Abstract
Cigarette butts (CBs) are among the dominant constituents of marine and beach litter. Few studies have been conducted, and the environmental effects of CBs on marine species are still poorly understood. This study aims to evaluate the ecotoxicological effects on marine organisms of both classic and electronic CBs. Three representative species of different trophic levels in marine ecosystems (Aliivibrio fischeri, bacteria; Phaeodactylum tricornutum, algae, primary producers; Paracentrotus lividus, echinoderms, consumers) were tested. The effects of natural ageing of CBs due to exposure to atmospheric conditions (natural sunlight vs. simulated rain) and for different times (1 vs. 2 weeks) were evaluated. The results were weighted together to obtain a synthetic hazard level to the environment (Class of Hazard) from Sediqualsoft®. Classic CBs (CCBs) performed the worst and posed a mild to moderate risk compared to electronic CBs (absent Class of Hazard). Smoked classic CBs posed a higher environmental risk than unsmoked. The highest risk was produced by classic CBs after one week of exposure in dry weather. Echinoderms and the body size reduction in normo-formed (72 h) plutei were shown to be the more sensitive organism and endpoint, respectively. We recommend the use of Sediqualsoft® software for risk assessment studies of sediments contaminated with contaminants of various types, especially in conjunction with a weight of evidence approach (WOE).
1. Introduction
Marine litter is defined as “any persistent, manufactured or processed material discarded, disposed of or abandoned in the marine and coastal environment” [1]. Since the first records of entanglement and ingestion of plastic items in the 1960s [2], marine litter went from being treated as a curiosity, to posing a risk to marine ecosystems [3] because of its ubiquity, persistence, and ability to interact with biota. The monitoring campaigns of litter accumulated along the coasts (also known as beach litter) were originally designed to raise public awareness. Over a thirty-year period, they have evolved into a monitoring tool to obtain an assessment of the extent of the problem [4]. To date, several studies have analysed the problem from the point of view of composition, density, and possible sources.
Regardless of the category of litter (cigarette butts, CBs, are classified as “plastic”, “isolated category”, or even “paper/cardboard” in the case of the Italian protocol; [5]), CBs represent a consistent component of marine litter. In 2019, the International Coastal Clean-up (ICC) world campaign collected a total of 32,485,488 litter items, of which 4,211,962 were cigarette butts (CBs), representing the second most abundant litter item after the category “food wrappers” [6]. Cigarette butts earn the first place in the top 10 list of most frequently collected items during beach clean-ups realized by volunteers [7]. Their relative abundance within marine litter on continental coasts is extremely variable and capable of reaching values >40% [8,9,10,11,12]. Many factors contribute to the transport and presence of CBs on beaches, including natural factors (prevalent wind, currents, rivers) and human aspects, such as the behaviour of smokers in public places near and on the beach itself, the density of and proximity to high population urban areas, and the frequency and efficacy of public cleaning services [13,14]. In addition to this, local authority clean-up efforts are quite successful at collecting larger pieces of beach litter (as reported in the study performed on Cyprus island; [15]); however, smaller pieces, such as cigarette butts and other plastic items related to recreational activities, may remain on the beaches or become a potential source of marine litter [15]. In the first case, they accumulate and become an integral part of the beach system: they can be buried, remain exposed to solar radiation, or encounter the seawater and rain, thus potentially releasing the large number of chemical substances known to be present in cigarettes.
This study focused on CB toxicity, simulating contamination from two different environmental matrices: marine water and beach sediment. In the first scenario, the toxicity of leachates from cigarette butts derived from traditional (CCBs) and electronic cigarettes (ECs) was investigated and compared. The experiment was conducted with both smoked (S-) and unsmoked (U-) cigarettes to investigate the role of combustion in determining toxicity. From the perspective of traditional cigarettes, many chemicals (including fungicides, herbicides, insecticides, and pesticides) are known to be used in the cultivation and processing of tobacco and in the manufacture of cigarettes [16]. As a result, over 5000 compounds are present in cigarettes. Of these, at least 150 (44 of which are in large quantities) are considered highly toxic, mainly because of their carcinogenic and mutagenic potential [17]. When burned, many of the chemicals in cigarettes form new compounds [18]; smoked cigarette butts contain nicotine, pinane, phenanthrene [19], and other chemicals such as polycyclic aromatic hydrocarbons. There are still many research gaps regarding electronic cigarettes [20]. Since the first prototype built in 2003 by Hon Lik [21], electronic cigarettes have greatly increased in both popularity and device complexity. Electronic cigarettes operate on the evaporation–condensation principle of aerosolization to produce an inhaled vapor containing a combination of nicotine, excipients (essentially propylene glycol and glycerol), and flavouring agents [22]. Among the various configurations of e-cigarettes that have been designed over the years, there are those that have a drip tip like classic cigarettes. That is, they have a real filter filmed on the outside to effectively emulate the filter of classic cigarettes. In fact, the manufacturer’s idea is to make the consumer feel like they have a traditional cigarette between their lips. The filters of e-cigarettes vary in composition: from 100 % cotton to polylactic acid (PLA), the most produced biodegradable plastic, obtained by fermenting sugar from corn, cane molasses, potatoes, sugar beets, etc. [23]. In 2020, about 26.6% of the smoking population in Italy preferred e-cigarettes as a tobacco product [24]. The relative consumption of these second generation (filtered) e-cigarettes is currently unknown; however, it is likely that electronic cigarette butts may enter marine and beach litter composition alongside conventional cigarette butts.
The second experiment was designed to replicate a much more complex and, in our opinion, unexplored interaction dynamic between butts, sandy sediments, and seawater. Attention was paid to the typology of CBs, which was shown to be more toxic by the previous step (i.e., CCB), exposing the different species to elutriates rather than leachates. In this study, natural sandy sediments were manually contaminated with CBs under laboratory conditions and then used to prepare the elutriates. It is known that cellulose acetate cigarette butts can last 18 months or longer under normal environmental conditions [25]. In this time frame, exposure of the butts to different weathering agents can affect the release dynamics of the contaminants they contain, which affects the final toxicity of the elutriates. To explore this mechanism, previously contaminated sediments were exposed to different simulated atmospheric conditions (simulated rain and natural sunlight) and time periods (1 and 2 weeks).
Although several studies have already demonstrated the harmfulness of compounds associated with CCBs to aquatic species [17,26,27,28,29], the ecological risk of CB elutriates to coastal and marine environments is still poorly understood [29]. To the best of our knowledge, no comprehensive study has been conducted to evaluate their integrated effect on marine ecosystems. In this study, results were collected using a battery of ecotoxicological bioassays (Aliivibrio fischeri, bacteria; Phaeodactylum tricornutum, algae, primary producers; Paracentrotus lividus, echinoderms, primary consumers) and integrated to estimate a synthetic ecotoxicological risk level by using the dedicated software Sediqualsoft®. Sediqualsoft® is a computer tool developed by ISPRA (the Italian Higher Institute for Environmental Protection and Research) in collaboration with Università Politecnica delle Marche. It was conceived in 2011 by Piva and collaborators [30], validated over the years, and finally used as a useful tool for the implementation of Ministerial Decree 173/2016, the Italian Regulation that establishes detailed rules and technical criteria for the authorization of seabed waste materials. The ecotoxicological classification of each sediment sample is based on the use of weighted integration criteria and results in a five-level ecotoxicological risk scale: absent, slight, moderate, major, and severe.
In summary, the specific aims of the present study were the following: (i) to compare the toxicity of leachates, derived from classic and electronic cigarette butts; (ii) to investigate the role of combustion in determining the toxicity of leachates; (iii) to study the influence of different atmospheric conditions (rain vs. dry) and time of weathering (1 vs. 2 weeks) in determining the toxicity of elutriates derived from sediments previously contaminated with cigarette butts; (iv) to evaluate the adequacy and usefulness of the Sediqualsoft® software in developing the ecotoxicological class of hazard for sediments contaminated under laboratory conditions.
2. Materials and Methods
2.1. Experimental Design
This study consisted of two separate multifactorial experiments (Figure 1). The first was used to determine which type of butt, the classic (CCB) or the electronic (ECB), was more toxic. To determine this, an experiment in the traditional and most direct form, namely the preparation of leachate, was conducted in artificial sea water (ASW; the composition can be found in the Supplementary Materials). Toxicity was assessed with both smoked (S-) and unsmoked (U-) cigarettes to investigate the role of combustion in determining toxicity. A three-factor nested experimental design was applied: “cigarette type” (ECB vs. CCB, two levels fixed); “combustion” (smoked vs. unsmoked, two levels fixed) and “beaker” (i.e., replicates, three levels, random).
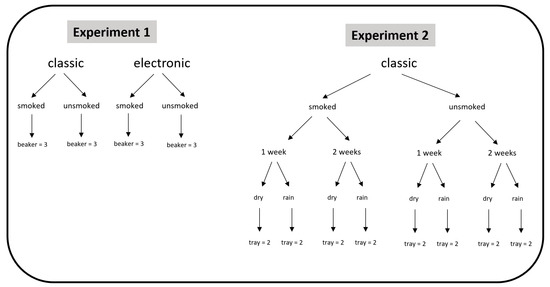
Figure 1.
Multifactorial experimental plans related to experiments 1 and 2.
In the second experiment, classic cigarette butts were used to contaminate natural sediments. After artificial contamination, the sandy samples were subjected to a weather simulation test. The weather simulation test was conducted by placing the contaminated sediments in a selected and protected area outside the laboratory in June 2020 (natural temperature ranging within 13–26 °C). The lab is in Tuscany (Italy), 1.5 km from the sea. The “dry” condition was simulated by exposing the samples outdoors to natural air and light, but protected from natural rain. To reproduce the “rain”, samples were wetted every 2 days with standard fresh water (SFW) through a drip sprayer with a sand/water ratio of 1:1 (v/v). The trays in which the sediments were placed were without holes at the bottom. Thus, the different species were exposed to elutriates rather than leachates. Four-factor nested experimental design was used: “combustion” (smoked vs. unsmoked, two levels fixed); “atmospheric conditions” (rain vs. dry, two levels fixed); “time” (1 week vs. 2 weeks, two levels fixed) and “tray” (i.e., replicates, two levels, random).
2.2. Production of the Cigarette Butts
Classic cigarettes were smoked artificially by placing a cigarette against a vacuum that was turned on and off to mimic the action of a smoker. The cigarettes were smoked to about 1 cm above the filter. The cigarette butts consisted of the filter plus the residual tobacco, paper, and ash.
The electronic cigarette used in this experiment vaporizes a liquid containing nicotine (0.5 mg of nicotine for ECB) and presents the taste of citrus fruit. The production of the butts of the electronic cigarette followed the same procedure as for traditional cigarettes: a vacuum was used until the cigarette was completely burned. According to the manufacturer, the filters are made of polylactic acid.
2.3. Preparation of the Leachates and Elutriates
Experiment 1: Ten CBs of each cigarette type (classic or electronic) were placed in separate glass bottles, each containing 1 L of ASW, agitated for 1 h at 100 rpm, and then filtered with a nitrocellulose membrane (with a pore size of 0.45 µm) to remove particulate matter. The control samples consisted of ASW only. In the absence of scientific data on the toxicity of ECBs, a relatively high non-environmental concentration (10 CB/L) was chosen. In fact, several studies have shown that a concentration of 10 classic CB/L is able to clearly induce toxicity in marine organisms such as benthic foraminifera [31], marine polychaetae, [28] and brackish water fish [29].
Experiment 2: Natural sandy sediments (NSSs) were collected from an unpolluted beach in Tuscany in March 2020 using a metal spoon, transported, and stored at 5 ± 1 °C (in the dark) until the time of analysis. The NSSs were tested before the start of the experiments and had an “Absent” ecotoxicological risk according to the same species battery and approach as in this study. On the day of the experiment, the NSSs were first homogenized by hand and then distributed into 25 × 25 cm trays, creating a 5 cm layer (with a total solid volume of 3125 cm3 per tray). Each tray was first artificially contaminated by adding classic CBs (0.11 CCB/L natural sediment) to the surface or by burial (randomly distributed) and then exposed to the different atmospheric conditions according to the design described previously. Selection of the concentration to be tested was difficult due to insufficient data in the literature. On this basis, a concentration was chosen that is two orders of magnitude lower than that used in Experiment 1; however, it remains difficult to judge whether it can be considered as environmentally relevant. After weathering, CCBs were removed from the sediment and elutriates were prepared following the protocol for marine sediments proposed in Italian Ministerial Decree 173/2016 [32]: ASW (salinity of 35 ± 1 PSU) at a ratio of 1:4 sediment-ASW, weight/volume (1 part sediment: 3 parts ASW) was added and immediately agitated for 1 h at 100 rpm. Then, the elutriates were filtered with a nitrocellulose membrane (with a pore size of 0.45 µm) and analysed for chemical-physical parameters (dissolved oxygen, salinity, and pH; Table 1). Prior to exposure to the target species, pH was corrected in some cases to meet the standardized requirements of the ecotoxicological tests (see Supplementary Materials, Table S1). Consequently, the effects observed in this study are exclusively due to the chemical composition of the solutions and not to possible changes in the physical-chemical parameters of the same due to the presence of CBs. The control samples were elutriates obtained from sediments never in contact with CBs.

Table 1.
Parameters recorded on leachates (CBs in water) and elutriates (CBs in sediments) before corrections.
2.4. Ecotoxicological Tests
Leachates and elutriates were tested with a standard battery of ecotoxicological assays on three species considered representative of different trophic levels in marine ecosystems and showing different sensitivity to toxic substances: (i) inhibition of bioluminescence of the marine bacterium Aliivibrio fischeri (acute; 15 and 30 min); (ii) inhibition of algal growth of the diatom Phaeodactylum tricornutum (chronic; 72 h); (iii) spermiotoxicity (acute; 20 min) in the sea urchin Paracentrotus lividus; (iv) embryo toxicity (chronic; 72 h) of the sea urchin Paracentrotus lividus. The bioassays were in accordance with Italian Law (D. Lgs. 173/2016; [32]).
2.4.1. Inhibition of Bioluminescence, Aliivibrio fischeri (UNI EN ISO 11348-3:2019)
The endpoint chosen was inhibition of bioluminescence emitted by marine bacteria A. fischeri when exposed to the sample. Bioluminescence was measured using a luminometer set at 430 nm. The test was performed in duplicate at 15 ± 1 °C for 15 and 30 min. The initial concentration of bacteria was 106 cells, and the maximum testable concentration of the sample was equal to 90%. During the test, a negative control (ASW) and a positive control (3.4 mg/L of 3,5-dichlorophenol) were applied in duplicate. The test was considered valid if the inhibition of the positive control was 20–80%.
2.4.2. Algal Growth Inhibition, Phaeodactylum tricornutum (UNI EN ISO 10253:2017)
Growth inhibition of the marine diatom P. tricornutum was chosen as the endpoint. The alga, which was in the exponential growth phase, was exposed to the various experimental conditions and placed under continuous light for 72 h to allow rapid growth. At time intervals of 24 h, the samples were shaken and measured using a spectrophotometer at a wavelength of 670 nm. The tests were performed in triplicate at 20 ± 2 °C. The initial concentration of the tested samples was 104 cells, and the tested sample concentration was 100%. Specific nutrients (S1 + S2 + S3; see Supplementary Materials for compositions) were added to each sample according to ISO (2016), except for the negative controls, as the algal culture medium was already nutrient-enriched. Positive controls were set up with potassium dichromate (n = 3). The test was considered valid if the algal concentration in the negative controls was 16 times the initial concentration after 72 h and if the EC50 of the positive controls was 20.1 ± 5.3 mg/L.
2.4.3. Fertilization Efficiency, Paracentrotus lividus (EPA/600/R-95-136/Section 16)
This method evaluates the spermiotoxicity in the sea urchin P. lividus. Male and female gametes emission was obtained by intraoral injection of 1 mL of 1 M potassium chloride from homogeneously sized adults. Spermatozoa were previously exposed to the leachates/elutriates for 20 min and then contacted with eggs to allow fertilization. The ratio of sperm and eggs, per mL of solution, was 15,000:1. After an additional 20 min, 2–3 drops of Lugol’s fixative were added to each sample to arrest cell division and verify the results microscopically. A total of 100 eggs per replicate were counted to determine the number of correctly fertilized eggs. The sample concentration tested was 50% as per ISPRA Guideline No. 11 (2017) [33], and the test was performed in triplicate. Negative control (ASW) and positive control (copper (II) nitrate) were tested to evaluate the quality of the results obtained. The test was considered valid when the negative control indicated >80% of normal fertilized eggs and when the EC50 of the positive control was between 21.69 and 68.18 µg/L Cu2+.
2.4.4. Larval Development (EPA/600/R-95-136/Section 15) and Larval Body-Size Variations, Paracentrotus lividus
The first endpoint of this analysis was the larval development in the sea urchin P. lividus after 72 h of exposure. Male and female gametes were obtained as described above. Eggs were fertilized with sperm, and after 20 min, the correct fertilized eggs were exposed to the test leachates/elutriates. After 72 h of development, 2–3 drops of Lugol’s fixative were added to each sample to stop cell division, and the results were examined by microscopy. After 72 h of normal development, fertilized eggs reached the larval stage of pluteus: to determine the % of abnormal larvae, 100 plutei were counted per each replicate. Larvae were considered abnormal if they exhibited arrested development, all arms were absent or of different lengths, there were additional arms or cross-lateral rods, they had asymmetrical body width, and other abnormalities were seen as reported in the literature [33]. The sample concentration tested was 50% according to ISPRA Guideline No. 11 (2017) [33], and the test was performed in triplicate. A negative control (ASW) and a positive control (copper (II) nitrate) were applied. The test was considered valid when the negative control had >80% normally developed larvae and when the EC50 values of the positive control were between 22.60 and 68.34 µg/L Cu2+.
Recent research recorded significant differences among body size (i.e., maximum arm lengths) of normo-formed larvae in a population of P. lividus exposed to chemicals compared to the body size of natural populations at the same developmental stage [34,35]. The second endpoint of this analysis used the same samples previously analysed for the detection of the anomalies in embryo development to evaluate the effects of the leachates/elutriates on larval body size of P. lividus, using stereomicroscopic measurement of their mean arm lengths (Nikon, SMZ-800 N equipped with Nikon’s software Nikon ACT-1). The measurements were performed on 10 normally developed plutei per each replicate and were expressed as percentage of body size reduction compared to the controls.
2.5. Quality Assurance and Quality Control
The Bioscience Research Center is a certified laboratory (ISO 9001:2015) and applies a severe control procedure under guidelines of the UNI EN ISO 17025:2018 to ensure the quality of produced data (ACCREDIA 1715L). QA/QC tests were performed as described by their reference methods. Specific variables of interest as defined by the applied method were standardized and monitored during tests (Table S1). Positive and negative controls were tested during the experiments, and results are reported in Supplementary Materials (Table S2).
2.6. Data Analyses
Statistics (mean, standard deviation, t-Test Student, and F-test), if appropriate, were calculated by Excel on experimental raw data, and the applied formulae were reported for each of the reference methods. The results of positive controls performed on opportune dilution of reference substances during tests on P. tricornutum and P. lividus were utilized to calculate the EC50 values and their related confidential limits. This calculation was performed using the US EPA Toxicity Relationship Analysis Program (TRAP) version 1.30, with a Gaussian distribution and logarithmic transformation of exposure variables. The positive control carried out on A. fischeri did not need the calculation of an EC50 value according to the reference method.
Recorded effects were uploaded in the Sediqualsoft® software to perform an integrated evaluation and a weighted risk assessment derived from cigarette butts’ leachates/elutriates. Ecotoxicological classification and the related Class of Hazard were elaborated by weigh integration of the results gathered by all the components of the biological battery. Weigh integration criteria applied by the software considered important aspects and specific characteristics of each applied biological assay (i.e., the statistical significance of the difference between sample and control, the strictness of the biological effect considered by the assay, the type of the exposition—acute or chronic, etc.). The limit, which represents the minimum variation, biologically significant for each experimental condition, is reported in Table 2 (extracted from Table A1 of Italian Ministerial Decree 173/2016). Furthermore, each assay was weighted with respect to (i) the biological endpoint measured, (ii) the exposition time, and (iii) the matrix (represented in Table 3 and extracted from Table A2 of Italian Ministerial Decree 173/2016, [32]). The criteria applied from Sediqualsoft® to weight ecotoxicological effects and to perform integrated classifications are detailed in Piva et al., 2011 [30].

Table 2.
Limits of biological assays required by Italian D.M. 173/2016 (translation of Table A1).

Table 3.
Weights assigned according to the relevance of the biological endpoint, the matrix, and the time of exposure.
3. Results
3.1. Effect of “Combustion” on Classic and Electronic CB Toxicity
A synthetic view of the results obtained is reported in Figure 2; in smoked classic CBs (S-CCBs) showed the highest negative score in terms of Battery Bioassay Hazard Quotient (HQ = 1.99) and the corresponding Class of Hazard (i.e., moderate).
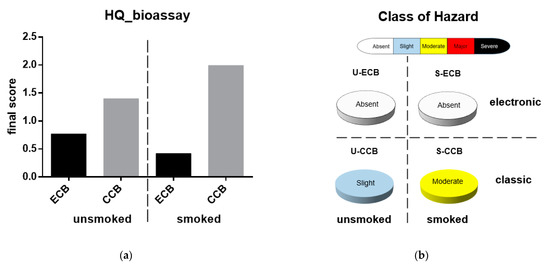
Figure 2.
Evaluation of ecotoxicological bioassay. Marine species were exposed to leachates of smoked (S-) and unsmoked (U-) cigarette butts from classic (CCB) and electronic (ECB) cigarettes. (a) Hazard Quotient of the battery of bioassays; (b) class of hazard. Calculations and categorization are those reported by Sediqualsoft®.
A more detailed report of the ecotoxicological responses for each tested species is described in Table 4. Exposure to the leachates induced the inhibition of the natural bioluminescence of bacteria (A. fischeri, values between 8.46% and 35.03%), the inhibition of growth in phytoplanktonic primary producers (P. tricornutum, between 7.01% and 32.43%), and the reduction in fertilization success of grazers (P. lividus, between 16.65% and 52.54%).

Table 4.
Ecotoxicological results recorded on tested species based on which HQ_battery and Class of Hazard were calculated.
P. lividus larvae 72 h after fertilization reported abnormal development in up to 14.09% of cases. The more sensitive organisms (namely, echinoderms) also reported a statistically significant reduction (p-value < 0.05) in the mean arm length of normo-formed plutei exposed to classic CCBs, with meaningless differences within the factor “combustion” (p-value < 0.01) (Figure 3). Specifically, larvae exposed to smoked CCBs reported a mean reduction of 21%; for unsmoked CCBs, it was 18.5%. No significant differences were induced by electronic cigarette elutriates.
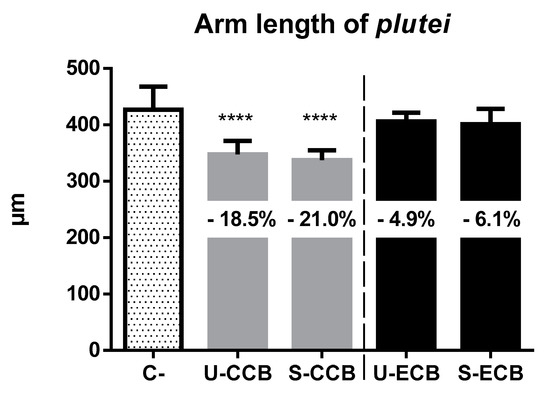
Figure 3.
Mean length (±SD) of arms in normo-formed 72 h old plutei of P. lividus exposed to leachates of smoked (S-) and unsmoked (U-) cigarette butts from classic (CCB) and electronic (ECB) cigarettes. C- = negative controls. Statically significant differences, compared to controls, were calculated in specimens exposed to CCBs (**** = p-value < 0.0001), and corresponded to an arm length reduction of up to 21.0% and meaningless differences within the factor “combustion”.
3.2. Effects of “Combustion”, “Time”, and “Atmospheric Condition” on Classic CB Toxicity
A synthetic view of the results obtained after 1 week of weathering is reported in Figure 4. Specifically, smoked classic CBs (S-CCBs) in “dry” conditions showed the highest score in terms of Battery Bioassay Hazard Quotient (HQ = 3.86) and Class of Hazard (namely, major). Such elutriates induced the inhibition of the bioluminescence in bacteria (A. fischeri, 68.87%), the inhibition of growth in phytoplanktonic primary producers (P. tricornutum, 47.7%), and the reduction in fertilization success of grazers (P. lividus, 62.12%). P. lividus larvae 72 h after fertilization reported abnormal development in up to 58.59% of cases. On the contrary, unsmoked CCBs in “dry” conditions corresponded to an “absent” Class of Hazard. CCBs pre-treated in “rain” conditions produced elutriates characterized by “moderate” (smoked-CCBs) and “slight” (unsmoked-CCBs) Class of Hazard. A more detailed report of the ecotoxicological responses for each tested species is described in Table 5.
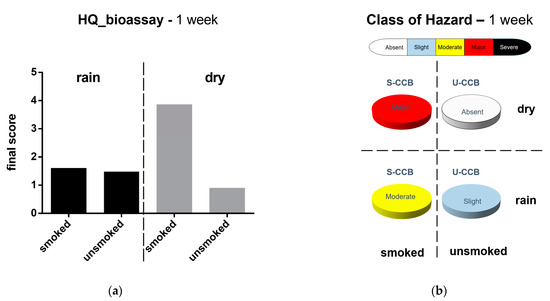
Figure 4.
Evaluation of ecotoxicological bioassay. Marine species were exposed to elutriates prepared from both smoked and unsmoked CCBs subjected to different atmospheric conditions (rain vs. dry) for 1 week. (a) Hazard Quotient of the battery of bioassays; (b) class of hazard. Calculations and categorization are those reported by Sediqualsoft®.

Table 5.
Ecotoxicological results recorded for tested species after 1 week of CCB conditioning under different atmospheric conditions (rain vs. dry).
After 2 weeks, the HQ and the ecotoxicological risk (Class of Hazard) changed in all treatments: in “dry” condition, S-CCBs passed from “major” to “slight”; U-CCBs from “absent” to “slight”. In “rain” condition, S-CCBs passed from “moderate” to “slight” and U-CCBs from “slight” to “absent”. The results are graphically reported in Figure 5 and detailed in Table 6.
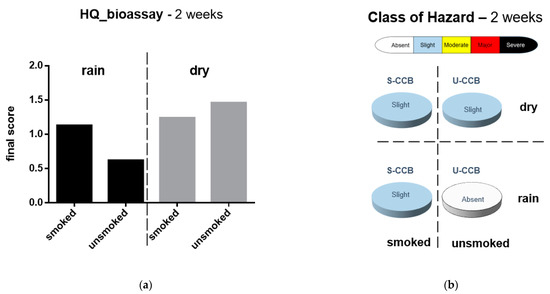
Figure 5.
Evaluation of ecotoxicological bioassay. Marine species were exposed to elutriates prepared from both smoked and unsmoked CCBs subjected to different atmospheric conditions (rain vs. dry) for 2 weeks. (a) Hazard Quotient of the battery of bioassays; (b) class of hazard. Calculations and categorization are those reported by Sediqualsoft®.

Table 6.
Ecotoxicological results recorded for tested species after 2 weeks of CCB conditioning under different atmospheric conditions (rain vs. dry).
4. Discussion and Conclusions
Leachates of cigarette butts, prepared in ASW at a high concentration (10 CB/L of ASW), were used to identify evident ecotoxicological responses in tested species and therefore facilitate the comparison between traditional and electronic cigarettes. Our results highlighted that classic cigarette butts, particularly the smoked ones, produced higher hazard and higher ecotoxicological risks compared to electronic cigarette butts. An “absent” Class of Hazard was assigned to electronic cigarettes; on the contrary, classic CBs represented a “slight” and “moderate” ecotoxicological risk, for unsmoked and smoked CBs, respectively. Smoked CCB leachates induced the inhibition of the bioluminescence in A. fischeri, the inhibition of algal growth, and the reduction in fertilization success of P. lividus. Smoked CCBs affected the development of plutei, increasing the percentage of abnormal larvae respect to controls, and also induced impairment in biometrics in normal-formed larvae. The body size reduction in normo-formed (72 h) plutei showed ecotoxicological efficacy for both smoked and unsmoked cigarette butts, thus confirming a sensitive endpoint, as reported in other studies [35]. These results are consistent with the literature showing that classic smoked cigarettes are worse than the unsmoked ones. As an example, Slaughter et al. (2011) [17] recorded in marine fish LC50 values of 1.8 CB/L of water and 5.1 CB/L of water, respectively, for smoked and unsmoked cigarette butts. Caridi and colleagues (2020) [31] showed that 4.0 CB/L of water is sufficient to determine shell decalcification and death in benthic foraminifera (Protista). The low ecotoxicological risk for electronic cigarettes is in accordance with results collected by Parker and Rayburn (2017) [36], who tested the potential developmental toxicities of three different cigarette butt leachates (regular cigarette butts, menthol, and electronic) in the frog embryo teratogenesis assay, Xenopus (FETAX). Xenopus laevis embryos were exposed to concentrations ranging from 0 to 10 ECB/L, and the ECB leachate was much less toxic than all other treatments, with an overall 96 h LC50 of 14.6 CB/L. ECB leachate was at least 10-fold less toxic than regular cigarette butts. On one hand, ECs do not release second-hand smoke, and thus they are thought of as being a safe alternative to traditional cigarettes [36]; on the other hand, researchers found impurities in both e-liquid and composition of the emitted vapour (e.g., lead, nickel, silver, silicate beads, and nanoparticles) [37], which are potentially toxic for aquatic organisms. Considering the paucity of data and the great variety in EC configurations (e.g., e-liquid composition in terms of % of nicotine, excipient, and flavouring agents), further studies are needed to better elucidate the toxicity of this new form of personal litter. Undoubtedly, butts derived from electronic cigarettes cannot be considered a prominent hazard today. However, future efforts should made, starting with the monitoring of their relative abundance in the environment. For example, a specific category of litter may be created in beach litter protocol to facilitate their analysis and trend tracking.
Considering the entirety of the study, the major environmental concern was associated with the elutriates derived from low levels of pollution (0.11 CB/L of sediment), in the presence of combusted cigarettes exposed, for a relatively short timeframe (1 week), to dry conditions. The increased toxicity of CBs subjected to combustion can be explained by the production of new compounds, such as polycyclic aromatic hydrocarbons [28], already proven in other studies to be toxic for aquatic organisms [38,39]. The highest score in terms of the Battery Bioassay Hazard Quotient (HQ = 3.86) and Class of Hazard (namely, major) for the treatment “1 week” + “dry” conditions showed that the mere exposure of butts to natural air and solar radiation is sufficient to cause the desorbing and leaching of chemical compounds able to affect the ecotoxicological responses of marine species. On the contrary, CCBs pre-treated under “rain” conditions produced elutriates characterized by “moderate” (smoked CCBs) and “slight” (unsmoked CCBs) Class of Hazard, representing an alternative source of variability in elutriate toxicity. Finally, the results of this study show that longer exposure times (2 weeks) reduced the ecotoxicity of classic CBs, probably due to a relevant dilution and/or inhibition of the toxicant substances by the rainwater.
The use of a battery of bioassays, involving species belonging to different trophic levels, makes our results of particular interest from an ecological point of view. Recorded impacts on tested species owning to bacteria, phytoplanktonic communities, and ecological groups of grazers could produce significant effects on their relative trophic webs. The decrease of Bacteroidates and Cyanobacteria Phyla in marine ecosystems, to the advantage of other bacterial groups, such as the Gamma-proteobacteria, Firmicutes, and Thermotogae, has been reported due to of the exposure to smoked and unsmoked classic CBs [40]. Research performed on Aliivibrio fischeri showed that about 0.03 CB/L can induce chronic toxicity, causing the inhibition of population growth [41]. Impacts on grazers in marine ecosystems are reported by the literature to affect algal communities, reduce grazer effects, and significantly impair ecosystem dynamics and species associations [42]. Based on the results recorded in the study, it emerges that classic cigarette butts can also represent indirect and long-term impacts for marine species by reducing reproductive success and body-size of the larval stages of grazers, reducing population growth of primary producers (algae), and unbalancing the transfer of energy into the marine trophic web.
This study sought to test the integration and synthesis capacity of Sediqualsoft® and its underlying theoretical approach in an experimental context that is unusual compared to the traditional use of the computer tool. This context was represented by the artificial contamination, under laboratory conditions, of environmental matrices. Traditionally, the software is suggested for the implementation of the Italian Ministerial Decree 173/2016 (the regulation laying down detailed rules and technical criteria for the re-use of marine, brackish, and coastal waste sediments). According to the law, before their re-use, sediments must be subject to a physical, chemical, and ecotoxicological characterization process; different destinations of the sediments, according to their quality, are then provided. The ecotoxicological analyses are evaluated at the level of “battery” (not of individual test), weighing the biological relevance of the measured effects, the statistical significance of the results, the ecological relevance of the tested matrix, and the type of exposure [43]. The final output of the software is a classification of sediment samples in a five-level ecotoxicological risk scale: absent, slight, moderate, major, and severe. From our point of view, the advantages of using Sediqualsoft® and its underlying theoretical approach are as follows: (i) it is a free software, obtainable on request (sediqualsoft109@isprambiente.it); (ii) it allows for the production of integrated, synthetic, and “in accordance with the law” data; (iii) thanks to the extended version of the software, it is possible to integrate data collected from the Line of Evidence (LOE) of Bioassay with the other LOEs, following a weight of evidence approach (WOE, [44]). Other LOEs may be derived from future studies on the chemical composition of the elutriates, the assessment of bioavailability, and the sub-lethal effects on battery of biomarkers. In this regard, the use of a weight of evidence approach (WOE, [44]) appears to be a powerful tool to support more complex processes of environmental risk assessment and furnish a comprehensive assessment of hazard associated with sediments polluted with contaminants of various kinds. A wide variety of literature is available on this aspect [43,44,45,46]. However, the Sediqualsoft® could be improved by making WOE-useful extensions easily available.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jmse9070734/s1, S1. Materials and Methods: Cigarette composition, Artificial sea water (ASW) composition, Composition of the nutrients used for P. tricornutum test. Table S1: Experimental conditions required by the applied methods; Table S2: Endpoint values obtained from the analyses of negative and positive controls during experiments.
Author Contributions
Conceptualization, M.R. and S.A.; methodology, validation, S.A.; formal analysis, M.P.; investigation, S.A., F.P., A.B.; resources, M.R.; writing—original draft preparation, M.P., M.R.; writing—review and editing, M.P., M.R., A.T.; visualization, M.P.; supervision, M.R.; project administration, M.R.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Bioscience Research Center.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Original data could be available on request under a specific agreement with BsRC.
Acknowledgments
Authors are grateful to Moira Sitta (Student from University of Trieste), who performed the initial bibliographic research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- UNEP. Marine Litter: A Global Challenge; UNEP: Nairobi, Kenya, 2009. [Google Scholar]
- Kenyon and Kridler. Layson Albatrosses Swallow Ingestible Matter. Auk 1969, 86, 339–343. [Google Scholar] [CrossRef]
- Bergmann, M.; Lars, G.; Klages, M. (Eds.) Marine Anthropogenic Litter; Springer Open: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Nelms, S.; Coombes, C.; Foster, L.; Galloway, T.; Godley, B.; Lindeque, P.; Witt, M. Marine Anthropogenic Litter on British Beaches: A 10-Year Nationwide Assessment Using Citizen Science Data. Sci. Total Environ. 2017, 579, 1399–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortibuoni, T.; Amadesi, B.; Vlachogianni, T. Composition and Abundance of Macrolitter along the Italian Coastline: The First Baseline Assessment within the European Marine Strategy Framework Directive—SUPPL. MAT. Environ. Pollut. 2021, 268, 115886. [Google Scholar] [CrossRef]
- Ocean Conservancy. Together, We Are Team Ocean 2020; Printivity: San Diego, CA, USA, 2020. [Google Scholar]
- Ocean Conservancy. The Beach and Beyond; Schmitz Press: Sparks, MD, USA, 2019. [Google Scholar] [CrossRef]
- Becherucci, M.E.; Seco Pon, J.P. What is left behind when the lights go off? Comparing the abundance and composition of litter in urban areas with different intensity of nightlife use in Mar Del plata, Argentina. Waste Manag. 2014, 34, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Blickley, L.C.; Currie, J.J.; Kaufman, G.D. Trends and Drivers of Debris Accumulation on Maui Shorelines: Implications for Local Mitigation Strategies. Mar. Pollut. Bull. 2016, 105, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Honorato-Zimmer, D.; Gatta-Rosemary, M.; Nuñez, P.; Hinojosa, I.A.; Thiel, M. Spatio-Temporal Variation of Anthropogenic Marine Debris on Chilean Beaches. Mar. Pollut. Bull. 2018, 126, 516–524. [Google Scholar] [CrossRef]
- Oigman-Pszczol, S.S.; Creed, J.C. Antagonistic Interactions between Fungal Pathogen and Leaf Surface Fungi of Onion. J. Coast. Res. 2007, 23, 421–428. [Google Scholar] [CrossRef]
- Santos, I.R.; Friedrich, A.C.; Wallner-Kersanach, M.; Fillmann, G. Influence of Socio-Economic Characteristics of Beach Users on Litter Generation. Ocean Coast. Manag. 2005, 48, 742–752. [Google Scholar] [CrossRef]
- Ariza, E.; Leatherman, S.P. No-Smoking Policies and Their Outcomes on U.S. Beaches. J. Coast. Res. 2012, 28, 143–147. [Google Scholar] [CrossRef]
- Araújo, M.C.B.; Costa, M.F. A Critical Review of the Issue of Cigarette Butt Pollution in Coastal Environments. Environ. Res. 2019, 172, 137–149. [Google Scholar] [CrossRef]
- Loizidou, X.I.; Loizides, M.I.; Orthodoxou, D.L. Persistent Marine Litter: Small Plastics and Cigarette Butts Remain on Beaches after Organized Beach Cleanups. Environ. Monit. Assess. 2018, 190, 414. [Google Scholar] [CrossRef]
- Glantz, S.A.; Slade, J.; Bero, L.A.; Hanauer, P.; Barnes, B. The Cigarette Papers; University of California Press: Berkeley, CA, USA, 1998. [Google Scholar]
- Slaughter, E.; Gersberg, R.M.; Watanabe, K.; Rudolph, J.; Stransky, C.; Novotny, T.E. Toxicity of Cigarette Butts, and Their Chemical Components, to Marine and Freshwater Fish. Tob. Control 2011, 20, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Kitajima, S.; Katahira, K. Waste on the Roadside, “poi-Sute” Waste: Its Distribution and Elution Potential of Pollutants into Environment. Waste Manag. 2009, 29, 1192–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savino, J.F.; Tanabe, L.L. Sublethal Effects of Phenanthrene, Nicotine, and Pinane on Daphnia Pulex. Bull. Environ. Contam. Toxicol. 1989, 42, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Spahn, J.E.; Stavchansky, S.A.; Cui, Z. Critical Research Gaps in Electronic Cigarette Devices and Nicotine Aerosols. Int. J. Pharm. 2021, 593, 120144. [Google Scholar] [CrossRef]
- Dutra, L.M.; Grana, R.; Glantz, S.A. Philip Morris Research on Precursors to the Modern E-Cigarette since 1990. Tob. Control 2016, 26, E97–E105. [Google Scholar] [CrossRef]
- Etter, J.F.; Bullen, C.; Flouris, A.D.; Laugesen, M.; Eissenberg, T. Electronic Nicotine Delivery Systems: A Research Agenda. Tob. Control 2011, 20, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.; Park, H.; Choi, O.; Sang, B.I. Anaerobic Co-Digestion of Bioplastics as a Sustainable Mode of Waste Management with Improved Energy Production—A Review. Bioresour. Technol. 2021, 322, 124537. [Google Scholar] [CrossRef]
- Kunst Alexander, Tobacco Product Usage in Italy 2020. Stastista. 2020. Available online: https://www.statista.com/forecasts/1000709/tobacco-product-usage-in-italy (accessed on 30 April 2021).
- Ach, A. Biodegradable Plastics Based on Cellulose Acetate. J. Macromol. Sci. Part A Pure Appl. Chem. 1993, 30, 733–740. [Google Scholar] [CrossRef]
- Booth, D.J.; Gribben, P.; Parkinson, K. Impact of Cigarette Butt Leachate on Tidepool Snails. Mar. Pollut. Bull. 2015, 95, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Micevska, T.; Warne, M.S.J.; Pablo, F.; Patra, R. Variation in, and Causes of, Toxicity of Cigarette Butts to a Cladoceran and Microtox. Arch. Environ. Contam. Toxicol. 2006, 50, 205–212. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Reid, M.J.; Thomas, K.V.; Galloway, T.S. Bioaccumulation and Biological Effects of Cigarette Litter in Marine Worms. Sci. Rep. 2015, 5, 14119. [Google Scholar] [CrossRef]
- Lee, W.; Lee, C.C. Developmental Toxicity of Cigarette Butts—An Underdeveloped Issue. Ecotoxicol. Environ. Saf. 2015, 113, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Piva, F.; Ciaprini, F.; Onorati, F.; Benedetti, M.; Fattorini, D.; Ausili, A.; Regoli, F. Assessing Sediment Hazard through a Weight of Evidence Approach with Bioindicator Organisms: A Practical Model to Elaborate Data from Sediment Chemistry, Bioavailability, Biomarkers and Ecotoxicological Bioassays. Chemosphere 2011, 83, 475–485. [Google Scholar] [CrossRef]
- Caridi, F.; Sabbatini, A.; Birarda, G.; Costanzi, E.; De Giudici, G.; Galeazzi, R.; Medas, D.; Mobbili, G.; Ricciutelli, M.; Letizia, M.; et al. Cigarette Butts, a Threat for Marine Environments: Lessons from Benthic Foraminifera (Protista). Mar. Environ. Res. 2020, 162, 105150. [Google Scholar] [CrossRef] [PubMed]
- Decreto Ministeriale 173/2016. Regolamento Recante Modalità e Criteri Tecnici per L’autorizzazione All’immersione in Mare dei Materiali di Escavo di Fondali Marini; Gazzetta Ufficiale: Rome, Italy, 2016; pp. 23–35. [Google Scholar]
- ISPRA. Saggio di Fecondazione e Saggio Di Sviluppo Embrionale Con Il Riccio di Mare Paracentrotus Lividus (Lamarck) (Echinodermata: Echinoidea); ISPRA: Livorno, Italy, 2017. [Google Scholar] [CrossRef]
- Messinetti, S.; Mercurio, S.; Parolini, M.; Sugni, M.; Pennati, R. Effects of Polystyrene Microplastics on Early Stages of Two Marine Invertebrates with Different Feeding Strategies. Environ. Pollut. 2018, 237, 1080–1087. [Google Scholar] [CrossRef]
- Piccardo, M.; Provenza, F.; Grazioli, E.; Cavallo, A.; Terlizzi, A.; Renzi, M. PET Microplastics Toxicity on Marine Key Species Is Influenced by PH, Particle Size and Food Variations. Sci. Total Environ. 2020, 715, 136947. [Google Scholar] [CrossRef]
- Parker, T.T.; Rayburn, J. A Comparison of Electronic and Traditional Cigarette Butt Leachate on the Development of Xenopus Laevis Embryos. Toxicol. Rep. 2017, 4, 77–82. [Google Scholar] [CrossRef]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and Silicate Particles Including Nanoparticles Are Present in Electronic Cigarette Cartomizer Fluid and Aerosol. PLoS ONE 2013, 8, e057987. [Google Scholar] [CrossRef] [Green Version]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, M.; Dai, Y.; Luo, Y.; Zhang, S. Health and ecotoxicological risk assessment for human and aquatic organism exposure to polycyclic aromatic hydrocarbons in the Baiyangdian Lake. Environ. Sci. Pollut. Res. 2021, 28, 574–586. [Google Scholar] [CrossRef]
- Quéméneur, M.; Chifflet, S.; Akrout, F.; Bellaaj-Zouari, A.; Belhassen, M. Impact of Cigarette Butts on Microbial Diversity and Dissolved Trace Metals in Coastal Marine Sediment. Estuar. Coast. Shelf Sci. 2020, 240, 106785. [Google Scholar] [CrossRef]
- Patra, R.W.; Cole, B. Toxicity and a Hazard Assessment of Cigarette Butts to aquatic Organisms [abstract]. In Interact 2002—Programme and Abstract Book, Proceedings of the Australasian Society of Ecotoxicology and the International Chemometrics Society 2002, 192 of the Conference “Interact 2002”, Sydney, NSW, Australia, 21–25 July 2002; The Royal Australian Society Chemical Institute: Sydney, Australia, 2002. [Google Scholar]
- Bulleri, F.; Benedetti-Cecchi, L.; Cinelli, F. Grazing by the Sea Urchins Arbacia Lixula L. and Paracentrotus Lividus Lam. in the Northwest Mediterranean. J. Exp. Mar. Biol. Ecol. 1999, 241, 81–95. [Google Scholar] [CrossRef]
- ISPRA. L’ecotossicologia Come Strumento di Gestione Degli Ambienti Acquatici e Terrestri; ISPRA: Livorno, Italy, 2019. [Google Scholar] [CrossRef]
- Regoli, F.; Pellegrini, D.; Cicero, A.M.; Nigro, M.; Benedetti, M.; Gorbi, S.; Fattorini, D.; D’Errico, G.; Di Carlo, M.; Nardi, A.; et al. A Multidisciplinary Weight of Evidence Approach for Environmental Risk Assessment at the Costa Concordia Wreck: Integrative Indices from Mussel Watch. Mar. Environ. Res. 2014, 96, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Maradonna, F.; Ancillai, D.; Notarstefano, V.; Valenti, A.; Leoni, T.; Carnevali, O. An Integrated Approach to Evaluate Port Sediment Quality: From Chemical Characterization to Multispecies Bioassays. Sci. Total Environ. 2020, 746, 141204. [Google Scholar] [CrossRef]
- Pittura, L.; Avio, C.G.; Giuliani, M.E.; D’Errico, G.; Keiter, S.H.; Cormier, B.; Gorbi, S.; Regoli, F. Microplastics as Vehicles of Environmental PAHs to Marine Organisms: Combined Chemical and Physical Hazards to the Mediterranean Mussels, Mytilus Galloprovincialis. Front. Mar. Sci. 2018, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).