Amplified Fluorescence by ZnO Nanoparticles vs. Quantum Dots for Bovine Mastitis Acute Phase Response Evaluation in Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of TEOS Modified ZnO-QDs
2.3. Fabrication of TEOS Modified ZnO-NPs
2.4. Milk Sampling
2.5. Quantification of NAGase Activity
2.6. Instrumentation
3. Results and Discussion
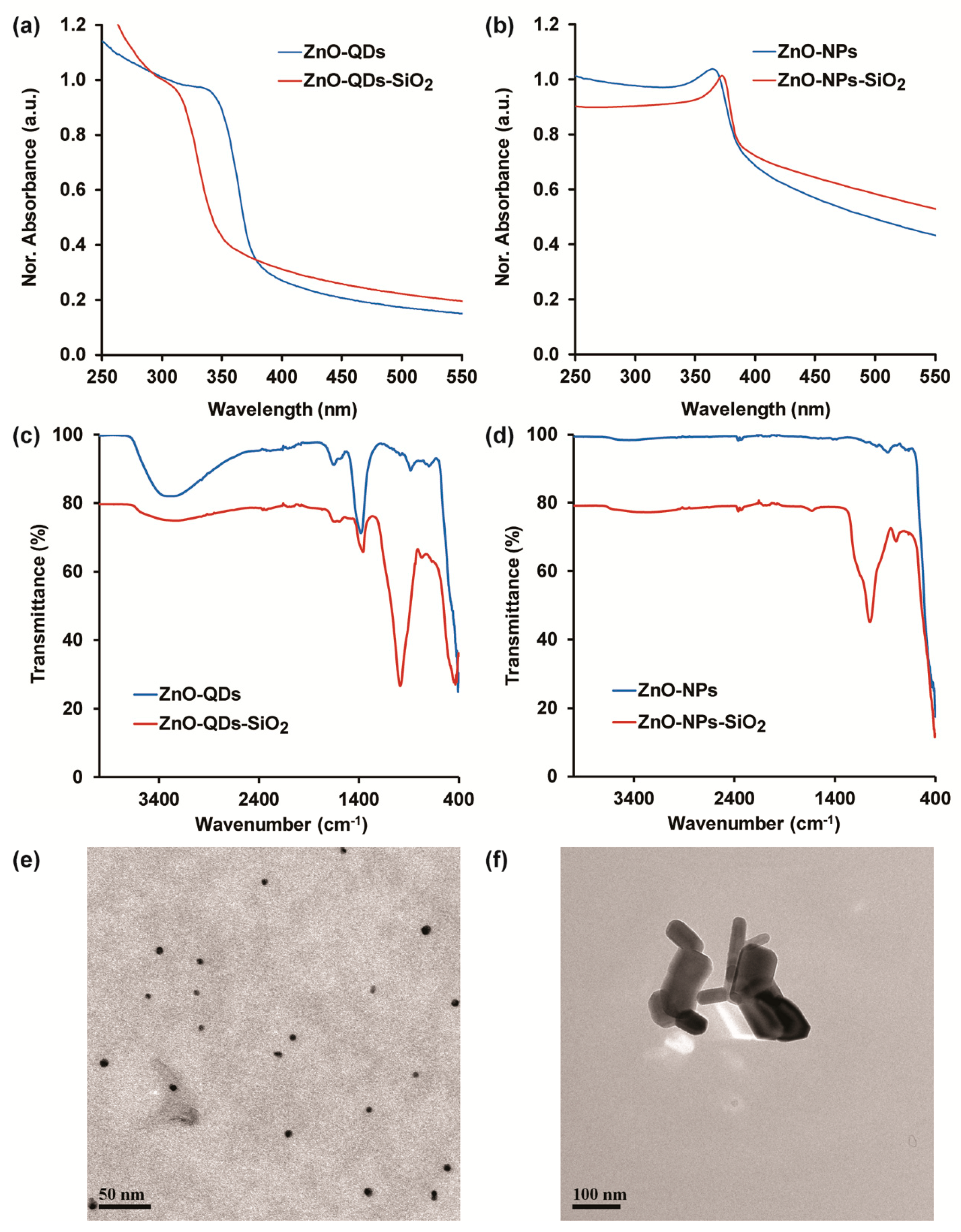
3.1. ZnO-Nanomaterials Characterization
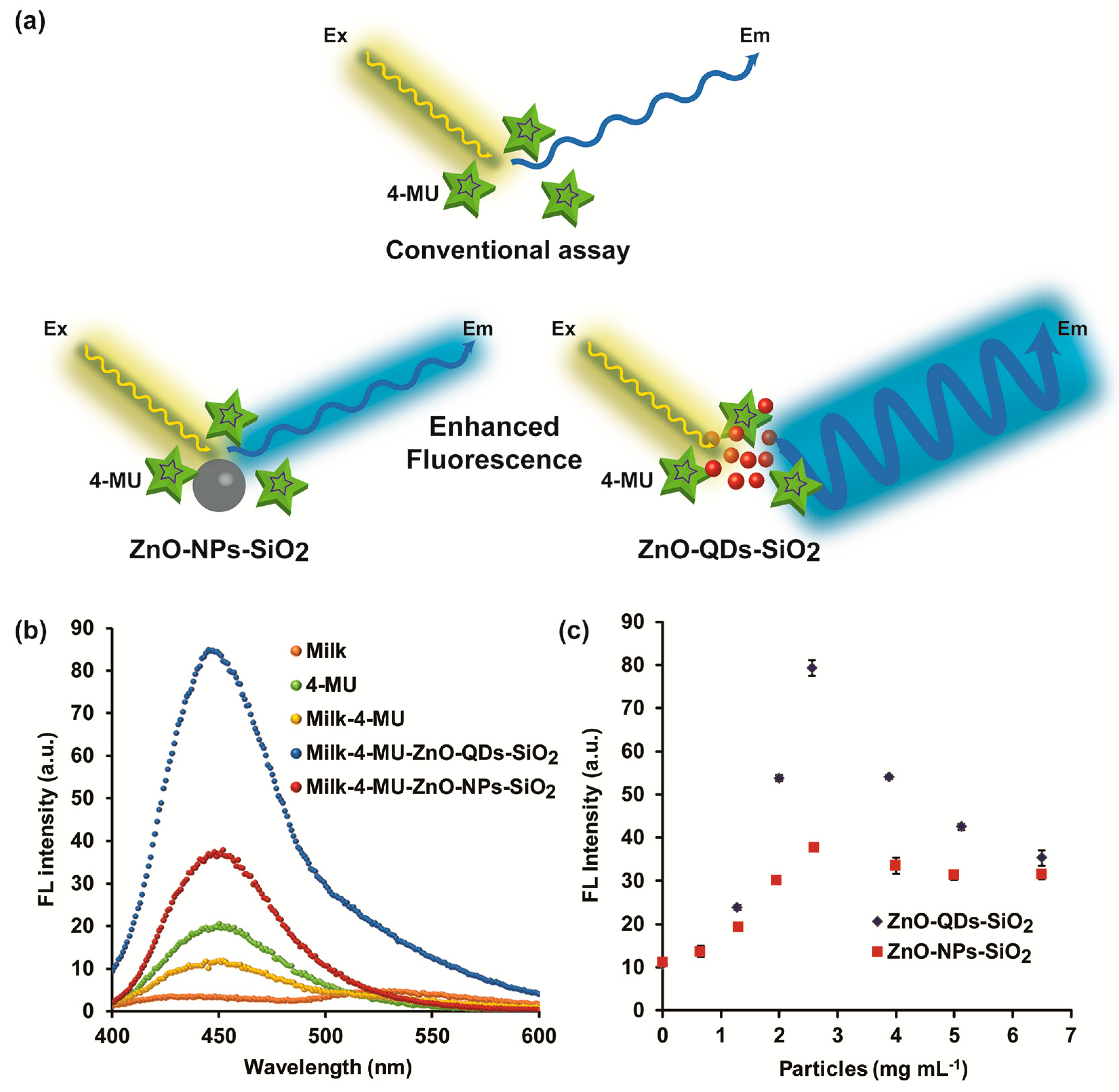
3.2. FL Amplification Characterization
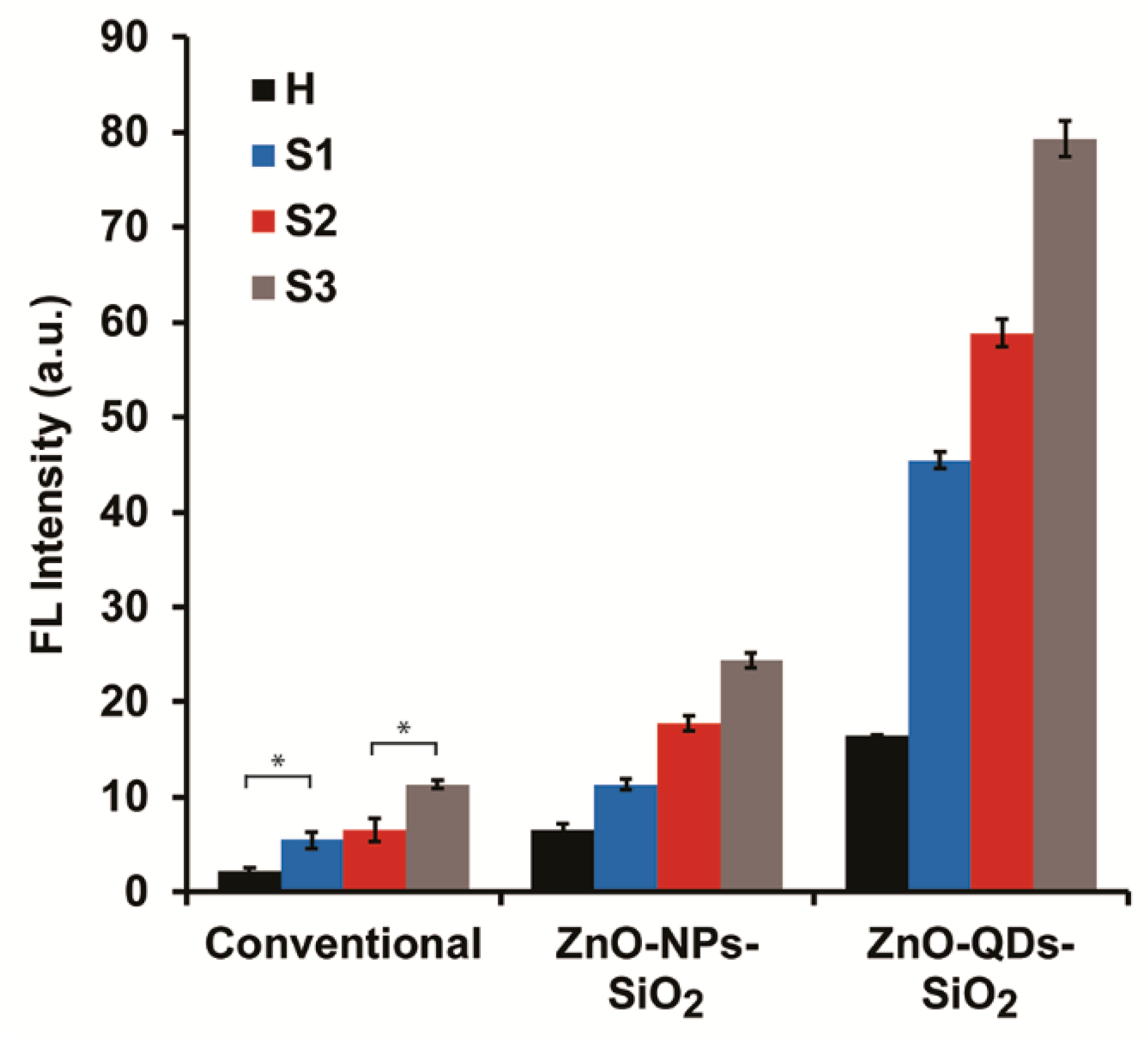
3.3. Comparative Studies of NAGase Activity with and without ZnO-Nanomaterials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalmus, P.; Simojoki, H.; Pyörälä, S.; Taponen, S.; Holopainen, J.; Orro, T. Milk haptoglobin, milk amyloid A, and N-acetyl-β-d-glucosaminidase activity in bovines with naturally occurring clinical mastitis diagnosed with a quantitative PCR test. J. Dairy Sci. 2013, 96, 3662–3670. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S.; Hovinen, M.; Simojoki, H.; Fitzpatrick, J.; Eckersall, P.; Orro, T. Acute phase proteins in milk in naturally acquired bovine mastitis caused by different pathogens. Vet. Rec. 2011, 168, 535. [Google Scholar] [CrossRef]
- Viguier, C.; Arora, S.; Gilmartin, N.; Welbeck, K.; O’Kennedy, R. Mastitis detection: Current trends and future perspectives. Trends Biotechnol. 2009, 27, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, L.H.; Moers, A.; Norde, W.; van Amerongen, A. Rapid mastitis detection assay on porous nitrocellulose membrane slides. Anal. Bioanal. Chem. 2013, 405, 7469–7476. [Google Scholar] [CrossRef] [PubMed]
- Hovinen, M.; Simojoki, H.; Pösö, R.; Suolaniemi, J.; Kalmus, P.; Suojala, L.; Pyörälä, S. N-acetyl-β-D-glucosaminidase activity in cow milk as an indicator of mastitis. J. Dairy Res. 2016, 83, 219–227. [Google Scholar] [CrossRef]
- Koess, C.; Hamann, J. Detection of mastitis in the bovine mammary gland by flow cytometry at early stages. J. Dairy Res. 2008, 75, 225–232. [Google Scholar] [CrossRef]
- Sears, P.M.; McCarthy, K.K. Diagnosis of mastitis for therapy decisions. Vet. Clin. N. Am. Large Anim. Pract. 2003, 19, 93–108. [Google Scholar] [CrossRef]
- Riffon, R.; Sayasith, K.; Khalil, H.; Dubreuil, P.; Drolet, M.; Lagacé, J. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J. Clin. Microbiol. 2001, 39, 2584–2589. [Google Scholar] [CrossRef]
- Nirala, N.R.; Pinker, N.; Desitti, C.; Shtenberg, G. Milk haptoglobin detection based on enhanced chemiluminescence of gold nanoparticles. Talanta 2019, 197, 257–263. [Google Scholar] [CrossRef]
- Nirala, N.R.; Shtenberg, G. Gold Nanoparticle Size-Dependent Enhanced Chemiluminescence for Ultra-Sensitive Haptoglobin Biomarker Detection. Biomolecules 2019, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, B.; Middleton, G.; Salmon, M. Bovine milk N-acetyl-β-D-glucosaminidase and its significance in the detection of abnormal udder secretions. J. Dairy Res. 1978, 45, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Shtenberg, G. Enhanced fluorescence of N-acetyl-β-D-glucosaminidase activity by ZnO quantum dots for early stage mastitis evaluation. Front. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Hassabo, A.G.; Mohamed, A.L.; Shaheen, T.I. Surface modification of SiO2 coated ZnO nanoparticles for multifunctional cotton fabrics. J. Colloid Interface Sci. 2017, 498, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Previte, M.J.; Zhang, Y.; Geddes, C.D. Metal-enhanced fluorescence from nanoparticulate zinc films. J. Phys. Chem. C 2008, 112, 18368–18375. [Google Scholar] [CrossRef] [PubMed]
- El-Nahhal, I.M.; Salem, J.K.; Kuhn, S.; Hammad, T.; Hempelmann, R.; Al Bhaisi, S. Synthesis & characterization of silica coated and functionalized silica coated zinc oxide nanomaterials. Powder Technol. 2016, 287, 439–446. [Google Scholar] [CrossRef]
- Hagura, N.; Ogi, T.; Shirahama, T.; Iskandar, F.; Okuyama, K. Highly luminescent silica-coated ZnO nanoparticles dispersed in an aqueous medium. J. Lumin. 2011, 131, 921–925. [Google Scholar] [CrossRef]
- Patra, M.; Manoth, M.; Singh, V.; Gowd, G.S.; Choudhry, V.; Vadera, S.; Kumar, N. Synthesis of stable dispersion of ZnO quantum dots in aqueous medium showing visible emission from bluish green to yellow. J. Lumin. 2009, 129, 320–324. [Google Scholar] [CrossRef]
- Wang, T.; Centeno, A.; Darvill, D.; Pang, J.S.; Ryan, M.P.; Xie, F. Tuneable fluorescence enhancement of nanostructured ZnO arrays with controlled morphology. Phys. Chem. Chem. Phys. 2018, 20, 14828–14834. [Google Scholar] [CrossRef]
- Zhao, D.; Song, H.; Hao, L.; Liu, X.; Zhang, L.; Lv, Y. Luminescent ZnO quantum dots for sensitive and selective detection of dopamine. Talanta 2013, 107, 133–139. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Wu, Y.L.; Tok, A.; Boey, F.Y.C.; Zeng, X.T.; Zhang, X.H. Surface modification of ZnO nanocrystals. Appl. Surf. Sci. 2007, 253, 5473–5479. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Fedorenko, V.; Smyntyna, V.; Konup, I.; Konup, A.; Eriksson, M.; Yakimova, R.; Ramanavicius, A.; Balme, S.; Bechelany, M. ZnO films formed by atomic layer deposition as an optical biosensor platform for the detection of Grapevine virus A-type proteins. Biosens. Bioelectron. 2017, 92, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shi, Y.; Liu, X.; Han, Z.; Zhao, Z.; Chen, Y.; Xie, W.; Li, X. Enhanced fluorescence detection of proteins using ZnO nanowires integrated inside microfluidic chips. Biosens. Bioelectron. 2018, 99, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Metkar, S.K.; Girigoswami, A.; Girigoswami, K. ZnO nanoflower based sensitive nano-biosensor for amyloid detection. Mater. Sci. Eng. C 2017, 78, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Shtenberg, G.; Massad-Ivanir, N.; Fruk, L.; Segal, E. Nanostructured porous Si optical biosensors: Effect of thermal oxidation on their performance and properties. ACS Appl. Mater. Interfaces 2014, 6, 16049–16055. [Google Scholar] [CrossRef]
- Fang, L.; Wang, W.; Liu, Y.; Xie, Z.; Chen, L. Janus nanostructures formed by mesoporous silica coating Au nanorods for near-infrared chemo–photothermal therapy. J. Mater. Chem. B 2017, 5, 8833–8838. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, R.; Park, S.R.; Lee, J.H.; Jung, H.; Lee, H.-S.; Lee, W. Highly sensitive and selective isoprene sensing performance of ZnO quantum dots for a breath analyzer. Sens. Actuators B 2019, 290, 258–266. [Google Scholar] [CrossRef]
- Tăbăcaru, A.; Muşat, V.; Ţigău, N.; Vasile, B.Ş.; Surdu, V.-A. Vinyltrimethoxysilane-modified zinc oxide quantum dots with tuned optical properties. Appl. Surf. Sci. 2015, 359, 766–773. [Google Scholar] [CrossRef]
- Biswick, T.; Jones, W.; Pacuła, A.; Serwicka, E.; Podobinski, J. The role of anhydrous zinc nitrate in the thermal decomposition of the zinc hydroxy nitrates Zn5(OH)8(NO3)2·2H2O and ZnOHNO3·H2O. J. Solid State Chem. 2007, 180, 1171–1179. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, T.; Ma, S.; Liu, Y.; Tian, Y.; Wang, R.; Jiang, Y.; Hou, D.; Wang, J. Fluorometric determination of the antibiotic kanamycin by aptamer-induced FRET quenching and recovery between MoS2 nanosheets and carbon dots. Microchim. Acta 2017, 184, 203–210. [Google Scholar] [CrossRef]
- Kitchen, B.J.; Kwee, W.S.; Middleton, G.; Andrews, R.J. Relationship between the level of N-acetyl-β-D-glucosaminidase (NAGase) in bovine milk and the presence of mastitis pathogens. J. Dairy Res. 1984, 51, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, X.; Guo, J.; Wang, R.; Wu, Y.; Zhang, M.; Li, C.; Han, Q.; Dong, J.; Zheng, H. Solution-based metal enhanced fluorescence with gold and gold/silver core–shell nanorods. Opt. Commun. 2015, 357, 156–160. [Google Scholar] [CrossRef]
- Gontero, D.; Veglia, A.V.; Bracamonte, A.G.; Boudreau, D. Synthesis of ultraluminescent gold core–shell nanoparticles as nanoimaging platforms for biosensing applications based on metal-enhanced fluorescence. RSC Adv. 2017, 7, 10252–10258. [Google Scholar] [CrossRef]
- Nirala, N.R.; Harel, Y.; Lellouche, J.-P.; Shtenberg, G. Ultrasensitive haptoglobin biomarker detection based on amplified chemiluminescence of magnetite nanoparticles. J. Nanobiotechnol. 2020, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Guan, P.; Zhang, L.; Fujita, T.; Chen, M. Size dependence of molecular fluorescence enhancement of nanoporous gold. Appl. Phys. Lett. 2010, 96, 73701. [Google Scholar] [CrossRef]
- Bardhan, R.; Grady, N.K.; Halas, N.J. Nanoscale Control of Near-Infrared Fluorescence Enhancement Using Au Nanoshells. Small 2008, 4, 1716–1722. [Google Scholar] [CrossRef]
- Mahjoub, M.A.; Monier, G.; Robert-Goumet, C.; Réveret, F.; Echabaane, M.; Chaudanson, D.; Petit, M.; Bideux, L.; Gruzza, B. Synthesis and Study of Stable and Size-Controlled ZnO–SiO2 Quantum Dots: Application as a Humidity Sensor. J. Phys. Chem. C 2016, 120, 11652–11662. [Google Scholar] [CrossRef]
- Ferrero, F.; Valledor, M.; Campo, J. Screening method for early detection of mastitis in cows. Measurement 2014, 47, 855–860. [Google Scholar] [CrossRef]
| Sample * | SCC (×103) Cells mL−1 | Bacteria |
|---|---|---|
| H | 60 | N/A |
| S1 | 350 | Strep. dysgalactiae |
| S2 | 800 | Strep. dysgalactiae |
| S3 | >1000 | Strep. dysgalactiae |
| Sample *,** | NAGase Activity Conventional Assay (µM min−1) | NAGase Activity with ZnO-NPs-SiO2 (µM min−1) | NAGase Activity with ZnO-QDs-SiO2 (µM min−1) |
|---|---|---|---|
| H | 0.30 ± 0.05 | 0.35 ± 0.04 | 0.33 ± 0.01 |
| S1 | 0.73 ± 0.12 | 0.68 ± 0.04 | 0.94 ± 0.02 |
| S2 | 0.86 ± 0.16 | 1.11 ± 0.05 | 1.21 ± 0.03 |
| S3 | 1.50 ± 0.06 | 1.56 ± 0.05 | 1.64 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nirala, N.R.; Shtenberg, G. Amplified Fluorescence by ZnO Nanoparticles vs. Quantum Dots for Bovine Mastitis Acute Phase Response Evaluation in Milk. Nanomaterials 2020, 10, 549. https://doi.org/10.3390/nano10030549
Nirala NR, Shtenberg G. Amplified Fluorescence by ZnO Nanoparticles vs. Quantum Dots for Bovine Mastitis Acute Phase Response Evaluation in Milk. Nanomaterials. 2020; 10(3):549. https://doi.org/10.3390/nano10030549
Chicago/Turabian StyleNirala, Narsingh R., and Giorgi Shtenberg. 2020. "Amplified Fluorescence by ZnO Nanoparticles vs. Quantum Dots for Bovine Mastitis Acute Phase Response Evaluation in Milk" Nanomaterials 10, no. 3: 549. https://doi.org/10.3390/nano10030549
APA StyleNirala, N. R., & Shtenberg, G. (2020). Amplified Fluorescence by ZnO Nanoparticles vs. Quantum Dots for Bovine Mastitis Acute Phase Response Evaluation in Milk. Nanomaterials, 10(3), 549. https://doi.org/10.3390/nano10030549