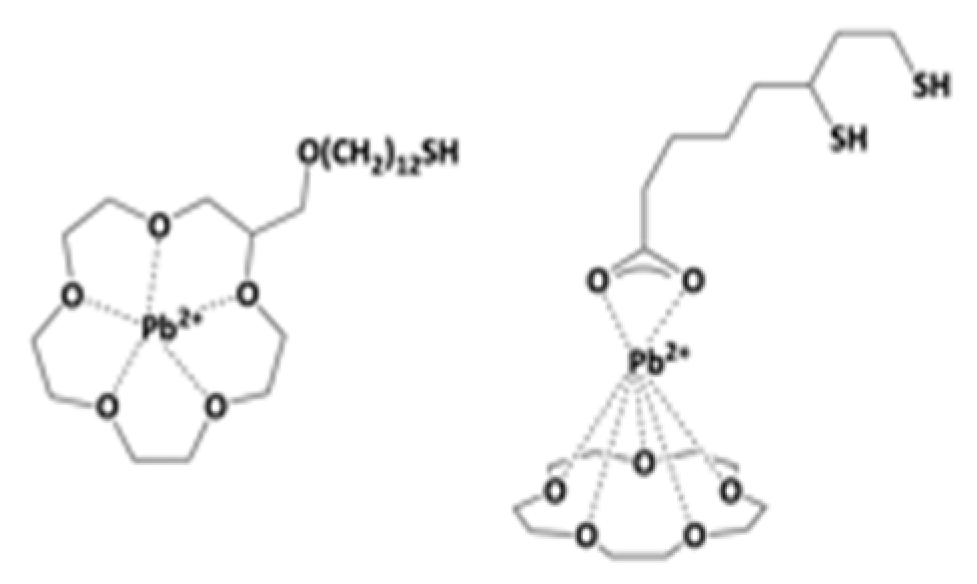
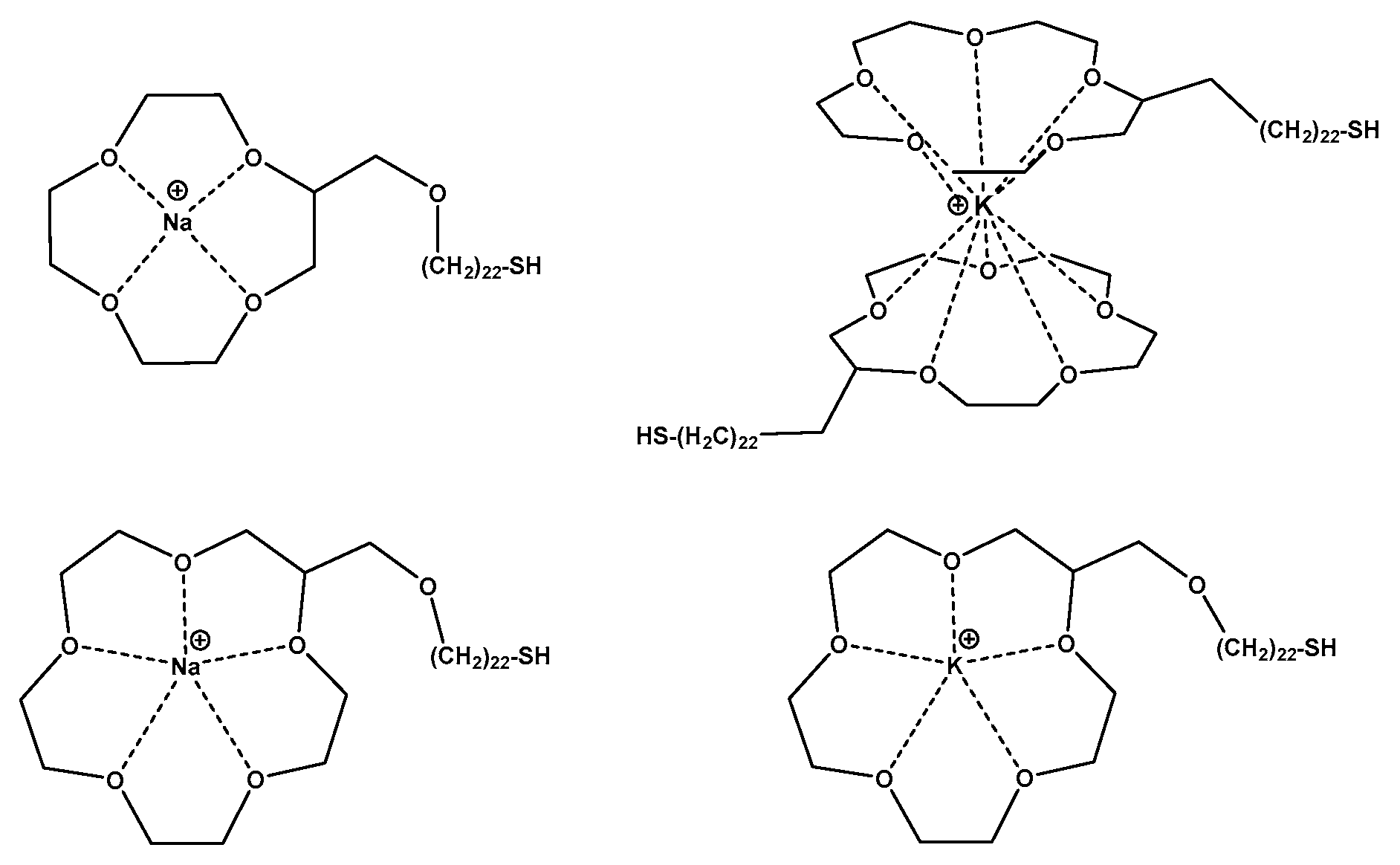
7.7. Crown Ethers Devices
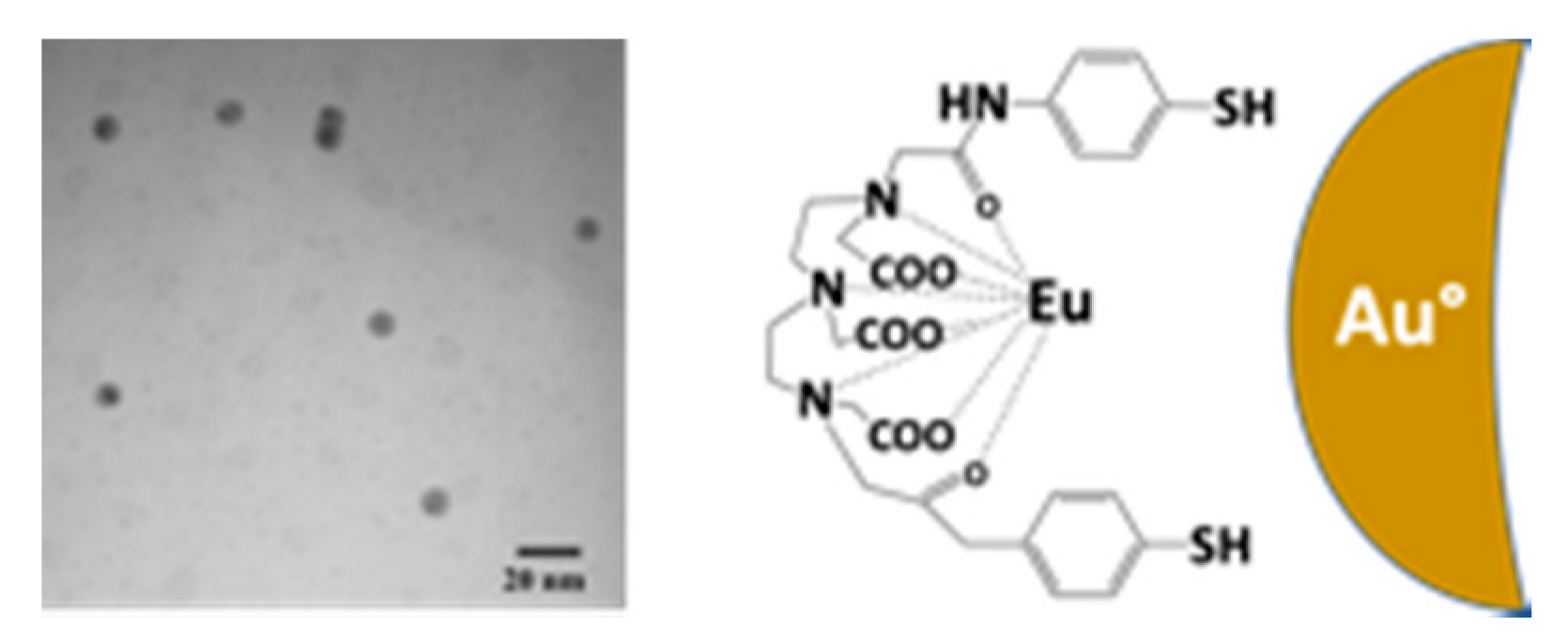
7.8. Functionalisation by Coordination Complexes of F-Block Elements
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Faraday Discuss. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J.D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate. Gold Cluster Molecules with Core Diameters from 1.5 to 5.2 nm: Core and Monolayer Properties as a Function of Core Size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef] [PubMed]
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. Chem. Phys. Chem. 2000, 1, 18–52. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M. Tailoring Surface Plasmons through the Morphology and Assembly of Metal Nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef]
- Yu, K.; Kelly, K.L.; Sakai, N.; Tatsuma, T. Tailoring Surface Plasmons through the Morphology and Assembly of Metal Nanoparticles. Langmuir 2008, 24, 5849–5854. [Google Scholar] [CrossRef]
- Hu, M.; Chen, J.; Li, Z.-Y.; Au, L.; Hartland, G.V.; Li, X.; Marquez, M.; Xia, Y. Gold nanostructures: Engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 2006, 35, 1084–1094. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Au, L.; Lu, X.; Li, X.; Xia, Y. Gold Nanocages for Biomedical Applications. Adv. Mater. 2007, 19, 3177–3184. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.; Lewis, A.; Sukenik, C. Controlled Deposition of Gold Nanowires on Semiconducting and Nonconducting Surfaces. Nano Lett. 2007, 7, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, S.; Knapp, D.; Lewis, N.S. Near-Ideal Photodiodes from Sintered Gold Nanoparticle Films on Methyl-Terminated Si (111) Surfaces. J. Am. Chem. Soc. 2008, 130, 3300–3301. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. Chem. Commun. 1995, 16, 1655. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D. Controlling the growth of charged-nanoparticle chains through interparticle electrostatic repulsion. Angew. Chem. Int. Ed. 2008, 47, 3984–3987. [Google Scholar] [CrossRef] [PubMed]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef]
- Donkers, R.L.; Song, Y.; Murray, R.W. Substituent Effects on the Exchange Dynamics of Ligands on 1.6 nm Diameter Gold Nanoparticles. Langmuir 2004, 20, 4703–4707. [Google Scholar] [CrossRef]
- Petroski, J.; Chou, M.H.; Creutz, C. Rapid Phosphine Exchange on 1.5-nm Gold Nanoparticles. Inorg. Chem. 2004, 43, 1597–1599. [Google Scholar] [CrossRef]
- Montalti, M.; Zaccheroni, N.; Prodi, L.; O’Reilly, N.; James, S.L. Enhanced Sensitized NIR Luminescence from Gold Nanoparticles via Energy Transfer from Surface-Bound Fluorophores. J. Am. Chem. Soc. 2007, 129, 2418–2419. [Google Scholar] [CrossRef]
- Lu, X.; Tuan, H.-Y.; Korgel, B.A.; Xia, Y. Facile Synthesis of Gold Nanoparticles with Narrow Size Distribution by Using AuCl or AuBr as the Precursor. Chem. Eur. J. 2008, 14, 1584–1591. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Mandal, S.; Pasricha, R.; Adyanthaya, S.D.; Sastry, M. One-step synthesis of hydrophobized gold nanoparticles of controllable size by the reduction of aqueous chloroaurate ions by hexadecylaniline at the liquid–liquid interface. Chem. Commun. 2002, 13, 1334–1335. [Google Scholar] [CrossRef]
- Gandubert, V.J.; Lennox, R.B. Assessment of 4-(Dimethylamino)pyridine as a Capping Agent for Gold Nanoparticles. Langmuir 2005, 21, 6532–6539. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Momozawa, O.; Hamatani, T.; Kimura, K. Stepwise Size-Selective Extraction of Carboxylate-Modified Gold Nanoparticles from an Aqueous Suspension into Toluene with Tetraoctylammonium Cations. Chem. Mater. 2001, 13, 4692–4697. [Google Scholar] [CrossRef]
- Roux, S.; Garcia, B.; Bridot, J.L.; Salome, M.; Marquette, C.; Lemelle, L.; Gillet, P.; Blum, L.; Perriat, P.; Tillement, O. Synthesis, Characterization of Dihydrolipoic Acid Capped Gold Nanoparticles, and Functionalization by the Electroluminescent Luminol. Langmuir 2005, 21, 2526–2536. [Google Scholar] [CrossRef]
- Vickers, M.S.; Cookson, J.; Beer, P.D.; Bishop, P.T.; Thiebaut, B. Dithiocarbamate ligand stabilised gold nanoparticles. J. Mater. Chem. 2006, 16, 209–215. [Google Scholar] [CrossRef]
- Tan, Y.; Li, Y.; Zhu, D. Fabrication of Gold Nanoparticles Using a Trithiol (Thiocyanuric Acid) as the Capping Agent. Langmuir 2002, 18, 3392–3395. [Google Scholar] [CrossRef]
- Zhang, H.L.; Evans, S.D.; Henderson, J.R.; Miles, R.E.; Shen, T. Spectroscopic Characterization of Gold Nanoparticles Passivated by Mercaptopyridine and Mercaptopyrimidine Derivatives. J. Phys. Chem. B 2003, 107, 6087–6095. [Google Scholar] [CrossRef]
- Kannan, P.; Abraham, J.S. Synthesis of mercaptothiadiazole-functionalized gold nanoparticles and their self-assembly on Au substrates. Nanotechnology 2008, 8, 19. [Google Scholar] [CrossRef]
- Dzwonek, M.; Załubiniak, D.; Piatek, P.; Cichowicz, G.; Meczynska-Wielgosz, S.; Stepkowski, S.; Kruszewski, M.; Wieckowska, A.; Bilewicz, R. Towards potent but less toxic nanopharmaceuticals–lipoic acid bioconjugates of ultrasmall gold nanoparticles with an anticancer drug and addressing unit. RSC Adv. 2018, 8, 14947–14957. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding Growth for Size Control of 5−40 nm Diameter Gold Nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Ji, X.; Song, X.; Li, J.; Bai, Y.; Yang, W.; Peng, X. Size Control of Gold Nanocrystals in Citrate Reduction: The Third Role of Citrate. J. Am. Chem. Soc. 2007, 129, 13939–13948. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.L.V.; Stoeva, S.I.; Sorensen, C.M.; Klabunde, K.J. Digestive Ripening of Thiolated Gold Nanoparticles: The Effect of Alkyl Chain Length. Langmuir 2002, 18, 7515–7520. [Google Scholar] [CrossRef]
- Rowe, M.P.; Plass, K.E.; Kim, K.; Kurdak, C.; Zellers, E.T.; Matzger, A.J. Single-Phase Synthesis of Functionalized Gold Nanoparticles. Chem. Mater. 2004, 16, 3513–3517. [Google Scholar] [CrossRef]
- Sakai, T.; Alexandridis, P. Mechanism of Gold Metal Ion Reduction, Nanoparticle Growth and Size Control in Aqueous Amphiphilic Block Copolymer Solutions at Ambient Conditions. J. Phys. Chem. B 2005, 109, 7766–7777. [Google Scholar] [CrossRef]
- Kim, B.; Tripp, S.L.; Wei, A. Self-Organization of Large Gold Nanoparticle Arrays. J. Am. Chem. Soc. 2001, 123, 7955–7956. [Google Scholar] [CrossRef]
- Xu, J.; Li, S.; Weng, J.; Wang, X.; Zhou, Z.; Yang, K.; Liu, M.; Chen, X.; Cui, Q.; Cao, M.; et al. Hydrothermal Syntheses of Gold Nanocrystals: From Icosahedral to Its Truncated Form. Adv. Funct. Mater. 2008, 18, 277–284. [Google Scholar] [CrossRef]
- Neveu, S.; Cabuil, V.; Mayer, C.R. 3D hybrid nanonetworks from gold functionalized nanoparticles. Adv. Mater. 2002, 14, 595–597. [Google Scholar]
- Kanehara, M.; Kodzuka, E.; Teranishi, T. Self-Assembly of Small Gold Nanoparticles through Interligand Interaction. J. Am. Chem. Soc. 2006, 128, 13084–13094. [Google Scholar] [CrossRef]
- Praharaj, S.; Ghosh, S.K.; Nath, S.; Kundu, S.; Panigrahi, S.; Basu, S.; Pal, T. Size-Selective Synthesis and Stabilization of Gold Organosol in CnTAC: Enhanced Molecular Fluorescence from Gold-Bound Fluorophores. J. Phys. Chem. B 2005, 109, 13166–13174. [Google Scholar] [CrossRef]
- Perez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzan, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Meyre, M.E.; Treguer-Delapierre, M.; Faure, C. Radiation-Induced Synthesis of Gold Nanoparticles within Lamellar Phases. Formation of Aligned Colloidal Gold by Radiolysis. Langmuir 2008, 24, 4421–4425. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ma, H.; Zhang, X.; Yong, F.; Feng, X.; Pan, W.; Wang, X.; Wang, Y.; Chen, S. Electrochemical Synthesis of Gold Nanocrystals and Their 1D and 2D Organization. J. Phys. Chem. B 2005, 109, 19823–19830. [Google Scholar] [CrossRef] [PubMed]
- Eustis, S.; Hsu, H.Y.; El-Sayed, M.A. Gold Nanoparticle Formation from Photochemical Reduction of Au3+ by Continuous Excitation in Colloidal Solutions. A Proposed Molecular Mechanism. J. Phys. Chem. B 2005, 109, 4811–4815. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Pal, U.; Martinez Gomez, L.; Agarwal, V. Size controlled green synthesis of gold nanoparticles using Coffea arabica seed extract and their catalytic performance in 4-nitrophenol reduction. RSC Adv. 2018, 8, 24819–24826. [Google Scholar] [CrossRef]
- Yasuda, K.; Sato, T.; Asakura, Y. Size-controlled synthesis of gold nanoparticles by ultrafine bubbles and pulsed ultrasound. Chem. Eng. Sci. 2020, 217, 115527. [Google Scholar] [CrossRef]
- Takahashi, F.; Yamamoto, N.; Todoriki, M.; Jin, J. Sonochemical preparation of gold nanoparticles for sensitive colorimetric determination of nereistoxin insecticides in environmental samples. Talanta. 2018, 188, 651–657. [Google Scholar] [CrossRef]
- Sanchez, C.; Soler-Illia, G.J.A.A.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed Hybrid Organic−Inorganic Nanocomposites from Functional Nanobuilding Blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Sommer, W.J.; Weck, M. Facile Functionalization of Gold Nanoparticles via Microwave-Assisted 1,3 Dipolar Cycloaddition. Langmuir 2007, 23, 11991–11995. [Google Scholar] [CrossRef]
- Zubarev, E.R.; Xu, J.; Sayyad, A.; Gibson, J.D. Novel Spherical Assembly of Gold Nanoparticles Mediated by a Tetradentate Thioether. J. Am. Chem. Soc. 2006, 128, 4958–4959. [Google Scholar] [CrossRef]
- Ikeda, M.; Tanifuji, N.; Yamaguchi, H.; Irie, M.; Matsuda, K. Photoswitching of conductance of diarylethene-Au nanoparticle network. Chem. Commun. 2007, 13, 1355–1357. [Google Scholar] [CrossRef]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-Functionalized Gold Nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef] [PubMed]
- Nerambourg, N.; Werts, M.H.V.; Charlot, M.; Blanchard-Desce, M. Quenching of Molecular Fluorescence on the Surface of Monolayer-Protected Gold Nanoparticles Investigated Using Place Exchange Equilibria. Langmuir 2007, 23, 5563–5570. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.L.; Hatzakis, N.S.; Tshikhudo, T.R.; Dirvianskyite, N.; Razumas, V.; Patkar, S.; Vind, J.; Svendsen, A.; Nolte, R.J.M.; Rowan, A.E.; et al. Bionanoconjugation via Click Chemistry: The Creation of Functional Hybrids of Lipases and Gold Nanoparticles. Bioconjugate Chem. 2006, 17, 1373–1375. [Google Scholar] [CrossRef]
- Thomas, K.G.; Kamat, P.V. Chromophore-Functionalized Gold Nanoparticles. Acc. Chem. Res. 2003, 36, 888–898. [Google Scholar] [CrossRef]
- Nehl, C.L.; Hafner, J.H. Shape-dependent plasmon resonances of gold nanoparticles. J. Mater. Chem. 2008, 18, 2415–2419. [Google Scholar] [CrossRef]
- Yang, C.-S.; Shih, M.-S.; Chang, F.-Y. Evolution study of photo-synthesized gold nanoparticles by spectral deconvolution model: A quantitative approach. New J. Chem. 2006, 30, 729–735. [Google Scholar] [CrossRef]
- Zhang, S.; Leem, G.; Srisombat, L.; Lee, T.R. Rationally Designed Ligands that Inhibit the Aggregation of Large Gold Nanoparticles in Solution. J. Am. Chem. Soc. 2008, 130, 113–120. [Google Scholar] [CrossRef]
- Esumi, K.; Akiyama, S.; Yoshimura, T. Multilayer Formation Using Oppositely Charged Gold- and Silver-Dendrimer Nanocomposites. Langmuir 2003, 19, 7679–7681. [Google Scholar] [CrossRef]
- Pengo, P.; Polizzi, S.; Battagliarin, M.; Pasquato, L.; Scrimin, P. Synthesis, characterization and properties of water-soluble gold nanoparticles with tunable core size. J. Mater. Chem. 2003, 13, 2471–2478. [Google Scholar] [CrossRef]
- Bergeron, D.E.; Hudgens, J.W. New Insights on the Nanoparticle Growth Mechanism in the Citrate Reduction of Gold(III) Salt: Formation of the Au Nanowire Intermediate and Its Nonlinear Optical Properties. J. Phys. Chem. C 2007, 111, 8195–8201. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Kneipp, J. SERS probing of proteins in gold nanoparticle agglomerates. Front. Chem. 2019, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-J.; Nan, F.; Liu, X.-L.; Hao, Z.-H.; Zhou, L.; Zeng, J.; Xu, H.-X.; Zhang, W.; Wang, Q.-Q. Plasmon-modulated excitation-dependent fluorescence from activated ctab molecules strongly coupled to gold nanoparticles. Sci. Rep. 2017, 7, 43282. [Google Scholar] [CrossRef] [PubMed]
- Dass, A.; Guo, R.; Tracy, J.B.; Balasubramanian, R.; Douglas, A.D.; Murray, R.W. Gold Nanoparticles with Perfluorothiolate Ligands. Langmuir 2008, 24, 310–315. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Nakamura, E.; Isobe, H. Functionalized Fullerenes in Water. The First 10 Years of Their Chemistry, Biology, and Nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar] [CrossRef]
- Brust, M.; Kiely, C.J.; Bethell, D.; Schiffrin, D.J. C-60 mediated aggregation of gold nanoparticles. J. Am. Chem. Soc. 1998, 120, 12367–12368. [Google Scholar] [CrossRef]
- Fujihara, H.; Nakai, H. Fullerenethiolate-Functionalized Gold Nanoparticles: A New Class of Surface-Confined Metal−C60 Nanocomposites. Langmuir 2001, 17, 6393–6395. [Google Scholar] [CrossRef]
- Sudeep, P.K.; Ipe, B.I.; Thomas, K.G.; George, M.V.; Barazzouk, S.; Hotchandani, S.; Kamat, P.V. Fullerene-Functionalized Gold Nanoparticles. A Self-Assembled Photoactive Antenna-Metal Nanocore Assembly. Nano Lett. 2002, 2, 29–35. [Google Scholar] [CrossRef]
- Shon, Y.-S.; Choo, H. [60]Fullerene-linked gold nanoparticles: Synthesis and layer-by-layer growth on a solid surface. Chem. Commun. 2002, 21, 2560–2561. [Google Scholar] [CrossRef]
- Liu, J.; Alvarez, J.; Ong, W.; Kaifer, A.E. Network aggregates formed by C60 and gold nanoparticles capped with γ-cyclodextrin hosts. Nano Lett. 2001, 1, 57–60. [Google Scholar] [CrossRef]
- Hasobe, T.; Imahori, H.; Kamat, P.V.; Fukuzumi, S. Quaternary Self-Organization of Porphyrin and Fullerene Units by Clusterization with Gold Nanoparticles on SnO2 Electrodes for Organic Solar Cells. J. Am. Chem. Soc. 2003, 125, 14962–14963. [Google Scholar] [CrossRef] [PubMed]
- Tariqul Islam, M.; Molugu, S.K.; Cooke, P.H.; Noveron, J.C. Fullerene stabilized gold nanoparticles. New J. Chem. 2015, 39, 5923–5926. [Google Scholar] [CrossRef]
- Frare, M.C.; Pilot, R.; de Filippo, C.C.; Weber, V.; Signorini, R.; Maggini, M.; Bozio, R. Fullerene functionalized gold nanoparticles for optical limiting of continuous wave lasers. Appl. Phys. B. 2019, 125, 47. [Google Scholar] [CrossRef]
- Plesek, J. Potential applications of the boron cluster compounds. Chem. Rev. 1992, 92, 269–278. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Maderna, A. Applications of Radiolabeled Boron Clusters to the Diagnosis and Treatment of Cancer. Chem. Rev. 1999, 99, 3421–3434. [Google Scholar] [CrossRef]
- Barberera, G.; Vaca, A.; Teixidor, F.; Sillanpaa, R.; Kivekas, R.; Vinas, C. Designed Synthesis of New ortho-Carborane Derivatives: From Mono- to Polysubstituted Frameworks. Inorg. Chem. 2008, 47, 7309–7316. [Google Scholar] [CrossRef]
- Dash, B.P.; Satapathy, R.; Maguire, J.A.; Hosmane, N.S. Synthesis of a New Class of Carborane-Containing Star-Shaped Molecules via Silicon Tetrachloride Promoted Cyclotrimerization Reactions. Org. Lett. 2008, 10, 2247–2250. [Google Scholar] [CrossRef]
- Smith, H.D.; Obenland, C.O.; Papetti, S. A New Series of Organoboranes. IX. The Preparation and Some Reactions of Sulfur-Carborane Derivatives. Inorg. Chem. 1966, 5, 1013–1015. [Google Scholar] [CrossRef]
- Plesek, J.; Hermanek, S. Syntheses and properties of substituted icosahedral carborane thiols. Collect. Czechoslov. Chem. Commun. 1981, 46, 687–692. [Google Scholar] [CrossRef]
- Base, T.; Bastl, Z.; Plzak, Z.; Grygar, T.; Plesek, J.; Carr, M.J.; Malina, V.; Subrt, J.; Bohacek, J.; Vecernikova, E.; et al. Carboranethiol-Modified Gold Surfaces. A Study and Comparison of Modified Cluster and Flat Surfaces. Langmuir 2005, 21, 7776–7785. [Google Scholar] [CrossRef] [PubMed]
- Baše, T.; Bastl, Z.; Šlouf, M.; Klementová, M.; Šubrt, J.; Vetushka, A.; Ledinský, M.; Fejfar, A.; Macháček, J.; Carr, M.J.; et al. Gold Micrometer Crystals Modified with Carboranethiol Derivatives. J. Phys. Chem. C. 2008, 112, 14446–14455. [Google Scholar] [CrossRef]
- Grzelczak, M.P.; Danks, S.P.; Klipp, R.C.; Belic, D.; Zaulet, A.; Kunstmann-Olsen, C.; Bradley, D.F.; Tsukuda, T.; Viñas, C.; Teixidor, F.; et al. Ion Transport across Biological Membranes by Carborane-Capped Gold Nanoparticles. ACS Nano 2017, 11, 12492–12499. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Ye, J.; Li, Z.; Jiang, H.; Yan, H.; Yu Stogniy, M.; Sivaev, I.B.; Bregadze, V.I.; Wang, X. Carborane derivative conjugated with gold nanoclusters for targeted cancer cell imaging. Biomacromolecules 2017, 18, 1466–1472. [Google Scholar] [CrossRef]
- Schmid, G.; Pugin, R.; Malm, J.O.; Bovin, J.O. Silsesquioxanes as Ligands for Gold Clusters. Eur. J. Inorg. Chem. 1998, 813–817. [Google Scholar] [CrossRef]
- Carroll, J.B.; Frankamp, B.L.; Rotello, V.M. Self-assembly of gold nanoparticles through tandem hydrogen bonding and polyoligosilsequioxane (POSS)–POSS recognition processes. Chem. Commun. 2002, 17, 1892–1893. [Google Scholar] [CrossRef]
- Carroll, J.B.; Frankamp, B.L.; Srivastava, S.; Rotello, V.M. Electrostatic self-assembly of structured gold nanoparticle/polyhedral oligomeric silsesquioxane (POSS) nanocomposites. J. Mater. Chem. 2004, 14, 690–694. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, J.; Xu, X.; Chen, X.; Wang, J. Protein corona-triggered catalytic inhibition of insufficient posspolymer-caged gold nanoparticles for sensitive colorimetric detection of metallothioneins. Anal. Chem. 2020, 92, 2080–2087. [Google Scholar] [CrossRef]
- Xia, S.; Yang, Y.; Zhu, W.; Lü, C. Quaternized polyhedral oligomeric silsesquioxanes stabilized Pd nanoparticles as efficient nanocatalysts for reduction reaction. Coll. Surf. A. 2020, 585, 124110. [Google Scholar] [CrossRef]
- Hanske, C.; Sanz-Ortiz, M.N.; Liz-Marzán, L.M. Silica-Coated Plasmonic Metal Nanoparticles in Action. Adv. Mater. 2018, 30, 1707003. [Google Scholar] [CrossRef]
- Chapman, B.S.; Wu, W.C.; Li, Q.; Holten-Andersen, N.; Tracy, J.B. Heteroaggregation approach for depositing magnetite nanoparticles onto Silica-overcoated gold nanorods. Chem. Mater. 2017, 29, 10362–10368. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M.; Giersig, M.; Mulvaney, P. Synthesis of Nanosized Gold−Silica Core−Shell Particles. Langmuir 1996, 12, 4329–4335. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, J.; Lee, S.S.; Ying, J.Y. Reverse Microemulsion-Mediated Synthesis of Silica-Coated Gold and Silver Nanoparticles. Langmuir 2008, 24, 5842–5848. [Google Scholar] [CrossRef] [PubMed]
- Casavola, M.; Buonsanti, R.; Caputo, G.; Cozzoli, P.D. Colloidal Strategies for Preparing Oxide-Based Hybrid Nanocrystals. Eur. J. Inorg. Chem. 2008, 837–854. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, J.; Pastoriza-Santos, I.; Perez-Juste, J.; GarciadeAbajo, F.J.; Liz-Marzan, L.M. The Effect of Silica Coating on the Optical Response of Sub-micrometer Gold Spheres. J. Phys. Chem. C. 2007, 111, 13361–13366. [Google Scholar] [CrossRef]
- Ruach-Nir, I.; Bendikov, T.A.; Doron-Mor, I.; Barkay, Z.; Vaskevich, A.; Rubinstein, I. Silica-Stabilized Gold Island Films for Transmission Localized Surface Plasmon Sensing. J. Am. Chem. Soc. 2007, 129, 84–92. [Google Scholar] [CrossRef]
- Liu, G.; Ji, H.; Yang, X.; Wang, Y. Synthesis of a Au/Silica/Polymer Trilayer Composite and the Corresponding Hollow Polymer Microsphere with a Movable Au Core. Langmuir 2008, 24, 1019–1025. [Google Scholar] [CrossRef]
- Tovmachenko, O.G.; Graf, C.; van den Heuvel, D.J.; van Blaaderen, A.; Gerritsen, H.C. Fluorescence Enhancement by Metal-Core/Silica-Shell Nanoparticles. Adv. Mater. 2006, 18, 91–95. [Google Scholar] [CrossRef]
- Kamata, K.; Lu, Y.; Xia, Y. Synthesis and Characterization of Monodispersed Core−Shell Spherical Colloids with Movable Cores. J. Am. Chem. Soc. 2003, 125, 2384–2385. [Google Scholar] [CrossRef]
- Iijima, S. Synthesis of Carbon Nanotubes. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Qian, D.; Rantell, T. Multiwall Carbon Nanotubes: Synthesis and Application. Acc. Chem. Res. 2002, 35, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.T.; Kim, Y.; Savagatrup, S.; Yoon, B.; Jeon, I.; Choi, S.J.; Kim, D.; Swager, T.M. Porous Ion Exchange Polymer Matrix for Ultrasmall Au Nanoparticle-Decorated Carbon Nanotube Chemiresistors. Chem. Mater. 2019, 31, 5413–5420. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Dhodamani, A.G.; Patil, S.M.; Mullani, S.B.; More, K.V.; Delekar, S.D. Interfacially Interactive Ternary Silver-Supported Polyaniline/ Multiwalled Carbon Nanotube Nanocomposites for Catalytic and Antibacterial Activity. ACS Omega 2020, 5, 219–227. [Google Scholar] [CrossRef]
- Xue, B.; Chen, P.; Hong, Q.; Lin, J.; Tan, K.L. Growth of Pd, Pt, Ag and Au nanoparticles on carbon nanotubes. J. Mater. Chem. 2001, 11, 2378–2381. [Google Scholar] [CrossRef]
- Han, L.; Wu, W.; Kirk, F.L.; Luo, J.; Maye, M.M.; Kariuki, N.N.; Lin, Y.; Wang, C.; Zhong, C.J. A Direct Route toward Assembly of Nanoparticle−Carbon Nanotube Composite Materials. Langmuir 2004, 20, 6019–6025. [Google Scholar] [CrossRef]
- Sainsbury, T.; Ikuno, T.; Okawa, D.; Pacile, D.; Frechet, J.M.J.; Zettl, A. Self-Assembly of Gold Nanoparticles at the Surface of Amine- and Thiol-Functionalized Boron Nitride Nanotubes. J. Phys. Chem. C. 2007, 111, 12992–12999. [Google Scholar] [CrossRef]
- Jiang, K.; Eitan, A.; Schadler, L.S.; Ajayan, P.M.; Siegel, R.W.; Grobert, N.; Mayne, M.; Reyes-Reyes, M.; Terrones, H.; Terrones, M. Selective Attachment of Gold Nanoparticles to Nitrogen-Doped Carbon Nanotubes. Nano Lett. 2003, 3, 275–277. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Liz-Marzán, L.M. Carbon Nanotubes as templates for one-dimensional nanoparticle assemblies. J. Mater. Chem. 2006, 16, 22–25. [Google Scholar] [CrossRef]
- Fu, Q.; Lu, C.; Liu, J. Selective Coating of Single Wall Carbon Nanotubes with Thin SiO2 Layer. Nano Lett. 2002, 2, 329–332. [Google Scholar] [CrossRef]
- Bottini, M.; Magrini, A.; Rosato, N.; Bergamaschi, A.; Mustelin, T. Dispersion of Pristine Single-walled Carbon Nanotubes in Water by a Thiolated Organosilane: Application in Supramolecular Nanoassemblies. J. Phys. Chem. B 2006, 110, 13685–13688. [Google Scholar] [CrossRef][Green Version]
- Ou, Y.Y.; Huang, M.H. High-Density Assembly of Gold Nanoparticles on Multiwalled Carbon Nanotubes Using 1-Pyrenemethylamine as Interlinker. J. Phys. Chem. B 2006, 110, 2031–2036. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L. Introduction: Polyoxometalates multicomponent molecular vehicles to probe fundamental issues and practical problems. Chem. Rev. 1998, 98, 1–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Neyman, A.; Arkhangelsky, E.; Gitis, V.; Meshi, L.; Weinstock, I. Self-Assembly and Structure of Directly Imaged Inorganic-Anion Monolayers on a Gold Nanoparticle. J. Am. Chem. Soc. 2009, 131, 17412–17422. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeiri, O.; Sharet, S.; Weinstock, I.A. Role of the Alkali-Metal Cation Size in the Self-Assembly of Polyoxometalate-Monolayer Shells on Gold Nanoparticles. Inorg. Chem. 2012, 51, 7436–7438. [Google Scholar] [CrossRef] [PubMed]
- Sutter, S.; Trepka, B.; Siroky, S.; Hagedorn, K.; Theiß, S.; Baum, P.; Polarz, S. Light-Triggered Boost of Activity of Catalytic Bola-Type Surfactants by a Plasmonic Metal−Support Interaction Effect. ACS Appl. Mater. Interfaces 2019, 11, 15936–15944. [Google Scholar] [CrossRef]
- Solarska, R.; Bienkowski, K.; Zoladek, S.; Majcher, A.; Stefaniuk, T.3; Kulesza, P.J.; Augustynski, J. Enhanced Water Splitting at Thin Film Tungsten Trioxide Photoanodes Bearing Plasmonic Gold-Polyoxometalate Particles. Angew. Chem. Int. Ed. Eng. 2014, 126, 14420–14424. [Google Scholar] [CrossRef]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid Organic-Inorganic Polyoxometalate Compounds: From Structural Diversity to Applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef]
- Mayer, C.R.; Neveu, S.; Cabuil, V. A nanoscale hybrid system based on gold nanoparticles and heteropolyanions. Angew. Chem. Int. Ed. Engl. 2002, 41, 501–503. [Google Scholar] [CrossRef]
- Shweta, H.; Satyawati, J.; Tulsi, M.; Sudhir, K. Formation of gold nanoparticles via a thiol functionalized polyoxometalate. Mater. Sci. Eng. C 2013, 33, 2332–2337. [Google Scholar]
- Martín, S.; Takashima, Y.; Lin, C.G.; Song, Y.F.; Miras, H.N.; Cronin, L. Integrated Synthesis of Gold Nanoparticles Coated with Polyoxometalate Clusters. Inorg. Chem. 2019, 58, 4110–4116. [Google Scholar] [CrossRef]
- Tomane, S.; Lopez-Maya, E.; Boujday, S.; Humblot, V.; Marrot, J.; Rabasso, N.; Castells-Gil, J.; Sicard, C.; Dolbecq, A.; Mialane, P.; et al. One-pot synthesis of a new generation of hybrid bisphosphonate polyoxometalate gold nanoparticles as antibiofilm agents. Nanoscale Adv. 2019, 1, 3400–3405. [Google Scholar] [CrossRef]
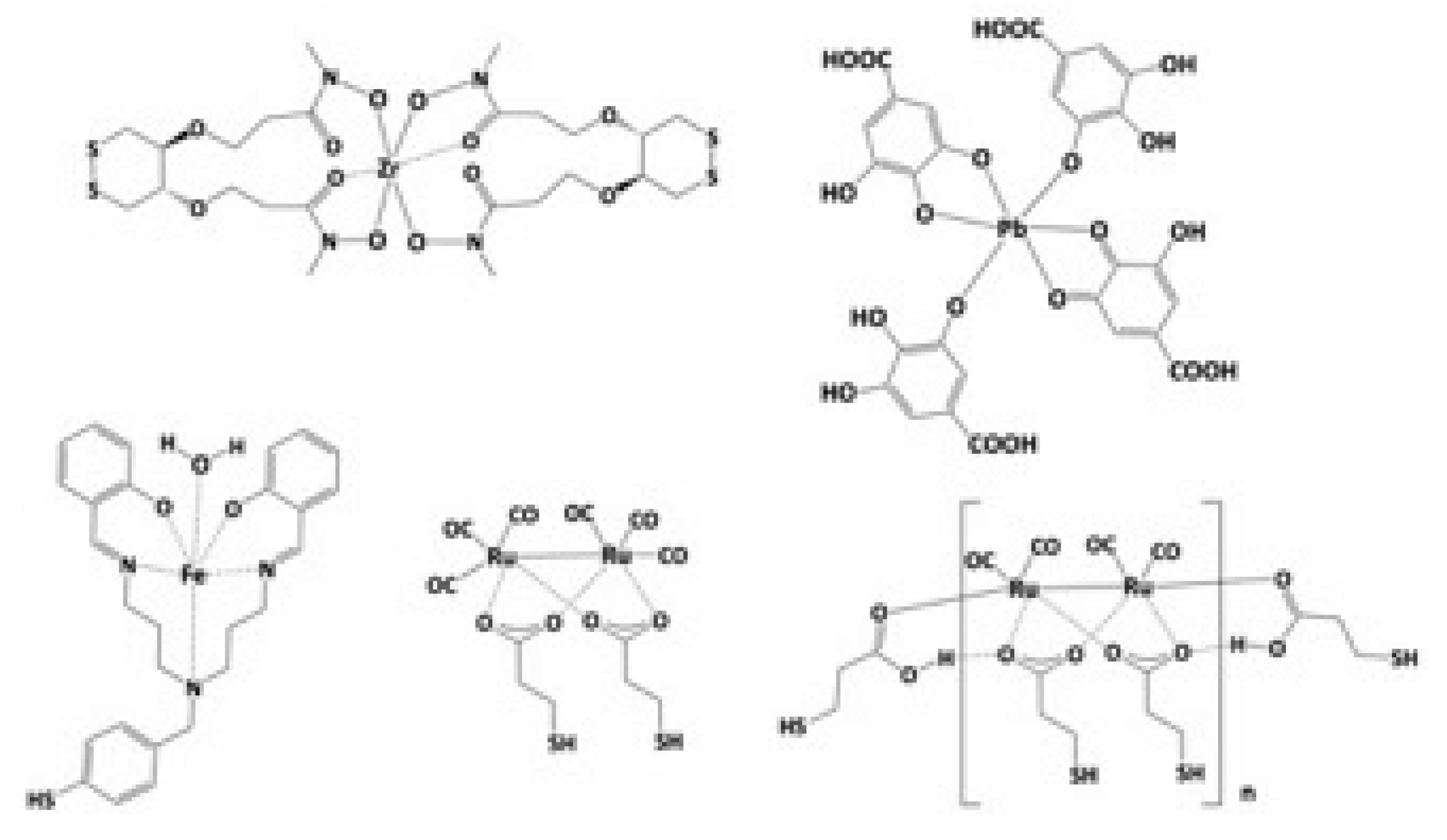
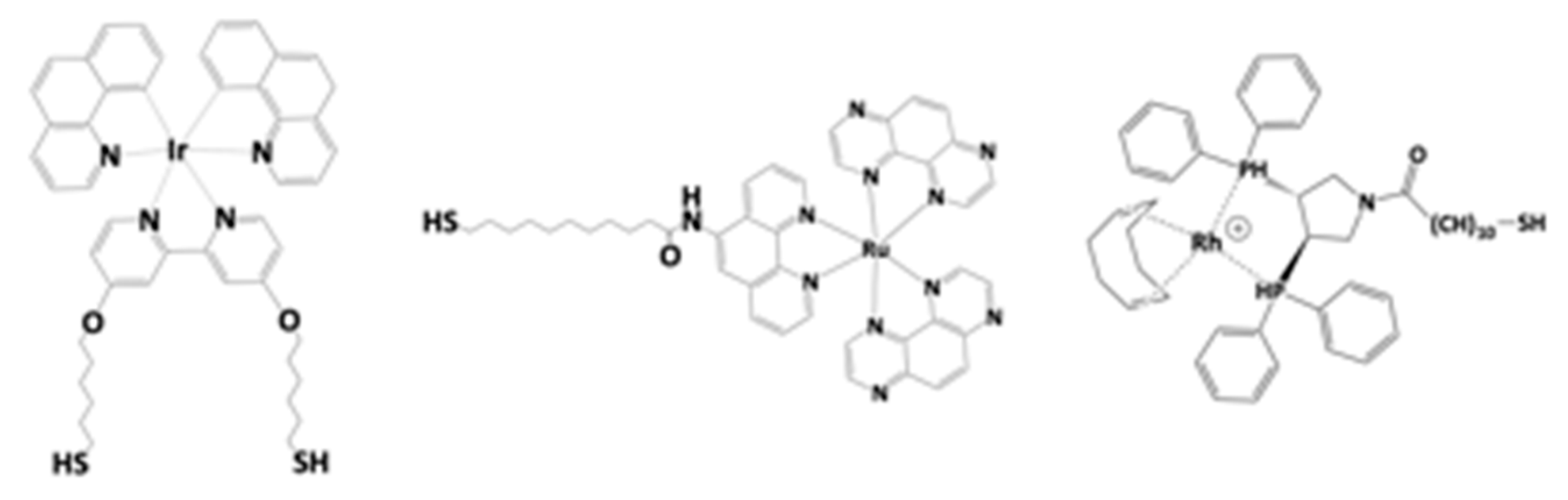
- Wilton-Ely, J.D.E.T. The surface functionalization of gold nanoparticles with metal complexes. Dalton Trans. 2008, 2008, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Stiles, R.L.; Balasubramanian, R.; Feldberg, S.W.; Murray, R.W. Anion-Induced Adsorption of Ferrocenated Nanoparticles. J. Am. Chem. Soc. 2008, 130, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.L.; Balasubramanian, R.; Tracy, J.B.; Murray, R.W. Fully Ferrocenated Hexanethiolate Monolayer-Protected Gold Clusters. Langmuir 2007, 23, 2247–2254. [Google Scholar] [CrossRef]
- Chen, S.; Murray, R.W. Arenethiolate Monolayer-Protected Gold Clusters. Langmuir 1999, 15, 682–689. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Li, J. Electrochemical study of 4-ferrocene thiophenol monolayers assembled on gold nanoparticles. Microelectron. Eng. 2003, 66, 91–94. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Jiang, J.; Li, J. Electroactive gold nanoparticles protected by 4-ferrocene thiophenol monolayer. J. Colloid Interface Sci. 2003, 264, 109–113. [Google Scholar] [CrossRef]
- Ingram, R.S.; Hostetler, M.J.; Murray, R.W. Poly-hetero-ω-functionalized Alkanethiolate-Stabilized Gold Cluster Compounds. J. Am. Chem. Soc. 1997, 119, 9175–9178. [Google Scholar] [CrossRef]
- Green, S.J.; Stokes, J.J.; Hostetler, M.J.; Pietron, J.; Murray, R.W. Three-Dimensional Monolayers: Nanometer-Sized Electrodes of Alkanethiolate-Stabilized Gold Cluster Molecules. J. Phys. Chem. B 1997, 101, 2663–2668. [Google Scholar] [CrossRef]
- Ornelas, C.; Mery, D.; Cloutet, E.; Aranzaes, J.R.; Astruc, D. Cross Olefin Metathesis for the Selective Functionalization, Ferrocenylation, and Solubilization in Water of Olefin-Terminated Dendrimers, Polymers, and Gold Nanoparticles and for a Divergent Dendrimer Construction. J. Am. Chem. Soc. 2008, 130, 1495–1506. [Google Scholar] [CrossRef]
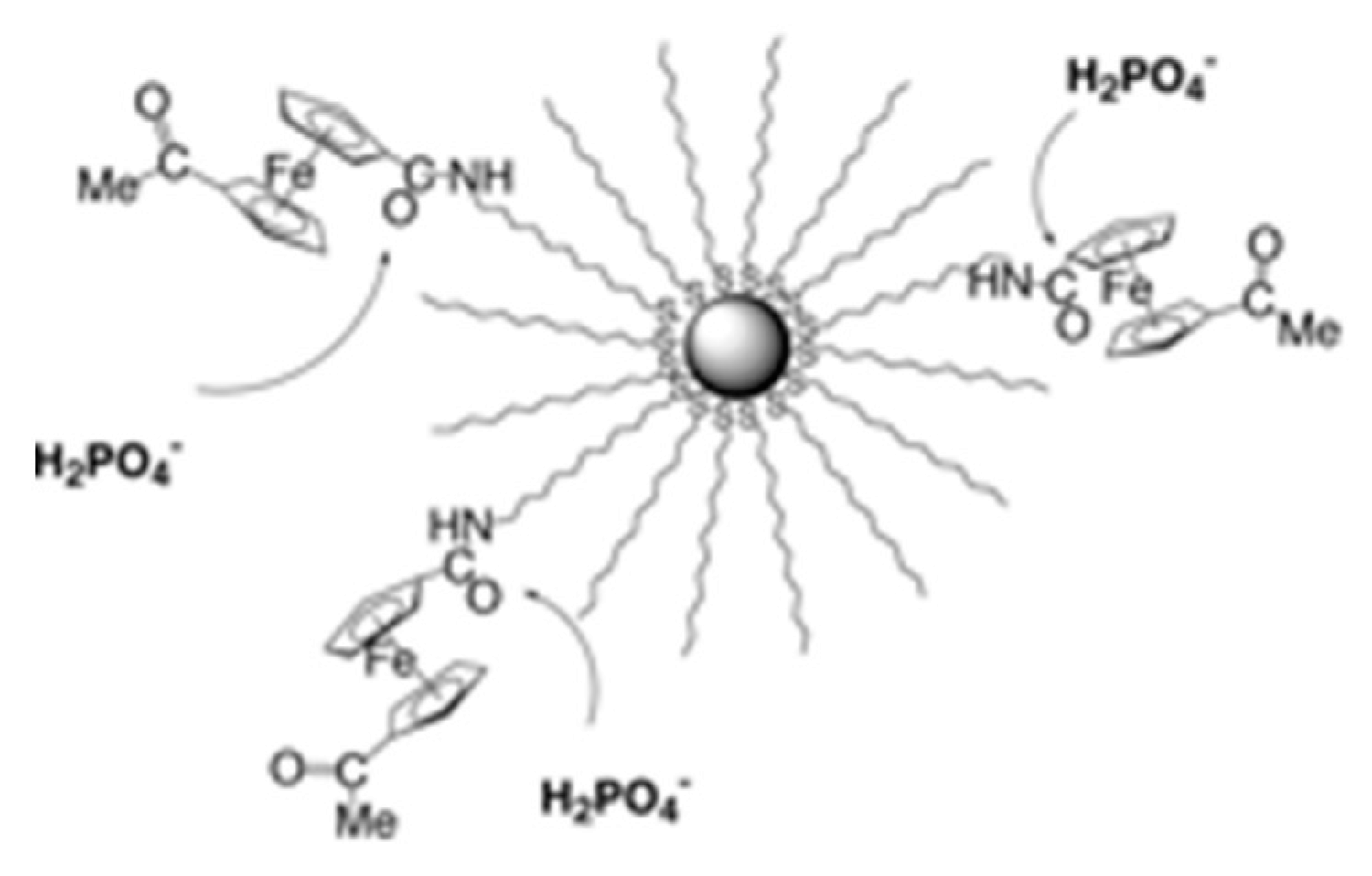
- Labande, A.; Astruc, D. Colloids as redox sensors: Recognition of H2PO4− and HSO4− by amidoferrocenylalkylthiol–gold nanoparticles. Chem. Commun. 2000, 1007–1008. [Google Scholar] [CrossRef]
- Wang, Y.; Salmon, L.; Ruiz, J.; Astruc, D. Metallodendrimers in three oxidation states with electronically interacting metals and stabilization of size-selected gold nanoparticles. Nat. Commun. 2014, 5, 3489. [Google Scholar] [CrossRef] [PubMed]
- Labande, A.; Ruiz, J.; Astruc, D. Supramolecular Gold Nanoparticles for the Redox Recognition of Oxoanions: Syntheses, Titrations, Stereoelectronic Effects, and Selectivity. J. Am. Chem. Soc. 2002, 124, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, T.; Itoh, M.; Kurihara, M.; Kubo, K.; Nishihara, H. Synthesis, redox behavior and electrodeposition of biferrocene-modified gold clusters. J. Electroanal. Chem. 1999, 473, 113–116. [Google Scholar] [CrossRef]
- Yamada, M.; Nishihara, H. Electrodeposition of Biferrocene Derivative-Attached Gold Nanoparticles: Solvent Effects and Lithographic Assembly. Langmuir 2003, 19, 8050–8056. [Google Scholar] [CrossRef]
- Yamada, M.; Tadera, T.; Kubo, K.; Nishihara, H. Quantized Capacitance Charging of Monolayer-Protected Au Clusters. J. Phys. Chem. B 2003, 107, 3703–3711. [Google Scholar] [CrossRef]
- Dong, T.Y.; Shih, H.W.; Chang, L.S. Synthesis and Redox Behavior of Biferrocenyl-Functionalized Ruthenium(II) Terpyridine Gold Clusters. Langmuir 2004, 20, 9340–9347. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Ruiz, J.; Nlate, S.; Palumbo, J.; Astruc, D.; Blais, J.-C. Gold nanoparticles containing redox-active supramolecular dendrons that recognize H2PO4−. Chem. Commun. 2001, 2000–2001. [Google Scholar] [CrossRef]
- Daniel, M.C.; Ruiz, J.; Nlate, S.; Blais, J.C.; Astruc, D. Nanoscopic Assemblies between Supramolecular Redox Active Metallodendrons and Gold Nanoparticles: Synthesis, Characterization, and Selective Recognition of H2PO4−, HSO4−, and Adenosine-5′-Triphosphate (ATP2−) Anions. J. Am. Chem. Soc. 2003, 125, 2617–2628. [Google Scholar] [CrossRef]
- Astruc, D.; Daniel, M.-C.; Ruiz, J. Dendrimers and gold nanoparticles as exo-receptors sensing biologically important anions. Chem. Commun. 2004, 2637–2649. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Astruc, D.; Gu, H. Dendronized triazolyl-containing ferrocenyl polymers as stabilizers of gold nanoparticles for recyclable two-phase reduction of 4-nitrophenol. J. Colloid Interf. Sci. 2019, 533, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Opuchlik, L.J.; Pawłowska, J.; Sęk, S.; Bilewicz, R. Ferrocenylated gold nanoparticles self-assemble at carbon surfaces to form stable films. J. Electroanal. Chem. 2018, 825, 22–29. [Google Scholar] [CrossRef]
- Vitale, F.; Vitaliano, R.; Battocchio, C.; Fratoddi, I.; Piscopiello, E.; Tapfer, L.; Russo, M.V. Synthesis and characterization of gold nanoparticles stabilized by palladium(II) phosphine thiol. J. Organomet. Chem. 2008, 693, 1043–1048. [Google Scholar] [CrossRef]
- Bartz, M.; Küther, J.; Seshadri, R.; Tremel, W. Colloid-Bound Catalysts for Ring-Opening Metathesis Polymerization: A Combination of Homogenous and Heterogeneous Properties. Angew. Chem. Int. Ed. 1998, 37, 2466–2468. [Google Scholar] [CrossRef]
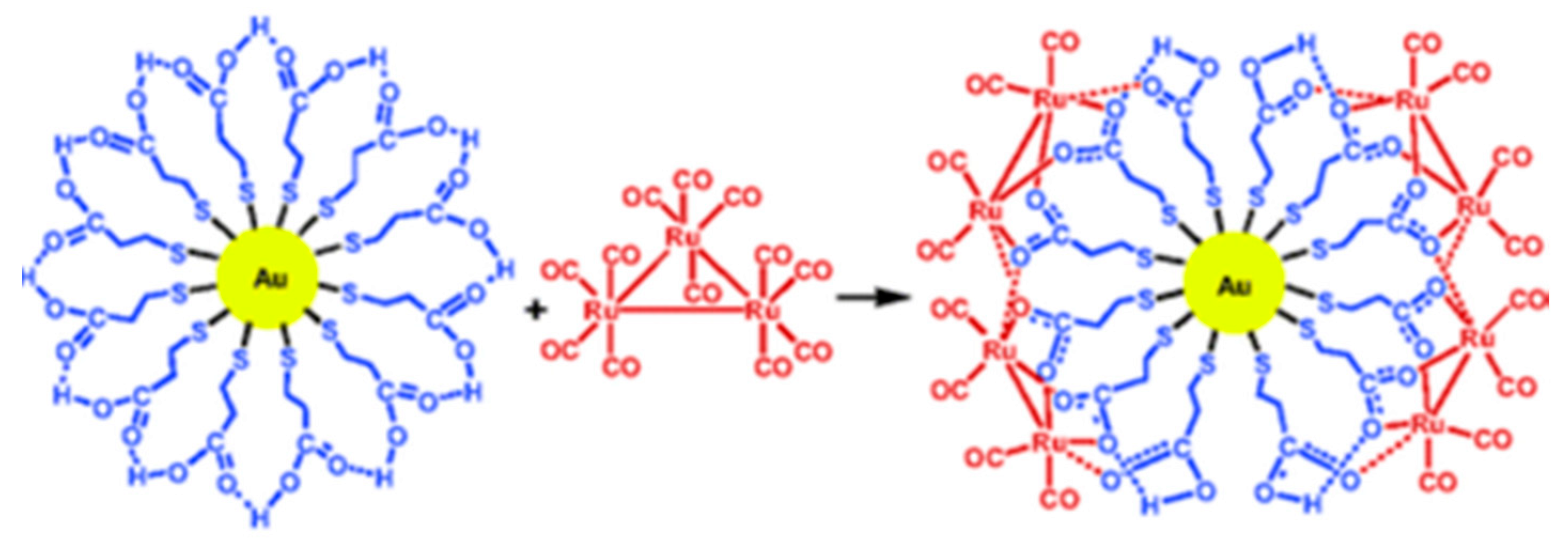
- Wang, S.; Sim, W.S. Au Nanoparticles Encapsulated in Ru Carbonyl Carboxylate Shells. Langmuir 2006, 22, 7861–7866. [Google Scholar] [CrossRef]
- Belser, T.; Stohr, M.; Pfaltz, A. Immobilization of Rhodium Complexes at Thiolate Monolayers on Gold Surfaces: Catalytic and Structural Studies. J. Am. Chem. Soc. 2005, 127, 8720–8731. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.S.; Joseph, J.; Phani, K.L. Novel Method for Deposition of Gold−Prussian Blue Nanocomposite Films Induced by Electrochemically Formed Gold Nanoparticles: Characterization and Application to Electrocatalysis. Chem. Mater. 2007, 19, 4722–4730. [Google Scholar] [CrossRef]
- Crespilho, F.N.; Zucolotto, V.; Brett, C.M.A.; Oliveira, O.N.; Nart, F.C. Unusual Interactions Binding Iron Tetrasulfonated Phthalocyanine and Poly(allylamine hydrochloride) in Layer-by-Layer Films. J. Phys. Chem. B 2006, 110, 17478–17483. [Google Scholar] [CrossRef]
- Qiu, J.D.; Peng, H.Z.; Liang, R.P.; Li, J.; Xia, X.H. Synthesis, Characterization, and Immobilization of Prussian Blue-Modified Au Nanoparticles: Application to Electrocatalytic Reduction of H2O2. Langmuir 2007, 23, 2133–2137. [Google Scholar] [CrossRef]
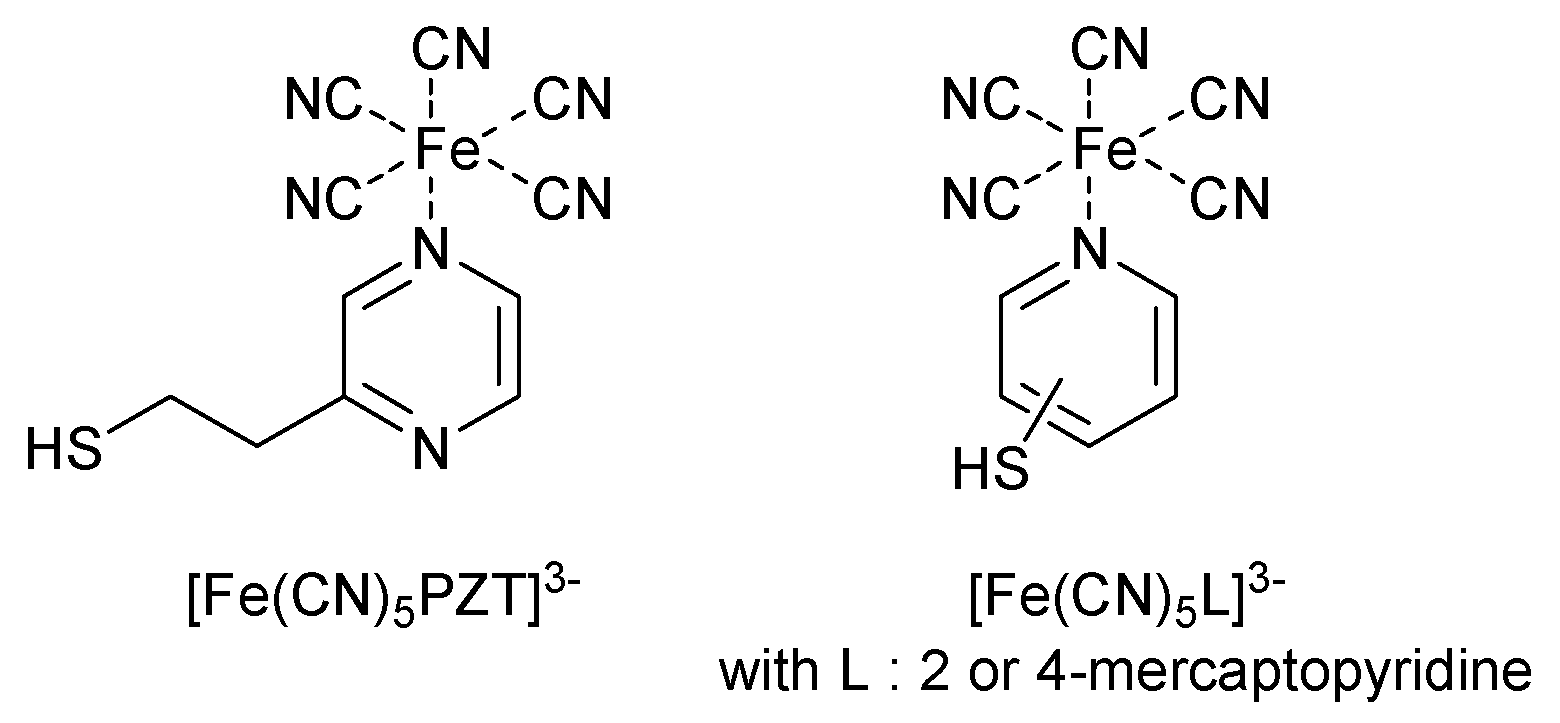
- Toma, S.H.; Bonacin, J.A.; Araki, K.; Toma, H.E. Controlled Stabilization and Flocculation of Gold Nanoparticles by Means of 2-Pyrazin-2-ylethanethiol and Pentacyanidoferrate(II) Complexes. Eur. J. Inorg. Chem. 2007, 3356–3364. [Google Scholar] [CrossRef]
- Nunes, F.S.; Bonifacio, L.D.S.; Araki, K.; Toma, H.E. Interaction of 2- and 4-Mercaptopyridine with Pentacyanoferrates and Gold Nanoparticle. Inorg. Chem. 2006, 45, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M.; Prodi, L.; Zaccheroni, N.; Beltrame, M.; Morotti, T.; Quici, S. Stabilization of terpyridine covered gold nanoparticles by metal ions complexation. New J. Chem. 2007, 31, 102–108. [Google Scholar] [CrossRef]
- Norsten, T.B.; Frankamp, B.L.; Rotello, V.M. Metal Directed Assembly of Terpyridine-Functionalized Gold Nanoparticles. Nano Lett. 2002, 2, 1345–1348. [Google Scholar] [CrossRef]
- Shenhar, R.; Jeoung, E.; Srivastava, S.; Norsten, T.B.; Rotello, V.M. Crosslinked Nanoparticle Stripes and Hexagonal Networks Obtained via Selective Patterning of Block Copolymer Thin Films. Adv. Mater. 2005, 17, 2206–2210. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Zhou, X.-P. From large 3D assembly to highly dispersed spherical assembly: Weak and strong coordination mediated self-aggregation of Au colloids. New J. Chem. 2006, 30, 706–711. [Google Scholar] [CrossRef]
- Hobara, D.; Kondo, S.; Choi, M.-S.; Ishioka, Y.; Hirata, S.; Murata, M.; Azuma, K.; Kasahara, J. Construction of a two-dimensional molecule–nanoparticle network using iron(II) bis(terpyridine) complex formation for molecular-device applications. Phys. Status Solidi Appl. Mater. Sci. 2007, 204, 1706–1711. [Google Scholar] [CrossRef]
- Ito, M.; Tsukatani, T.; Fujihara, H. Preparation and characterization of gold nanoparticles with a ruthenium-terpyridyl complex, and electropolymerization of their pyrrole-modified metal nanocomposites. J. Mater. Chem. 2005, 15, 960–964. [Google Scholar] [CrossRef]
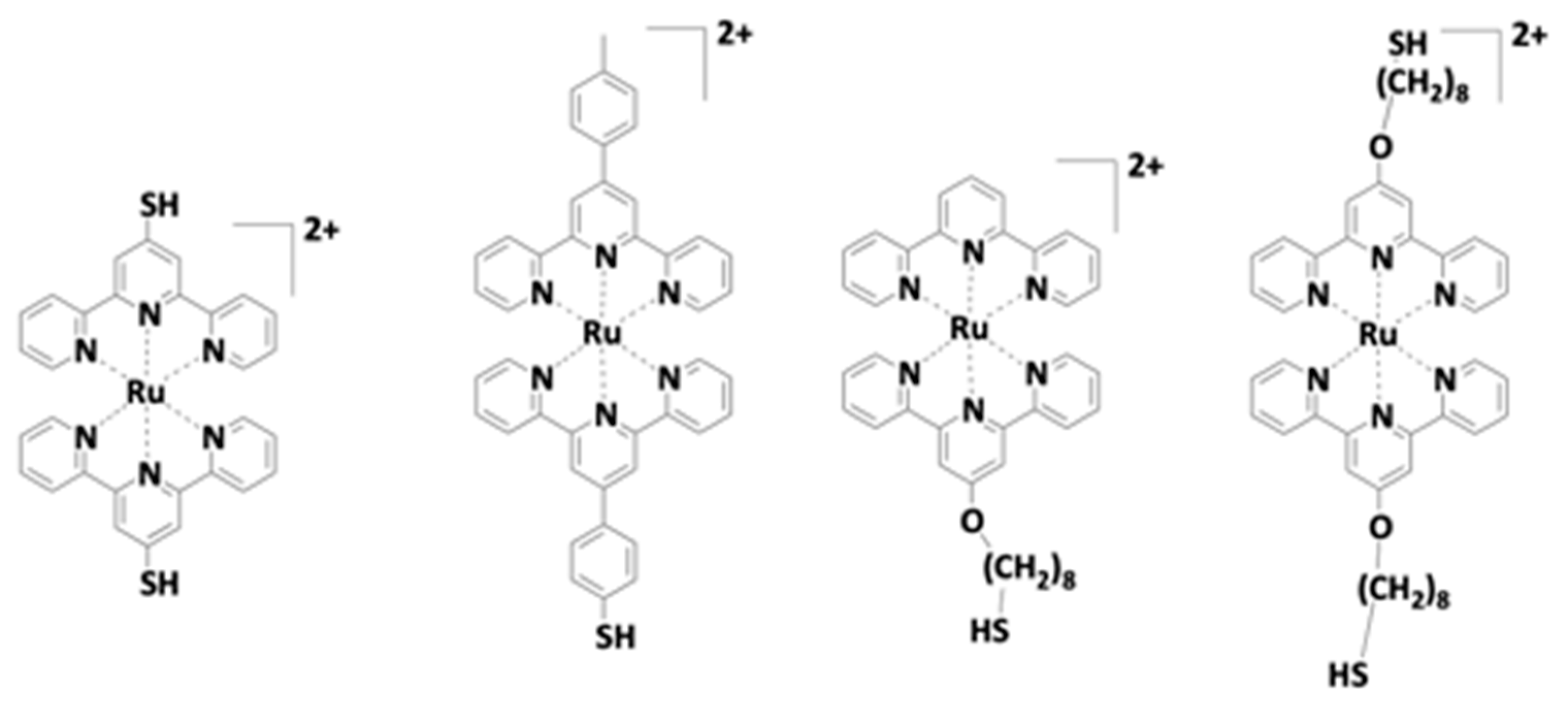
- Mayer, C.R.; Dumas, E.; Michel, A.; Sécheresse, F. Gold nanocomposites with rigid fully conjugated heteroditopic ligands shell as nanobuilding blocks for coordination chemistry. Chem. Commun. 2006, 40, 4183. [Google Scholar] [CrossRef]
- Seo, K.; Konchenko, A.V.; Lee, J.; Bang, G.S.; Lee, H. Molecular Conductance Switch-On of Single Ruthenium Complex Molecules. J. Am. Chem. Soc. 2008, 130, 2553–2559. [Google Scholar] [CrossRef]
- Mayer, C.R.; Dumas, E.; Sécheresse, F. Size controlled formation of silver nanoparticles by direct bonding of ruthenium complexes bearing a terminal mono- or –bi-pyridyl group. Chem. Commun. 2005, 345–347. [Google Scholar] [CrossRef]
- Guerlin, A.; Le Pleux, L.; Selvakannan, P.R.; Lehoux, A.; Dumur, F.; Pellegrin, Y.; Blart, E.; Dumas, E.; Méallet-Renault, R.; Miomandre, F.; et al. Mutual Influence of Gold and Silver Nanoparticles on Tris-(2,2′bipyridine)-Ru(II) Core Complexes: Post-functionalization Processes, Optical and Electrochemical Investigations. Appl. Surf. Sci. 2020, 499, 143847. [Google Scholar]
- Cheng, P.P.H.; Silvester, D.; Wang, G.; Kalyuzhny, G.; Douglas, A.; Murray, R.W. Dynamic and Static Quenching of Fluorescence by 1–4 nm Diameter Gold Monolayer-Protected Clusters. J. Phys. Chem. B 2006, 110, 4637–4644. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.H.; Glomm, W.R.; Johnson, M.C.; Knag, M.K.; Franzen, S. Probing BSA Binding to Citrate-Coated Gold Nanoparticles and Surfaces. Langmuir 2005, 21, 9303–9307. [Google Scholar] [CrossRef]
- Xu, X.-H.N.; Huang, S.; Brownlow, W.; Salaita, K.; Jeffers, R.B. Size and Temperature Dependence of Surface Plasmon Absorption of Gold Nanoparticles Induced by Tris(2,2′-bipyridine)ruthenium(II). J. Phys. Chem. B 2004, 108, 15543–15551. [Google Scholar] [CrossRef]
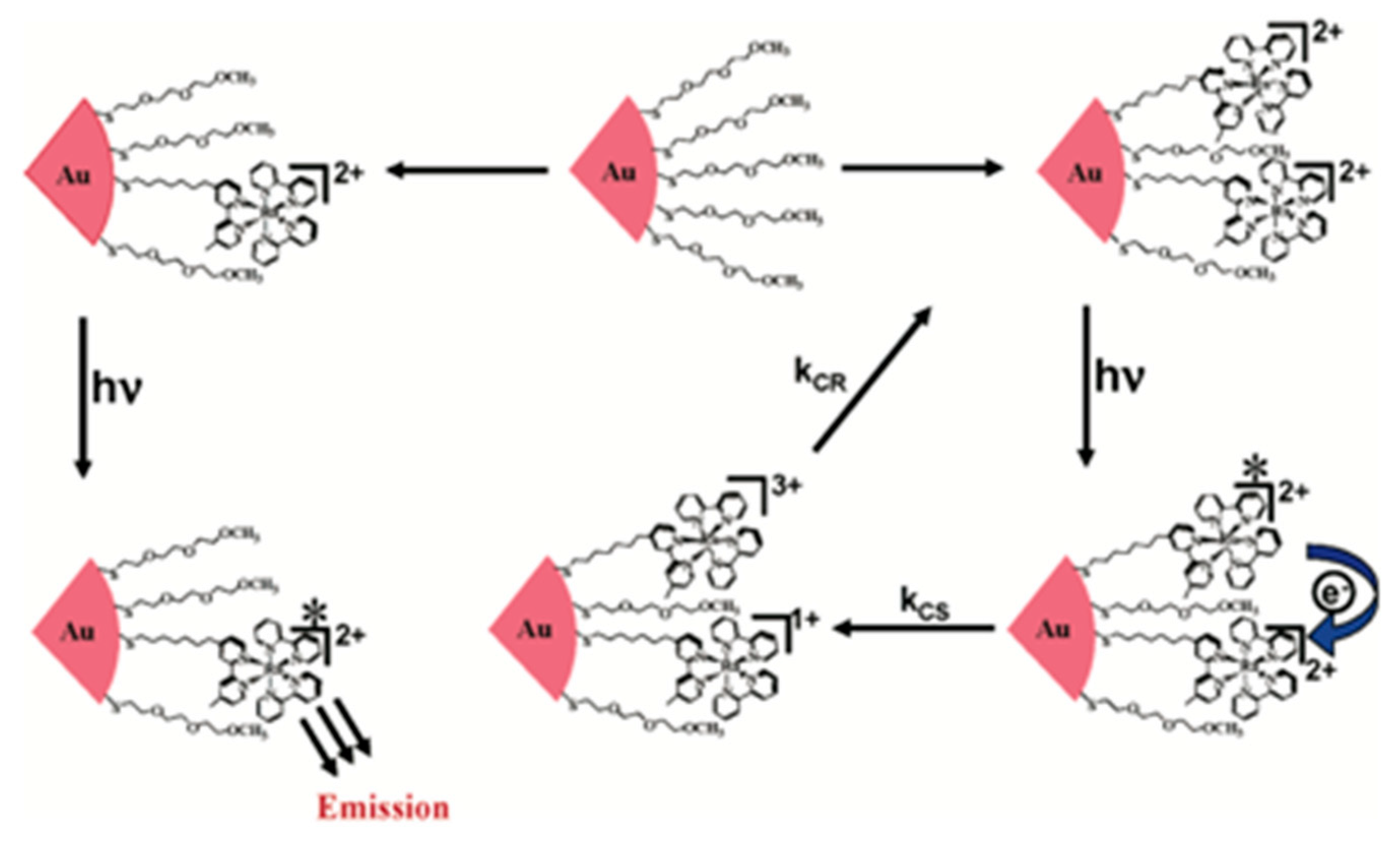
- Pramod, P.; Sudeep, P.K.; Thomas, K.G.; Kamat, P.V. Photochemistry of Ruthenium Tris-bipyridine Functionalized on Gold Nanoparticles. J. Phys. Chem. B 2006, 110, 20737–20741. [Google Scholar] [CrossRef] [PubMed]
- Jebb, M.; Sudeep, P.K.; Pramod, P.; Thomas, K.G.; Kamat, P.V. Ruthenium(II) Tris-bipyridine Functionalized Gold Nanorods. Morphological Changes and Excited-State Interactions. J. Phys. Chem. B 2007, 111, 6839–6844. [Google Scholar] [CrossRef]
- Sun, X.; Du, Y.; Dong, S.; Wang, E. Method for Effective Immobilization of Ru(bpy)32+ on an Electrode Surface for Solid-State Electrochemiluminescene Detection. Anal. Chem. 2005, 77, 8166–8169. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, W.; Liu, Q.; Yao, S. Study on the enhancement of Ru(bpy)32+ electrochemiluminescence by nanogold and its application for pentoxyverine detection. Electrophoresis 2005, 26, 4468–4477. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Akiyama, T.; Yamada, S. Construction of gold nanoparticle-ruthenium (II) tris(2,2′-bipyridine) self-assembled multistructures and their photocurrent responses. Thin Solid Films 2001, 393, 273–277. [Google Scholar] [CrossRef]
- Akiyama, T.; Yutaka, K.; Terasaki, K.N.; Niidome, Y.; Yamada, S. Particle-size effects on the photocurrent efficiency of nanostructured assemblies consisting of gold nanoparticles and a ruthenium complex–viologen linked thiol. J. Electroanal. Chem. 2003, 550, 303–307. [Google Scholar] [CrossRef]
- Yamada, S.; Tasaki, T.; Akiyama, T.; Terasaki, N.; Nitahara, S. Gold nanoparticle–porphyrin self-assembled multistructures for photoelectric conversion. Thin Solid Films 2003, 438, 70–74. [Google Scholar] [CrossRef]
- Akiyama, T.; Inoue, K.; Kuwahara, Y.; Niidome, Y.; Terasaki, N.; Nitahara, S.; Yamada, S. Facile Fabrication of Morphology-Controlled Gold Nanoparticle Architectures by Electrolyte-Induced Agglomeration and Their Photoelectrochemical Applications. Langmuir 2005, 21, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Lahav, M.; Heleg-Shabtai, V.; Wasserman, J.; Katz, E.; Willner, I.; Dürr, H.; Hu, Y.-Z.; Bossmann, S.H. Photoelectrochemistry with Integrated Photosensitizer−Electron Acceptor and Au-Nanoparticle Arrays. J. Am. Chem. Soc. 2000, 122, 11480–11487. [Google Scholar] [CrossRef]
- Shipway, A.N.; Lahav, M.; Willner, I. Nanostructured Gold Colloid Electrodes. Adv. Mater. 2000, 12, 993–998. [Google Scholar] [CrossRef]
- Chen, S.; Pei, R.; Zhao, T.; Dyer, D.J. Gold Nanoparticle Assemblies by Metal Ion−Pyridine Complexation and Their Rectified Quantized Charging in Aqueous Solutions. J. Phys. Chem. B 2002, 106, 1903–1908. [Google Scholar] [CrossRef]
- Soller, T.; Ringler, M.; Wunderlich, M.; Klar, T.A.; Feldmann, J.; Josel, H.-P.; Markert, Y.; Nichtl, A.; Kürzinger, K. Radiative and Nonradiative Rates of Phosphors Attached to Gold Nanoparticles. Nano Lett. 2007, 7, 1941–1946. [Google Scholar] [CrossRef]
- Slim, M.; Durisic, N.; Grutter, P.; Sleiman, H.F. DNA–Protein Noncovalent Cross-Linking: Ruthenium Dipyridophenazine Biotin Complex for the Assembly of Proteins and Gold Nanoparticles on DNA Template. ChemBioChem 2007, 8, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Shultz, D.A.; Kumar, R.K.; Bin-Salamon, S.; Kirk, M.L. Valence tautomerization and exchange coupling in a cobalt–nitronylnitroxide–semiquinone complex. Polyhedron 2005, 24, 2876–2879. [Google Scholar] [CrossRef]
- Mayer, C.R.; Dumas, E.; Miomandre, F.; Méallet-Renault, R.; Warmont, F.; Vigneron, J.; Pansu, R.; Etcheberry, A.; Sécheresse, F. Polypyridyl ruthenium complexes as coating agent for the formation of gold and silver nanocomposites in different media. Preliminary luminescence and electrochemical studies. New. J. Chem. 2006, 30, 1628. [Google Scholar] [CrossRef]
- Pérez, L.C.; Kador, L.; Peng, B.; Thelakkat, M. Influence of the Solvent on the Surface-Enhanced Raman Spectra of Ruthenium(II) Bipyridyl Complexes. J. Phys. Chem. B 2005, 109, 5783–5789. [Google Scholar] [CrossRef]
- Huang, W.; Tanaka, H.; Ogawa, T. Effects of Metal−Ion Complexation for the Self-Assembled Nanocomposite Films Composed of Gold Nanoparticles and 3,8-Bis(terthiophenyl)phenanthroline-Based Dithiols Bridging 1 μm Gap Gold Electrodes: Morphology, Temperature Dependent Electronic Conduction, and Photoresponse. J. Phys. Chem. C 2008, 112, 11513–11526. [Google Scholar]
- Ono, F.; Kanemasa, S.; Tanaka, J. Reusable nano-sized chiral bisoxazoline catalysts. Tetrahedron Lett. 2005, 46, 7623–7626. [Google Scholar] [CrossRef]
- Hone, D.C.; Walker, P.I.; Evans-Gowing, R.; FitzGerald, S.; Beeby, A.; Chambrier, I.; Cook, M.J.; Russell, D.A. Generation of Cytotoxic Singlet Oxygen via Phthalocyanine-Stabilized Gold Nanoparticles: A Potential Delivery Vehicle for Photodynamic Therapy. Langmuir 2002, 18, 2985–2987. [Google Scholar] [CrossRef]
- Beer, P.D.; Cormode, D.P.; Davis, J.J. Zinc metalloporphyrin-functionalised nanoparticle anion sensors. Chem. Commun. 2004, 4, 414–415. [Google Scholar] [CrossRef]
- Ozawa, H.; Kawao, M.; Tanaka, H.; Ogawa, T. Synthesis of dendron-protected porphyrin wires and preparation of a one-dimensional assembly of gold nanoparticles chemically linked to the π-conjugated wires. Langmuir 2007, 23, 6365–6371. [Google Scholar] [CrossRef]
- Aubin-Tam, M.-E.; Hamad-Schifferli, K. Gold Nanoparticle−Cytochrome c Complexes: The Effect of Nanoparticle Ligand Charge on Protein Structure. Langmuir 2005, 21, 12080–12084. [Google Scholar] [CrossRef]
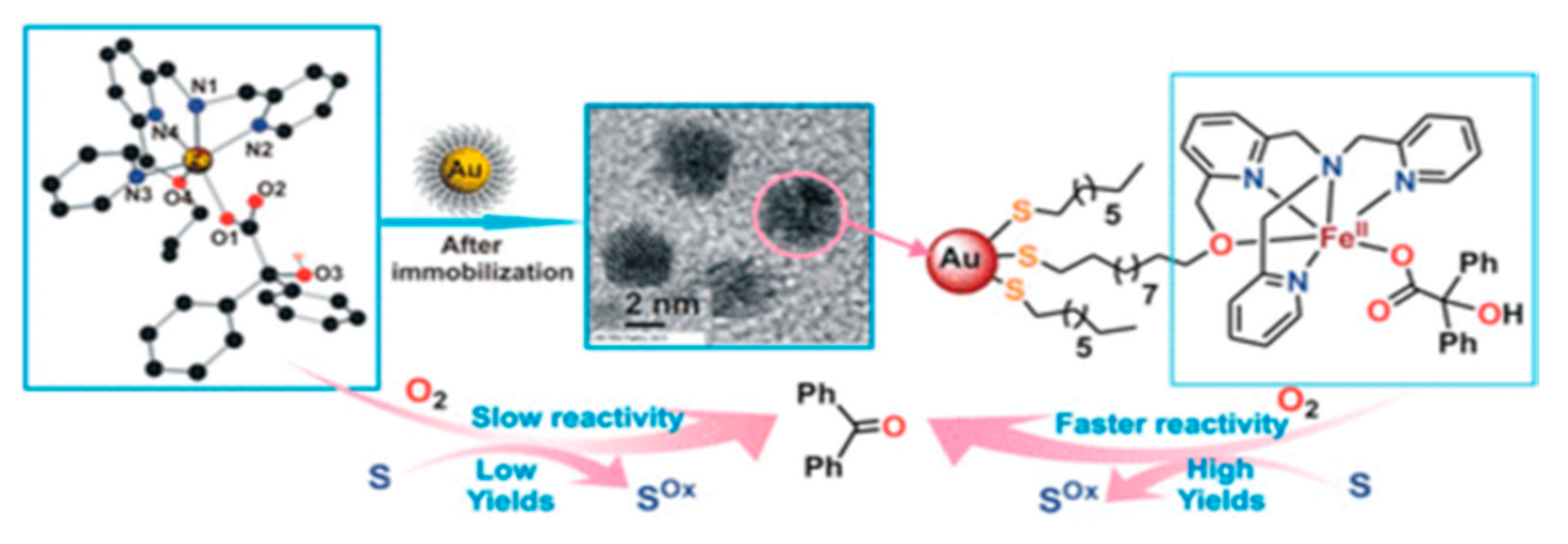
- Sheet, D.; Bera, A.; Jana, R.D.; Paine, T.K. Oxidizing Ability of a Dioxygen-Activating Nonheme Iron(II)-Benzilate Complex Immobilized on Gold Nanoparticles. Inorg. Chem. 2019, 58, 4828–4841. [Google Scholar] [CrossRef]
- Huang, C.-C.; Yang, Z.; Lee, K.-H.; Chang, H.-T. Synthesis of highly fluorescent gold nanoparticles for sensing mercury(II). Angew. Chem. Int. Ed. 2007, 46, 6824–6828. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chang, H.-T. Parameters for selective colorimetric sensing of mercury (II) in aqueous solutions using mercaptopropionic acid-modified gold nanoparticles. Chem. Commun. 2007, 12, 1215–1217. [Google Scholar] [CrossRef]
- Zamborini, F.P.; Hicks, J.F.; Murray, R.W. Quantized Double Layer Charging of Nanoparticle Films Assembled Using Carboxylate/(Cu2+ or Zn2+)/Carboxylate Bridges. J. Am. Chem. Soc. 2000, 122, 4514–4515. [Google Scholar] [CrossRef]
- Zamborini, F.P.; Leopold, M.C.; Hicks, J.F.; Kulesza, P.J.; Malik, M.A.; Murray, R.W. Electron hopping conductivity and vapor sensing properties of flexible network polymer films of metal nanoparticles. J. Am. Chem. Soc. 2002, 124, 8958–8964. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.F.; Zamborini, F.P.; Murray, R.W. Dynamics of Electron Transfers between Electrodes and Monolayers of Nanoparticles. J. Phys. Chem. B 2002, 106, 7751–7757. [Google Scholar] [CrossRef]
- Sheibley, D.; Tognarelli, D.J.; Szymanik, R.; Leopold, M.C. Ultra-fast formation and characterization of stable nanoparticle film assemblies. J. Mater. Chem. 2005, 15, 491–498. [Google Scholar] [CrossRef]
- Xu, R.; Wang, D.; Zhang, J.; Li, Y. Shape-dependent catalytic activity of silver nanoparticles for the oxidation of styrene. Chem. An Asian J. 2006, 1, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Wanunu, M.; Popovitz-Biro, R.; Cohen, H.; Vaskevich, A.; Rubinstein, I. Coordination-based gold nanoparticle layers. J. Am. Chem. Soc. 2005, 127, 9207–9215. [Google Scholar] [CrossRef]
- Mayer, C.R.; Cucchiaro, G.; Jullien, J.; Dumur, F.; Marrot, J.; Dumas, E.; Sécheresse, F. Functionalization of gold nanoparticles by iron (III) complexes derived from Schiff base ligands. Eur. J. Inorg. Chem. 2008, 3614. [Google Scholar] [CrossRef]
- Guo, C.; Boullanger, P.; Jiang, L.; Liu, T. Highly sensitive gold nanoparticles biosensor chips modified with a self-assembled bilayer for detection of Con A. Biosens. Bioelectr. 2007, 22, 1830–1834. [Google Scholar] [CrossRef]
- Turkekul, K.; Uzer, A.; Can, Z.; Ercag, E.; Apak, R. Colorimetric Sensing of the Insensitive Energetic Material 3-Nitro-1,2,4-triazol-5-one (NTO) Using l-Cysteine Stabilized Gold Nanoparticles and Copper(II). Anal. Lett. 2019, 52, 2809–2821. [Google Scholar] [CrossRef]
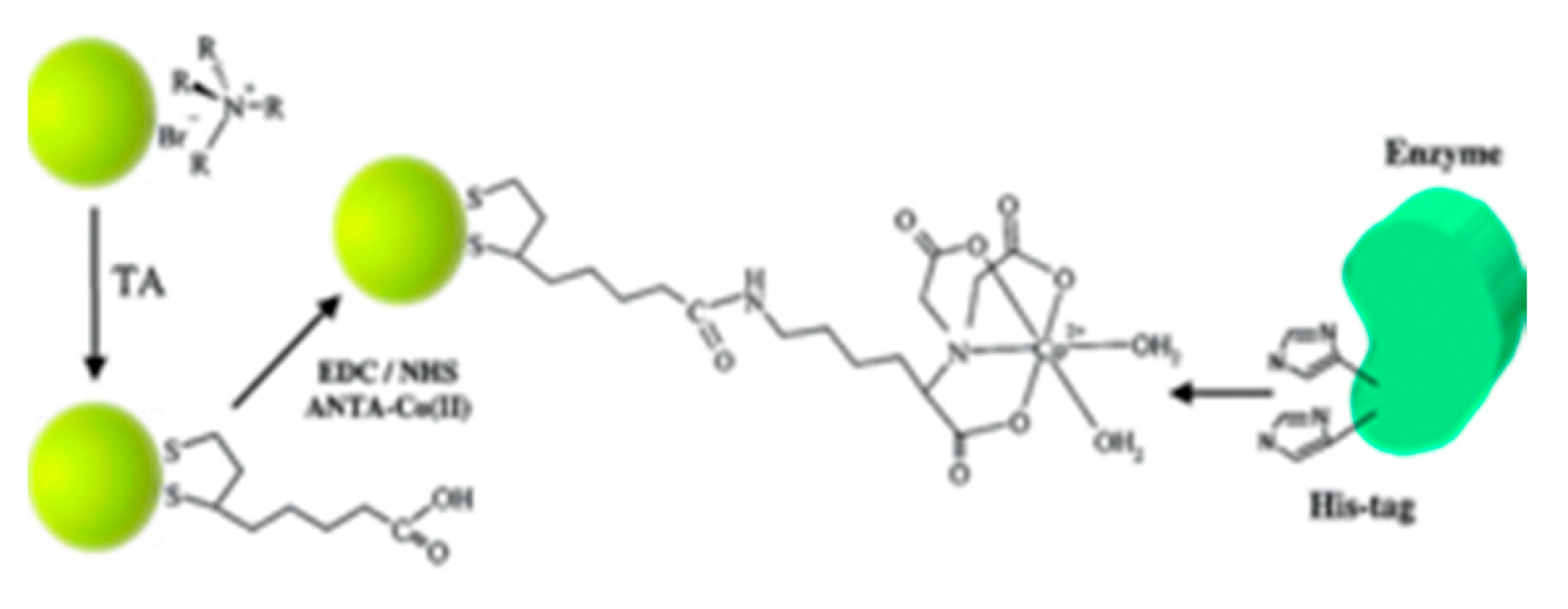
- Abad, J.M.; Mertens, S.F.L.; Pita, M.; Fernandez, V.M.; Schiffrin, D.J. Functionalization of Thioctic Acid-Capped Gold Nanoparticles for Specific Immobilization of Histidine-Tagged Proteins. J. Am. Chem. Soc. 2005, 127, 5689–5694. [Google Scholar] [CrossRef]
- Fuente, J.M.D.L.; Barrientos, A.G.; Rojas, T.C.; Rojo, J.; Cañada, J.; Fernández, A.; Penadés, S. Gold glyconanoparticles as water-soluble polyvalent models to study carbohydrate interactions. Angew. Chem. Int. Ed. 2001, 40, 2257–2261. [Google Scholar] [CrossRef]
- Reynolds, A.J.; Haines, A.H.; Russell, D.A. Gold glyconanoparticles for mimics and measurement of metal ion-mediated carbohydrate—Carbohydrate interactions. Langmuir 2006, 22, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Kotal, A.; Mandal, T.K. One-dimensional assembly of peptide-functionalized gold nanoparticles: An approach toward mercury ion sensing. J. Phys. Chem. C. 2007, 111, 1248–1255. [Google Scholar] [CrossRef]
- Lee, J.-S.; Han, M.S.; Mirkin, C.A. Colorimetric Detection of Mercuric Ion (Hg2+) in Aqueous Media using DNA-Functionalized Gold Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y. A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J. Am. Chem. Soc. 2003, 125, 6642–6643. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Accelerated Color Change of Gold Nanoparticles Assembled by DNAzymes for Simple and Fast Colorimetric Pb2+ Detection. J. Am. Chem. Soc. 2004, 126, 12298–12305. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Optimization of a Pb2+-Directed Gold Nanoparticle/DNAzyme Assembly and Its Application as a Colorimetric Biosensor for Pb2+. Chem. Mater. 2004, 16, 3231–3238. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Stimuli-responsive disassembly of nanoparticle aggregates for light-up colorimetric sensing. J. Am. Chem. Soc. 2005, 127, 12677–12683. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Smart nanomaterials inspired by biology: Dynamic assembly of error-free nanomaterials in response to multiple chemical and biological stimuli. Acc. Chem. Res. 2007, 40, 315–323. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Colorimetric Cu2+ detection with a ligation DNAzyme and nanoparticles. Chem. Commun. 2007, 4872–4874. [Google Scholar] [CrossRef]
- Marubayashi, K.; Takizawa, S.; Kawakusu, T.; Arai, T.; Sasai, H. Monolayer-Protected Au Cluster (MPC)-Supported Ti− BINOLate Complex. Org. Lett. 2003, 5, 4409–4412. [Google Scholar] [CrossRef]
- Rogers, N.J.; Claire, S.; Harris, R.M.; Farabi, S.; Zikeli, G.; Styles, I.B.; Hodges, N.J.; Pikramenou, Z. High coating of Ru(II) complexes on gold nanoparticles for single particle luminescence imaging in cells. Chem. Commun. 2014, 50, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, N.; Jayakumar, I.; Ravichandran, M.; Ganesan, V.V.; Nair, B.U. Photocrosslinking of collagen using Ru(II)-polypyridyl complex functionalized gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Kittredge, K.W.; Ca, D.V. Measurement platforms fabricated by layer-by-layer assembly of crown ether functionalized gold nanoclusters. J. Solid State Electrochem. 2004, 8, 722–726. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wu, S.H.; Chen, C. A simple strategy for prompt visual sensing by gold nanoparticles: General applications of interparticle hydrogen bonds. Angew. Chem. Int. Ed. Engl. 2006, 45, 4948–4951. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C. Detection of chemical pollutants in water using gold nanoparticles as sensors: A review. Rev. Anal. Chem. 2013, 32, 1–14. [Google Scholar] [CrossRef]
- Yamauchi, A.; Hayashita, T.; Nishizawa, S.; Watanabe, M.; Teramae, N. Benzo-15-crown-5 Fluoroionophore/γ-Cyclodextrin Complex with Remarkably High Potassium Ion Sensitivity and Selectivity in Water. J. Am. Chem. Soc. 1999, 121, 2319–2320. [Google Scholar] [CrossRef]
- Xia, W.-S.; Schmehl, R.H.; Li, C.-J. A Highly Selective Fluorescent Chemosensor for K+ from a Bis-15-Crown-5 Derivative. J. Am. Chem. Soc. 1999, 121, 5599–5600. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Liu, S.-W.; Lin, C.M.; Chen, C.H. Recognition of Potassium Ion in Water by 15-Crown-5 Functionalized Gold Nanoparticles. Anal. Chem. 2002, 74, 330–335. [Google Scholar] [CrossRef]
- Pompano, R.R.; Wortley, P.G.; Moatz, L.M.; Tognarelli, D.J.; Kittredge, K.W.; Leopold, M.C. Crown ether-metal “sandwiches” as linking mechanisms in assembled nanoparticle films. Thin Solid Films 2006, 510, 311–319. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chen, C.-H.; Lin, M.-C.; Hsu, H.-F. A Cooperative Effect of Bifunctionalized Nanoparticles on Recognition: Sensing Alkali Ions by Crown and Carboxylate Moieties in Aqueous Media. Anal. Chem. 2005, 77, 4821–4828. [Google Scholar] [CrossRef]
- Gao, J.; Fu, J.; Lin, C.; Lin, J.; Han, Y.; Yu, X.; Pan, C. Formation and Photoluminescence of Silver Nanoparticles Stabilized by a Two-Armed Polymer with a Crown Ether Core. Langmuir 2004, 20, 9775–9779. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Chen, W.; Yan, W.; Xu, L.; Zhu, Y.; Liu, L.; Chu, H.; Peng, C.; Wang, L.; Kotov, N.A.; et al. Crown ether assembly of gold nanoparticles: Melamine sensor. Biosens. Bioelectr. 2011, 26, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Holmes, H.; Wainwright, L.; Toscani, A.; Stasiuk, G.J.; White, A.J.P.; Bell, J.D.; Wilton-Ely, J.D.E.T. Multimetallic Complexes and Functionalized Gold Nanoparticles Based on a Combination of d- and f-Elements. Inorg. Chem. 2014, 53, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Truman, L.K.; Bradberry, S.J.; Comby, S.; Kotova, O.; Gunnlaugsson, T. Surface-Modified Gold Nanoparticles Possessing Two-Channel Responsive Eu-III/Tb-III Cyclen Complexes as Luminescent Logic Gate Mimics. ChemPhysChem 2017, 18, 1746–1751. [Google Scholar] [CrossRef]
- Lewis, D.J.; Day, T.M.; MacPherson, J.V.; Pikramenou, Z. Luminescent nanobeads: Attachment of surface reactive Eu(III) complexes to gold nanoparticles. Chem. Commun. 2006, 1433–1435. [Google Scholar] [CrossRef]
- Massue, J.; Quinn, S.J.; Gunnlaugsson, T. Lanthanide luminescent displacement assays: The sensing of phosphate anions using Eu(III)− Cyclen-conjugated gold nanoparticles in aqueous solution. J. Am. Chem. Soc. 2008, 130, 6900–6901. [Google Scholar] [CrossRef]
- Ipe, B.I.; Yoosaf, K.; Thomas, K.G. Functionalized gold as Phosphorescent Nanomaterials and Sensors. J. Am. Chem. Soc. 2006, 128, 1907–1913. [Google Scholar] [CrossRef]
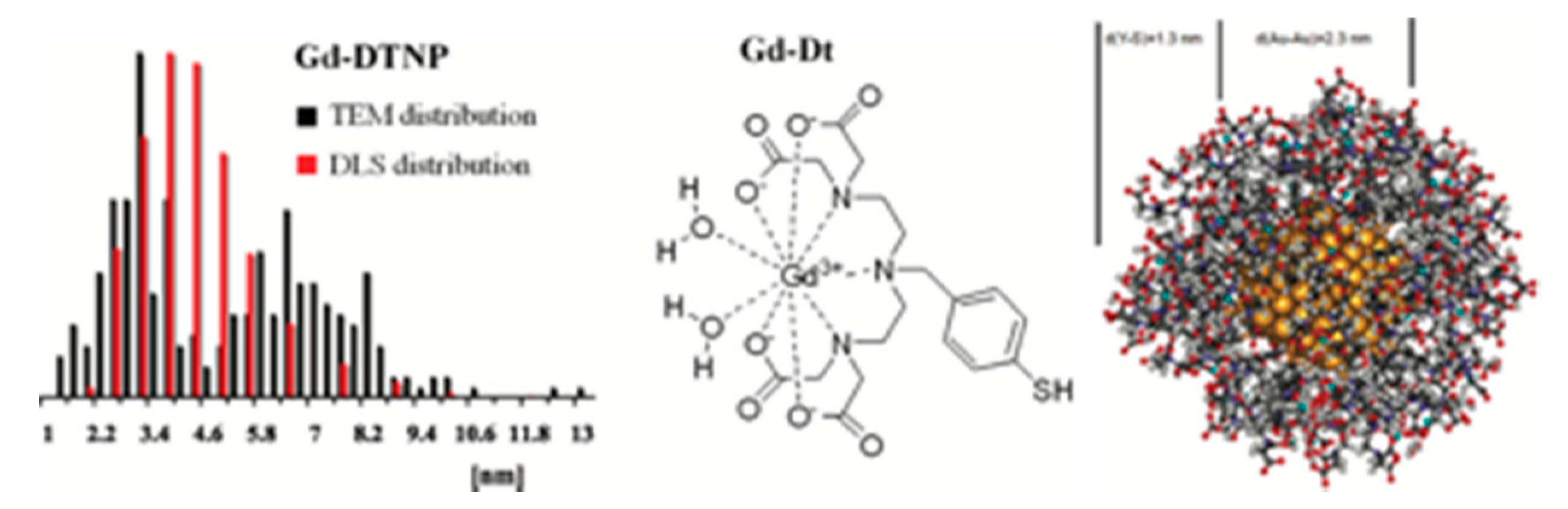
- Alric, C.; TaleB, J.; Le Duc, G.; Mandon, C.; Billotey, C.; Le Meur-Herland, A.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P.; et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both X-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915. [Google Scholar] [CrossRef]
- Debouttière, P.-J.; Roux, S.; Vocanson, F.; Billotey, C.; Beuf, O.; Favre-Réguillon, A.; Lin, Y.; Pellet-Rostaing, S.; Lamartine, R.; Perriat, P.; et al. Design of gold nanoparticles for magnetic resonance imaging. Adv. Funct. Mat. 2006, 16, 2330–2339. [Google Scholar] [CrossRef]
- Moriggi, L.; Cannizzo, C.; Dumas, E.; Mayer, C.R.; Ulianov, A.; Helm, L. Gold Nanoparticles Functionalized with Gadolinium Chelates as High Relaxivity MRI Contrast Agents. J. Am. Chem. Soc. 2009, 131, 10828. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Z.; Zhang, J.; Peng, C.; Klajnert-Maculewicz, B.; Shen, M.; Shi, X. Zwitterionic Gadolinium(III)- Complexed Dendrimer-Entrapped Gold Nanoparticles for Enhanced Computed Tomography/Magnetic Resonance Imaging of Lung Cancer Metastasis. Acs Appl. Mater. Interfaces 2019, 11, 15212–15221. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.F.; Goncalves, J.; Mousavi, B.; Prata, M.I.M.; Rodrigues, S.P.J.; Calle, D.; Lopez-Larrubia, P.; Cerdan, S.; Rodrigues, T.B.; Ferreira, P.M.; et al. Gold nanoparticles functionalised with fast water exchanging Gd3+ chelates: Linker effects on the relaxivity. Dalton Trans. 2015, 44, 4016–4031. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumur, F.; Dumas, E.; Mayer, C.R. Functionalization of Gold Nanoparticles by Inorganic Entities. Nanomaterials 2020, 10, 548. https://doi.org/10.3390/nano10030548
Dumur F, Dumas E, Mayer CR. Functionalization of Gold Nanoparticles by Inorganic Entities. Nanomaterials. 2020; 10(3):548. https://doi.org/10.3390/nano10030548
Chicago/Turabian StyleDumur, Frédéric, Eddy Dumas, and Cédric R. Mayer. 2020. "Functionalization of Gold Nanoparticles by Inorganic Entities" Nanomaterials 10, no. 3: 548. https://doi.org/10.3390/nano10030548
APA StyleDumur, F., Dumas, E., & Mayer, C. R. (2020). Functionalization of Gold Nanoparticles by Inorganic Entities. Nanomaterials, 10(3), 548. https://doi.org/10.3390/nano10030548