Abstract
Light intensity and spectral quality play crucial roles in microalgal growth and biochemical biosynthesis. This study investigates the effects of different light intensities (3000, 8000 and 15,000 lux) and colors (red, white, yellow and green) on the growth and metabolites of Nephroselmis sp. Moderate intensity (8000 lux) of white light is sufficient to produce this microalga. The colors of light strongly affect the parameters of the growth of Nephroselmis under each light intensity (p < 0.05). The yellow and green light supported the highest growth rates for the three intensities. Blue and green light at 15,000 Lux stimulates high levels of chl-a corresponding to antenna size 2.80 and 2.46. Nephroselmis illuminated with red light synthesizes carotenoids reaching 13 µg mL−1 at 15,000 lux. This latter for each color stops the proliferation of Nephroselmis, and cells shift their metabolism towards the accumulation of protein. Nephroselmis accumulates more protein, followed by carbohydrates, lipids and polyphenols. Nephroselmis exhibited the highest protein (64% D.W) content when cultured under white light, and the green at 15,000 lux enhanced their production. Nephroselmis is rich in carbohydrates, which accounted for more than 20% D.W under all combinations of light intensities and colors. The accumulation of polyphenols and carotenoids under high-intensity red and white light may reflect an oxidative stress response, suggesting their role as protective antioxidants. The capacity of Nephroselmis sp. to thrive and synthesize valuable metabolites under variable light regimes underscores its potential as a robust candidate for the production of various molecules.
1. Introduction
Light is a critical parameter that influences the growth, pigments and metabolic activity in microalgae [1,2]. Photons are absorbed by photosynthetic pigment–protein antenna complexes, which are composed of chlorophylls (Chl), phycobiliproteins (PBP) and carotenoids in cyanobacteria and eukaryotic microalgae [3]. Chl-a, the most abundant pigment for light harvesting, is composed of a porphyrin ring and a hydrophobic chain, which can produce other chlorophylls at the end of the photosynthetic process [4]. Chl-b is restricted largely or entirely to Chlorophyceae [3]. Moreover, microalgae biosynthesize various primary metabolites such as proteins, lipids, vitamins, and essential minerals [5]. Carotenoids chromophores secondary pigments are usually either hydrocarbons (α-carotene, β-carotene) or oxygenated hydrocarbons (xanthophylls, e.g., lutein, violaxanthin, zeaxanthin, fucoxanthin, peridinin) [6].
The metabolism of microalgae cells is important to understand during the growth phase. Pigments and biochemical compounds have been widely used to monitor the physiological status of cells, helping to optimize culture conditions [7].
Chlorophyceae have gained considerable interest for biotechnological purposes mainly in the fields of bioenergy, animal and human nutrition, waste water treatment and CO2 mitigation [5,8,9]. Chlorella, Scenedesmus and Nannochloropsis microalgae show potential in the sequestration of several organic and inorganic pollutants from water [10,11]. Policymakers and scientists are constantly looking for novel strains of microalgae in response to the increase in the level of polluted water so as to improve wastewater treatment [12,13].
The impact of light intensity and colors has been studied in several microalgae groups, mainly diatoms [14,15,16], cyanobacteria [17,18,19] and chlorophyceae [20,21,22]. Studies have shown that both the growth and the metabolite production patterns of microalgae are significantly influenced and modeled by light intensity and color. The review of Sharmila et al. [18] reported that blue light seems to enhance the accumulation of biomass, pigments and lipids [21,23] for most microalgal species, but it decreases the synthesis of protein. However, microalgae such as Spirulina platensis, Chlorlella vulgaris and Nannochloropsis sp. recorded the highest growth rate when cultivated in red light [24]. Studies related to the effects of the color and intensity of light on chlorophyceae have been conducted on Chlorella, Nannochloropsis, Chlamydomonas and Tetraselmis. The chlorophyta Nephroselmis sp. is recognized to absorb CO2 [25]. The growth of this microalga was assessed at different light intensities [26]. However, the impacts of different colors of light combined with different intensities on the growth and metabolism of Nephroselmis were not studied.
This work aims to study the impacts of light of different colors and intensities on the growth and metabolome of Nephroselmis sp., which can provide valuable information on the response of marine microalgae to environmental variations.
2. Materials and Methods
2.1. Growth Conditions
Nephroselmis sp. was isolated from lagoons in Western Greece, and identified based on morphological observations and DNA sequencing data [27]. Nephroselmis was cultured in batch mode in 1.5 L sterilized plastic bottles containing enriched seawater using Walne medium [28], with a salinity of 40 ppt, under controlled room conditions at 22 °C (Figure 1). The cultures were permanently mixed by bubbling ordinary air (approximately 0.04% CO2), prefiltered through a 0.45 µm filter, at a flow rate of 0.5 L/min. The cultures were exposed to different light intensities of 3000, 8000 and 15,000 lux and various light colors, including white, red, green, blue and yellow, by covering the bottles with colored paper (Figure 1). White light was used as the control.

Figure 1.
Experiments on the culturing of Nephroselmis sp. under combined conditions of different light intensities (3000, 8000 and 15,000 Lux) and colors (white, red, green, yellow and blue).
The pH, temperature and optical density of the cultures at 750 nm were measured daily using a spectrophotometer to monitor cell density. In addition, we performed spectral measurements every day using UV Probe software (Version 2.3). At the end of each experiment, the biomass was collected and lyophilized for the biochemical analysis.
2.2. Growth Parameters
The kinetic growth of Nephroselmis was assessed by the determination of the specific growth rate (day−1), generation time (hours) and productivity (g mL−1 day−1). The maximum specific growth rate was determined during the exponential growth phase, where N1 and N2 are cell concentrations at days t1 and t2 [29].
2.3. Pigments Analysis
Photosynthetic pigments in Nephroselmis were analyzed at the beginning (T1) and the end (T2) of culturing using methanol as a solvent. Here, 1 mL of culture was centrifuged for 5 min at 4000 rpm. The pellet was then suspended in 2 mL of methanol and kept in the dark for 20 min for extraction, with shaking every 5 min. After that, the samples were centrifuged again for 5 min at 4000 rpm. The optical density of the extracts was measured at 470 nm, 653 nm and 666 nm using a spectrophotometer (UVmini-1240 UV-visible, Shimadzu, Kyoto, Japon) to determine pigment concentrations (µg mL−1). The spectral data were analyzed using a UV Probe software to obtain chlorophyll-a, b and carotenoid concentrations using standard equations,
Antenna size, which is representative of photosystem II, was assessed during growth by calculation of the ratio ch-a/ch-b [30].
2.4. Biochemical Analysis
Biochemical compounds were assessed in the stationary phase of Nephroselmis cultured under different conditions of intensities and colors of light. The total lipids content was determined after extraction with a solvent mixture of chloroform, methanol (Sigma-Aldrich, St. Louis, MO, USA) and water in a 2:2:1.8 ratio (v/v/v) according to Bligh and Dyer [31]. The total protein concentration was determined using the method of Lowry et al. [32]. However, the carbohydrates accumulated by cells were quantified using the phenol-sulfuric acid method and glucose as the reference standard [33]. The determination of total polyphenol was performed using Folin–Ciocalteu’s phenol reagent and gallic acid standard (Sigma-Aldrich, St. Louis, MO, USA) according to the Singleton colorimetric method [34]. Values were expressed as a percentage of the total dry weight (D.W).
2.5. Statistical Analysis
The normality of the data was assessed using the Shapiro–Wilk test and the variance was stabilized by applying a logarithmic transformation of the values (Log (X + 1)). Data were expressed as mean ± S.D (standard deviation) when needed. In order to understand the effect of light spectrum and intensity, values corresponding to the growth and biochemical parameters of Nephroselmis were submitted to one-way analysis of variance (ANOVA) followed by pairwise comparison using Bonferroni post hoc correction. The paired Student-t test was used to evaluate the effect of light intensity and color on the amounts of pigment accumulated at the beginning and end of the culture. The means were significantly different at the 95% confidence level. Growth parameters and biochemical analyses were performed in duplicate, while the pigments were quantified three times along with the degree of freedom corresponding to the number of values (N) − 1. All statistical analyses were conducted using XLstat-2019 (Version 2.2).
3. Results
3.1. Kintetic Growth of Nephroselmis
Figure 2 depicts the growth curves of Nephroselmis sp. and the daily pH values for each combination of light intensity and color treatment. After an initial adaptation-delay period of 3 days, all curves showed an intense and prolonged exponential phase, which was more intense under high light (15,000 lux), but lasted only until the 11th day, while under low light it lasted up to the 13th day. The pH fluctuated in the alkaline range, with an initial value of 8.7 that quickly rose to 9.86 under high light.
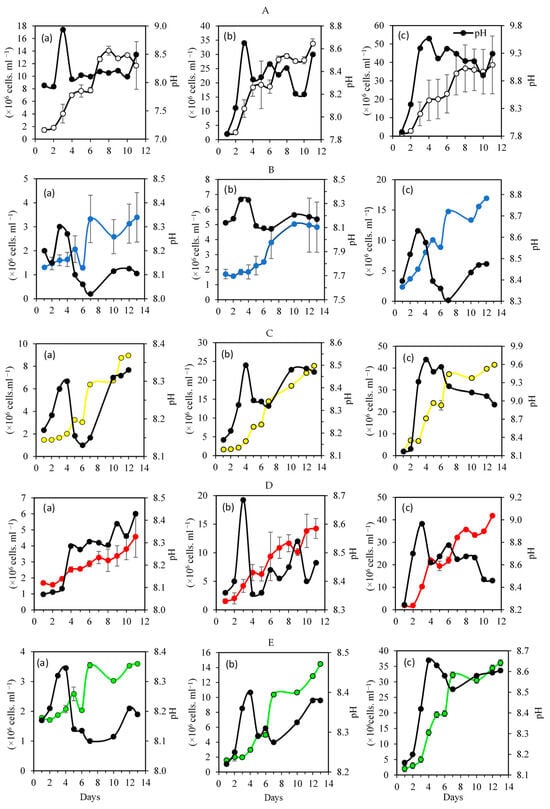
Figure 2.
Kinetic growth of Nephroselmis sp. reared under different combined color and intensity values: (A) white light; (B) blue light; (C) yellow light; (D) red light; (E) green light. (a) 3000 lux, (b) 8000 lux, (c) 15,000 lux. The black line with solid black points in each subfigure corresponds to the pH, while the colored lines correspond to the number of cells.
Under the white color, the growth of Nephroselmis increased with light intensity (Figure 2A). The specific growth rate (µ) ranged from 1.47 ± 0.0 to 2.09 day−1 at 3000 to 15,000 lux, respectively (Table 1). In the stationary phase, the cell density reached 33.00 ± 11.70 × 106 cells mL−1, but this corresponded to a low productivity of 0.12 ± 0.02 g mL−1. d−1 (Table 1). This may be due to the low generation time recorded at 15,000 lux, which was about 0.33 h (Table 1). The one-way ANOVA showed that the intensities significantly affected µ (p = 0.003), but the productivity did not change significantly (Table 1). The pH above 9 confirms a high photosynthetic activity at 15,000 lux (Figure 2A(c)).

Table 1.
Effects of colors (white, blue, yellow, red and green) and intensity (3000, 8000 and 15,000 Lux) of light on growth parameters and biochemical compounds in Nephroselmis sp. grown in artificial seawater. Means ± SD (n = 2).
Under blue light, the growth of Nephroselmis increased with intensity, and the maximum cell density reached 18.00 ± 0.41 × 106 cells mL−1 (Figure 2B, Table 1). The specific growth rate varied significantly (ANOVA, p = 0.029) between 1.79 ± 0.07 and 2.02 ± 0.00 day−1 under 3000 and 15,000 lux, respectively. Under low light intensity, the pH started at 8.4 and remained stable around that value for 15 days (Figure 2B). Under 15,000 lux, the pH value was higher, ranging from 8.32 to 8.82 for 3000 lux and 8000 lux. This was the result of the intense metabolic activity that protects cells from stress. The highest productivity was recorded at 8000 lux, averaging 0.12 ± 0.02 day−1, but it did not exceed 0.05 day−1 for the two other intensities (Table 1).
Under low-intensity yellow light, the maximum concentration of cells of Nephroselmis sp. was low, at 9 × 106 cells mL−1 recorded on day 15 (Figure 2C(a)). However, at 15,000 lux, the concentration reached 40 × 106 cells mL−1 in 5 days (Figure 2C(c)), corresponding to a high specific growth rate of 2.15 ± 0.03 day−1 (Table 1). Nephroselmis recorded the lowest productivity of 0.02 g mL−1 d−1 when cultured under 3000 and 15,000 lux, but exhibited the highest productivity under 8000 lux, reaching 0.21 ± 0.09 g mL−1d−1 (Table 1). The ANOVA revealed that the light intensities significantly changed the growth parameters, except for productivity (Table 1).
The culture incubated under yellow light and 15,000 lux recorded the highest pH averaging 9.7 (Figure 2C(c)), suggesting a high photosynthetic activity.
Under the red color, the growth curve of Nephroselmis showed the same pattern, with the highest density of cells reached on the 10th day (Figure 2D). However, increasing the light intensity significantly enhanced (ANOVA: p = 0.003) the proliferation of Nephroselmis, with a maximum yield of 32 ± 0.03 × 106 cells mL−1 recorded at 15,000 lux (Figure 2D(c), Table 1). Similarly to blue, the productivity recorded for the Nephroselmis reared under red light was low, and did not exceed 0.06 g mL−1 d−1 at the lowest intensity (Table 1). At 3000 lux, the pH increased proportionally with the cell’s concentration, reaching 8.44 at ten days (Figure 2D(a)).
Under green light, the specific growth rate and the density of cells increased significantly with light intensity (p < 0.01) (Figure 2E, Table 1).
Moreover, the quality of light (color) strongly affects the growth parameters of the Nephroselmis under each intensity level (p < 0.05) (Table 1).
3.2. Pigments
Figure 3 illustrates the influences of different light intensities (3000, 8000 and 15,000 Lux) and light colors (white, blue, green, yellow, and red) on the pigment production of Chl-a, Chl-b and Carotenoids in Nephroselmis sp. during the lag (T1) and stationary (T2) phases. In general, the pigment contents were significantly higher during T1 compared to T2 across all treatments, suggesting active pigment biosynthesis during early growth.
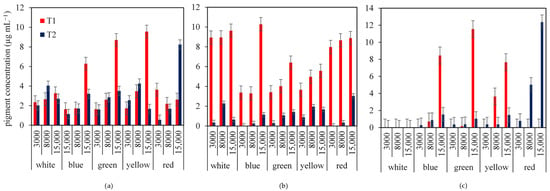
Figure 3.
Effects of combined light intensities (3000, 8000, and 15,000 lux) and colors (white, blue, green, yellow and red) on pigments biosynthesis in Nephroselmis sp. at the lag (T1) and the stationary (T2) phases of culture: (a) chlorophyll-a, (b) chlorophyll-b, (c) carotenoids.
Chl-a concentrations peaked under yellow light at 15,000 Lux during the lag phase, exceeding 10 μg/mL. Notably, blue and green light at 15,000 Lux also stimulated high levels of chl-a corresponding to antenna sizes of 2.80 and 2.46, respectively (Table 1). However, during the stationary phase, pigment levels were more balanced, with red light at 15,000 Lux promoting the highest chl-a concentration, indicating a possible photo-adaptive response (Figure 3a). Chl-b exhibited the highest productivity when Nephroselmis was cultured under white and red colors to reach 10 µg mL−1 at the latency phase (Figure 3b). However, at T2, the concentration of this pigment decreased and did not exceed 2 µg mL−1 (Figure 3b). Carotenoids were absent during T1 under white and red light. However, under blue, green and yellow colors, carotenoid production started at 8000 and 15,000 lux to reach 8 and 12 µg mL−1, respectively, then decreased drastically at the stationary phase (Figure 3c). At T2, Nephroselmis synthesized carotenoids under red light, and the concentrations increased with intensity, reaching 13 µg mL−1 at 15,000 lux (Figure 3c). Nephroselmis exhibited the highest antenna size of the photosystem above 5 for white (3000 lux), white (15,000 lux), blue (8000 lux), red (8000 lux) and green (3000 lux) (Table 1). The yellow light exhibited the lowest antenna size, which did not exceed 2.86 (Table 1).
3.3. Biosynthesis of Biochemical Compounds
The heatmaps show the combined effects of light colors and intensities on the synthesis of proteins, carbohydrates, lipids and polyphenols in Nephroselmis (Figure 4).
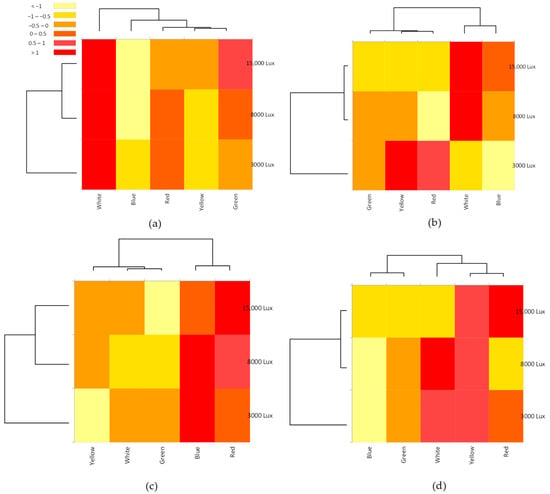
Figure 4.
Heatmaps of the combined effect of colors (white, blue, red, yellow and green) and high-intensity light (3000, 8000 and 15,000 lux) on the protein (a), carbohydrate (b), lipids (c) and polyphenols (d) biosynthesized by Nephroselmis sp. The color scale represents concentration levels from yellow (low) to red (high).
Nephroselmis exhibited the highest protein content when cultured under white light, with significant increases at 3000, 8000 and 15,000 lux (ANOVA: F = 26.77; d.f. = 5; p = 0.012) (Figure 4a, Table 1). The Bonferroni post-hoc test revealed that the highest intensities (8000 and 15,000 lux) did not affect protein contents (Table 1). Blue light inhibited protein synthesis that did not exceed 33.83% of total dry weight (Figure 4a, Table 1). The green light at 15,000 lux seems to have enhanced the production of protein (Figure 4a).
Carbohydrates were accumulated when Nephroselmis was cultured under white light at 8000 lux and 15,000 lux, and yellow and red lights at 3000 lux (Figure 4b). One-way ANOVA showed that the intensities significantly affected carbohydrate synthesis (Table 1). Although the amounts of carbohydrates increased with light intensity for white and blue light, the contents of this molecule were negatively affected with increases in the intensities of yellow, red and green lights (Table 1). Nephroselmis synthesizes more protein than carbohydrates under yellow, red and green light (Table 1).
Our results show that the light intensities did not significantly affect the lipid and polyphenol contents (p > 0.05) in Nehproselsmis for each color except for the blue at 15,000 lux, which enhances polyphenol accumulation (F = 40.49, d.f. = 5, p = 0.007) (Table 1). Nephroselmis reared under blue and red light accumulated lipids that reached 19.00 ± 1.41% DW at 8000 lux and 21.00 2.83% DW at 15,000 lux, respectively (Figure 4c, Table 1). The heatmaps show that the highest polyphenols contents were recorded under white, yellow and red light colors (Figure 4d). Blue light at 3000 and 8000 inhibited the polyphenol accumulation (Figure 4d).
4. Discussion
4.1. Growth of Nephroselmis
This study investigates the photo-physiological and metabolic responses of the chlorophyceae Nephroselmis sp. when exposed to different light intensities and spectral qualities. To the best of our knowledge, there have been no works published on the metabolite contents of Nephroselmis under various light spectra. Our results clearly indicate that both light parameters significantly modulate growth kinetics, pigment synthesis, and the accumulation of key biochemical compounds, suggesting the capacity of this Chlorophyceae to adapt to the fluctuation of the light environment.
Our strain grows well under various combinations of high light intensities (3000, 8000 and 15,000 lux) and light colors (white, blue, yellow, red and green) (Table 1, Figure 2). Several studies have reported that microalgae grow best in terms of maximum cell density, specific growth rate, and biomass yield under high illumination [20,26,35]. Nevertheless, the doubling time decreases with increasing light intensity under all spectral colors. Hotos and Avramidou [26], who cultured the same strain at 2000 and 8000 lux under white light at 40 ppt salinity, recorded 35 × 106 cells mL−1 at ten days of growth. This density was similar to the cell density that we obtained under the same conditions (Figure 2A(b)). Moreover, ANOVA showed that 15,000 lux of white light did not significantly increase the number of cells of Nephroselmis (p > 0.05; Table 1). We assumed that a moderate intensity (8000 lux) of white light is sufficient for producing this microalga at a laboratory scale. This was confirmed by the decrease in productivity recorded at the highest light intensity, which did not exceed 0.12 ± 0.02 g mL−1 d−1 (Table 1). However, Nephroselmis reared under yellow, red and green, but not blue, required 15,000 lux to reach the highest density, averaging 30 × 106 cells mL−1. The ANOVA completed by the Bonferroni post-hoc test revealed that any light color at 15,000 lux did not significantly affect the maximum cell density of Nephroselmis (F = 4.14; d.f. = 9; p = 0.075), but had a significant impact at 3000 and 8000 lux (p = 0.002) (Table 1). While the high light intensity of 15,000 lux did not augment the cell density, it significantly improved the specific growth rate, which exceeded 2 day−1 for all light colors except red (≈1.3 day−1) (Table 1). Interestingly, yellow and green light supported the highest growth rates across intensities, suggesting that these spectra may be optimal for cultivating Nephroselmis at the laboratory scale, as previously noted in Dunaliella and Nannochloropsis cultures [2,21]. He et al. [36] denoted that green light, compared to other color spectra, could trigger the microalgae to establish an efficient system for energy and carbon storage. De Mooij et al. [37] stated that yellow light increases the biomass of Chlamydomonas reinhardtii more than when cultured under blue and red spectra. Nephroselmis reared under blue light exhibited the lowest density (Max = 13 × 106 cells mL−1) and productivity (Max = 0.12 g mL−1 d−1) (Table 1). The higher photon energy of blue light may generate photo-oxidative stress and reduce carbon assimilation efficiency [18,24]. However, other studies have found that the biomass of some species of diatoms (Nitzschia frigida, Cyclotella cryptica) [14,15], chlorophyceae (Chlorella pyrenoidosa, Nannochloropsis sp.) [22,38], cyanobacteria (Oscillatoria sp.) [18], and the dinoflagellates Alexandrium tamarense [16] increases under blue light. Nevertheless, red and white light enhance the biomass production of the Chlorophyceae Ankistrodesmus sp., Tetraselmis suecica and Chlorella vulgaris [18,39,40], and the cyanobacteria Spirulina platensis [17].
4.2. Light Quality Effect on Pigments Biosynthesis
The pigment profile of Nephroselmis revealed a distinct phase-dependent pattern, with higher chlorophyll and carotenoid content during the early (lag) phase, followed by a reduction in the stationary phase. We suggested a down-regulation of light-harvesting photosystems as cells transition from active growth towards the stationary phase [3]. This study has shown that yellow light was particularly effective in enhancing chlorophyll-a biosynthesis in Nephroselmis, especially at 3000 and 8000 lux [2,6]. Mohsenpour and Willoughby [41] reported that green light increases the chlorophyll content in Chlorella vulgaris compared to blue, yellow, orange and red. However, Tetraselmis suecica synthesizes more chlorophyll when illuminated with blue light [39]. Interestingly, red light induced carotenoid accumulation in the xanthophyll cycle of Nephroselmis with a high chl-a content during the stationary phase at the highest intensities (Figure 2c), suggesting the protective effects of carotenoids on chlorophyll. Carotenoids, as secondary metabolites, particularly in green microalgae, are recognized by their internal antioxidant properties, such as reducing the oxidative damage of the photosynthetic apparatus (i.e., photoinhibition) by non-photochemical quenching through the dissipation of excess energy in the form of heat [42,43,44]. Photoinhibition enhanced the production of highly reactive oxygen species (ROS), such as O2.−, H2O2, and OH. At low concentrations, these metabolic byproducts act as signaling molecules, triggering various mechanisms against light stress by quenching ROS [45,46]. However, high levels of ROS can be harmful to cellular structures, physiological functions, and biochemical compounds [46,47]. In response to oxidative stress, microalgae produce various small non-enzymatic antioxidant compounds, such as glutathione, vitamins, alkaloids, flavonoids, and carotenoids, which neutralize the excess ROS [45]. In Nephroselmis, when exposed to red light at a high intensity of 15,000 lux, the highest lipids level was recorded, reaching 21 ± 2.83 % (Table 1), confirming that this wavelength was the most stressful for this microalga, as reported by Shi et al. [48]. Our results corroborate the finding of Baba et al. [49], who illuminated the green alga Botryococcus braunii with red light. However, the blue wavelength enhances the production of carotenoids compared to the red in Dunaliella salina [20]. The increase in the Chl-a/Chl-b ratio under red light, at 8000 to 15,000 lux (Table 1), suggests a potential adjustment of the photosynthetic antenna size, likely as a photoacclimation mechanism that optimizes photon capture under high irradiance [30]. This acclimation relies on the biosynthesis of specific pigments through the xanthophyll cycle and the function of the stress-related light-harvesting complex proteins LI818, which are known as LHCSR in Chlorophyceae [50,51]. This plasticity could be advantageous for biotechnological applications, where the yield of a mixture of pigments (Chl-a and carotenoids) is the objective.
4.3. Macromolecule Modulation
The analysis of macromolecules in Nephroselmis during growth demonstrated its ability to modulate its biochemical profile in response to different light spectra and irradiance. Under our light conditions, Nephroselmis accumulated more protein, followed by carbohydrates, lipids and polyphenols (Table 1).
The highest protein content was obtained under white and green light (Figure 4a), which may reflect an optimal balance between energy input and photosynthetic efficiency [5]. The lowest protein content was obtained with blue light. This pattern is quite similar to that obtained with Arthrospira platensis reared in a photobioreactor under various colors of light diodes [23,52]. However, the works of Chen et al. [53] and Raqiba and Sibi [54] show that Isochrysis sp., Arthospira platensis, Chlorella vulgaris and Scenedesmus obliquus illuminated with blue light produce more protein compared to white light. ANOVA has displayed that both colors and intensities significantly affect (p < 0.01) the protein amount accumulated in Nephroselmis (Table 1). Our study has indicated that the highest light intensity for each color inhibited the proliferation of Nephroselmis cells, leading to a metabolic shift towards protein accumulation [54]. The increase in protein content in Nephroselmis may be ascribed to the fact that white and green light spectra promote the expression of the light-inducible protein (LIP) gene, which contains three responsible motifs sensitive to light [55,56]. In contrast, carbohydrate accumulation was more variable and seemed to depend more on light quality than on intensity. Yellow light strongly stimulated carbohydrate synthesis when Nephroselmis was cultivated at the lowest intensity [18]. Overall, Nephroselmis appears to be rich in carbohydrates, which accounted for more than 20% of the dry weight under all combinations of light intensity and quality. Our light intensities and spectra promoted the expression of ribulose 1, 5-bisphosphate carboxylase/oxygenase (RuBisCO) large subunit (rbcL gene), which catalyzes the fixation of carbon by Rubisco during the dark reaction of the Calvin cycle [57,58]. Carbohydrates derived from microalgae represent a potential feedstock for producing bio-alcohols, such as bioethanol and biobutanol [36]. Microalgae utilize atmospheric CO2 and modulate Rubisco to produce energy-rich molecules, including carbohydrate and lipids [59].
The lipid content in Nephroselmis did not significantly change with increasing light intensities (p > 0.05) across all light colors (Table 1). However, it was enhanced under red and blue light at 8000–15,000 lux. This finding aligns with those groom previous studies showing that stress conditions, including high light exposure, can stimulate lipid synthesis as a photoprotective strategy [23,24]. Both red and blue light may activate the acetyl co-enzyme A carboxylase (ACC) gene, which encodes the rate-controlling enzyme for fatty acid biosynthesis, thereby enhancing the cellular lipid content [60,61,62]. ACCase, a key enzyme for lipid accumulation, can catalyze the conversion of acetyl-CoA to malonyl-CoA and cause it to enter the lipid biosynthesis pathway [57]. Nephroselmis reared under red and blue light could represent an available product for biodiesel production. Similarly, the accumulation of polyphenols under high-intensity red and white light can also reflect an oxidative stress response, suggesting their role as protective antioxidants as well as carotenoids [6,7,63].
Author Contributions
Conceptualization, G.N.H. and H.A.; methodology, I.S.; software, I.S. and W.G.; validation, W.G. and M.E.-k.; formal analysis, I.S.; investigation, I.S.; resources, G.N.H.; data curation, I.S. and W.G.; writing—original draft preparation, I.S.; writing—review and editing, W.G.; visualization, I.S. and W.G.; supervision, H.A. and G.N.H.; project administration, G.N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
This work was conducted as part of a collaborative project between the University of Sfax (Tunisia) and the University of Patras (Greece). This study was supported by the Tunisian Ministry of Scientific Research and Technology and the Greek ministry of high education. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| d.f. | Degree of freedom |
| D.W | Dry weight |
| d | Day |
| ROS | Reactive Oxygen Species |
References
- Esteves, A.F.; Salgado, E.M.; Vilar, V.J.P.; Gonçalves, A.L.; Pires, J.C.M. A Growth Phase Analysis on the Influence of Light Intensity on Microalgal Stress and Potential Biofuel Production. Energy Convers. Manag. 2024, 311, 118511. [Google Scholar] [CrossRef]
- Hotos, G.N. Quantity and Quality of Light on Growth and Pigment Content of Dunaliella sp. and Anabaena sp. Cultures and the Use of Their Absorption Spectra as a Proxy Method for Assessment. JMSE 2023, 11, 1673. [Google Scholar] [CrossRef]
- Ángeles, R.; Carvalho, J.; Hernández-Martínez, I.; Morales-Ibarría, M.; Fradinho, J.C.; Reis, M.A.M.; Lebrero, R. Harnessing Nature’s Palette: Exploring Photosynthetic Pigments for Sustainable Biotechnology. New Biotechnol. 2025, 85, 84–102. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.; Sun, J.; Wei, J.; Ma, Q.; Gao, X. Recent Advances in Microbial Synthesis of Free Heme. Appl. Microbiol. Biotechnol. 2024, 108, 68. [Google Scholar] [CrossRef]
- Costa, M.M.; Spínola, M.P.; Prates, J.A.M. Microalgae as an Alternative Mineral Source in Poultry Nutrition. Vet. Sci. 2024, 11, 44. [Google Scholar] [CrossRef]
- Razzak, S.A. Comprehensive Overview of Microalgae-Derived Carotenoids and Their Applications in Diverse Industries. Algal Res. 2024, 78, 103422. [Google Scholar] [CrossRef]
- Guermazi, W.; Masmoudi, S.; Trabelsi, N.A.; Gammoudi, S.; Ayadi, H.; Morant-Manceau, A.; Hotos, G.N. Physiological and Biochemical Responses in Microalgae Dunaliella Salina, Cylindrotheca Closterium and Phormidium Versicolor NCC466 Exposed to High Salinity and Irradiation. Life 2023, 13, 313. [Google Scholar] [CrossRef] [PubMed]
- Aljabory, M.N.; Alhaboubi, N.A. Green Solutions for CO2 Mitigation: Exploring Microalgae-Based Carbon Capture and Utilization Technologies. J. Biotechnol. Res. Cent. 2025, 19, 52–64. [Google Scholar] [CrossRef]
- Akubude, V.C.; Nwaigwe, K.N.; Dintwa, E. Production of Biodiesel from Microalgae via Nanocatalyzed Transesterification Process: A Review. Mater. Sci. Energy Technol. 2019, 2, 216–225. [Google Scholar] [CrossRef]
- Abuhasheesh, Y.; Ghazal, A.; Tang, D.Y.Y.; Banat, F.; Hasan, S.W.; Show, P.L. Advances in Chlorella Microalgae for Sustainable Wastewater Treatment and Bioproduction. Chem. Eng. J. Adv. 2025, 22, 100715. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for High-Value Products: A Way towards Green Nutraceutical and Pharmaceutical Compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef]
- Yu, K.L.; Ong, H.C.; Zaman, H.B. Integrated Energy Informatics Technology on Microalgae-Based Wastewater Treatment to Bioenergy Production: A Review. J. Environ. Manag. 2024, 368, 122085. [Google Scholar] [CrossRef]
- Dai, C.; Wang, F. Potential Applications of Microalgae–Bacteria Consortia in Wastewater Treatment and Biorefinery. Bioresour. Technol. 2024, 393, 130019. [Google Scholar] [CrossRef]
- Shih, S.C.C.; Mufti, N.S.; Chamberlain, M.D.; Kim, J.; Wheeler, A.R. A Droplet-Based Screen for Wavelength-Dependent Lipid Production in Algae. Energy Environ. Sci. 2014, 7, 2366. [Google Scholar] [CrossRef]
- Rochet, M.; Legendre, L.; Demers, S. Photosynthetic and Pigment Responses of Sea-Ice Microalgae to Changes in Light Intensity and Quality. J. Exp. Mar. Biol. Ecol. 1986, 101, 211–226. [Google Scholar] [CrossRef]
- Kwon, H.K.; Oh, S.J.; Yang, H.; Kim, D.; Kang, I.J.; Oshima, Y. Laboratory Study for the Phytoremediation of Eutrophic Coastal Sediment Using Benthic Microalgae and Light Emitting Diode (LED). J. Fac. Agric. Kyushu Univ. 2013, 58, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-B.; Wu, J.-Y.; Wang, C.-F.; Fu, C.-C.; Shieh, C.-J.; Chen, C.-I.; Wang, C.-Y.; Liu, Y.-C. Modeling on Chlorophyll a and Phycocyanin Production by Spirulina platensis under Various Light-Emitting Diodes. Biochem. Eng. J. 2010, 53, 52–56. [Google Scholar] [CrossRef]
- Sharmila, D.; Suresh, A.; Indhumathi, J.; Gowtham, K.; Velmurugan, N. Impact of Various Color Filtered LED Lights on Microalgae Growth, Pigments and Lipid Production. Eur. J. Biotechnol. Biosci. 2018, 6, 1–7. [Google Scholar]
- Hotos, G.N.; Antoniadis, T.I. The Effect of Colored and White Light on Growth and Phycobiliproteins, Chlorophyll and Carotenoids Content of the Marine Cyanobacteria Phormidium sp. and Cyanothece sp. in Batch Cultures. Life 2022, 12, 837. [Google Scholar] [CrossRef]
- Fu, W.; Guðmundsson, Ó.; Paglia, G.; Herjólfsson, G.; Andrésson, Ó.S.; Palsson, B.Ø.; Brynjólfsson, S. Enhancement of Carotenoid Biosynthesis in the Green Microalga Dunaliella salina with Light-Emitting Diodes and Adaptive Laboratory Evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef]
- Pérez-Pazos, J.-V.; Fernández-Izquierdo, P. Synthesis of Neutral Lipids in Chlorella sp. under Different Light and Carbonate Conditions. CTF-Cienc. Tecnol. Y Futuro 2011, 4, 47–58. [Google Scholar] [CrossRef]
- Asuthkar, M.; Gunti, Y.; Rao, R.; Rao, C.S.; Yadavalli, R. Effect of Different Wavelengths of Light on the Growth of Chlorella pyrenoidosa. Int. J. Pharm. Sci. Res 2016, 7, 847–851. [Google Scholar]
- Markou, G. Effect of Various Colors of Light-Emitting Diodes (LEDs) on the Biomass Composition of Arthrospira platensis Cultivated in Semi-Continuous Mode. Appl. Biochem. Biotechnol. 2014, 172, 2758–2768. [Google Scholar] [CrossRef]
- Wang, S.; Stiles, A.R.; Guo, C.; Liu, C. Microalgae Cultivation in Photobioreactors: An Overview of Light Characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar] [CrossRef]
- Qilu, C.; Ligen, X.; Fangmin, C.; Gang, P.; Qifa, Z. Bicarbonate-Rich Wastewater as a Carbon Fertilizer for Culture of Dictyosphaerium sp. of a Giant Pyrenoid. J. Clean. Prod. 2018, 202, 439–443. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D. The Effect of Various Salinities and Light Intensities on the Growth Performance of Five Locally Isolated Microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (Var. Red Pappas), Asteromonas gracilis and Dunaliella sp.] in Laboratory Batch Cultures. J. Mar. Sci. Eng. 2021, 9, 1275. [Google Scholar] [CrossRef]
- Hotos, G.; Avramidou, D.; Mastropetros, S.G.; Tsigkou, K.; Kouvara, K.; Makridis, P.; Kornaros, M. Isolation, Identification, and Chemical Composition Analysis of Nine Microalgal and Cyanobacterial Species Isolated in Lagoons of Western Greece. Algal Res. 2023, 69, 102935. [Google Scholar] [CrossRef]
- Laing, I. Cultivation of Marine Unicellular Algae; Ministry of Agriculture, Fisheries and Food Conwy: Conwy, UK, 1991.
- Perni, S.; Andrew, P.W.; Shama, G. Estimating the Maximum Growth Rate from Microbial Growth Curves: Definition Is Everything. Food Microbiol. 2005, 22, 491–495. [Google Scholar] [CrossRef]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of Photosynthetic Light Energy Utilization by Microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Palanisamy, K.M.; Rahim, M.H.A.; Govindan, N.; Ramaraj, R.; Kuppusamy, P.; Maniam, G.P. Effect of Blue Light Intensity and Photoperiods on the Growth of Diatom Thalassiosira pseudonana. Bioresour. Technol. Rep. 2022, 19, 101152. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of Light Intensity on Physiological Changes, Carbon Allocation and Neutral Lipid Accumulation in Oleaginous Microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef]
- De Mooij, T.; De Vries, G.; Latsos, C.; Wijffels, R.H.; Janssen, M. Impact of Light Color on Photobioreactor Productivity. Algal Res. 2016, 15, 32–42. [Google Scholar] [CrossRef]
- Das, P.; Lei, W.; Aziz, S.S.; Obbard, J.P. Enhanced Algae Growth in Both Phototrophic and Mixotrophic Culture under Blue Light. Bioresour. Technol. 2011, 102, 3883–3887. [Google Scholar] [CrossRef] [PubMed]
- Abiusi, F.; Sampietro, G.; Marturano, G.; Biondi, N.; Rodolfi, L.; D’Ottavio, M.; Tredici, M.R. Growth, Photosynthetic Efficiency, and Biochemical Composition of Tetraselmis suecica F&M-M33 Grown with LEDs of Different Colors. Biotech. Bioeng. 2014, 111, 956–964. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Chow, K.P.; Yung, K.K.L. Effect of Different Light Sources on Algal Biomass and Lipid Production in Internal Leds-Illuminated Photobioreactor. J. Mar. Biol. Aquac 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Willoughby, N. Luminescent Photobioreactor Design for Improved Algal Growth and Photosynthetic Pigment Production through Spectral Conversion of Light. Bioresour. Technol. 2013, 142, 147–153. [Google Scholar] [CrossRef]
- Choochote, W.; Suklampoo, L.; Ochaikul, D. Evaluation of Antioxidant Capacities of Green Microalgae. J. Appl. Phycol. 2014, 26, 43–48. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Recent Advances in Lutein Production from Microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative Stress: Molecular Perception and Transduction of Signals Triggering Antioxidant Gene Defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, K.; Kulikovskiy, M.; Maltseva, S. Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition. Biology 2021, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of Gene Expression by Reactive Oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef]
- Shi, T.-Q.; Wang, L.-R.; Zhang, Z.-X.; Sun, X.-M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef]
- Baba, M.; Kikuta, F.; Suzuki, I.; Watanabe, M.M.; Shiraiwa, Y. Wavelength Specificity of Growth, Photosynthesis, and Hydrocarbon Production in the Oil-Producing Green Alga Botryococcus braunii. Bioresour. Technol. 2012, 109, 266–270. [Google Scholar] [CrossRef]
- Zhu, S.-H.; Guo, J.; Maldonado, M.T.; Green, B.R. Effects of Iron and Copper Deficiency on the Expression of Members of the Light-Harvesting Family in the Diatom Thalassiosira pseudonana (Bacillariophyceae)1: Fe-Cu Deficiency and Lhc Expression. J. Phycol. 2010, 46, 974–981. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, H.; Lin, S. Enhancement of Non-Photochemical Quenching as an Adaptive Strategy under Phosphorus Deprivation in the Dinoflagellate Karlodinium veneficum. Front. Microbiol. 2017, 8, 404. [Google Scholar] [CrossRef]
- Ravelonandro, P.H.; Ratianarivo, D.H.; Joannis-Cassan, C.; Isambert, A.; Raherimandimby, M. Influence of Light Quality and Intensity in the Cultivation of Spirulina platensis from Toliara (Madagascar) in a Closed System. J. Chem. Tech. Biotech. 2008, 83, 842–848. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, Y.-C.; Huang, H.-C.; Huang, C.-C.; Lee, W.-L.; Chang, J.-S. Engineering Strategies for Enhancing the Production of Eicosapentaenoic Acid (EPA) from an Isolated Microalga Nannochloropsis oceanica CY2. Bioresour. Technol. 2013, 147, 160–167. [Google Scholar] [CrossRef]
- Raqiba, H.; Sibi, G. Light Emitting Diode (LED) Illumination for Enhanced Growth and Cellular Composition in Three Microalgae. Adv. Microbiol. Res. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Doron, L.; Segal, N.; Shapira, M. Transgene Expression in Microalgae—From Tools to Applications. Front. Plant Sci. 2016, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.; Lee, Y.; Nam, O.; Park, S.; Sim, S.J.; Jin, E. Introducing Dunaliella LIP Promoter Containing Light-inducible Motifs Improves Transgenic Expression in Chlamydomonas reinhardtii. Biotechnol. J. 2016, 11, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Liu, P.; Xia, J.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Nie, Z.; Qiu, G. The Effect of Mixotrophy on Microalgal Growth, Lipid Content, and Expression Levels of Three Pathway Genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Miyagi, A.; Aoki, S.; Ito, M.; Kadono, Y.; Kosuge, K. Molecular Adaptation of rbcL in the Heterophyllous Aquatic Plant Potamogeton. PLoS ONE 2009, 4, e4633. [Google Scholar] [CrossRef]
- Portis, A.R.; Parry, M.A.J. Discoveries in Rubisco (Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase): A Historical Perspective. Photosynth Res. 2007, 94, 121–143. [Google Scholar] [CrossRef]
- Lopez, J.M.; Bennett, M.K.; Sanchez, H.B.; Rosenfeld, J.M.; Osborne, T.E. Sterol Regulation of Acetyl Coenzyme A Carboxylase: A Mechanism for Coordinate Control of Cellular Lipid. Proc. Natl. Acad. Sci. USA 1996, 93, 1049–1053. [Google Scholar] [CrossRef]
- Bianchi, A.; Evans, J.L.; Nordlund, A.; Watts, T.D.; Witters, L.A. Acetyl-CoA Carboxylase in Reuber Hepatoma Cells: Variation in Enzyme Activity, Insulin Regulation, and Cellular Lipid Content. J. Cell. Biochem. 1992, 48, 86–97. [Google Scholar] [CrossRef]
- Modiri, S.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Evaluation of Transcription Profile of Acetyl-CoA Carboxylase (ACCase) and Acyl-ACP Synthetase (AAS) to Reveal Their Roles in Induced Lipid Accumulation of Synechococcus sp. HS01. Renew. Energy 2018, 129, 347–356. [Google Scholar] [CrossRef]
- Faraloni, C.; Di Lorenzo, T.; Bonetti, A. Impact of Light Stress on the Synthesis of Both Antioxidants Polyphenols and Carotenoids, as Fast Photoprotective Response in Chlamydomonas reinhardtii: New Prospective for Biotechnological Potential of This Microalga. Symmetry 2021, 13, 2220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).