The Aggregate-Mediated Restoration of Degraded Black Soil via Biochar and Straw Additions: Emphasizing Microbial Community Interactions and Functions
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiment Description
2.2. Soil Sampling and Analysis
2.3. Illumina Sequencing and Bioinformatic Analysis
2.4. Statistical Analysis
3. Results
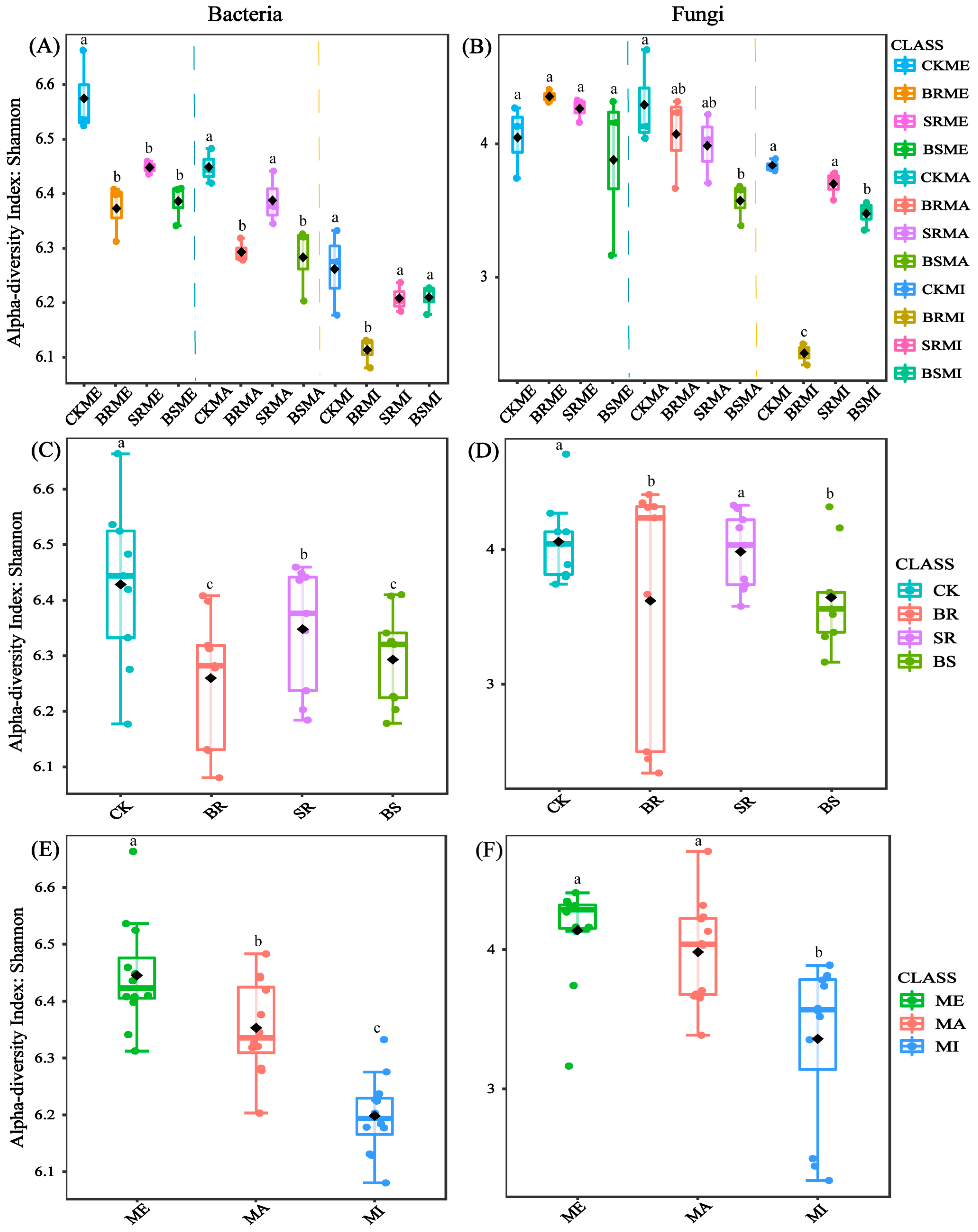
3.1. Microbial Community Diversity
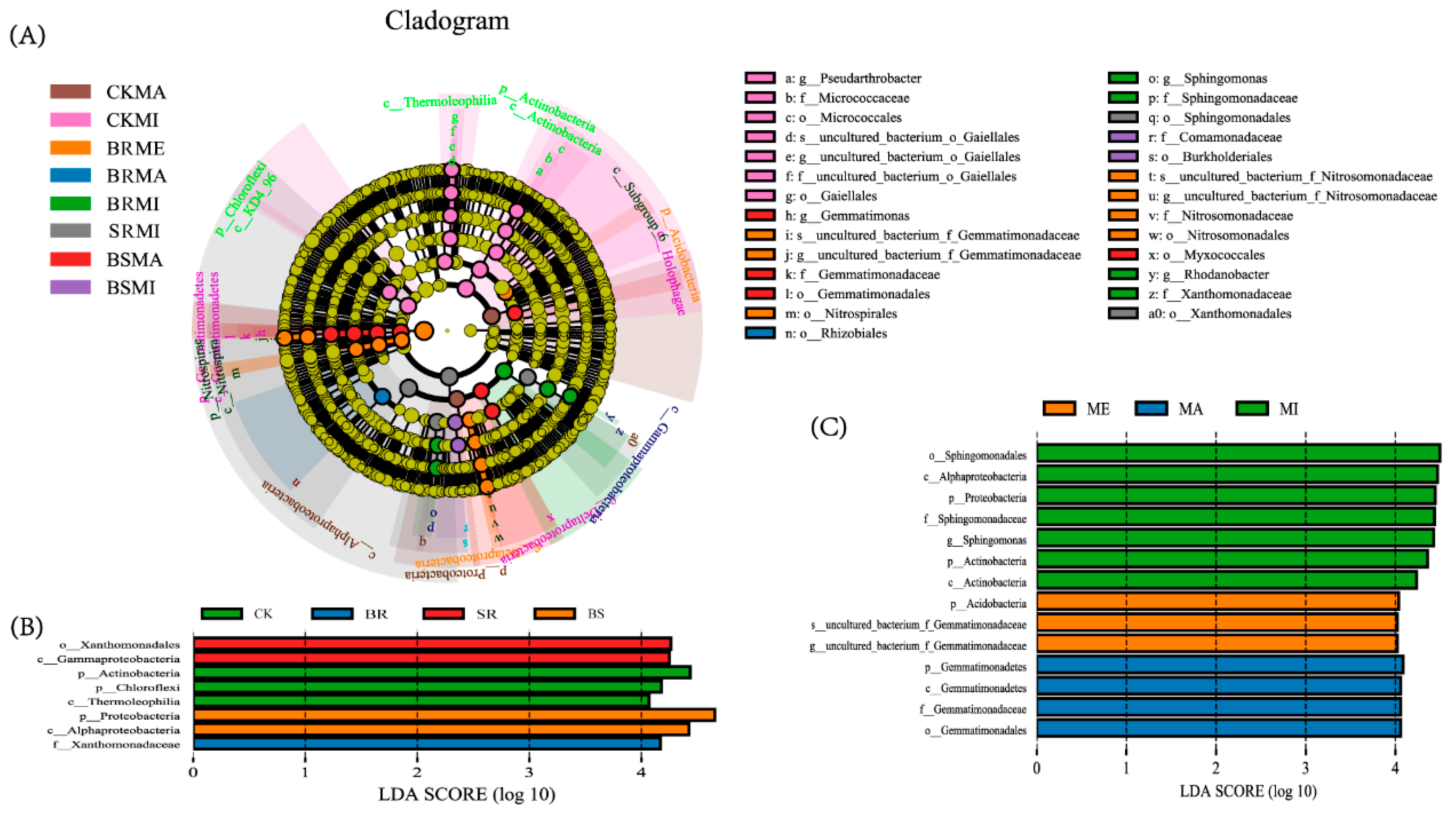
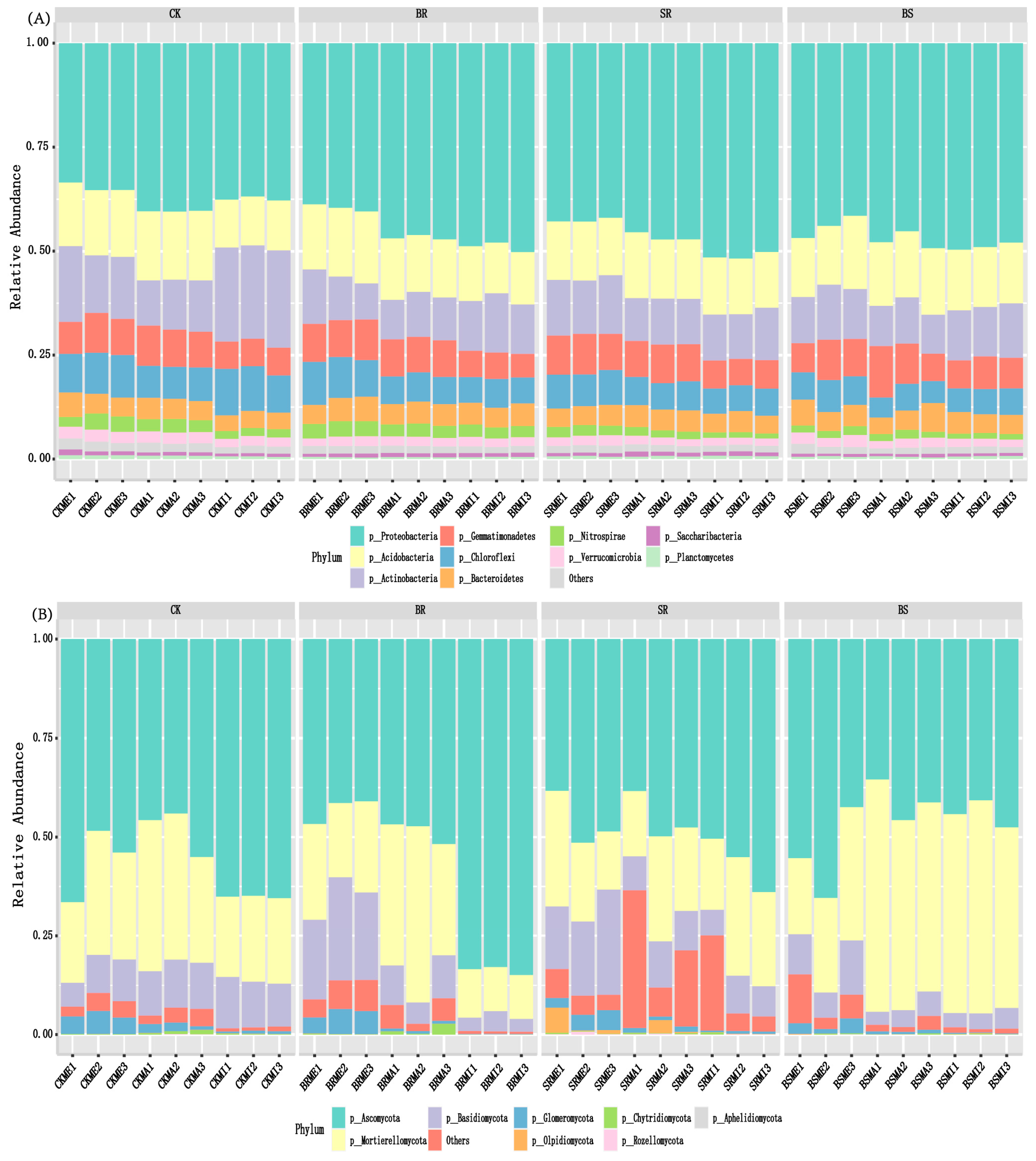
3.2. Bacterial Community Composition
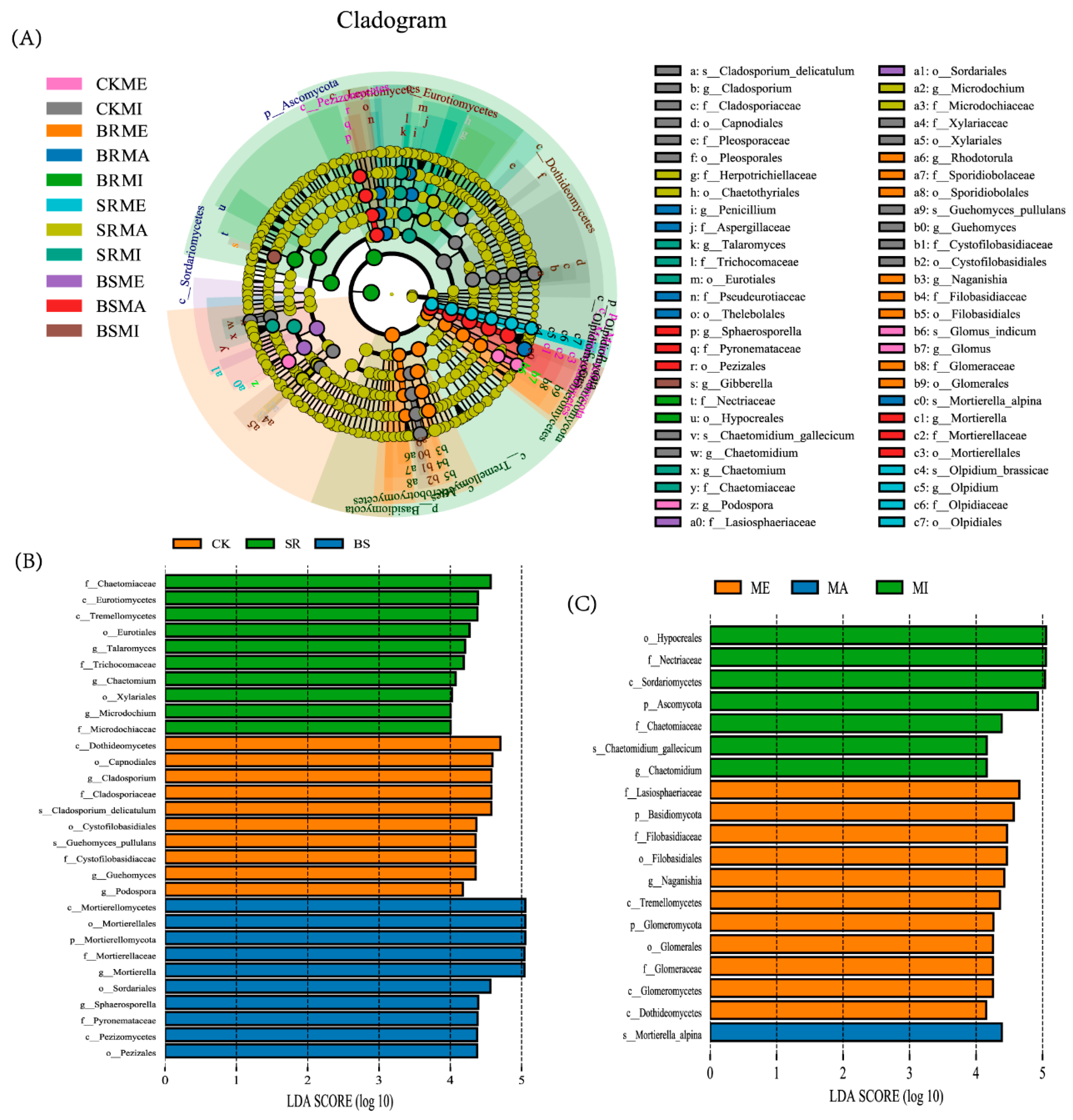
3.3. Fungal Community Composition
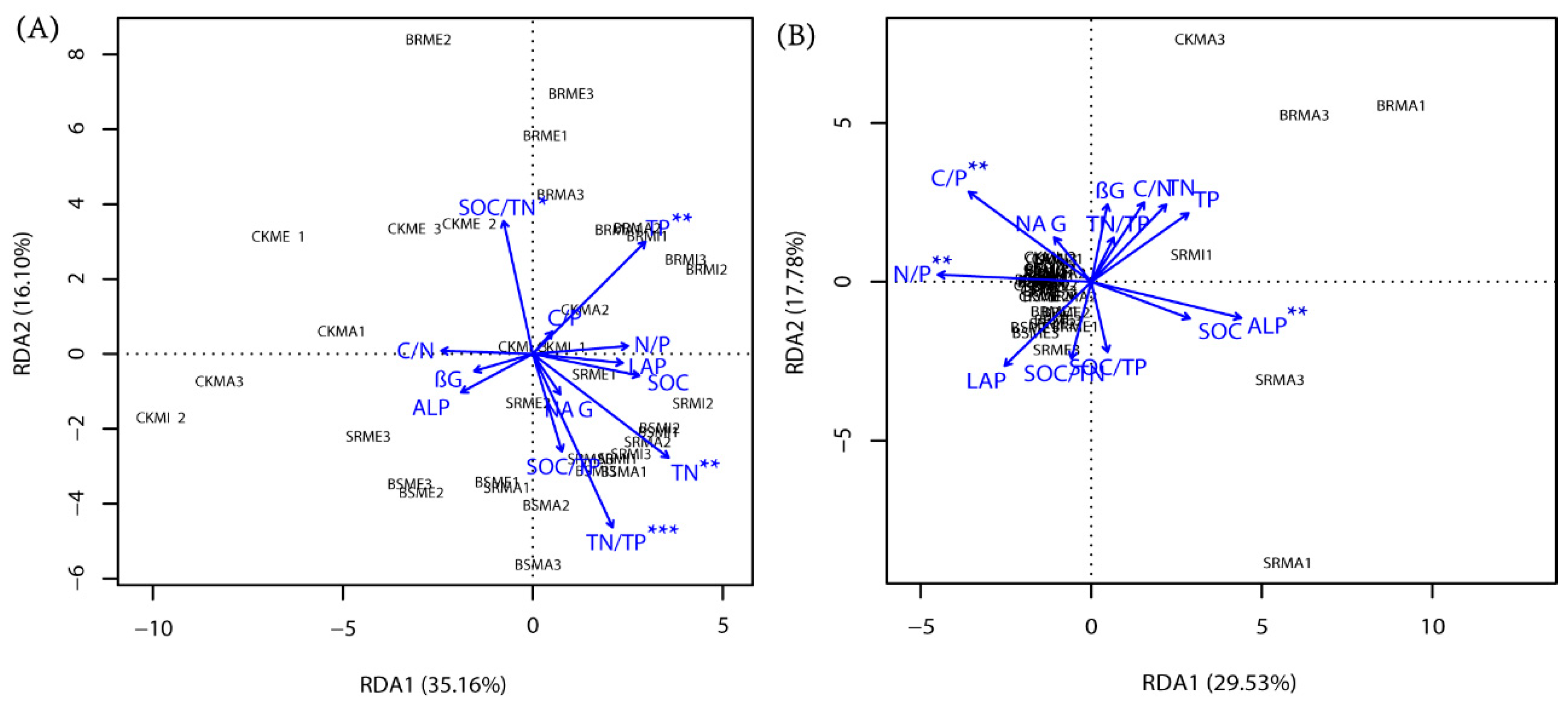
3.4. Redundancy Analyses
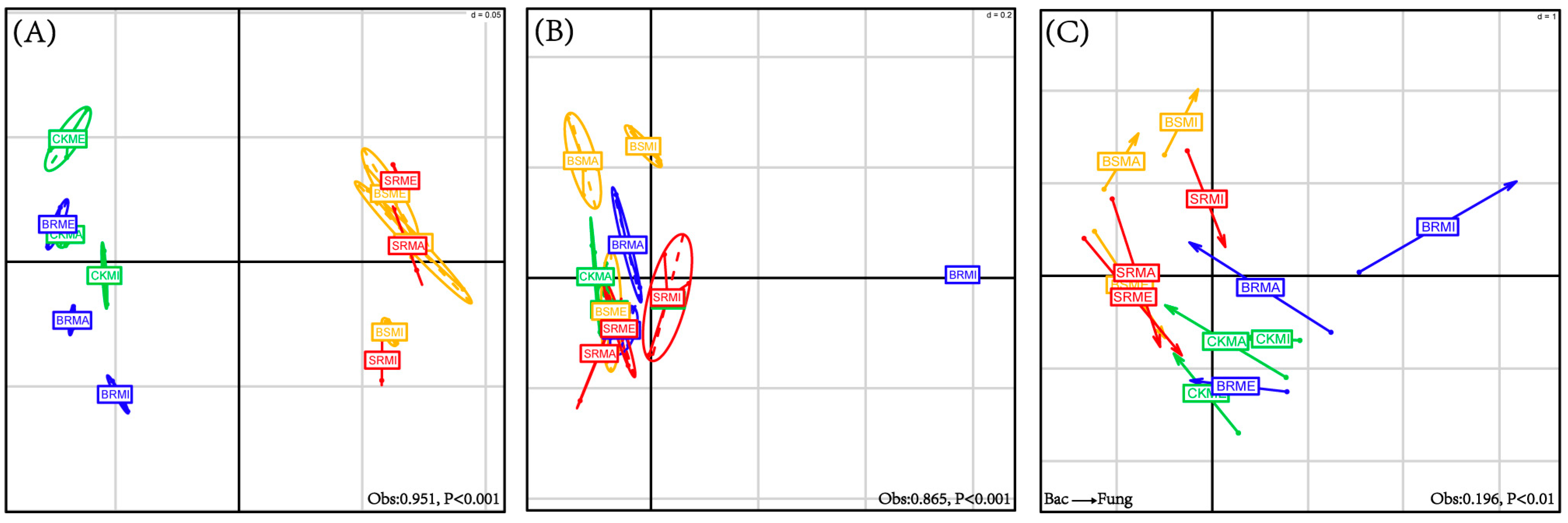
3.5. Between-Class and Co-Inertia Analyses
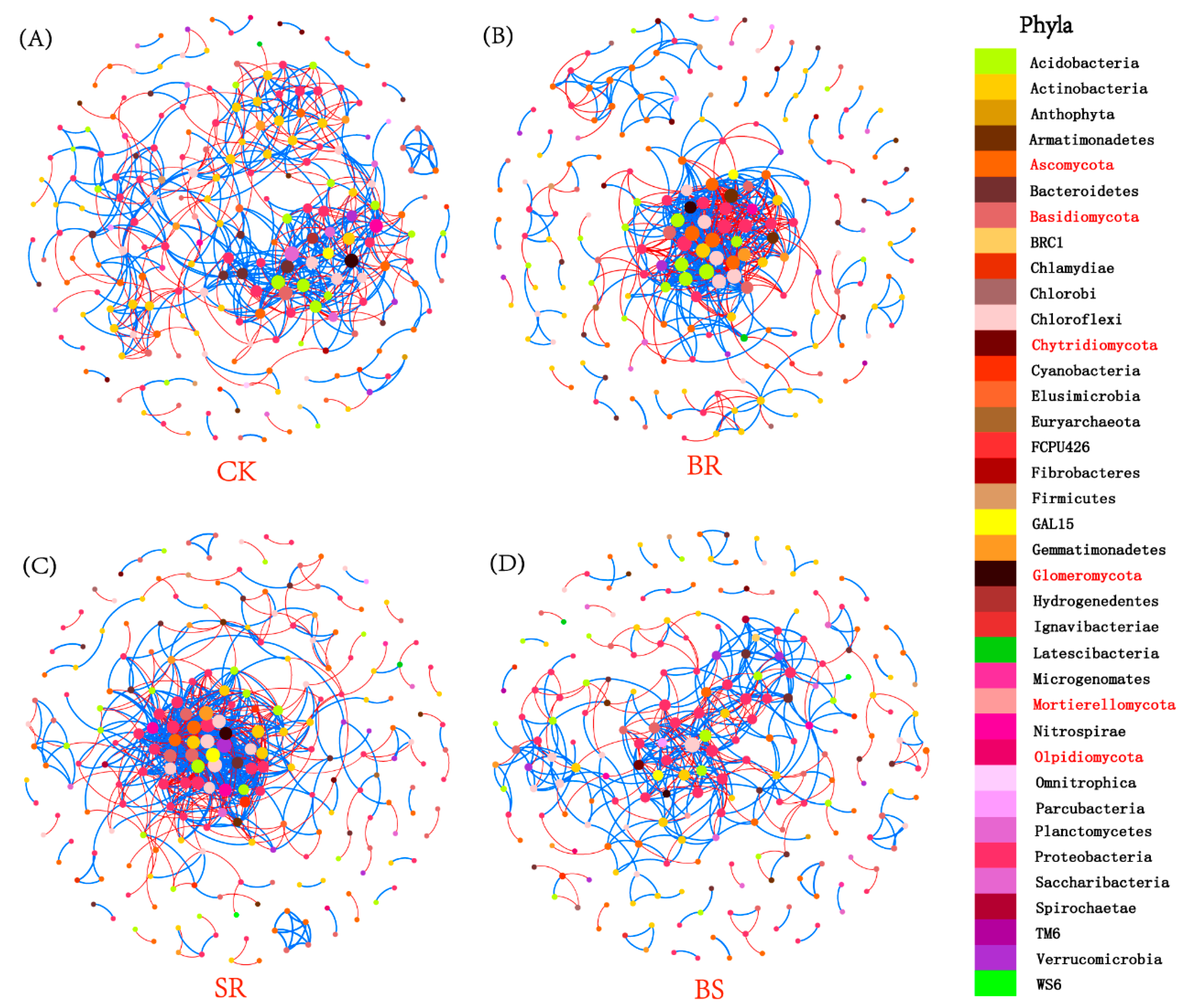
3.6. Microbial Community Co-Occurrence Networks
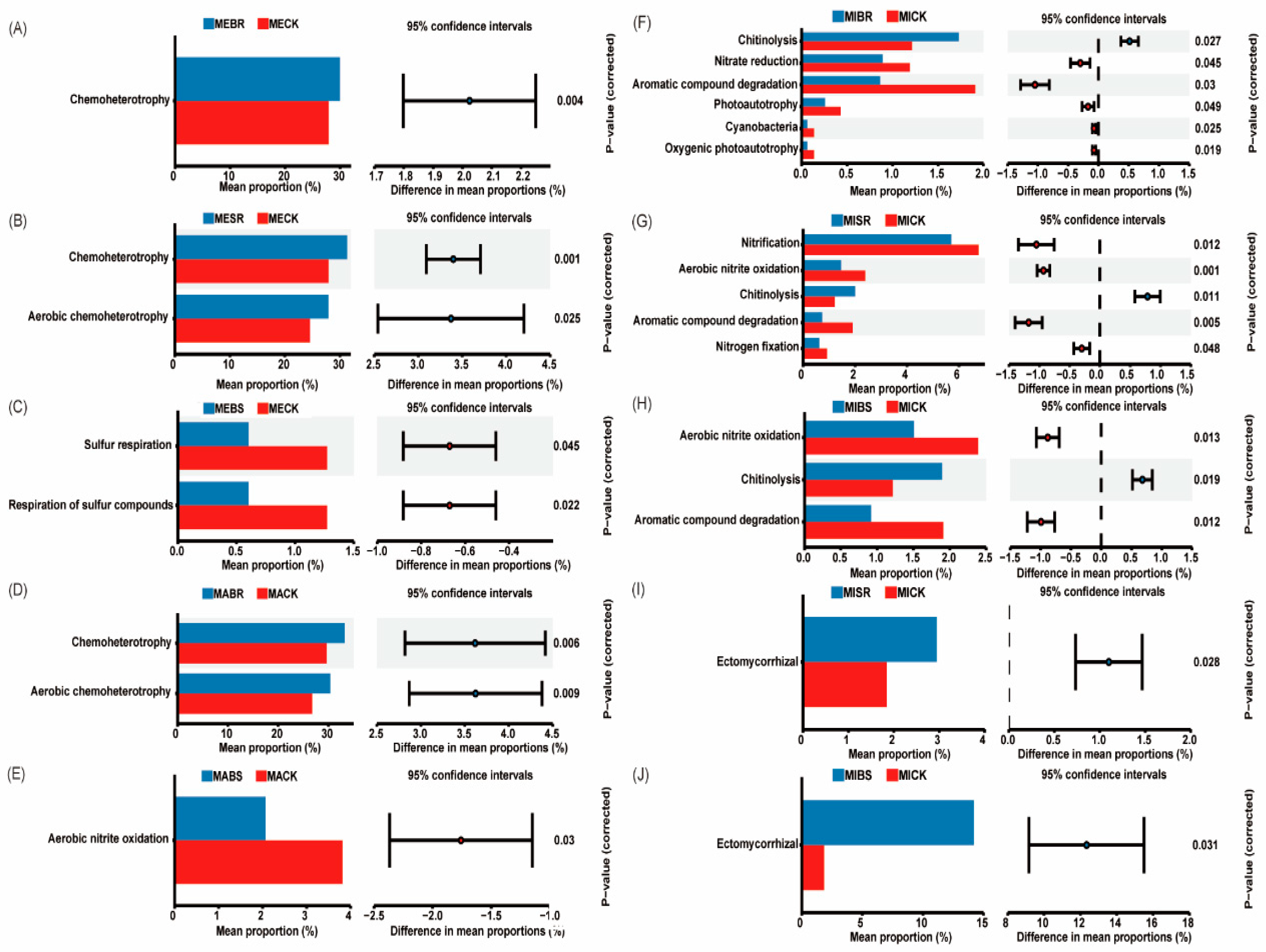
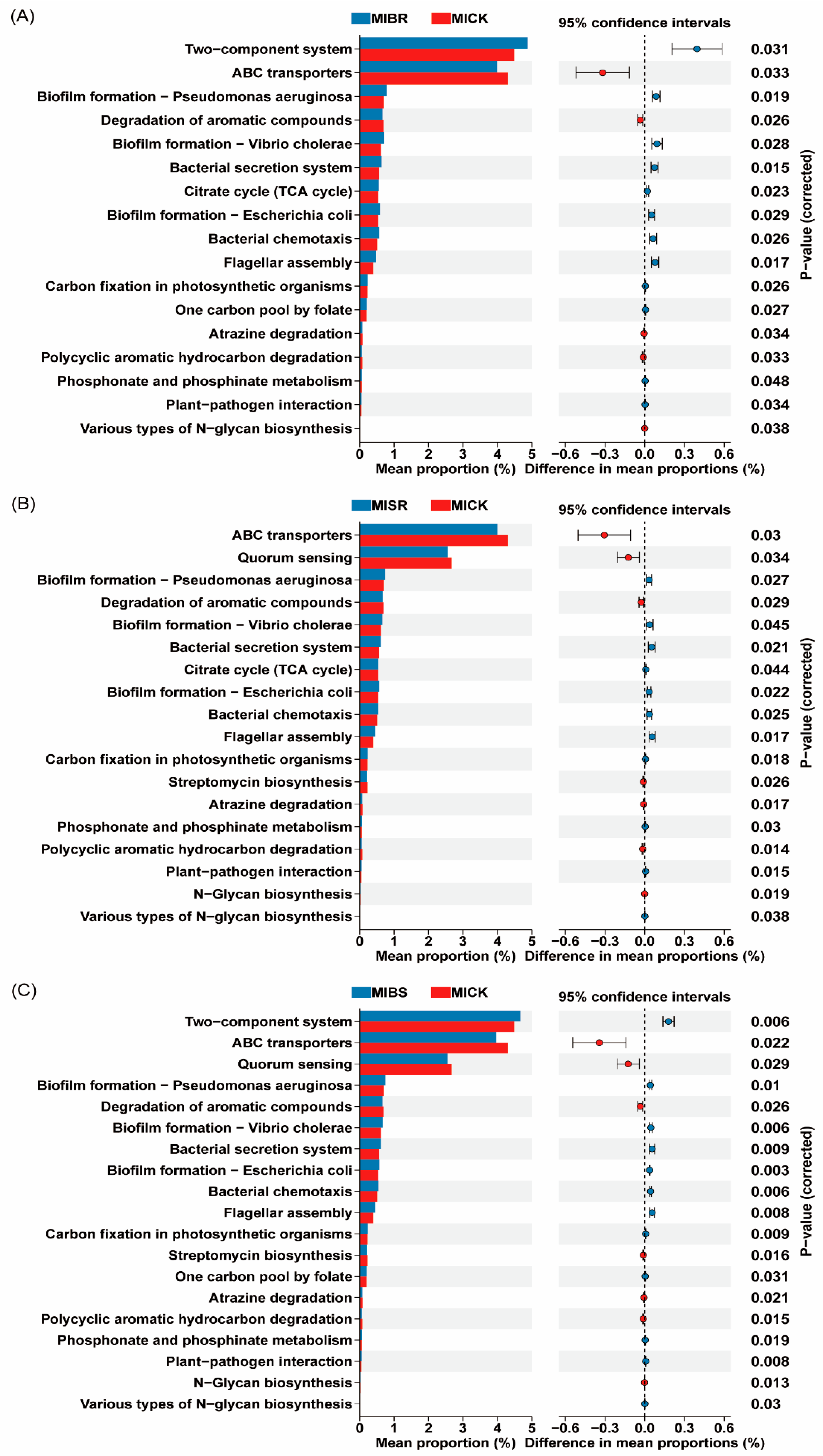
3.7. Function Prediction
4. Discussion
4.1. Straw-Returning Method and Aggregate Fraction Change Soil Microbial Community Structure
4.2. Straw-Returning Method and Aggregate Fraction Change Soil Bacterial Community Composition
4.3. Straw-Returning Method and Aggregate Fraction Change Soil Fungal Community Composition
4.4. Straw-Returning Method and Aggregate Fraction Affect the Interaction of Microbial Community
4.5. Straw-Returning Method and Aggregate Fraction Affect the Function of Microbial Community
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Aggregates | Treatments | SOC (g kg−1) | TN (g kg−1) | TP (g kg−1) | SOC/TN | SOC/TP | TN/TP | βG (IU g−1) | LAP (IU g−1) | NAG (IU g−1) | ALP (IU g−1) | C/N | C/P | N/P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ME | CK | 7.31 c | 0.95 b | 0.49 a | 7.71 c | 14.95 c | 1.94 b | 328.00 a | 1728.00 a | 2848.67 a | 414.67 a | 0.07 a | 0.80 a | 11.17 a |
| BR | 10.31 a | 0.54 c | 0.52 a | 18.97 a | 19.98 b | 1.05 c | 362.67 a | 1859.00 a | 2591.33 a | 427.33 a | 0.08 a | 0.85 a | 10.57 a | |
| SR | 10.47 a | 1.03 a | 0.44 b | 10.23 b | 24.05 a | 2.35 a | 301.33 a | 1843.33 a | 2485.00 a | 411.00 a | 0.07 a | 0.73 a | 10.57 a | |
| BS | 9.34 b | 0.97 b | 0.52 a | 9.64 b | 18.10 b | 1.88 b | 307.67 a | 1792.67 a | 2485.00 a | 372.67 a | 0.07 a | 0.82 a | 11.57 a | |
| MA | CK | 7.73 c | 1.05 b | 0.45 bc | 7.36 b | 17.75 c | 2.41 a | 360.00 a | 1555.00 a | 2412.00 a | 383.67 b | 0.09 a | 0.95 a | 10.53 a |
| BR | 11.61 a | 1.42 a | 0.58 a | 8.18 b | 20.29 bc | 2.48 a | 326.33 a | 1453.67 a | 2516.00 a | 402.67 ab | 0.08 a | 0.82 ab | 10.03 a | |
| SR | 11.12 ab | 1.10 b | 0.50 ab | 10.14 a | 22.24 b | 2.20 a | 340.00 a | 1651.33 a | 2589.00 a | 511.00 a | 0.08 a | 0.67 b | 8.33 a | |
| BS | 10.47 b | 1.05 b | 0.40 c | 9.94 a | 26.51 a | 2.67 a | 396.67 a | 1605.33 a | 2775.33 a | 440.00 ab | 0.09 a | 0.91 a | 10.00 a | |
| MI | CK | 8.72 b | 1.02 c | 0.47 c | 8.51 a | 18.61 a | 2.18 b | 346.67 a | 1349.67 b | 2659.67 a | 435.33 a | 0.09 a | 0.80 a | 9.30 a |
| BR | 8.60 b | 1.25 b | 0.55 a | 6.95 b | 15.52 b | 2.25 b | 302.00 a | 1710.00 ab | 2579.67 a | 375.67 a | 0.07 ab | 0.81 a | 11.60 a | |
| SR | 7.33 c | 1.23 b | 0.51 b | 5.96 b | 14.45 b | 2.43 b | 355.67 a | 1938.33 a | 2808.33 a | 396.00 a | 0.08 ab | 0.90 a | 12.13 a | |
| BS | 10.94 a | 1.54 a | 0.55 a | 7.12 b | 20.17 a | 2.85 a | 299.00 a | 1843.33 a | 2914.67 a | 373.00 a | 0.06 b | 0.80 a | 12.83 a |
References
- Zhang, C.; Zhao, X.; Liang, A.J.; Li, Y.Y.; Song, Q.Y.; Li, X.Y.; Li, D.P.; Hou, N. Insight into the soil aggregate-mediated restoration mechanism of degraded black soil via biochar addition: Emphasizing the driving role of core microbial communities and nutrient cycling. Environ. Res. 2023, 228, 115895. [Google Scholar] [CrossRef]
- Zhang, J.B. Improving inherent soil productivity underpins agricultural sustainability. Pedosphere 2023, 33, 3–5. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.K.; Zhao, B.S.; Ma, M.C.; Guan, D.W.; Li, J.; Chen, S.F.; Cao, F.M.; Shen, D.L.; et al. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Zhang, J.B.; Yin, J.; Huang, S.M. Bacterial community structure after long-term organic and inorganic fertilization reveals important associations between soil nutrients and specific taxa involved in nutrient transformations. Front. Microbiol. 2017, 8, 187. [Google Scholar] [CrossRef]
- Peng, X.H.; Zhu, Q.H.; Zhang, Z.B.; Hallett, P.D. Combined turnover of carbon and soil aggregates using rare earth oxides and isotopically labelled carbon as tracers. Soil Biol. Biochem. 2017, 109, 81–94. [Google Scholar] [CrossRef]
- Cheng, J.; Lin, B.J.; Chen, J.S.; Duan, H.X.; Sun, Y.F.; Zhao, X.; Dang, Y.P.; Xu, Z.Y.; Zhang, H.L. Strategies for crop straw management in China’s major grain regions: Yield-driven conditions and factors influencing the effectiveness of straw return. Resour. Conserv. Recycl. 2025, 212, 107941. [Google Scholar] [CrossRef]
- Liu, R.; Tang, M.; Luo, Z.H.; Zhang, C.; Liao, C.Y.; Feng, S.Y. Straw returning proves advantageous for regulating water and salt levels, facilitating nutrient accumulation, and promoting crop growth in coastal saline soils. Agronomy 2024, 14, 1196. [Google Scholar] [CrossRef]
- Li, H.; Dai, M.W.; Dai, S.L.; Dong, X.J. Current status and environment impact of direct straw return in China’s cropland–A review. Ecotoxicol. Environ. Saf. 2018, 159, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK, 2015. [Google Scholar]
- Sun, Q.; Yang, X.; Meng, J.; Lan, Y.; Han, X.; Chen, W.; Huang, Y. Long-term effects of straw and straw-derived biochar on humic substances and aggregate-associated humic substances in brown earth soil. Front. Environ. Sci. 2022, 10, 899935. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.; Nabel, M.; Roß-Nickoll, M.; Van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef]
- Xu, P.D.; Wang, Q.; Duan, C.J.; Huang, G.Y.; Dong, K.H.; Wang, C.H. Biochar addition promotes soil organic carbon sequestration dominantly contributed by macro-aggregates in agricultural ecosystems of China. J. Environ. Manag. 2024, 359, 121042. [Google Scholar] [CrossRef]
- Chen, L.M.; Sun, S.L.; Yao, B.; Peng, Y.T.; Gao, C.F.; Qin, T.; Zhou, Y.Y.; Sun, C.R.; Quan, W. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 2022, 13, 986763. [Google Scholar] [CrossRef]
- Xiu, L.Q.; Zhang, W.M.; Sun, Y.Y.; Wu, D.; Meng, J.; Chen, W.F. Effects of biochar and straw returning on the key cultivation limitations of Albic soil and soybean growth over 2 years. Catena 2019, 173, 481–493. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, G.; Feng, H.; Sun, B.H.; Zhao, Y.; Chen, H.X.; Chen, J.; Dyck, M.; Wang, X.D.; Zhang, J.G.; et al. Effects of straw and biochar amendments on aggregate stability, soil organic carbon, and enzyme activities in the Loess Plateau, China. Environ. Sci. Pollut. Res. 2017, 24, 10108–10120. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.L.; Wu, F.P.; Zeng, D.H.; Chang, S.X. Wheat straw and its biochar had contrasting effects on soil C and N cycling two growing seasons after addition to a Black Chernozemic soil planted to barley. Biol. Fertil. Soils 2014, 50, 1291–1299. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.L.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Trivedi, P.; Delgado-Baquerizo, M.; Jeffries, T.C.; Trivedi, C.; Anderson, I.C.; Lai, K.; McNee, M.; Flower, K.; Singh, B.P.; Minkey, D.; et al. Soil aggregation and associated microbial communities modify the impact of agricultural management on carbon content. Environ. Microbiol. 2017, 19, 3070–3086. [Google Scholar] [CrossRef]
- Liao, N.; Li, Q.; Zhang, W.; Zhou, G.W.; Ma, L.J.; Min, W.; Ye, J.; Hou, Z.N. Effects of biochar on soil microbial community composition and activity in drip-irrigated desert soil. Eur. J. Soil Biol. 2016, 72, 27–34. [Google Scholar] [CrossRef]
- Chen, Z.M.; Wang, H.Y.; Liu, X.W.; Zhao, X.L.; Lu, D.J.; Zhou, J.M.; Li, C.Z. Changes in soil microbial community and organic carbon fractions under short-term straw return in a rice–wheat cropping system. Soil Tillage Res. 2017, 165, 121–127. [Google Scholar] [CrossRef]
- Han, G.M.; Chen, Q.Q.; Zhang, S.X.; Li, G.R.; Yi, X.D.; Feng, C.H.; Wang, X.G.; Yu, C.; Lan, J.Y. Biochar effects on bacterial community and metabolic pathways in continuously cotton-cropped soil. J. Soil Sci. Plant Nutr. 2019, 19, 249–261. [Google Scholar] [CrossRef]
- Bai, N.L.; Zhang, H.L.; Li, S.X.; Zheng, X.Q.; Zhang, J.Q.; Zhang, H.Y.; Zhou, S.; Sun, H.F.; Lv, W.G. Long-term effects of straw and straw-derived biochar on soil aggregation and fungal community in a rice–wheat rotation system. PeerJ 2019, 6, e6171. [Google Scholar] [CrossRef]
- Xu, Y.X.; He, L.L.; Chen, J.Y.; Lyu, H.H.; Wang, Y.Y.; Yang, L.; Yang, S.M.; Liu, Y.X. Long-Term Successive Biochar Amendments Alter the Composition and α-Diversity of Bacterial Community of Paddy Soil in Rice-Wheat Rotation. Front. Environ. Sci. 2022, 10, 921766. [Google Scholar] [CrossRef]
- Wei, Z.B.; Han, X.R.; Wang, Y.H.; Zhang, L.L.; Gong, P.; Shi, Y.L. Effects of biochar, dual inhibitor, and straw return on maize yield, soil physicochemical properties, and microbial system under fertilization conditions. Front. Microbiol. 2025, 16, 1570237. [Google Scholar] [CrossRef]
- Song, T.S.; Wang, J.K.; Xu, X.Y.; Sun, C.X.; Sun, C.; Chen, Z.H.; Zhang, Y.L.; Hao, L.Y. Microbial community and network differently reshaped by crushed straw or biochar incorporation and associated with nitrogen fertilizer level. GCB Bioenergy 2023, 15, 1255–1272. [Google Scholar] [CrossRef]
- Lei, C.T.; Lu, T.; Qian, H.F.; Liu, Y.X. Machine learning models reveal how biochar amendment affects soil microbial communities. Biochar 2023, 5, 89. [Google Scholar] [CrossRef]
- Bai, J.Z.; Huang, Y.M.; Bai, Y.X.; Chen, D.Y.; Haider, S.; Song, J.J.; Moreira, B.R.D.A.; Ren, G.X.; Yang, G.H.; Feng, Y.Z.; et al. Impact of straw-biochar amendments on microbial activity and soil carbon dynamics in wheat-maize system. Soil Tillage Res. 2024, 244, 106284. [Google Scholar] [CrossRef]
- Meng, W.S.; An, N.; Guan, S.; Dou, S.; Zhang, B.W.; Zhu, W.J.; Yue, J.H. Unraveling mechanisms of carbon enrichment via straw and biochar application to enhance soil fertility and improve maize yield. Eur. J. Agron. 2025, 169, 127673. [Google Scholar] [CrossRef]
- Xie, N.H.; Fan, Y.C.; Duan, N.; Yang, L.; Radosevich, M.; Zhang, Y.; Wang, Y.F.; Wang, J.K.; Liang, X.L. Interactive effects of straw and biochar amendments on soil organic carbon stabilization and bacterial community dynamics. Biol. Fertil. Soils 2025, 61, 1423–1437. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, J.W.; Wu, H.; Zheng, Q.; Song, D.L.; Wang, X.B.; Zhang, S.Q. Organic carbon, total nitrogen, and microbial community distributions within aggregates of calcareous soil treated with biochar. Agric. Ecosyst. Environ. 2021, 314, 107408. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Du, L.; Zheng, Z.C.; Li, T.X.; Wang, Y.D.; Huang, H.G.; Yu, H.Y.; Ye, D.H.; Liu, T.; Zhang, X.Z. Aggregate-associated carbon compositions explain the variation of carbon sequestration in soils after long-term planting of different tea varieties. Sci. Total Environ. 2023, 856, 159227. [Google Scholar] [CrossRef]
- Bai, N.L.; Zhang, H.L.; Zhou, S.; Sun, H.F.; Zhao, Y.H.; Zheng, X.Q.; Li, S.X.; Zhang, J.Q.; Lv, W.G. Long-term effects of straw return and straw-derived biochar amendment on bacterial communities in soil aggregates. Sci. Rep. 2020, 10, 7891. [Google Scholar] [CrossRef]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil Aggregate Microbial Communities: Towards Understanding Microbiome Interactions at Biologically Relevant Scales. Appl. Environ. Microbiol. 2019, 85, e00324-e19. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E. Insights into soil and biochar variations and their contribution to soil aggregate status–A meta-analysis. Soil Tillage Res. 2024, 244, 106282. [Google Scholar] [CrossRef]
- Luo, S.S.; Wang, S.J.; Tian, L.; Shi, S.H.; Xu, S.Q.; Yang, F.; Li, X.J.; Wang, Z.C.; Tian, C.J. Aggregate-related changes in soil microbial communities under different ameliorant applications in saline-sodic soils. Geoderma 2018, 329, 108–117. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, S.J.; Zhang, J.X.; Tian, C.J.; Luo, S.S. Biochar application enhances microbial interactions in mega-aggregates of farmland black soil. Soil Tillage Res. 2021, 213, 105145. [Google Scholar] [CrossRef]
- Luo, S.S.; Wang, S.J.; Tian, L.; Li, S.Q.; Li, X.J.; Shen, Y.F.; Tian, C.J. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl. Soil Ecol. 2017, 117–118, 10–15. [Google Scholar] [CrossRef]
- Ding, S.J.; Guo, D.D.; Zhang, S.Q.; Song, X.; Zhang, K.K.; Guo, T.F.; Yue, K.; Huang, S.M.; Zhou, G.Q. Effects of 31-year long-term fertilization on the evolution of trace elements in fluvo-aquic soil and their uptake by wheat. J. Plant Nutr. Fertil. 2024, 30, 1858–1871. [Google Scholar]
- Ren, H.W.; Feng, Y.P.; Pei, J.W.; Li, J.P.; Wang, Z.Y.; Fu, S.F.; Zheng, Y.; Li, Z.Z.; Peng, Z.P. Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef]
- Chen, L.M.; Sun, S.L.; Zhou, Y.Y.; Zhang, B.X.; Peng, Y.T.; Zhuo, Y.C.; Ai, W.K.; Gao, C.F.; Wu, B.; Liu, D.W.; et al. Straw and straw biochar differently affect fractions of soil organic carbon and microorganisms in farmland soil under different water regimes. Environ. Technol. Innov. 2023, 32, 103412. [Google Scholar] [CrossRef]
- Zhao, C.S.; Zhang, Y.P.; Liu, X.B.; Ma, X.W.; Meng, Y.T.; Li, X.Q.; Quan, X.; Shan, J.R.; Zhao, W.; Wang, H.Y. Comparing the effects of biochar and straw amendment on soil carbon pools and bacterial community structure in degraded soil. J. Soil Sci. Plant Nutr. 2020, 20, 751–760. [Google Scholar] [CrossRef]
- Tian, X.P.; Wang, L.; Hou, Y.H.; Wang, H.; Tsang, Y.F.; Wu, J.H. Responses of soil microbial community structure and activity to incorporation of straws and straw biochars and their effects on soil respiration and soil organic carbon turnover. Pedosphere 2019, 29, 492–503. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.B.; Zhao, B.J.; Yan, P.; Zhou, G.X.; Xin, X.L. Effects of straw amendment and moisture on microbial communities in Chinese fluvo-aquic soil. J. Soils Sediments 2014, 14, 1829–1840. [Google Scholar] [CrossRef]
- Yang, Y.L.; Bao, X.L.; Xie, H.T.; He, H.B.; Zhang, X.D.; Shao, P.S.; Zhu, X.F.; Jiang, Y.J.; Liang, C. Frequent stover mulching builds healthy soil and sustainable agriculture in Mollisols. Agric. Ecosyst. Environ. 2022, 326, 107815. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.R.; Gao, W.H.; Guo, Z.H.; Xue, C.; Pang, J.Y.; Shu, L.Z. Effects of biochar amendment on bacterial and fungal communities in the reclaimed soil from a mining subsidence area. Environ. Sci. Pollut. Res. 2019, 26, 34368–34376. [Google Scholar] [CrossRef]
- Lu, H.; Yan, M.; Wong, M.H.; Mo, W.Y.; Wang, Y.; Chen, X.W.; Wang, J.J. Effects of biochar on soil microbial community and functional genes of a landfill cover three years after ecological restoration. Sci. Total Environ. 2020, 717, 137133. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xin, L.; Liu, J.T.; Yuan, M.Z.; Liu, S.T.; Jiang, W.; Chen, J.P. Changes in bacterial community of soil induced by long-term straw returning. Sci. Agric. 2017, 74, 349–356. [Google Scholar] [CrossRef]
- Su, P.; Lou, J.; Brookes, P.C.; Luo, Y.; He, Y.; Xu, J.M. Taxon-specific responses of soil microbial communities to different soil priming effects induced by addition of plant residues and their biochars. J. Soils Sediments 2017, 17, 674–684. [Google Scholar] [CrossRef]
- Rahimlou, S.; Bahram, M.; Tedersoo, L. Phylogenomics reveals the evolution of root nodulating alpha-and beta-Proteobacteria (rhizobia). Microbiol. Res. 2021, 250, 126788. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shao, C.; Sun, H.; Liu, Z.B.; Guan, Y.M.; Wu, L.J.; Zhang, L.L.; Pan, X.X.; Zhang, Z.H.; Zhang, Y.Y.; et al. Arbuscular mycorrhizal fungi biofertilizer improves American ginseng (Panax quinquefolius L.) growth under the continuous cropping regime. Geoderma 2020, 363, 114155. [Google Scholar] [CrossRef]
- Palomo, A.; Jane Fowler, S.; Gülay, A.; Rasmussen, S.; Sicheritz-Ponten, T.; Smets, B.F. Metagenomic analysis of rapid gravity sand filter microbial communities suggests novel physiology of Nitrospira spp. ISME J. 2016, 10, 2569–2581. [Google Scholar] [CrossRef]
- Im, W.T.; Kim, S.H.; Kim, M.K.; Ten, L.N.; Lee, S.T. Pleomorphomonas koreensis sp. nov., a nitrogen-fixing species in the order Rhizobiales. Int. J. Syst. Evol. Microbiol. 2006, 56, 1663–1666. [Google Scholar] [CrossRef]
- Kojima, H.R.; Tokizawa, R.; Fukui, M. Mizugakiibacter sediminis gen. nov., sp. nov., isolated from a freshwater lake. Int. J. Syst. Evol. Microbiol. 2014, 64, 3983–3987. [Google Scholar] [CrossRef] [PubMed]
- Su, J.Q.; Ding, L.J.; Xue, K.; Yao, H.Y.; Quensen, J.; Bai, S.J.; Wei, W.X.; Wu, J.S.; Zhou, J.Z.; Tiedje, J.M.; et al. Long-term balanced fertilization increases the soil microbial functional diversity in a phosphorus-limited paddy soil. Mol. Ecol. 2015, 24, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, J.Q.; Yuan, Q.Y.; Li, C.; Yan, X.; Hong, Q.; Li, S.P. Characterization of the propanil biodegradation pathway in Sphingomonas sp Y57 and cloning of the propanil hydrolase gene prpH. J. Hazard. Mater. 2011, 196, 412–419. [Google Scholar] [CrossRef]
- Wang, W.H.; Luo, X.; Ye, X.F.; Chen, Y.; Wang, H.; Wang, L.; Wang, Y.B.; Yang, Y.Y.; Li, Z.K.; Cao, H.; et al. Predatory Myxococcales are widely distributed in and closely correlated with the bacterial community structure of agricultural land. Appl. Soil Ecol. 2020, 146, 103365. [Google Scholar] [CrossRef]
- Li, H.; Man, H.L.; Han, J.; Jia, X.X.; Wang, L.; Yang, H.Y.; Shi, G.Y. Soil Microorganism Interactions under Biological Fumigations Compared with Chemical Fumigation. Microorganisms 2024, 12, 2044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.N.; Wang, Y.; Sun, L.T.; Qiu, C.; Ding, Y.Q.; Gu, H.L.; Wang, L.J.; Wang, Z.S.; Ding, Z.T. Organic mulching positively regulates the soil microbial communities and ecosystem functions in tea plantation. BMC Microbiol. 2020, 20, 103. [Google Scholar] [CrossRef]
- Zhang, X.P.; Li, Q.L.; Zhong, Z.K.; Huang, Z.Y.; Bian, F.Y.; Yang, C.B.; Wen, X. Changes in Soil Organic Carbon Fractions and Fungal Communities, Subsequent to Different Management Practices in Moso Bamboo Plantations. J. Fungi 2022, 8, 640. [Google Scholar] [CrossRef]
- Oliveira, M.C.O.; Alves, A.; Fidalgo, C.; de Freitas, J.G.; Pinheiro de Carvalho, M.A. Variations in the structure and function of the soil fungal communities in the traditional cropping systems from Madeira Island. Front. Microbiol. 2024, 15, 1426957. [Google Scholar] [CrossRef]
- Liu, J.J.; Sui, Y.Y.; Yu, Z.H.; Shi, Y.; Chu, H.Y.; Jin, J.; Liu, X.B.; Wang, G.H. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Redmile-Gordon, M.; Zhang, J.B.; Zhang, C.Z.; Ning, Q.; Li, W. Mortierella elongata’s roles in organic agriculture and crop growth promotion in a mineral soil. Land Degrad. Dev. 2018, 29, 1642–1651. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Zhao, J.N.; Wang, L.L.; Yang, D.L.; Li, G.; Xiu, W.M. Variation of soil bacterial and fungal communities from fluvo-aquic soil under chemical fertilizer reduction combined with organic materials in North China Plain. J. Soil Sci. Plant Nutr. 2021, 21, 349–363. [Google Scholar] [CrossRef]
- Lange, L.; Barrett, K.; Pilgaard, B.; Gleason, F.; Tsang, A. Enzymes of early-diverging, zoosporic fungi. Appl. Microbiol. Biotechnol. 2019, 103, 6885–6902. [Google Scholar] [CrossRef]
- Liu, Y.J.; Shi, G.X.; Mao, L.; Cheng, G.; Jiang, S.J.; Ma, X.J.; An, L.Z.; Du, G.Z.; Johnson, N.C.; Feng, H.Y. Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol. 2012, 194, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Denis, M.; Gené, J.; Sutton, D.A.; Wiederhold, N.P.; Cano-Lira, J.F.; Guarro, J. New species of Cladosporium associated with human and animal infections. Persoonia-Mol. Phylogeny Evol. Fungi 2016, 36, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.W.; Suo, M.; Qiu, Z.J.; Wu, H.; Zhao, M.; Yang, H.Y. Regulating root fungal community using Mortierella alpina for Fusarium oxysporum resistance in Panax ginseng. Front. Microbiol. 2022, 13, 850917. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Bo, B.; Malec, P.; Fu, P. The effect of a consortium of Penicillium sp. and Bacillus spp. in suppressing banana fungal diseases caused by fusarium sp. and Alternaria sp. J. Appl. Microbiol. 2021, 131, 1890–1908. [Google Scholar] [CrossRef] [PubMed]
- Osorio, N.W.; Habte, M. Synergistic effect of a phosphate-solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in an Oxisol fertilized with rock phosphate. Botany 2013, 91, 274–281. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kang, S.M.; Baek, I.Y.; Lee, I.J. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J. Plant Interact. 2014, 9, 754–762. [Google Scholar] [CrossRef]
- Weber, R.W.; Webster, J. Teaching techniques for mycology: 9 Olpidium and Rhizophlyctis (Chytridiomycetes). Mycologist 2000, 14, 17–20. [Google Scholar] [CrossRef]
- Lay, C.Y.; Hamel, C.; St-Arnaud, M. Taxonomy and pathogenicity of Olpidium brassicae and its allied species. Fungal Biol. 2018, 122, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zachow, C.; Raaijmakers, J.M.; De Bruijn, I. Elucidating the diversity of aquatic Microdochium and Trichoderma species and their activity against the fish pathogen Saprolegnia diclina. Int. J. Mol. Sci. 2016, 17, 140. [Google Scholar] [CrossRef]
- Ma, X.J.; Zhang, Y.L.; Wei, F.; Zhao, L.H.; Zhou, J.L.; Qi, G.R.; Ma, Z.; Zhu, H.Q.; Feng, H.J.; Feng, Z.L. Applications of Chaetomium globosum CEF-082 improve soil health and mitigate the continuous cropping obstacles for Gossypium hirsutum. Ind. Crop. Prod. 2023, 197, 116586. [Google Scholar] [CrossRef]
- Dagher, D.J.; Pitre, F.E.; Hijri, M. Ectomycorrhizal fungal inoculation of sphaerosporella brunnea significantly increased stem biomass of salix miyabeana and decreased lead, tin, and zinc, soil concentrations during the phytoremediation of an industrial landfill. J. Fungi 2020, 6, 87. [Google Scholar] [CrossRef]
- Herren, C.M.; McMahon, K.D. Keystone taxa predict compositional change in microbial communities. Environ. Microbiol. 2018, 20, 2207–2217. [Google Scholar] [CrossRef]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling interactions in the microbiome: A network perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jing, X.Y.; Guan, Y.P.; Zhai, C.; Wang, T.; Shi, D.Y.; Sun, W.P.; Gu, S.Y. Response of fungal communities and co-occurrence network patterns to compost amendment in black soil of Northeast China. Front. Microbiol. 2019, 10, 1562. [Google Scholar] [CrossRef]
- Schlemper, T.R.; van Veen, J.A.; Kuramae, E.E. Co-variation of bacterial and fungal communities in different sorghum cultivars and growth stages is soil dependent. Microb. Ecol. 2018, 76, 205–214. [Google Scholar] [CrossRef]
- Freches, A.; Fradinho, J.C. The biotechnological potential of the Chloroflexota phylum. Appl. Environ. Microbiol. 2024, 90, e0175623. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Dilfuza, E. Role of microorganisms in nitrogen cycling in soils. In Soil Nutrients; Nova Publishers: New York, NY, USA, 2011; pp. 159–176. [Google Scholar]
- Hayatsu, M.; Tago, K.; Saito, M. Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Twagirayezu, G.; Cheng, H.; Wu, Y.; Lu, H.; Huang, S.; Fang, X.; Irumva, O. Insights into the influences of biochar on the fate and transport of pesticides in the soil environment: A critical review. Biochar 2024, 6, 9. [Google Scholar] [CrossRef]
| CK | BR | SR | BS | ||
|---|---|---|---|---|---|
| Topological features | Nodes | 221 | 207 | 226 | 218 |
| Links | 634 | 692 | 873 | 456 | |
| Positive | 60.60% | 59.10% | 60.60% | 62.70% | |
| Negative | 39.40% | 40.90% | 39.40% | 37.30% | |
| Average degree | 5.738 | 6.686 | 7.726 | 4.183 | |
| Modularity | 1.954 | 1.558 | 1.344 | 2.155 | |
| Module hubs | Bacterial phyla | Planctomycetes | Chloroflexi | Chloroflexi | Chloroflexi |
| Acidobacteria | Acidobacteria | GAL15 | Proteobacteria | ||
| Nitrospirae | Proteobacteria | Verrucomicrobia | Acidobacteria | ||
| Proteobacteria | |||||
| Gemmatimonadetes | |||||
| Fungal phyla | Glomeromycota | Ascomycota | Ascomycota | Basidiomycota | |
| Basidiomycota | |||||
| Keystone taxa | Family | Phycisphaeraceae | uncultured_bacterium_c_P2-11E | ||
| Blastocatellaceae_[Subgroup_4] | uncultured_bacterium_c_JG30-KF-CM66 | uncultured_bacterium_c_JG37-AG-4 | |||
| Glomeraceae | Acidobacteriaceae_[Subgroup_1] | DA111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, S.; Wen, Y.; Hao, W.; Zhao, Y.; Luo, S. The Aggregate-Mediated Restoration of Degraded Black Soil via Biochar and Straw Additions: Emphasizing Microbial Community Interactions and Functions. Agriculture 2025, 15, 2342. https://doi.org/10.3390/agriculture15222342
Wang S, Liu S, Wen Y, Hao W, Zhao Y, Luo S. The Aggregate-Mediated Restoration of Degraded Black Soil via Biochar and Straw Additions: Emphasizing Microbial Community Interactions and Functions. Agriculture. 2025; 15(22):2342. https://doi.org/10.3390/agriculture15222342
Chicago/Turabian StyleWang, Shaojie, Siyang Liu, Yingqi Wen, Wenjun Hao, Yiyi Zhao, and Shasha Luo. 2025. "The Aggregate-Mediated Restoration of Degraded Black Soil via Biochar and Straw Additions: Emphasizing Microbial Community Interactions and Functions" Agriculture 15, no. 22: 2342. https://doi.org/10.3390/agriculture15222342
APA StyleWang, S., Liu, S., Wen, Y., Hao, W., Zhao, Y., & Luo, S. (2025). The Aggregate-Mediated Restoration of Degraded Black Soil via Biochar and Straw Additions: Emphasizing Microbial Community Interactions and Functions. Agriculture, 15(22), 2342. https://doi.org/10.3390/agriculture15222342




