Evaluation of Composites Comprising Spherical, Porous, Sintered β-Tricalcium Phosphate Particles and Cyanoacrylate as Bone Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Sintering of Materials
2.1.1. Preparation of Raw Powder
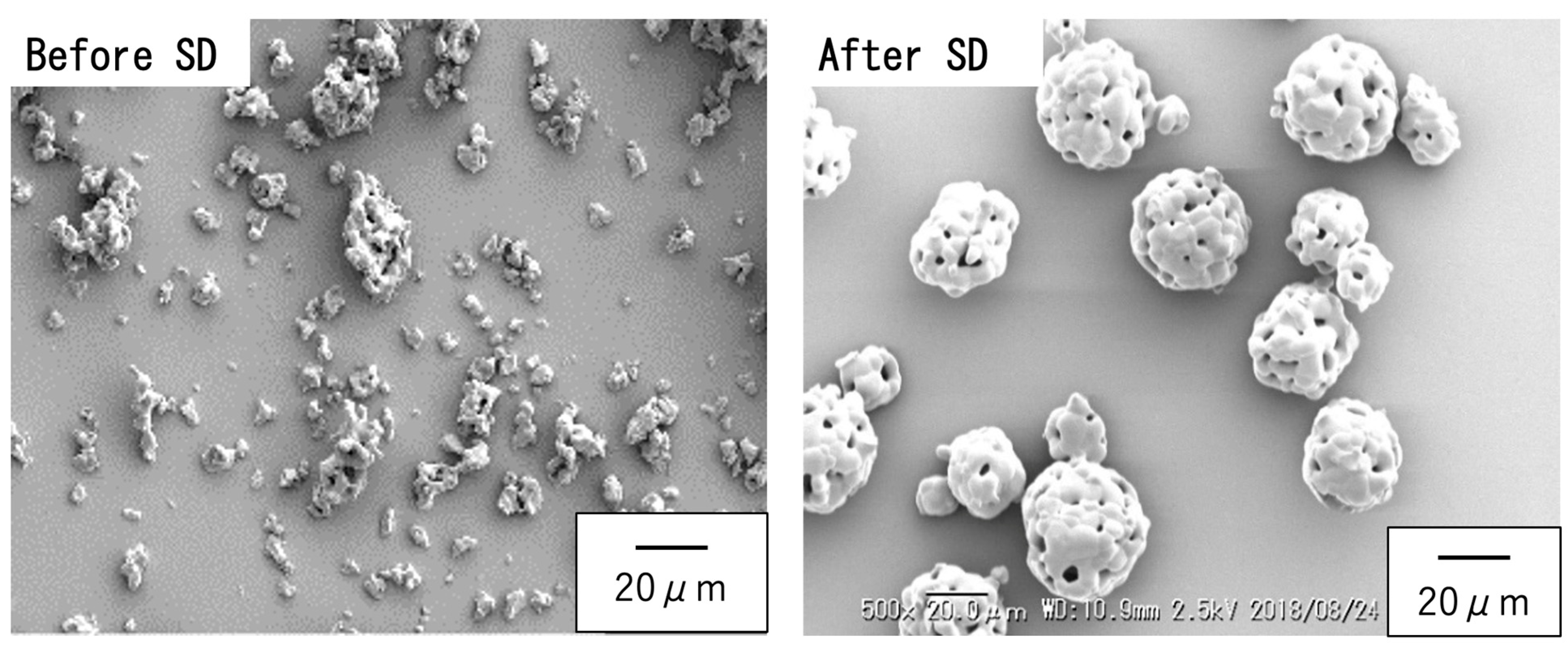
2.1.2. Preparation of Spherical Sintered Particles
2.2. Setting Tests
2.3. Semi-Quantification of Acid Sites on Sintered Particles
2.4. Water Stability of BC Paste
2.5. Mechanical Properties of Cured BC Specimens
2.6. Implant Fixation and Bone Grafting Evaluation
2.7. Tibial Tray Fixation Strength Comparison
2.8. Statistical Analysis
3. Results and Discussion
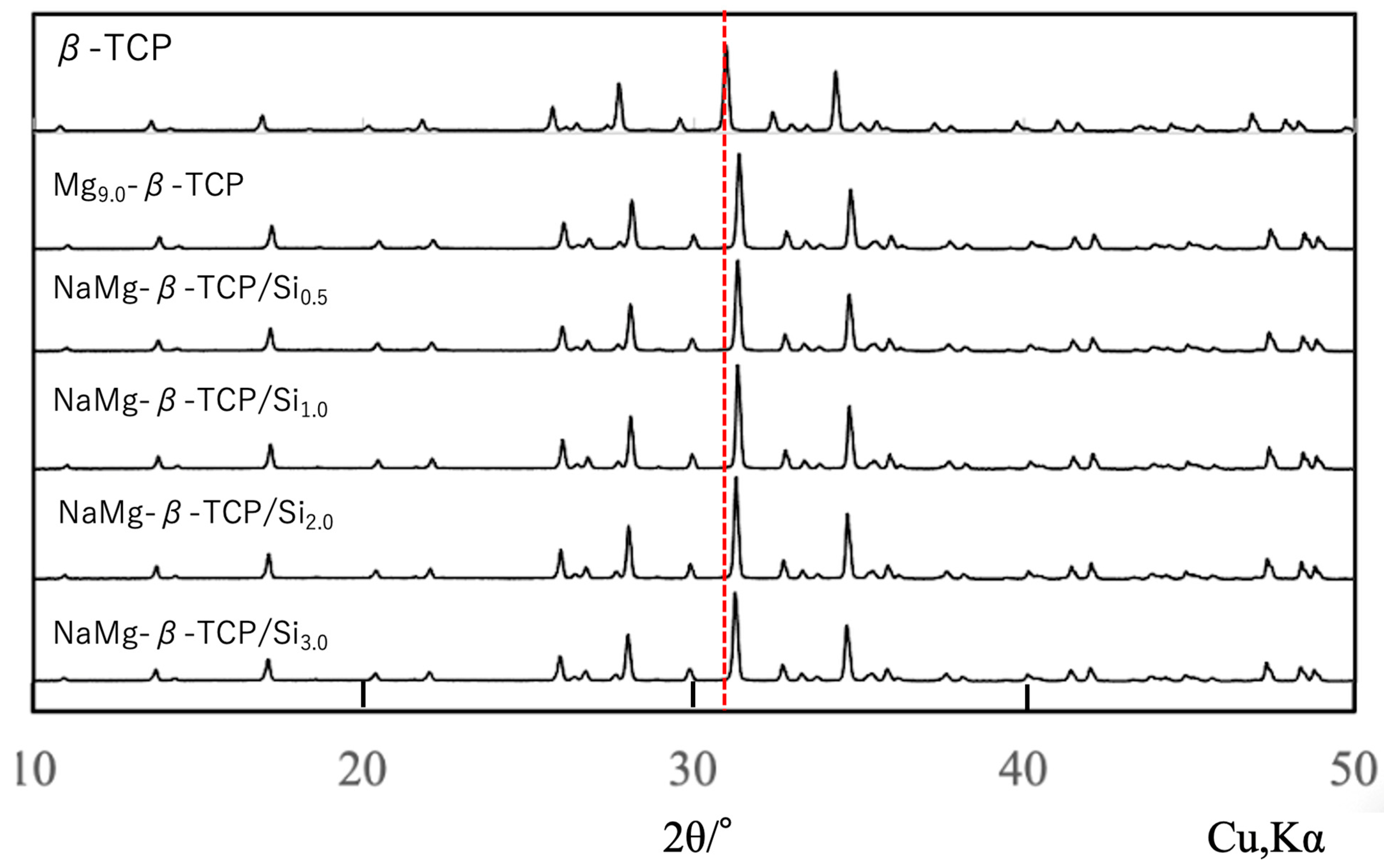
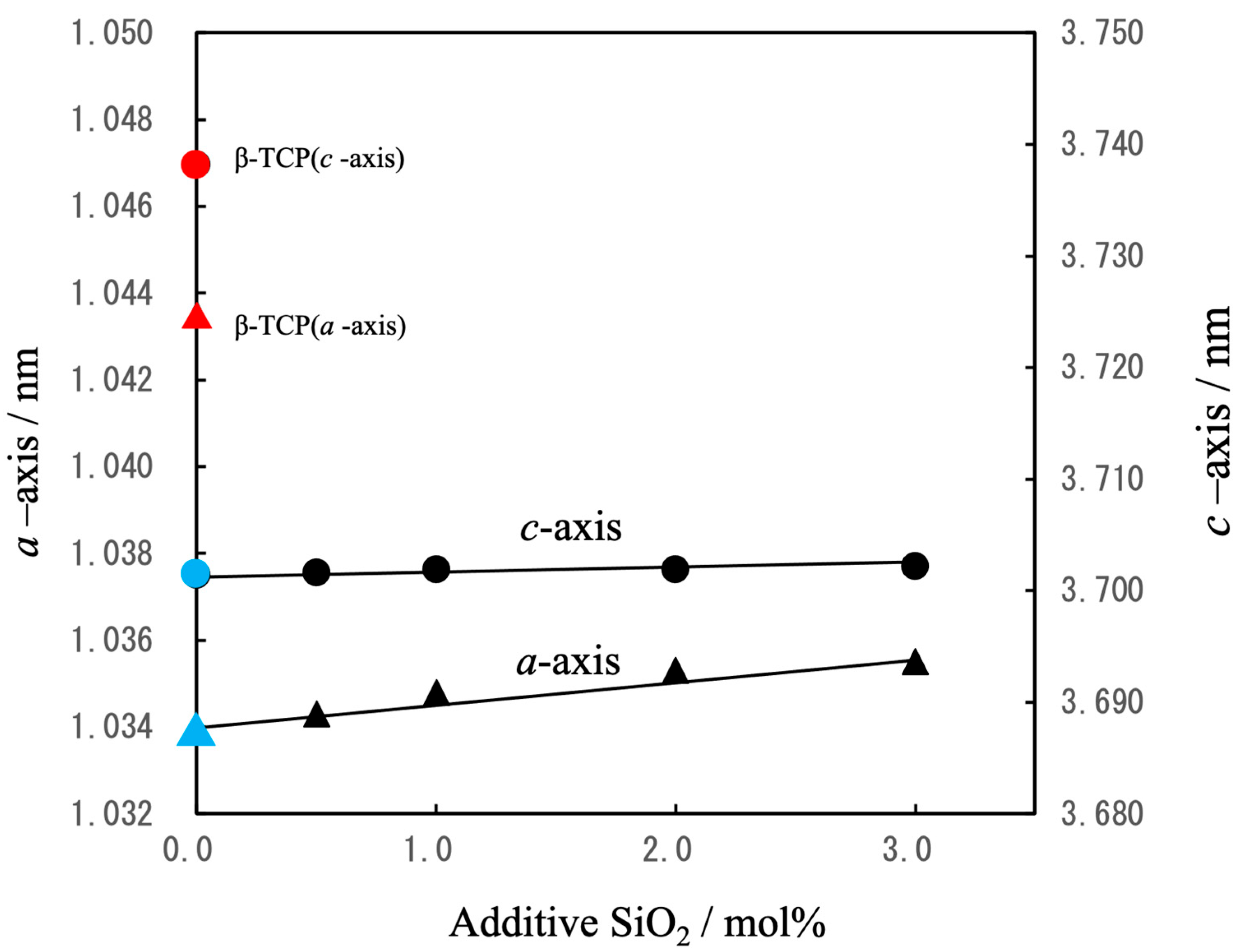
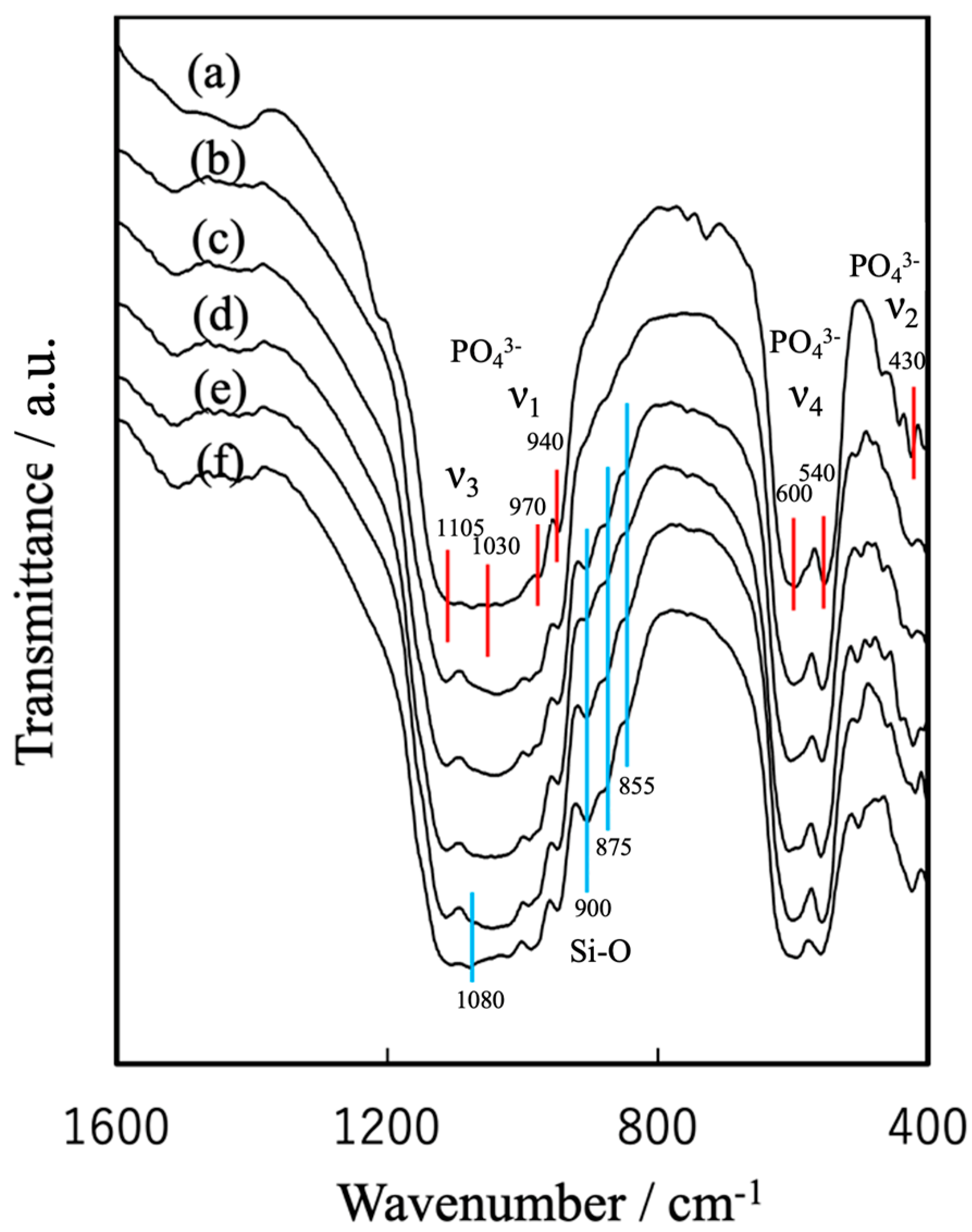
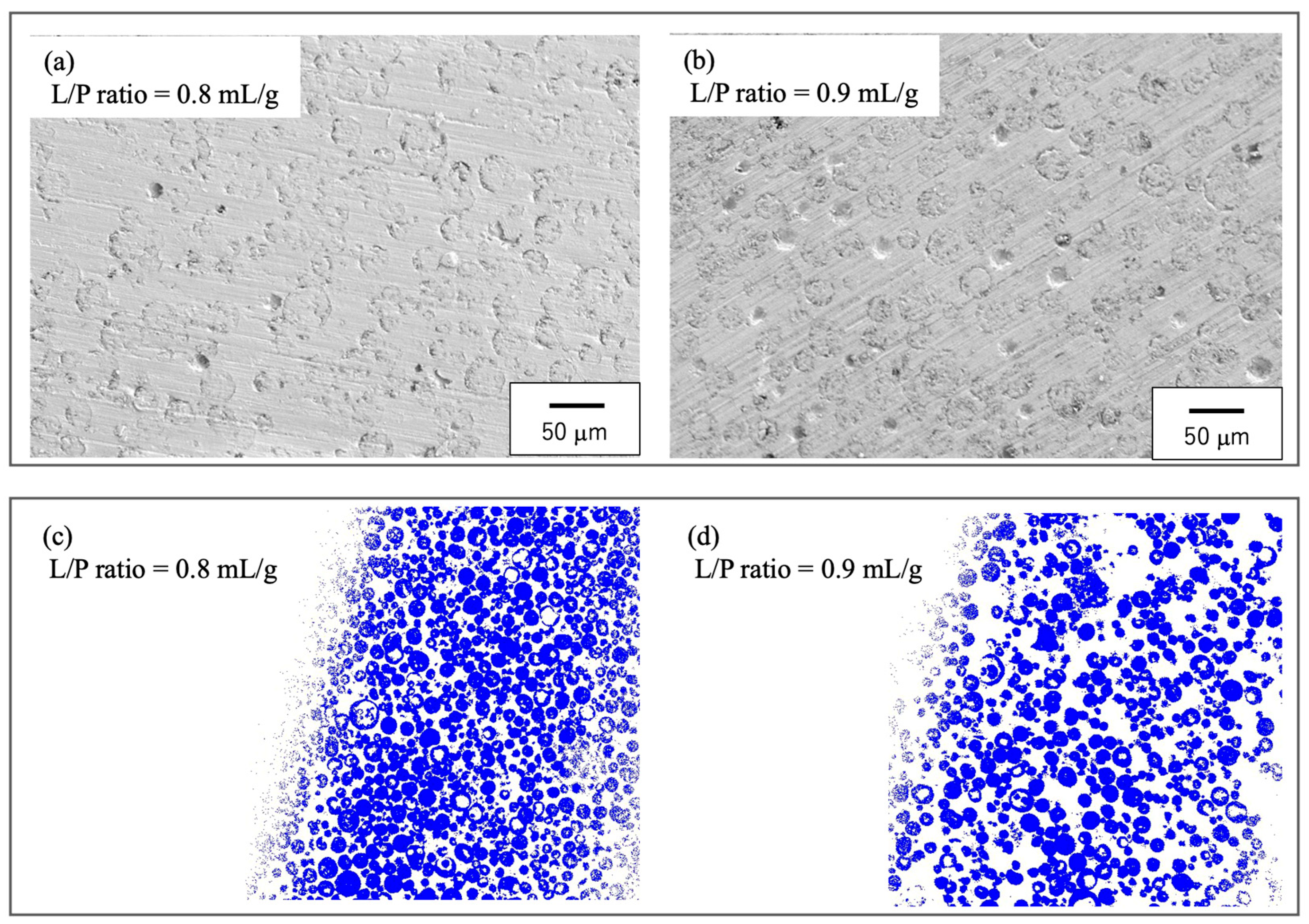
3.1. Preparation of Raw Materials for BC
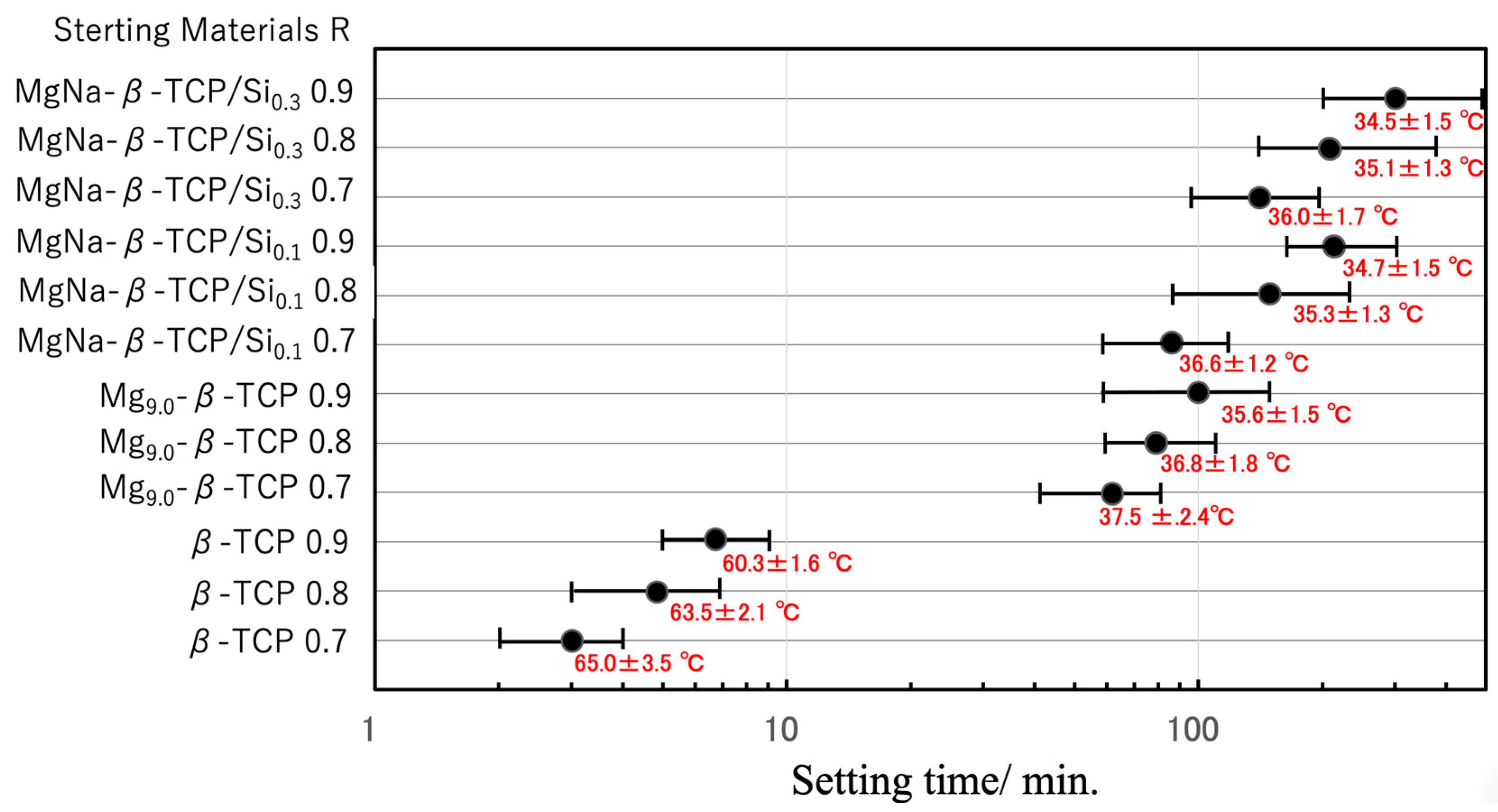
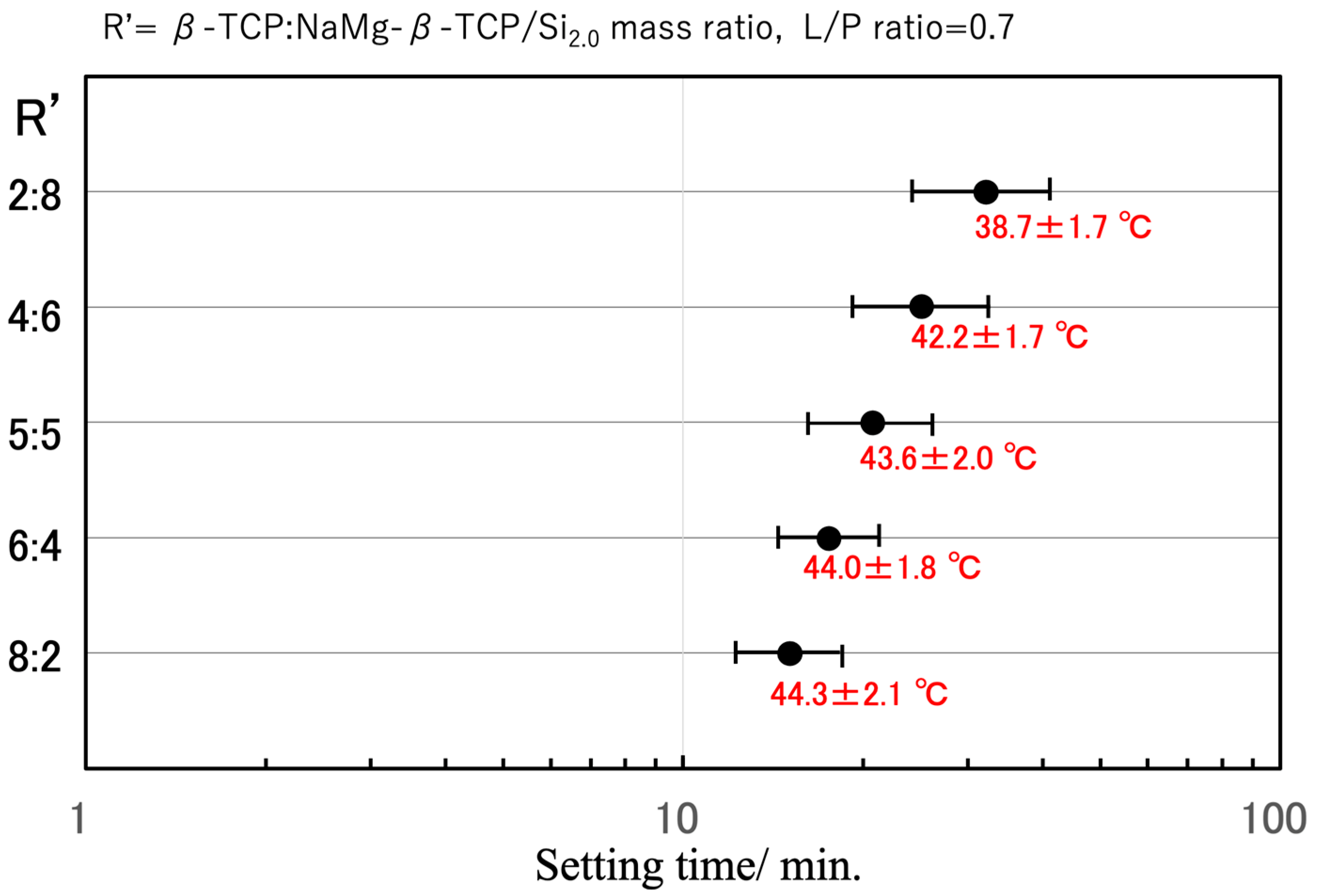
3.2. BC Setting Time and Peak Temperature
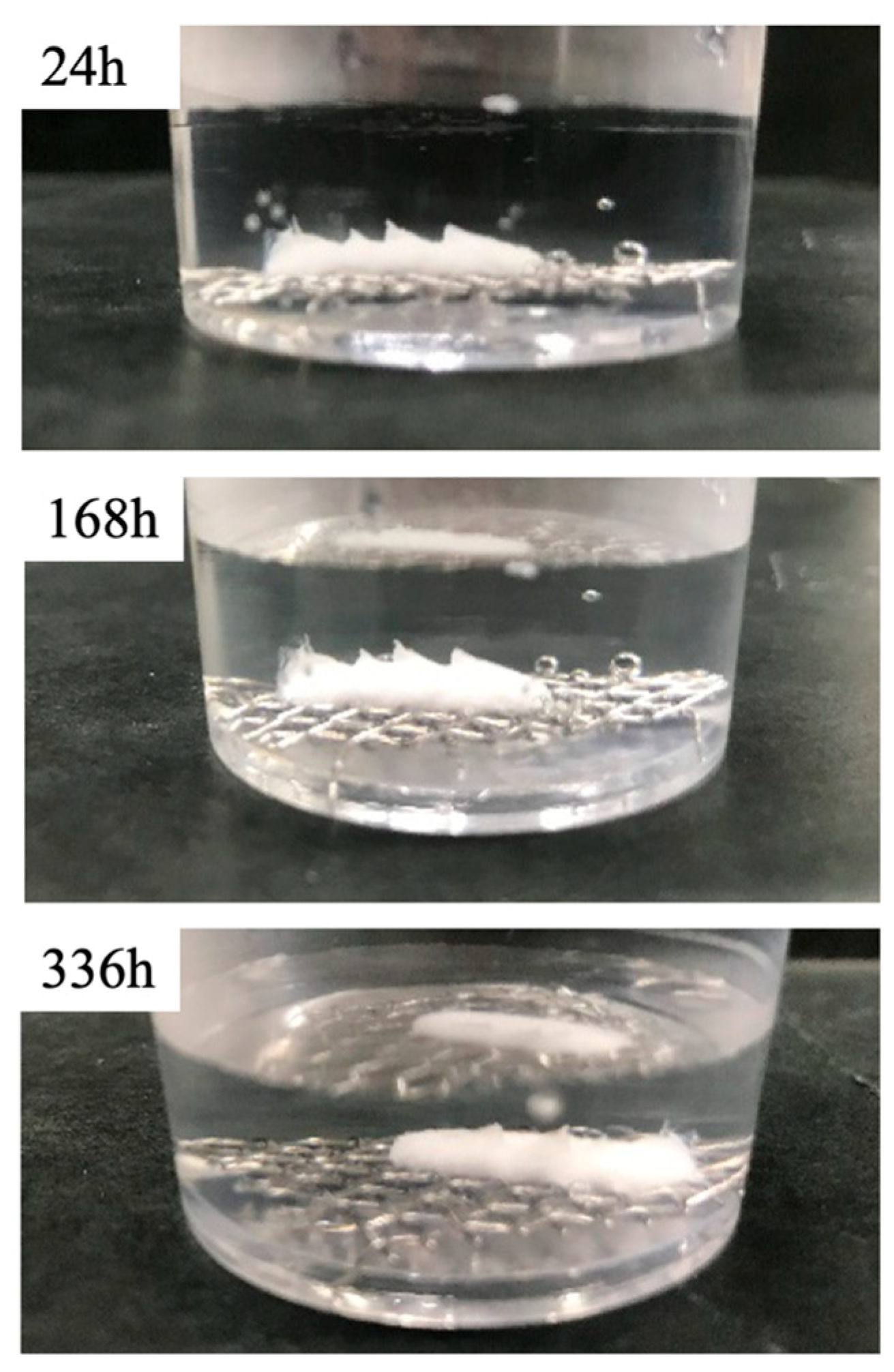
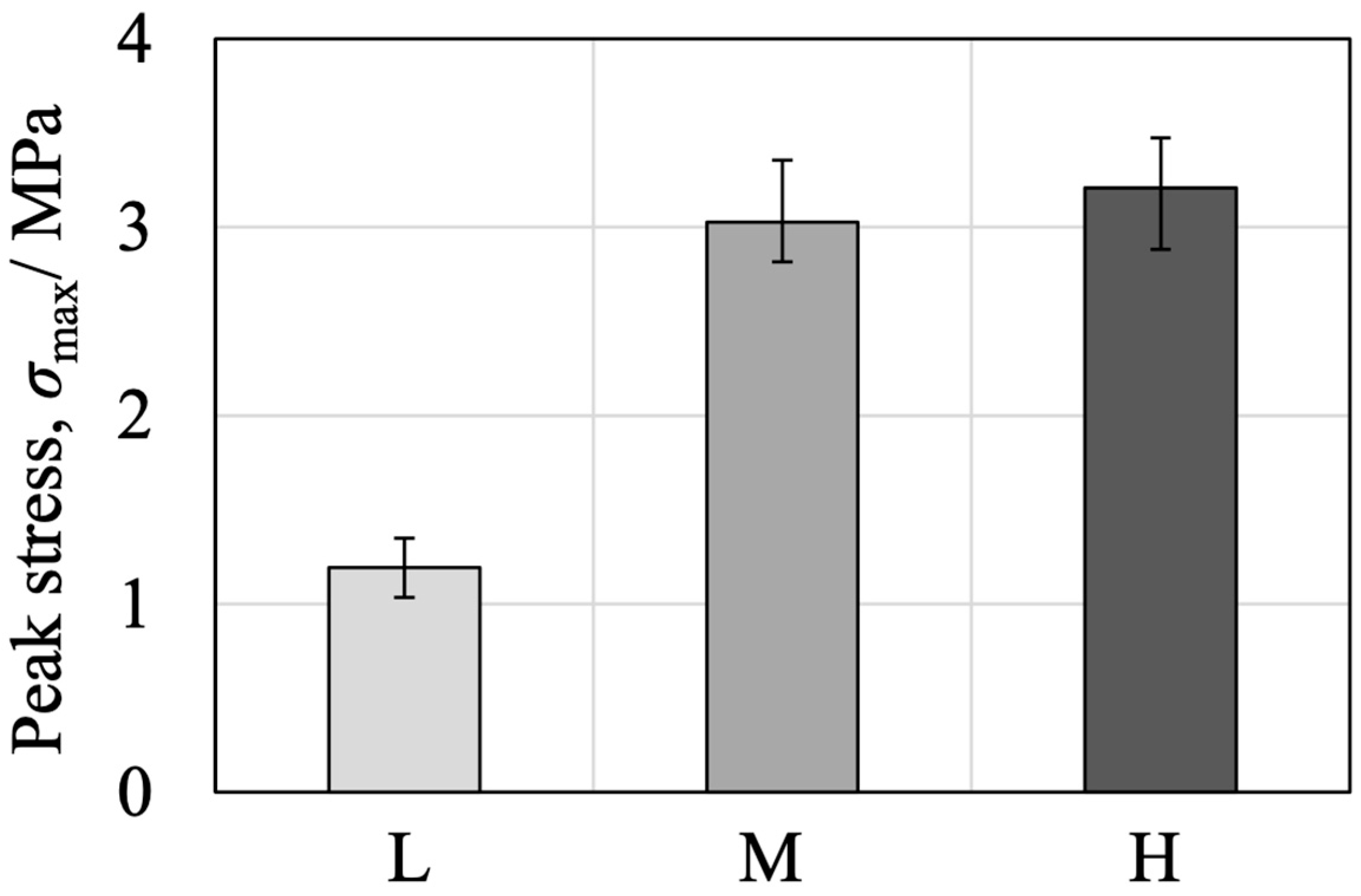
3.3. BC Disintegration Resistance and Injectability
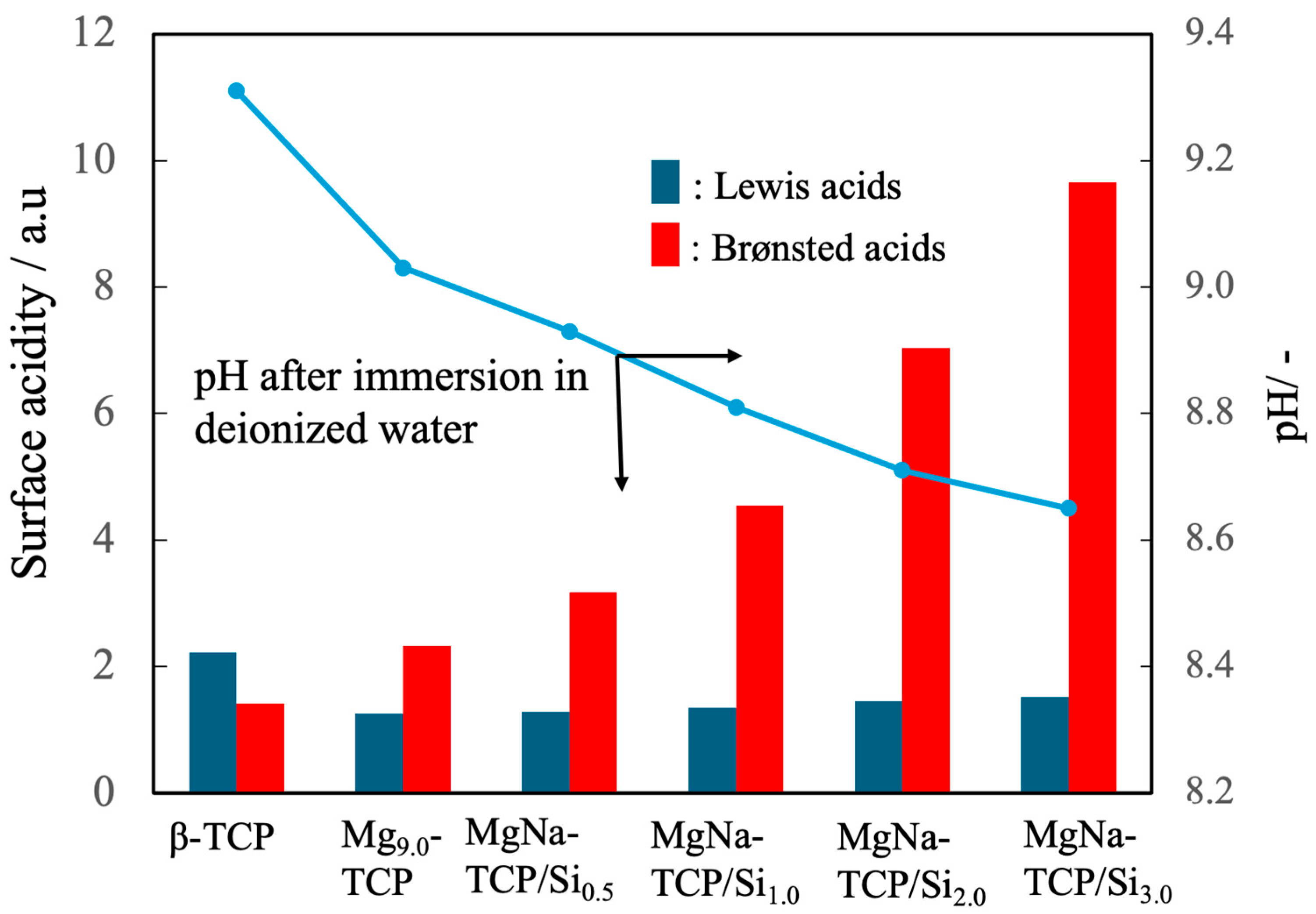
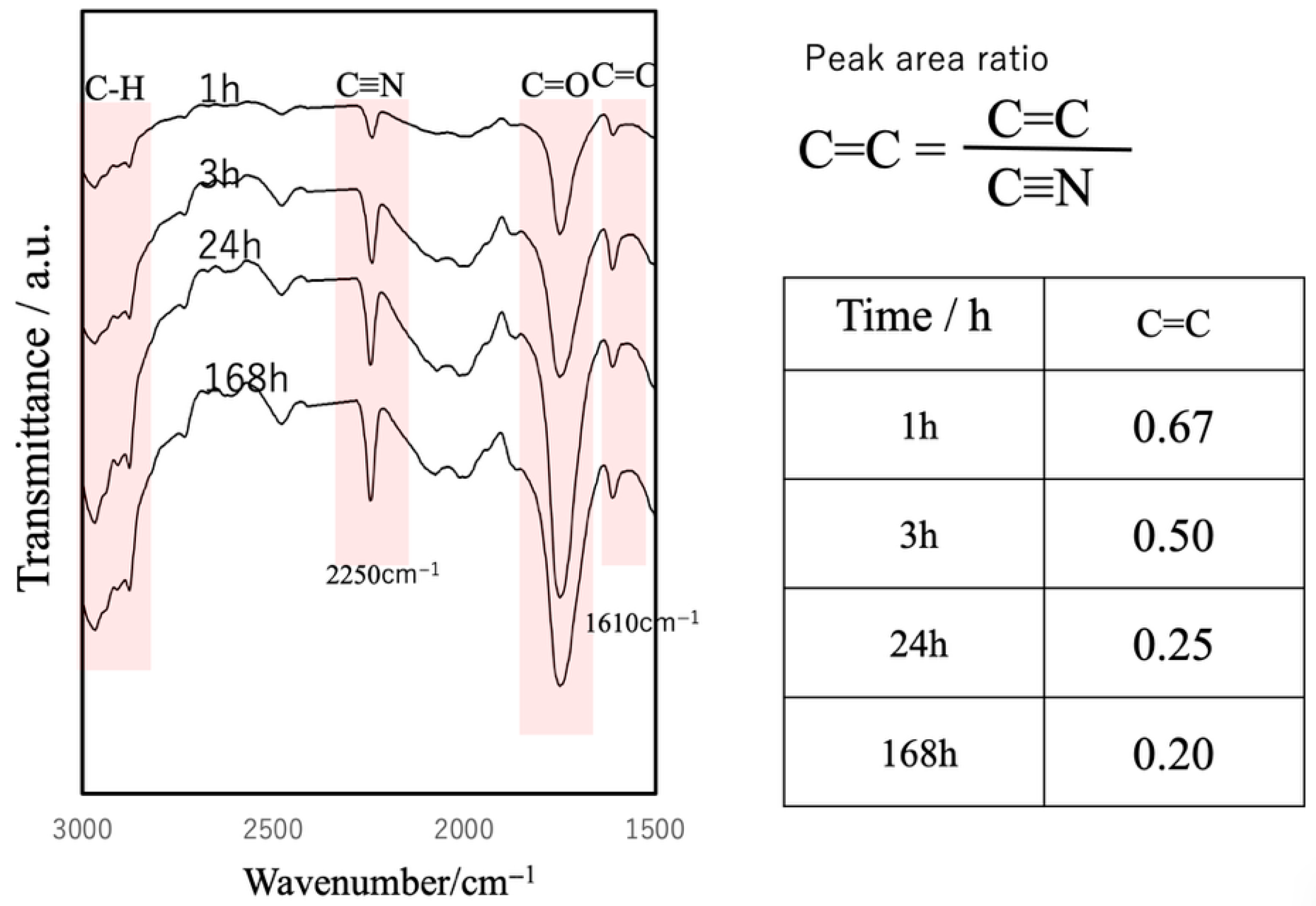
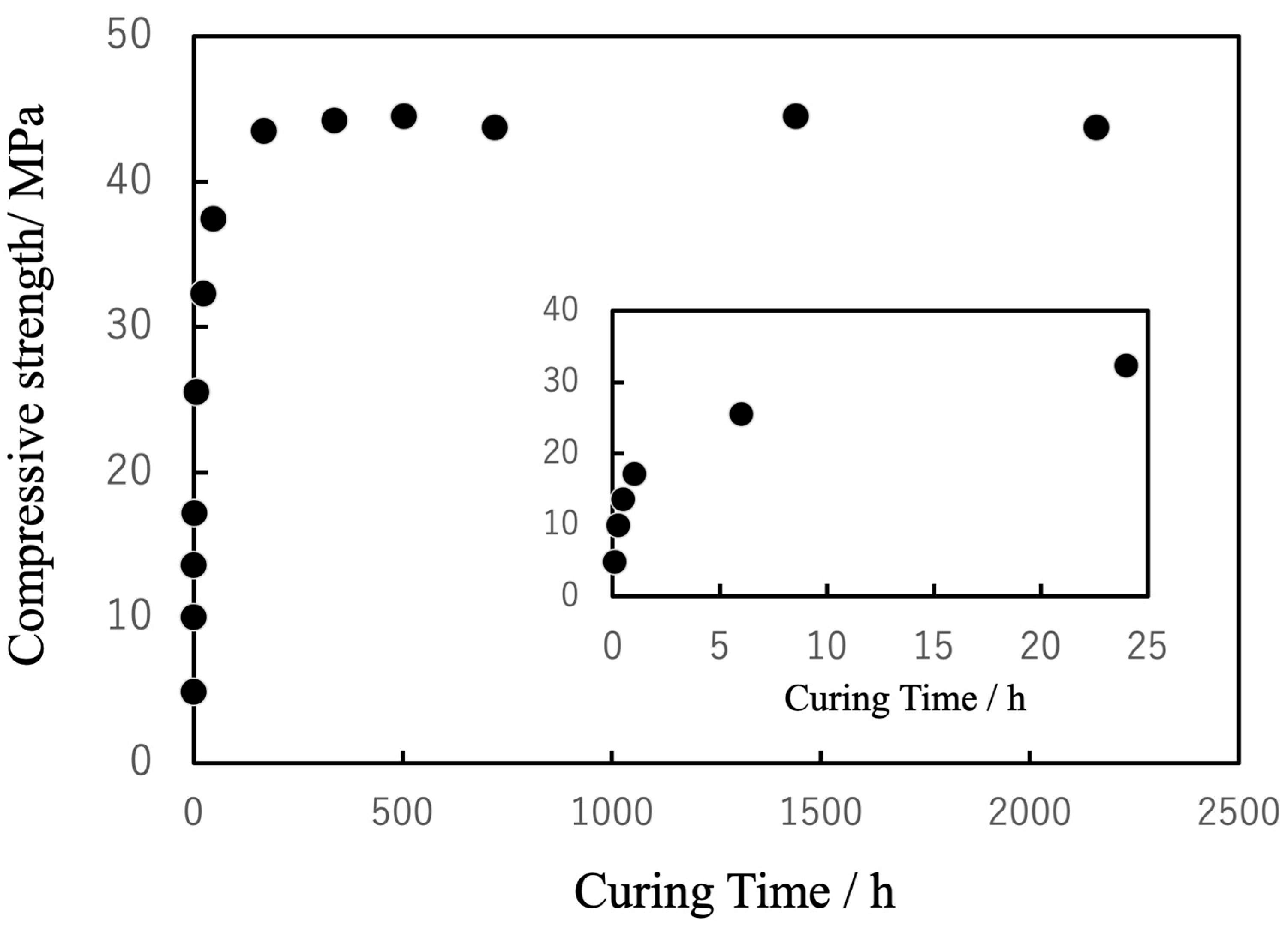
3.4. Curing Behavior of the Composite
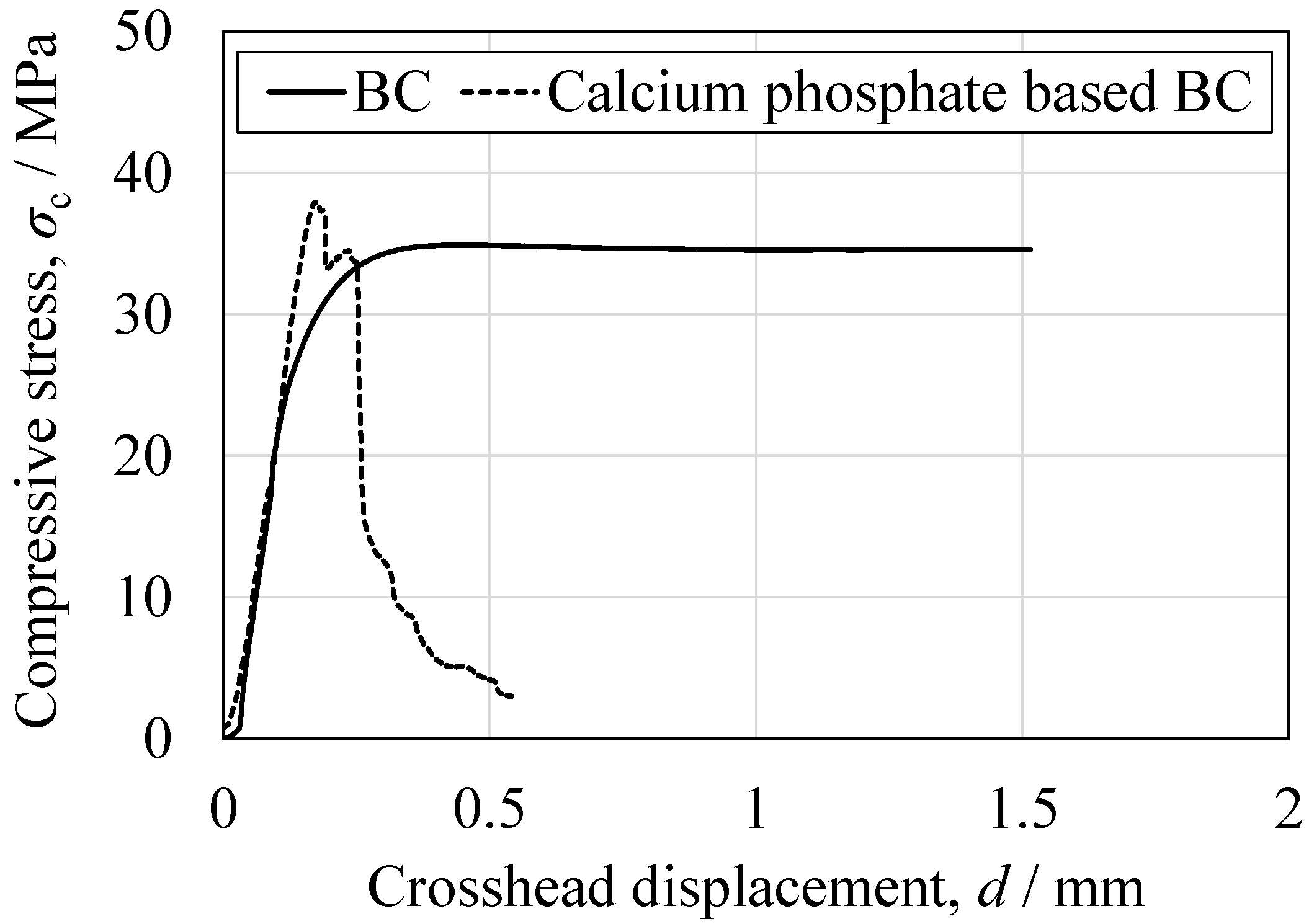
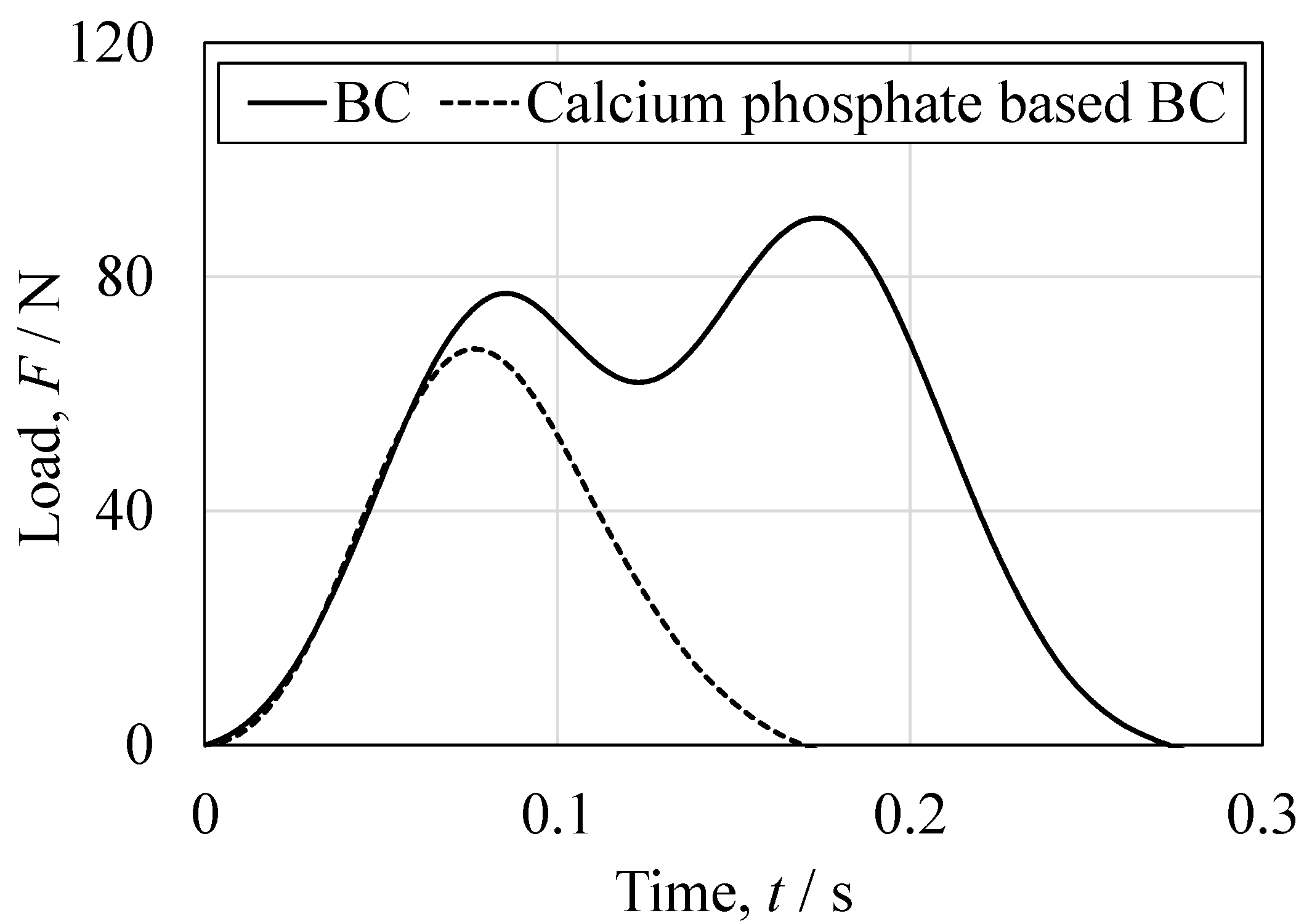
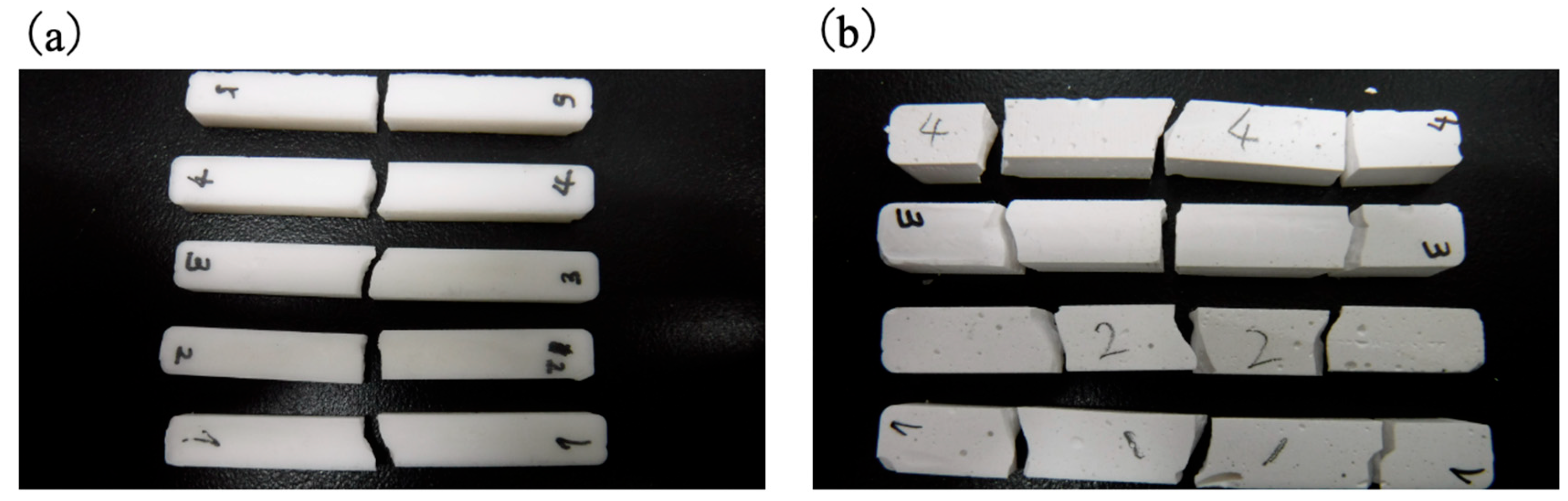
3.5. Mechanical Properties of Cured BC
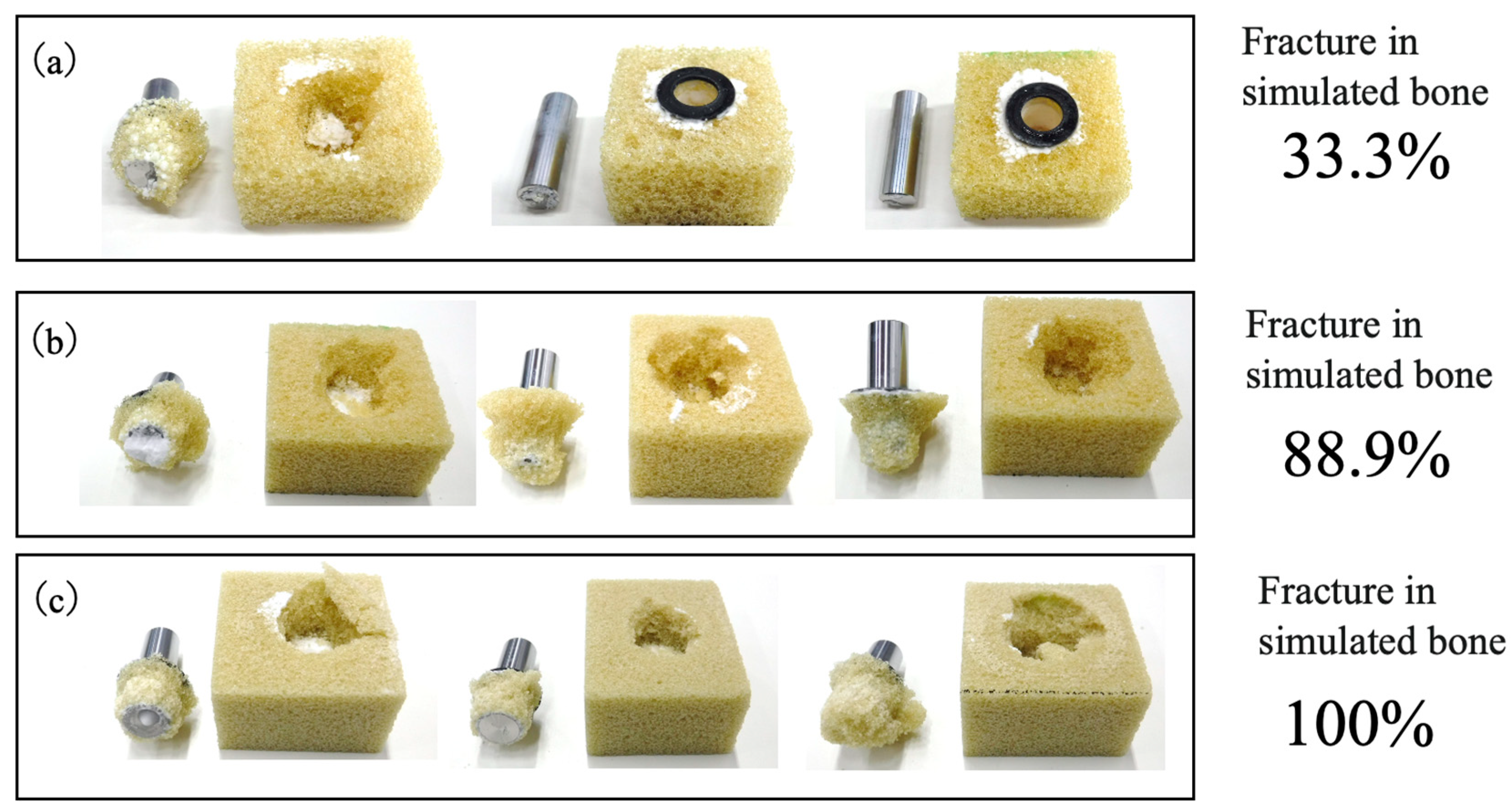
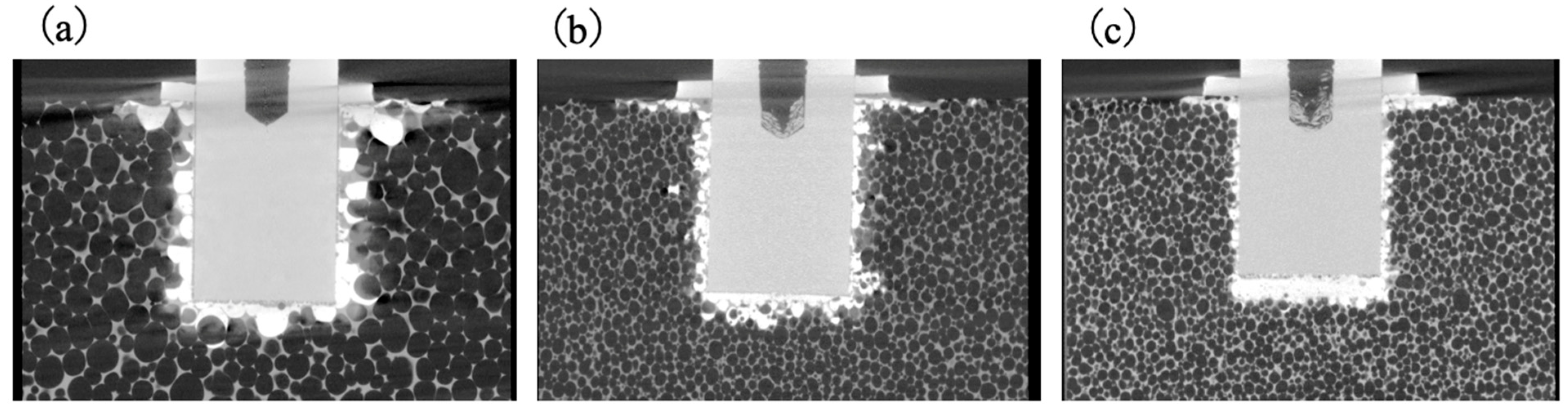
3.6. Pull-Out Strengths of Implant–Bone Complexes Fabricated Using Simulated Bone Blocks
3.7. Comparison of Fixation Strength in Tibial Tray–Simulated Bone Complexes Using Various Bone Substitute Materials
4. Conclusions
- Successful fabrication of an injectable β-TCP/cyanoacrylate composite bone cement.
- Ion substitution (Mg, Na, Si) enabled controlled cyanoacrylate polymerization, extending setting time and reducing exothermic heat.
- Excellent injectability and chemical stability suitable for minimally invasive procedures.
- Compressive strength exceeding cancellous bone, with enhanced toughness-related properties such as ductility, energy absorption, and impact resistance.
- Clear advantages over PMMA-based cements (lack of bioactivity, thermal risks) and CPCs (brittleness, limited injectability).
- Remaining limitations include insufficient tensile/fatigue performance and the need to clarify potential inflammatory responses associated with adhesive degradation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Vail, T.P.; Berry, D.J. The epidemiology of revision total hip arthroplasty in the United States. J. Bone Jt. Surg. Am. 2009, 91, 128–133. [Google Scholar] [CrossRef]
- Goodman, S.B.; Gallo, J. Periprosthetic osteolysis: Mechanisms, prevention and treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Berry, D.J. Epidemiology: Hip and knee. Orthop. Clin. N. Am. 1999, 30, 183–190. [Google Scholar] [CrossRef]
- Abdel, M.P.; Berry, D.J. Current practice trends in primary hip and knee arthroplasties among members of the American Association of Hip and Knee Surgeons: A long-term update. J. Arthroplast. 2019, 34, S24–S27. [Google Scholar] [CrossRef]
- Satalich, J.R.; Lombardo, D.J.; Newman, S.; Golladay, G.J.; Patel, N.K. Cementation in total hip arthroplasty: History, principles, and technique. EFORT Open Rev. 2022, 7, 747–757. [Google Scholar] [CrossRef]
- Emara, A.K.; Ng, M.; Krebs, V.E.; Bloomfield, M.; Molloy, R.M.; Piuzzi, N.S. Femoral stem cementation in hip arthroplasty: The know-how of a “Lost” Art. Curr. Rev. Musculoskelet. Med. 2021, 14, 47–59. [Google Scholar] [CrossRef]
- Giebel, G.; Hardt, S.; Perka, C.; Ascherl, R. The cemented stem in hip arthroplasty—State of the art technique and recommendations. EFORT Open Rev. 2024, 9, 1047–1059. [Google Scholar] [CrossRef]
- Sardar, I. Cemented total hip arthroplasty. In Complex Primary Total Hip Arthroplasty; Haidukewych, G., Berry, D.J., Eds.; Springer: Cham, Switzerland, 2024; pp. 123–136. [Google Scholar]
- Xará-Leite, F.; Pereira, A.D.; Andrade, R.; Sarmento, A.; Sousa, R.; Ayeni, O.R.; Espregueira-Mendes, J.; Soares, D. The cement-in-cement technique is a reliable option in hip arthroplasty revision surgery: A systematic review. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 7–22. [Google Scholar] [CrossRef]
- Gie, G.A.; Linder, L.; Ling, R.S.; Simon, J.P.; Slooff, T.J.; Timperley, A.J. Impacted cancellous allografts and cement for revision total hip arthroplasty. J. Bone Jt. Surg. Br. 1993, 75, 14–21. [Google Scholar] [CrossRef]
- Wahlig, H.; Dingeldein, E.; Bergmann, R.; Reuss, K. The release of gentamicin from polymethylmethacrylate beads. J. Bone Jt. Surg. Br. 1978, 60, 270–275. [Google Scholar] [CrossRef]
- Moojen, D.J.; Hentenaar, B.; Vogely, H.C.; Verbout, A.J.; Castelein, R.M.; Dhert, W.J. In vitro release of antibiotics from commercial PMMA beads and articulating hip spacers. J. Arthroplast. 2008, 23, 1152–1156. [Google Scholar] [CrossRef]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Burlet, N. Osteoporosis: A still increasing prevalence. Bone 2006, 38, S4–S9. [Google Scholar] [CrossRef]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Lindsay, R.; Silverman, S.L.; Cooper, C.; Hanley, D.A.; Barton, I.; Broy, S.B.; Licata, A.; Benhamou, L.; Geusens, P.; Flowers, K.; et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001, 285, 320–323. [Google Scholar] [CrossRef]
- Felsenberg, D.; Silman, A.J.; Lunt, M.; Armbrecht, G.; Ismail, A.A.; Finn, J.D.; Cockerill, W.C.; Banzer, D.; Benevolenskaya, L.I.; Bhalla, A.; et al. Incidence of vertebral fracture in Europe: Results from the European Prospective Osteoporosis Study (EPOS). J. Bone Miner. Res. 2002, 17, 716–724. [Google Scholar] [CrossRef]
- Klazen, C.A.; Lohle, P.N.; de Vries, J.; Jansen, F.H.; Tielbeek, A.V.; Blonk, M.C.; Venmans, A.; van Rooij, W.J.J.; Schoemaker, M.C.; Juttmann, J.R.; et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (VERTOS II): An open-label randomized trial. Lancet 2010, 376, 1085–1092. [Google Scholar] [CrossRef]
- Garfin, S.R.; Yuan, H.A.; Reiley, M.A. New technologies in spine: Kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 2001, 26, 1511–1515. [Google Scholar] [CrossRef]
- Lewis, G. Injectable bone cements for use in vertebroplasty and kyphoplasty: State-of-the-art review. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.D. Bone Cements: Up-to-Date Comparison of Physical and Chemical Properties of Commercial Materials; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular bone modulus–density relationships depend on anatomic site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef]
- Lewis, G. Properties of acrylic bone cement: State of the art review. J. Biomed. Mater. Res. 1997, 38, 155–182. [Google Scholar] [CrossRef]
- Webb, J.C.; Spencer, R.F. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J. Bone Jt. Surg. Br. 2007, 89, 851–857. [Google Scholar] [CrossRef]
- Constantz, B.R.; Ison, I.C.; Fulmer, M.T.; Poser, R.D.; Smith, S.T.; Van Wagoner, M.; Ross, J.; Goldstein, S.A.; Jupiter, J.B.; Rosenthal, D.I. Skeletal repair by in situ formation of the mineral phase of bone. Science 1995, 267, 1796–1799. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31, D37–D47. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Chow, L.C.; Takagi, S. A natural bone cement—A laboratory novelty led to the development of revolutionary new biomaterials. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1029–1033. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Rilliard, A.; Fernández, E.; Elvira, C.; San Román, J.; Planell, J.A. Mechanical and rheological improvement of calcium phosphate cements by the addition of a polymeric drug. J. Biomed. Mater. Res. 2001, 57, 113–118. [Google Scholar] [CrossRef]
- Wang, X.-H.; Jia, S.-J.; Hao, D.-J. Advances in the modification of injectable calcium-phosphate-based bone cements for clinical application. Chin. Med. J. 2020, 133, 2610–2612. [Google Scholar] [CrossRef]
- Wong, S.K.; Wong, Y.H.; Chin, K.-Y.; Ima-Nirwana, S. A review on the enhancement of calcium phosphate cement with biological materials in bone defect healing. Polymers 2021, 13, 3075. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, J.; Fan, P.; Zhao, Z.; Deng, H.; Li, J.; Wang, Y.; Wang, Y. Biomaterial-based strategies for bone cement: Modulating the bone microenvironment and promoting regeneration. J. Nanobiotechnol. 2025, 23, 343. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Ohtsuki, C.; Miyazaki, T. Behavior of ceramic biomaterials derived from tricalcium phosphate in physiological condition. J. Biomater. Appl. 2008, 23, 197–212. [Google Scholar] [CrossRef]
- Hashimoto, K.; Imai, T.; Shibata, H. Preparation of silicon-substituted beta-tricalcium phosphate by the polymerized complex method. Phosphorus Res. Bull. 2023, 39, 14–22. [Google Scholar] [CrossRef]
- Grupp, T.M.; Schilling, C.; Schwiesau, J.; Pfaff, A.; Altermann, B.; Mihalko, W.M. Tibial implant fixation behavior in total knee arthroplasty: A study with five different bone cements. J. Arthroplast. 2020, 35, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Dickens, B.; Schroeder, L.W.; Brown, W.E. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2. The crystal structure of pure β-Ca3(PO4)2. J. Solid State Chem. 1974, 10, 232–248. [Google Scholar] [CrossRef]
- Massit, A.; Fathi, M.; El Yacoubi, A.; El Youbi, M.S.; El Idrissi, B.C. Structural properties analysis of Mg-β-TCP by X-ray powder diffraction with Rietveld refinement. Lett. Appl. NanoBioSci. 2020, 9, 1562–1568. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Jillavenkatesa, A.; Condrate, R.A.S. The infrared and Raman spectra of β- and α-tricalcium phosphate (Ca3(PO4)2). Spectrosc. Lett. 1998, 31, 1619–1634. [Google Scholar] [CrossRef]
- Raja, P.R. Cyanoacrylate adhesives: A critical review. Rev. Adhes. Adhes. 2016, 4, 398–416. [Google Scholar] [CrossRef]
- Klemarczyk, P.; Guthrie, J. Advances in anaerobic and cyanoacrylate adhesives. In Advances in Structural Adhesive Bonding; Dillard, D.A., Ed.; Woodhead Publishing: Cambridge, UK, 2010; pp. 96–131. [Google Scholar]
- Wekwejt, M.; Jesiołkiewicz, R.; Mielewczyk-Gryń, A.; Kozień, D.; Ronowska, A.; Kozłowska, J.; Gbureck, U. Injectable bone cement based on magnesium potassium phosphate and cross-linked alginate hydrogel designed for minimally invasive orthopedic procedures. Sci. Rep. 2024, 14, 20279. [Google Scholar] [CrossRef] [PubMed]
- ISO 5833:2002; Implants for Surgery—Acrylic Resin Cements. International Organization for Standardization (ISO): Geneva, Switzerland, 2002.
- Augat, P.; Claes, L. Mechanical characteristics of bone and bone substitute materials. Injury 2006, 37 (Suppl. S2), S31–S35. [Google Scholar]
- Bercier, A.; Gonçalves, S.; Lignon, O.; Fitremann, J. Calcium phosphate bone cements including sugar surfactants: Part one—Porosity, setting times and compressive strength. Materials 2010, 3, 4695–4709. [Google Scholar] [CrossRef]
- Robo, C.; Öhman-Mägi, C.; Persson, C. Fatigue behaviour of low-modulus bone cements under cyclic loading. J. Mech. Behav. Biomed. Mater. 2021, 123, 104764. [Google Scholar] [CrossRef]
- Anderson, T.L. Fracture Mechanics: Fundamentals and Applications, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Yousefi, A.-M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, Article ID 2280800019872594. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Yang, X.; Zou, W.; Chen, X.; Deng, H.; Zhang, Q.; Yan, Y. A Bioactive Degradable Composite Bone Cement Based on Calcium Sulfate and Magnesium Polyphosphate. Materials 2024, 17, 1861. [Google Scholar] [CrossRef]
- Abd El-Hamid, H.K.; Farag, M.M.; Abdelraof, M.; Elwan, R.L. Regulation of the antibiotic elution profile from tricalcium phosphate bone cement by addition of bioactive glass. Sci. Rep. 2024, 14, 2804. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Wu, Y.; Li, M.; Xie, H.; Shen, B. A comprehensive comparison between cementless and cemented fixation in the total knee arthroplasty: An updated systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 176. [Google Scholar] [CrossRef]
- Moya-Angeler, J.; Akkaya, M.; Innocenti, M.; Bergadano, D.; Martin-Alguacil, J.; León-Muñoz, V. Fixation options for total knee arthroplasty: A comprehensive literature review. J. Orthop. Surg. Res. 2024, 19, 463. [Google Scholar] [CrossRef]
- Toksvig-Larsen, S.; Ryd, L.; Lindstrand, A. Fixation of total knee arthroplasties: A comparison between cemented and uncemented fixation. J. Arthroplast. 1995, 10, 217–222. [Google Scholar]
- Wilczyński, M.; Bieniek, M.; Krakowski, P.; Karpiński, R. Cemented vs. cementless fixation in primary knee replacement: A narrative review. Materials 2024, 17, 1136. [Google Scholar] [CrossRef]




| Sample | Blending Ratio | Chemical Formula | ||||||
|---|---|---|---|---|---|---|---|---|
| CaCO3 /mol | (NH4)2HPO4 /mol | NaNO3 /mol | MgO /mol | SiO2 /mol | Vacancy:□ /mol | (Ca + Na + Mg + □)/(P + S) Molar Ratio | ||
| Β-TCP | 3.0000 | 2.0000 | - | - | - | - (0.1429) | 1.50 (1.571) | β-Ca3(PO4)2 (β-Ca21.0□1.0(PO4)14) |
| Mg9.0-β-TCP | 2.7143 | 2.000 | - | 0.2857 | - | 0.1429 | 1.571 | Ca19.0Mg2.0□1.0(PO4)14.0 |
| NaMg-β-TCP/Si0.5 | 2.7143 | 1.9900 | 0.010 | 0.2857 | 0.010 | 0.1419 | 1.571 | Ca19.0Mg2.0Na0.07□0.93(PO4)13.93(SiO4)0.07 |
| NaMg-β-TCP/Si1.0 | 2.7143 | 1.9800 | 0.020 | 0.2857 | 0.020 | 0.1409 | 1.571 | Ca19.0Mg2.0Na0.14□0.86(PO4)13.86(SiO4)0.14 |
| NaMg-β-TCP/Si2.0 | 2.7143 | 1.9600 | 0.040 | 0.2857 | 0.040 | 0.1389 | 1.571 | Ca19.0Mg2.0Na0.28□0.72(PO4)13.72(SiO4)0.28 |
| NaMg-β-TCP/Si3.0 | 2.7143 | 1.9400 | 0.060 | 0.2857 | 0.060 | 0.1369 | 1.571 | Ca19.0Mg2.0Na0.42□0.68(PO4)13.58(SiO4)0.42 |
| Test Method | Mechanical Properties | Results (Mean ± SD) | p-Value (Welch’s t-Test) Significance Level α = 0.05 | |
|---|---|---|---|---|
| BC | Calcium Phosphate Based BC | |||
| Tensile | Peak tensile stress (MPa) | 10.2 ± 1.2 | ― | ― |
| Elastic modulus (MPa) | 4787 ± 590 | ― | ― | |
| Compression | Peak compressive stress (MPa) | 36.0 ± 1.4 | 35.4 ± 9.6 | 0.893 |
| Elastic modulus (MPa) | 3448 ± 390 | 4590 ± 459 | 0.017 | |
| Bending | Peak bending stress (MPa) | 29.3 ± 8.5 | 9.29 ± 3.1 | 0.004 |
| Elastic modulus (MPa) | 2169 ± 287 | 1353 ± 413 | 0.008 | |
| Torsion | Peak shear stress (MPa) | 17.5 ± 3.3 | ― | ― |
| Fatigue | fatigue life at 5 million cycles (MPa) | 3.00 | ― | ― |
| Type of Fixation | Fixation Load (Mean ± SD) | p-Value (Welch’s t-Test) Significance Level α = 0.05 | ||
|---|---|---|---|---|
| BC | PMMA-Based BC | Calcium Phosphate Based BC | ||
| Cemented fixation | 4.57 ± 0.92 | 6.42 ± 0.57 | ― | 0.006 |
| Cementless fixation | 6.14 ± 0.43 | ― | 2.28 ± 0.24 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, K.; Aida, S.; Takigawa, I.; Shibata, H.; Kobune, S.; Konishi, T.; Meguro, T.; Fukuyama, S.; Tanaka, S. Evaluation of Composites Comprising Spherical, Porous, Sintered β-Tricalcium Phosphate Particles and Cyanoacrylate as Bone Cement. J. Funct. Biomater. 2025, 16, 458. https://doi.org/10.3390/jfb16120458
Hashimoto K, Aida S, Takigawa I, Shibata H, Kobune S, Konishi T, Meguro T, Fukuyama S, Tanaka S. Evaluation of Composites Comprising Spherical, Porous, Sintered β-Tricalcium Phosphate Particles and Cyanoacrylate as Bone Cement. Journal of Functional Biomaterials. 2025; 16(12):458. https://doi.org/10.3390/jfb16120458
Chicago/Turabian StyleHashimoto, Kazuaki, Shuhei Aida, Iori Takigawa, Hirobumi Shibata, Satoshi Kobune, Toshiisa Konishi, Takashi Meguro, Shigeo Fukuyama, and Shinya Tanaka. 2025. "Evaluation of Composites Comprising Spherical, Porous, Sintered β-Tricalcium Phosphate Particles and Cyanoacrylate as Bone Cement" Journal of Functional Biomaterials 16, no. 12: 458. https://doi.org/10.3390/jfb16120458
APA StyleHashimoto, K., Aida, S., Takigawa, I., Shibata, H., Kobune, S., Konishi, T., Meguro, T., Fukuyama, S., & Tanaka, S. (2025). Evaluation of Composites Comprising Spherical, Porous, Sintered β-Tricalcium Phosphate Particles and Cyanoacrylate as Bone Cement. Journal of Functional Biomaterials, 16(12), 458. https://doi.org/10.3390/jfb16120458





