Abstract
Elaeagnus multiflora Thunb., also known as “cherry silverberry”, “cherry elaeagnus”, and “goumi” has been used for a long time in traditional Chinese medicine as a phytosterol-rich plant. Today, the fruits of this species are also becoming more popular as a “superfood” in Europe, but the cultivation of these plants is not yet carried out on a large commercial scale. The aim of this study was to evaluate the yield and morphological quality of the fruit of nine E. multiflora biotypes and two cultivars, ‘Jahidka’ and ‘Sweet Scarlet’, to determine their suitability for cultivation in the climatic conditions of Poland. The lowest yields (an average of 0.49 kg per bush) were recorded in 2021. In this year, the fruits of the biotypes and cultivars were distinguished by the highest mean fruit weight, fruit-to-seed weight ratio, and total soluble solids content. Our research shows that due to the greatest weight of fruits, cultivar ‘Jahidka’ and the biotype B11 can be recommended for cultivation in north-eastern Poland. Biotype B11 was distinguished by the highest yield (an average of 4.02 kg per bush). The smallest share of stone in relation to the weight of the fruit was shown for the cultivars ‘Jahidka’, and biotype B4.
1. Introduction
Today climate changes are of a global nature and affect plant production in various parts of the world [1,2]. The range of species that grow naturally in warmer climate zones is changing, and in the near future, these species may replace the current traditional cultivated plants in horticulture. Understanding phenotypic variation is an indispensable first step for developing breeding strategies [3]. Consideration of the effect of climate conditions on yielding and quality of fruit is particularly important as it will permit proper regionalization of the cultivation and choice of cultivars adapted to the diversified environmental conditions of production area [4]. In the countries of north-eastern Europe, the range of species that can be introduced to commercial cultivation is limited. The area of north-eastern Poland is regarded as the least favourable in terms of agroclimatic conditions, with lower air temperatures, higher rainfall and a shorter growing season than in other parts of Poland [4]. The successful cultivation of introduced plant species, in the northern Polish region would represent a significant expansion to the range of their cultivation. Fruit tree planting is an important part of agricultural production [5]. The phenotypic variation of plants shows their adaptation to different environmental pressures [6,7]. The variation in fruit morphology has been used to determine the pattern of fruit geographic distribution and its response to climate change [7,8]. In some cases, studies on the phenotypes of fruit trees have shown a great reference value for accurate irrigation [5,9,10], disease control [5,11], and quality evaluation [5,12].
Fruit production is an important branch of agriculture in Poland [13,14,15], and species whose fruits are a rich source of bioactive substances are of particular importance [16,17,18,19,20,21,22,23,24]. Producers in these regions are interested in growing plants that are resistant to frost and spring ground frosts. Consumers prefer tasty, natural food produced in an unpolluted environment. The species with fruits containing many bioactive substances are particularly highly valued [25]. Elaeagnus multiflora Thunb., also known as cherry silverberry, cherry elaeagnus, and “goumi”, perfectly matches the current trends in horticulture to promote the cultivation of plants which can be sold in special markets for health-oriented consumers.
Elaeagnus multiflora belongs to the genus Elaeagnus L. and the family Elaeagnaceae Juss. The genus Elaeagnus includes approximately 70–80 plant species, of which only sea buckthorn is native to Poland, while the others have been introduced into cultivation in Polish climatic conditions [26,27,28,29].
Trees of the genus Elaeagnus can enrich the soil with nutrients as a result of symbiosis with bacteria of the genus Actinomycetes that produce root nodules and fix atmospheric nitrogen [16,30,31,32,33,34,35]. A symbiosis with nitrogen-fixing actinomycetes makes Elaeagnus multiflora a pioneer soil fertilising species [16,36]. The study of biodiversity in the context of food security and health promotion through diet indicates that E. multiflora is a species that meets these requirements [26].
Elaeagnus multiflora has long been grown in China, Korea, and Japan and has been cultured for centuries as a decorative plant, as well as a food and medicinal plant [16,36,37,38,39]. Today, cherry silverberry is grown in the eastern part of the United States and Europe, not only as an ornamental plant but also as a plant that can be used for home processing [26,36,39]. To date, there is no information in the international literature on the commercial cultivation and economic value of E. multiflora. However, its health value is known, as shown by Bieniek et al. [16]. Various parts of E. multiflora, such as the fruits, leaves, and young branches, can be used as phenolic antioxidant additives and dietary supplements [26,27,36,40,41,42,43,44,45,46,47,48,49], as well as natural remedies for diarrhea, cough, gastrointestinal disorders, itch, bone diseases, and cancer [26,27,49]. According to the available literature [19,25,36], Elaeagnus multiflora fruit contain pectin, carbohydrates, and acids. In addition, there are lipids in the fruit along with dissolved sterols, vitamins, and mineral compounds [27,28,29].
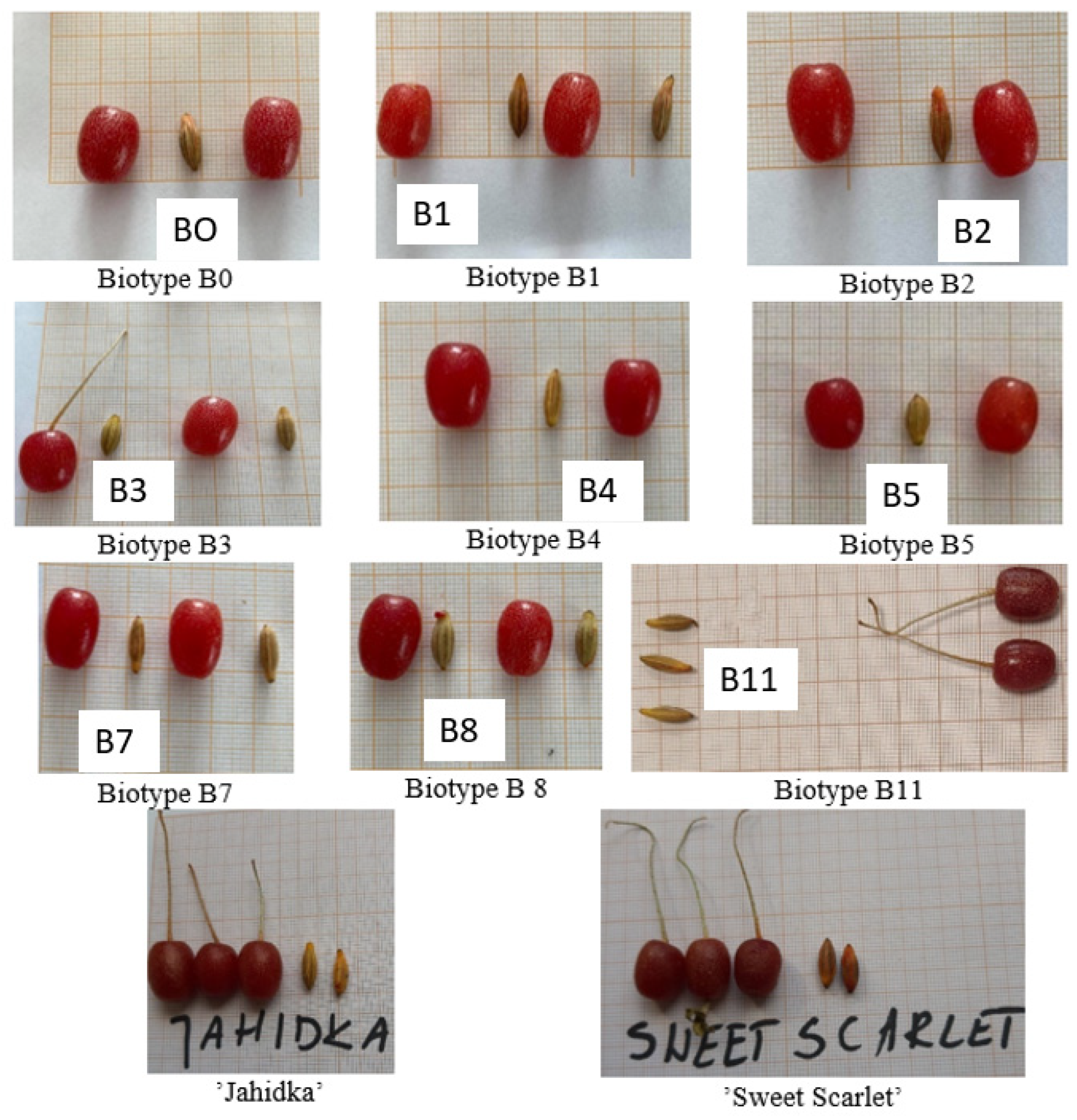
Elaeagnus multiflora produce ellipsoidal, drupe-like fruit, up to 1 cm long, and set on stems (Figure 1). They are red in colour, juicy, and sour [16,18]. An additional advantage of E. multiflora berries is that they can be eaten straight from the bush because in taste and colour they resemble red currant [16,18]. Colour is considered an important parameter of fruit quality [50,51,52,53,54,55,56], but the most important feature is the fruit’s chemical composition [18]. The Elaeagnus multiflora fruit was found to contain several carotenoids, which are versatile dietary compounds with additional roles as natural pigments and as precursors of vitamin retinoids [29,56,57]. One of the main dietary carotenoids for humans is lycopene [16,29,38,58]. As shown in the literature [16,29,48,57,58,59,60], fruits of E. multiflora contain significant amounts of this carotenoid, which is responsible for their red colour and is known for its anticarcinogenic effects.

Figure 1.
Fruits and seeds of the biotypes and cultivars of Elaeagnus multiflora studied.
The research of Lachowicz et al. [18], which was carried out on several cultivars and new biotypes of Elaeagnus multiflora selected at the University of Warmia and Mazury in Olsztyn, showed a large diversity in terms of chemical composition and antioxidant activity. The fruit of Elaeagnus multiflora is also characterized by high antidiabetic activity. The average inhibition of α-amylase and α-glucosidase is about 20% more effective than red currant, two times more than apples and five times more than cherries. For the first time, 63 new compounds (in fruits, leaves and seeds) have been identified that can be used to create functional foods and/or nutritional supplements [16]. Despite the unique composition and the well-studied properties of E. multiflora, the morphological variability of E. multiflora fruits remains to be studied [16,61]. Since the 1990s, the Department of Horticulture (currently Agroecosystems and Horticulture) of the UWM in Olsztyn has conducted the detailed research necessary to select the best forms of Elaeagnus multiflora for orchard cultivation [16,26,39]. Currently, experiments are being carried out with several dozen seedlings to select optimal biotypes that could be grown in Poland and other countries. Elaeagnus multiflora can be grown on a small scale and cultivated commercially with the use of combine harvesters [16,18]. However, more planting material is needed. Polish nurseries sell cultivars such as ‘Sweet Scarlet’ and ‘SSP’, a seedling found in Austria. The first ripening cultivar is ‘Sweet Scarlet’. This cultivar has darker and sweeter berries than other cultivars (Figure 1). Noteworthy is also cv. ‘Jahidka’, a shrub whose branches are lower than those of the above-mentioned cultivars, grows up to 1.5 m, and which produces red oval fruits weighing 1–1.5 g [16].
The aim of this research was to assess the yield and morphological parameters of the fruit of two cultivars, ‘Jahidka’ and ‘Sweet Scarlet’, and nine biotypes of Elaeagnus multiflora, and to determine their suitability for cultivation in the climatic conditions of Poland. The most valuable biotypes will be recommended for cultivation and used as parental forms and donors of valuable traits in the breeding programme carried out at the Department of Agroecosystems and Horticulture of the University of Warmia and Mazury in Olsztyn (Poland). An additional task was to investigate whether the morphological characteristics of fruits of the tested biotypes and E. multiflora cultivars vary from season to season due to climatic conditions.
2. Materials and Methods
2.1. Plant Material
The experiments were carried out in 2019–2022 in the experimental garden and laboratory of the University of Warmia and Mazury in Olsztyn (north-eastern Poland, latitude: 53°50′ N, 20°31′ E). The climate of Olsztyn is typical for Lakeland areas and is influenced by local elements of the environment, i.e., the land form and numerous lakes and forests.
The research involved nine biotypes (referred to as B0, B1, B2, B3, B4, B5, B7, B8, and B11) and two cultivars (‘Jahidka’ and ‘Sweet Scalret’) of Elaeagnus multiflora (Figure 1). The study used three biotypes (B1, B2 and B3) obtained from the Institute of Fruit Growing in Samokhvalovitchy in Belarus from the E-2 breeding form and six biotypes (B0, B4, B5, B7, B8, and B11) obtained from the seeds of the biotype growing in gardens in Olsztyn (latitude: 53°50 N, 20°31 E). The tested biotypes were selected from a population of over 1000 seedlings. The biotypes assessed were plants that have been obtained through 12 years of selection from a population of 40 plants for desirable features for potential cultivation (fruit size, shape and taste, ripening date and number of fruits per bush). From these plants, shoots for rooting were selected. The biotypes were compared with the already established and patented cultivars ‘Jahidka’ and ‘Sweet Scarlet’, which were bought from a nursey in central Poland. Three-year-old plants of the biotypes and cultivars were planted in 2007 in Albic Luvisolix (Arenic), flat deep soil, produced from clays of pH in KCl 6.8 [62], at a spacing of 4 × 2 m. The pomological characteristics were assessed with 4 replications consisting of 100 fruits per biotype/cultivar. No additional irrigation or protection against pests or diseases was applied.
2.2. Quantity and Quality of Yield
The yield was collected when fruits achieved the harvest maturity stage, separately for each plant. The yield was weighed to withing an accuracy of 0.1 kg. After collecting all the data, the yield was converted to kg per bush. The harvest dates were 2–7 July 2019, 7–10 July 2020, 5–15 July 2021 and 12–13 July 2022.
The quality of fruit was assessed based on a representative sample of 100 fruits in 4 replications of each biotype and cultivar. The research was conducted on fruits collected in the consumption ripeness stage when the fruits were fully red.
The fruit was weighed by means of an electronic scale (with an accuracy of 0.01 g), determining the mass of a single fruit (g). The seeds of the fruit were hand-mined from fresh fruit that had been previously weighed and measured. The fresh seeds were then weighed on the same scale as the fruits. The biometric features of the fruits and seeds were measured with calipers. The aforementioned parameters provided the basis for calculation of the mass of seed in the total mass of fruit. The shape parameters of the fruits and seeds were also calculated (length/diameter).
Laboratory analyses were performed in 2019–2022 to determine the content of extract (total soluble solids—TSS) by refractometry. The TSS, expressed as Brix scale (%), was quantified with the aid of a digital refractometer (Digital Abbe Refractometer DR-A1, ATAGO, Tokyo, Japan). The Brix scale (%) is a standard sugar content scale recommended by ICUMSA, and its measurement value (percentage) is expressed as “% mass (sucrose)” in international units (SI units). The results are presented as an average of ten repetitions per biotype or cultivar and expressed as percentage values.
2.3. Climatic Conditions
The weather conditions (mean daily temperatures and total precipitation) that prevailed in 2018–2022 (Table 1) and the spring ground frost in Olsztyn in 2019–2022 (Table 2) were based on daily meteorological data obtained and processed from the station of the Institute of Meteorology and Water Management (IMGW-PIB), Poland. The climatic conditions of the area of north-eastern Poland were described by meteorological data from the thirty-year research period for the years 1981–2010 from the IMGW—PIB stations located in the study area.

Table 1.
The mean monthly values of weather parameters in Olsztyn (north-eastern Poland) in 2018–2022 and the multiannual mean (data obtained and processed from the IMGW-PIB station of the Institute of Meteorology and Water Management).

Table 2.
Spring ground frosts in Olsztyn (north-eastern Poland) in 2019–2022 (data obtained and processed from the station of the Institute of Meteorology and Water Management IMGW-PIB).
2.4. Statistical Analysis of the Data
All data included in this study related to the yield measurements, TSS contend, morphological characteristics of fruits and seeds of the biotypes and cultivars are presented as the mean value ± standard deviation. Statistical analyses using one-way ANOVA were conducted using STATISTICA software version 13.3 (TIBO Stat Soft Inc. 1984–2017, Kraków, Poland). Significant differences (p < 0.05) between mean values were evaluated by one-way ANOVA and Tukey’s multiple range test. Pearson’s correlations were determined using Microsoft Excel 2019.
3. Results and Discussion
3.1. Climatic Conditions
A selection of the climatic conditions (average temperatures and precipitation) at the experimental location in the study years and in the multi-year period of 1981–2010 are presented in Table 1 and Figure 2 In Table 2, we present data for spring ground frosts in 2019–2022.

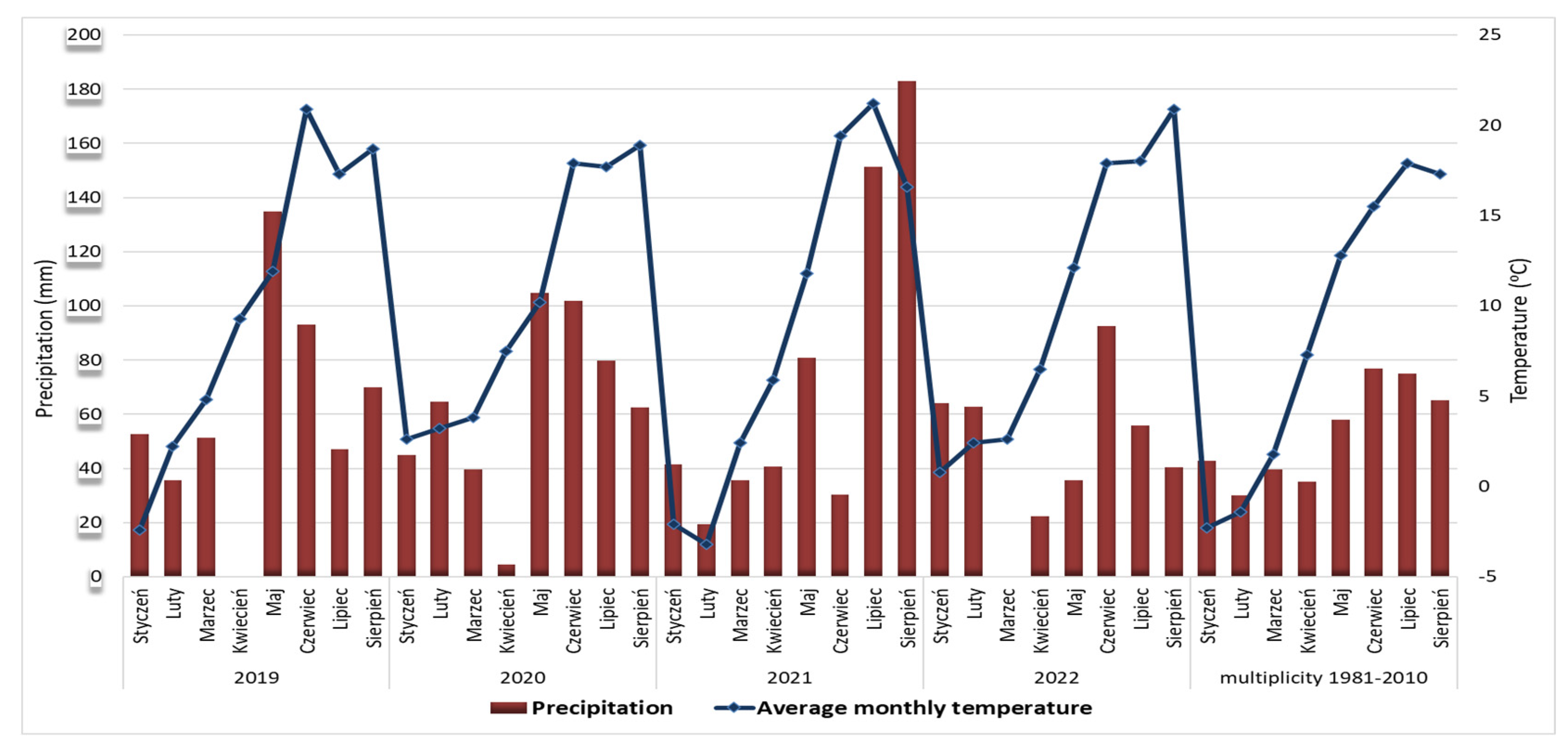
Figure 2.
Precipitation and monthly average temperatures in Olsztyn (north-eastern Poland) from January to August in 2019–2022 and the multiannual period 1981–2010 (data obtained and processed from the station of the Institute of Meteorology and Water Management IMGW-PIB).
In Poland, Elaeagnus multiflora blooms from April to the end of May and ripens at the end of June or at the beginning of July [16]; therefore, the distribution of meteorological conditions in this period is significant.
The analyses of the climatic conditions in the particular years the research was conducted on the yields and morphological characteristics of selected biotypes and cultivars found that the highest mean temperature of the vegetative season from flowering (April) to fruit harvest (July) (Table 1) occurred in 2019 and reached 14.8 °C. The lowest mean temperature during the vegetative season (13.3 °C) was recorded in 2020. In the year 2021, especially low temperatures were reported in winter months: −3.2 °C in February and −1.6 °C in December. In turn, the temperatures of the summer months June and July were the highest in 2021, and the summer temperature during the research period (20.3 °C, mean of two months) was 3.6 °C higher compared with the multiannual period.
During the four-year experiment, the mean temperatures recorded in April ranged from 5.9 °C in 2021 to 9.3 °C in 2019. The closest value to the multiannual mean, which was 7.3 °C, was recorded in 2020 (7.5 °C). In all experimental years, the mean temperatures recorded in May were lower than the multiannual mean of 1981–2010, which was 12.8 °C. The highest mean temperature in May (12.1 °C) was recorded in 2022, and the lowest in 2020 (10.2 °C). In June, on the other hand, in all the analysed monthly temperatures were higher than the multiannual mean (15.5 °C) and ranged from 17.9 °C in 2020 and 2022 to 20.9 °C in 2019.
The Table 2 shows spring ground frost occurring in April and May. The beginning of Elaeagnus multiflora flowering was noted in late April or early May and lasts from 15 to 20 days. In each of these months in 2019, there were 18 (April) and 4 (May) days with a temperature below 0 °C. Similarly, in subsequent years, i.e., 2020, 2021 and 2022, the number of days with ground frosts in April and May were 20 and 6, 16 and 4, and 14 and 7, respectively.
Minimum temperatures below zero on three consecutive days in May were recorded in 2019 as follows: −5.1, −2.8 and −3.4 °C. According to Faust [63], when occurring during the bloom or post-bloom stages, temperatures from −2 to −5 °C injure the flowers or young fruits. In 2019, the latest frost also occurred, which was on 30th of May (−1 °C). The ground frosts (−2.2 °C and −4.5 °C) on 14th and 15th of May in 2020 threatened to freeze flowers. In 2021, the fewest days with frost were recorded. In 2022, slight frosts (from −0.5 to −1.3 °C) were recorded from 3 May to 5 May, and on 10 May, a frost of −2.5 °C posed a high risk. In this year, the frosts occurred in the second half of May, and on 19th of May, the minimum temperature at the ground was recorded at −1.1 °C.
Large differences in precipitation during the vegetative season (from April to July) were observed in different years (Table 1). In April 2019, no precipitation was recorded, whereas in May of the same year, the monthly precipitation was the highest and amounted to 134.8 mm. Low precipitation (4.5 mm) was also recorded in April 2020, whereas in May, the total precipitation level significantly exceeded the multiannual mean and reached 104.8 mm. In 2019 and 2020, the precipitation, both during the growing season from April to July and throughout the year, was higher than the mean precipitation for these periods between 1981 and 2010.
In March 2022, no precipitation was recorded, and the total precipitation in the season from April to July was the lowest in the experimental years and was also lower than data for the period 1981–2010 (Table 1, Figure 2).
The course of the climatic conditions from April to July had a strong influence on the yield and some morphological features of the biotypes and cultivars of the Elaeagnus multiflora fruits tested (Table 3 and Table 4).

Table 3.
Correlation coefficients between yield, morphological characteristic of fruits, and temperatures in the months of vegetation from April to July in 2019–2022.

Table 4.
Correlation coefficients between yield and morphological characteristics of fruits and seeds, and total precipitation in the months of vegetation from April to July in 2019–2022.
Precipitation in June had a negative influence (p < 0.01) on the weight, length, and width of fruits for the tested biotypes and cultivars of Elaeagnus multiflora (Table 4). On the other hand, temperatures in May had a significant (p < 0.05) positive effect on the length of the fruit and the length-to-width ratio of the fruit. In the discussed vegetative season, both temperature and precipitation had a significant positive influence on the width of fruit (Table 3). The strongest positive impact of these weather factors on the width of fruit was demonstrated for July, that is, just before fruit harvest. A statistically significant (p < 0.01) positive influence of precipitation during June and July on the width of the seeds was found (Table 3 and Table 4). Precipitation in April had a significant positive influence on the ratio of seed length to width (Table 4). The dependencies testify to the relatively high water needs of the studied species, and high rainfall affects the quality parameters of the fruit.
Table 3 and Table 4 demonstrate significant negative correlations between temperatures and precipitation of this season and yield. There was a highly significant negative influence on yield from temperatures in June (p < 0.001) and July (p < 0.01) (Table 3) and precipitation in May (p < 0.001), and in June and July (p < 0.01) (Table 4). Environmental conditions influence flower bud development. The summer temperature may also determine the rate of flower bud initiation. In apples in shaded areas where less than 30% of the sunlight penetrates, practically no flower bud development occurs [63]. Heide et al. [64] showed that at 12 °C, flowering in apple seems to be limited by low temperature depression of growth and leaf production, whereas at 27 °C, flowering is blocked by inhibition of the floral initiation itself. Intermediate temperatures of 18–21 °C, on the other hand, seem to satisfy the requirements for both processes. For good yield and development, Elaeagnus multiflora requires large amounts of sunlight [16,36].
Elaeagnus multiflora has a long vegetative season, and leaf loss begins immediately after the first autumn frosts. Table 5 shows the effects of temperatures and precipitation in the months after fruit harvest from August to March on the yield in the following year. The correlation analysis shows that the temperatures from August to October had a significant negative impact on yield in the next year. However, temperatures in November and March, did not have a significant effect on yield. The correlation analysis also shows that the temperatures in the winter months from December to February had a significantly negative impact on the yielding of the tested biotypes and cultivars of E. multiflora grown in the conditions of north-eastern Poland. Analysis of the interaction of precipitation with E. multiflora yields in the following year shows that precipitation in November had a particularly significant positive impact, whereas precipitation in October had a significantly negative impact on yield (Table 5). Analysing the interaction of temperature and precipitation throughout the year (January to December) prior to fruiting on yield, a significantly negative effect of temperature and statistically significant (p < 0.05) effect of precipitation (r = 0.36) were found (Table 5).

Table 5.
Correlation coefficients between temperatures (°C) and precipitation (mm) in months after fruit harvest in 2018–2022 and the production of tested biotypes and cultivars of Elaeagnus multiflora in the following years.
A study conducted in Ukraine [65] showed that in November, the fall of leaves can be caused by a significant night frost. All leaves shed infrequently. Usually, one or more leaves can remain on the tops of annual shoots for a very long time. The late fall can cause shoots to freeze during severe winters. E. multiflora is a plant with low resistance to frost and high regenerative properties [36]. According to Grygorieva et al. [65], shrub shoots, even completely frozen, regenerate well and grow from the root neck in significant amounts. After cold winters, there are many young shoots with vegetative buds on the bush, which thicken the bushes, thereby protecting the buds that form on older shoots from the external environment. In E. multiflora, flower buds occasionally develop one or two at a time on the axils of the lower leaves of replacement shoots (Figure 3).

Figure 3.
Elaeagnus multiflora shoot during the flowering period.
On one shoot of E. multiflora, flower buds are formed gradually, synchronously with its growth and development. On the same shoot, as well as on the same tree, the fruits ripen at different times, which corresponds to the gradual development of generative buds.
According to the data presented in Table 3, Table 4 and Table 5, the climatic conditions of Poland during the growing season from April to July, as well as in the year before the fruiting from August to October, had a significant impact on the yield of the biotypes and cultivars of Elaeagnus multiflora. Environmental factors affect flower development. The period of flower bud formation in E. multiflora. begins after fruit harvest in Poland’s climatic conditions, usually in July and during flowering in April and May, and any severe heat or moisture stress hinders normal flower growth. Inadequate winter chilling limits cell division and spring development, or in severe cases, the flower buds simply drop from the tree [66]. Rodrigo and Herro [67] showed that flower drop can vary not only between cultivars, but also for the same cultivar, depending on the year or site. After dormancy and during the pre-blossom period, flower buds are exposed to variable climatic conditions from the end of winter to the beginning of spring. In this period, frost temperatures can easily occur in most temperate zones, and the stage of development is the most important factor in the resistance to frost injury [68]. Therefore, most of the work conducted on frost damage in the reproductive organs of fruit trees concentrates on either the endodormancy [69] or post flowering periods [70].
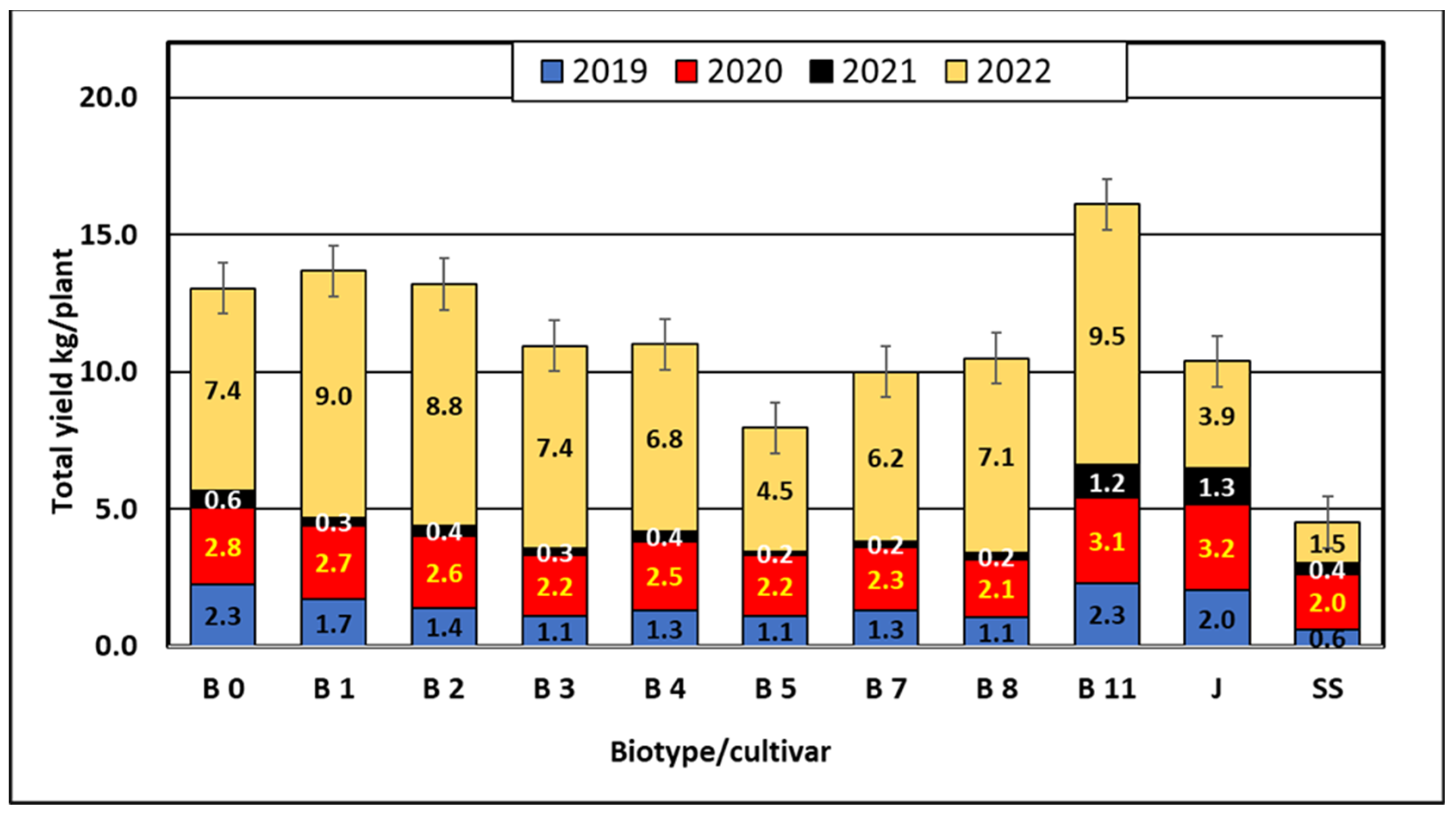
The data in Table 1 show that in the period preceding fruiting in 2019 and 2021, that is, from August to September 2018 and 2020, the average monthly temperatures were higher than the multiannual mean of 1981–2010. In 2019 and 2021, the yields were lower than in 2020 and 2022, which may indicate that the differentiation of flower buds E. multiflora requires lower temperatures and more precipitation (Table 1 and Table 5). According to Westwood [66], biennial bearing of most tree fruits results from poor flower initiation during a heavy crop year, which can also be seen in our research (Figure 4). Research conducted by scientists in Ukraine [65] shows that E. multiflora has abundant and regular fruiting. In our experiment we observed relatively low yields of Elaeagnus multiflora cultivars and biotypes (Table 6, Figure 4). The lowest yields were obtained in 2021 and the highest (6.5 kg per shrub) in 2022. The mean yields of biotypes and cultivars from the period 2019–2022 ranged from 1.13 kg for the cultivar ‘Sweet Scarlet’ to 4.02 kg for biotype B11. Biotype B11 was significantly different in terms of yield from the other biotypes and cultivars tested (Table 6). Statistical analysis of the yield results showed that six biotypes (B0, B3, B4, B8, B7, B8) and two cultivars (‘Jahidka’ and ‘Sweet Scarlet’) form the largest homogeneous group, with the lowest mean values of 1.13 to 3.26 kg (Table 6). Slightly more than 3 kg of fruit were harvested from the shrubs of the B1, B2 and B0 biotypes, but the least-yielding biotype was B5 with 1.99 kg per the shrub, and the ‘Jahidka’ cultivar yielded 2.59 kg per the shrub (Figure 4). E. multiflora begins to bear fruit in the fourth to fifth year. The most productive fruiting occurs at the age of 8 and lasts at least 12–15 years [61]. In our investigation, the biotypes and cultivars were at production age. The shrubs started to fructify in the third year after planting, so it was their ninth year of fructification in 2019. In this experiment we obtained relatively low yields.
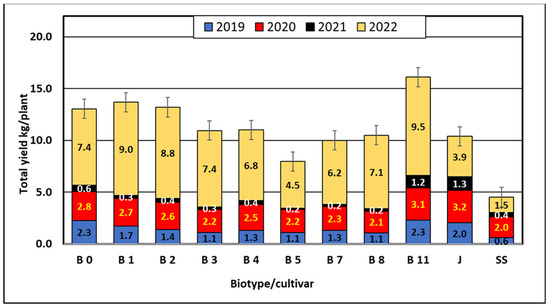
Figure 4.
Cumulative yields (kg·plant−1) of Elaeagnus multiflora biotypes and cultivars in 2019–2022.

Table 6.
Yield (kg·plant−1) of Elaeagnus multiflora biotypes and cultivars.
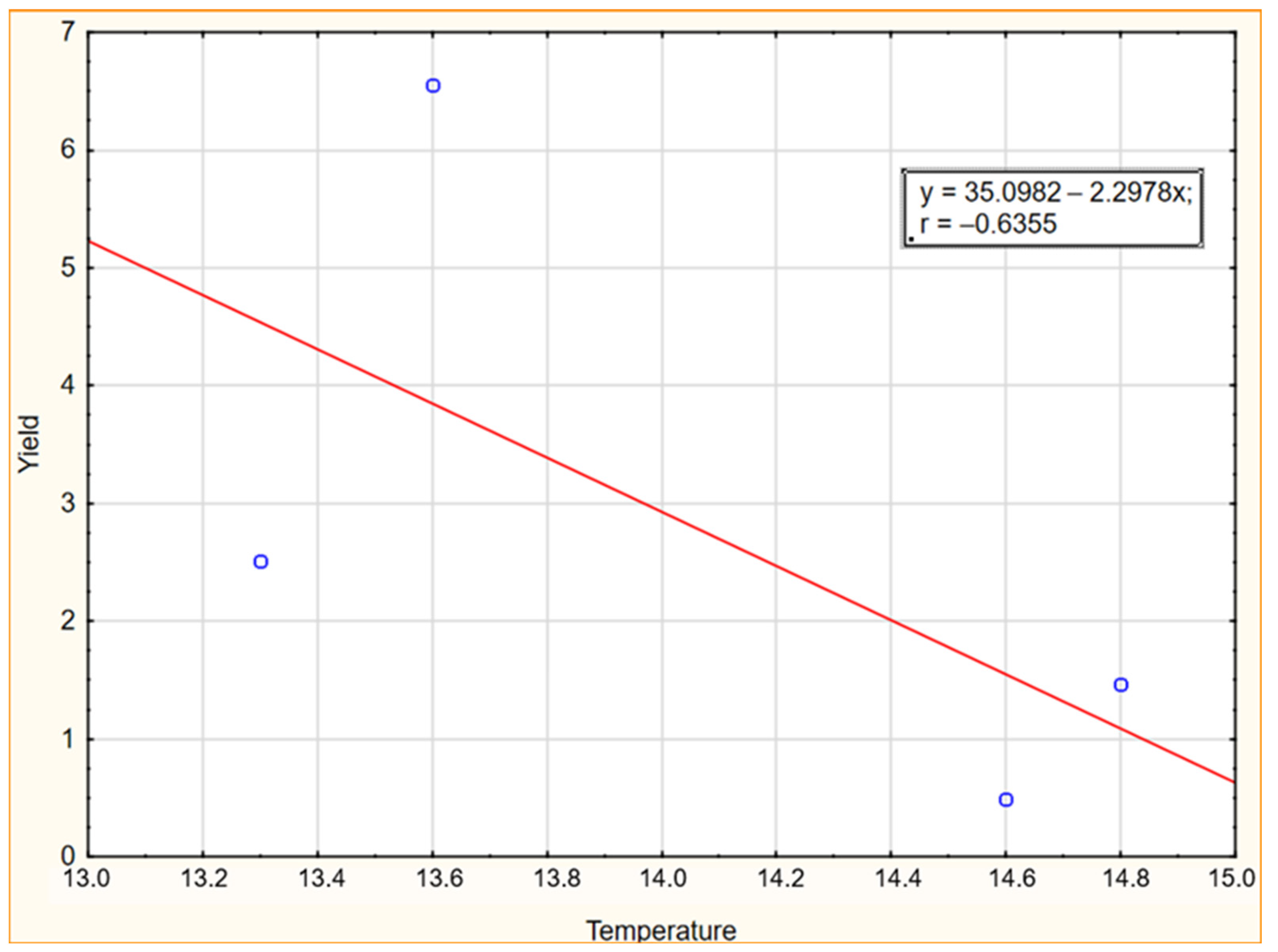
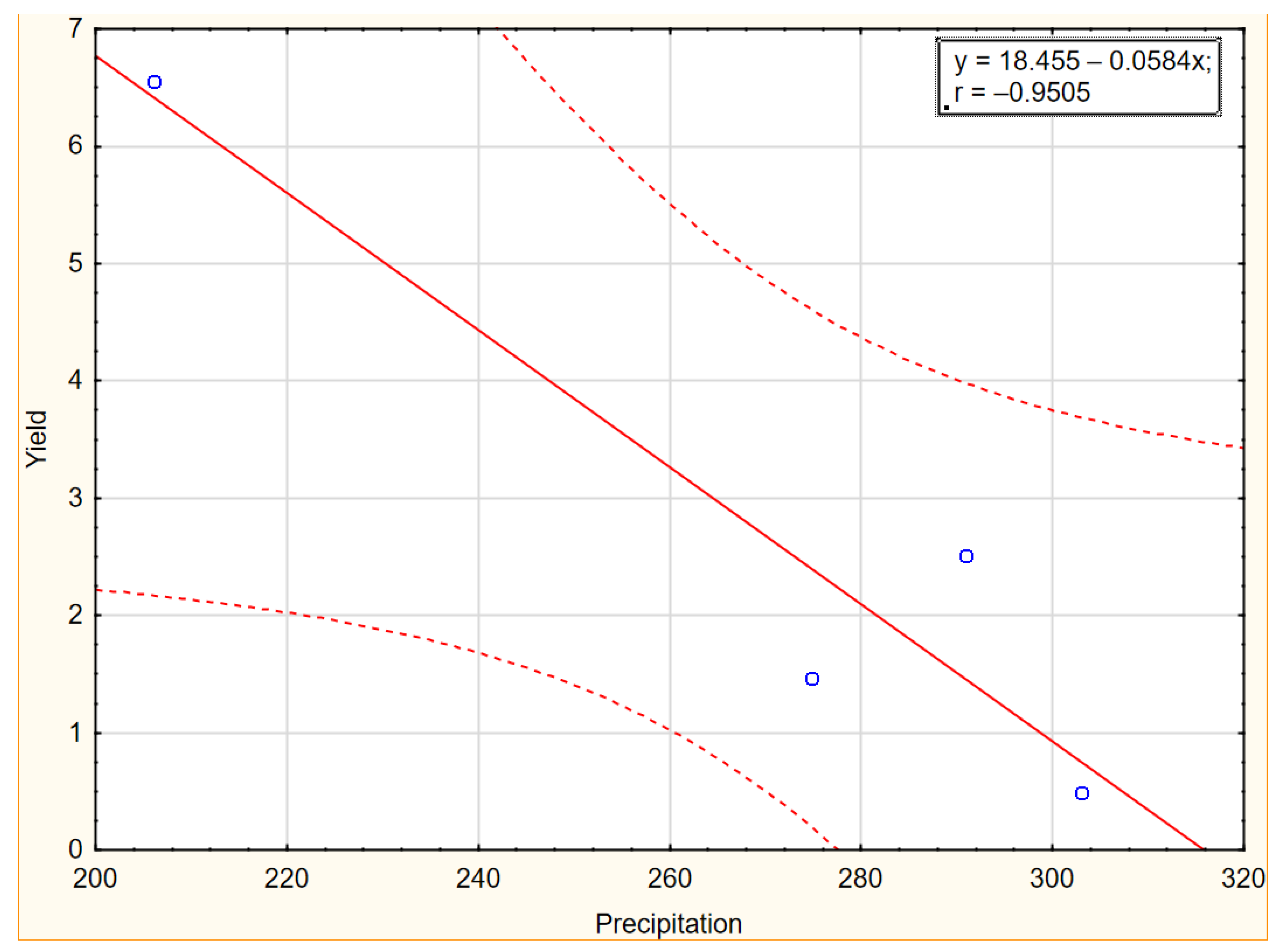
As shown in Figure 5 and Figure 6, the influence of temperatures and precipitation in the experimental years were negatively correlated with yield. The graphs of the linear relationship show a stronger negative impact of precipitation than temperatures in the years of the research, indicating that higher rainfall reduces the yield of Elaeagnus multiflora. According to Chawla et al. [71], rain during flowering washes out the pollen from the stigmas of flowers, resulting in poor or no fruit setting. Heavy rainfall in areas of poor drainage reduces oxygen availability in the soil, leading to reduced growth of beneficial microorganisms. Additionally, due to water-logged conditions, many insect-pests and diseases occur which affect crop yield.

Figure 5.
Scatter plot with correlation coefficient for yield and temperature in 2019–2022.
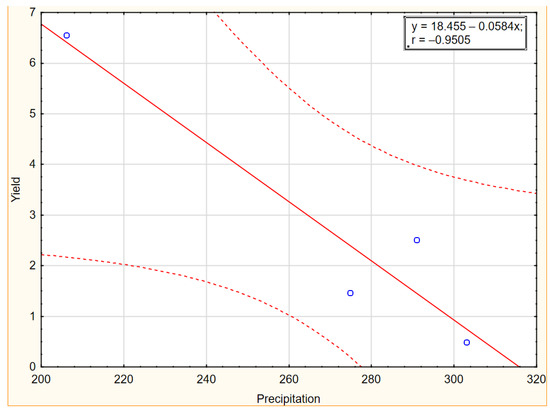
Figure 6.
Scatter plot with correlation coefficient for yield and precipitation in 2019–2022.
The harvest yields of fruit plants are dependent on both genetic characteristics and the climatic conditions in a given vegetative season [4,22,36,72,73,74,75,76,77]. The climate of north-eastern Poland, which is determined mainly by air masses flowing in from the eastern border of the country, has been observed to change in recent years. One of the reasons for this change is global warming, which has led to an increase in the mean annual temperature and, consequently, to decreases in the total annual precipitation and soil humidity [57]. According to the available literature [78,79], climate change and the potential for more extreme temperature events will affect the phenology and productivity of plants. Consideration of the effects of climate conditions on yielding and quality of fruit of Elaeagnus multiflora are particularly important to enable proper regionalization of cultivation, and choice of cultivars adapted to the diversified environmental conditions of the north-eastern European production area.
3.2. Fruit Quality
3.2.1. Fruit Weight
The mean fruit weight for biotypes and cultivars in 2019–2022 (Table 7) in our study ranged from 0.84 g (B5) to 1.57 g (‘Jahidka’). The biotype B11 had the highest weight (1.16 g) among the biotypes. In 2021 (when the lowest yields were obtained), the average fruit weight (1.21 g) of the analysed biotypes and cultivars was significantly higher than in the other years of the study. In turn, in 2022, the lowest average fruit weight (1.03 g) was recorded. An earlier investigation by Bieniek et al. [36] measured a range of fruit weights for biotypes from 1.03 to 1.29 g. Bieniek et al. [36] found that cultivation conditions, as well as climatic factors during the plant vegetation period, regardless of genetic factors, had a significant effect on yield and qualitative characteristics of fruits. Many authors [74,75] have shown that the average weight of the fruit depends on the age of the orchard.

Table 7.
Mean fruit weight (g) of Elaeagnus multiflora biotypes and cultivars.
3.2.2. Shape Parameters
The shape of an object can be characterized by certain shape parameters, i.e., the length to width ratio. Table 8 presents data for the shape of fruits and seeds. The fruit length to width ratio was found to be in the range of 1.23 (B 5) to 1.50 (B 11) (Table 8).

Table 8.
Fruit length to width ratio of Elaeagnus multiflora biotypes and cultivars.
The highest variation in fruit length to width ratio was observed for the ‘Jahidka’ cultivar in 2019 and biotype B5 in 2020. In our experiment, it was shown that the most elongated fruits were characteristic of the cultivar ‘Jahidka’ and biotype B11 (Table 8). The biotypes analysed in our research demonstrate significant variability in shape of the fruit and seed (Table 8 and Table 9). These parameters can be used for the identification of the biotypes.

Table 9.
Seed length to width ratio of Elaeagnus multiflora biotypes and cultivars.
The size of the fruit is a genetic trait but also depends on the yield of trees. To date, no research on thinning has been conducted, but probably, as in the case of other stone trees, the response would be similar [22,76].
The seed length-width ratio ranged from 2.61 (B 7) to 3.54 (B 11), so the biotypes demonstrated significant variability in the seed shape, as seen in Table 9. These parameters can be used for the identification of biotypes. The shape index of the fruit in Grygorieva et al. [37] ranged from 1.25 to 1.56, and the shape index of the seed ranged from 2.90 to 4.04.
3.2.3. Fruit-to-Seed Weight Ratio
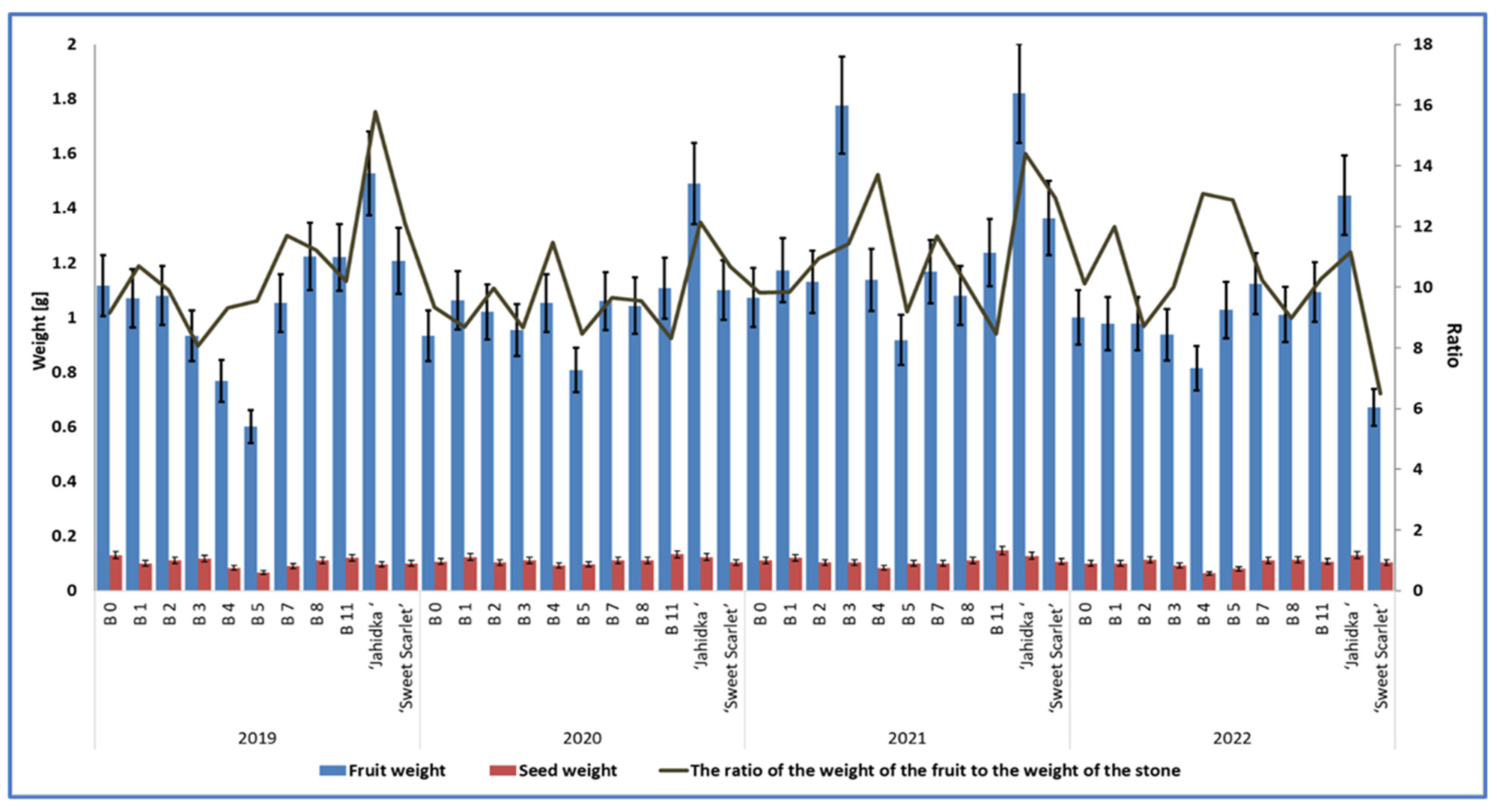
What is of high importance in the evaluation of fruit quality, particularly in the context of their usability for processing, is the relation of the fruit weight to the seed weight, according to Bieniek et al. [36]. In our study, the highest ratio of fruit weight to seed weight was found in cv. ‘Jahidka’, with the average ratio from 4 years for this cultivar being 13.37 (Table 10, Figure 7). Among the biotypes analysed in our study, biotype B4 was distinguished by the highest proportion of pulp relative to seed, with a fruit-to-seed weight ratio of 11.89 (Table 10, Figure 7). Cultivar ‘Sweet Scarlet’ and biotype B1 had a similar ratios, which were 10.54 and 10.30, respectively. The experiment by Bieniek et al. [36] did not show any significant differences between four biotypes with respect to this parameter, which ranged from 9.69 to 11.79. Szot and Łysiak [22] reported that the cultivars of Cornus mas with large stones are used to produce oil which is rich in unsaturated fatty acids.

Table 10.
Fruit-to-seed weight ratios of biotypes and cultivars.
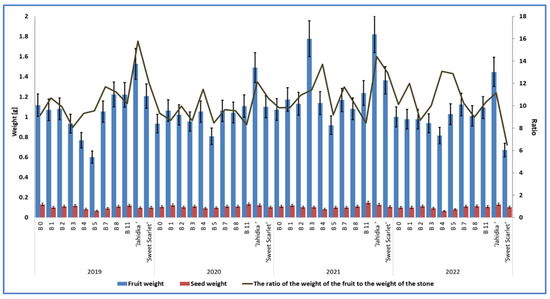
Figure 7.
The mean weights of fruits and seeds and the fruit-to-seed weight ratios of 9 biotypes and cultivars of E. multiflora in 2019–2022.
3.2.4. Total Soluble Solids
The extract of total soluble solids includes the organic compounds dissolved in the cell sap.
As shown in Table 11, the content of TSS in E. multiflora fruit was also influenced by the climatic conditions in a given research season. In 2021, the average TSS content in the Elaeagnus multiflora fruit was 16.38 Brix%, which was 1.54 Brix% higher than in 2022.

Table 11.
TSS extracts (%) in fruits of tested biotypes and cultivars of Elaeagnus multiflora.
The content of the TSS in the fruit could have been influenced by weather conditions during fruit ripening, mainly in June. As shown in the data presented in Table 1, in 2021, the temperatures in June were higher than the multiannual average by 3.9 degrees Celsius, and the precipitation was lower than the multiannual mean 1981–2010 by 46.6 mm. This indicates that there were high temperature and drought conditions, which could have resulted in an increase in the TSS content in the fruits of Elaeagnus multiflora. The results show that the average TSS content in the fruit of the Elaeagnus multiflora cultivars and biotypes ranged from 14.25 Brix% for biotype B11 to 17.43 Brix% for biotype B1. According to the statistical analysis, B11 together with the biotype B5 and the cv. ‘Jahidka’ formed a homogeneous group with the lowest values of TSS. The fruits of the ‘Sweet Scarlet’ cultivar contained 15.92% TSS, which is similar to the TSS content in the fruits of biotype B2 (15.68%) (Table 11).
The TSS values in fruits of the tested biotypes and cultivars of Elaegnus multiflora were close to the mean value (16.23 ± 0.15° Brix) obtained for Elaeagnus umbellate berries [80]. From the available data in the literature [81,82,83,84] on the content of TSS in the fruit of E. multiflora, it appears that the differences in TSS content are also caused by the different locations of the crops. A study of six Elaeagnus umbellate grown in Cookeville (TN, USA) recorded TSS values ranging from 10.6 to 18.4° Brix [83]. According to Walkowiak-Tomczak et al. [84], changes in TSS also depend on the duration and conditions of transport, storage and on the genetic properties of the cultivar. The content of the total soluble solids (TSS) is the basic characteristic used to estimate the quality of fruit intended for direct consumption and processing [22]. Therefore, the TSS results, in addition to the morphological characteristics of the fruit, such as weight and the ratio of the fruit weight to the seed weight, help to assess whether a given biotype is suitable for processing or for direct consumption as a dessert.
4. Conclusions
The morphological features of fruits of biotypes and cultivars of Elaeagnus multiflora grown in north–eastern Polish climatic conditions are significantly affected by weather conditions in the year preceding fruiting and in the growing season from April to July.
The yields of the Elaeagnus multiflora cultivars and biotypes varied significantly, both between different years and between different biotypes. The lowest yields were recorded in 2021. In this year, the fruits of the studied biotypes and cultivars were shown to be distinguished by the highest mean fruit weight, fruit weight to seed weight ratio and TSS content. The fruits were larger because there were fewer of them. In June before the harvest, there were high temperatures and low rainfall, which may have increased TSS in the fruit. The temperatures and precipitation in the vegetative season were significantly negatively correlated with the yields of biotypes an cultivars of E. multiflora. A highly significant negative influence of precipitation in June on the yield was observed. Precipitation in June caused the decrease of weight, length and width of fruits in the biotypes and cultivars of Elaeagnus multiflora. Temperatures in May significantly increased the length of the fruit and the length-to-width ratio of the fruit. Both temperature and precipitation significantly increase influenced the width of fruit. For cultivation in Poland, we recommend biotype B11 due to the highest crop yields, and the cultivar ‘Jahidka’ and the biotype B11 due the highest fruit weight. The smallest share of stone in relation to the weight of the fruit was observed for the cultivars ‘Jahidka’ and biotype B4. The group of dessert fruits with the highest TSS content included biotypes B1 and B0.
The successful cultivation of Elaeagnus multiflora in north–eastern Poland suggests it may be suitable for introduction to broader cultivation in other countries of the Baltic Sea region with a similar climate, and also perhaps, for wider range of cultivation in other regions of the world.
Author Contributions
Methodology, A.B. (Anna Bieniek); investigation, A.B. (Anna Bieniek), A.B. (Arkadiusz Bieniek) and N.B.; resources, A.B. (Anna Bieniek); data curation, A.B. (Anna Bieniek); writing—original draft preparation, A.B. (Anna Bieniek); writing—review and editing, A.B. (Anna Bieniek); visualization, A.B. (Anna Bieniek), A.B. (Arkadiusz Bieniek) and N.B; supervision, A.B. (Anna Bieniek); project administration, A.B. (Anna Bieniek), A.B. (Arkadiusz Bieniek) and N.B.; funding acquisition, A.B. (Anna Bieniek). All authors have read and agreed to the published version of the manuscript.
Funding
The results presented in this paper were obtained as part of a comprehensive study financed by the University of Warmia and Mazury in Olsztyn, Faculty of Agriculture and Forestry, Department of Agroecosystems and Horticulture, 30.610.016-110.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
The authors thanks to Krzysztof Rutkowski for help with data analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Christensen, J.H.; Christensen, O.B. A summary of the PRUDENCE model projections of changes in European climate by the end of this century. Clim. Chang. 2007, 81, 7–30. [Google Scholar] [CrossRef]
- Christensen, J.H.; Hewiston, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, I.; Jones, R.; Kolli, R.K.; Kwon, W.T.; Laprise, R.; et al. Regional Climate Projections. In The Physical Science Basis; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K., Tignor, M., Miller, H.L., Eds.; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, NY, USA, 2007. [Google Scholar]
- Sun, W.; Yuan, X.; Liu, Z.-J.; Lan, S.; Tsai, W.-c.; Zou, S.-Q. Multivariate analysis reveals phenotypic diversity of Euscaphis japonica population. PLoS ONE 2019, 14, e0219046. [Google Scholar] [CrossRef]
- Bieniek, A.; Dragańska, E.; Pranckietis, V. Assesment of climatic conditions for Actinidia arguta cultivation in north-eastern Poland. Zemdirb.-Agric. 2016, 103, 311–318. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, Z.; Li, D.; Liu, X. Phenotypic techniques and applications in fruit trees: A review. Plant Methods 2020, 16, 107. [Google Scholar] [CrossRef]
- Alcàntara-Ayala, O.; Oyama, K.; Rios-Muñoz, C.A.; Rivas, G.; Remirez-Barahona, S.; Luna-Vega, I. Morphological variation of leaf traits in the Ternstroemia lineta species complex (Ericales: Penthaphylacaceae) in response to geographic and climatic variation. PeerJ 2020, 8, e8307. [Google Scholar] [CrossRef]
- Gao, S.; Wang, B.; Liu, F.; Zhao, J.; Yuan, J.; Xiao, S.; Masabni, J.; Zou, F.; Yuan, D. Variation in fruit Morphology and seed oil fatty acid composition of Camellia oleifera collected from diverse region in Southern China. Horticulturae 2022, 8, 818. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.Q.; Yu, M.K.; Han, Y.Z.; Wu, T.G. Variation in seed size and seed mass related to tree growth over 5 years for 23 provenances of Quercus acutissima from across China. J. For. Res. 2017, 28, 917–923. [Google Scholar] [CrossRef]
- Chetty, K.; Govender, M.; Bulcock, H. A review of hyperspectral remote sensing and its application in vegetation and water resource studies. Water Sa 2007, 33, 145–151. [Google Scholar]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 55, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Alemu, K. Detection of diseases, identification and diversity of viruses: A review. J. Biol. Agric. Health 2015, 5, 204–213. [Google Scholar]
- Ali, M.M.; Bachik, N.A.; Bachik, N.A.; Muhadi, N.A.; Yusof, T.N.T.; Gomes, C. Non-destructive techniques of detecting plant diseases: A review. Physiol. Mol. Plant Pathol. 2019, 108, 101426. [Google Scholar] [CrossRef]
- Szpadzik, E.; Krupa, T.; Niemiec, W.; Jadczuk-Tobjas, E. Yielding and fruit quality of selected sweet cherry (Prunus avium) cultivars in the conditions of central Poland. Acta Sci. Pol. Hortorum Cultus 2019, 18, 117–126. [Google Scholar] [CrossRef]
- Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Zaleski, T.; Bogdał, S.; Bieniasz, M.; Błaszczyk, J.; Knaga, J.; Nawrocki, J.; Pniak, M. Variability of nutrients in the leachates from everbearing strawberry cultivated in soilless conditions on gutters. Acta Sci. Pol. Form. Circumiectus 2019, 18, 13–23. [Google Scholar] [CrossRef]
- Sitarek, M. Evaluation of selected apricot cultivars based on many years of research in the collection of RIH in Skierniewice, Poland. Acta Hortic. 2020, 1290, 155–158. [Google Scholar] [CrossRef]
- Bieniek, A.; Lachowicz-Wiśniewska, S.; Bojarska, J. The Bioactive Profile, Nutritional Value, Health Benefits and Agronomic Requirements of Cherry Silverberry (Elaeagnus multiflora Thunb.): A Review. Molecules 2022, 27, 2719. [Google Scholar] [CrossRef]
- Krupa, T.; Tomala, K. Effect of Oxygen and Carbon Dioxide Concentration on the Quality of Minikiwi Fruits after Storage. Agronomy 2021, 11, 2251. [Google Scholar] [CrossRef]
- Lachowicz, S.; Bieniek, A.; Gil, Z.; Bielska, N.; Markuszewski, B. Phytochemical parameters and antioxidant activity of new cherry silverberry biotypes (Elaeagnus multiflora Thunb.). Eur. Food Res. Technol. 2019, 245, 1997–2005. [Google Scholar] [CrossRef]
- Bieniek, A.A.; Grygorieva, O.; Bielska, N. Biological Properties of Honeysuckle (Lonicera caerulea L.): A Review: The nutrition, health properties of honeysuckle. Agrobiodiversity Improv. Nutr. Health Life Qual. 2021, 5, 287–295. [Google Scholar] [CrossRef]
- Mech-Nowak, A.; Kruczek, M.; Kaszycki, P.; Bieniasz, M.; Kostecka-Gugała, A. Polyphenols, carboxylic hydroxyacids and carotenoids in berries of blue honeysuckle (Lonicera coerulea var. kamtschatica) Polifenole, hydroksykwasy karboksylowe i karotenoidy w owocach suchodrzewu jadalnego (Lonicera coerulea var. kamtschatica). Przemysł Chem. 2014, 93, 948–953. [Google Scholar] [CrossRef]
- Sosna, I. Evaluation of several Asian pear cultivars in the climatic conditions of lower Silesia. Acta Sci. Pol. Hortorum Cultus 2018, 17, 107–114. [Google Scholar] [CrossRef]
- Szot, I.; Łysiak, G.P. Effect of the Climatic Conditions in Central Europe on the growth and Yield of Cornelian Cherry Cultivars. Agriculture 2022, 12, 1295. [Google Scholar] [CrossRef]
- Antoniewska-Krzeska, A.; Ivanišová, E.; Klymenko, S.; Bieniek, A.A.; Fatrcová-Šramková, K.; Brindza, J. Nutrients content and composition in different morphological parts of Cornelian cherry (Cornus mas L.). Agrobiodiversity Improv. Nutr. Health Life Qual. 2022, 6, 1–10. [Google Scholar] [CrossRef]
- Bienisz, M.; Konieczny, A.; Błaszczyk, J.; Nawrocki, J.; Kopeć, M.; Mierzwa-Hereszek, M.; Gondek, K.; Zaleski, T.; Knaga, J.; Pniak, M. Titanium Organic Complex Improves Pollination and Fruit Development of Remontant Strawberry Cultivars under High-Temperature Conditions. Agriculture 2022, 12, 1795. [Google Scholar] [CrossRef]
- Bieniek, A.; Dragańska, E. Content of macroelements in fruits of Ukrainian cultivars of hardy kiwifruit and Actinidia charta depending on the weather conditions during the phonological phases. J. Elem. 2013, 18, 23–38. [Google Scholar] [CrossRef]
- Lachowicz, S.; Kapusta, I.; Świeca, M.; Stinco, C.M.; Meléndez-Martínez, A.J.; Bieniek, A. In vitro Antioxidant and Antidiabetic potency of fruits and leaves of Elaeagnus multiflora Thunb. and their isoprenoids and polyphenolics profile. Antioxidants 2020, 9, 436. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, Y.K.; Park, O.J. Cherry silverberry (Elaeagnus multiflora) extracts exere anti-inflammatory effects by inhibiting COX-2 and Akt signals in HT-29 colon cancer cells. Food Sci. Biotechnol. 2010, 19, 1673–1677. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, W.T.; Cho, K.M. Determination of phytochemical contents and biological activities from the fruits of Elaeagnus multiflora. Int. J. Food Sci. Nutr. 2011, 16, 29–36. [Google Scholar] [CrossRef]
- Nowak, K.W.; Mielnik, P.; Sięda, M.; Staniszewska, I.; Bieniek, A. The effect of ultrasound treatment on the extraction of lycopene and β-carotene from cherry silverberry fruits. AIMS Agric. Food 2021, 6, 247–254. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar] [CrossRef]
- Chwil, M.; Wereszko-Chmielewska, E. Micromorphology of the floral elements, the structure of nectary, and the apicultural value of Elaeagnus commutata Bernh. Ex. Rydb. Acta Agrobot. 2011, 64, 27–34. [Google Scholar] [CrossRef]
- Caru, M.; Mosquera, G.; Bravo, L.; Guevara, R.; Sepulveda, D.; Cabello, A. Infectivity and effectivity of Frankia strains from the Rhamnaceae family on different actinorhizal plants. Plant Soil 2003, 251, 219–225. [Google Scholar] [CrossRef]
- Clawson, M.L.; Bourret, A.; Bensona, D.R. Assessing the phylogeny of Frankia-actinorhizal plant nitrogen-fixing root nodule symbioses with Frankia 16S rRNA and glutamine synthetase gene sequences. Mol. Phylogenetics Evol. 2004, 31, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Khamzina, A.; Lamers, J.P.A.; Martius, C.h.; Worbes, M.; Vlek, P.L.G. Potential of nine multipurpose tree species to reduce saline groundwater tables in the lower Amu Darya River region of Uzbekistan. Agrofor. Syst. 2006, 68, 151–165. [Google Scholar] [CrossRef]
- Follstad Shah, J.J.; Harner, M.J.; Tibbets, T.M. Elaeagnus angustifolia elevates soil inorganic nitrogen pools in riparian ecosystems. Ecosystems 2010, 13, 46–61. [Google Scholar] [CrossRef]
- Bieniek, A.; Piłat, B.; Szałkiewicz, M.; Markuszewski, B.; Gojło, E. Evaluation of yield, morphology and quality of (Elaeagnus multiflora Thunb.) biotypes under conditions of north-eastern Poland. Pol. J. Nat. Sci. 2017, 32, 61–70. [Google Scholar]
- Grygorieva, O.; Klymenko, S.; Ilinska, A.; Brindza, J. Variation of fruits morphometric parameters of Elaeagnus multiflora Thunb. germplasm collection. Potravin. Slovak J. Food Sci. 2018, 12, 527–532. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Kim, K.B.; An, C.S.; Kim, J.H.; Song, S.D. Geographical Distribution and Soil Characteristics of Elaeagnus Plants in Korea. Korean J. Ecol. 1994, 17, 159–170. [Google Scholar]
- Lachowicz-Wiśniewska, S.; Kapusta, I.; Stinco, C.M.; Meléndez-Martínez, A.J.; Bieniek, A.; Ochmian, I.; Gil, Z. Distribution of Polyphenolic and Isoprenoid Compounds and Biological Activity Differences between in the Fruit Skin+ Pulp. Seeds. and Leaves of New Biotypes of Elaeagnus multiflora Thunb. Antioxidants 2021, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Hussain, M.; Mahar, S.; Iqbal, S. Investigation on Total Phenolic Contents of Elaeagnus multiflora. Asian J. Chem. 2015, 27, 4587–4590. [Google Scholar] [CrossRef]
- Shin, S.R.; Hong, J.Y.; Yoon, K.Y. Antioxidant properties and total phenolic contents of cherry Elaeagnus (Elaeagnus multiflora Thunb.) leaf extracts. Food Sci. Biotechnol. 2008, 17, 608–612. [Google Scholar]
- Kim, S.A.; Oh, S.I.; Lee, M.S. Antioxidative and cytotoxic effects of solvent fractions from Elaeagnus multiflora. Korean J. Food Nutr. 2007, 20, 134–142. [Google Scholar]
- Kim, S.T.; Kim, S.W.; Ha, J.; Gal, S.W. Elaeagnus multiflora fruit extract inhibits melanin biosynthesis via regulation of tyrosinase gene on translational level. Res. J. Biotechnol. 2014, 9, 1–6. [Google Scholar]
- Lee, Y.S.; Chang, Z.Q.; Oh, B.C.; Park, S.C.; Shin, S.R.; Kim, N.W. Antioxidant activity, anti-inflammatory activity, and whitening effects of extracts of Elaeagnus multiflora Thunb. J. Med. Food 2007, 10, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; OH, S.; Lee, M. Antioxidative and Cytoxic Effects of Ethanol Extracts from Elaeagnus multiflora. Korean J. Food Nutr. 2008, 21, 403–409. [Google Scholar]
- Houng, J.Y.; Nam, H.S.; Lee, Y.S.; Yoon, K.Y.; Kim, N.W.; Shin, S.R. Study on the antioxidant activity of extracts from the fruit of Elaeagnus multiflora Thunb. Korean J. Food Preserv. 2006, 13, 413–419. [Google Scholar]
- Chang, Z.Q.; Park, S.C.; Oh, B.C.; Lee, Y.S.; Shin, S.R.; Kim, N. Antiplatet aggregation and antiinflammatory activity for extracts of Elaeagnus multiflora. Korean J. Med. Crop Sci. 2006, 51, 516–517. [Google Scholar]
- Chinnici, F.; Spinabelli, U.; Riponi, C.; Amati, A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J. Food Compos. Anal. 2005, 18, 121–130. [Google Scholar] [CrossRef]
- Patel, S. Plant genus Elaeagnus: Underutilized lycopene and linoleic acid reserve with permaculture potential. Fruits 2015, 70, 191–199. [Google Scholar] [CrossRef]
- Kurlus, R.; Rutkowski, K.; Łysiak, G.P. Improving of Cherry Fruit Quality and earing Regularity by Chemical Thinning with Fertilizer. Agronomy 2020, 10, 1281. [Google Scholar] [CrossRef]
- Figiel-Korczyńska, M.; Ochmian, I.; Lachowicz, S.; Krupa-Małkiewicz, M.; Wróbel, J.; Gamrat, R. Actinidia (Mini Kiwi) Fruit uality in Relation to Summer Cutting. Agronomy 2021, 11, 964. [Google Scholar] [CrossRef]
- Łysiak, G.; Kurlus, R.; Zydlik, Z.; Walkowiak-Tomczak, D. Apple Skin Colour Changes during Harvest as An Indicator of Maturity. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–83. [Google Scholar]
- Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupińska, A.; Kruczyńska, D.E. Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits. Molecules 2020, 25, 4444. [Google Scholar] [CrossRef]
- Tomala, K.; Grzęda, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. The Effects of Preharvest 1-Methylcyclopropene (1-MCP) Treatment on the Fruit Quality Parameters of Cold-Stored ‘Szampion’ Cultivar Apples. Agriculture 2020, 10, 80. [Google Scholar] [CrossRef]
- Szot, I.; Szot, P.; Lipa, T.; Sosnowska, B.; Dobrzański, B. Determination of physical and chemical properties of Cornelian cherry (Cornus mas L.) fruits depending on degree of ripening and ecotypes. Acta Sci. Pol. Hortorum Cultus 2019, 18, 251–262. [Google Scholar] [CrossRef]
- Bokszczanin, K.Ł.; Wrona, D.; Przybyłko, S. Influence of an Alternative Soil Management System to Herbicide Use on Tree Vigor, Yield, and Quality of Apple Fruit. Agronomy 2021, 11, 58. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids and vitamin A in agro-food, nutrition, health and disease. Mol. Nutr. Food Res. 2019, 63, e1801045. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin carotenoids in public health and nutricosmetics: The emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Ahmadiani, A.; Hosseiny, J.; Semnanian, S.; Javan, M.; Saeedi, F.; Kamalinejad, M.; Saremi, S. Antinociceptive and antiflammatory effects of Elaeagnus angustifolia fruit extract. J. Ethnopharmacol. 2000, 72, 287–292. [Google Scholar] [CrossRef]
- Grygorieva, O.; Klymenko, S.; Ilinska, A.; Ivanišová, E.; Bieniek, A.A.; Antoniewska, A. Morphometric analysis of fruits and seeds of Elaeagnus multiflora Thunb. In Proceedings of the Global consequences of plant introduction in conditions of climate change, Proceedings of the International Scientific Conference Is Dedicated to the 30-th Anniversary of Independence of Ukraine, Kyiv, Ukraine, 5–7 October 2021; pp. 81–82, ISBN 978-617-520-173-2. [Google Scholar]
- World reference base for soil resources 2014. In World Soil Resources Reports; IUSS Working Group WRB, FAO: Rome, Italy, 2014; Volume 106, p. 6.
- Faust, M. Physiology of Temperate Zone Fruit Trees; John Wiley & Sons, Inc.: New York, NY, USA, 1989; p. 338. ISBN 0-471-81781-3. [Google Scholar]
- Heide, O.M.; Rivero, R.; Sønsteby, A. Temperature control of shoot growth and foral initiation in apple (Malus × domestica Borkh.). CABI Agric. Biosci. 2020, 1, 8. [Google Scholar] [CrossRef]
- Grygorieva, O.; Ilyniska, A.; Zhurba, M.; Klymenko, S.; Kalista, M. Phenological growth stages according to BBCH scale Elaeagnus multiflora Thunb. Agrobiodiversity Improv. Nutr. Health Life Qual. 2022, 2, 229–241. [Google Scholar] [CrossRef]
- Westwood, M.N. Temperate-Zone Pomology: Physiology and Culture; Timber Press, Inc.: Portland, OR, USA, 1993; p. 552. ISBN 0-88192-253-6. [Google Scholar]
- Rodrigo, J.; Herrero, M. The onset of fruiting in apricot (Prunus armeniaca L.). J. Appl. Bot. 2002, 76, 13–19. [Google Scholar]
- Proebsting, E.L.; Mills, H.H. Low Temperature Resistance of Developing Flower Buds of Six Deciduous Fruit Species. J. Amer. Soc. Hortic. Sci. 1978, 103, 192–198. [Google Scholar] [CrossRef]
- Ashworth, E.N.; Wisniewski, M.E. Response of fruit tree tissues to freezing temperatures. HortScience 1991, 26, 501–504. [Google Scholar] [CrossRef]
- Rodrigo, J. Spring frosts in deciduous fruit trees—Morphological damage and flower hardiness. Sci. Hortic. 2000, 85, 155–173. [Google Scholar] [CrossRef]
- Chawla, R.; Sheokand, A.; Rai, M.; Sadawarti, R.K. Impact of climate change on fruit production and various approaches to mitigate these impacts. Pharma Innov. J. 2021, 10, 564–571. [Google Scholar]
- Tomala, K. Orchard factors affecting fruit storage quality and prediction of harvest date of apples. Acta Hortic. 1999, 485, 373–382. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Weather Conditions, Orchard Age and Nitrogen Fertilization Influences Yield and Quality of ‘Łutówka’ Sour Cherry Fruit. Agriculture 2022, 12, 2008. [Google Scholar] [CrossRef]
- Lipa, T.; Szot, I. Effect of Fertilization Methods on Growth of Pear Trees, Yielding and Fruit Quality. Mod. Phytomorphology 2013, 4, 55–58. [Google Scholar]
- Rutkowski, K.; Łysiak, G. Thinning methods to regulate sweet cherry crops—A review. Appl. Sci. 2022, 12, 1280. [Google Scholar] [CrossRef]
- Kawecki, Z.; Bieniek, A. Influance of climatic conditions of northeastern Poland on growth of bower actinidia. Sci. Work. Lith. Inst. Hortic. Lith. Univ. Agriculture. Sodininkystè Daržininkystè 2008, 27, 307–318. [Google Scholar]
- Łysiak, G.P. Degree Days as a Method to Estimate the Optimal Harvest Date of ‘Conference’ Pears. Agriculture 2022, 12, 1803. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Adelina, R.; Suliansyah, I.; Syarif, A.; Warnita. Phenology of Flowering and Fruit Set in Snake Fruit (Salacca Sumatrana Becc.). Acta Agrobot. 2021, 74, 742. [Google Scholar] [CrossRef]
- Hong, J.Y.; Cha, H.S.; Shin, S.R.; Jeong, Y.J.; Youn, K.S.; Kim, M.H.; Kim, N.W. Optimization of manufacturing condition and physicochemical properties for mixing beverage added extract of Elaeagnus multiflora Thunb. fruits. Korean J. Food Preserv. 2007, 14, 269–275. [Google Scholar]
- Gamba, G.; Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Randriamampionona, D.; Beccaro, G.L. Phytochemical Characterization and Bioactivity Evaluation of Autumn Olive (Elaeagnus umbellata Thunb.) Pseudodrupes as Potential Sources of Health-Promoting Compounds. Appl. Sci. 2020, 10, 4354. [Google Scholar] [CrossRef]
- Hussain, I. Physiochemical and sensory characteristics of Elaeagnus umbellata (Thunb) fruit from Rawalakot (Azad Kashmir) Pakistan. Afr. J. Food Sci. 2011, 2, 151–156. [Google Scholar]
- Wang, S.Y.; Fordham, I.M. Differences in chemical composition and antioxidant capacity among different genotypes of Autumn Olive (Elaeagnus umbellate Thunb.). Food Technol. Biotechnol. 2007, 45, 402. [Google Scholar]
- Walkowiak-Tomczak, D.; Idaszewska, N.; Łysiak, G.P.; Bieńczak, K. The Effect of Mechanical Vibration during Transport under Model Conditions on the Shelf-Life, Quality and Physico-Chemical Parameters of Four Apple Cultivars. Agronomy 2021, 11, 81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).