Abstract
Coal-derived soil amendments have been shown to improve soil physicochemical properties and promote plant growth; however, their effects on rhizosphere microbial communities remain insufficiently understood. In this study, a comprehensive assessment of the impacts of a lignite coal-based, microbially processed amendment on lettuce (Lactuca sativa) growth, soil properties, and rhizosphere microbiota was conducted. Application of the coal-based amendment resulted in a more than two-fold increase in plant fresh weight compared to untreated soil. The amendment significantly improved soil organic matter content but did not increase phosphate or potassium levels. Rhizosphere bacterial and fungal communities were profiled using 16S rRNA and ITS gene sequencing. Principal coordinate analysis revealed that the coal-based amendment, commercial organic fertilizer, and raw coal each induced distinct shifts in microbial community structure. Notably, treatment with the coal-based amendment reduced the relative abundance of Proteobacteria while increasing Acidobacteriota and Chloroflexi in the bacterial community. In fungal communities, the amendment decreased Basidiomycota and enriched Ascomycota. These results suggest that the observed enhancement in plant growth is closely linked to changes in rhizosphere microbial composition and soil organic matter content, highlighting the potential of microbially processed coal products as sustainable soil amendments in agriculture.
1. Introduction
The global population has quadrupled in the last century, from 1.8 billion in 1915 to 7.8 billion in 2020, and there is an 80% probability that the world population will increase to between 9.6 billion and 12.3 billion in 2100 [1]. The world faces the challenge of producing sufficient crops to meet growing food demand for future generations. While the use of chemical fertilizers has played a key role in the significant increase in crop production, soil health has deteriorated severely due to excessive use of chemical fertilizers in farming [2]. The long-term application of chemical fertilizers can lead to organic matter and nutrient depletion, deterioration of the rhizosphere environment, and negative effects on soil health, such as soil structure deterioration and acidification [2]. Methods to improve crop production to meet continued population growth while developing sustainable agriculture practices are urgently needed. Organic fertilizers and amendments derived from natural sources such as biochar, livestock and poultry manure, plant residues, anaerobic digestion, and agricultural by-products are becoming more important in sustainable agriculture owing to their advantages over chemical fertilizers [3]. In addition to providing the essential nutrients for plant growth, organic fertilizers and amendments have been shown to increase soil organic matter, improve soil texture and water holding capacity, reduce soil crusting problems and erosion, and maintain and improve soil microbiome diversity and activity, thus supporting both food security and long-term agricultural sustainability [3].
Low-rank coals (e.g., lignite and subbituminous coal) have a low heating value and pose environmental threats when used as fuel. With intensified concerns about climate change, the coal industry is facing serious challenges. As a natural resource, low-rank coals are rich in large and stable carbonaceous content, such as humic substances, which can potentially be used as a soil amendment to increase soil organic matter content [4]. Different types of low-rank coals applied to soil can help ameliorate soil structure and improve nutrient mobility as short- to medium-term benefits and serve as a stable source of soil organic matter, potentially long-term [4]. Due to their broad geographical availability, relatively low cost, and existing production process, low-rank coals can serve as a candidate soil amendment. However, coal compounds are typically not readily biodegradable and available for soil microorganisms or plants due to their stable polyphenolic skeleton and complex branching structures. Moreover, some coal contains hazardous components such as heavy metals that are toxic to both soil microorganisms and plants [5,6]. Therefore, the effectiveness of low-rank coals as soil amendment varies substantially and it has been prevented from being widely used.
Recent studies have demonstrated that low-rank coal and the solid waste from its mining and washing processes could be microbiologically transformed into an effective soil amendment to increase soil organic matter content, benefit soil microbial populations, and enhance plant growth [7]. Fallgren et al. applied effluent solids from a process that partially digested the coal waste and found substantially higher yields of Kentucky bluegrass and chives in sandy soils amended with the product, when compared to a commercial organic fertilizer, indicating that the microbial digestion of coal to produce organic soil amendment is a viable alternative and beneficial use of coal [7]. Chen et al. conducted a study with the same microbially digested coal product that determined its effects on sunflower growth in saline–sodic soil in the Inner Mongolia Autonomous Region, China [8]. The results of this study indicated that saline–sodic soil amended with digested coal product resulted in improved sunflower root and overall growth, water productivity, and partial nitrogen productivity [8]. The improvement in root growth and partial nitrogen productivity are indicators of positive effects on soil microbial activity by the digested coal product amendment. Other studies have been conducted to investigate the influence of low-rank coals on soil quality and plant growth [9,10,11]; however, investigations of the effects of low-rank coal-based products on soil microbial communities are scarce.
A study conducted by Guan et al. used the same microbially digested coal product as Fallgren et al. [7] and Chen et al. [8] to determine its impact on Tibetan barley growth and soil microbial activity [12]. The study indicated that the soil amended with both the digested coal product and inorganic fertilizer resulted in the promotion of rhizosphere microorganism growth, enhancement of root-zone microbial interactions, and resistance to pathogens and environmental stress [12].
Since an evaluation of the changes in the rhizosphere microbiome and soil properties might provide insights into the mechanism of the observations of plant growth promotion by the microbiologically transformed coal product, the objectives of this greenhouse study were to measure these effects using lettuce (Lactuca sativa) as a model crop.
2. Materials and Methods
2.1. Materials
The lignite coal-derived product was prepared by a biochemical growth and fragmentation (BGF) process, as described in Fallgren et al. [5], and was provided by Advanced Environmental Technologies, LLC (Fort Collins, CO, USA). The BGF product, commercially available as “Ginate®”, contained 2% N, 2% P, 3% K, and 72.5% organic matter (OM). The commercial organic fertilizer used was GRO-WELL Proven Organics All-Purpose (Tempe, AZ, USA) containing 7% N (total), 5% P (P2O5), 7% K (K2O), and 85% OM. The ground raw coal used was lignite from Underwood, North Dakota containing 5% N (total), 8.3% ash, and 65% OM. This lignite, which was also the starting point for the production of BGF, had arsenic concentration < 10 mg/kg, cadmium concentration < 20 mg/kg, lead concentration < 90 mg/kg, and mercury concentration < 2 mg/kg. These values pass the Organic Materials Review Institute organic fertilizer/soil amendment standards.
The soil used for the experiments was collected from a farm in western Nebraska (USA). It was a sandy loam with high pH (8.1) and low organic matter content (0.9%). Soil N (total) and P (total) were 4.0 and 4.3 mg/kg, respectively.
2.2. Lettuce Growth Experiments
The experiments were conducted in 2020–2021 at the CSU greenhouse facility. To determine the effects on lettuce growth (Lactuca sativa), the BGF product was mixed into the base soil before planting at two doses: 4 and 15 g/kg soil. Based on a previous study using 7.5 t/ha [8], the doses of 0.4% and 1.5% w/w correspond to 7.8 and 29 t/ha (0–15 cm, bulk density 1.3 g·cm−3).
The same amount of the commercial organic fertilizer and an equivalent amount of ground raw coal were applied in different treatments as reference treatments. Seedlings were raised in Profile Porous Ceramic Greens Grade and fertilized during the nursery phase with Murashige and Skoog medium (1 L per tray). Lettuce seedlings at the two-true-leaves stage were transplanted into the 1-US gallon (3.785 L) treatment pots, approximately 4.92 kg soil per pot. One plant per pot and three biological replicates per treatment (n = 5 pots). All lettuce plants were cultivated under uniform greenhouse conditions and randomly arranged on the bench during the experimental period at Colorado State University (Fort Collins, CO, USA). No additional nutrients were applied. Plants were watered every 2 days during the growth period, approximately 200 mL water per pot. The greenhouse chamber was maintained under a photoperiod of 12–16 h of light with a photosynthetic photon flux density of 200–600 µmol m−2 s−1. Relative humidity was 50% to 75%, and the temperature was controlled at 22–28 °C in the day and 18–22 °C at night.
Lettuce plants were harvested after 5 weeks in the greenhouse. Plant fresh weight, shoot fresh weight, and root fresh weight were measured. Then, all the lettuce plants and roots were oven-dried at 65 °C for 3 days, and the dry weights of shoot and root were recorded.
To assess root morphological traits, lettuce roots were separated from the soils, washed, and scanned using WinRHIZO root-scanning equipment (Epson Expression 1100 XL, Epson America, Inc., Long Beach, CA, USA) and software (Regent Instruments, Inc. Quebec, QC, Canada) [13].
2.3. Soil Sampling and Analysis
Soil samples (7 treatments and n = 3 per treatment) were collected immediately before transplanting (with no post-mixing incubation) and immediately after harvest from all treatment pots to determine the influence of different treatments on soil parameters. The soil pH, electrical conductivity (EC), OM, and the contents of nitrate-nitrogen (NO3-N), phosphorus (P), and potassium (K) of all 7 treatments (n = 3 per treatment) were measured at the Soil, Water and Plant Testing Laboratory at Colorado State University (https://agsci.colostate.edu/divi-soiltestinglab/wp-content/uploads/sites/140/2023/02/Soil-Test-Interpretation.pdf, accessed in 1 January 2024). Soil pH and EC were measured using a 1:1 ratio of air-dried soil–water. The soil OM content was determined using the loss-on-ignition method. The NO3 −N content was determined using the KCl extraction method and reported in parts per million units (mg kg−1). Soil P and K were extracted by Olsen’s bicarbonate and ammonium acetate (CH3CO2NH4) methods, respectively, and reported in parts per million units (mg kg−1).
2.4. Rhizosphere Microbiome Analysis
2.4.1. Rhizosphere Soil Sampling and DNA Extraction
Soil samples mixed with different treatments prior to lettuce planting (7 treatments and n = 3 per treatment) were collected and stored at −80 °C until DNA isolation. To collect the rhizosphere soil samples, the roots of the lettuce plants (7 treatments and n = 3 per treatment) were vigorously shaken by hand for 10 min until all non-adhering soil particles were removed. Then, each root sample was submerged in 50 mL of phosphate-buffered saline (PBS) and vortexed vigorously for 5 min to remove the adhering soils. The slurry was centrifuged at 4000× g and 4 °C for 10 min, the supernatant was discarded, and the soil pellet was stored at −80 °C until DNA isolation.
The total soil DNA was extracted from 0.5 g of soil sample (both soil samples prior to lettuce planting and rhizosphere soil samples after lettuce harvesting; n = 14 treatments × 3 replicates = 42 samples) using a Quick-DNA Fecal/Soil Microbe Miniprep Kit (D6010; Zymo Research Corp., Irvine, CA, USA) according to the manufacturer’s instructions. The DNA quality was checked on 1% agarose gels and was quantified using a spectrophotometer (Thermo Scientific NanoDrop 2000c, Vernon Hills, IL, USA). All isolated DNA samples had an absorbance ratio (A260/A280) between 1.8 and 2.0.
2.4.2. Sequencing Libraries Preparation, 16S rRNA, and ITS Sequencing
Sample pool sequencing libraries were constructed by PCR amplification of the V4 region of the prokaryotic 16S rRNA gene using primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) [14]. The internal transcribed spacer (ITS) regions of fungal gene were amplified using primers ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [15]. Briefly, amplicon libraries containing Illumina adaptors and barcodes were generated for each sample. Then the PCR products were cleaned using AmPure beads (A63881; Beckman Coulter Life Sciences, Indianapolis, IN, USA), quantified with Quant-iT™ PicoGreen™ dsDNA Assay Kit (P7581; Invitrogen, Waltham, MA, USA), and pooled in equimolar ratios prior to sequencing using a 2 × 250 MiSeq flow cell (Illumina, San Diego, CA, USA) in the Genomics Core on the University of Colorado Anschutz Medical Campus.
2.4.3. Sequencing Data Analysis
The raw 16S rRNA and ITS gene sequencing reads were demultiplexed, quality-filtered by Trimmomatic (v0.36) [16], and merged by FLASH (v1.20) [17], with the following criteria: (i) an average quality score of <20 over a 50 bp sliding window and (ii) only overlapping sequences longer than 10 bp were assembled according to their overlapped sequence. Reads shorter than 120 bp were discarded, as were reads containing ambiguous characters. The maximum mismatch ratio of the overlap region was 0.1. Barcodes and primers were removed from the raw sequences to obtain clean sequences. The operational taxonomic units (OTUs) were obtained using Qiime (V1.8.0) [18] using clean sequences. The OTUs with 97% similarity cutoff were clustered using UPARSE version 7.1 [19], and chimeric sequences were identified and removed. The taxonomy of each OTU representative sequence was analyzed by RDP Classifier version 2.2 [20] against the 16S rRNA database (Release 128/132/138 http://www.arb-silva.de; accessed on 15 January 2022) and ITS database (Unite Release 8.2 http://unite.ut.ee/index.php; accessed on 15 January 2022).
Bioinformatic analyses were primarily performed in QIIME2 (V1.8.0) [18] and R (v4.1.2). Alpha diversity indices, including the Chao1 and Shannon indices, were calculated and visualized as boxplots in QIIME 2. Microbial taxonomic composition and relative abundance were also analyzed within QIIME 2. Beta diversity was evaluated using principal coordinates analysis (PCoA) based on Bray-Curtis dissimilarity matrices. The group differences were tested with PERMANOVA (adonis) using permutations, while multigroup dispersion was checked with betadisper and p-values were adjusted for multiple testing using the Benjamini–Hochberg procedure. The differences in community composition were visualized in two-dimensional ordination plots. The qvalue package (v2.22.0) in R was used to estimate false discovery rates (FDR), and statistical differences among groups were determined using one-way ANOVA, Kruskal–Wallis, and Duncan’s multiple comparison tests at a significance level of α = 0.05. Linear discriminant analysis Effect Size (LEfSe) analysis and Linear Discriminant Analysis (LDA) were conducted using microbiomeMarker (R package, 2022) to identify biomarkers with significant differences (p < 0.05, LDA score = 3), which are visualized as cladograms.
2.5. Statistical Analysis
The effects of different treatments on plant growth were analyzed using one-way ANOVA with Dunnett’s post-hoc test to compare treatments against the untreated reference. Each measured variable (e.g., height, biomass, root surface area, and root volume) was first evaluated for normality using the Shapiro–Wilk test and for homogeneity of variances using Levene’s test, both performed with the Real Statistics add-in (http://real-statistics.com, Release 7.5 (late-2022)) for Excel. For the effects on soil properties measured at two time points, p-values were adjusted for multiple comparisons using false discovery rate (FDR, Benjamini–Hochberg) method, implemented with the Real Statistics add-in for Excel. Results were reported as mean ± standard deviation, with statistically significant differences from the untreated reference indicated at p < 0.05.
3. Results
3.1. Soil Properties
To determine the influence of the different treatments on soil properties, soil samples from all treatment pots, both prior to lettuce planting and after lettuce harvesting, were collected and analyzed (Table 1).

Table 1.
Physical and chemical properties of soils from different treatments before lettuce planting and at the time the lettuce was harvested. n = 3 per treatment.
The soil pH was in the range of 5.7 to 7.1 in different treatments before planting and in the range of 6.8 to 7.4 after harvesting. The addition of BGF product did not change the soil pH significantly, but the organic fertilizer at both dosages (0.4 and 1.5% w/w) and raw coal at 1.5% resulted in significantly lower pH prior to planting in comparison to that of the reference soil. Notably, the pH in all the treatments was not significantly different when lettuce plants were harvested.
The soil electrical conductivity did not change significantly following additions of BGF product and raw coal. In contrast, the addition of organic fertilizer at both low and high dosages resulted in significantly higher EC (approximately three-fold and five-fold) than the reference. When the lettuce plants were harvested, the soil EC values of the BGF product and organic fertilizer treatments at 0.4% were not significantly different to those of the untreated soil. However, soils treated with the BGF product and organic fertilizer at 1.5% had about two-fold the EC of the untreated soil.
Furthermore, the addition of BGF product, organic fertilizer, and raw coal, especially at 1.5%, significantly improved the soil organic matter content in the soils. After lettuce plants were harvested, the OM content in all the treatments was not significantly higher than that of the untreated soil, apart from the organic fertilizer treatments at 1.5%.
The addition of BGF products and raw coal did not change the soil NO3-N content significantly, but soils treated with organic fertilizer (0.4% and 1.5% w/w) contained significantly higher NO3-N than the reference. There was no significant difference in the NO3-N content among the treatments at the time of lettuce harvesting. Furthermore, the addition of BGF products and raw coal into the soils did not significantly change the phosphate and potassium content, except soil treated with 1.5% BGF, while the organic fertilizer treatments led to soil with significantly higher phosphate and potassium in comparison to the untreated soil. However, the phosphate and potassium content in all the treatments were at a similar level to the untreated soil after lettuce plants were harvested.
3.2. Effects on Lettuce Growth
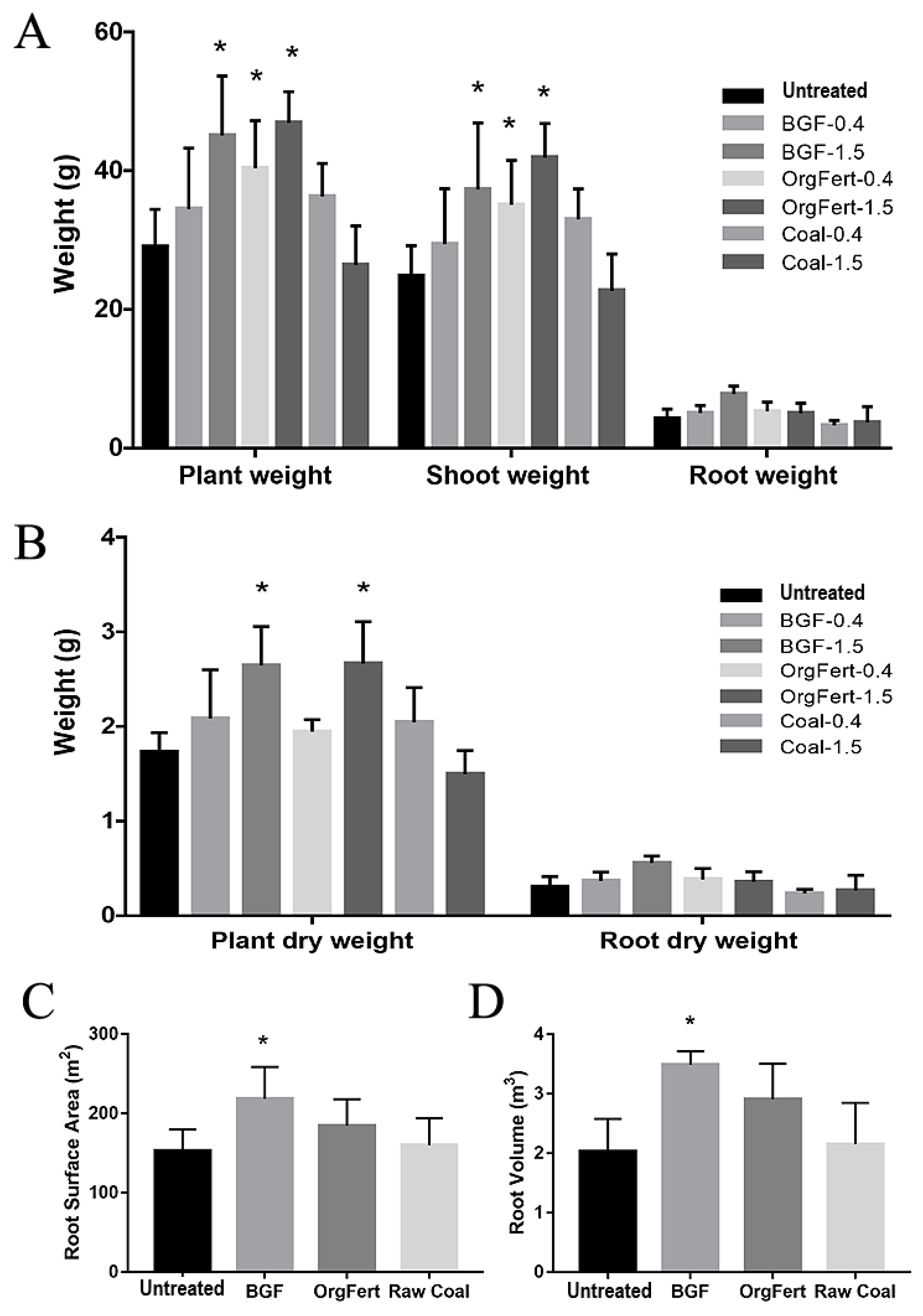
The addition of both BGF product and organic fertilizer to the soil promoted the growth of lettuce plants relative to untreated soil (Figure 1). The addition of BGF product at 1.5% (w/w) and organic fertilizer at both 0.4% and 1.5% (w/w) resulted in significantly higher plant weight, shoot weight, and root weight in comparison to plants in the untreated soil (Figure 1A,B). Moreover, lettuce in soil with the BGF product at 1.5% (w/w) had significantly higher root weight in comparison to the untreated soil, unlike any other treatment. Lettuce grown in soils treated with BGF product and organic fertilizer at 1.5% (w/w) had significantly higher plant dry weight than lettuce in the untreated soil. However, only the addition of the BGF product at 1.5% (w/w) resulted in higher dry root weight than the untreated soil (Figure 1A,B). Furthermore, the application of raw coal at both 0.4% and 1.5% (w/w) did not significantly affect plant growth in terms of either fresh weight or dry weight relative to untreated soil.
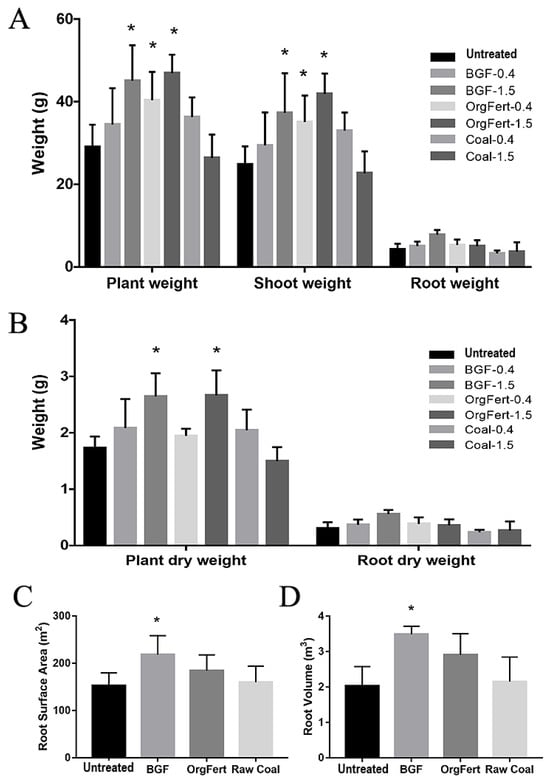
Figure 1.
Lettuce growth results. (A) Plant weight, shoot weight, and root weight of lettuce plants in soil with different treatments (BGF, organic fertilizer, and raw coal at 0.4 and 1.5% w/w) in comparison to untreated soils. (B) Plant dry weight and root dry weight of lettuce plants in different treatments (BGF, organic fertilizer, and raw coal at 0.4 and 1.5% w/w). (C) Root surface area of lettuce plants in different treatments (BGF, organic fertilizer, and raw coal at 1.5% w/w). (D) Root volume of lettuce plants in different treatments (BGF, organic fertilizer, and raw coal at 1.5% w/w). n = 5 per treatment. Error bars represent one standard deviation. One-way ANOVA with Dunnett’s post-hoc test was conducted to compare the effects of different treatments on plant growth. The asterisks above the bars indicate significance relative to the control (untreated) at the p < 0.05 level.
The root analysis revealed that the application of the BGF product resulted in more developed lettuce root systems compared to the reference (Figure 1C,D and Supplementary Figure S1). The lettuce plants in soils treated with BGF had more than 40% higher root surface area and 75% higher root volume in comparison to those of the reference treatment. Neither organic fertilizer nor raw coal treatments changed the root surface area or root volume significantly.
3.3. Rhizosphere Bacterial Community Analysis
3.3.1. Abundance and Diversity of Rhizosphere Bacteria
Bacterial community profiling of untreated soil, soils with three treatments (BGF, organic fertilizer, raw coal) at two levels (0.4% and 1.5% w/w), and the rhizosphere soil samples of lettuce after harvest after all these treatments was performed. In total, 2.2 M high-quality sequences were obtained from 42 samples (14 treatments with 3 biological replicates of each). The number of high-quality sequences across all 42 samples ranged from 17.5 K to 98.4 K, with an average of 52.6 K. A total of 11,057 bacterial OTUs were identified at 97% sequence similarity cutoff, and 2734 OTUs on average across all samples. There were 8868 OTUs from the soils mixed with different treatments and 9171 OTUs from the rhizosphere soils of lettuce plants. In total, 6981 out of the 11,057 OTUs were shared in soils of different treatments before planting and the rhizosphere soils of lettuce after harvest, while 1886 OTUs could only be found in the soils before planting, and 2190 OTUs were only found in the rhizosphere soils.
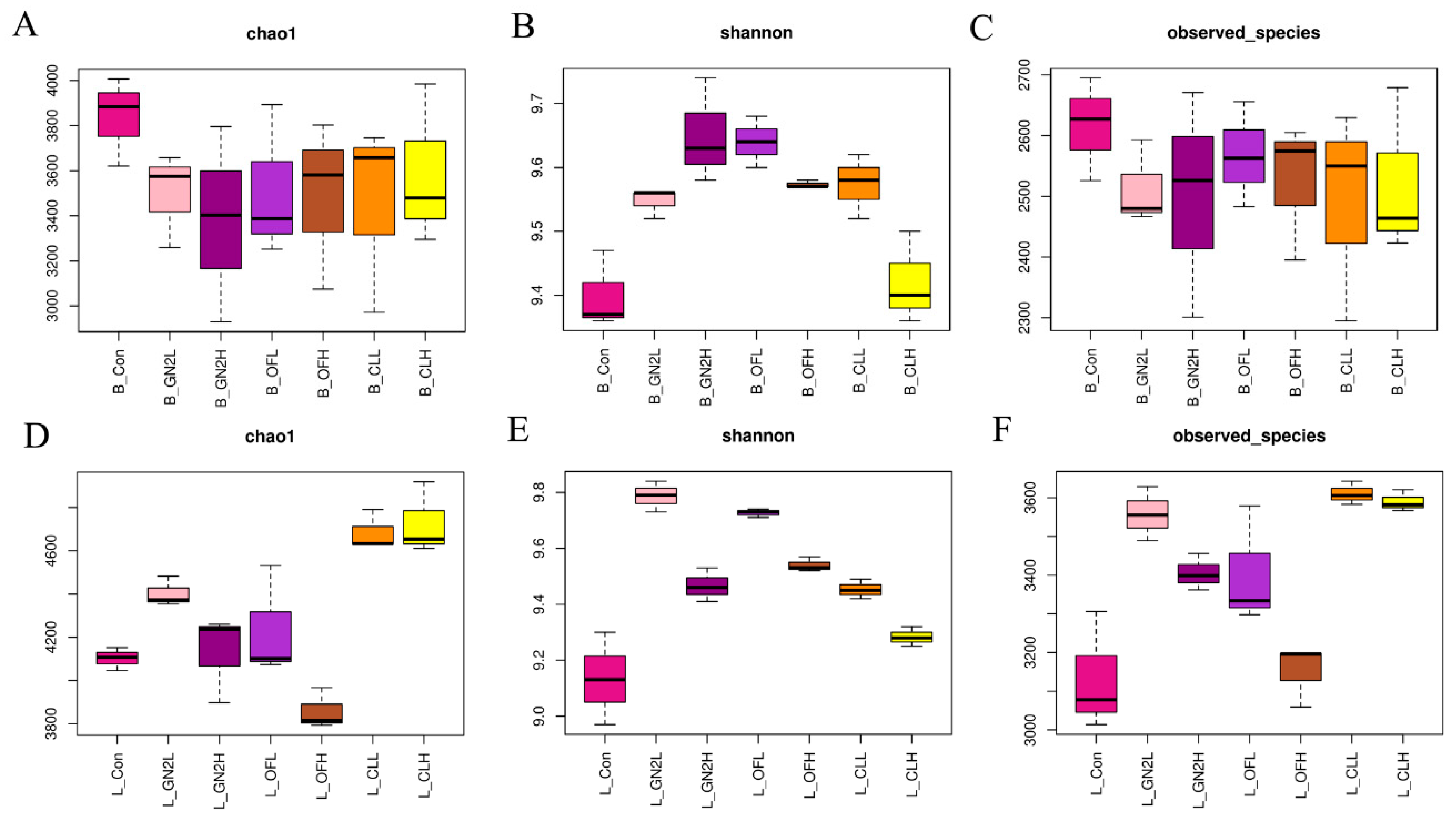
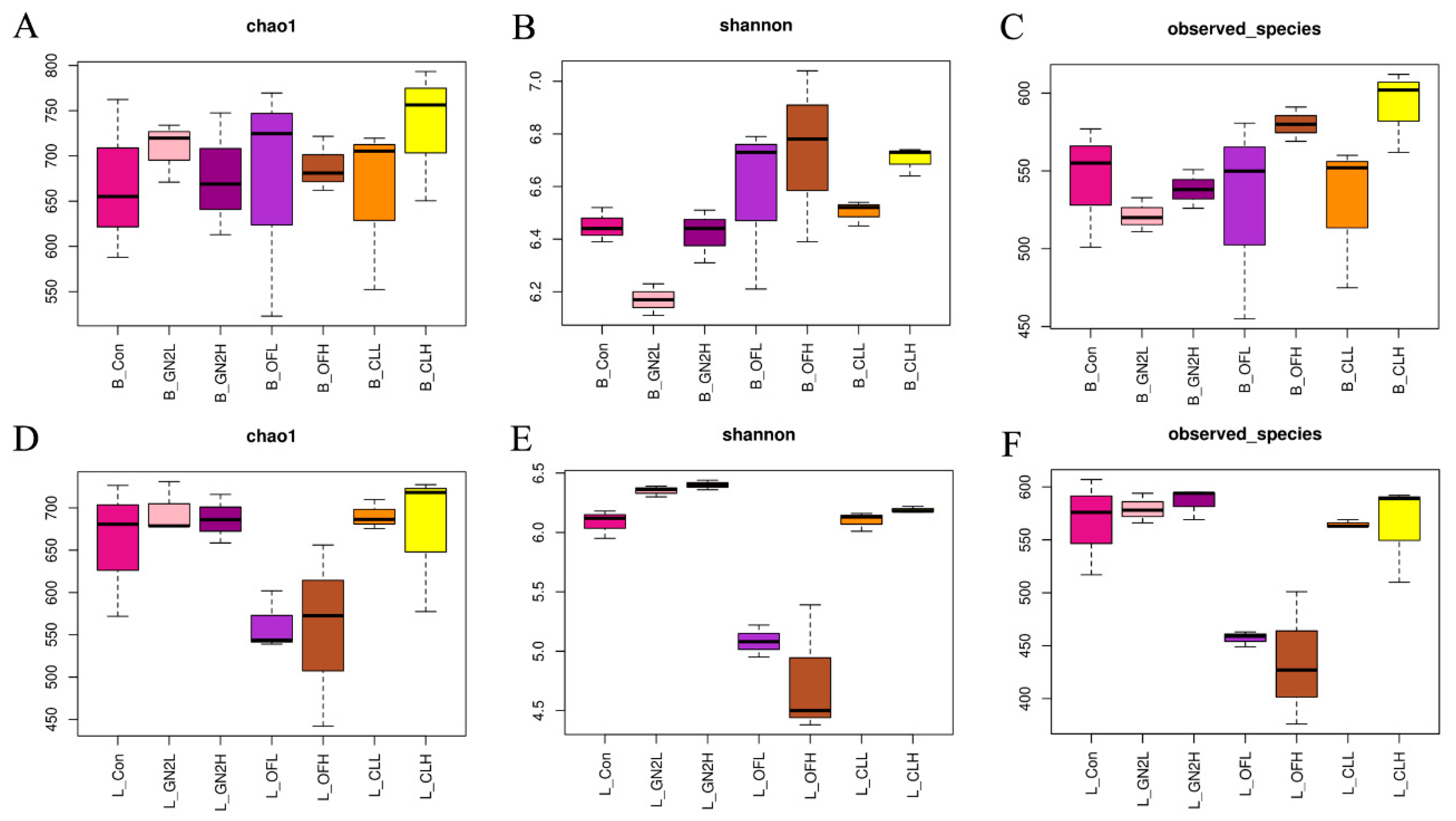
The rarefaction curves of all samples do not plateau, indicating that additional sequencing would have revealed additional bacterial diversity (Supplementary Figure S2). Nonetheless, the sequences covered most of the bacterial diversity within the samples. The average coverage of the bacterial community was 0.92 to 0.94 across all samples (Supplementary Table S1). Three measures of the bacterial community diversity revealed differences among treatments (Figure 2 and Supplementary Table S1). The community richness (Chao1) in the samples before planting was statistically similar (Figure 2A). However, treatments with the BGF product at 0.4% and the raw coal treatments led to rhizosphere communities with significantly higher Chao1 values, while treatment with organic fertilizer at 1.5% produced communities with significantly lower richness than that of the untreated soils (Figure 2D). The Shannon index, providing an estimation of alpha diversity in each sample, ranged from 9.57 to 9.81 in the soil samples before planting and from 9.13 to 9.69 in the rhizosphere soil samples. The application of BGF product and organic fertilizer at both dosages resulted in significantly higher alpha diversity than in the untreated soil before planting and at the time of harvest (Figure 2B,E). While the number of observed bacterial species was essentially the same across treatments before planting, treatment with the BGF product and raw coal at both levels led to significantly higher observed bacterial species in the rhizosphere soils after lettuce harvest, while no significant differences were observed in the organic fertilizer treatments (Figure 2C,F).
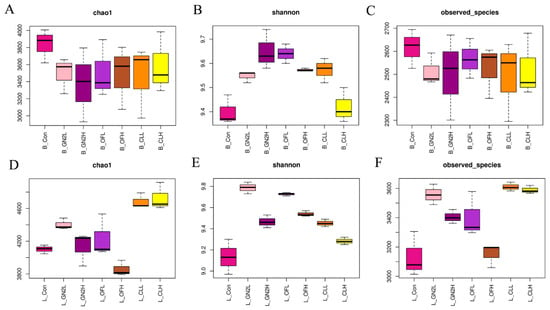
Figure 2.
The richness and diversity of bacterial communities. Values are the average of three replicates (n = 3). (A–C) Chao1, Shannon index, and number of observed species in the soil samples before planting. (D–F) Chao1, Shannon index, and number of observed species in the rhizosphere samples after lettuce harvest. B_: soil mix before planting; L_: lettuce rhizosphere after harvested; Con: control (untreated) in dark pink; GN2L: 0.4% BGF in pink; GN2H: 1.5% BGF in dark purple; OFL: 0.4% organic fertilizer in purple; OFH: 1.5% organic fertilizer in brown; CLL: 0.4% coal in orange; CLH: 0.4% coal in yellow; H: high dosage (1.5% w/w); L: low dosage (0.4% w/w). The error bars represent one standard deviation.
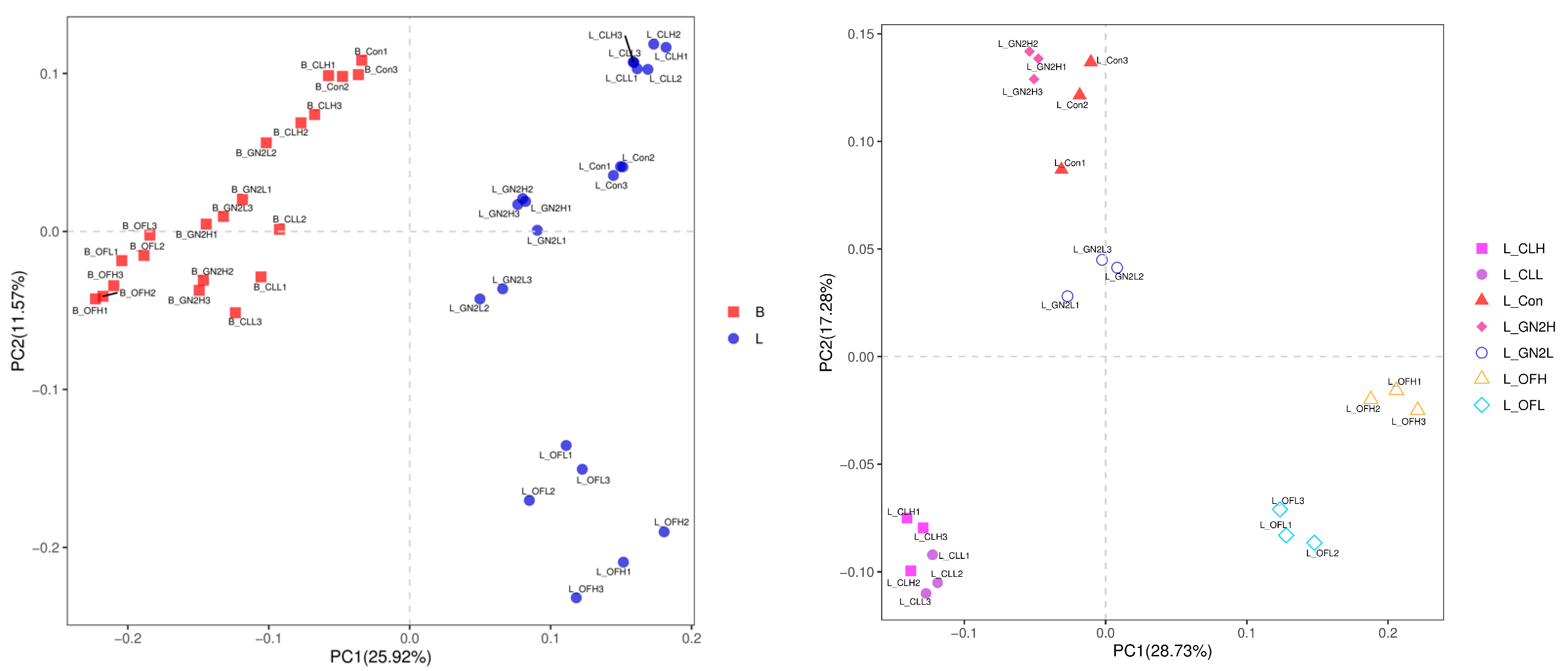
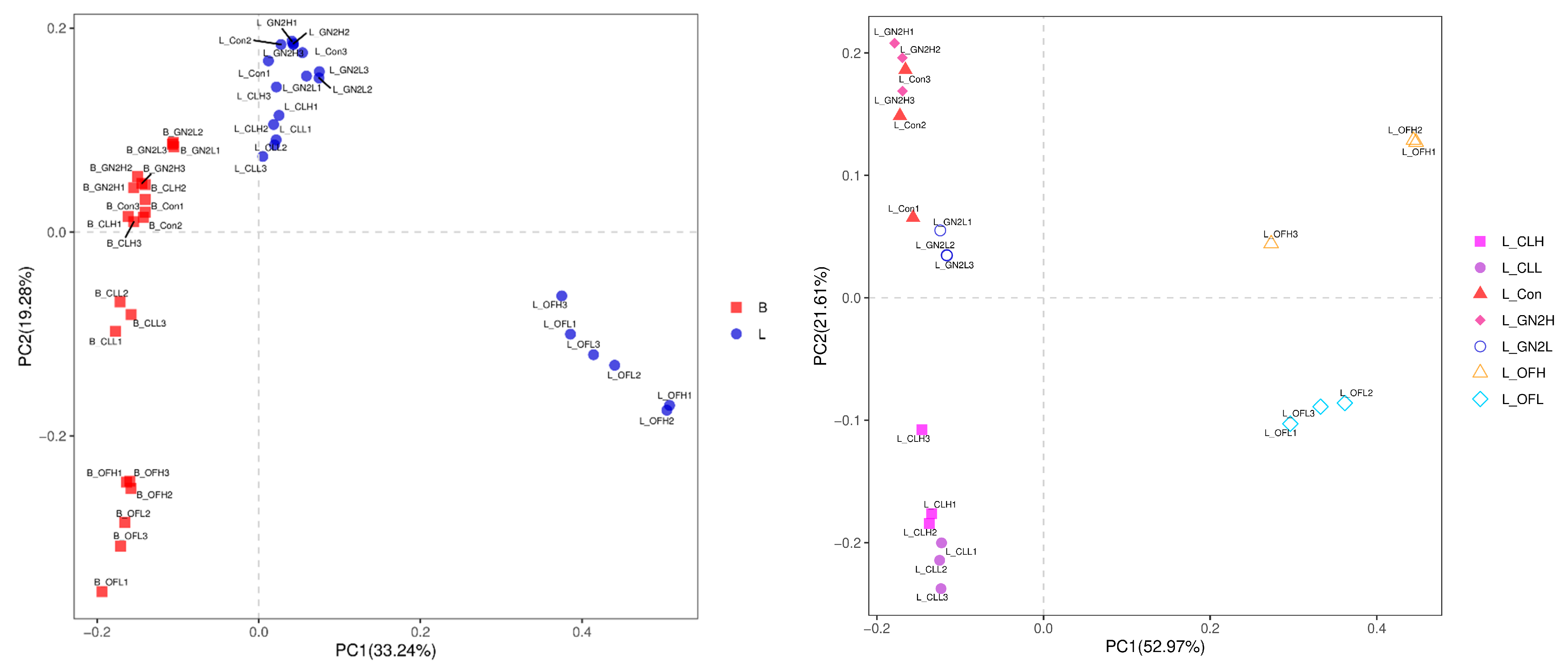
The Bray–Curtis distances among the bacteria in the soil samples reveal substantial changes in the soil microbiomes following plant growth (Figure 3, left). Furthermore, the rhizosphere soil samples from different treatments formed distinct clusters (Figure 3, right), although the distance between high and low dosages of the same treatment were not well separated except for in the case of organic fertilizer.
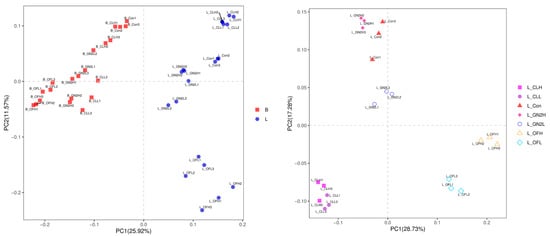
Figure 3.
Principal coordinates analysis for visualization of pairwise bacterial community similarities (Bray–Curtis index). (Left): Comparison of bacterial communities before planting vs. after harvest. (Right): Comparison of bacterial communities from different treatments after harvest. B_: soil mix before planting; L_: lettuce rhizosphere after harvest; Con: control (untreated); CL: coal; GN2: BGF; OF: organic fertilizer; H: high dosage (1.5% w/w); L: low dosage (0.4% w/w).
3.3.2. The Community Structure and Composition of the Rhizosphere Bacteria
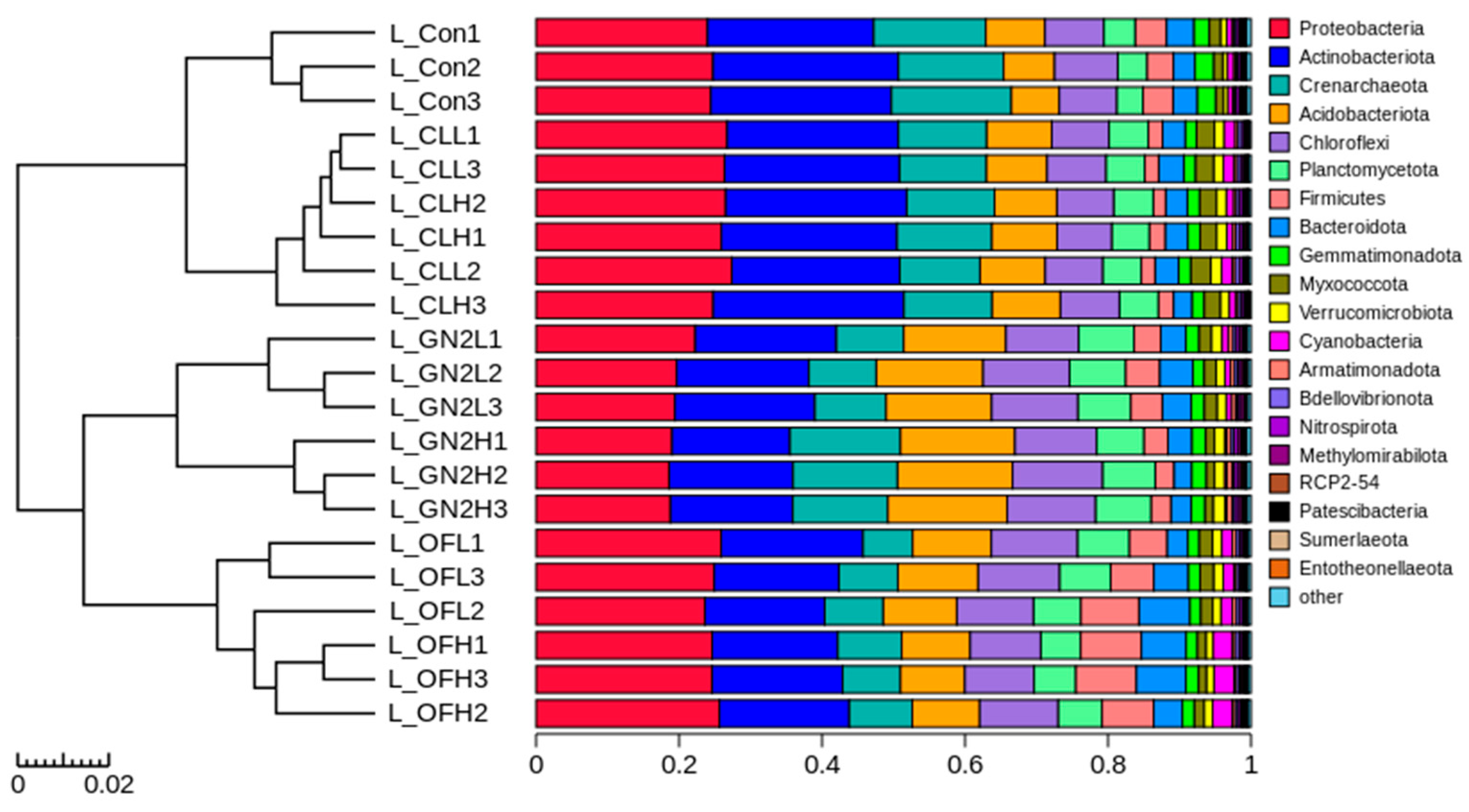
Different treatments had different effects on the bacterial community structure in the rhizosphere soil samples (Figure 4). The structure of the communities from each treatment at both low and high levels clustered closely. The raw coal treatments were closest to the reference, while BGF product and organic fertilizer treatments were separated.
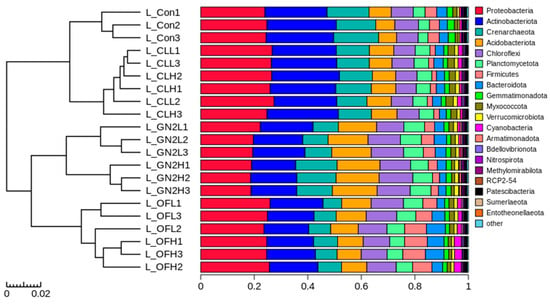
Figure 4.
Analysis of bacterial community structure from rhizosphere soil samples following lettuce harvest. Hierarchical clustering at the phylum level. L_: lettuce rhizosphere after harvest; Con: control (untreated); CL: coal; GN2: BGF; OF: organic fertilizer; H: high dosage (1.5% w/w); L: low dosage (0.4% w/w).
At the phylum level, the five most abundant phyla were Actinobacteriota, Proteobacteria, Acidobacteriota, Chloroflexi, and Crenarchaeota, forming approximately 80% of the bacterial community in the soil samples before planting and the rhizosphere samples (Supplementary Figure S3). In the rhizosphere samples, the BGF treatments at low and high dosages led to lower relative abundances of Actinobacteriota and Proteobacteria and increased Acidobacteriota and Chloroflexi in comparison to the untreated soil (Supplementary Figures S3B and S4). Organic fertilizer treatments resulted in lower abundances of Actinobacteriota and Crenarchaeota and significantly increased Acidobacteriota and Chloroflexi compared to the untreated soil. The only significant change caused by the raw coal treatments was a higher relative abundance of Proteobacteria (Supplementary Figures S3B and S4). At the family level, Nitrososphaeraceae, Sphingomonadaceae, and Nocardioidaceae were significantly lower and Vicinamibacteraceae, Pirellulaceae, and Burkholderiaceae were significantly higher in rhizosphere soils from all treatments in comparison to those of the untreated soil. Furthermore, the rhizosphere soils of BGF treatments had a significantly higher relative abundance of Pyrinomonadaceae and Comamonadaceae than the untreated soil (Supplementary Figure S6).
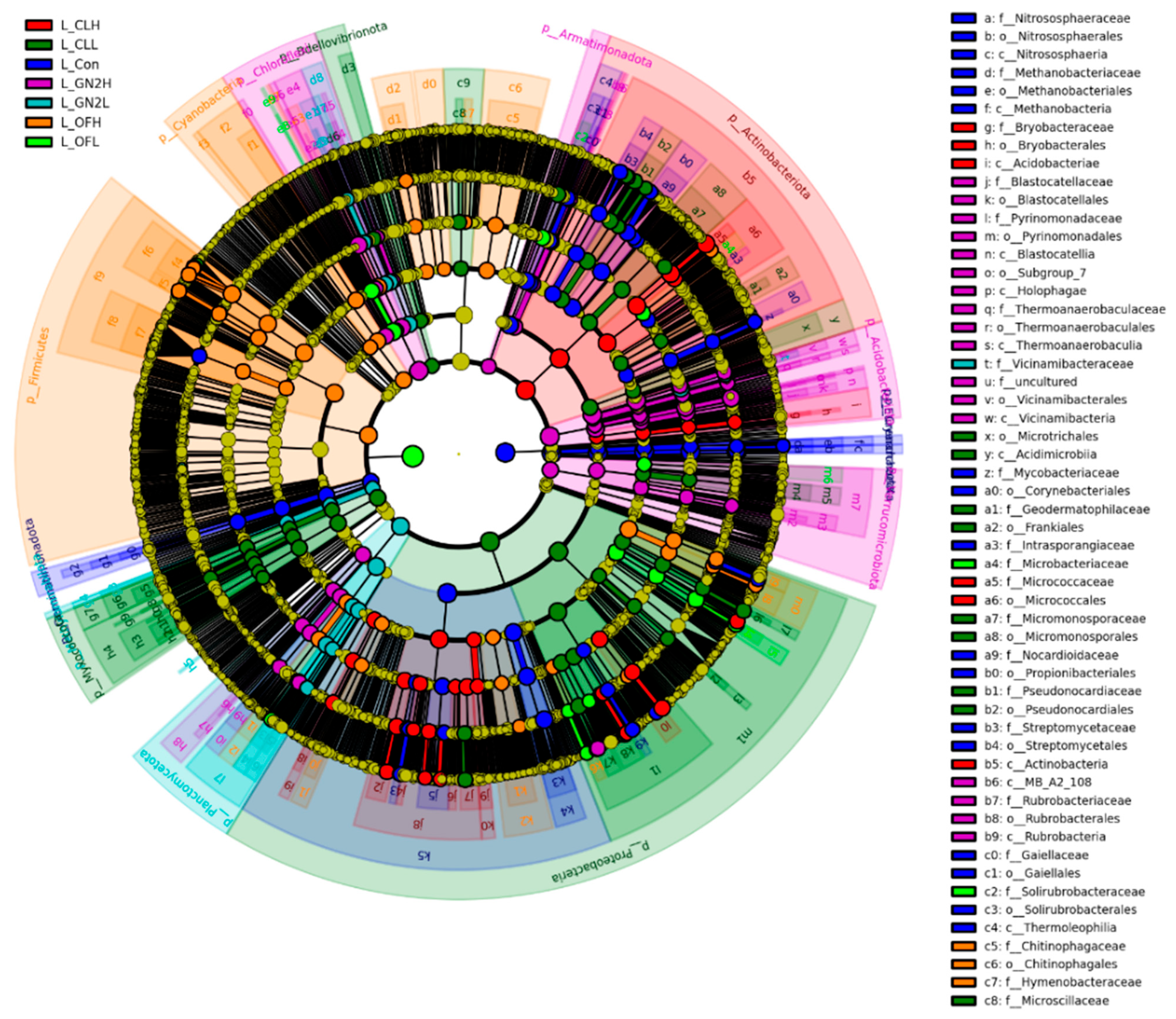
Linear discriminant analysis Effect Size (LEfSe) and Linear Discriminant Analysis (LDA) identified biomarkers with significant differences (p < 0.05, LDA score = 3) (Figure 5). Several biomarkers were identified in BGF treatments at the family level, including Vicinamibacteraceae, Pyrinomonadaceae, and Rubrobacteriaceae, which were consistent with the results of relative abundance analysis.
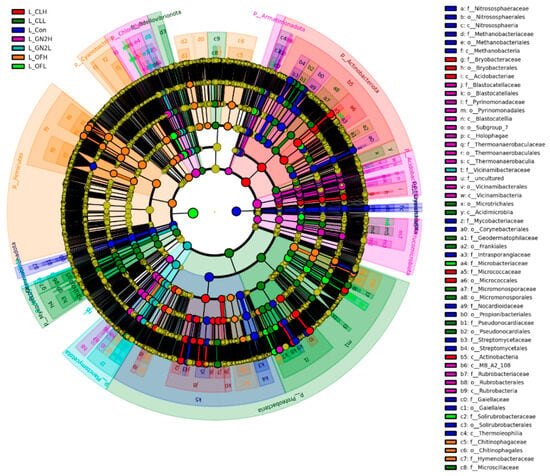
Figure 5.
LEfSe cladogram analysis of the bacterial communities in lettuce rhizosphere samples. From inside to outside, rings represent the phylum, class, order, family, and genus taxonomic levels. The different color nodes on the ring represent significant changes in taxonomic composition in the different treatments. L_: lettuce rhizosphere after harvest; Con: control (untreated); CL: coal; GN2: BGF; OF: organic fertilizer; H: high dosage (1.5% w/w); L: low dosage (0.4% w/w).
3.4. Rhizosphere Fungal Community
3.4.1. Abundance and Diversity of Rhizosphere Fungi
The fungal community structures in soils from the various treatments were investigated to study the effects of the treatments. In total, 2.19 M high-quality sequences were obtained from all 42 samples (14 treatments with three biological replicates). The number of high-quality sequences across the 42 samples ranged from 19 K to 115 K with an average of 52 K. A total of 2185 fungal OTUs were identified at 97% sequence similarity cutoff and 536 OTUs on average across all samples. There were 1772 OTUs from the soils from different treatments before planting and 1629 OTUs from the rhizosphere soils of lettuce plants after harvest. Out of the 2185 OTUs, 1216 were common in soils of different treatments before planting and the rhizosphere soils, while 556 OTUs could only be found in the soils before planting and 413 OTUs were only found in the rhizosphere soils.
The rarefaction curves of treatments did not plateau, indicating that additional sequencing would have continued to reveal additional fungal diversity (Supplementary Figure S5). However, the average coverage of the fungal community was 0.98 to 0.99 across all samples. The Shannon index, providing an estimation of alpha diversity in each sample, ranged from 4.85 to 6.67 with an average of 6.22. The richness (Chao1) ranged from 559 to 781, with an average of 688 (Supplementary Table S2).
The fungal communities in soil samples before planting were found to have the same richness (Chao1) and number of observed fungal species (Figure 6 and Supplementary Table S2). The only differences in the Shannon index for fungi prior to planting were found for the BGF product treatment at 0.4% (lower) and the raw coal treatment at 1.5% (higher) (Figure 6B). In the rhizosphere soil samples, the organic fertilizer treatment at both levels had significantly lower richness (Chao1), Shannon index, and number of observed fungal species than the untreated soil community, while the soils with BGF product had a significantly higher Shannon index than the untreated soil but no statistical difference in richness and number of observed species (Figure 6D–F).
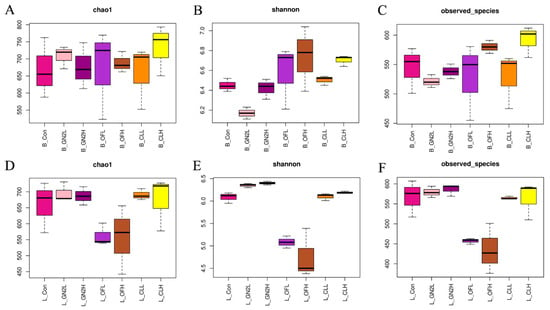
Figure 6.
The richness and diversity of fungal communities in soil samples. Values are the average of three replicates (n = 3). (A–C) Chao1 value, Shannon index, and number of observed species in the soil samples before planting. (D–F) Chao1 value, Shannon index, and number of observed species in the rhizosphere samples after lettuce harvest. B_: soil mix before planting; L_: lettuce rhizosphere after harvested; Con: control (untreated) in dark pink; GN2L: 0.4% BGF in pink; GN2H: 1.5% BGF in dark purple; OFL: 0.4% organic fertilizer in purple; OFH: 1.5% organic fertilizer in brown; CLL: 0.4% coal in orange; CLH: 0.4% coal in yellow; H: high dosage (1.5% w/w); L: low dosage (0.4% w/w). Error bars represent one standard deviation.
The Bray–Curtis distances among all the samples were determined (Figure 7). As with the soil bacteria, substantial changes in the communities were observed post-harvest. In the rhizosphere soils, the fungal communities in the BGF product and raw coal-treated soils clustered closely to the untreated samples, while the organic fertilizer treatments were very different (Figure 7, right).
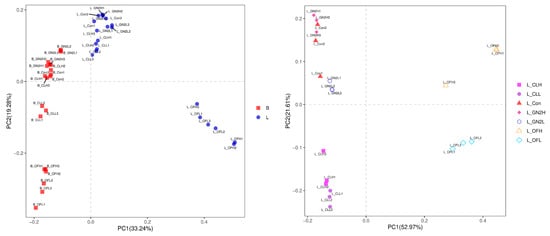
Figure 7.
Principal coordinates analysis for visualization of pairwise fungal community similarities (Bray–Curtis index). (Left): Comparison of bacterial communities before planting vs. after harvest. (Right): Comparison of bacterial communities from different treatments after harvest. B_: soil mix before planting; L_: lettuce rhizosphere after harvest; Con: control (untreated); CL: coal; GN2: BGF; OF: organic fertilizer; H: high dosage (1.5% w/w); L: low dosage (0.4% w/w).
3.4.2. The Community Structure and Composition of the Rhizosphere Fungi
The fungal communities of the soil samples before planting and after harvest were analyzed at different taxonomic levels. At the phylum level, Ascomycota (about 80%) and Basidiomycota (about 13%) dominated the fungal community in all the soil samples before planting lettuce (Supplementary Figure S6). Different treatments resulted in different fungal community structures in the rhizosphere soils of lettuce plants. In comparison to the reference, the BGF product treatments both at low and high levels decreased the relative abundance of Basidiomycota and increased Ascomycota abundance (Supplementary Figures S6 and S7). The use of organic fertilizer at both low and high dosages led to significant lower Basidiomycota and Ascomycota but increased the abundance of Mucoromycota in comparison to the reference and other treatments (Supplementary Figures S6 and S7). At family level, the relative abundances of Aspergillaceae, Thyridariaceae, Onygenaceae, Coniochaetaceae, Plectosphaerellaceae, Clavicipitaceae, Hypocreaceae were significantly different in the BGF product treatments in comparison to the abundances of those organisms in the untreated soil.
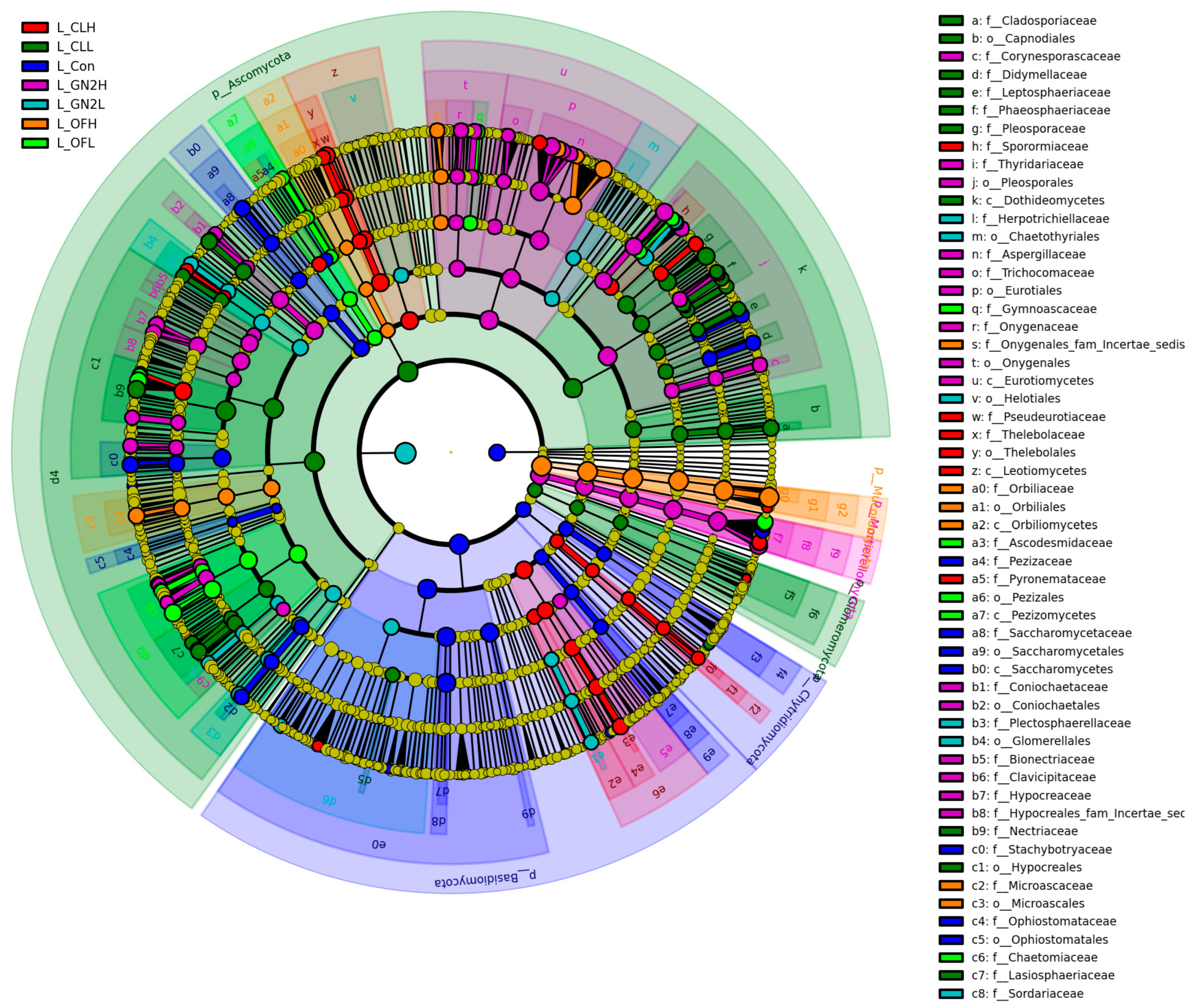
LEfSe analysis has been conducted to identify biomarkers which showed significant differences (p < 0.05, LDA score = 3) (Figure 8). Several biomarkers were identified in BGF treatments including Aspergillaceae, Thyridariaceae, Onygenaceae, Coniochaetaceae, Plectosphaerellaceae, Clavicipitaceae, and Hypocreaceae at the family level.
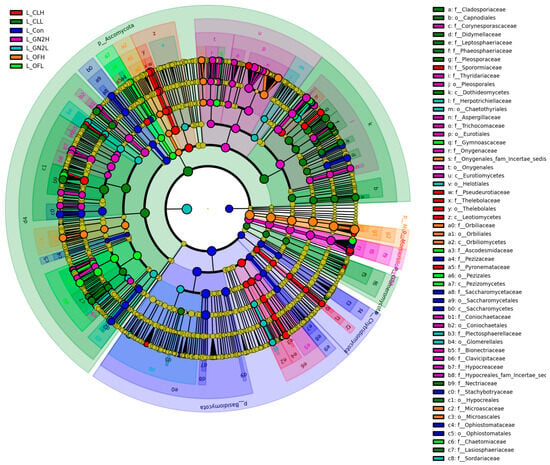
Figure 8.
LEfSe cladogram analysis of the fungal communities in the lettuce rhizosphere samples. From inside to outside, rings represent the phylum, class, order, family, and genus taxonomic levels. The different color nodes on the ring represent significant changes in taxonomic composition in the different treatments. L_: lettuce rhizosphere after harvest; Con: control (untreated); CL: coal; GN2: BGF; OF: organic fertilizer; H: high dosage (1.5% w/w); L: low dosage (0.4% w/w).
4. Discussion
4.1. The Effects of the Coal-Derived (BGF) Product on Plant Growth
Low-rank coal and coal-derived products were developed as soil amendments due to the positive effects on plant growth. Commercial products derived from lignite (brown coal) are widely promoted as plant growth stimulants leading to higher crop yields [21]. However, the actual efficacy of coal products as soil amendments on promoting plant growth can vary dramatically depending on the used coal type, the tested plant and soil, as well as environmental conditions. The application of low-rank coal and coal-derived products has shown improvement on plant growth and development in various studies on different plants including sunflower [8], potato [9], sugar beet [22], wheat [23,24], bean [25], soybean [26], and pepper [27]. For instance, a leonardite-based soil amendment had a significant positive effect on potato growth (54.9%) and tuber yield (66.4%) when compared to the control [28] and a foliar application of a leonardite-based product on sugar beet (Beta vulgaris L.) plants promoted plant growth and sugar yield [22]. The humic acid derived from low-rank coal has been reported to be a promising natural resource showing persistent effects on plant growth promotion, nutrient uptake, and soil nutrient status. Arjumend et al. found that the application of humic acid improved soil nutrient status by increasing organic matter (9%), total N (30%), available P (166%) and available K (52%) and increased wheat growth in terms of shoot length (18%), root length (29%), shoot dry weight (76%), root dry weight (100%) and chlorophyll content (96%) [29]. Another study reported a positive benefit for arnica (Arnica montana L.) from leonardite additions. Leonardite application not only significantly increased the number of flowering stems and inflorescences per plant, but also affected the activity of enzymes catalyzing the transformation of the most important processes of soil organic matter [30].
The results from this study appear to be equivalent or outperform the above referenced studies. The addition of the BGF product resulted in significantly higher plant, shoot, and root weights in comparison to those of plants in untreated soil. Moreover, the application of the BGF product resulted in a more developed root system with more than 40% higher root surface area and 75% higher root volume, in comparison to those of the plants in untreated soil (Figure 1C,D). This is consistent with the results from a field test on sunflower in a previous study [8]. Chen et al. found that microbially digested lignite promoted sunflower growth including plant height, leaf area index, and dry biomass respectively, in comparison with the reference treatment. Moreover, the root length, root surface area, root volume, average yield, water productivity, partial nitrogen productivity, and economic gain in the lignite bioorganic fertilizer treatments were substantially higher than those of the control (CK) treatment [8].
4.2. The Effects of the Coal-Derived (BGF) Product on Soil Properties
The application of low-rank coal and its derivatives has been found to improve the soil organic matter in several previous studies due to their high contents of organic matter [23,25,31,32,33]. In the present study, we found that the addition of the BGF product, especially at 1.5% w/w, significantly improved the soil organic matter content in the amended soils to a level approximately 50% higher than the reference soil (Table 1). In addition to increased SOM, previous studies have shown that low-rank coals and derived products can improve water retention ability, aggregate stability/porosity, aeration, and bulk density. The addition of highly humified organic matter such as coal-derived humic substances can improve the structural and water retention properties of degraded arable soils [24]. Ciarkowska et al. studied the effects of a lignite-derived humic acid product on medium and coarse-textured soil properties, finding that almost all soil parameters, including sorption complex characteristics, organic matter quality and dehydrogenase activity, were improved after humic acids application in both soils [31]. Furthermore, low-rank coal has been modified to improve its pore structure and nutrient absorption, making it suitable for developing slow-release fertilizer [34,35,36].
Low-rank coal contains large amounts of humic substances that can effectively improve the physicochemical, biological, and ecological characteristics of soils. Studies have shown that low-rank coals and its derivatives can positively impact the soil properties and organic matter turnover [4].
The positive effect of humic acids on plant roots is well known including for bell pepper (Capsicum annuum L. cv. revolution) [26], barley [37], Zea mays [38], riceberry [39], snap bean (Phaseolus vulgaris L.) [40], and wheat [41]. However, the mechanism responsible for this effect of humic substances is poorly understood. Qian et al. characterized different fractions of humic acid derived from leonhardite and evaluated their effects on the seedling growth and nutrient uptake of snap bean (Phaseolus vulgaris L.). They found that a low-molecular-weight fraction of humic acid appeared to promote the production of snap bean due to an enhancement in the physical growth of the leaf and root [40]. Another study found that a low-molecular-size (LMS < 3500 Da) fraction of leonhardite organic matter is the major candidate for determining the positive effects on plant growth because the can easily reach the plasmalemma of higher plant cells and be taken up into them while a high molecular size fraction (HMS > 3500 Da) is not absorbed and can interact only with the cell wall [42]. In the present study, soil amendment with the BGF product promoted the growth and root system of lettuce. The BGF product was prepared by microbial digestion of lignite, and the product contains organic matter with different molecular sizes ranging from humic substances to small soluble organic acids [7]. It is possible that the molecular size range of the organic matter in the BGF product is like that of the LMS fraction of the humic acid derived from leonhardite. However, further studies are needed to characterize the functional groups of compounds in the BGF products for plant growth.
Chen et al. [8] conducted studies using the BGF product and found that soil amended with BGF product resulted in improved crop root and overall growth, water productivity, and partial nitrogen productivity. The improvement in root growth and partial nitrogen productivity are indicators of positive effects of the digested coal product amendment on soil microbial.
4.3. The Effects of the Coal-Derived (BGF) Product on the Plant Rhizosphere Microbiome
Previous studies have shown the application of low-rank coal and derived products affected the soil microbiome. For instance, the addition of 0.5 and 1.0 kg ha−1 humic acid increased the bacterial population by 355% to 476% and the fungal population 610 to 716% in a study by Sharif et al. on maize (Zea mays L. kissan) [43]. Similarly, leonhardite treatment increased soil microbial biomass [33]. However, raw lignite was found to have only a minor, temporary impact on soil microbial activity and community composition [44].
A metagenomic analysis in potato soils showed that the application of humic acid resulted in higher microbial diversity and richness compared to the control, indicating that humic acid may serve as a nutrient source for microbial communities to stimulate their growth [28].
Although the present study primarily reports correlative changes in the rhizosphere microbiome, several lines of evidence suggest plausible mechanistic links between the observed community restructuring and the pronounced lettuce-growth response [45,46,47].
The enrichment of Acidobacteriota and Chloroflexi may be associated with carbon turnover and auxin-like signaling. The coal-derived amendment substantially increased the relative abundance of Acidobacteriota (by 38%) and Chloroflexi (by 24%). Members of both phyla possess broad repertoires of carbohydrate-active enzymes and are efficient degraders of recalcitrant organic matter [45]. By accelerating lignocellulose and humic-substance depolymerization, these taxa likely release low-molecular-weight carboxylates, phenolics, and amino acids that can serve as readily assimilable nutrients for the plant and act as signaling molecules that modulate root architecture. Several Acidobacteriota genomes encode indole-3-acetic acid (IAA) biosynthetic pathways [47]; increased IAA flux from the rhizosphere can explain the 40% expansion in root surface area and the 75% rise in root volume measured in the amendment treatment.
Shifts from Basidiomycota to Ascomycota may indicate disease suppression and nutrient mobilization. Fungal profiling revealed a decrease in Basidiomycota (−31%) and a concomitant enrichment of Ascomycota (+27%). Many Basidiomycetes are saprotrophs that compete with plants for mineral nitrogen, whereas Ascomycetous genera such as Trichoderma, Chaetomium, and Penicillium produce an arsenal of chitinases, β-glucanases, and antibiotics that suppress root pathogens [46]. LEfSe analysis (Figure 8) did indeed highlight Trichoderma spp. as indicator taxa in the amended rhizosphere. Their proliferation could have reduced pathogen pressure and freed plant resources for growth.
Size-exclusion chromatography showed that ~70% of the dissolved organic carbon in the BGF product studied is <3.5 kDa. Such small, labile molecules preferentially fuel fast-growing copiotrophs, including many Acidobacteriota sub-groups and Ascomycota, thereby steering the community toward taxa that recycle nutrients rapidly and secrete growth-promoting metabolites. This selective enrichment offers a coherent explanation for the tight coupling between microbiome composition and plant performance recorded here.
Taken together, these observations support a working model in which the coal-derived amendment shapes the rhizosphere microbiome toward a consortium that mineralizes complex organics, supplies auxinic and antifungal metabolites, and accelerates nutrient cycling. Future work plans to employ stable-isotope tracing and metabolomics to confirm carbon flow from amendment to microbe to plant, and gnotobiotic experiments to dissect the individual roles of key indicator taxa.
5. Conclusions
This study demonstrates that a lignite-derived, microbially digested amendment (BGF product, Ginate®) can enhance lettuce productivity through coordinated effects on soil chemistry, root architecture, and the rhizosphere microbiome. BGF application doubled shoot biomass and, unlike the commercial organic fertilizer, uniquely expanded root mass, surface area, and volume—traits that underpin greater water and nutrient capture.
Soil analyses revealed a marked rise in total organic matter after BGF treatment, comparable to the organic fertilizer control, yet the accompanying shifts in microbial consortia were distinct. BGF selectively enriched bacterial taxa such as Acidobacteriota and Chloroflexi, and fungal groups within the Ascomycota, lineages frequently linked to carbon mineralization, auxin production, and pathogen suppression. These community changes, coupled with the amendment’s high proportion of low-molecular-weight organics, likely accelerate nutrient cycling and supply plant-growth-promoting metabolites, thereby explaining the superior root and shoot development observed.
In summary, our results indicate that this coal-derived amendment boosts soil organic matter and steers the rhizosphere toward a microbiome conducive to vigorous plant growth, without directly increasing the NPK content of the soil. These dual benefits position the BGF product as a viable, circular-economy alternative to conventional organic fertilizers, warranting further field validation and mechanistic studies across diverse crops and soil types.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15212310/s1, Supplementary Table S1: The richness and diversity of bacterial communities; Supplementary Table S2: The richness and diversity of fungal communities; Supplementary Figure S1: Lettuce root structure in the soils with different treatments (BGF, organic fertilizer, and raw coal at 1.5% w/w) in comparison to untreated soils; Supplementary Figure S2: Rarefaction curves of the samples for bacterial community analysis; Supplementary Figure S3: Histograms of the relative abundance of bacteria at the phylum level; Supplementary Figure S4: Relative abundance of bacteria in the rhizosphere soil samples at phylum (top) and family (bottom) levels; Supplementary Figure S5: Rarefaction curves of the samples for fungal community analysis; Supplementary Figure S6: Histograms showing the relative abundance of fungi at phylum level for soils before planting (top) and after harvest (bottom); Supplementary Figure S7: Relative abundance of fungi in the rhizosphere soil samples at phylum (top) and family (bottom) levels.
Author Contributions
X.-F.H., K.F.R., P.H.F. and S.J. conceived the presented idea and planned the experiment. X.-F.H. and P.H.F. carried out the experiments. X.-F.H. analyzed the data. X.-F.H., wrote the manuscript in consultation with K.F.R., P.H.F. and S.J., K.F.R. and S.J. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The soil analysis and plant growth data presented in this study are included in the article. The data presented in this study are available on request from the corresponding author due to the fact that we have not deposited the microbiome data in a publicly accessible repository yet.
Acknowledgments
The authors thank Patrick Maag (Scottsbluff, Nebraska) for providing, collecting and delivering the soil used in the experiments.
Conflicts of Interest
Authors Paul H. Fallgren and Song Jin were employed by the company Advanced Environmental Technologies, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Gerland, P.; Raftery, A.E.; Ševčíková, H.; Li, N.; Gu, D.; Spoorenberg, T.; Alkema, L.; Fosdick, B.K.; Chunn, J.; Lalic, N.; et al. World population stabilization unlikely this century. Science 2014, 346, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health; Springer: Cham, Switzerland, 2021; Volume 2. [Google Scholar]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Akimbekov, N.S.; Digel, I.; Tastambek, K.T.; Sherelkhan, D.K.; Jussupova, D.B.; Altynbay, N.P. Low-Rank Coal as a Source of Humic Substances for Soil Amendment and Fertility Management. Agriculture 2021, 11, 1261. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Rouhani, A.; Skousen, J.; Tack, F.M.G. An Overview of Soil Pollution and Remediation Strategies in Coal Mining Regions. Minerals 2023, 13, 1064. [Google Scholar] [CrossRef]
- Fallgren, P.H.; Chen, L.; Peng, M.; Urynowicz, M.A.; Jin, S. Facultative-anaerobic microbial digestion of coal preparation waste and use of effluent solids to enhance plant growth in a sandy soil. Int. J. Coal Sci. Technol. 2021, 8, 767–779. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Zhang, X.; Xiong, Y.; Huang, Q.; Jin, S.; Sun, S.; Chi, D.; Huang, G. Effects of lignite bioorganic product on sunflower growth, water and nitrogen productivity in saline-sodic farmlands at Northwest China. Agric. Water Manag. 2022, 271, 107806. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Fenton, O.; Szara, E.; Thornton, S.F.; Malina, G. Holistic Assessment of Biochar and Brown Coal Waste as Organic Amendments in Sustainable Environmental and Agricultural Applications. Water Air Soil Pollut. 2021, 232, 106. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Jeong, H.J.; Cha, J.-Y.; Choi, M.; Jang, K.-S.; Kim, W.-Y.; Kim, M.G.; Jeon, J.-R. Structural variation of humic-like substances and its impact on plant stimulation: Implication for structure-function relationship of soil organic matters. Sci. Total Environ. 2020, 725, 138409. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. Chapter Two—A Meta-Analysis and Review of Plant-Growth Response to Humic Substances: Practical Implications for Agriculture. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 124, pp. 37–89. [Google Scholar]
- Guan, X.; Cheng, Z.; Li, Y.; Wang, J.; Zhao, R.; Guo, Z.; Zhao, T.; Huang, L.; Qiu, C.; Shi, W.; et al. Mixed organic and inorganic amendments enhance soil microbial interactions and environmental stress resistance of Tibetan barley on plateau farmland. J Environ. Manag. 2023, 330, 117137. [Google Scholar] [CrossRef]
- Miller, S.B.; Heuberger, A.L.; Broeckling, C.D.; Jahn, C.E. Non-Targeted Metabolomics Reveals Sorghum Rhizosphere-Associated Exudates are Influenced by the Belowground Interaction of Substrate and Sorghum Genotype. Int. J. Mol. Sci. 2019, 20, 431. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Little, K.R.; Rose, M.T.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Do lignite-derived organic amendments improve early-stage pasture growth and key soil biological and physicochemical properties? Crop Pasture Sci. 2014, 65, 899–910. [Google Scholar] [CrossRef]
- Della Lucia, M.C.; Bertoldo, G.; Broccanello, C.; Maretto, L.; Ravi, S.; Marinello, F.; Sartori, L.; Marsilio, G.; Baglieri, A.; Romano, A.; et al. Novel Effects of Leonardite-Based Applications on Sugar Beet. Front. Plant Sci. 2021, 12, 646025. [Google Scholar] [CrossRef]
- Olego, M.Á.; Cuesta Lasso, M.; Quiroga, M.J.; Visconti, F.; López, R.; Garzón-Jimeno, E. Effects of Leonardite Amendments on Vineyard Calcareous Soil Fertility, Vine Nutrition and Grape Quality. Plants 2022, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, A.; Pietramellara, G.; Mbagwu, J.S.C. Effects of coal derived humic substances on water retention and structural stability of Mediterranean soils. Soil Use Manag. 1996, 12, 209–213. [Google Scholar] [CrossRef]
- Ece, A.; Saltali, K.; Eryigit, N.; Uysal, F. The Effects of Leonardite Applications on Climbing Bean (Phaseolus vulgaris L.) Yield and the Some Soil Properties. J. Agron. 2007, 6, 480–483. [Google Scholar] [CrossRef]
- da Silva, M.S.R.d.A.; de Carvalho, L.A.L.; Braos, L.B.; de Sousa Antunes, L.F.; da Silva, C.S.R.d.A.; da Silva, C.G.N.; Pinheiro, D.G.; Correia, M.E.F.; Araújo, E.d.S.; Colnago, L.A.; et al. Effect of the application of vermicompost and millicompost humic acids about the soybean microbiome under water restriction conditions. Front. Microbiol. 2022, 13, 1000222. [Google Scholar] [CrossRef]
- Qin, K.; Leskovar, D.I. Lignite-derived humic substances modulate pepper and soil-biota growth under water deficit stress. J. Plant Nutr. Soil Sci. 2018, 181, 655–663. [Google Scholar] [CrossRef]
- Akimbekov, N.; Qiao, X.; Digel, I.; Abdieva, G.; Ualieva, P.; Zhubanova, A. The Effect of Leonardite-Derived Amendments on Soil Microbiome Structure and Potato Yield. Agriculture 2020, 10, 147. [Google Scholar] [CrossRef]
- Arjumend, T.; Abbasi, M.K.; Rafique, E. Effects of lignite-derived humic acid on some selected soil properties, growth and nutrient uptake of wheat (Triticum aestivum L.) grown under greenhouse conditions. Pak. J. Bot 2015, 47, 2231–2238. [Google Scholar]
- Sugier, D.; Kołodziej, B.; Bielińska, E. The effect of leonardite application on Arnica montana L. yielding and chosen chemical properties and enzymatic activity of the soil. J. Geochem. Explor. 2013, 129, 76–81. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Sołek-Podwika, K.; Filipek-Mazur, B.; Tabak, M. Comparative effects of lignite-derived humic acids and FYM on soil properties and vegetable yield. Geoderma 2017, 303, 85–92. [Google Scholar] [CrossRef]
- Cubillos Hinojosa, J.G.; Valero Valero, N.O.; Peralta, A.d.J. Effect of a low rank coal inoculated with coal solubilizing bacteria for the rehabilitation of a saline-sodic soil in field conditions. Rev. Fac. Nac. Agron. Medellín 2017, 70, 8271–8283. [Google Scholar] [CrossRef]
- Bekele, A.; Roy, J.L.; Young, M.A. Use of biochar and oxidized lignite for reconstructing functioning agronomic topsoil: Effects on soil properties in a greenhouse study. Can. J. Soil Sci. 2015, 95, 269–285. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Cheng, D. Activated-Lignite-Based Super Large Granular Slow-Release Fertilizers Improve Apple Tree Growth: Synthesis, Characterizations, and Laboratory and Field Evaluations. J. Agric. Food Chem. 2017, 65, 5879–5889. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Rose, M.T.; Wong, V.; Cavagnaro, T.R.; Patti, A.F. Hybrid brown coal-urea fertiliser reduces nitrogen loss compared to urea alone. Sci. Total Environ. 2017, 601–602, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Rose, M.T.; Wong, V.N.L.; Cavagnaro, T.R.; Patti, A.F. Nitrogen Dynamics in Soil Fertilized with Slow Release Brown Coal-Urea Fertilizers. Sci. Rep. 2018, 8, 14577. [Google Scholar] [CrossRef]
- Nagasawa, K.; Wang, B.; Nishiya, K.; Ushijima, K.; Zhu, Q.; Fukushima, M.; Ichijo, T. Effects of humic acids derived from lignite and cattle manure on antioxidant enzymatic activities of barley root. J. Environ. Sci. Health Part B 2016, 51, 81–89. [Google Scholar] [CrossRef]
- David, J.; Šmejkalová, D.; Hudecová, Š.; Zmeškal, O.; von Wandruszka, R.; Gregor, T.; Kučerík, J. The physico-chemical properties and biostimulative activities of humic substances regenerated from lignite. SpringerPlus 2014, 3, 156. [Google Scholar] [CrossRef]
- Jomhataikool, B.; Faungnawakij, K.; Kuboon, S.; Kraithong, W.; Chutipaichit, S.; Fuji, M.; Eiad-Ua, A. Effect of humic acid extracted from Thailand’s leonardite on rice growth. J. Met. Mater. Miner. 2019, 29, 1–7. [Google Scholar]
- Qian, S.; Ding, W.; Li, Y.; Liu, G.; Sun, J.; Ding, Q. Characterization of humic acids derived from Leonardite using a solid-state NMR spectroscopy and effects of humic acids on growth and nutrient uptake of snap bean. Chem. Speciat. Bioavailab. 2015, 27, 156–161. [Google Scholar] [CrossRef]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Kazmi, M.H. Lignite-Derived Humic Acid Effect on Growth of Wheat Plants in Different Soils. Pedosphere 2011, 21, 124–131. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Sharif, M.; Khattak, R.A.; Sarir, M.S. Effect of different levels of lignitic coal derived humic acid on growth of maize plants. Commun. Soil Sci. Plant Anal. 2002, 33, 3567–3580. [Google Scholar] [CrossRef]
- Kim Thi Tran, C.; Rose, M.T.; Cavagnaro, T.R.; Patti, A.F. Lignite amendment has limited impacts on soil microbial communities and mineral nitrogen availability. Appl. Soil Ecol. 2015, 95, 140–150. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Trojan, D.; Roux, S.; Herbold, C.; Rattei, T.; Woebken, D. Genomic insights into the Acidobacteria reveal strategies for their success in terrestrial environments. Env. Microbiol 2018, 20, 1041–1063. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The Ecology of Acidobacteria: Moving beyond Genes and Genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).