Plant Desiccation and Root Rot in Rosemary: Insight into Macrophomina phaseolina, Ceratobasidium sp. and Fusarium falciforme Roles in Co-Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Inspection and Sampling
2.2. Pathogens Isolation and Morphological Characterization
2.3. Molecular Identification and Phylogenetic Analysis
| Pathogen-Type Colony | Region | Primers | Annealing T (°C) | References |
|---|---|---|---|---|
| Rhizoctonia sp. | Internal transcribing spacer | ITS1-4 | 55 | [19] |
| Macrophomina sp. | Internal transcribing spacer | ITS1-4 | 55 | [19] |
| Translation elongation factor 1α | TEF 728-986 | 58 | [20] | |
| Fusarium sp. | Internal transcribing spacer | ITS1-4 | 55 | [19] |
| Translation elongation factor 1α | TEF 688-1251 | 58 | [21] | |
| β-tubulin | T1/β-tub2b | 58 | [22,23] | |
| Actin | ACT512-783 | 59 | [20] |
2.4. In Vitro Interaction Analysis
2.5. Pathogenicity Tests and Pathogen In Vivo Interaction
2.6. Statistical Analysis
3. Results
3.1. Isolation and Morphological Identification
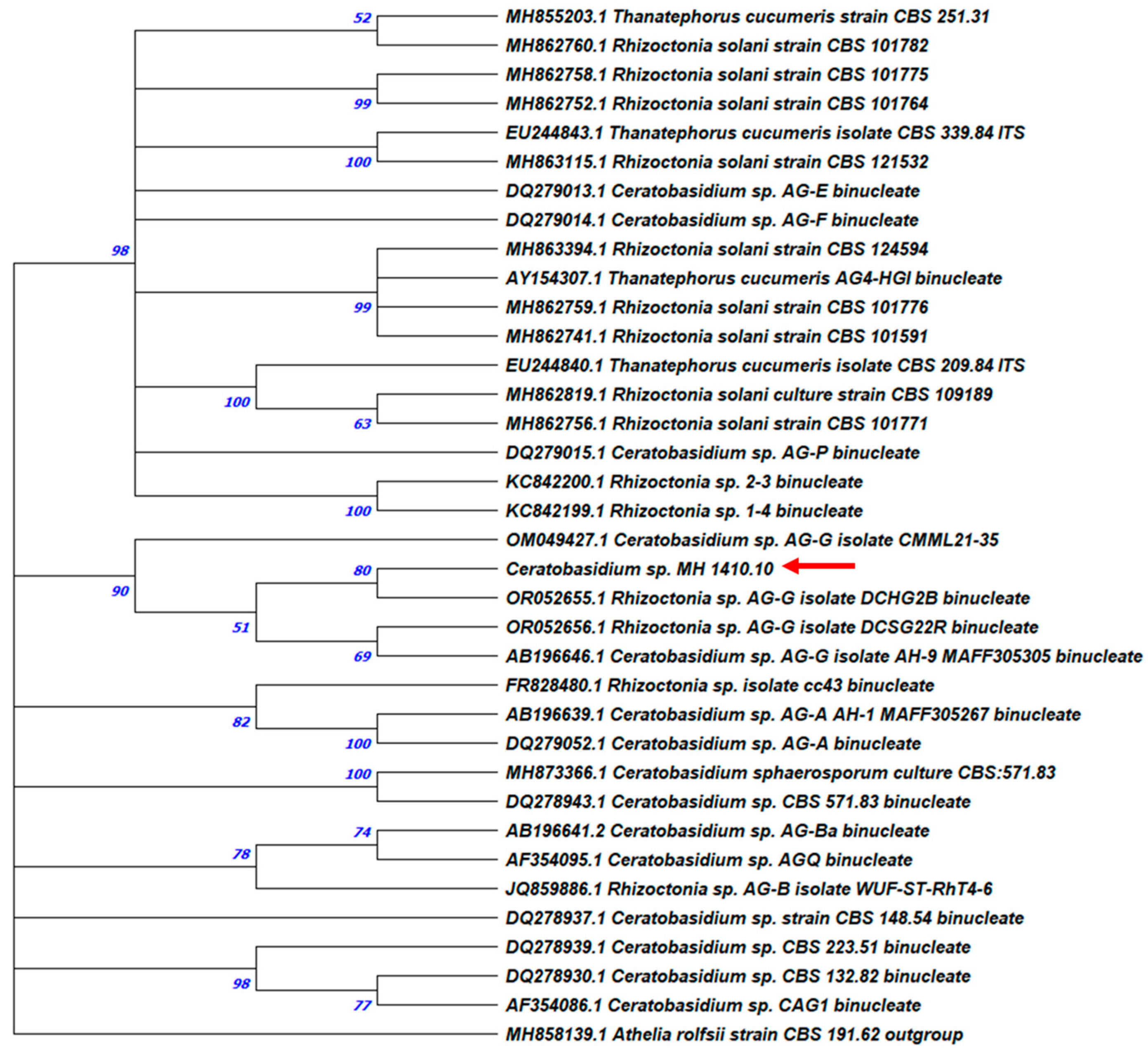
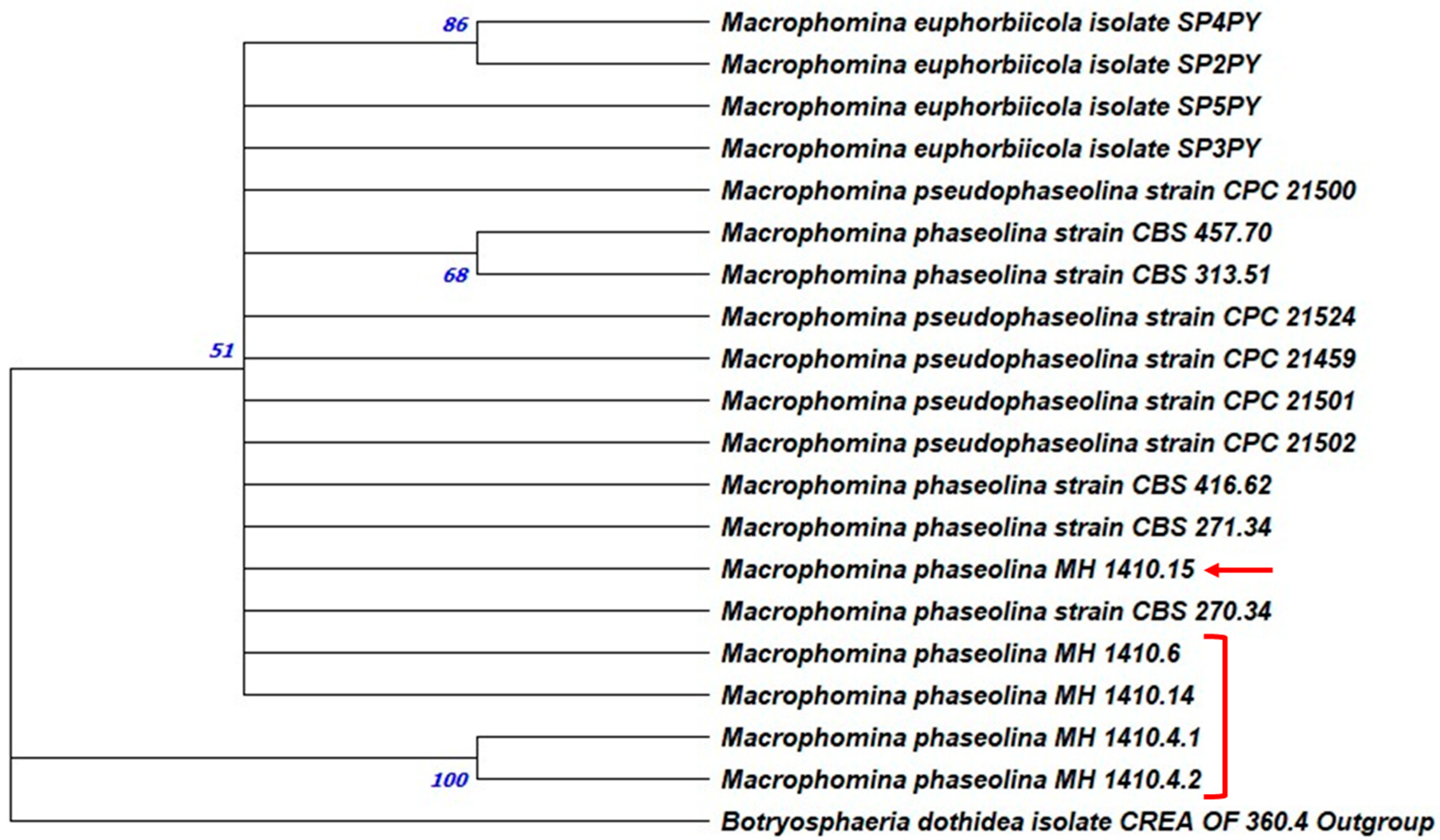
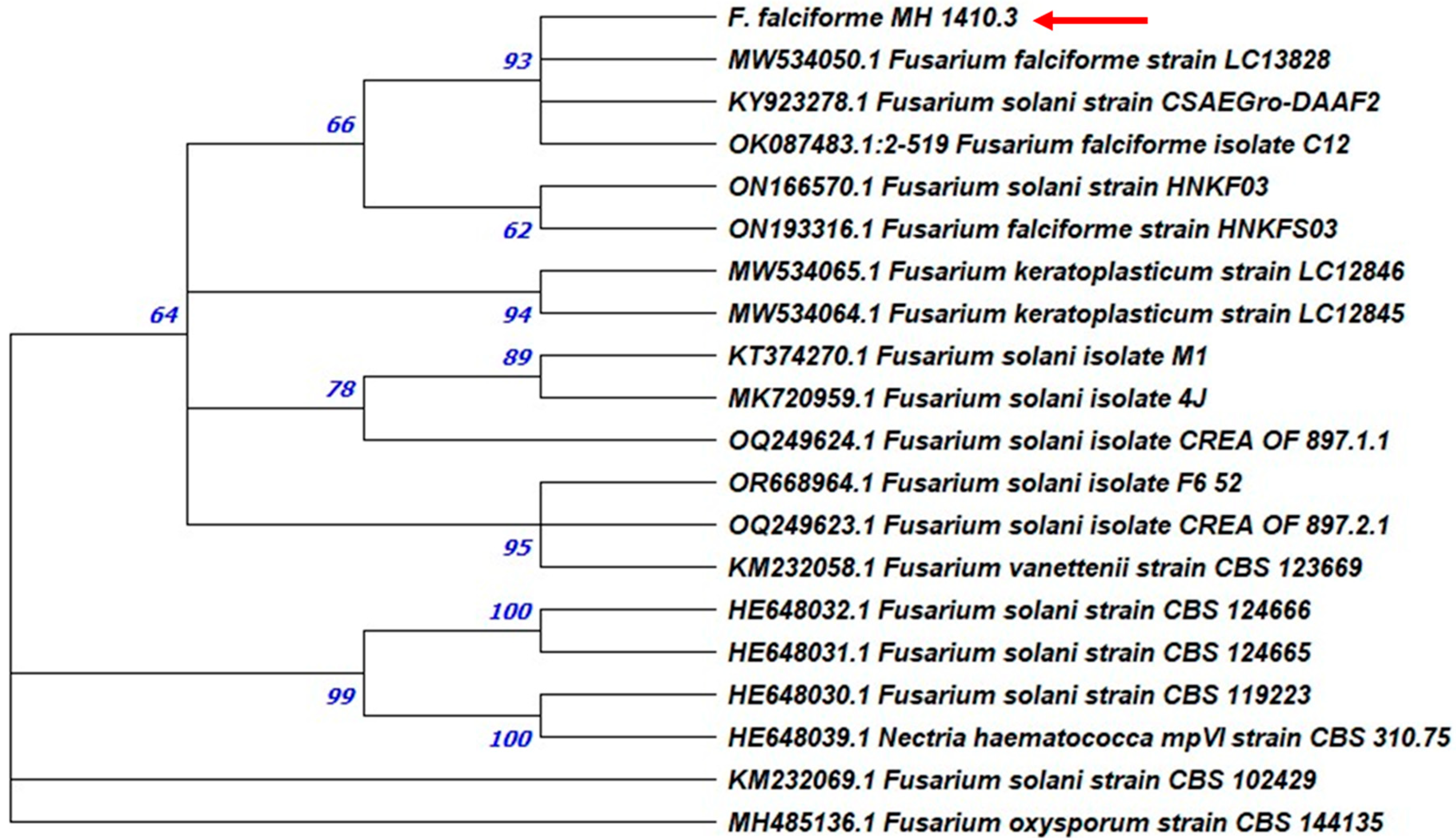
3.2. Molecular Identification and Phylogenetic Analysis
3.3. In Vitro Pathogen Interaction
3.4. Pathogenicity Test and In Vivo Pathogen Interaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mwithiga, G.; Maina, S.; Gitari, J.; Muturi, P. Rosemary (Rosmarinus officinalis L.) growth rate, oil yield and oil quality under differing soil amendments. Heliyon 2022, 8, e09277. [Google Scholar] [CrossRef]
- Datiles, M.J.; Acevedo-Rodríguez, P. Rosmarinus officinalis (rosemary). CABI Compend. 2022. [Google Scholar] [CrossRef]
- Gurbuz, B.; Bagdat, R.B.; Uyanik, M.; Rezaeieh, K.A.P. Rosemary (Rosmarinus officinalis L.) cultivation studies under Ankara ecological conditions. Ind. Crops Prod. 2016, 88, 12–16. [Google Scholar] [CrossRef]
- Macaluso, D.; Licciardo, F.; Carbone, K. Farming of Medicinal and Aromatic Plants in Italy: Structural Features and Economic Results. Agriculture 2024, 14, 151. [Google Scholar] [CrossRef]
- Park, M.J.; Han, J.G.; Shin, H.D. First Korean report of rosemary powdery mildew caused by Golovinomyces biocellatus. Plant Pathol. 2010, 59, 408. [Google Scholar] [CrossRef]
- Wichura, A.; Braun, U.; Weber, R.W.S.; Hildebrands, A. Golovinomyces orontii and other powdery mildews on Rosmarinus officinalis. Plant Pathol. 2012, 2, 162–166. [Google Scholar] [CrossRef]
- Suthaparan, A.; Solhaug, K.A.; Bjugstad, N.; Gislerød, H.R.; Gadoury, D.M.; Stensvand, A. Suppression of powdery Mildews by UV-B: Application frequency and timing, dose, reflectance, and automation. Plant Dis. 2016, 100, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, L.A.; Pérez-Sierra, A.; Armengol, J.; García-Jiménez, J. Characterization of Phytophthora nicotianae isolates causing collar and root rot of lavender and rosemary in Spain. J. Plant Pathol. 2007, 89, 261–264. [Google Scholar]
- Zarandi, D.M.; Rezaee, S.; Aminaee, M.M.; Sharzei, A. First report of Fusarium sambucinum on rosemary plant in Iran. New Dis. Rep. 2014, 29, 16. [Google Scholar] [CrossRef]
- Putnam, M.L. First report of stem rot of rosemary caused by Sclerotinia sclerotiorum in the United States. Plant Pathol. 2004, 53, 252. [Google Scholar] [CrossRef]
- Garibaldi, A.; Bertetti, D.; Pensa, P.; Matić, S.; Gullino, M.L. First Report of white mould caused by Sclerotinia sclerotiorum on Rosemary (Rosmarinus officinalis L.) in Italy. J. Plant Pathol. 2018, 99, 543. [Google Scholar]
- Garibaldi, A.; Bertetti, D.; Pensa, P.; Poli, A.; Gullino, M.L. First report of web blight on rosemary (Rosmarinus officinalis) caused by Rhizoctonia solani AG-1-IA in Italy. Plant Dis. 2013, 97, 844. [Google Scholar] [CrossRef] [PubMed]
- Azcona, J.S.; Woodhall, J.W.; Perkins, K.; Henderson, D.; Barnes, A.V.; Wharton, P.S.; Henry, C. First report of Rhizoctonia solani AG1-IB on Rosmarinus officinalis in the United Kingdom. New Dis. Rep. 2017, 35, 12. [Google Scholar] [CrossRef]
- González, V.; Garcés-Claver, A.; Ibarra, N. First report of root rot on Rosmarinus officinalis caused by Ceratorhiza fragariae (Binucleate rhizoctonia) in Spain. Plant Dis. 2017, 101, 1542. [Google Scholar] [CrossRef]
- Mekonnen, M.; Wariyo, A.; Hilu, G. Antifungal activities of some essential oils against Fusarium oxysporum of rosemary and sage plants. Adv. Crop Sci. Tech. 2019, 7, 419. [Google Scholar] [CrossRef]
- Pscheidt, J.W. Rosemary (Rosmarinus officinalis)–Botrytis Stem Canker. In Pacific Northwest Pest Management Handbooks, Plant Disease; 2021; Available online: https://pnwhandbooks.org/plantdisease/host-disease/rosemary-rosmarinus-officinalis-botrytis-stem-canker (accessed on 7 June 2025).
- Rachid Lahlali, R.; Taoussi, M.; Laasli, S.E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Yamamoto, D.T.; Uchida, J.Y. Rapid Nuclear Staining of Rhizoctonia solani and Related Fungi with Acridine Orange and with Safranin O. Mycologia 1982, 74, 145–149. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. ISBN 9780123721808. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Nosratabadi, M.; Kachuei, R.; Rezaie, S.; Harchegani, A.B. Beta-tubulin gene in the differentiation of Fusarium species by PCR-RFLP analysis. Infez. Med. 2018, 26, 52–60. [Google Scholar] [PubMed]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Sonawane, A.; Mahjan, M.; Renake, S. Antifungal activity of a fungal isolate against pomegranate wilt pathogen Fusarium. Int. J. Curr. Microbiol. Appl. Sci. 2015, 2, 48–57. [Google Scholar]
- McKinney, H.H. A new system of grading plant diseases. Agric. Res. 1923, 26, 95–98. [Google Scholar]
- Anonymous. PM 7/129 (2) DNA barcoding as an identification tool for a number of regulated pests. EPPO Bull. 2021, 51, 100–143. [Google Scholar] [CrossRef]
- Sharon, M.; Kuninaga, S.; Hyakumachi, M.; Naito, S.; Sneh, B. Classification of Rhizoctonia spp. using rDNA-ITS sequence analysis supports the genetic basis of the classical anastomosis grouping. Mycoscience 2008, 49, 93–114. [Google Scholar] [CrossRef]
- Dell’Olmo, E.; Tripodi, P.; Zaccardelli, M.; Sigillo, L. Occurrence of Macrophomina phaseolina on Chickpea in Italy: Pathogen Identification and Characterization. Pathogens 2022, 11, 842. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J. The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Mol. Plant Pathol. 2016, 17, 146–158. [Google Scholar] [CrossRef]
- Sabahi, F.; Banihashemi, Z.; Mirtalebi, M.; Rep, M.; Cacciola, S.O. Molecular Variability of the Fusarium solani Species Complex Associated with Fusarium Wilt of Melon in Iran. J. Fungi. 2023, 9, 486. [Google Scholar] [CrossRef]
- Rentería-Martínez, M.E.; Guerra-Camacho, M.A.; OchoaMeza, A.; Moreno-Salazar, S.F.; Varela-Romero, A.; Gutiérrez-Millán, L.E.; Meza-Moller, A.C. Multilocus phylogenetic analysis of fungal complex associated with root rot watermelon in Sonora, Mexico. Rev. Mex. De Fitopatol. 2018, 36, 233–255. [Google Scholar] [CrossRef]
- Nishad, I.; Srivastava, A.K.; Saroj, A.; Babu, B.K.; Samad, A. First Report of Root Rot of Nepeta cataria Caused by Macrophomina phaseolina in India. Plant Dis. 2018, 102, 2380. [Google Scholar] [CrossRef]
- Koşar, İ.; Güney, İ.G.; Derviş, S.; Kırlı, O.; Özer, G. First report of Macrophomina phaseolina causing charcoal rot on common sage (Salvia officinalis) in Turkey. J. Plant Pathol. 2021, 103, 1371. [Google Scholar] [CrossRef]
- Koşar, I.; Güney, I.G.; Üner, S.E.; Özer, G.; Derviş, S. First report of Macrophomina phaseolina causing charcoal rot on lemon balm (Melissa officinalis) in Turkey. J. Plant Pathol. 2022, 104, 895. [Google Scholar] [CrossRef]
- Palacıoğlu, G.; Ören, E.; Baran, B.; Orak, A.B. First report of Macrophomina phaseolina causing charcoal rot on lavender (Lavandula angustifolia) in Turkey. J. Plant Pathol. 2024, 107, 743. [Google Scholar] [CrossRef]
- Rena, Y.Z.; Tana, H.; Lib, Z.J.; Dua, J.; Lia, H. First report of lavender wilt caused by Fusarium solani in China. Plant Pathol. 2008, 57, 377. [Google Scholar] [CrossRef]
- Sunanda, C.R. Studies on Root Rot of Sage (Salvia officinalis L.) and Its Management. Master’s Thesis, University of Agricultural Science, Bangalore, India, 2000; p. 89. [Google Scholar]
- Omar, A.M.; Ahmed, A.I.S. Antagonistic and Inhibitory Effect of Some Plant Rhizo-Bacteria Against Different Fusarium Isolates on Salvia officinalis. Am.-Eurasian J. Agric. Environ. Sci. 2014, 12, 1437–1446. [Google Scholar]
- Mallesh, S.B.; Narendrappa, T.; Kumari. Management of Root Rot of Sage (Salvia officinalis) Caused by Fusarium solani and Rhizoctonia solani. Int. J. Plant Prot. 2009, 2, 261–264. [Google Scholar]
- Cara, M.; Merkuri, J.; Salliu, A.; Vojvodić, M.; Knežević, I.; Grkinić, M.; Bulajić, A. Binucleate rhizoctonia AG-A causing black root rot of strawberry in Albania. J. Phytopathol. 2024, 172, e13265. [Google Scholar] [CrossRef]
- Baiswar, P.; Ngachan, S.V. First Report of Root and Collar Rot of Strawberry (Fragaria × ananassa) Caused by Ceratobasidium sp. AG-B(o) (Binucleate rhizoctonia) in India. Plant Dis. 2018, 102, 1035. [Google Scholar] [CrossRef]
- Erper, I.; Ozer, G.; Yildirim, E.; Zholdoshbekova, S.; Turkkan, M. First report of root rot on strawberry caused by Binucleate rhizoctonia AG-G and AG-K in Kyrgyzstan. J. Plant Pathol. 2022, 104, 387–388. [Google Scholar] [CrossRef]
- Borrero, C.; Avilés-García, I.; López, N.; Avilés, M. First Report of Root Rot on Strawberry Caused by Binucleate rhizoctonia AG-K in Spain. Plant Dis. 2018, 103, 376. [Google Scholar] [CrossRef]
- Mosquera-Espinosa, A.T.; Bayman, P.; Prado, G.A.; Gómez-Carabalí, A.; Otero, J.T. The double life of Ceratobasidium: Orchid mycorrhizal fungi and their potential for biocontrol of Rhizoctonia solani sheath blight of rice. Mycologia 2013, 105, 141–150. [Google Scholar] [CrossRef]
- Manrique-Barros, S.; Flanagan, N.S.; Ramírez-Bejarano, E.; Mosquera-Espinosa, A.T. Evaluation of Tulasnella and Ceratobasidium as Biocontrol Agents of Fusarium Wilt on Vanilla planifolia. Agronomy 2023, 13, 2425. [Google Scholar] [CrossRef]
- Khan, F.U.; Nelson, B.D.; Helms, T.C. Greenhouse evaluation of Binucleate rhizoctonia for control of R. solani in soybean. Plant Dis. 2005, 89, 373–379. [Google Scholar] [CrossRef]
- Wen, K.; Seguin, P.; St.-Arnaud, M.; Jabaji-Hare, S. Real-Time Quantitative RT-PCR of Defense-Associated Gene Transcripts of Rhizoctonia solani-Infected Bean Seedlings in Response to Inoculation with a Non pathogenic Binucleate rhizoctonia Isolate. phytopathology 2005, 95, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Siwek, K.; Harris, A.R.; Scott, E.S. Mycoparasitism of Pythium ultimum by Antagonistic Binucleate rhizoctonia Isolates in Agar Media and on Capsicum Seeds. J. Phytopathol. 2008, 10, 417–423. [Google Scholar] [CrossRef]
- Nascimento, P.G.M.L.; Ambrósio, M.M.Q.; Freitas, F.C.L.; Cruz, B.L.S.; Dantas, A.M.M.; Sales Junior, R.; da Silva, W.L. Incidence of root rot of muskmelon in different soil management practices. Eur. J. Plant Pathol. 2018, 152, 433–446. [Google Scholar] [CrossRef]
- Dutt, A.; Andrivon, D.; Le May, C. Multi-infections, competitive interactions, and pathogen coexistence. Plant Pathol. 2022, 71, 5–22. [Google Scholar] [CrossRef]
- Ashrafi, S.J.; Rastegar, M.F.; Saremi, H. Rosemary wilting disease and its management by soil solarization technique in Iran. Afr. J. Biotechnol. 2010, 9, 7048–7057. [Google Scholar]
- Dura, S.; Hanson, S.F. Rosemary Decline, an Apparent Disease of Unknown Etiology Affecting the Southwestern US. WJASS 2024, 9, 1–4. [Google Scholar]


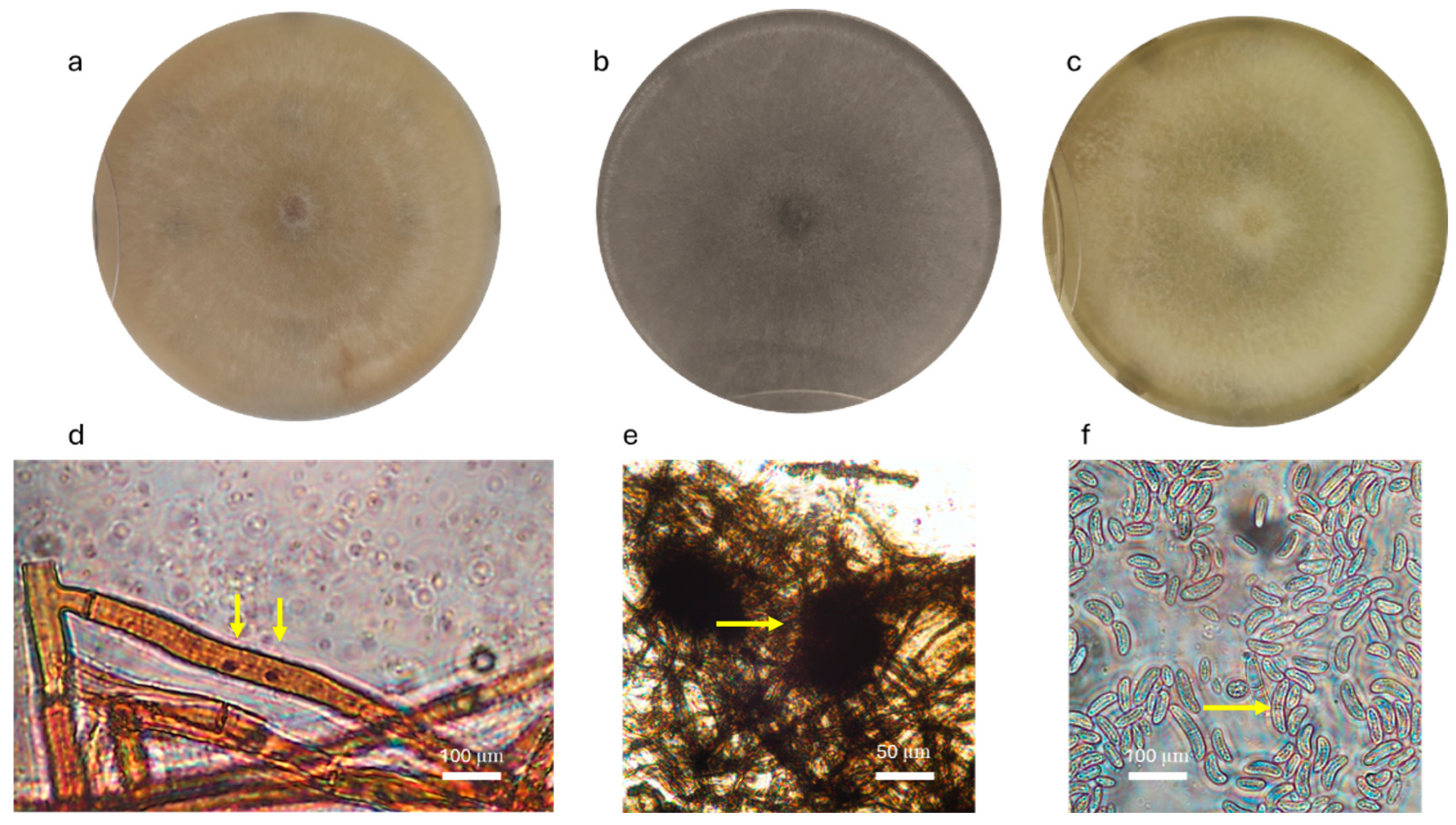

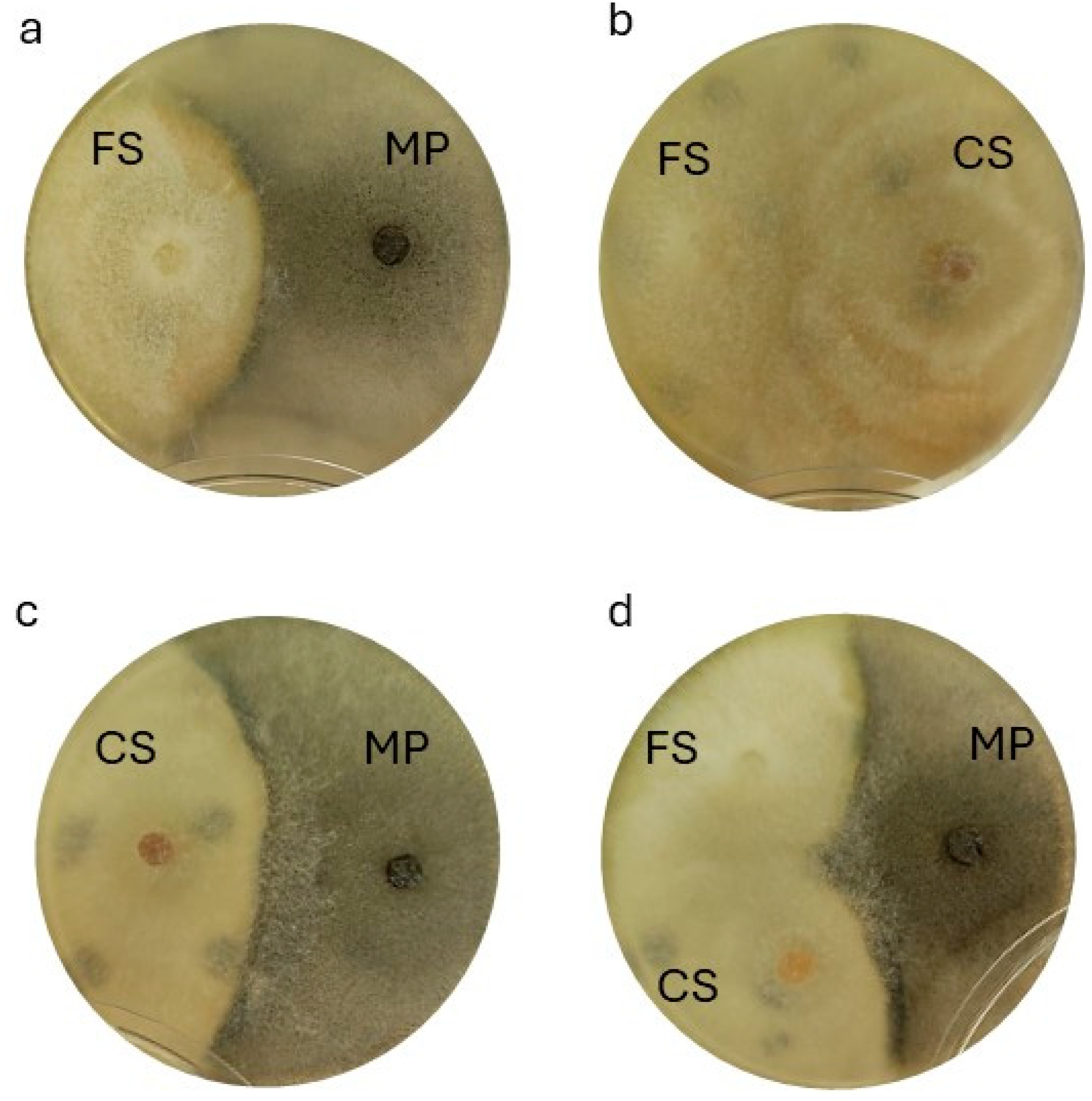
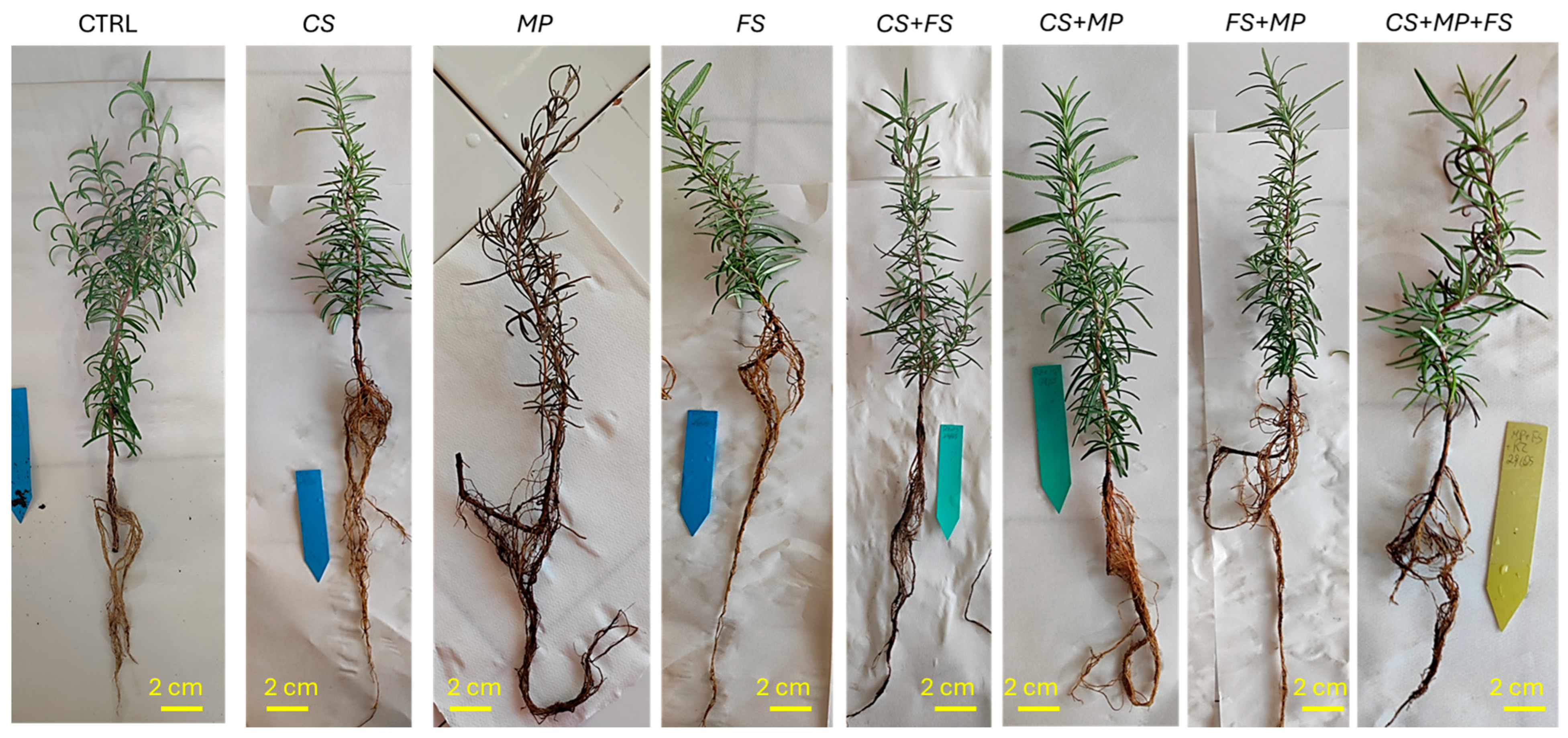
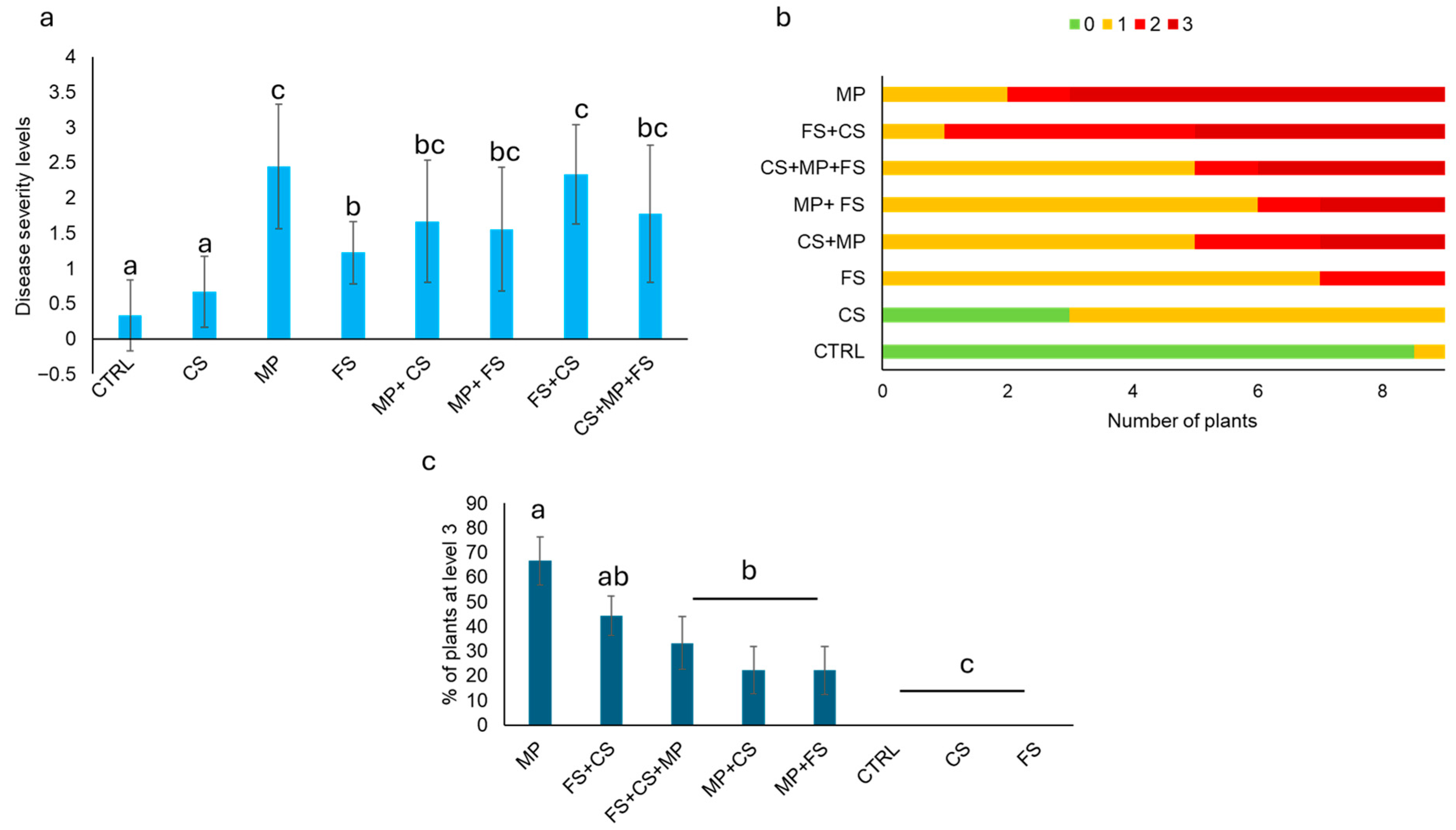
| Treatment | Inoculum |
|---|---|
| CS | 2 CS rice grains |
| FS | 2 FS rice grains |
| MP | 2 MP rice grains |
| FS + CS | 2 FS rice grains + 2 CS rice grains |
| MP + CS | 2 MP rice grains + 2 CS rice grains |
| MP + FS | 2 MP rice grains + 2 FS rice grains |
| CS + MP + FS | 2 MP rice grains + 2 FS rice grains + 2 CS rice grains |
| Isolate Code | Sequenced Gene Region | Sequence Accessions No. Uploaded in GenBank | Accession Sequences Retrieved from GenBank | Similarity (%) | Species Identified |
|---|---|---|---|---|---|
| MH CREA OF 1410.10 | ITS | PP508396 | KJ573103 | 93.75% | Ceratobasidium sp. AG-G |
| MH CREA OF 1410.4.1 | ITS | PP506663 | OP815340 | 93.40% | M. phaseolina |
| MH CREA OF 1410.4.1 | TEF1-α | PP517404 | MN318093 | 99.22% | M. phaseolina |
| MH CREA OF 1410.4.2 | ITS | PP506664 | OP815340 | 93.40% | M. phaseolina |
| MH CREA OF 1410.4.2 | TEF1-α | PP517405 | MN318093 | 99.22% | M. phaseolina |
| MH CREA OF 1410.6 | ITS | PP506665 | MK757624 | 97.82% | M. phaseolina |
| MH CREA OF 1410.6 | TEF1-α | PP517406 | MN318093 | 100.00% | M. phaseolina |
| MH CREA OF 1410.14 | ITS | PP506666 | MK757624 | 97.82% | M. phaseolina |
| MH CREA OF 1410.14 | TEF1-α | PP517407 | PP460507 | 99.61% | M. phaseolina |
| MH CREA OF 1410.15 | ITS | PP506666 | MK757624 | 97.82% | M. phaseolina |
| MH CREA OF 1410.15 | TEF1-α | PP517407 | KX400853 | 99.61% | M. phaseolina |
| MH CREA OF 1410.3 | ITS | PP506679 | ON181990 | 98.43% | F. solani |
| MH CREA OF 1410.3 | TEF1-α | PP517409 | OQ122079 | 99.63% | F. falciforme |
| MH CREA OF 1410.3 | TUB | PP517410 | MF662660 | 100% | F. solani |
| MH CREA OF 1410.3 | ACT | PP517411 | MK968888 | 99.27% | F. solani |
| PIRG of Isolates When Tested in Pairs (%) | |||
|---|---|---|---|
| Isolates on dual plate | MP | FS | CS |
| MP + FS | 37.53 ± 0.05 d | 57.57 ± 0.10 a | / |
| MP + CS | 27.53 ± 0.05 f | / | 50.10 ± 0.06 b |
| FS + CS | / | 37.50 ± 0.10 d | 0.00 ± 0.00 e |
| PIRG between two isolates when tested in three (%) | |||
| MP + FS | 37.47 ± 0.15 d | 49.93 ± 0.02 b | / |
| MP + CS | 37.60 ± 0.15 d | / | 67.47 ± 0.05 c |
| FS + CS | / | 50.17 ± 0.25 b | 50.07 ± 0.10 b |
| Control | Isolates Inoculated in Rosemary Plants | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CS | FS | MP | MP + CS | FS + CS | MP + FS | CS + MP + FS | |||
| Reisolation (%) | CS | 0 | 88.8 | / | / | 42.8 | 50.0 | / | 12.5 |
| FS | 0 | / | 100.0 | / | / | 100.0 | 100.0 | 62.5 | |
| MP | 0 | / | 0 | 100 | 57.1 | / | 50.0 | 87.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Olmo, E.; Serratore, G.; Mataffo, A.; Ragosta, G.; Napoletano, G.; Sigillo, L. Plant Desiccation and Root Rot in Rosemary: Insight into Macrophomina phaseolina, Ceratobasidium sp. and Fusarium falciforme Roles in Co-Infection. Agriculture 2025, 15, 2309. https://doi.org/10.3390/agriculture15212309
Dell’Olmo E, Serratore G, Mataffo A, Ragosta G, Napoletano G, Sigillo L. Plant Desiccation and Root Rot in Rosemary: Insight into Macrophomina phaseolina, Ceratobasidium sp. and Fusarium falciforme Roles in Co-Infection. Agriculture. 2025; 15(21):2309. https://doi.org/10.3390/agriculture15212309
Chicago/Turabian StyleDell’Olmo, Eliana, Giovanna Serratore, Alessandro Mataffo, Giovanni Ragosta, Giovanna Napoletano, and Loredana Sigillo. 2025. "Plant Desiccation and Root Rot in Rosemary: Insight into Macrophomina phaseolina, Ceratobasidium sp. and Fusarium falciforme Roles in Co-Infection" Agriculture 15, no. 21: 2309. https://doi.org/10.3390/agriculture15212309
APA StyleDell’Olmo, E., Serratore, G., Mataffo, A., Ragosta, G., Napoletano, G., & Sigillo, L. (2025). Plant Desiccation and Root Rot in Rosemary: Insight into Macrophomina phaseolina, Ceratobasidium sp. and Fusarium falciforme Roles in Co-Infection. Agriculture, 15(21), 2309. https://doi.org/10.3390/agriculture15212309









