Abstract
Aim: This study aimed to compare mortality risks across uric acid (UA) levels between non-diabetes adults and participants with diabetes and to investigate the association between hyperuricemia and mortality risks in low-risk adults. Methods: We analyzed data from adults aged >18 years without coronary heart disease and chronic kidney disease (n = 29,226) from the National Health and Nutrition Examination Survey (1999–2010) and the associated mortality data (up to December 2011). We used the Cox proportional hazards models to examine the risk of all-cause and cause-specific (cardiovascular disease (CVD) and cancer) mortality at different UA levels between adults with and without diabetes. Results: Over a median follow-up of 6.6 years, 2069 participants died (495 from CVD and 520 from cancers). In non-diabetes adults at UA ≥ 5 mg/dL, all-cause and CVD mortality risks increased across higher UA levels (p-for-trend = 0.037 and 0.058, respectively). The lowest all-cause mortality risk in participants with diabetes was at the UA level of 5–7 mg/dL. We set the non-diabetes participants with UA levels of <7 mg/dL as a reference group. Without considering the effect of glycemic control, the all-cause mortality risk in non-diabetes participants with UA levels of ≥7 mg/dL was equivalent to risk among diabetes adults with UA levels of <7 mg/dL (hazard ratio = 1.44 vs. 1.57, p = 0.49). A similar result was shown in CVD mortality risk (hazard ratio = 1.80 vs. 2.06, p = 0.56). Conclusion: Hyperuricemia may be an indicator to manage multifaceted cardiovascular risk factors in low-risk adults without diabetes, but further studies and replication are warranted.
1. Introduction
Studies in adults of various ethnicities reported that gout was associated with a higher risk of death from all causes and cardiovascular disease (CVD) [,]. Hyperuricemia is an important factor of gout, but it is to be determined whether hyperuricemia is a causal risk factor or just a marker for mortality. In the United States, hyperuricemia prevalence was >20% in both men and women (definition of hyperuricemia: Serum urate level of >7.0 mg/dL in men and >5.7 mg/dL in women) [], even higher than diabetes prevalence. Asymptomatic hyperuricemia was reported to be associated with systemic inflammatory markers [] and could even predict higher C-reactive protein levels when tested during a period of three years []. It has been demonstrated that gout may induce refractory inflammation that contributes to CVD [], similar to inflammation that induces atherosclerosis change in rheumatoid arthritis [] and systemic lupus erythematosus []. However, it was not evident that urate-lowering therapy (ULT) could sufficiently lower CVD risk [] or reduce the risk of decline of renal function in patients with chronic kidney disease (CKD) and hyperuricemia [,].
Mortality trends have changed in diabetes patients and there was no difference in cardiac death between participants with and without diabetes during 2005–2010 in the United States []. However, the proportion of cardiac mortality in people without diabetes did not decrease as it did with the diabetes population []. Hyperuricemia, which can be affected by diet, life habits, and genetic factors, is a problem similar to hyperglycemia. Although hyperuricemia is a potential risk factor/marker for mortality, it is not suggested to be controlled aggressively like diabetes, hyperlipidemia, or hypertension in clinical guidelines [,].
In this study, we compared mortality risks between participants with and without diabetes across different uric acid (UA) levels. By comparing mortality risks between non-diabetes adults and participants with diabetes, we aimed to investigate the potential role of hyperuricemia associated with mortality risks in adults without diabetes.
2. Materials and Methods
2.1. Data Source and Study Population
The National Health and Nutrition Examination Survey (NHANES) data was constructed by the National Center for Health Statistics (NCHS). The NHANES examinations included a standardized medical examination and questionnaires to address different health-related records. We analyzed data from a variety of records within NHANES (1999–2010, n = 62,160) and their linked National Death Index mortality data (through 31 December 2011). The participants without data of serum UA levels were excluded. Furthermore, the participants with CKD or coronary heart disease (CHD) were excluded. The definition of CKD was impaired glomerular filtration rate (GFR) of <60 ml/min/1.73 m2. Estimated GFR was calculated using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation []. The participants were classified into having CHD if they answered yes to the following question: Have you ever been told you had coronary heart disease? In total, 29,226 participants aged ≥18 years were included (Supplementary Figure S1).
2.2. Definition of Diabetes
There are only 29,174 participants with glycated hemoglobin (HbA1c) data enrolled in this study. The participants who self-reported a physician’s diagnosis of diabetes or self-reported taking insulin or diabetic pills were categorized as having diabetes mellitus (DM). Overall, 2506 participants were classified as having diabetes. The type of DM was not accounted for adjustment or exclusion.
2.3. Mortality Outcomes
Mortality outcomes of interest in this study include all-cause mortality, cardiovascular death, and cancer death, based on ICD-10 (10th revision of the International Statistical Classification of Diseases and Related Health Problems) codes defined in NHANES. To identify causes of death in participants, the specific codes were the following: I00–I09, I11, I13, I20–I51, and I60–I69 were categorized as cardiovascular death, while the codes of C00–C97 were causes of death from malignant neoplasms (cancer death). Mortality status for NCHS survey participants was ascertained primarily through probabilistic record matching with the National Death Index (NDI) death certificate records [].
2.4. Statistical Analysis
We utilized chi-square and analysis of variance (ANOVA) tests to examine significant differences in baseline demographics and characteristics across levels of uric acid and diabetes status. Cox proportional hazards regression models were used to compare the hazard ratios (HRs) and 95% CI (confidence interval) for the association of UA levels with all-cause, cardiovascular, and cancer mortality, separately in participants with and without DM. Additionally, we set the non-diabetes participants with UA levels of 5–7 mg/dL as the reference, which allowed mortality risks at every UA category in non-diabetes and diabetes groups to be compared. Similarly, different UA categorizations were set (<7, 7–9, ≥9, and <7, ≥7) in order to compare different mortality risks across UA levels between non-diabetes and diabetes groups. In addition, age, sex, race/ethnicity, body mass index (BMI), high-density lipoprotein cholesterol (HDL-C), systolic blood pressure (SBP), creatinine, and smoking state were adjusted in all survival analyses. All variables were from baseline data. Due to the complex survey design of the NHANES study, all analyses were adequately weighted to represent the US population. The weighted data were calculated according to analytic guidelines []. The un-weighted HRs were also presented as sensitivity analysis. All analyses were conducted using the Statistical Analysis System survey procedures (SAS version 9.4, 2013, Cary, NC, USA). p-values < 0.05 were considered statistically significant.
3. Results
To determine if elevated levels of uric acid are a risk factor for diabetes mellitus, we analyzed uric acid levels in both diabetic and non-diabetic adults. Table 1 and Table 2 document the characteristics of the subjects according to diabetic status and uric acid level at baseline, respectively. In the overall population, non-diabetes participants were associated with younger age (43.4 ± 0.2 vs. 56.7 ± 0.4, p < 0.001), lower prevalence of cancers (7.1% vs. 12.5%, p < 0.001), and hypertension (23.2% vs. 61.2%, p < 0.001), lower BMI (27.9 ± 0.1 vs. 32.5 ± 0.2, p < 0.001), lower SBP (121 ± 0.2 mmHg versus 131 ± 0.7 mmHg, p < 0.001), higher HDL-C (53.2 ± 0.2 mg/dL vs. 48.2 ± 0.4 mg/dL, p < 0.001), higher total cholesterol (199.6 ± 0.4 mg/dL vs. 196.9 ± 1.5 mg/dL, p < 0.001), and triglyceride (143.5 ± 1.2 mg/dL vs. 205.2 ± 7.1 mg/dL, p < 0.001). In non-diabetes participants, there were older age, higher BMI, higher prevalence of hypertension, lower HDL-C, higher total cholesterol, higher triglyceride, higher fasting blood glucose, and higher serum creatinine levels across higher UA categories (Table 2). Diabetes participants, however, consisted of more non-Hispanic Black participants, and were associated with older age, higher BMI, lower HDL-C, higher triglyceride, higher fasting blood glucose, and higher serum creatine levels across higher UA categories. In both the non-diabetes and diabetes groups, the cancer prevalence was insignificantly different across UA categories (p-value = 0.31 and 0.91, respectively).

Table 1.
Baseline characteristics by diabetic status.

Table 2.
Baseline characteristics by uric acid levels in non-DM and DM groups.
The median follow-up period was 6.6 years. Among those participants who died (n = 2069, 7.9 per 1000 person-years), 495 (23.9%) died due to CVD, and 520 (25.1%) died due to cancer. Table 3 shows the adjusted un-weighted and weighted all-cause and cause-specific mortality risks across UA levels, separately for adults without diabetes and with diabetes. Different models are shown in Supplementary Table S1. For non-diabetes persons, when serum UA levels are above 5 mg/dL, the weighted HRs for all-cause mortality and CVD mortality increase significantly across higher UA levels (p-for-trend = 0.003 and 0.058, respectively). Among participants with diabetes, the lowest weighted all-cause mortality risk is at the UA level of 5–7 mg/dL. Regarding CVD mortality risk in participants with diabetes at different UA levels, no significant difference is found between UA levels of <5 mg/dL and 5–7 mg/dL, but the highest HR (2.53, 95% CI 1.18–5.41) is at UA levels of 7–9 mg/dL, when compared to UA levels of 5–7 mg/dL. Compared to the mortality risk at UA level of 5–7 mg/dL in non-diabetes adults, HRs at every UA category in non-diabetes and diabetes groups are shown in Table 4 and Supplementary Table S2. At UA levels of 5–7 mg/dL, CVD mortality risk is significantly higher (HR = 2.25, 95% CI 1.25–4.06) in participants with diabetes, but all-cause and cancer mortality risks are not. The significantly higher HRs for all-cause mortality risk in non-diabetes adults at UA levels of 7–9 mg/dL and ≥9 mg/dL are 1.40 and 2.35, respectively. In participants with diabetes, HRs for all-cause mortality risk at UA levels of <5 mg/dL, 7–9 mg/dL, and ≥9 mg/dL are significantly higher at 2.01, 2.87, and 2.79, respectively. CVD mortality risk in non-diabetes adults with UA levels of ≥9 mg/dL approximates the risk at UA levels of 7–9 mg/dL in diabetes patients (HR = 5.07 versus 4.99) and is even higher than those with diabetes and UA levels of 5–7 mg/dL (HR = 5.07 versus 2.25). However, the HRs for cancer-specific mortality did not differ significantly across UA levels among non-diabetes and diabetes participants.

Table 3.
Relative HRs (95% CI) compared to the group with UA level of 5–7 mg/dL for the association between mortality risks and uric acid levels among adults without diabetes and with diabetes.

Table 4.
Relative HRs (95% CI) of mortality risks compared with non-diabetes participants with UA 5–7 mg/dL among participants without diabetes and with diabetes.
Figure 1a shows the weighted all-cause mortality risk after adjustment across UA levels in both non-diabetes and diabetes groups. It indicates that all-cause mortality risks in non-diabetes adults with UA levels of 7–9 mg/dL and ≥9 mg/dL are equivalent to the risk in those with diabetes and a UA level of <7 mg/dL (p = 0.33 and 0.17, respectively). The non-diabetes participants with a UA level of ≥9 mg/dL even have approximate risk to participants with diabetes with a UA level of 7–9 mg/dL or ≥9 mg/dL (p = 0.49 and 0.72, respectively). Figure 1b exhibits the comparison of weighted risk of CVD death after adjustment across UA levels between non-diabetes and diabetes groups. The CVD mortality risk in non-diabetes participants with a UA level of ≥9 mg/dL is comparable to the risk in those with diabetes and UA levels of 7–9 mg/dL (p = 0.98). The CVD mortality risks in non-diabetes participants with UA levels of 7–9mg/dl and diabetes participants with UA level of <7 mg/dl are nearly the same (HR = 1.63 versus 2.06, p = 0.31). Both groups have a significantly higher risk than non-diabetes participants with a UA level of <7 mg/dL (p = 0.008 and 0.002, respectively).
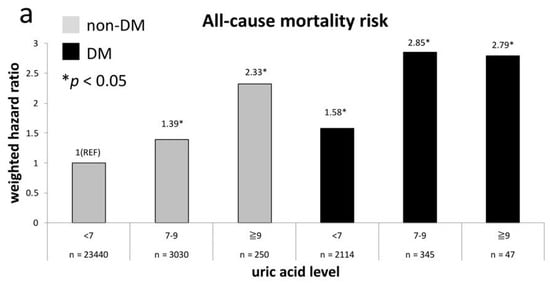
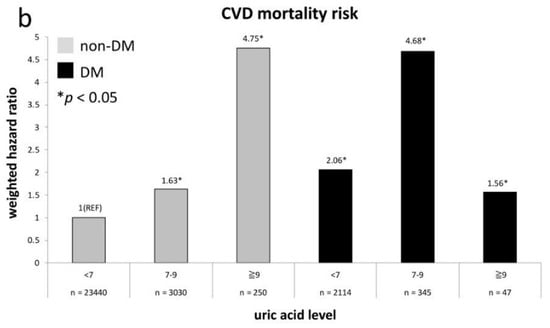
Figure 1.
All-cause (a) and CVD (b) mortality risks at every uric acid (UA) category compared to non-diabetes mellitus (DM) participants with UA level of <7 mg/dL.
The HRs regarding risks of all-cause death and CVD death in relation to UA levels (UA < 7 mg/dL and ≥7 mg/dL) and history of DM at baseline are exhibited in Figure 2. The comparative reference is also non-diabetes participants with a UA level of <7 mg/dL. We demonstrate that all-cause mortality risks in another three groups are all significantly higher than the reference group (Figure 2a) and the risk is equivalent between non-diabetes adults with a UA level of ≥7 mg/dL and diabetes adults with a UA level of <7 mg/dL (p = 0.48). Figure 2b exhibits the similar results in terms of CVD mortality risks.
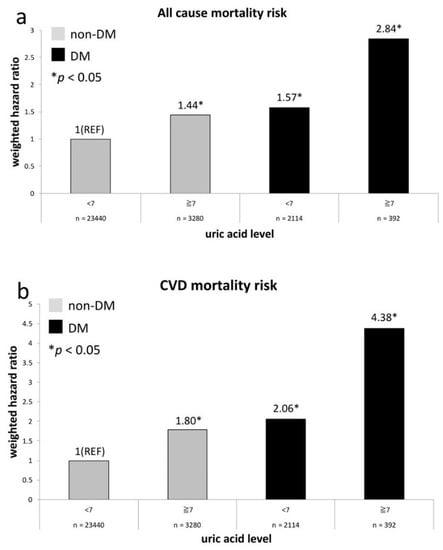
Figure 2.
All-cause (a) and CVD (b) mortality risks between diabetic and non-diabetic groups compared with non-DM participants of UA level <7 mg/dL.
4. Discussion
In this study, all participants were without chronic kidney disease or coronary heart disease at baseline. The results demonstrate that the mortality risks in non-diabetes adults are significantly higher at UA level of ≥7.0 mg/dL and all-cause and CVD mortality risks in non-diabetes participants with a UA level of ≥9.0 mg/dL are approximate to, or even higher than, those with diabetes and a UA level of <9.0 mg/dL. Hyperuricemia was associated with a statistically significant increased risk of CVD and all-cause mortality but did not exhibit a significant increase in terms of cancer mortality risk. Similar results have been reported [,], but a comparison between hyperuricemia and diabetes status is not yet elucidated. In the present study, Table 4 and Figure 1 and Figure 2 show the joint effects of DM and different UA level.
We found that the trend of all-cause mortality risk across higher UA levels is similar to CVD mortality risk in non-diabetes adults. However, the major difference between the current study and other reports from South Korea [] and Japan [] are that both the Asian data show a U-shaped association between mortality risks and UA level. The lowest studied UA levels were further lower than the present study. Furthermore, diabetic status was not addressed in the two studies. Low UA level may be associated with malnutrition [] and weak antioxidant protection from endothelial injury and oxidative stress [,]. The more critical issue is whether non-diabetes adults with hyperuricemia have a mortality risk comparable to that of diabetes adults. If this is true, it suggests a similar baseline risk of CVD mortality, and management of cardiovascular risk factors in adults with hyperuricemia may be similar to diabetes patients. Our data indicate all-cause and CVD mortality risks elevated across UA levels in non-diabetes adults, but the U-shaped relationship between UA level and all-cause mortality is reflected in the participants with diabetes (Table 3). It is still debated whether hyperuricemia is an independent risk factor for CVD or merely associated with other risk factors, including hypertension [], renal disease [], hyperlipidemia [], and diabetes []. In patients with gout, ULT would be cost-effective []. In adults with asymptomatic hyperuricemia, routine ULT is not recommended in current guidelines [,]. In the Losartan Intervention For Endpoint reduction in hypertension (LIFE) trial, major CV protection was derived from inhibition of the renin-angiotensin-aldosterone system, despite the UA-lowering effect of Losartan explaining 29% of the positive effect on the primary composite end points of cardiovascular death, myocardial infarction, and stroke []. Inflammation and oxidative stress might be causes of higher mortality risks associated with hyperuricemia [,,]. In healthy people, sodium-glucose-linked transporter 2 inhibitor with cardiovascular benefit was reported to increase urate excretion contrary to loop-diuretic [], but current evidence could not tell us when and how starting ULT is helpful to CV risk in hyperuricemic adults. Although asymptomatic hyperuricemia was associated with increased risk of CKD [,], which has been found to increase all-cause and CVD mortality risks [], ULT could not prevent kidneys from developing CKD with single-faceted treatment in people with normal renal function. In contrast, in patients with high low-density lipoprotein cholesterol, statin therapy can reduce mortality risk [,], no matter whether in diabetes or non-diabetes patients []. Management of risk factors of CKD progression and CVD in hyperuricemic adults should not only focus on reduction of serum UA level, but also on the potency of multifaceted risk control, similar to what clinicians have done for diabetes population.
Fang and Alderman have reported that ischemic heart disease mortality rate was 8.14/1000 person-years in males with a UA level of >7.0 mg/dL, who had no myocardial infarction, stroke, or gout at baseline []. In middle-aged men without history of CVD, followed up for 17 years, the CV mortality rate was 10.3/1000 person-years in participants with gout, implying an approximately 30% greater risk than those without gout (8.0/1000 person-years) []. In the trial comparing cardiovascular safety of febuxostat and allopurinol in patients with gout and a history of major cardiovascular disease, overall CV mortality rate of 14.2/1000 person-years was published []. In this study, the all-cause mortality and CV mortality in non-diabetes adults at a UA level of ≥9.0 mg/dL are 19.4/1000 person-years and 5.3/1000 person-years, respectively. The mortality risks are comparable to those with diabetes and a UA level of <7.0 mg/dL (20.4/1000 person-years and 5.1/1000 person-years). Exclusion of kidney disease and coronary history at baseline, as well as broader use of statins and angiotensin-converting-enzyme inhibitors/angiotensin II receptor blockers after the 2000s than in the 1990s, may be the causes of the lower proportion of CVD mortality in the present study. Although diabetes population had reduced CV mortality from >40% during 1999–2004 to approximately 20% during 2005–2010, the non-DM population had an increased proportion of cardiac death []. These results indicate that aggressive management of CV risk factors in hyperuricemic adults should be more important than only focusing on the effects of ULT. This study extends previous findings to further support that the mortality risk between diabetes and non-diabetes adults with high UA levels is similar, and so the same comprehensive treatment to manage other CV risk factors in both groups is reasonable.
Certain limitations deserve a mention. First, the time-varying changes in UA levels during the follow-up period of 6.6 years were not considered in this study. This was limited by the cross-sectional structure of NHANES data. In this kind of study, it is difficult to differentiate whether UA is just a marker or a leading cause of mortality. Second, the much smaller participant number in the diabetes group with a UA level of ≥9 mg/dL may produce biased change in mortality analyses across UA categories. Third, glycemic control may affect mortality risk, but glycated hemoglobin level was unable to be collected in all participants in NHANES data. Although indexes of glycemic control were not considered in this study, both CVD mortality and all-cause mortality were higher in participants with diabetes than in the non-diabetes group. One of the strengths of this study is due to it being the first study to the best of our knowledge, to compare the mortality risk across UA categories between diabetes and non-diabetes adults. Additionally, we completed the preliminary and multiethnic study to examine the mortality risk based on the nationally representative data in the United States. We utilized the large sample size to examine the relative HRs across subgroups where mortality risk is varying across UA categories. Furthermore, the participants without diabetes, CKD, and CHD in this study generally had the lowest mortality risk, and mortality risks in these adults are easily neglected in the setting of a clinical trial design. The epidemiological results in this study provided the material to examine the impact of hyperuricemia on these lowest-risk adults.
In conclusion, we have shown that non-diabetes adults with a UA level of ≥7 mg/dL, who have not had CHD or CKD, have all-cause and CVD mortality risks similar to those among diabetes participants with a UA level of <7 mg/dL. This result may suggest that low-risk adults with hyperuricemia (UA level ≥ 7.0 mg/dL) could be managed as if they have DM. Adopting a more aggressive strategy to prevent mortality risk factors may be beneficial for adults with hyperuricemia. We propose that a stricter clinical trial to investigate the effects of comprehensive management of cardiovascular risk factors on hyperuricemic adults with lower risk should be conducted.
5. Conclusions
In summary, higher UA level of ≥7 mg/dL in the low-risk non-DM adults may indicate clinicians to address the multifaceted risk factors because their all-cause and CVD mortality risks would be similar to those with diabetes.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/8/12/2127/s1, Figure S1: Flowchart of enrollment of study population, Table S1: Stepwise analysis of relative HRs (95%CI) for the association between mortality risks and uric acid levels among adults without diabetes and with diabetes., Table S2: Stepwise analysis of relative HRs (95%CI) of mortality risks compared with non-diabetes participants with UA 5–7 mg/dL among participants without diabetes and with diabetes. Table S3: Racial differences in relative HRs (95%CI) of mortality risks compared with non-diabetes participants with UA 5–7 mg/dL.
Author Contributions
C.-L.L. and C.-H.C. had full access to the data in the study and take full responsibility for the integrity of the data and accuracy of the data analysis. C.-L.L. and P.-H.C. guided the data analysis and conceptualized the study. P.-H.C., Y.-W.C. and W.-J.L. performed the data analysis and interpretation. P.-H.C., S.-W.H., C.-H.C. and C.-L.L. contributed to the drafting of the manuscript and interpretation. All authors approved the final manuscript.
Funding
This work was supported by a research grant from the Taichung Veterans General Hospital, Taichung, Taiwan (TCVGH-1087301C, 2019) as well as in part by a research grant from Dialysis Clinic, Inc. (DCI #C-3917).
Acknowledgments
The authors thank the National Health Interview Survey (NHIS) for providing information on the health status of the noninstitutionalized U.S. civilian population.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- Kuo, C.F.; See, L.C.; Luo, S.F.; Ko, Y.S.; Lin, Y.S.; Hwang, J.S.; Lin, C.M.; Chen, H.W.; Yu, K.H. Gout: An independent risk factor for all-cause and cardiovascular mortality. Rheumatology 2010, 49, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Curhan, G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007, 116, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pandya, B.J.; Choi, H.K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 2011, 63, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Cherubini, A.; Ble, A.; Bos, A.J.; Maggio, M.; Dixit, V.D.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Uric acid and inflammatory markers. Eur. Heart J. 2006, 27, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Cherubini, A.; Miller, E.; Maggio, M.; Najjar, S.S.; Lauretani, F.; Bandinelli, S.; Senin, U.; Ferrucci, L. Usefulness of uric acid to predict changes in C-reactive protein and interleukin-6 in 3-year period in Italians aged 21 to 98 years. Am. J. Cardiol. 2007, 100, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kienhorst, L.B.; van Lochem, E.; Kievit, W.; Dalbeth, N.; Merriman, M.E.; Phipps-Green, A.; Loof, A.; van Heerde, W.; Vermeulen, S.; Stamp, L.K.; et al. Gout Is a Chronic Inflammatory Disease in Which High Levels of Interleukin-8 (CXCL8), Myeloid-Related Protein 8/Myeloid-Related Protein 14 Complex, and an Altered Proteome Are Associated with Diabetes Mellitus and Cardiovascular Disease. Arthritis Rheumatol. 2015, 67, 3303–3313. [Google Scholar] [CrossRef]
- Snow, M.H.; Mikuls, T.R. Rheumatoid arthritis and cardiovascular disease: The role of systemic inflammation and evolving strategies of prevention. Curr. Opin. Rheumatol. 2005, 17, 234–241. [Google Scholar] [CrossRef]
- Full, L.E.; Ruisanchez, C.; Monaco, C. The inextricable link between atherosclerosis and prototypical inflammatory diseases rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res. Ther. 2009, 11, 217. [Google Scholar] [CrossRef]
- Richette, P.; Latourte, A.; Bardin, T. Cardiac and renal protective effects of urate-lowering therapy. Rheumatology 2018, 57, i47–i50. [Google Scholar] [CrossRef]
- Ramirez, M.E.G.; Bargman, J.M. Treatment of asymptomatic hyperuricemia in chronic kidney disease: A new target in an old enemy–A review. J. Adv. Res. 2017, 8, 551–554. [Google Scholar] [CrossRef]
- Kimura, K.; Hosoya, T.; Uchida, S.; Inaba, M.; Makino, H.; Maruyama, S.; Ito, S.; Yamamoto, T.; Tomino, Y.; Ohno, I.; et al. Febuxostat Therapy for Patients with Stage 3 CKD and Asymptomatic Hyperuricemia: A Randomized Trial. Am. J. Kidney Dis. 2018, 72, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Favourable changes in mortality in people with diabetes: US NHANES 1999–2010. Diabetes Obes. Metab. 2018, 20, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.; Grainge, M.J.; Mallen, C.; Zhang, W.; Doherty, M. Rising burden of gout in the UK but continuing suboptimal management: A nationwide population study. Ann. Rheum. Dis. 2015, 74, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Graf, S.W.; Whittle, S.L.; Wechalekar, M.D.; Moi, J.H.; Barrett, C.; Hill, C.L.; Littlejohn, G.; Lynch, N.; Major, G.; Taylor, A.L.; et al. Australian and New Zealand recommendations for the diagnosis and management of gout: Integrating systematic literature review and expert opinion in the 3e Initiative. Int. J. Rheum. Dis. 2015, 18, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- NCHS 2011 Linked Mortality Files Matching Methodology; National Center for Health Statistics, Office of Analysis and Epidemiology: Hyattsville, MD, USA, 2013. Available online: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/linkage_methods_analytical_support/2011_linked_mortality_file_matching_methodology.pdf (accessed on 29 October 2019).
- National Health and Nutrition Examination Survey: Analytic Guidelines, 2011–2014 and 2015–2016. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011–2012/analyticguidelines/analytic_guidelines_11_16.pdf (accessed on 29 October 2019).
- Zuo, T.; Liu, X.; Jiang, L.; Mao, S.; Yin, X.; Guo, L. Hyperuricemia and coronary heart disease mortality: A meta-analysis of prospective cohort studies. BMC Cardiovasc. Disord. 2016, 16, 207. [Google Scholar] [CrossRef]
- Lehto, S.; Niskanen, L.; Ronnemaa, T.; Laakso, M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke 1998, 29, 635–639. [Google Scholar] [CrossRef]
- Cho, S.K.; Chang, Y.; Kim, I.; Ryu, S. U-Shaped Association between Serum Uric Acid Level and Risk of Mortality: A Cohort Study. Arthritis Rheumatol. 2018, 70, 1122–1132. [Google Scholar] [CrossRef]
- Zhang, W.; Iso, H.; Murakami, Y.; Miura, K.; Nagai, M.; Sugiyama, D.; Ueshima, H.; Okamura, T.; Epoch-Japan, G. Serum Uric Acid and Mortality Form Cardiovascular Disease: EPOCH-JAPAN Study. J. Atheroscler. Thromb. 2016, 23, 692–703. [Google Scholar] [CrossRef]
- Beberashvili, I.; Sinuani, I.; Azar, A.; Shapiro, G.; Feldman, L.; Stav, K.; Sandbank, J.; Averbukh, Z. Serum uric acid as a clinically useful nutritional marker and predictor of outcome in maintenance hemodialysis patients. Nutrition 2015, 31, 138–147. [Google Scholar] [CrossRef]
- Cutler, R.G. Urate and ascorbate: Their possible roles as antioxidants in determining longevity of mammalian species. Arch. Gerontol. Geriatr. 1984, 3, 321–348. [Google Scholar] [CrossRef]
- Wakasugi, M.; Kazama, J.J.; Narita, I.; Konta, T.; Fujimoto, S.; Iseki, K.; Moriyama, T.; Yamagata, K.; Tsuruya, K.; Asahi, K.; et al. Association between hypouricemia and reduced kidney function: A cross-sectional population-based study in Japan. Am. J. Nephrol. 2015, 41, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Iliesiu, A.; Campeanu, A.; Dusceac, D. Serum uric acid and cardiovascular disease. Maedica 2010, 5, 186–192. [Google Scholar] [PubMed]
- Eleftheriadis, T.; Golphinopoulos, S.; Pissas, G.; Stefanidis, I. Asymptomatic hyperuricemia and chronic kidney disease: Narrative review of a treatment controversial. J. Adv. Res. 2017, 8, 555–560. [Google Scholar] [CrossRef]
- Peng, T.C.; Wang, C.C.; Kao, T.W.; Chan, J.Y.; Yang, Y.H.; Chang, Y.W.; Chen, W.L. Relationship between hyperuricemia and lipid profiles in US adults. Biomed. Res. Int. 2015, 2015, 127596. [Google Scholar] [CrossRef]
- Krishnan, E.; Akhras, K.S.; Sharma, H.; Marynchenko, M.; Wu, E.Q.; Tawk, R.; Liu, J.; Shi, L. Relative and attributable diabetes risk associated with hyperuricemia in US veterans with gout. QJM 2013, 106, 721–729. [Google Scholar] [CrossRef][Green Version]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis. Care Res. 2012, 64, 1431–1446. [Google Scholar] [CrossRef]
- Richette, P.; Doherty, M.; Pascual, E.; Barskova, V.; Becce, F.; Castaneda-Sanabria, J.; Coyfish, M.; Guillo, S.; Jansen, T.L.; Janssens, H.; et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann. Rheum. Dis. 2017, 76, 29–42. [Google Scholar] [CrossRef]
- Hoieggen, A.; Alderman, M.H.; Kjeldsen, S.E.; Julius, S.; Devereux, R.B.; De Faire, U.; Fyhrquist, F.; Ibsen, H.; Kristianson, K.; Lederballe-Pedersen, O.; et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004, 65, 1041–1049. [Google Scholar] [CrossRef]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef]
- Yu, M.A.; Sanchez-Lozada, L.G.; Johnson, R.J.; Kang, D.H. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 2010, 28, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S.; Shen, W.; Boulton, D.W.; Leslie, B.R.; Griffen, S.C. Interaction between the Sodium-Glucose-Linked Transporter 2 Inhibitor Dapagliflozin and the Loop Diuretic Bumetanide in Normal Human Subjects. J. Am. Heart. Assoc. 2018, 7, e007046. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Niwa, K.; Hisatome, I.; Nakagawa, T.; Roncal-Jimenez, C.A.; Andres-Hernando, A.; Bjornstad, P.; Jensen, T.; Sato, Y.; Milagres, T.; et al. Asymptomatic Hyperuricemia Without Comorbidities Predicts Cardiometabolic Diseases: Five-Year Japanese Cohort Study. Hypertension 2017, 69, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.W.; Lin, S.Y.; Kuo, C.C.; Huang, C.C. Serum Uric Acid and Progression of Kidney Disease: A Longitudinal Analysis and Mini-Review. PLoS ONE 2017, 12, e0170393. [Google Scholar] [CrossRef] [PubMed]
- Chronic Kidney Disease Prognosis, Consortium; Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T.; Levey, A.S. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W., Jr.; Garcia, F.A.; Gillman, M.W.; Kemper, A.R.; Krist, A.H. Statin Use for the Primary Prevention of Cardiovascular Disease in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 316, 1997–2007. [Google Scholar]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Opie, L.H.; Dalby, A.J. Cardiovascular prevention: Lifestyle and statins–competitors or companions? S. Afr. Med. 2014, 104, 168–173. [Google Scholar] [CrossRef][Green Version]
- Fang, J.; Alderman, M.H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 2000, 283, 2404–2410. [Google Scholar] [CrossRef]
- Krishnan, E.; Svendsen, K.; Neaton, J.D.; Grandits, G.; Kuller, L.H.; Group, M.R. Long-term cardiovascular mortality among middle-aged men with gout. Arch. Intern. Med. 2008, 168, 1104–1110. [Google Scholar] [CrossRef]
- White, W.B.; Saag, K.G.; Becker, M.A.; Borer, J.S.; Gorelick, P.B.; Whelton, A.; Hunt, B.; Castillo, M.; Gunawardhana, L.; Investigators, C. Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout. N. Engl. J. Med. 2018, 378, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).