Effect of Zinc and Magnesium Compounds and Nano-Hydroxyapatite on the Physicochemical Properties and Biological Activity of Alginate and Gelatin Scaffolds for Osteochondral Defects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Scaffolds Fabrication
2.3. Characterization
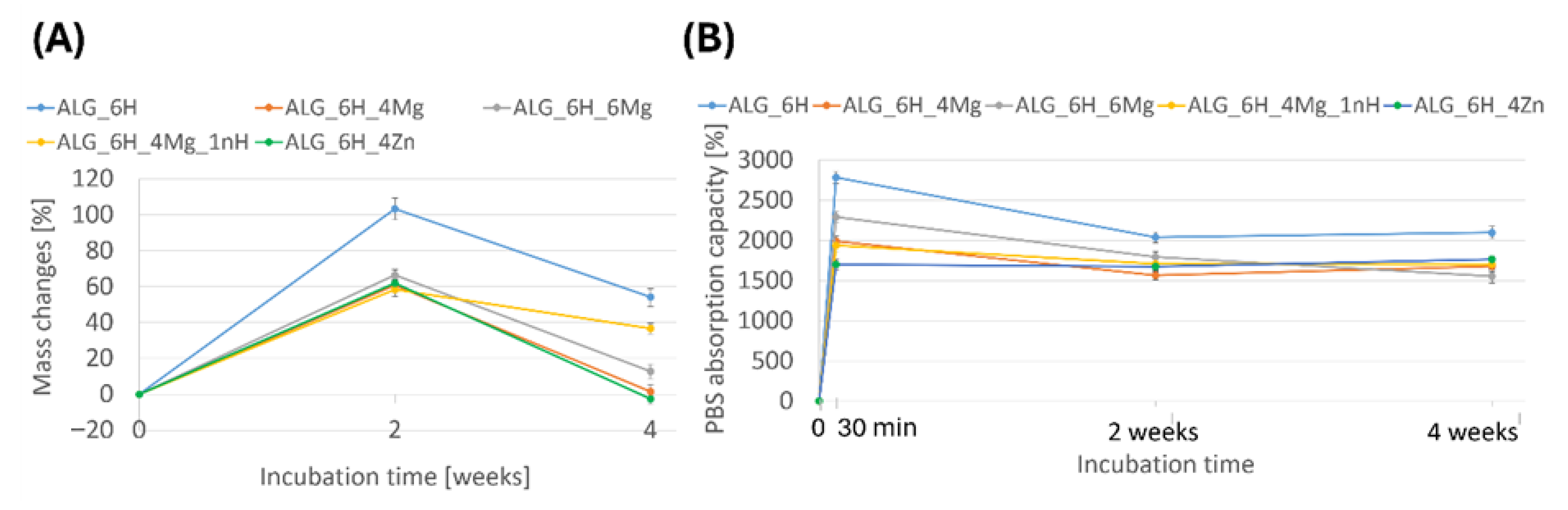
2.3.1. The Initial Water Content, PBS Absorption Capacity (Swelling Ability), and Mass Changes During Incubation in PBS
2.3.2. Zinc and Magnesium Release
2.3.3. Antibacterial Activity Tests
2.3.4. Mechanical Tests
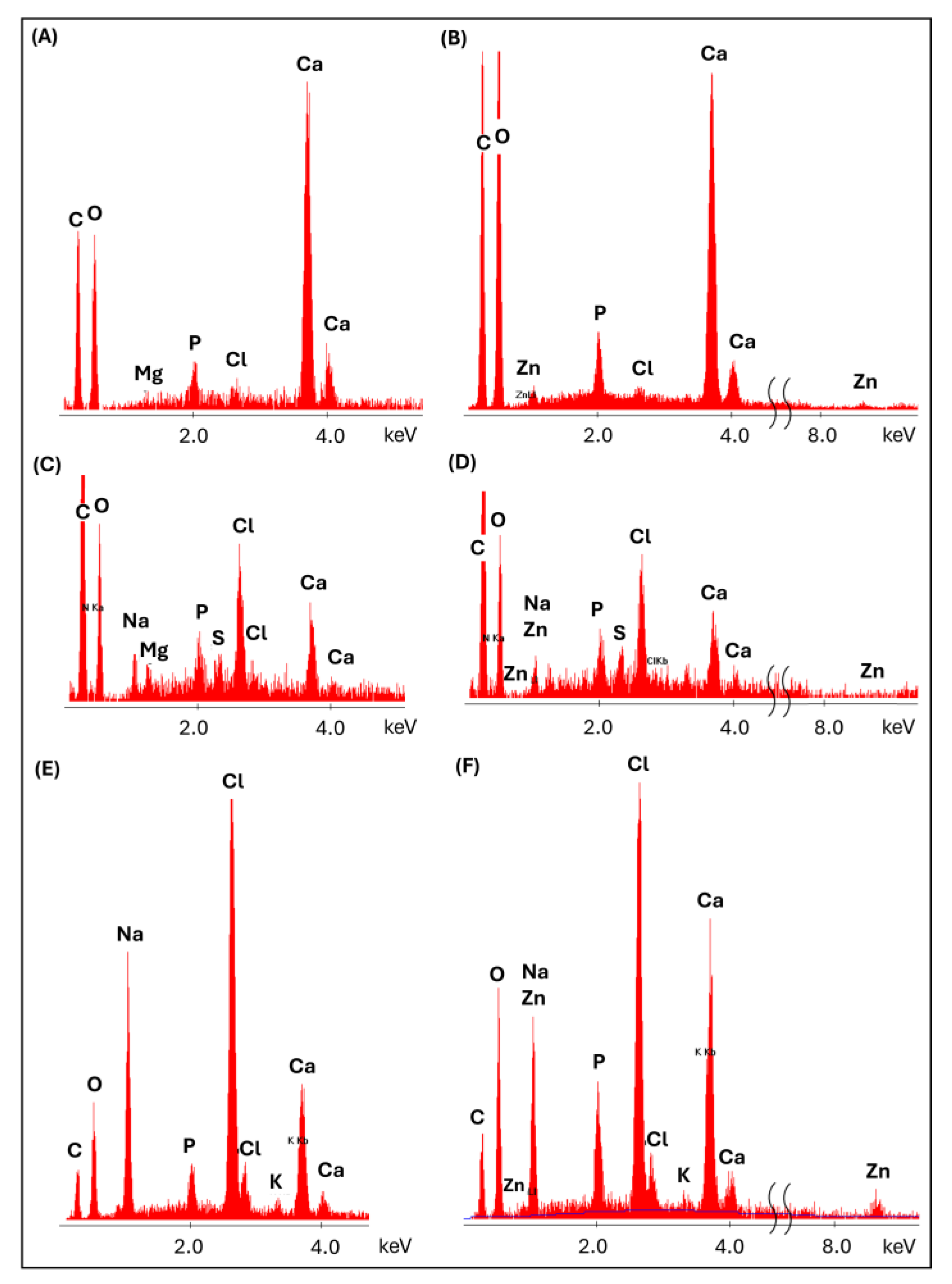
2.3.5. Microstructural Observations with Elemental Analysis (SEM/EDS)
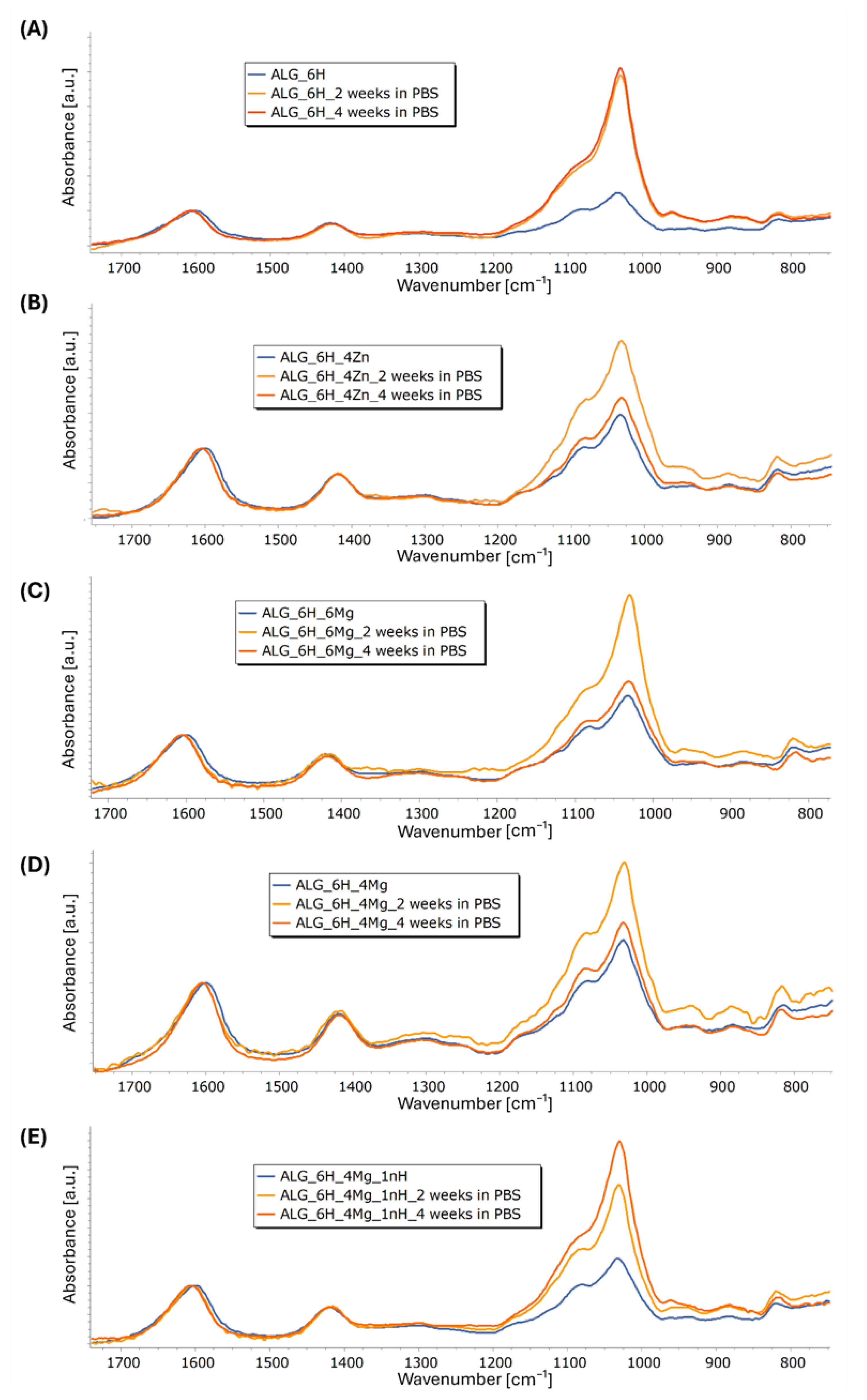
2.3.6. Infrared Structural Analysis (FTIR-ATR)
2.3.7. Size Distribution of the ZnO Particles
2.3.8. Statistical Analysis
3. Results and Discussion
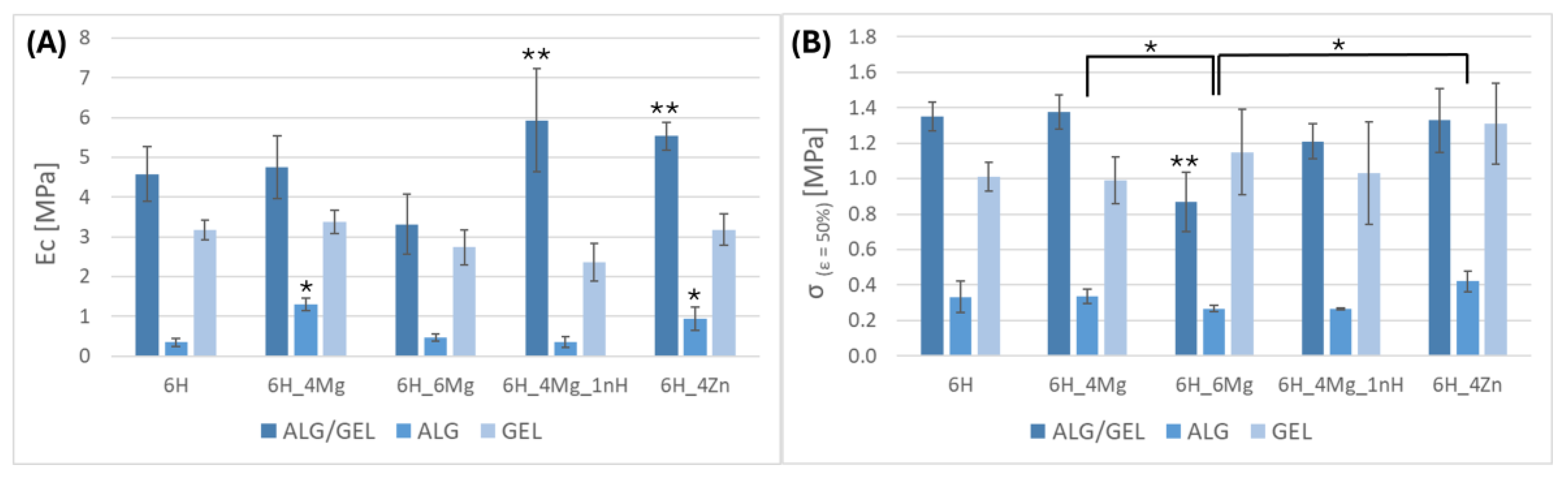
3.1. Mechanical Properties
3.2. The Initial Water Content in Hydrogel Scaffolds
3.3. Release of Mg2+ and Zn2+ Ion from Scaffolds
3.4. Antibacterial Activity
3.5. Behavior of Scaffolds in a Simulated Biological Environment
3.5.1. Changes in Scaffold Weight After Incubation in PBS
3.5.2. Changes of the Mechanical Parameters of Scaffolds After Incubation in PBS
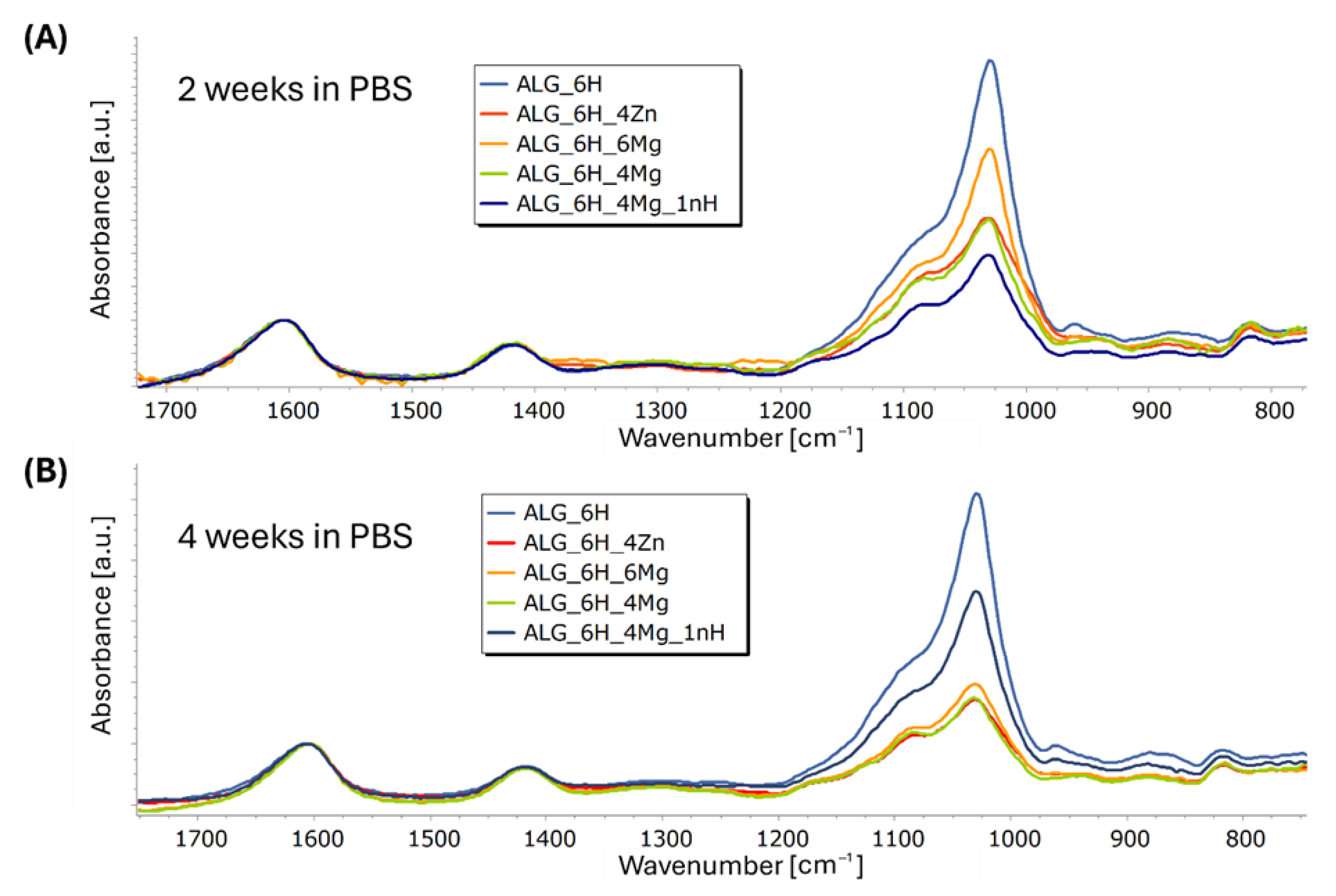
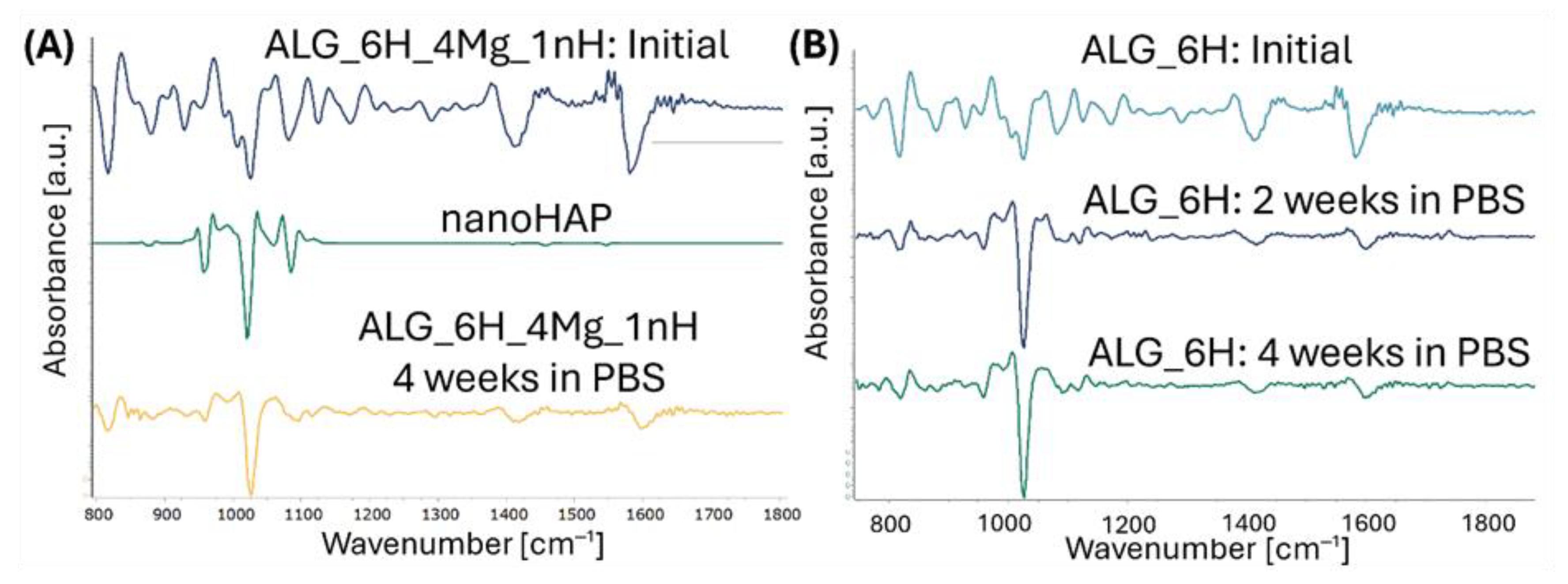
3.5.3. Structural and Microstructural Changes of Scaffolds After Incubation in PBS (FTIR, SEM/EDS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsujii, A.; Ohori, T.; Hanai, H.; Nakamura, N. Next Generation Approaches for Cartilage Repair and Joint Preservation. J. Cartil. Jt. Preserv. 2024, 4, 100177. [Google Scholar] [CrossRef]
- Cong, B.; Sun, T.; Zhao, Y.; Chen, M. Current and Novel Therapeutics for Articular Cartilage Repair and Regeneration. Ther. Clin. Risk Manag. 2023, 19, 485. [Google Scholar] [CrossRef]
- Haung, S.-M.; Lin, Y.-T.; Liu, S.-M.; Chen, J.-C.; Chen, W.-C. In Vitro Evaluation of a Composite Gelatin–Hyaluronic Acid–Alginate Porous Scaffold with Different Pore Distributions for Cartilage Regeneration. Gels 2021, 7, 165. [Google Scholar] [CrossRef]
- Sharma, S.; Bhende, M.; Goel, A. A Review: Polysaccharide-Based Hydrogels and Their Biomedical Applications. Polym. Bull. 2024, 81, 8573–8594. [Google Scholar] [CrossRef]
- Tomić, S.L.; Nikodinović-Runić, J.; Vukomanović, M.; Babić, M.M.; Vuković, J.S. Novel Hydrogel Scaffolds Based on Alginate, Gelatin, 2-Hydroxyethyl Methacrylate, and Hydroxyapatite. Polymers 2021, 13, 932. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-Polymer Biocomposites for Bone Regeneration: A Review of Current Trends. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Kavitha Sri, A.; Arthi, C.; Neya, N.R.; Hikku, G.S. Nano-Hydroxyapatite/Collagen Composite as Scaffold Material for Bone Regeneration. Biomed. Mater. 2023, 18, 032002. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, B.; Song, Y.; Cao, Y.; Liu, Y.; Zhou, D.; Zhou, Q.; Xie, F.; Huang, W.; Li, X.; et al. Nanohydroxyapatite and Liposomes-Coated Integral Bilayer Scaffold for Osteochondral Repair via Mimicking the Dual Differentiation Microenvironment of BMSCs. Nano Mater. Sci. 2025. In Press. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Fatima, G.; Dzupina, A.; Alhmadi, H.B.; Magomedova, A.; Siddiqui, Z.; Mehdi, A.; Hadi, N. Magnesium Matters: A Comprehensive Review of Its Vital Role in Health and Diseases. Cureus 2024, 16, e71392. [Google Scholar] [CrossRef]
- Yuan, Z.; Lyu, Z.; Liu, X.; Zhang, J.; Wang, Y.; Yuan, Z.; Lyu, Z.; Liu, X.; Zhang, J.; Wang, Y. Mg-BGNs/DCECM Composite Scaffold for Cartilage Regeneration: A Preliminary In Vitro Study. Pharmaceutics 2021, 13, 1550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, H.; Wang, L.; Jiang, D.; Wang, W.; Yuan, G.; Pei, J.; Jia, W. The Beneficial Potential of Magnesium-Based Scaffolds to Promote Chondrogenesis through Controlled Mg2+ Release in Eliminating the Destructive Effect of Activated Macrophages on Chondrocytes. Biomater. Adv. 2022, 134, 112719. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, N.; Guo, Y.; Zhao, S.; Tan, J.; Wang, L.; Li, G.; Wu, J.; Yang, Y.; Xu, W.; et al. Additively Manufactured Biodegradable Porous Magnesium Implants for Elimination of Implant-Related Infections: An in Vitro and in Vivo Study. Bioact. Mater. 2022, 8, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, P.O.; Sossa, K.; Contreras, D.; Urrutia, H.; Nocker, A. Antimicrobial Properties of Magnesium Chloride at Low PH in the Presence of Anionic Bases. Magnes. Res. 2014, 27, 57–68. [Google Scholar] [CrossRef]
- Mousa, H.M.; Abdal-Hay, A.; Bartnikowski, M.; Mohamed, I.M.A.; Yasin, A.S.; Ivanovski, S.; Park, C.H.; Kim, C.S. A Multifunctional Zinc Oxide/Poly(Lactic Acid) Nanocomposite Layer Coated on Magnesium Alloys for Controlled Degradation and Antibacterial Function. ACS Biomater. Sci. Eng. 2018, 4, 2169–2180. [Google Scholar] [CrossRef]
- Shitole, A.A.; Raut, P.W.; Sharma, N.; Giram, P.; Khandwekar, A.P.; Garnaik, B. Electrospun Polycaprolactone/Hydroxyapatite/ZnO Nanofibers as Potential Biomaterials for Bone Tissue Regeneration. J. Mater. Sci. Mater. Med. 2019, 30, 51. [Google Scholar] [CrossRef]
- Nair, S.; Sasidharan, A.; Divya Rani, V.V.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of Size Scale of ZnO Nanoparticles and Microparticles on Toxicity toward Bacteria and Osteoblast Cancer Cells. J. Mater. Sci. Mater. Med. 2009, 20, 235–241. [Google Scholar] [CrossRef]
- Bashir, S.; Awan, M.S.; Farrukh, M.A.; Naidu, R.; Khan, S.A.; Rafique, N.; Ali, S.; Hayat, I.; Hussain, I.; Khan, M.Z. In-Vivo (Albino Mice) and in-Vitro Assimilation and Toxicity of Zinc Oxide Nanoparticles in Food Materials. Int. J. Nanomed. 2022, 17, 4073. [Google Scholar] [CrossRef]
- Kwiecień, K.; Knap, K.; Heida, R.; Czajkowski, J.; Gorter, A.; Ochońska, D.; Mielczarek, P.; Dorosz, A.; Niewolik, D.; Reczyńska-Kolman, K.; et al. Novel Copolymers of Poly(Sebacic Anhydride) and Poly(Ethylene Glycol) as Azithromycin Carriers to the Lungs. Biocybern. Biomed. Eng. 2025, 45, 114–136. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Liao, Y.; Wang, H.; Sun, X.; Chen, X.; Yan, H.; Lin, Q. Design and Properties of Alginate/Gelatin/Cellulose Nanocrystals Interpenetrating Polymer Network Composite Hydrogels Based on in Situ Cross-Linking. Eur. Polym. J. 2023, 201, 112556. [Google Scholar] [CrossRef]
- Ko, C.L.; Wu, H.Y.; Lin, Y.S.; Yang, C.H.; Chen, J.C.; Chen, W.C. Modulating the Release of Proteins from Aloaded Carrier of Alginate/Gelatin Porous Spheres Immersed in Different Solutions. Biomed. Mater. Eng. 2017, 28, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of Hydrogel Biomaterials with Controlled Mechanical Properties for Tissue Engineering Applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of Alginate-Based Hydrogels: Crosslinking Strategies and Biomedical Applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zi, Y.; Kan, G.; Li, L.; Shi, C.; Wang, X.; Zhong, J. Preparation of Food-Grade EDC/NHS-Crosslinked Gelatin Nanoparticles and Their Application for Pickering Emulsion Stabilization. Food Chem. 2024, 436, 137700. [Google Scholar] [CrossRef]
- Chen, P.R.; Kang, P.L.; Su, W.Y.; Lin, F.H.; Chen, M.H. The Evaluation of Thermal Properties and in Vitro Test of Carbodiimide or Glutaraldehyde Cross-Linked Gelatin for PC 12 Cells Culture. Biomed. Eng.-Appl. Basis Commun. 2005, 17, 101–107. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; de Claville Christiansen, J. Mechanical Properties of Alginate Hydrogels Cross-Linked with Multivalent Cations. Polymers 2023, 15, 3012. [Google Scholar] [CrossRef] [PubMed]
- Innocentini, M.D.D.M.; Rasteira, V.D.; Potoczek, M.; Chmielarz, A.; Kocyło, E. Physical, Fluid Dynamic and Mechanical Properties of Alumina Gel-Cast Foams Manufactured Using Agarose or Ovalbumin as Gelling Agents. J. Mater. Res. 2017, 32, 2810–2818. [Google Scholar] [CrossRef]
- Shaheen, A.; Maswal, M.; Dar, A.A. Synergistic Effect of Various Metal Ions on the Mechanical, Thixotropic, Self-Healing, Swelling and Water Retention Properties of Bimetallic Hydrogels of Alginate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127223. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Fernández-Calderón, M.C.; Pacha-Olivenza, M.A.; Pérez-Giraldo, C.; Gallardo-Moreno, A.M.; González-Martín, M.L. The Role of Magnesium in Biomaterials Related Infections. Colloids Surf. B Biointerfaces 2020, 191, 110996. [Google Scholar] [CrossRef]
- Kang, S.N.; Jeong, C.M.; Jeon, Y.C.; Byon, E.S.; Jeong, Y.S.; Cho, L.R. Effects of Mg-Ion and Ca-Ion Implantations on P. Gingivalis and F. Nucleatum Adhesion. Tissue Eng. Regen. Med. 2014, 11, 39–46. [Google Scholar] [CrossRef]
- De Kerchove, A.J.; Elimelech, M. Calcium and Magnesium Cations Enhance the Adhesion of Motile and Nonmotile Pseudomonas Aeruginosa on Alginate Films. Langmuir 2008, 24, 3392–3399. [Google Scholar] [CrossRef]
- Meißner, T.; Oelschlägel, K.; Potthoff, A. Implications of the Stability Behavior of Zinc Oxide Nanoparticles for Toxicological Studies. Int. Nano Lett. 2014, 4, 116. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, P.; Huang, Y.; Zhang, W.; Chen, F.; Zhang, X.; Mao, J.; Chen, D.; Cai, Q.; Yang, X. Injectable PLGA Microspheres with Tunable Magnesium Ion Release for Promoting Bone Regeneration. Acta Biomater. 2019, 85, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Valadbeigi, H.; Sadeghifard, N.; Kaviar, V.H.; Haddadi, M.H.; Ghafourian, S.; Maleki, A. Effect of ZnO Nanoparticles on Biofilm Formation and Gene Expression of the Toxin-Antitoxin System in Clinical Isolates of Pseudomonas Aeruginosa. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 89. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.; Ren, L.; Tu, B.; Wang, W.; Fu, Z. Theoretical Study on Composition-Dependent Properties of ZnO·nAl2O3 Spinels. Part II: Mechanical and Thermophysical. J. Am. Ceram. Soc. 2021, 104, 6455–6466. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Liu, Y.; Zhang, G.; Zhu, P. Characterization and Functional Assessment of Alginate Fibers Prepared by Metal-Calcium Ion Complex Coagulation Bath. Carbohydr. Polym. 2020, 232, 115693. [Google Scholar] [CrossRef]
- Teerakanok, S.; Zhao, M.; Giordano, R.; Fan, Y. Interaction of Doped Magnesium, Zinc and Fluoride Ions on Hydroxyapatite Crystals Grown on Etched Human Enamel. J. Cryst. Growth 2021, 571, 126262. [Google Scholar] [CrossRef]
- Yang, X.; Xie, B.; Wang, L.; Qin, Y.; Henneman, Z.J.; Nancollas, G.H. Influence of Magnesium Ions and Amino Acids on the Nucleation and Growth of Hydroxyapatite. CrystEngComm 2011, 13, 1153–1158. [Google Scholar] [CrossRef]
- Alessandri Bonetti, G.; Pazzi, E.; Zanarini, M.; Marchionni, S.; Checchi, L. The Effect of Zinc-Carbonate Hydroxyapatite versus Fluoride on Enamel Surfaces after Interproximal Reduction. Scanning 2014, 36, 356–361. [Google Scholar] [CrossRef]
- Lelli, M.; Putignano, A.; Marchetti, M.; Foltran, I.; Mangani, F.; Procaccini, M.; Roveri, N.; Orsini, G. Remineralisation and Repair of Enamel Surface by Biomimetic Zn-Carbonate Hydroxyapatite Containing Toothpaste: A Comparative in Vivo Study. Front. Physiol. 2014, 5, 333. [Google Scholar] [CrossRef] [PubMed]
- Gani, M.A.; Lee, G.; Ardianto, C.; Rantam, F.A.; Lestari, M.L.A.D.; Addimaysqi, R.; Ketut Adnyana, I.; Lee, K.; Khotib, J. Comparative Study of Bovine and Synthetic Hydroxyapatite in Micro- and Nanosized on Osteoblasts Action and Bone Growth. PLoS ONE 2025, 20, e0311652. [Google Scholar] [CrossRef] [PubMed]
- de Mello Innocentini, M.D.; Fuzatto Bueno, B.R.; Urbaś, A.; Morawska-Chochół, A. Microstructural, Fluid Dynamic, and Mechanical Characterization of Zinc Oxide and Magnesium Chloride-Modified Hydrogel Scaffolds. ACS Biomater. Sci. Eng. 2024, 10, 4791–4801. [Google Scholar] [CrossRef] [PubMed]








| No | Code | Composition with wt.% |
|---|---|---|
| 1 | ALG_6H | alginate + 6% HAp |
| 2 | ALG_6H_4Mg | alginate + 6% HAp + 1.90% MgCl2 |
| 3 | ALG_6H_6Mg | alginate + 6% HAp + 2.88% MgCl2 |
| 4 | ALG_6H_4Mg_1nH | alginate + 6% HAp + 1.90% MgCl2 + 1% nHAp |
| 5 | ALG_6H_4Zn | alginate + 6% HAp + 4% ZnO |
| 1 | GEL_6H | gelatin + 6% HAp |
| 2 | GEL_6H_4Mg | gelatin + 6% HAp + 1.90% MgCl2 |
| 3 | GEL_6H_6Mg | gelatin + 6% HAp + 2.88% MgCl2 |
| 4 | GEL_6H_4Mg_1nH | gelatin + 6% HAp + 1.90% MgCl2 + 1% nHAp |
| 5 | GEL_6H_4Zn | gelatin + 6% HAp + 4% ZnO |
| 1 | ALG/GEL_6H | alginate/gelatin + 6% HAp |
| 2 | ALG/GEL_6H_4Mg | alginate/gelatin + 6% HAp + 1.90% MgCl2 |
| 3 | ALG/GEL_6H_6Mg | alginate/gelatin + 6% HAp + 2.88% MgCl2 |
| 4 | ALG/GEL_6H_4Mg_1nH | alginate/gelatin + 6% HAp + 1.90% MgCl2 + 1% nHAp |
| 5 | ALG/GEL_6H_4Zn | alginate/gelatin + 6% HAp + 4% ZnO |
| Matrix | Inhibition Zone [mm] |
|---|---|
| ALG_6H | 3.2 ± 0.3 |
| ALG_6H_6Mg | 3.0 ± 1.7 |
| ALG_6H_4Zn | 5.0 ± 0 |
| ALG/GEL_6H | 1.0 ± 0 |
| ALG/GEL_6H_6Mg | 0.6 ± 0.3 |
| ALG/GEL_6H_4Zn | 1.0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawska-Chochół, A.; Urbaś, A.; Reczyński, W.; Kwiecień, E.; Rzewuska, M. Effect of Zinc and Magnesium Compounds and Nano-Hydroxyapatite on the Physicochemical Properties and Biological Activity of Alginate and Gelatin Scaffolds for Osteochondral Defects. J. Funct. Biomater. 2025, 16, 300. https://doi.org/10.3390/jfb16080300
Morawska-Chochół A, Urbaś A, Reczyński W, Kwiecień E, Rzewuska M. Effect of Zinc and Magnesium Compounds and Nano-Hydroxyapatite on the Physicochemical Properties and Biological Activity of Alginate and Gelatin Scaffolds for Osteochondral Defects. Journal of Functional Biomaterials. 2025; 16(8):300. https://doi.org/10.3390/jfb16080300
Chicago/Turabian StyleMorawska-Chochół, Anna, Agnieszka Urbaś, Witold Reczyński, Ewelina Kwiecień, and Magdalena Rzewuska. 2025. "Effect of Zinc and Magnesium Compounds and Nano-Hydroxyapatite on the Physicochemical Properties and Biological Activity of Alginate and Gelatin Scaffolds for Osteochondral Defects" Journal of Functional Biomaterials 16, no. 8: 300. https://doi.org/10.3390/jfb16080300
APA StyleMorawska-Chochół, A., Urbaś, A., Reczyński, W., Kwiecień, E., & Rzewuska, M. (2025). Effect of Zinc and Magnesium Compounds and Nano-Hydroxyapatite on the Physicochemical Properties and Biological Activity of Alginate and Gelatin Scaffolds for Osteochondral Defects. Journal of Functional Biomaterials, 16(8), 300. https://doi.org/10.3390/jfb16080300









