Quantitative Analysis of the Seminal Plasma Proteome in Secondary Hypogonadism
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Hormonal Study
2.3. Semen Analysis and In-solution Digestion
2.4. Proteomic Analysis
2.5. Data Analysis
2.6. Western Blot
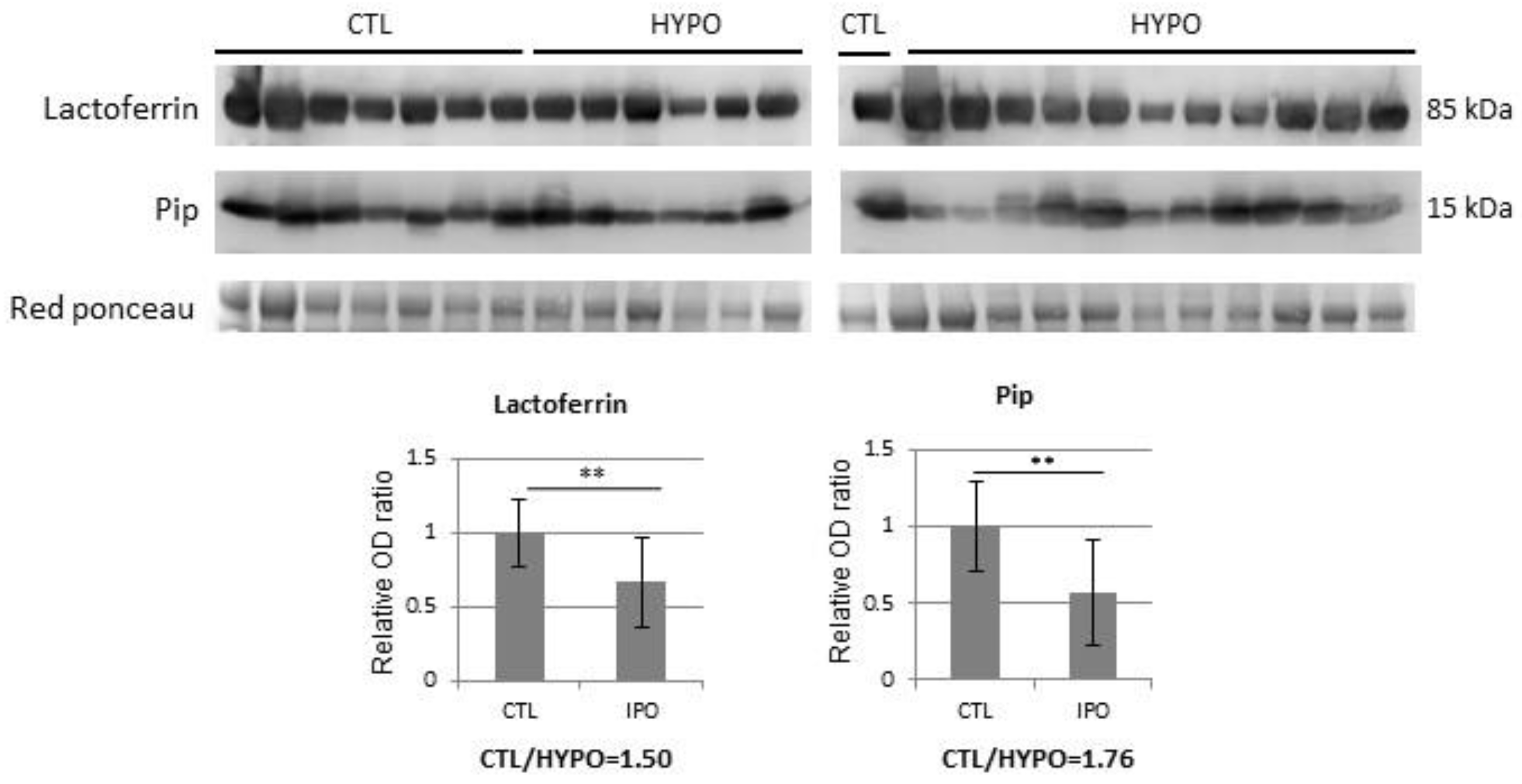
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Petak, S.M.; Nankin, H.R.; Spark, R.F.; Swerdloff, R.S.; Rodriguez-Rigau, L.J. American Association of Clinical Endocrinologists American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients—2002 update. Endocr. Pract. 2002, 8, 440–456. [Google Scholar]
- Arver, S.; Lehtihet, M. Current Guidelines for the Diagnosis of Testosterone Deficiency. In Advances in the Management of Testosterone Deficiency; KARGER: Basel, Switzerland, 2008; Volume 37, pp. 5–20. [Google Scholar]
- La Vignera, S.; Condorelli, R.A.; Cimino, L.; Russo, G.I.; Morgia, G.; Calogero, A.E. Late-onset hypogonadism: The advantages of treatment with human chorionic gonadotropin rather than testosterone. Aging Male 2016, 19, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.M. Hormonal therapy of male hypogonadism. Endocrinol. Metab. Clin. N. Am. 1994, 23, 857–875. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Vignozzi, L.; Maggi, M. Emerging medication for the treatment of male hypogonadism. Expert Opin. Emerg. Drugs 2012, 17, 239–259. [Google Scholar] [CrossRef]
- Yılmazel, F.K.; Karabulut, İ.; Yılmaz, A.H.; Keskin, E.; Bedir, F.; Özbey, İ. A review of hypogonadotropic hypogonadism cases followed up in our clinic in the last decade. Urol. J. 2019. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone Therapy in Men with Androgen Deficiency Syndromes: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.; McGill, J.; Agarwal, A. Prostatitis and male infertility. J. Reprod. Immunol. 2013, 100, 30–36. [Google Scholar] [CrossRef]
- Brooks, D.E. Influence of androgens on the weights of the male accessory reproductive organs and on the activities of mitochondrial enzymes in the epididymis of the rat. J. Endocrinol. 1979, 82, 293–303. [Google Scholar] [CrossRef]
- Green, S.M.; Mostaghel, E.A.; Nelson, P.S. Androgen action and metabolism in prostate cancer. Mol. Cell. Endocrinol. 2012, 360, 3–13. [Google Scholar] [CrossRef]
- Ma, C.; Yoshioka, M.; Boivin, A.; Gan, L.; Takase, Y.; Labrie, F.; St-Amand, J. Atlas of dihydrotestosterone actions on the transcriptome of prostate in vivo. Prostate 2009, 69, 293–316. [Google Scholar] [CrossRef]
- Barrachina, F.; Jodar, M.; Delgado-Dueñas, D.; Soler-Ventura, A.; Estanyol, J.M.; Mallofré, C.; Ballescà, J.L.; Oliva, R. Stable-Protein Pair Analysis as a Novel Strategy to Identify Proteomic Signatures: Application to Seminal Plasma from Infertile Patients. Mol. Cell. Proteom. 2019, 18, S77–S90. [Google Scholar] [CrossRef] [PubMed]
- Cayer, D.M.; Nazor, K.L.; Schork, N.J. Mission critical: The need for proteomics in the era of next-generation sequencing and precision medicine. Hum. Mol. Genet. 2016, 25, R182–R189. [Google Scholar] [CrossRef]
- Castillo, J.; Jodar, M.; Oliva, R. The contribution of human sperm proteins to the development and epigenome of the preimplantation embryo. Hum. Reprod. Update 2018, 24, 535–555. [Google Scholar] [CrossRef] [PubMed]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Giampietro, A.; Messana, I.; Castagnola, M.; Marana, R.; De Marinis, L.; Pontecorvi, A. Novel Biomarkers of Androgen Deficiency from Seminal Plasma Profiling Using High-Resolution Mass Spectrometry. J. Clin. Endocrinol. Metab. 2014, 99, 2813–2820. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Michalski, A.; Damoc, E.; Lange, O.; Denisov, E.; Nolting, D.; Mü, M.; Viner, R.; Schwartz, J.; Remes, P.; Belford, M.; et al. Ultra High Resolution Linear Ion Trap Orbitrap Mass Spectrometer (Orbitrap Elite) Facilitates Top Down LC MS/MS and Versatile Peptide Fragmentation Modes. Mol. Cell. Proteom. 2012, 11, O111-013698. [Google Scholar] [CrossRef]
- Lan, N.; Montelione, G.T.; Gerstein, M. Ontologies for proteomics: Towards a systematic definition of structure and function that scales to the genome level. Curr. Opin. Chem. Biol. 2003, 7, 44–54. [Google Scholar] [CrossRef]
- Behre, H.M.; Kliesch, S.; Schädel, F.; Nieschlag, E. Clinical relevance of scrotal and transrectal ultrasonography in andrological patients. Int. J. Androl. 1995, 18 (Suppl. 2), 27–31. [Google Scholar]
- World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Xu, S.; Jiang, J.; Zhang, Y.; Chen, T.; Zhu, M.; Fang, C.; Mi, Y. Discovery of potential plasma protein biomarkers for acute myocardial infarction via proteomics. J. Thorac. Dis. 2019, 11, 3962–3972. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, R.; Wei, J.; Ye, J.; He, Q.; Ye, J.; Li, Y.; Liu, Z.; Lin, Y. Identification of Candidate Biomarkers in Malignant Ascites from Patients with Hepatocellular Carcinoma by iTRAQ-Based Quantitative Proteomic Analysis. BioMed Res. Int. 2018, 2018, 5484976. [Google Scholar] [CrossRef]
- Grande, G.; Vincenzoni, F.; Mancini, F.; Baroni, S.; Luca, G.; Calafiore, R.; Marana, R.; Castagnola, M.; Pontecorvi, A.; Milardi, D. Semen proteomics reveals the impact of enterococcus faecalis on male fertility. Protein Pept. Lett. 2018, 25, 472–477. [Google Scholar] [CrossRef]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Uhrin, P.; Schöfer, C.; Zaujec, J.; Ryban, L.; Hilpert, M.; Weipoltshammer, K.; Jerabek, I.; Pirtzkall, I.; Furtmüller, M.; Dewerchin, M.; et al. Male fertility and protein C inhibitor/plasminogen activator inhibitor-3 (PCI): Localization of PCI in mouse testis and failure of single plasminogen activator knockout to restore spermatogenesis in PCI-deficient mice. Fertil. Steril. 2007, 88, 1049–1057. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marlar, R.A.; Griffin, J.H. Deficiency of protein C inhibitor in combined factor V/VIII deficiency disease. J. Clin. Investig. 1980, 66, 1186–1189. [Google Scholar] [CrossRef]
- Suzuki, K.; Nishioka, J.; Hashimoto, S. Protein C inhibitor. Purification from human plasma and characterization. J. Biol. Chem. 1983, 258, 163–168. [Google Scholar] [PubMed]
- España, F.; Berrettini, M.; Griffin, J.H. Purification and characterization of plasma protein C inhibitor. Thromb. Res. 1989, 55, 369–384. [Google Scholar] [CrossRef]
- Meijers, J.C.; Kanters, D.H.; Vlooswijk, R.A.; van Erp, H.E.; Hessing, M.; Bouma, B.N. Inactivation of human plasma kallikrein and factor XIa by protein C inhibitor. Biochemistry 1988, 27, 4231–4237. [Google Scholar] [CrossRef]
- Rezaie, A.R.; Cooper, S.T.; Church, F.C.; Esmon, C.T. Protein C inhibitor is a potent inhibitor of the thrombin-thrombomodulin complex. J. Biol. Chem. 1995, 270, 25336–25339. [Google Scholar] [CrossRef]
- Geiger, M.; Huber, K.; Wojta, J.; Stingl, L.; Espana, F.; Griffin, J.H.; Binder, B.R. Complex formation between urokinase and plasma protein C inhibitor in vitro and in vivo. Blood 1989, 74, 722–728. [Google Scholar] [CrossRef]
- España, F.; Estellés, A.; Fernández, P.J.; Gilabert, J.; Sánchez-Cuenca, J.; Griffin, J.H. Evidence for the regulation of urokinase and tissue type plasminogen activators by the serpin, protein C inhibitor, in semen and blood plasma. Thromb. Haemost. 1993, 70, 989–994. [Google Scholar] [CrossRef]
- Hermans, J.M.; Jones, R.; Stone, S.R. Rapid inhibition of the sperm protease acrosin by protein C inhibitor. Biochemistry 1994, 33, 5440–5444. [Google Scholar] [CrossRef]
- Zheng, X.; Geiger, M.; Ecke, S.; Bielek, E.; Donner, P.; Eberspächer, U.; Schleuning, W.D.; Binder, B.R. Inhibition of acrosin by protein C inhibitor and localization of protein C inhibitor to spermatozoa. Am. J. Physiol. Cell Physiol 1994, 267, C466–C472. [Google Scholar] [CrossRef] [PubMed]
- Ecke, S.; Geiger, M.; Resch, I.; Jerabek, I.; Sting, L.; Maier, M.; Binder, B.R. Inhibition of tissue kallikrein by protein C inhibitor. Evidence for identity of protein C inhibitor with the kallikrein binding protein. J. Biol. Chem. 1992, 267, 7048–7052. [Google Scholar] [PubMed]
- España, F.; Gilabert, J.; Estellés, A.; Romeu, A.; Aznar, J.; Cabo, A. Functionally active protein C inhibitor/plasminogen activator inhibitor-3 (PCI/PAI-3) is secreted in seminal vesicles, occurs at high concentrations in human seminal plasma and complexes with prostate-specific antigen. Thromb. Res. 1991, 64, 309–320. [Google Scholar] [CrossRef]
- Christensson, A.; Lilja, H. Complex formation between protein C inhibitor and prostate-specific antigen in vitro and in human semen. Eur. J. Biochem. 1994, 220, 45–53. [Google Scholar] [CrossRef]
- Uhrin, P.; Dewerchin, M.; Hilpert, M.; Chrenek, P.; Schöfer, C.; Zechmeister-Machhart, M.; Krönke, G.; Vales, A.; Carmeliet, P.; Binder, B.R.; et al. Disruption of the protein C inhibitor gene results in impaired spermatogenesis and male infertility. J. Clin. Investig. 2000, 106, 1531–1539. [Google Scholar] [CrossRef]
- Szecsi, P.B.; Lilja, H. Gastricsin-mediated proteolytic degradation of human seminal fluid proteins at pH levels found in the human vagina. J. Androl. 1993, 14, 351–358. [Google Scholar]
- Szecsi, P.B.; Dalgaard, D.; Stakemann, G.; Wagner, G.; Foltmann, B. The concentration of pepsinogen C in human semen and the physiological activation of zymogen in the vagina. Biol. Reprod. 1989, 40, 653–659. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Gram, L.; Johnsen, A.H.; Andersson, E.; Bangsbøll, S.; Tjabringa, G.S.; Hiemstra, P.S.; Malm, J.; Egesten, A.; Borregaard, N. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: A novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 2003, 278, 28540–28546. [Google Scholar] [CrossRef]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; Favilla, V.; Cimino, S.; Russo, G.I.; Morgia, G.; La Vignera, S. Male accessory gland infection: Relevance of serum total testosterone levels. Int. J. Endocrinol. 2014, 2014, 915752. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Autilio, C.; Mancini, F.; De Marinis, L.; Marana, R.; Zuppi, C.; Urbani, A.; Pontecorvi, A.; Baroni, S. Seminal suPAR Levels as Marker of Abacterial Male Accessory Gland Inflammation in Hypogonadism. Protein Pept. Lett. 2018, 25, 478–482. [Google Scholar] [CrossRef]
- Dubé, J.Y.; Pelletier, G.; Gagnon, P.; Tremblay, R.R. Immunohistochemical localization of a prostatic secretory protein of 94 amino acids in normal prostatic tissue, in primary prostatic tumors and in their metastases. J. Urol. 1987, 138, 883–887. [Google Scholar] [CrossRef]
- Lilja, H.; Abrahamsson, P.A. Three predominant proteins secreted by the human prostate gland. Prostate 1988, 12, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Anahí Franchi, N.; Avendaño, C.; Molina, R.I.; Tissera, A.D.; Maldonado, C.A.; Oehninger, S.; Coronel, C.E. beta-Microseminoprotein in human spermatozoa and its potential role in male fertility. Reproduction 2008, 136, 157–166. [Google Scholar] [CrossRef]
- Jagtap, D.D.; Narahari, A.; Swamy, M.J.; Mahale, S.D. Disulphide bond reduction and S-carboxamidomethylation of PSP94 affects its conformation but not the ability to bind immunoglobulin. Biochim. Biophys. Acta 2007, 1774, 723–731. [Google Scholar] [CrossRef]
- Kamada, M.; Mori, H.; Maeda, N.; Yamamoto, S.; Kunimi, K.; Takikawa, M.; Maegawa, M.; Aono, T.; Futaki, S.; Koide, S.S. beta-Microseminoprotein/prostatic secretory protein is a member of immunoglobulin binding factor family. Biochim. Biophys. Acta 1998, 1388, 101–110. [Google Scholar] [CrossRef]
- Udby, L.; Lundwall, Å.; Johnsen, A.H.; Fernlund, P.; Valtonen-André, C.; Blom, A.M.; Lilja, H.; Borregaard, N.; Kjeldsen, L.; Bjartell, A. β-Microseminoprotein binds CRISP-3 in human seminal plasma. Biochem. Biophys. Res. Commun. 2005, 333, 555–561. [Google Scholar] [CrossRef]
- Yadav, V.K.; Kumar, V.; Chhikara, N.; Kumar, S.; Manral, P.; Kashav, T.; Saini, S.; Srinivasan, A.; Singh, S.; Singh, T.P.; et al. Purification and characterization of a native zinc-binding high molecular weight multiprotein complex from human seminal plasma. J. Sep. Sci. 2011, 34, 1076–1083. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Messana, I.; Pontecorvi, A.; De Marinis, L.; Castagnola, M.; Marana, R. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil. Steril. 2012, 97, 67–73. [Google Scholar] [CrossRef]
- Piomboni, P.; Gambera, L.; Serafini, F.; Campanella, G.; Morgante, G.; De Leo, V. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J. Androl. 2008, 10, 201–206. [Google Scholar] [CrossRef]
- Ban, R.W.; Cooper, J.F.; Imfeld, H.; Foti, A. Hormonal effects on prostatic acid phosphatase synthesis in tissue culture. Investig. Urol. 1974, 11, 308–311. [Google Scholar]
- Simon, A.M.; Veyssière, G.; Jean, C. Structure and sequence of a mouse gene encoding an androgen-regulated protein: A new member of the seminal vesicle secretory protein family. J. Mol. Endocrinol. 1995, 15, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Tomar, A.K.; Sooch, B.S.; Raj, I.; Singh, S.; Yadav, S. Interaction analysis identifies semenogelin I fragments as new binding partners of PIP in human seminal plasma. Int. J. Biol. Macromol. 2013, 52, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.I.; Waheed, A.; Yadav, S.; Singh, T.P.; Ahmad, F. Prolactin inducible protein in cancer, fertility and immunoregulation: Structure, function and its clinical implications. Cell. Mol. Life Sci. 2009, 66, 447–459. [Google Scholar] [CrossRef]
- Martinez-Heredia, J.; de Mateo, S.; Vidal-Taboada, J.M.; Ballesca, J.L.; Oliva, R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum. Reprod. 2008, 23, 783–791. [Google Scholar] [CrossRef]
| Hypogonadic Patients (n = 10) | Controls (n = 10) | Range Values | |
|---|---|---|---|
| Age | 37.5 ± 9.1 | 39.6 ± 8.3 | |
| Testosterone (T) | 1.87 ± 0.64 | 4.9 ± 0.9 * | 2.5–8.4 ng/mL |
| Estradiol (E2) | 23.25 ± 4.72 | 27.9 ± 9.4 | 15–44 pg/mL |
| SHBG | 19.67 ± 4.34 | 38.2± 9.3 | 16–80 nmol/L |
| FSH | 1.72 ± 1.14 | 2.6 ± 1.3 * | 1.0–8.0 mU/mL |
| LH | 0.90 ± 0.50 | 2.9 ± 0.8 * | 2.5–10.0 mU/mL |
| Seminal volume | 1.75 ± 1.13 mL | 2.9 ± 0.9 * | |
| Sperm concentration | 2.61 ± 4.83 × 106/mL | 74.2 ± 28.3 × 106/mL * | |
| Total sperm motility | 8.3 ± 12.90% | 52.6 ± 8.3% * | |
| Normal morphology | 3.2 ± 4.1% | 8.9 ± 2.0% * |
| After TRT (n = 5) | After TRT (n = 5) | ||
|---|---|---|---|
| Testosterone (T) | 1.24 ± 0.76 | 3.63 ± 0.48 * | 2.5–8.4 ng/mL |
| Estradiol (E2) | 21.47 ± 5.72 | 29.48 ± 8.73 | 15–44 pg/mL |
| Accession Number | Protein Description | Gene | Ratio C/HH |
|---|---|---|---|
| P05154 | Plasma serine protease inhibitor | IPSP | 2.89 |
| P12273 | Prolactin-inducible protein | PIP | 2.32 |
| P54107 | Cysteine-rich secretory protein 1 | CRIS1 | 2.21 |
| P20142 | Gastricsin | PEPC | 2.19 |
| P04279 | Semenogelin-1 | SEMG1 | 2.12 |
| P08118 | Beta-microseminoprotein | MSMB | 2.04 |
| Q02383 | Semenogelin-2 | SEMG2 | 1.92 |
| P07288 | Prostate-specific antigen | KLK3 | 1.65 |
| P07602 | Prosaposin | SAP | 1.64 |
| P15309 | Prostatic acid phosphatase | PPAP | 1.54 |
| P02788 | Lactotransferrin | TRFL | 1.52 |
| Accession Number | Protein Description | Gene | Ratio After/Before TRT |
|---|---|---|---|
| P04279 | Semenogelin-1 | SEMG1 | 2.42 |
| Q02383 | Semenogelin-2 | SEMG2 | 2.15 |
| Q6W4X9 | Mucin-6 | MUC6 | 1.87 |
| P12273 | Prolactin-inducible protein | PIP | 1.76 |
| P15309 | Prostatic acid phosphatase | PPAP | 1.64 |
| P02788 | Lactotransferrin | TRFL | 1.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, G.; Vincenzoni, F.; Mancini, F.; Barrachina, F.; Giampietro, A.; Castagnola, M.; Urbani, A.; Oliva, R.; Milardi, D.; Pontecorvi, A. Quantitative Analysis of the Seminal Plasma Proteome in Secondary Hypogonadism. J. Clin. Med. 2019, 8, 2128. https://doi.org/10.3390/jcm8122128
Grande G, Vincenzoni F, Mancini F, Barrachina F, Giampietro A, Castagnola M, Urbani A, Oliva R, Milardi D, Pontecorvi A. Quantitative Analysis of the Seminal Plasma Proteome in Secondary Hypogonadism. Journal of Clinical Medicine. 2019; 8(12):2128. https://doi.org/10.3390/jcm8122128
Chicago/Turabian StyleGrande, Giuseppe, Federica Vincenzoni, Francesca Mancini, Ferran Barrachina, Antonella Giampietro, Massimo Castagnola, Andrea Urbani, Rafael Oliva, Domenico Milardi, and Alfredo Pontecorvi. 2019. "Quantitative Analysis of the Seminal Plasma Proteome in Secondary Hypogonadism" Journal of Clinical Medicine 8, no. 12: 2128. https://doi.org/10.3390/jcm8122128
APA StyleGrande, G., Vincenzoni, F., Mancini, F., Barrachina, F., Giampietro, A., Castagnola, M., Urbani, A., Oliva, R., Milardi, D., & Pontecorvi, A. (2019). Quantitative Analysis of the Seminal Plasma Proteome in Secondary Hypogonadism. Journal of Clinical Medicine, 8(12), 2128. https://doi.org/10.3390/jcm8122128






