Abstract
Abundant and functionally diverse earthworm communities in perennials deliver ecosystem services like increasing nutrient availability by incorporating organic matter. This study aimed to analyse the decomposition of annual and perennial energy crop residues, and the subsequent nutrient release, depending on earthworm functional diversity. In a laboratory experiment, two ecological earthworm groups—anecics (detritivorous Lumbricus terrestris (L.)) and endogeics (geophagous Aporrectodea caliginosa and A. rosea)—were incubated with wilted cup plant (Silphium perfoliatum) and maize (Zea mays) litter for 4 or 8 weeks. Decomposition and consumption rates were calculated. The C, N and P in litter and casts were analysed. Mineralisation was a function of earthworm biomass and the number of individuals. Functional diversity had no beneficial effect. Decomposition was found to be highest in treatments with detritivorous earthworms, i.e., higher earthworm biomass, yet consumption and nutrient turnover in relation to earthworm biomass were higher in treatments with geophages indicating enhanced competition. N limitation became apparent in both litter treatments and was predominant with cup plant litter. N limitation and recalcitrant cell wall compounds affected consumption rates and the egestion of total C and P. While N in casts was lower than expected, P was increased. We observed that the effects manifested at different stages of decomposition of maize and cup plant, highlighting differences in temporal development of decomposition and nutrient turnover between litter types. Our results indicate that earthworms promote decomposition of recalcitrant litter and nutrient turnover, but N limitation may hamper nutrient release. Cup plant systems offer a suitable habitat for soil-dwelling organisms, but management approaches must consider the adequate input of organic matter as an energy and nutrient source to enhance ecosystem service provision.
1. Introduction
Biogas production is a much-debated topic when it comes to sustainable energy generation. While it represents a valuable alternative to fossil fuels, the used substrate is a crucial factor regarding sustainability [1,2,3]. The most common substrate is annual maize (Zea mays), due to its high methane yield of up to 10,200 m3N ha−1 [4]. However, the sustainability of annuals like maize for biogas production is often questioned because of high external inputs of fertilizer, fossil fuels and pesticides [1,2]. Second-generation energy crops open up new possibilities for sustainable biogas production as they may be managed as low-input systems reducing resource input. While this management approach may impact the overall yield in comparison to maize, there are valuable assets to second-generation energy crops, which increase their competitiveness and act as important incentives for farmers. Perennial energy crops are particularly beneficial for soil health and the subsequent crop [5] and, in contrast to maize, may counteract soil erosion [6]. In this context, the cup plant (Silphium perfoliatum) has shown great potential, as it combines relatively high yields [7] with an array of positive effects on soil and biodiversity. Its yellow flowers provide a valuable food source for pollinators in a prolonged blooming period from July to late September [8,9,10]. In the first year after establishment, weed control is crucial, but in the following years, the need for pesticide input is typically low [8,11]. The omission of tillage and the extensive root system of the cup plant protect the soil against erosion [12]. Additionally, cup plant cultivation provides favourable habitat conditions for soil-dwelling organisms like earthworms [13,14]. Earthworms generally benefit from no-till regimes [15].
The cup plant, for example, may have some notable disadvantages compared to maize, like lower water use efficiency and lower yield [10,16]. Yet, the development of larger earthworm populations under cup plant cultivation potentially grants access to numerous ecosystem services compensating for its disadvantages. For example, earthworms mitigate soil erosion, counteract soil compaction by improving water infiltration as well as gas exchange and thus, improve plant growth [17,18,19]. Bertrand et al. [20] and Blouin et al. [21] report on the role of earthworms in decomposition of organic matter, nutrient cycling and soil organic carbon stabilisation. The processes underlying earthworm-controlled mineralisation and C sequestration are complex and depend on the local and temporal context, as well as on organic matter input and earthworm community structure. For instance, earthworms may cause a decline in soil C stocks [20,21,22]. In perennial cropping systems, a higher potential to increase C sequestration can be assumed and in the presence of earthworms, a distribution of protected C into the subsoil is likely [23,24,25]. As a matter of fact, both mineralisation and sequestration of soil C, occur simultaneously; yet several factors, such as the provision of organic matter, C incorporation into aggregates and eventually plant growth will affect the balance of those two processes [22]. However, with respect to energy cropping systems, it is fundamental to assess whether earthworm communities and above-ground organic residue production synchronise to promote C sequestration or trigger SOM mineralisation. Taken together, the abovementioned examples show that it is important to be aware of the complex relationships, which drive earthworm-controlled processes towards ecosystem service and which circumstances will shift them towards disservices.
The anecic species Lumbricus terrestris L., and the endogeic species Aporrectodea caliginosa and Aporrectodea rosea, are of particular interest because they are commonly found in arable soils and represent two different feeding strategies. Detritivorous anecics feed on fresh surface residues that they drag into their burrows for consumption, while geophagous endogeics feed on already decayed organic matter within the soil [26]. Detritivorous earthworms are subsequently called primary decomposers and geophagous earthworms are called secondary decomposers. The combination of both guilds can lead to positive complementary effects on mineralisation and nutrient turnover, as demonstrated by Ernst et al. [27], who observed in a microcosm study with winter rape, maize, miscanthus and oat litter that L. terrestris increased easily available soil organic matter and positively affected endogeics, i.e., secondary decomposers. A study by Postma-Blaauw et al. [28] showed that a combination of decomposer guilds, similar to those in our study, enhanced bacterial growth. A complex interaction of A. caliginosa and L. terrestris was reported by Laossi et al. [29], who found beneficial effects on plant biomass depending on the plant species.
Furthermore, earthworms are broadly known to accumulate nutrients in their casts [21,26]. A recent meta-analysis by Van Groenigen et al. [30] found that earthworms increase the total organic C, total N, and P by 40–48% in casts compared to the bulk soil. In a grassland study by Decaëns et al. [31], earthworm casts contained 40–60% more total N and 40–90% more total C than the surrounding bulk soil. Another grassland study by Graff [32] revealed about 50% more N and a fivefold increase in plant-available P in casts compared to soil. Even when not provided with any external food source, earthworm casts contained slightly higher nutrient levels then the soil, but differences were mainly insignificant [33,34]. This implies that earthworms may play a crucial role for nutrient cycling in low-input systems like perennials.
Therefore, this study aims to focus on earthworm decomposition services in annual and perennial energy crops (maize versus cup plant) and its effects on mineralisation and nutrient turnover, which are two of the most important ecosystem services in agriculture. We hypothesize that (i) a higher functional earthworm diversity of primary and secondary decomposers enhances mineralisation of organic residues (litter) and nutrient release; (ii) the quality of the applied litter controls earthworm activity and therefore, the extent of the mineralisation and nutrient release. A laboratory experiment was conducted, in which soil was amended with wilted cup plant and maize litter and to which either anecics or endogeics or a mix of both ecological groups were added. The experiment was conducted with two different incubation periods (4 and 8 weeks). Decomposition and consumption rates were calculated and the C, N and P in casts and litter were evaluated to test our hypotheses.
2. Material and Methods
2.1. Soil
The soil was obtained from the Ap horizon of a commercially cultivated maize field near St. Wendel (49.45° N, 7.13° E) in southwest Germany. The soil was identified as a Hypereutric Mollic Stagnosol [35] and categorised as silt loam (SiL; 17.7% sand, 59.3% silt, 23.0% clay). Determination of particle size distribution was conducted with air-dried soil using the Köhn method with a Sedimat 4–12 (Umweltgerätetechnik UGT, Müncheberg, Germany) [36]. Soil was defaunated by a threefold 24 h cycle of freezing (−18°C) and thawing (room temperature). Afterwards, the soil was dried, sieved (2 mm) and rewetted. The soil was mixed thoroughly the next day and stored for one week to ensure even moisture distribution. The gravimetric water content was set to 33.0 ± 0.7%, which was close to field capacity, as advised by Fründ et al. [37], to ensure suitable moisture conditions for earthworms. Plant-available phosphate P [mg kg−1] in soil material was extracted with a calcium-acetate-lactate (CAL) solution and measured spectrometrically via ICP-OES iCAP 7400 (Thermo Fisher Scientific, Waltham, MA, USA), according to VDLUFA [38]. Total phosphate Ptotal [mg kg−1] in plant material was extracted by aqua regia digestion and measured via Agilent 725 ICP-OES (Agilent Technologies, Santa Clara, CA, USA), according to ISO 11885 [39]. For measurements of C and N see the litter section below. Soil pH was measured in a CaCl2 solution with a SI Analytics Lab 850 pH-meter (Thermo Fisher Scientific, Waltham, MA, USA), in accordance with ISO 10,390 [40]. Before incubation the soil contained 19.84 ± 0.76 g kg−1 total C, 2.09 ± 0.04 g kg−1 total N, and 56.99 ± 0.36 mg kg−1 plant-available CAL-P at pH 6.6. Pre-incubation C/N ratio of the experimental soil was 9.5.
2.2. Litter
Litter of cup plant (Silphium perfoliatum) and maize (Zea mays) was collected from commercially cultivated fields near St. Wendel (49.51° N, 7.07° E) in late summer. Cup plant litter was collected from the ground. Maize litter was collected as wilting senescent leaves from the bottom part of the maize plants. Both, cup plant and maize litter were dried in an oven at 60 °C and chopped into 2 cm fragments. Stems were excluded. The chemical composition, i.e., total C [g kg−1], N [g kg−1] and P [g kg−1] as well as cellulose [%], hemicellulose [%] and lignin [%] are listed in Table 1. Fibre composition of maize and cup plant litter was measured using a Fibretherm FT12 system (C. Gerhardt GmbH, Königswinter, Germany). Single plant composites, such as cellulose, hemicellulose and lignin, were analysed according to VDLUFA [41,42,43]. Total carbon and nitrogen C and N in the initial litter as well as in the remaining litter, soil and casts were measured by dry combustion with a TruMac CN Analysator (LECO, St. Joseph, MO, USA).

Table 1.
Initial total C and N content [g kg−1] and total P [g kg−1] and plant structural substances hemicellulose, cellulose and lignin [%] of dried maize and cup plant litter (mean ± SE).
2.3. Earthworms
Primary decomposer earthworms, Lumbricus terrestris L., were purchased from a commercial supplier (Herrmann & Herrmann GbR, Braunschweig, Germany). Secondary decomposer earthworms, Aporrectodea caliginosa (Savigny, 1826) and Aporrectodea rosea (Savigny, 1826), were collected from grassland at the Thünen campus in Braunschweig (Germany). Adult and subadult individuals were washed in tap water and adapted to the experimental conditions by keeping them in the experimental soil and providing experimental plant material ad libitum at least one week prior to the experiment. During adaptation, the earthworms were kept in darkness at 15 °C (±1 °C), which is the optimum temperature for earthworm activity.
2.4. Experimental Design
The experiment was set up with the following treatments with five replicates each resulting in a total of 80 units: four earthworm treatments (primary decomposer, secondary decomposer, mixed primary and secondary decomposer, non-earthworm control), two litter treatments (cup plant, maize), and two incubation periods (4 weeks, 8 weeks). The experimental set up as well as the mean raw earthworm biomass before incubation are shown in Table 2; the non-earthworm control is not listed in Table 2. In the following sections, earthworm biomass is given as raw weight [g]. Earthworm density equalled 105 individuals per m2 (primary decomposers), 263 individuals per m2 (mix of primary and secondary decomposers) and 421 individuals per m2 (secondary decomposers).

Table 2.
Initial raw earthworm biomass [g] (means ± SE, n = 5) and individuals per replicate for cup plant and maize treatments for incubations of 4 and 8 weeks. Earthworm treatments: primary = primary decomposer L. terrestris; secondary = secondary decomposers A. caliginosa and A. rosea; mix = primary and secondary decomposers.
2.5. Incubation Experiment and Sampling of Soil, Casts and Litter
Plastic boxes (length: 20 cm, width: 9.5 cm, height: 8.5 cm) were filled with 1 kg of moist soil to a bulk density of 0.9 g cm−3, which corresponded to a water filled pore space of about 45.0%. Earthworms were added to the boxes according to Table 2. Earthworm biomasses were set to be similar among the different litter treatments and duration periods: primary ca. 13 g; mix ca. 8 g; secondary ca. 4 g (Table 2). To encourage earthworms to burrow into the soil, small holes of 1 cm depth were pushed into the soil surface and earthworms were inserted headfirst. Afterwards, 12 g of dried litter was evenly distributed on the soil surface and gently sprayed with tap water. The boxes were closed with a lid and kept in the dark at 15 °C (±1 °C) for 4 and 8 weeks, respectively. During the incubation, the boxes were controlled three times a week to check for moisture levels and mould development. After the incubation, the total remaining litter was removed from the boxes, and the surface casts were carefully collected and manually separated from any adherent residual litter. The earthworms were carefully removed from the soil and transferred into tap water to remove adherent soil, organic material and mucus. After briefly putting the earthworms on dry tissue, their biomass was determined by weighing (±0.01 g). Before and after the experiment, gut emptying was avoided in order to maintain earthworm vitality. Further, clearly identifiable casts as well as bulk soil was sampled. Collected cast material was combined per sample without further differentiation. Bulk soil and cast samples were dried at 105 °C for 24 h, weighed, sieved (2 mm) and subsequently ground for measurements of total C and N and plant-available P. The remaining litter was cautiously rinsed in tap water if necessary to remove soil and dried at 60 °C, weighed and ground for analysis of total C and N and total P afterwards. Since remaining plant material was scarce after incubation, the five replicates were pooled so that after incubation, three measurements of total Clitter [mg], total Nlitter [mg] and total Plitter [mg] per treatment were possible.
2.6. Calculations
2.6.1. Decomposition and Consumption
To calculate the decomposition rate k, we applied a transposed version of Olson’s model for a system with decay without production [44] (Equation (1)).
For the calculation of k, the ln of the quotient of the remaining litter fraction X [g] and the initial litter quantity X0 [g] is divided by the duration of the incubation t [d]. To connect the decomposition to earthworm biomass, we further calculated the weekly consumption rate C [g g−1] by modifying the method postulated by Daniel [45] (Equation (2)).
The consumption rate C is determined using X and X0 again and by considering the mean of the initial and final fresh earthworm biomass, i.e., raw earthworm weight W [g], the duration d in weeks and a fraction of the loss L by microbial activity without earthworms. To determine the fraction of the loss L, we differed from Daniel [45] and used the final litter biomass Xnone from the respective control treatments (litter control without earthworms) (Equation (3)).
For better assessment of nutrient turnover in relation earthworm activity, the cast nutrient contents Ccast, Ncast and CAL-Pcast were divided by the mean of the initial and final fresh earthworm biomass i.e., raw earthworm weight W [g], similarly to the consumption rate.
2.6.2. Litter-Derived Total C, N and P
For the quantification of nutrient release, litter-derived amounts Ylitter of the total Clitter [g], total Nlitter [mg] and total Plitter [mg] were calculated using the initial litter quantity X0 [g] and the remaining litter fraction X [g] as well as the initial contents yinitial of the total Cinitial [%], total Ninitial [%] and total Pintital [mg kg−1] of the litter and the final contents yfinal of the total Cfinal [%], total Nfinal [%] and total Pfinal [mg kg−1] of the remaining litter (Equation (4)).
Since samples were pooled, maximum standard errors for Clitter, Nlitter and Plitter were calculated and combined, according to Higgins et al. [46], before standard errors were calculated.
2.7. Statistical Evaluation
For each of the dependent variables, k, C as well as cast C, N and CAL-P, we built linear models using the R function “lm” in R version 4.0.3 [47] and tested the significance of the treatment effects with type III ANOVA using the “car” package [48]. Earthworm treatment, duration and litter treatment were explanatory variables for all models. Subsequently, the significance of the explanatory variables is indicated by the ratio of variance within groups (F) and the respective p-value (Pr > F). Furthermore, nutrient turnover was more thoroughly assessed by dividing cast C, N and CAL-P by the mean earthworm raw weight, i.e., the mean of the initial and final raw earthworm biomass. The resulting quotients were used as dependent variables, with earthworm treatment, duration and litter treatment as explanatory variables in a type III ANOVA. Litter-derived C, N and P were evaluated via a generalised linear mixed model using the “glmmTMB” function [49] combined with a type III ANOVA. Explanatory variables were the same as in the linear models, but the group that describes the specific combination of treatments (earthworm treatment + litter treatment + duration) was added as a random factor to account for the pooling and subsequent drawing of samples for measurement, as described in Section 2.5. The significance of the explanatory variables is indicated by the difference between the variables (Chisq) and the respective p-value (Pr > Chisq). To assess earthworm biomass and number, we created separate models for the dependent variables decomposition rate and consumption rate with the following predictors: litter and duration treatment, and either the combination of mean earthworm biomass + (mean earthworm biomass)2 or earthworm number + (earthworm number)2. The dependent variables were log-transformed where necessary. The normal distribution and homogeneity of the variance of the model residuals were checked via the Shapiro–Wilk test and visual inspection. Post hoc testing was conducted with Tukey’s HSD using “emmeans” [50]. The compact letter display in figures was generated with the package “agricolae” using the R function “HSD.test” [51]. Further, the package “multcomp” [52] was used. To contrast the final litter biomasses as well as the nutrients in the soil and cast, a two-sided two-sample Wilcoxon rank-sum test was applied, using the R function “wilcox.test” from the package “stats” [47]. Scatterplots were created using the packages “ggplot2” [53] and “ggpubr” [54]. For comparison of the initial and final earthworm biomass, a paired, two-sided two-sample Wilcoxon rank-sum test was used. Possible correlations were examined with the Spearman’s rank correlation.
3. Results
3.1. Earthworm Survival
Total earthworm survival was 99% for maize treatments and 97% for cup plant treatments. On day one of incubation, one individual was found dead in a 4-week replicate of the cup plant treatment with primary decomposers and in a 4-week replicate of the cup plant treatment with primary and secondary decomposers, respectively. The dead earthworms were immediately replaced by individuals of similar biomass. In one 8-week replicate of the cup plant treatment with primary decomposers, all animals perished during incubation. This replicate was excluded from the data evaluation. The formation of mould was observed. In two 8-week replicates of the maize treatment, one without earthworms and one with primary decomposers, mould was slightly torn down because it filled the headspace almost entirely. An adverse effect of this procedure on final parameters like litter biomass or earthworm biomass was not observed. In total, 3% and 10% of all endogeic earthworms were found to be in aestivation in maize and cup plant treatments, respectively. The earthworm biomass changed in both treatments. Cup plant treatments induced an insignificant biomass decrease of <1%. A 7% increase of earthworm biomass in maize treatments was significant (p < 0.001). Stronger biomass decreases were observed in treatments with primary decomposers. In treatments with secondary decomposers, the earthworm biomass was more likely to increase (Supplementary Information, Table S1).
3.2. Mineralisation of Litter Biomass
Table 3 shows the results of the statistical evaluation for k, C as well as litter derived nutrients and cast nutrients, which are presented in detail in the following sections. The remaining litter biomass may be found in Table S2 in the Supplementary Information. The regression coefficients and the respective standard error for each model may be found in Table S6 of the Supplementary Information.

Table 3.
ANOVA significance levels as expressed by F and Pr(>F) for the decomposition rate k, consumption rate C, total C, N and P released from the litter (Clitter, Nlitter, Plitter) and total C and N and CAL-P of the casts (Ccast, Ncast, CAL-Pcast). Earthworm treatment, litter treatment and duration were used as dependent variables. F and Pr(>F) values for the decomposition rate k, consumption rate C and the total C and N and CAL-P of the casts (Ccast, Ncast, CAL-Pcast) as well as Chisq and Pr(>Chisq) values for the total C, N and P released from the litter (Clitter, Nlitter, Plitter) are given for interaction effects of the earthworm treatment and litter treatment (earthworm:litter), earthworm treatment and duration (earthworm:duration) and litter treatment and duration (litter:duration).
3.2.1. Decomposition Rate k
Significant differences in the decomposition rate k were found between litter treatments (Figure 1, Table 3; Supplementary Information Table S2). Decomposition rates in cup plant treatments were up to twice as high as in the respective maize treatment (Figure 1). Furthermore, the duration of the experiment significantly influenced the decomposition rates (Table 3; Supplementary Information Table S2). After 8 weeks of incubation, decomposition rates were predominantly higher than after 4 weeks. Changes in the decomposition rates induced by duration depended on the litter treatment, as can be seen in Figure 1 and Table 3 (litter:duration). Differences in k caused by the length of the incubation period were stronger in maize treatments. Here, k was increased by 19.3% in treatments without earthworms and by 96.1% in mixed earthworm treatments after 8 weeks. Cup plant treatments resulted only in slight alterations, apart from a clear decrease in non-earthworm control treatments (−30.4%).
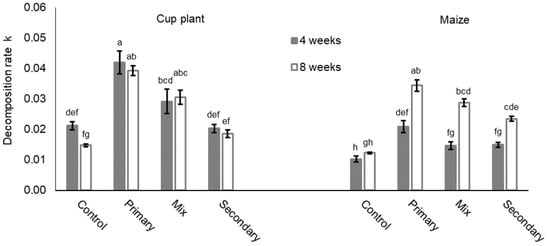
Figure 1.
Decomposition rate k (means ± SE, n = 5) for cup plant and maize treatments after 4 and 8 weeks of incubation. Control = without earthworms; primary = primary decomposer L. terrestris; secondary = secondary decomposers A. caliginosa and A. rosea; mix = primary and secondary decomposers. For statistics see Table 3 and Supplementary Information Table S6; for further information see Supplementary Information, Tables S1 and S2.
Generally, the earthworm activity significantly enhanced k and further supported higher decomposition rates after 8 weeks of incubation, where treatments without earthworms showed declining or stagnant trends (Table 3, earthworm:duration). The highest decomposition rates were found in litter treatments with primary decomposers (here: L. terrestris). Secondary decomposers A. caliginosa and A. rosea induced lower decomposition rates, while the mix of both decomposer guilds led to intermediate decomposition rates. Statistical evaluation verified the significant effect of earthworm treatments (Table 3). Moreover, a negative Spearman’s rank correlation between litter biomass and earthworm biomass was found for cup plant treatments (R −0.53; p > 0.01, Supplementary Information Figure S1). A similar relationship in maize treatments was not significant (Supplementary Information Figure S1). Our model validated the effect of earthworm weight (mean earthworm biomass; F 16.279 Pr(>F) > 0.001). On the other hand, the number of earthworms per replicate had no significant effect.
3.2.2. Consumption Rate C
Consumption rates C varied particularly among earthworm treatments. The highest consumption rates were calculated for treatments with the endogeic species A. caliginosa and A. rosea. The lowest consumption rates were found in treatments with the primary decomposer L. terrestris (Figure 2). Consumption rates within the earthworm treatments were significantly influenced by the litter treatment (Table 3, earthworm:litter), which was most evident in treatments with primary decomposer L. terrestris. The 4-week treatment with primary decomposers and the mixed treatment both resulted in 47.5% to 49.1% lower consumption rates, respectively, when treated with maize litter in comparison to the respective cup plant treatment. Corresponding differences in the treatments with secondary decomposers are less pronounced. Statistical analysis further indicated an additional linear quadratic effect of the number of individuals per replicate within earthworm treatments on the consumption rates (earthworm number2; F 5.9 Pr(>F) 0.019). Evaluation of earthworm biomass per replicate indicated a linear link between lower earthworm biomass and consumption (mean earthworm biomass; F −2.9 Pr(>F) 0.005). The litter treatment in general showed only insignificant differences in consumption rates, but in context with the duration, effects were highly significant (Table 3). Maize treatments showed lower consumption rates compared to cup plant treatments after a short incubation (4 weeks), as illustrated by Figure 2. After 8 weeks of incubation, consumption rates in both treatments were similar and only very small differences remained (see also Supplementary Material Table S2). Especially in maize treatments with secondary decomposers, consumption rates nearly doubled. In cup plant treatments, a 31.6% increase in replicates with secondary decomposers was found as well, but in the mixed treatment with both decomposer guilds, consumption rates did not change after 8 weeks. In the 8-week cup plant treatments with primary decomposer, consumption rates dropped by 20.7% compared to the 4-week incubation.
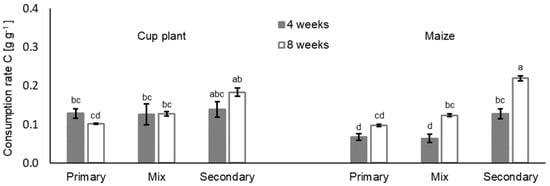
Figure 2.
Consumption rate C [g g−1] (means ± SE, n = 5) of primary decomposers L. terrestris (primary), secondary decomposers A. caliginosa and A. rosea (secondary) and a mix of both decomposer guilds (mix) in cup plant and maize treatments after 4 and 8 weeks of incubation. For statistics see Table 3 and Supplementary Information Table S6; for further information see Supplementary Information, Tables S1 and S2.
3.3. Nutrient Release
3.3.1. Carbon, Nitrogen and Phosphate from Litter
Up to three times more Nlitter was released from maize litter after 4 and 8 weeks relative to cup plant (Table S3, Supplementary Material). In the 4-week cup plant treatments with earthworms, 25.1% to 31.7% more Clitter was released. The litter treatment had significant effects on the Clitter, Nlitter and Plitter (Table 3). More Clitter, Nlitter and Plitter was found as mineralisation progressed. The magnitude of the nutrient release was subject to significant temporal variability depending on the litter type (Table 3). For example, the Clitter in 8-week cup plant treatments was on average 30.6% higher compared to the 4-week cup plant treatments, while the Clitter in 8-week maize treatments was 87.4% higher. Our data further show that treatments with primary decomposers led to the highest amounts of Clitter, Nlitter and Plitter (Table S3, Supplementary Material). The earthworm treatment was a significant factor for all three litter-derived nutrients (Table 3).
3.3.2. Carbon, Nitrogen and Phosphate in Earthworm Casts
The earthworm effect on the fate of litter-derived total Clitter, Nlitter and Plitter was assessed by analysing the Ccast, Ncast and CAL-Pcast in the casts of each earthworm treatment and by analysing cast C, N and CAL-P per mean earthworm raw body weight in [g] as a measurement for nutrient turnover (Table 4, Supplementary Material Figures S2–S4 and Table S5).

Table 4.
Total Ccast, total Ncast [g kg−1] and total CAL-Pcast [10−6 g kg−1] in casts per mean earthworm biomass [g] (means ± SE, n = 5) in cup plant and maize treatments for 4 and 8 weeks of incubation for earthworm treatments. Primary = primary decomposer L. terrestris; secondary = secondary decomposers A. caliginosa and A. rosea; mix = primary and secondary decomposers. For statistics see Figures S2–S4 and Table S5 of the Supplementary Information.
Litter treatment significantly influenced the overall Ncast (Figure 3), but regarding Ncast per earthworm body weight, no significant differences were found (F 0.01 Pr(>F) 0.928). Differences regarding the Ccast and CAL-Pcast, which were found in the respective litter treatment, were not significant (Table 3). However, the differences in C and CAL-P turnover per earthworm biomass was significant in both cases (FCcast 7.65 Pr(>F)Ccast 0.009; FCAL-Pcast 8.05 Pr(>F)CAL-Pcast 0.007). In general, the cast nutrients per earthworm raw biomass [g] showed a similar course to that of the consumption rate. Among litter treatments, the Ccast and CAL-Pcast as well as the Ncast varied over time, as shown in Figure 3, Figure 4 and Figure 5.
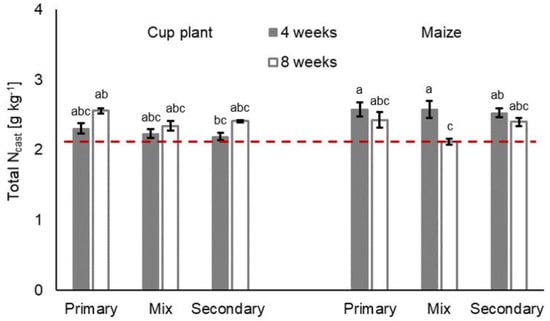
Figure 3.
Total nitrogen Ncast [g kg−1] (means ± SE, n = 5) in casts in treatments with primary decomposers L. terrestris (‘primary’), secondary decomposers A. caliginosa and A. rosea (‘secondary’) and a mix of primary and secondary decomposers (‘mix’) in cup plant and maize treatments after 4 and 8 weeks of incubation. Initial total soil nitrogen content is expressed by the dashed red line. For statistics see Table 3 and Supplementary Information Table S6; for further information see Supplementary Information, Table S4.
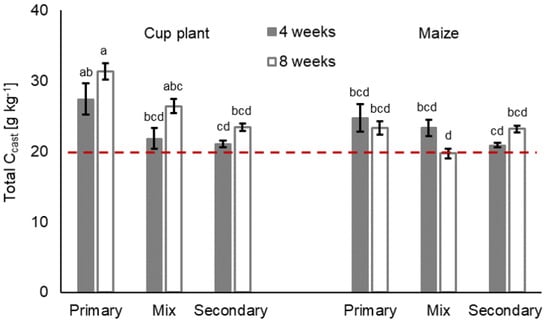
Figure 4.
Total carbon Ccast [g kg−1] (means ± SE, n = 5) in casts in treatments with primary decomposers L. terrestris (‘primary’), secondary decomposers A. caliginosa and A. rosea (‘secondary’) and a mix of primary and secondary decomposers (‘mix’) in cup plant and maize treatments after 4 and 8 weeks of incubation. Initial total soil carbon content is expressed by the dashed red line. For statistics see Table 3 and Supplementary Information Table S6; for further information see Supplementary Information, Table S4.
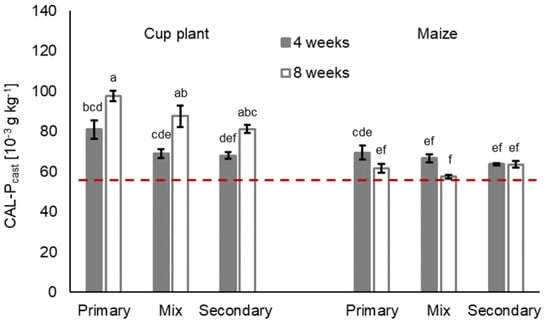
Figure 5.
Plant-available, i.e., CAL-extractable Pcast [10−3 g kg−1] (means ± SE, n = 5) in casts in treatments with primary decomposers L. terrestris (‘primary’), secondary decomposers A. caliginosa and A. rosea (‘secondary’) and a mix of primary and secondary decomposers (‘mix’) in cup plant and maize treatments after 4 and 8 weeks of incubation. Initial CAL-P content of the soil is expressed by the dashed red line. For statistics see Table 3 and Supplementary Information Table S6; for further information see Supplementary Information, Table S4.
The interaction of litter treatment and duration was highly significant for all three nutrients (Table 3, litter:duration) and for cast nutrient per earthworm raw biomass as well (Table S5, Supplementary Material). In 4-week treatments, for instance, earthworm casts in maize treatments showed 10.4% to 13.3% more total N than their respective cup plant counterparts. After 8 weeks of incubation, however, casts in cup plant treatments contained more total N. The total C and CAL-P in casts varied less among litter treatments after 4 weeks of incubation. After 8 weeks, casts in cup plant treatments contained 1.6% to 25.6% more total C and 21.8% to 36.8% more CAL-Pcast than the respective maize treatment. The Ccast and CAL-Pcast performed similarly and were found to positively correlate (Spearman’s rank correlation; Rcup plant 0.89, Pcup plant < 0.0001; Rmaize 0.71, Pmaize < 0.0001).
Earthworm treatments had no significant effect on the cast nutrient contents, but the affected Ccast and CAL-Pcast depended on the litter type (Table 3, earthworm:litter). This became evident in cup plant treatments, where the highest Ccast as well as the highest CAL-Pcast were found with primary decomposer L. terrestris (Figure 3 and Figure 5). Less total C and CAL-P were found in the casts of secondary decomposers A. caliginosa and A. rosea. Regarding cast nutrient turnover per earthworm raw biomass, the C, N and CAL-P were two to three times as high in treatments with secondary decomposers as in the treatments with primary decomposers or in mixed treatments (FC > 155.41 Pr(>FC) < 0.001; FN > 279.36 Pr(>FN) < 0.001; FCAL-P > 235.38 Pr(>FCAL-P) < 0.001) (Table 4; Supplementary Material Table S5).
After the incubation, especially in 8-week treatments, the bulk soil was heavily traversed by earthworm burrows, which complicated sampling of the virgin bulk soil. As a result, the measurement results of the nutrient content in post-incubation bulk soil had to be questioned and were consequently not reported in this study. When compared to the initial nutrient contents of the soil (Figure 3, Figure 4 and Figure 5, red dashed lines), the largest Ccast and CAL-Pcast accumulations with on average 26.5% and 42.0%, respectively, are attributed to cup plant treatments. In maize treatments, the Ccast and CAL-Pcast increased by an overall mean of 13.7% and 11.8%, compared to the initial soil. Slightly higher Ncast enrichments were attributed to maize treatments (avg. 15.9%; cup plant treatments: avg. 11.3%). All earthworm treatments in all the litter treatments displayed nutrient accumulation to some extent, except for maize treatments with mixed decomposer guilds (Mix) after 8 weeks, which made virtually no difference to the soil’s initial total C, total N or CAL-P content.
4. Discussion
4.1. Earthworm Functional Groups
In our laboratory study, the highest overall decomposition was linked to primary decomposers and the lowest to secondary decomposers, and vice versa for consumption rates. The decomposition and consumption of earthworm treatments with both primary and secondary decomposers (mix) laid in-between. Inter- and intraspecies competition for space may have played a role, notably we used a soil volume of a little less than a litre [33,55]. Unlike Eriksen-Hamel & Whalen [56], we did not notice any significant earthworm biomass loss. Nevertheless, earthworm density expressed as individuals per m2 was higher than it would be in the field. Noting the feeding behaviour of the earthworm species in our experiment and the territorial behaviour of L. terrestris [57], competition either has occurred to a limited extent due the short incubation period or would have been more pronounced during longer incubation. However, we found that a higher earthworm biomass led to a higher decomposition rate, but not to a higher consumption rate. A higher number of earthworms, however, was linked to higher consumption rates. This implies that L. terrestris promotes decomposition to a stronger extent than the secondary decomposers, although they are linked to a higher consumption per g of their biomass. There are two underlying reasons for this observation: (i) the biomass of the primary decomposer was much higher than the secondary decomposer biomass, hence, even though more litter was decomposed, the consumption by primary decomposers was lower; (ii) consumption in the treatments with the secondary decomposers may only appear to be higher. Due to the more intensive burrowing and casting activity that is associated with geophagous earthworms, it may be assumed that they could have created a larger hot spot area, in which more microbial decomposition occurred [26]. Hence, consumption by earthworms alone may not account for the measured litter decay, but earthworms may have stimulated soil microorganisms depending on the earthworm density and activity. Further investigation of the detritivorous community and the complex relationship between soil microorganisms and earthworms is required. Our findings, however, imply that decomposition as well as consumption may be a function of earthworm abundance, rather than decomposer guild or functional group [58]. Therefore, our hypothesis that a higher functional earthworm diversity enhances mineralisation, cannot be verified.
The earthworms in general responded differently to the crop type and quality of the applied litter. Litter decomposition was subject to complex treatment-dependent temporal dynamics. Cup plant treatments showed much higher rates than maize treatments after 4 weeks, but remained at that level, while rates in maize treatments significantly increased after 8 weeks. Three reasons may be possible for this effect: (i) cup plant litter was collected from the soil surface, while maize litter only partially touching the soil was cut off the plant. Hence, the microbial pre-incubation effect on maize litter was less pronounced diminishing palatability for earthworms [58,59,60,61]. In addition, the litter in our experiment was surface applied, thus microbial attack and mineralisation was initially limited by contact and finally determined by earthworm-controlled incorporation and possibly feeding behaviour [59]. (ii) Maize litter contained substantial amounts of fibre and cup plant litter contained more than 15% lignin. These cell-wall components can hamper accessibility and palatability for earthworms because they require intricate hydrolytic enzymatic action and the presence of specific fungi for decomposition [62,63,64,65]. Lignin may also immobilise N contributing to (iii) N limitation [66]. It is broadly acknowledged that earthworms as well as soil microorganisms prefer N-rich food sources to meet their nutritional demands [27,58,65,67,68,69]. Litter in our study had wide C/N ratios (cup plant: 53.97; maize: 27.55) unfavourable to soil biota, which prefer C/N ratios well below 25 [69]. C/N ratios alone are unsuitable proxies for earthworm-controlled decomposition as studies have shown that the lignin content, phenolic compounds and parameters like litter toughness also play an important role [62,67,70,71]. We postulate that maize litter, even though it contained more N, had to be inoculated more thoroughly first, due to its fibre content and thickness, causing initially slower mineralisation. Cup plant litter, on the other hand, was collected from the soil surface, which implies that cup plant litter was already exposed to a longer, more thorough inoculation by soil microorganisms in the field. Therefore, cup plant litter exhibited higher decomposition and consumption rates despite the lower nutritional value. Regarding this and the duration of our experiment, we assume that the influence of the litter quality overlaid any beneficial effect of the combination of both decomposer guilds in the short-term, although long-term effects may not be excluded [57].
The overall low food quality and accessibility, which is known to trigger a shift in earthworm feeding behaviour and niche overlapping, may have increased inter- and intraspecies competition [55,57,72]. Behavioural changes became evident in the treatments with only one decomposer guild. In the 4-week experiment with cup plant litter and secondary decomposers, A. caliginosa and A. rosea appeared to inhibit decomposition rates, as decomposition rates in these treatments were even lower than in the control treatment without earthworms. A. caliginosa and A. rosea are geophages and usually feed on already decomposed organic matter within the soil [26]. Soil organic carbon levels of the soil, however, were low, which may have caused a shift to grazing on soil microorganisms, thereby inhibiting microbial-controlled decomposition [26,73,74]. In the later stages of incubation, the secondary decomposers started to slowly consume the offered litter on the surface as also mentioned by Jégou et al. [72] and Felten and Emmerling [75]. This behaviour is usually attributed to anecic earthworms like L. terrestris.
However, the overall earthworm activity promoted decomposition of the litter and presumably the recalcitrant residue due to priming effects. Priming effects are strong short-term changes in the turnover of the soil organic matter and may be caused by for e.g., the addition of organic C or the mechanical treatment of the soil [76]. Earthworms promote the comminution and mixing of the litter with the soil and add easily degradable substances like mucus activating microorganisms and triggering priming effects in hot spots [20,21,69,76,77,78,79]. Other than gradual mineralisation, priming effects are marked by peaks in microbial activity and decomposition that counteracts N immobilisation by enhancing the decay of recalcitrant residues via co-metabolism and N and C mining from soil organic matter [66,69,76,77,80]. Priming effects, however, were found to vary, depending on the quality and quantity of the added organic substances [79]. Due to the experimental duration and the addition of litter and earthworms, this study presumably observed priming effects. L. terrestris and A. caliginosa are both known to trigger this process [77,81], but effect size in relation to earthworm density, species and functional group association and substrate, requires further investigation. Apart from that, the rewetting of the used soil and plant biomass may have further added to the observed priming effects. Furthermore, it must be mentioned that both the decomposing plant biomass as well as the earthworms may alter pH levels in the short-term and, thus, affect enzyme activity and mineralisation [82,83]. These effects may be short-lived and require a more sophisticated and thorough investigation in further studies.
4.2. Cast Nutrients
Overall, cast nutrient levels after both incubations (4 and 8 weeks) were comparatively low. Even though substantially more N was introduced by the maize litter, the maximum total N accumulation in the casts was only 22.6 % higher (4-week treatments with primary decomposers) than the initial soil total N. The maximum Ncast accumulation in the cup treatments was 21.5% (8-week treatments with primary decomposers), while the means of Ccast and CAL-Pcast accumulation were almost twice and quadruple the amount found in casts in maize treatments, respectively. Small fractions of the introduced N may have been subject to volatilisation. Earthworms are able to enhance N2O emissions from the soil, but this process is heavily controlled by the amount of available N [84,85]. Considering the N limitation in our case, assimilation and competition were recognised as controlling factors for nutrient cycling, and we hypothesise that the N-limited system and low-quality litter led to competition of the earthworms, but also the soil microorganisms, and caused N-depleted casts and egestion of excess C and P. Hence, the higher Ccast and CAL-Pcast were found in the more N-limited cup plant treatment [27,69,70,86]. Therefore, our second hypothesis that the quality of the applied litter controls earthworm activity as well as the extent of mineralisation and nutrient release, was verified. These findings further imply that earthworms increase nutrient release only, when the amount of nutrients offered in the food source exceeds the amount needed for earthworm survival.
The C, N and P in casts allow for assumptions regarding the adaption of earthworm feeding behaviour. The Ccast was mostly higher in treatments with primary decomposer L. terrestris, since they derive most of their food from the applied litter, while the C in casts of secondary decomposers like A. caliginosa and A. rosea is typically lower, because they feed on soil with decayed organic matter, assimilate a portion of the ingested C and egest C-depleted casts [33,62]. It may be assumed, however, that secondary decomposers had to turn over more soil in relation to their body size in order to retrieve enough nutrients, since they could not immediately feed on the litter directly. Hence, the nutrient turnover per earthworm raw biomass was greater in the treatments with secondary decomposers. Nutrient limitation and adaption mechanisms like enhanced turnover became evident in both litter treatments. While N limitation was predominantly evident in cup plant treatments, in 8-week maize treatments with both decomposer guilds present, the N and C barely differed from the initial soil contents, thus implying competitive behaviour. In this case, endogeic secondary decomposers likely fed on the enriched burrow linings of the primary decomposer L. terrestris as a form of commensalism [75], and also both decomposer guilds ingest more soil, watering down the C and N contents in the casts [28,57,62,70,72,87]. Low cast nutrient levels and further N limitation may have been impaired by the fibre and alkaloid content, especially in the case of the lignin-rich cup plant litter [62,66].
The CAL-Pcast and Ccast correlated and higher CAL-P was found in the casts of the primary decomposers. A similar relationship was found by Van Groenigen et al. [30]. Their findings suggest that elevated CAL-Pcast was not only caused by litter decomposition and subsequent transformation of organic P, but also by earthworm-controlled incorporation of organic matter, which resulted in competition between the newly introduced organic compounds and P for sorption sites [30,88,89]. Inorganic P is rapidly immobilised by sorption and in microbial biomass, causing initially high contents of P in earthworm casts to decrease drastically during aging [30,31,34,88]. This makes its availability a crucial factor for plant growth. In this context, we assume that earthworm-controlled mineralisation and organic matter incorporation could play a key role by facilitating continuous P remobilisation and plant availability. Especially in low-input systems, i.e., no-till systems with low pesticide input, which depend on reliable nutrient cycling due to low external input, earthworms could help secure the provision of plant residue-derived nutrients [90]. Nevertheless, a study by Damon et al. [88] found that crop residues would only significantly affect plant-available P if applied in large amounts. Litter fall and earthworm activity in cup plant is much higher than in maize [14], but a more thorough investigation is needed to analyse the scope of this effect in a low-input soil–plant system with perennials.
Moreover, relationships between earthworms and soil microorganisms must be considered, as the latter competes for the available nutrients as well [27]. Their relationship and its effect on N and P availability, as well as soil organic matter decomposition, however, are highly complex [28,91]. The nature of this relationship is likely to be altered by the aforementioned priming effect that not only affects C and N cycling, but presumably also P cycling [76,79,80,92]. With this in mind, it must also be assumed that casts contain an unknown portion of nutrients that was not derived from litter, but from soil organic matter and microbial biomass [65,79]. While priming effects have to be distinguished from the process of gradual mineralisation, the C losses they induce from already C-depleted soil are problematic, because they may reduce the microbial decomposer community and thereby lead to the inhibition of decomposition [65,66]. In this context, the amplification of 13C tracer experiments on energy cropping systems could be a useful tool to examine the fate of soil organic matter, distinguish C sources and elucidate the role of priming effects contrary to gradual decomposition.
4.3. Applicability under Field Conditions
In our study, we used earthworm densities that exceeded field conditions to a certain extent. Emmerling et al. [13] found an average of 43 earthworms per m2 in maize and 90 earthworms per m2 in cup plant, while our earthworm numbers ranged from 105 to 421 earthworms per m2. Schorpp & Schrader [14] have noted that they found far more than the average 20 to 100 endogenics per m2 in some plots, but that was due to a high number of juveniles. Furthermore, the earthworms were restricted to limited space, which was mentioned above. Both of these circumstances imply that the effects of limitation may be less pronounced in the field. Nevertheless, we argue that limitation still may occur under field conditions and especially in low-input systems; hence, the implications of our findings may be transferable. Furthermore, defaunation of the soil may have affected the soil microorganisms and triggered C and M mineralisation. Even though freezing and thawing cycles occur in the field as well, temperature changes may be less drastic. However, the incubation started more than two weeks after defaunation and limitations of both entities were observed. It may be assumed that the equilibrium in the system may have been restored by the time of our experiment, hence minor effects of the procedure may be assumed in this case. Also, we conducted the experiment under the exclusion of plants and, due to defaunation, other soil fauna. Future studies may elucidate earthworm-controlled ecosystem services in energy cropping systems with respect to soil faunal diversity and plant and root growth.
5. Conclusions
While our findings show that the litter type affects the decomposition and nutrient turnover, functional earthworm diversity had no short-term beneficial effect. Nevertheless, we found evidence of enhanced nutrient turnover and inter- and intraspecies competition as a result of nutrient limitation. Furthermore, although earthworm-controlled service delivery was observed, ecosystem services depend on the respective framework of boundary conditions consisting of abiotic factors, such as soil moisture and temperature, but also on the availability of C and N and interactions with soil microorganisms. Earthworms may provide valuable ecosystem services and are capable of turning over large amounts of soil and, thus, nutrients. However, this is only the case when boundary conditions, such as nutrient levels, are beneficial. If boundary conditions are unsuitable, these services may turn into disservices. Our study hinted at the potential for earthworms to accelerate decomposition and nutrient turnover, but also indicated potential short-term C loss under N limiting conditions. A well-adapted organic fertilisation that allows for nutrient release and simultaneous C sequestration may aide this circumstance. Perennial energy crops like cup plant in fact provide advantageous conditions for soil biota due to no-till practices and early canopy closure [8,13,14], and there is a substantial number of other ecosystem services and disservices to be assessed in an appropriate setting. For instance, the asymmetrical organic matter input and C cycling in annual and perennial energy crops, as well as the response of soil biota, require longer and more thorough field investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture13020494/s1.
Author Contributions
L.W. and S.S. designed the experiment. L.W. performed the experiment, collected and evaluated the data. J.T. supervised the statistical evaluation. L.W. wrote the first manuscript draft. T.R., C.E., J.T. and S.S. commented on, reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fachagentur Nachwachsende Rohstoffe e.V. (FNR, Agency for Renewable Resources, Germany) through the project BESTLAND (FKZ: 22403618 and 22404217).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Sabine El Sayed, Marion Krause and Arne Heitkamp for their support in the laboratory. Furthermore, we thank the farmers for their permission to sample their sites as well as Andreas Kirch for support during sampling. Furthermore, we thank all reviewers for their constructive input.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herrmann, A. Biogas Production from Maize: Current State, Challenges and Prospects. 2. Agronomic and Environmental Aspects. BioEnergy Res. 2013, 6, 372–387. [Google Scholar] [CrossRef]
- Hijazi, O.; Munro, S.; Zerhusen, B.; Effenberger, M. Review of life cycle assessment for biogas production in Europe. Renew. Sustain. Energy Rev. 2016, 54, 1291–1300. [Google Scholar] [CrossRef]
- Lijó, L.; González-García, S.; Bacenetti, J.; Fiala, M.; Feijoo, G.; Lema, J.M.; Moreira, M.T. Life Cycle Assessment of electricity production in Italy from anaerobic co-digestion of pig slurry and energy crops. Renew. Energy 2014, 68, 625–635. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H. Others Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef] [PubMed]
- Schrama, M.; Vandecasteele, B.; Carvalho, S.; Muylle, H.; van der Putten, W.H. Effects of first- and second-generation bioenergy crops on soil processes and legacy effects on a subsequent crop. GCB Bioenergy 2016, 8, 136–147. [Google Scholar] [CrossRef]
- Ruf, T.; Gilcher, M.; Udelhoven, T.; Emmerling, C. Implications of Bioenergy Cropping for Soil: Remote Sensing Identification of Silage Maize Cultivation and Risk Assessment Concerning Soil Erosion and Compaction. Land 2021, 10, 128. [Google Scholar] [CrossRef]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff-Hönninger, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crops Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crops Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Mueller, A.L.; Berger, C.A.; Schittenhelm, S.; Stever-Schoo, B.; Dauber, J. Water availability affects nectar sugar production and insect visitation of the cup plant Silphium perfoliatum L. (Asteraceae). J. Agron. Crop Sci. 2020, 206, 529–537. [Google Scholar] [CrossRef]
- Schorpp, Q.; Müller, A.; Schrader, S.; Dauber, J. Agrarökologisches Potential der Durchwachsenen Silphie (Silphium perfoliatum L.) aus Sicht biologischer Vielfalt— Agro-ecological potential of the cup plant (Silphium perfoliatum L.) from a biodiversity perspective. J. Kulturpflanzen 2016, 68, 412–422. [Google Scholar] [CrossRef]
- Cumplido-Marin, L.; Graves, A.R.; Burgess, P.J.; Morhart, C.; Paris, P.; Jablonowski, N.D.; Facciotto, G.; Bury, M.; Martens, R.; Nahm, M. Two Novel Energy Crops: Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L.—State of Knowledge. Agron. J. 2020, 10, 928. [Google Scholar] [CrossRef]
- Grunwald, D.; Panten, K.; Schwarz, A.; Bischoff, W.A.; Schittenhelm, S. Comparison of maize, permanent cup plant and a perennial grass mixture with regard to soil and water protection. GCB Bioenergy 2020, 12, 694–705. [Google Scholar] [CrossRef]
- Emmerling, C.; Ruf, T.; Audu, V.; Werner, W.; Udelhoven, T. Earthworm communities are supported by perennial bioenergy cropping systems. Eur. J. Soil Biol. 2021, 105, 103331. [Google Scholar] [CrossRef]
- Schorpp, Q.; Schrader, S. Earthworm functional groups respond to the perennial energy cropping system of the cup plant (Silphium perfoliatum L.). Biomass Bioenergy 2016, 87, 61–68. [Google Scholar] [CrossRef]
- Van Capelle, C.; Schrader, S.; Brunotte, J. Tillage-induced changes in the functional diversity of soil biota—A review with a focus on German data. Eur. J. Soil Biol. 2012, 50, 165–181. [Google Scholar] [CrossRef]
- Schoo, B.; Wittich, K.P.; Böttcher, U.; Kage, H.; Schittenhelm, S. Drought Tolerance and Water-Use Efficiency of Biogas Crops: A Comparison of Cup Plant, Maize and Lucerne-Grass. J. Agron. Crop Sci. 2017, 203, 117–130. [Google Scholar] [CrossRef]
- Andriuzzi, W.S.; Pulleman, M.M.; Schmidt, O.; Faber, J.H.; Brussaard, L. Anecic earthworms (Lumbricus terrestris) alleviate negative effects of extreme rainfall events on soil and plants in field mesocosms. Plant Soil 2015, 397, 103–113. [Google Scholar] [CrossRef]
- Capowiez, Y.; Cadoux, S.; Bouchant, P.; Ruy, S.; Roger-Estrade, J.; Richard, G.; Boizard, H. The effect of tillage type and cropping system on earthworm communities, macroporosity and water infiltration. Soil Tillage Res. 2009, 105, 209–216. [Google Scholar] [CrossRef]
- Ernst, G.; Felten, D.; Vohland, M.; Emmerling, C. Impact of ecologically different earthworm species on soil water characteristics. Eur. J. Soil Biol. 2009, 45, 207–213. [Google Scholar] [CrossRef]
- Bertrand, M.; Barot, S.; Blouin, M.; Whalen, J.; de Oliveira, T.; Roger-Estrade, J. Earthworm services for cropping systems. A review. Agron. Sustain. Dev. 2015, 35, 553–567. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, L.E.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Lubbers, I.M.; Pulleman, M.M.; Van Groenigen, J.W. Can earthworms simultaneously enhance decomposition and stabilization of plant residue carbon? Soil Biol. Biochem. 2017, 105, 12–24. [Google Scholar] [CrossRef]
- Agostini, F.; Gregory, A.S.; Richter, G.M. Carbon Sequestration by Perennial Energy Crops: Is the Jury Still Out? BioEnergy Res. 2015, 8, 1057–1080. [Google Scholar] [CrossRef]
- Chimento, C.; Almagro, M.; Amaducci, S. Carbon sequestration potential in perennial bioenergy crops: The importance of organic matter inputs and its physical protection. GCB Bioenergy 2016, 8, 111–121. [Google Scholar] [CrossRef]
- Don, A.; Steinberg, B.; Schöning, I.; Pritsch, K.; Joschko, M.; Gleixner, G.; Schulze, E.D. Organic carbon sequestration in earthworm burrows. Soil Biol. Biochem. 2008, 40, 1803–1812. [Google Scholar] [CrossRef]
- Lee, K.E. Earthworms: Their Ecology and Relationships with Soils and Land Use; Academic Press Inc.: Sydney, Australia, 1985. [Google Scholar]
- Ernst, G.; Henseler, I.; Felten, D.; Emmerling, C. Decomposition and mineralization of energy crop residues governed by earthworms. Soil Biol. Biochem. 2009, 41, 1548–1554. [Google Scholar] [CrossRef]
- Postma-Blaauw, M.B.; Bloem, J.; Faber, J.H.; van Groenigen, J.W.; de Goede, R.G.M.; Brussaard, L. Earthworm species composition affects the soil bacterial community and net nitrogen mineralization. Pedobiologia 2006, 50, 243–256. [Google Scholar] [CrossRef]
- Laossi, K.R.; Ginot, A.; Noguera, D.C.; Blouin, M.; Barot, S. Earthworm effects on plant growth do not necessarily decrease with soil fertility. Plant Soil 2010, 328, 109–118. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Van Groenigen, K.J.; Koopmans, G.F.; Stokkermans, L.; Vos, H.M.J.; Lubbers, I.M. How fertile are earthworm casts? A meta-analysis. Geoderma 2019, 338, 525–535. [Google Scholar] [CrossRef]
- Decaëns, T.; Rangel, A.F.; Asakawa, N.; Thomas, R.J. Carbon and nitrogen dynamics in ageing earthworm casts in grasslands of the eastern plains of Colombia. Biol. Fertil. Soils 1999, 30, 20–28. [Google Scholar] [CrossRef]
- Graff, O. Stickstoff, Phosphor und Kalium in der Regenwurmlosung auf der Wiesenversuchsfläche des Sollingprojektes. Ann. Zool. 1971, 4, 503–512. [Google Scholar]
- Abail, Z.; Sampedro, L.; Whalen, J.K. Short-term carbon mineralization from endogeic earthworm casts as influenced by properties of the ingested soil material. Appl. Soil Ecol. 2017, 116, 79–86. [Google Scholar] [CrossRef]
- Scheu, S. Microbial activity and nutrient dynamics in earthworm casts (Lumbricidae). Biol. Fertil. Soils 1987, 5, 230–234. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- ISO 11277; ISO 11277:1998/Cor 1:2002 Soil Quality—Determination of Particle Size Distribution in Mineral soil Material—Method by Sieving and Sedimentation. International Organization for Standardization (ISO): Geneva, Switzerland, 2002. Available online: https://www.iso.org/standard/36291.html (accessed on 16 December 2020).
- Fründ, H.C.; Butt, K.; Capowiez, Y.; Eisenhauer, N.; Emmerling, C.; Ernst, G.; Potthoff, M.; Schädler, M.; Schrader, S. Using earthworms as model organisms in the laboratory: Recommendations for experimental implementations. Pedobiologia 2010, 53, 119–125. [Google Scholar] [CrossRef]
- VDLUFA, Methode A 6.2.1.1, Bestimmung von Phosphor und Kalium im Calcium-Acetat-Lactat-Auszug. In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch); Bd. I Die Untersuchung von Böden; 4. Aufl., 6. Teillfg. 2012; VDLUFA-Verlag: Darmstadt, Germany, 2012.
- ISO 11885; Water quality—Determination of selected elements by inductively coupled plasma optical emission spectrometry (ICP-OES). International Organization for Standardization (ISO): Geneva, Switzerland, 2007. Available online: https://www.iso.org/standard/36250.html (accessed on 16 December 2020).
- ISO 10390; Soil quality—Determination of pH. International Organization for Standardization (ISO): Geneva, Switzerland, 2005. Available online: https://www.iso.org/standard/40879.html#:~:text=ISO%2010390%3A2005%20specifies%20an,(pH%20in%20CaCl2) (accessed on 16 December 2020).
- VDLUFA, Methode A 6.5.1, Bestimmung der Neutral-Detergenzien-Faser nach Amylasebehandlung (aNDF) sowie nach Amylasebehandlung und Veraschung (aNDFom). In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III Die chemische Untersuchung von Futtermitteln; 3. Aufl., 8. Erg. 2012; VDLUFA-Verlag: Darmstadt, Germany, 2012.
- VDLUFA (2012c) Methode A 6.5.2, Bestimmung der Säure-Detergenzien-Faser (ADF) und der Säure-Detergenzien-Faser nach Veraschung (ADFom). In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III Die chemische Untersuchung von Futtermitteln; 3. Aufl., 8. Erg. 2012; VDLUFA-Verlag: Darmstadt, Germany, 2012.
- VDLUFA, Methode A 6.5.3, Bestimmung des Säure-Detergenzien-Lignins (ADL). In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III Die chemische Untersuchung von Futtermitteln; 3. Aufl., 8. Erg. 2012; VDLUFA-Verlag: Darmstadt, Germany, 2012.
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Daniel, O. Leaf-litter consumption and assimilation by juveniles of Lumbricus terrestris L. (Oligochaeta, Lumbricidae) under different environmental conditions. Biol. Fertil. Soils 1991, 12, 202–208. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.1. 2020. Available online: www.training.cochrane.org/handbook (accessed on 16 December 2020).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 29 June 2020).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 29 June 2020).
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.5.2-1. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 29 June 2020).
- De Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research. R package version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 29 June 2020).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346––363. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. Ggpubr: ’Ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 29 June 2020).
- Lowe, C.; Butt, K. Interspecific interactions between earthworms: A laboratory-based investigation. Pedobiologia 1999, 43, 808–817. [Google Scholar]
- Eriksen-Hamel, N.S.; Whalen, J.K. Competitive interactions affect the growth of Aporrectodea caliginosa and Lumbricus terrestris (Oligochaeta: Lumbricidae) in single- and mixed-species laboratory cultures. Eur. J. Soil Biol. 2007, 43, 142–150. [Google Scholar] [CrossRef]
- Uvarov, A.V. Inter- and intraspecific interactions in lumbricid earthworms: Their role for earthworm performance and ecosystem functioning. Pedobiologia 2009, 53, 1–27. [Google Scholar] [CrossRef]
- Huang, W.; González, G.; Zou, X. Earthworm abundance and functional group diversity regulate plant litter decay and soil organic carbon level: A global meta-analysis. Appl. Soil Ecol. 2020, 150, 103473. [Google Scholar] [CrossRef]
- Cortez, J.; Bouché, M.B. Field decomposition of leaf litters: Earthworm–microorganism interactions—The ploughing-in effect. Soil Biol. Biochem. 1998, 30, 795–804. [Google Scholar] [CrossRef]
- Lavelle, P.; Lattaud, C.; Trigo, D.; Barois, I. Mutualism and biodiversity in soils. Plant Soil 1995, 170, 23–33. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; Blackwell: Oxford, UK, 1979. [Google Scholar]
- Buck, C.; Langmaack, M.; Schrader, S. Nutrient content of earthworm casts influenced by different mulch types. Eur. J. Soil Biol. 1999, 35, 23–30. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; de la Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Vidal, A.; Watteau, F.; Remusat, L.; Mueller, C.W.; Nguyen Tu, T.T.; Buegger, F.; Derenne, S.; Quenea, K. Earthworm Cast Formation and Development: A Shift From Plant Litter to Mineral Associated Organic Matter. Front. Environ. Sci. 2019, 7, 55. [Google Scholar] [CrossRef]
- McDaniel, M.D.; Grandy, A.S.; Tiemann, L.K.; Weintraub, M.N. Crop rotation complexity regulates the decomposition of high and low quality residues. Soil Biol. Biochem. 2014, 78, 243–254. [Google Scholar] [CrossRef]
- Abail, Z.; Whalen, J.K. Corn residue inputs influence earthworm population dynamics in a no-till corn-soybean rotation. Appl. Soil Ecol. 2018, 127, 120–128. [Google Scholar] [CrossRef]
- Bohlen, P.J.; Parmelee, R.W.; McCartney, D.A.; Edwards, C.A. Earthworm effects on carbon and nitrogen dynamics of surface litter in corn agroecosystems. Ecol. Appl. 1997, 7, 1341–1349. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Muhammad, S.; Zhou, A.; Hao, R.; Wu, Y. How do earthworms affect decomposition of residues with different quality apart from fragmentation and incorporation? Geoderma 2018, 326, 68–75. [Google Scholar] [CrossRef]
- Flegel, M.; Schrader, S. Importance of food quality on selected enzyme activities in earthworm casts (Dendrobaena octaedra, Lumbricidae). Soil Biol. Biochem. 2000, 32, 1191–1196. [Google Scholar] [CrossRef]
- Patoine, G.; Thakur, M.P.; Friese, J.; Nock, C.; Hönig, L.; Haase, J.; Scherer-Lorenzen, M.; Eisenhauer, N. Plant litter functional diversity effects on litter mass loss depend on the macro-detritivore community. Pedobiologia 2017, 65, 29–42. [Google Scholar] [CrossRef]
- Jégou, D.; Capowiez, Y.; Cluzeau, D. Interactions between earthworm species in artificial soil cores assessed through the 3D reconstruction of the burrow systems. Geoderma 2001, 102, 123–137. [Google Scholar] [CrossRef]
- Butenschön, O. Regulation of Soil organic Matter Dynamics and Microbial Activity by Endogeic Earthworms. Dissertation, Degree: Dr rer nat (doctor rerum naturalium) ; Technische Universität Darmstadt: Darmstadt, Germany, 2007. Available online: http://elib.tu-darmstadt.de/diss/000820 (accessed on 9 February 2021).
- Song, K.; Sun, Y.; Qin, Q.; Sun, L.; Zheng, X.; Terzaghi, W.; Lv, W.; Xue, Y. The Effects of Earthworms on Fungal Diversity and Community Structure in Farmland Soil with Returned Straw. Front. Microbiol. 2020, 11, 594265. [Google Scholar] [CrossRef] [PubMed]
- Felten, D.; Emmerling, C. Earthworm burrowing behaviour in 2D terraria with single-and multi-species assemblages. Biol. Fertil. Soils 2009, 45, 789–797. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Maiorov, E.I.; Orlova, N.E. The priming effects induced by earthworm mucus on mineralization and humification of plant residues. Eur. J. Soil Biol. 2012, 50, 1–6. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Bernard, L.; Chapuis-Lardy, L.; Razafimbelo, T.; Razafindrakoto, M.; Pablo, A.L.; Legname, E.; Poulain, J.; Brüls, T.; O’Donohue, M.; Brauman, A.; et al. Endogeic earthworms shape bacterial functional communities and affect organic matter mineralization in a tropical soil. ISME J. 2012, 6, 213–222. [Google Scholar] [CrossRef]
- Hoang, D.T.T.; Bauke, S.L.; Kuzyakov, Y.; Pausch, J. Rolling in the deep: Priming effects in earthworm biopores in topsoil and subsoil. Soil Biol. Biochem. 2017, 114, 59–71. [Google Scholar] [CrossRef]
- Schrader, S. Influence of earthworms on the pH conditions of their environment by cutaneous mucus secretion. Zool. Anz. 1994, 233, 211–219. [Google Scholar]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 5794869. [Google Scholar] [CrossRef]
- Lubbers, I.M.; González, E.L.; Hummelink, E.W.J.; Van Groenigen, J.W. Earthworms can increase nitrous oxide emissions from managed grassland: A field study. Agric. Ecosyst. Environ. 2013, 174, 40–48. [Google Scholar] [CrossRef]
- Lubbers, I.M.; van Groenigen, K.J.; Fonte, S.; Six, J.; Brussaard, L.; Van Groenigen, J.W. Greenhouse gas emissions from soils increased by earthworms. Nat. Clim. Change 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Chang, C.H.; Szlavecz, K.; Buyer, J.S. Species-specific effects of earthworms on microbial communities and the fate of litter-derived carbon. Soil Biol. Biochem. 2016, 100, 129–139. [Google Scholar] [CrossRef]
- Frazão, J.; de Goede, R.G.M.; Capowiez, Y.; Pulleman, M.M. Soil structure formation and organic matter distribution as affected by earthworm species interactions and crop residue placement. Geoderma 2019, 338, 453–463. [Google Scholar] [CrossRef]
- Damon, P.M.; Bowden, B.; Rose, T.; Rengel, Z. Crop residue contributions to phosphorus pools in agricultural soils: A review. Soil Biol. Biochem. 2014, 74, 127–137. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Ruf, T.; Emmerling, C. Different life-form strategies of perennial energy crops and related nutrient exports require a differentiating view specifically concerning a sustainable cultivation on marginal land. GCB Bioenergy 2021, 13, 893–904. [Google Scholar] [CrossRef]
- Bohlen, P.J.; Parmelee, R.W.; Allen, M.F.; Ketterings, Q.M. Differential Effects of Earthworms on Nitrogen Cycling from Various Nitrogen-15-Labeled Substrates. Soil Sci. Soc. Am. J. 1999, 63, 882–890. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).