Abstract
Background/Objectives: Functional limitations are common among older cancer survivors and tend to increase with age and survivorship duration. Physical activity (PA) associates with better functional outcomes, but little is known about how these associations vary as time passes post-diagnosis. This study examined how years since diagnosis, three types of physical activity, and their interactions associate with functional limitations in older cancer survivors. Methods: Data drawn from the 2021 National Health Interview Survey (NHIS), representing adults aged 55+ and with a prior cancer diagnosis (n = 9356; mean age = 72.17 ± 8.5 years), were studied. A four-item self-reported difficulty index (i.e., washing/dressing, walking one block, climbing stairs, and picking up/opening objects) was summed to measure functional limitations. PA was assessed using the items aligned with the United States PA Guidelines. Hierarchical regression was used to evaluate associations between functional limitations and years since diagnosis, vigorous physical activity, moderate physical activity, and strength training. Interaction effects of years since diagnosis and each activity type were also examined. Covariates were age, sex, BMI, and educational attainment. Results: Elapsed time since cancer diagnosis positively associated with functional limitations in interaction with physical behaviors, while moderate physical activity and strength training negatively associated with functional limitations. Interactions of years since diagnosis and both moderate physical activity and strength training revealed smaller increases in functional limitations. No interaction effects were observed for vigorous physical activity. Conclusions: Among older cancer survivors, the association between survivorship duration and functional limitations differs by engagement in moderate and resistance-based physical activity. These findings support the clinical importance of promoting sustainable, non-vigorous physical activity in long-term survivorship care.
1. Introduction
Cancer survivorship in the United States has increased substantially over recent decades due to improvements in early detection and treatment, with more than 18 million survivors currently living nationwide [1]. As cancer survivors age, functional limitations become increasingly salient. Older adult cancer survivors experience higher rates of mobility difficulty, reduced physical functioning, and greater impairment in activities of daily living compared with individuals without a cancer history [2,3,4]. These and other functional limitations can persist for many years after diagnosis and substantially affect survivors’ independence, quality of life, and healthcare costs [5,6,7]. Functional limitations reflect multiple biological and behavioral influences, including treatment-related toxicities and fatigue, accelerated aging, systemic inflammation, and multimorbidity [8,9,10,11,12].
Functional limitations among cancer survivors can vary considerably over time. While some cancer survivors experience gradual recovery, others exhibit progressive functional decline as long-term treatment effects accumulate [13]. Understanding whether functional limitations differ according to years since diagnosis (YSD) is therefore essential for identifying periods of heightened vulnerability across the survivorship trajectory. Additionally, physical activity (PA) has been consistently associated with fewer functional limitations in older adults [14]. Among cancer survivors, engagement in moderate-intensity aerobic activity and strength training has been linked to lower disability, improved mobility, enhanced muscle function, and reduced treatment-related side effects [15,16,17]. However, much of this evidence is derived from intervention trials or small cohort studies that may not fully represent the broader survivor population. Moreover, little is known about whether the association between PA and functional limitations varies as a function of survivorship duration. It is plausible that PA is more strongly associated with functional preservation later in survivorship, when acute treatment effects have diminished and age-related functional decline becomes more prominent.
This study extends survivorship research by demonstrating that associations between PA and functional limitations are conditional on survivorship duration in a large, nationally representative sample. Large-scale, population-level analyses linking PA, YSD, and functional limitations among older cancer survivors remain scarce. The National Health Interview Survey (NHIS) assesses functional limitations using standardized difficulty ratings for routine mobility and activities of daily living [18]. Recent updates to the NHIS incorporated multiple standardized measures of PA and functional limitations [19], providing a unique opportunity to examine these associations in a nationally representative sample. Accordingly, the purpose of this study was to examine associations between YSD, PA intensity, and functional limitations in older adult cancer survivors, while controlling for basic demographic and health characteristics. Specifically, this study evaluated: (1) whether YSD was associated with functional limitations independent of age, sex, body mass index, and education; (2) whether three forms of PA—moderate-intensity activity, vigorous activity, and strength training—were directly associated with functional limitations; and (3) whether interactions between PA type and YSD were associated with functional limitations. Clarifying these relationships may help identify when, and for whom, PA is most strongly associated with preserved functioning among older adult cancer survivors.
2. Materials and Methods
2.1. Study Design and Data Source
This cross-sectional study used data from the 2021 NHIS Sample Adult file, accessed through the Integrated Public Use Microdata Series (IPUMS) version 7.5 Health Surveys database [18]. The NHIS employs a multistage, stratified sampling design to obtain a nationally representative sample of non-institutionalized adults in the United States. The 2021 cycle was selected because it includes standardized assessments of PA and functional limitation relevant to the study aims. All data were publicly available and fully de-identified (See Appendix A); therefore, institutional review board approval was not required.
2.2. Participants
Participants were eligible if they were aged 55 years or older and reported at least one prior cancer diagnosis other than non-melanoma skin cancer. Exclusion criteria included age younger than 55 years, implausible PA values, current pregnancy, lack of a reported cancer diagnosis, or excessive missing responses to multiple functional limitation items or covariates. Implausible PA values were defined using logical thresholds, specifically weekly totals exceeding 1680 min per week for aerobic activity and strength/resistance training sessions exceeding 14 per week. After applying these criteria, the analytic sample included 9356 older adults with a history of cancer. Sensitivity analyses retaining these extreme PA values did not meaningfully alter regression coefficients, statistical significance patterns, or overall conclusions.
2.3. Measures
2.3.1. Functional Limitations
Functional limitations served as the dependent variable. NHIS includes a wide set of mobility and daily functioning questions; however, many items overlap conceptually. To avoid redundancy and multicollinearity, four non-overlapping items were selected to represent distinct functional domains. Respondents reported the level of difficulty they experienced when washing or dressing, walking one block on level ground, climbing a flight of stairs, and picking up or opening small objects. Each item used a four-point scale ranging from “no difficulty” to “unable to perform”. These four items were summed to create a researcher-defined composite index, with higher scores indicating greater functional limitation. The composite demonstrated acceptable internal consistency in this study (Cronbach’s α > 0.7). Although each item is ordinal (four response categories), the summed index is commonly treated as approximately continuous in large population-based studies, enabling interpretable estimation of associations and interaction effects. Sensitivity analyses using alternative ordinal and factor-based specifications yielded substantively similar conclusions and justify the linear modeling approach. As a sensitivity check, models were re-estimated using ordinal regression treating each item as ordered categorical and using factor-score specifications derived from exploratory factor analysis; in all cases, interaction effects between years since diagnosis and PA remained directionally consistent.
2.3.2. Years Since Cancer Diagnosis
YSD was defined as the difference between the NHIS survey year (2021) and the reported year of earliest cancer diagnosis, representing survivorship duration in full years. The earliest diagnosis was selected for participants with multiple cancers to reflect the maximum survivorship duration. YSD was expressed in full years, consistent with NHIS reporting conventions. Chronological age and YSD capture distinct constructs—biological aging versus survivorship duration—and were included simultaneously to estimate the unique association of survivorship duration with functional limitations while adjusting for age. Correlation between age and YSD was moderate (r = 0.107), and variance inflation factors remained below accepted thresholds (maximum VIF = 1.19), indicating acceptable collinearity.
2.3.3. PA Variables
PA was assessed using NHIS items aligned with the United States PA Guidelines. Respondents reported the total weekly duration of moderate-intensity aerobic activity and vigorous-intensity aerobic activity, and the total number of weekly strength training sessions. Weekly minutes of moderate and vigorous activity were computed separately, and strength training was recorded as the number of weekly sessions. All PA variables were treated as continuous measures.
2.3.4. Covariates
Age in years, sex, educational attainment, and body mass index (BMI) were included as covariates due to their established associations with physical functioning. BMI was calculated from self-reported height and weight. Age was included as a covariate alongside YSD to statistically distinguish survivorship duration effects from effects attributable to chronological aging.
2.4. Statistical Analysis
Hierarchical multiple linear regression was used to examine the associations among demographic factors, YSD, PA variables, and functional limitations. The dependent variable was functional limitations. Model 1 included the covariates, age, sex, education, and BMI, as baseline predictors. Model 2 added YSD. Model 3 added moderate PA, vigorous PA, and strength training frequency. Model 4 included the interaction terms between YSD and each PA variable. All continuous predictors were standardized prior to computing interaction terms to place variables on comparable scales and to reduce non-essential multicollinearity.
Although NHIS employs a complex survey design with sampling weights, strata, and clustering, unweighted regression models were used in this study. Preliminary weighted analyses incorporating interaction terms produced model instability and convergence issues. Consistent with prior NHIS-based studies examining analytic subpopulations and interaction effects, unweighted models were therefore selected to prioritize stable estimation of associations. While this approach primarily limits population-level generalizability, parameter estimates and standard errors may also be modestly affected by the absence of survey weights. Similar analytic decisions have been adopted in prior NHIS research focusing on interaction effects and subgroup inference (e.g., [2,13]).
Regression assumptions included standard diagnostic procedures. Variance inflation factors were below accepted thresholds (all VIFs < 3.5), indicating no problematic multicollinearity. Visual inspection of residual-versus-fitted plots indicated homoscedasticity, and residual distributions demonstrated acceptable normality. Model fit was evaluated using R2 and ΔR2, standardized β coefficients were reported as effect-size estimates. Because the hierarchical models represented sequential adjustments rather than independent hypothesis tests, no Bonferroni or family-wise correction was applied. Given the large analytic sample (n = 9356), the study had sufficient power (>90%) to detect small effect sizes in multivariable models. Analyses were conducted using IBM SPSS Statistics Version 29.0.
3. Results
Descriptive characteristics of the sample are summarized in Table 1. Hierarchical regression results examining associations between demographic factors, YSD, PA variables, and functional limitation scores are presented in Table 2.

Table 1.
Demographic Characteristics of Sample (n = 9356).

Table 2.
Hierarchical Regression Results.
Model 1 (Demographic and Health Covariates): In the initial model, older age (β = 0.244, p < 0.01) and higher BMI (β = 0.217, p < 0.01) were associated with greater functional limitations. Male sex was associated with fewer limitations compared with females (β = −0.098, p < 0.01), and higher educational attainment was associated with lower functional limitation (β = −0.138, p < 0.01). Model 1 accounted for a noteworthy proportion of the variance in functional limitations (R2 = 0.126).
Model 2 (Covariates + YSD): The addition of YSD to the base model did not yield a significant main effect (β = 0.015, p > 0.05) and resulted in only a trivial improvement in model fit (R2 = 0.127; ΔR2 = 0.001). All demographic predictors remained significant and in the same direction.
Model 3 (Covariates + YSD + PA Variables): Moderate-intensity activity (β = 0.02, p > 0.05), vigorous-intensity activity (β = −0.04, p > 0.05), and strength training frequency (β = −0.081, p > 0.05), were entered into a third model alongside the covariates and YSD. Results suggest no direct associations between PA and functional limitations. The inclusion of PA variables increased overall model fit (R2 = 0.153, ΔR2 = 0.026), although education was no longer statistically significant in this model. The increase in explained variance despite non-significant individual PA coefficients likely reflects shared variance and suppression effects, whereby PA variables contribute collectively rather than independently to functional limitation outcomes.
Model 4 (Full model with Interaction Effects): The addition of interaction terms yielded a meaningful improvement in model fit (R2 = 0.186; ΔR2 = 0.033). Two interactions were statistically significant. Moderate-intensity activity moderates the association between YSD and functional limitations, such that greater moderate activity was linked to lower limitations among long-term survivors (β = −0.28, p < 0.05). Strength training showed an even stronger moderating effect, with higher frequency associated with fewer limitations as survivorship duration increased (β = −0.363, p < 0.05). The interaction between vigorous activity and YSD was not statistically significant (β = 0.205, p = 0.094). With interaction terms included, YSD emerged as a significant positive predictor of functional limitations (β = 0.363, p < 0.05). Importantly, once interaction terms are included, the YSD main effect should not be interpreted as a population-average association with functional limitations, but rather as a conditional effect evaluated at mean-centered levels of PA. The emergence of YSD as a significant predictor in Model 4 reflects its conditional association with functional limitations at varying levels of PA. When interaction terms are present, main effects represent conditional associations evaluated at mean-centered levels of the interacting variables and should not be interpreted as average marginal effects across all PA levels. To aid interpretation of these interaction effects, Figure 1 presents predicted functional limitation scores across YSD at the lowest 25%, middle 50%, and highest 25% levels of moderate PA and strength training.
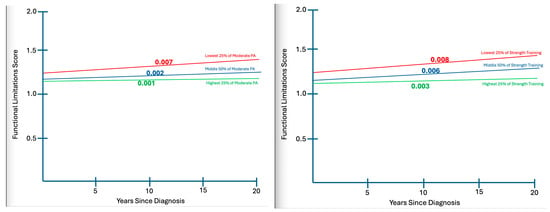
Figure 1.
Interaction effects of YSD and PA on functional limitations.
As seen in Figure 1, PA types of interest, participants in the lowest 25% range demonstrate substantially more limitation (5–6%) more limitation than those in the highest quartile of participation as each decade of survivorship passes.
4. Discussion
This study examined the associations among YSD, PA behaviors, and functional limitations in a nationally representative sample of older adult cancer survivors. Three primary findings emerged. First, demographic factors—particularly age, sex, BMI, and education attainment—were strong and consistent predictors of functional limitations, supporting a null hypothesis that sociodemographic factors would contribute substantially to functional outcomes. Second, YSD alone was not directly associated with functional limitations when evaluated at mean-centered levels of PA, indicating that survivorship duration is not, in isolation, a reliable marker of functional limitations status. Third, although moderate and vigorous PA and strength training did not show significant direct associations with functional limitations, both moderate aerobic PA and strength training exhibited significant interactions with YSD. These interaction effects indicated that moderate-intensity aerobic PA and strength training were increasingly associated with fewer functional limitations as survivorship duration increased. In other words, the functional advantages of these PA behaviors became more pronounced among long-term survivors. By contrast, vigorous PA did not exhibit a significant interaction with YSD, suggesting that its association with function does not vary meaningfully across survivorship stages.
4.1. Interpretation of Key Findings
Demographic predictors demonstrated strong associations with functional limitations, aligning with prior research that advanced age, higher BMI, and lower educational attainment are linked to mobility impairments and reduced functional reserve among cancer survivors [20]. These findings confirm expectations that demographic covariates account for substantial variance in functional limitations.
Contrary to our hypothesis, YSD alone was not directly associated with functional limitations. Survivorship trajectories vary widely due to cancer type, treatment exposure, comorbidities, and socioeconomic resources, resulting in divergent long-term functional outcomes [21]. Some survivors experience functional recovery over time, while others develop persistent or late-emerging impairments linked to treatment toxicity, cardiometabolic dysregulation, or psychological burden [4]. This heterogeneity likely obscured any linear relationship between YSD and functional status.
The absence of direct associations between PA and functional limitations may reflect methodological and behavioral factors. Self-reported PA is prone to recall error and social desirability bias, often leading to misclassification and attenuation of true associations [14,22,23]. Additionally, the NHIS PA measures lack specificity about type, duration, and context, introducing further error variance. Functional benefits of PA accumulate over years, yet cross-sectional surveys capture only a snapshot of demonstrated behaviors. Older survivors also face barriers to PA engagement—including fatigue, pain, and limited access to appropriate exercise environments—which can reduce consistency and diminish observed associations [22].
The significant interactions between YSD and both moderate PA and strength training are particularly noteworthy. These findings suggest that these forms of PA become increasingly protective as survivorship progresses. Long-term survivors exhibit physiological changes resembling accelerated aging, including chronic inflammation, mitochondrial dysfunction, reduced muscle mass, and declining neuromuscular efficiency [24]. Moderate aerobic PA and strength training directly counter these mechanisms by improving mitochondrial function, preserving lean muscle mass, reducing inflammatory burden, and enhancing physical resilience [25]. These biological pathways provide a clear rationale for why moderate PA and strength training showed stronger associations with functional limitations among long-term survivors: those with the greatest vulnerability may benefit the most.
In contrast, vigorous PA did not significantly interact with YSD in abating functional limitations. Older adults, particularly those with chronic conditions or treatment-related sequelae, engage in vigorous PA infrequently, resulting in limited variability and reduced statistical power. It is also the least accurately reported PA domain, further complicating interpretation. Additionally, very high-intensity exercise may exceed physiological tolerance in older survivors, leading to poor adherence or limited benefit [26]. Moderate and resistance-based PA appear to be the most feasible and impactful modalities for this population.
These findings align with meta-analyses showing that moderate aerobic PA and resistance training improved mobility, reduced fatigue, and better functional performance in cancer survivors [27]. Combined aerobic-resistance programs appear particularly effective across treatment and survivorship stages [28]. However, most evidence originates from controlled intervention studies with limited generalizability [29,30,31]. Through our analysis of the NHIS data, this study extends those findings to a broader survivor population and highlights how different types of PA may interact with survivorship duration to influence functional outcomes in real-world contexts.
4.2. Reverse Causation and Alternative Explanations
Reverse causation remains a critical alternative explanation to consider. Survivors with fewer functional limitations may be more likely to engage in PA, rather than PA reducing functional decline. Prior studies have shown that functional impairment predicts lower PA participation among cancer survivors [23]. Because cross-sectional data cannot disentangle these temporal pathways, longitudinal studies are needed to clarify directionality. Accordingly, the observed interaction effects should be interpreted as conditional associations rather than evidence that PA causally preserves function.
4.3. Clinical and Public Health Implications
These findings hold meaningful implications for survivorship care. The observed interaction effects suggest that moderate-intensity PA and strength training may be particularly important for long-term survivors who are biologically more vulnerable to decline. Clinical guidelines, including those from the American College of Sports Medicine, emphasize the role of continued PA in reducing treatment-related symptoms and supporting functional health throughout survivorship [32]. Routine PA assessment and functional screening should be incorporated into survivorship care plans, with referrals to accessible, age-appropriate exercise programs. Public health efforts should prioritize PA programs that are safe, feasible, and tailored to older adults given the benefits of regular participation in PA for cancer survivors [33,34], including resistance training and low- to moderate-intensity aerobic PA. These findings underscore the need for longitudinal and intervention-based studies that test whether targeted moderate-intensity aerobic PA and resistance training programs can causally preserve functional independence among long-term cancer survivors.
4.4. Limitations and Future Directions
This study has several limitations that should be considered when interpreting the findings. First, the cross-sectional design precludes causal inference. Because preserved function may also enable greater participation in PA, reverse causation cannot be ruled out. Additionally, survivorship bias may be present, as individuals who survive longer and remain sufficiently healthy to engage in PA are likely to represent a more resilient subset of cancer survivors. Second, PA measures relied on self-reported, increasing risk of bias and underestimation of true physical behaviors. Third, the functional limitation composite, though based on standardized NHIS items, does not constitute a validated clinical measure. Fourth, key cancer-related characteristics, cancer type, stage, treatment exposure, treatment end data, and comorbidity burden, were unavailable, likely introducing residual confounding. Finally, NHIS excludes institutionalized adults, long-term care, and individuals unable to complete interviews, potentially underestimating impairment among the most vulnerable survivors.
Future research should prioritize longitudinal datasets, objective PA measurement (e.g., accelerometry), validated functional assessments, and inclusion of clinical treatment history. Examining psychosocial, behavioral, and environmental determinants of PA in long-term survivorship may further clarify mechanisms linking PA and functional health.
5. Conclusions
In summary, this study found that moderate-intensity PA and strength training were more strongly associated with lower functional limitations among long-term cancer survivors, despite the absence of direct effects in the overall sample. These findings underscore the potential value of sustained, feasible PA behaviors in preserving functional ability as survivorship progresses. Tailoring PA recommendations to survivorship duration may enhance the effectiveness of clinical and public health strategies aimed at maintaining independence and quality of life in older cancer survivors [35].
Author Contributions
A.K.A.: Writing—original draft, analysis, review and editing; Z.A.: Review and editing; Z.G.: Review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All data were publicly available and fully de-identified; therefore, institutional review board approval was not required.
Informed Consent Statement
This study used fully de-identified, publicly available secondary data obtained from the IPUMS Health database. The original data collection was conducted by the National Center for Health Statistics (NCHS), which obtained informed consent from all participants at the time of data collection under approved federal protocols. The authors did not have access to any direct identifiers, nor did they interact with human subjects.
Data Availability Statement
Data can be freely downloaded from www.ipums.org.
Acknowledgments
The authors thank Jessh Mavoungou of the University of Tennessee—Knoxville for his helpful comments in the earlier stages of the manuscript development and for mentoring the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
- Variable Definitions and NHIS Variable Codes
Age: Age in years [IPUMS/NHIS code: AGE].
Sex: Biological sex at birth, 0 = female, 1 = male [IPUMS/NHIS code: SEX].
Education Attainment: categorical, with 1 = grades 1–11, 11 = terminal or professional degree [IPUMS/NHIS code: EDUC].
BMI: Body Mass Index, calculated from publicly released height and weight variables [IPUMS/NHIS code: BMI].
Years Since Diagnosis: Years since a patient’s initial cancer diagnosis (calculated as survey year minus diagnosis year, in full years) [IPUMS/NHIS code: BIRTHYR, CNXXXXAG].
Moderate PA: Moderate PA in minutes per week [IPUMS/NHIS code: MOD10DMIN].
Vigorous PA: Vigorous PA in minutes per week [IPUMS/NHIS code: VIG10DMIN].
Strength training: Strength/resistance training in sessions per week [IPUMS/NHIS code: STRONGFWK].
(DV) Functional Limitations: Four-item index measure calculated as the sum of four items:
DP1: Amount of difficulty washing or dressing (1 = none, 4 = extreme) [IPUMS/NHIS code: LAWASHDRRESDIF].
DP2: Amount of difficulty walking one block on level ground (1 = none, 4 = extreme) [IPUMS/NHIS code: WALKDIF1BL1].
DP3: Amount of difficulty walking up or down 12 steps (1 = none, 4 = extreme) [IPUMS/NHIS code: WALKDIF12SD1].
DP4: Amount of difficulty picking up objects or opening bottles (1 = none, 4 = extreme) [IPUMS/NHIS code: LAHANDDIF].
References
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022–2024; American Cancer Society: Atlanta, GA, USA, 2025. [Google Scholar]
- Brown, J.C.; Yang, S. Physical activity and mobility disability in older adult cancer survivors. JNCI Cancer Spectr. 2025, 9, pkaf084. [Google Scholar] [CrossRef] [PubMed]
- Ness, K.K.; Armstrong, G.T.; Kundu, M.; Wilson, C.L.; Tchkonia, T.; Kirkland, J.L. Frailty in childhood cancer survivors. Cancer 2015, 121, 1540–1547. [Google Scholar] [CrossRef]
- Stubblefield, M.D.; Keole, N. Physical functioning and rehabilitation in cancer survivors. CA Cancer J. Clin. 2014, 64, 43–64. [Google Scholar] [CrossRef]
- Alwhaibi, M.; Al-Ruthia, Y.; Sales, I. The impact of depression and anxiety on adult cancer patients’ health-related quality of life. J. Clin. Med. 2023, 12, 2196. [Google Scholar] [CrossRef]
- Misiąg, W.; Piszczyk, A.; Szymańska-Chabowska, A.; Chabowski, M. Physical activity and cancer care—A review. Cancers 2022, 14, 4154. [Google Scholar] [CrossRef]
- Tak, H.J.; Horner, R.D.; Lee, M.S.; Shih, Y.T. Impact of functional disability on health-care use and medical costs among cancer survivors. JNCI Cancer Spectr. 2023, 7, pkad059. [Google Scholar] [CrossRef]
- Ahmad, T.A.; Gopal, D.P.; Chelala, C.; Ullah, A.Z.D.; Taylor, S.J. Multimorbidity in people living with and beyond cancer: A scoping review. Am. J. Cancer Res. 2023, 13, 4346. [Google Scholar] [PubMed]
- Baltussen, J.C.; de Glas, N.A.; van Holstein, Y.; van der Elst, M.; Trompet, S.; den Boogaard, A.U.; van der Plas-Krijgsman, W.; Labots, G.; Holterhues, C.; van der Bol, J.M.; et al. Chemotherapy-related toxic effects and quality of life and physical functioning in older patients. JAMA Netw. Open 2023, 6, e2339116. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, A.; Vaz-Luis, I. Systemic inflammation and cancer-related frailty: Shifting the paradigm toward precision survivorship medicine. ESMO Open 2024, 9, 102205. [Google Scholar] [CrossRef]
- Locquet, M. Cancer-treatment-induced accelerated aging in older adult cancer survivors: A call for actions for future perspectives in geriatric oncology. Arch. Gerontol. Geriatr. 2025, 134, 105858. [Google Scholar] [CrossRef]
- Wang, X.S.; Woodruff, J.F. Cancer-related and treatment-related fatigue. Gynecol. Oncol. 2015, 136, 446–452. [Google Scholar] [CrossRef]
- Bellizzi, K.M.; Rowland, J.H.; Jeffery, D.D.; McNeel, T. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. J. Clin. Oncol. 2005, 23, 8884–8893. [Google Scholar] [CrossRef]
- Hardcastle, S.J.; Maxwell-Smith, C.; Cavalheri, V.; Boyle, T.; Román, M.L.; Platell, C.; Levitt, M.; Saunders, C.; Sardelic, F.; Nightingale, S.; et al. A randomized controlled trial of Promoting Physical Activity in Regional and Remote Cancer Survivors (PPARCS). J. Sport Health Sci. 2024, 13, 819. [Google Scholar] [CrossRef]
- Fong, D.Y.T.; Ho, J.W.C.; Hui, B.P.H.; Lee, A.M.; Macfarlane, D.J.; Leung, S.S.K.; Cheng, K.K.; Cerin, E.; Chan, W.Y.Y.; Leung, I.P.F.; et al. Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. BMJ 2012, 344, e70. [Google Scholar] [CrossRef]
- Liska, T.M.; Kolen, A.M. The role of physical activity in cancer survivors’ quality of life. Health Qual. Life Outcomes 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.; Niermann, C.; Jekauc, D.; Woll, A. Long-term health benefits of physical activity—A systematic review of longitudinal studies. BMC Public Health 2013, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Blewett, L.A.; Drew, J.; King, M.L.; Williams, K.C.W.; Lin, S. IPUMS Health Surveys: National Health Interview Survey; Version 7.5; IPUMS: Minneapolis, MN, USA, 2023; Available online: https://www.ipums.org/projects/ipums-health-surveys/d070.v7.1 (accessed on 11 December 2025).
- National Center for Health Statistics. National Health Interview Survey, 2021; National Center for Health Statistics: Hyattsville, MD, USA, 2021. Available online: https://www.cdc.gov/nchs/nhis.htm (accessed on 23 July 2025).
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Patient-reported versus objectively measured physical function and mortality risk among cancer survivors. J. Geriatr. Oncol. 2016, 7, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.D.; Syrjala, K.L.; Andrykowski, M.A. Physical and psychological long-term and late effects of cancer. Cancer 2008, 112, 2577–2592. [Google Scholar] [CrossRef]
- Blaney, J.M.; Lowe-Strong, A.; Rankin-Watt, J.; Campbell, A.; Gracey, J.H. Cancer survivors’ exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: A questionnaire survey. Psycho-Oncology 2013, 22, 186–194. [Google Scholar] [CrossRef]
- Blanchard, C.M.; Courneya, K.S.; Stein, K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: Results from the American Cancer Society’s SCS-II. J. Clin. Oncol. 2008, 26, 2198–2204. [Google Scholar] [CrossRef]
- Cupit-Link, M.C.; Kirkland, J.L.; Ness, K.K.; Armstrong, G.T.; Tchkonia, T.; LeBrasseur, N.K.; Armenian, S.H.; Ruddy, K.J.; Hashmi, S.K. Biology of premature ageing in survivors of cancer. ESMO Open 2017, 2, e000250. [Google Scholar] [CrossRef]
- Stene, G.B.; Helbostad, J.L.; Balstad, T.R.; Riphagen, I.I.; Kaasa, S.; Oldervoll, L.M. Effect of physical exercise on muscle mass and strength in cancer patients during treatment: A systematic review. Crit. Rev. Oncol. Hematol. 2013, 88, 573–593. [Google Scholar] [CrossRef]
- Giallauria, F.; Cirillo, P.; Pacileo, M.; Piepoli, M.F.; Vigorito, C. Exercise training in elderly cancer patients: A systematic review. Cancers 2023, 15, 1671. [Google Scholar] [CrossRef]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.T.; Hartland, M.C.; Maloney, L.T.; Davison, K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: A systematic review of meta-analyses of clinical trials. Br. J. Sports Med. 2018, 52, 1311. [Google Scholar] [CrossRef] [PubMed]
- Bettariga, F.; Taaffe, D.R.; Galvao, D.A.; Bishop, C.; Kim, J.-S.; Newton, R.U. Suppressive effects of exercise-conditioned se-rum on cancer cells: A narrative review of the influence of exercise mode, volume, and intensity. J. Sport Health Sci. 2024, 13, 48498. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ryu, S.; Zhou, W.; Adams, K.; Hassan, M.; Zhang, R.; Blaes, A.; Wolfson, J.; Sun, J. Effects of personalized exercise prescriptions and social media delivered through mobile health on cancer survivors’ physical activity and quality of life. J. Sport Health Sci. 2023, 12, 705–714. [Google Scholar] [CrossRef]
- Courneya, K.S. Exercise as a cancer treatment: New evidence from preclinical and early phase clinical studies. J. Sport Health Sci. 2025, 14, 101066. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Stamatakis, E.; Ahmadi, M.N.; Elphick, T.-L.; Huang, B.-H.; Paudel, S.; Teixeira-Pinto, A.; Chen, L.-J.; Cruz, B.d.P.; Lai, Y.-J.; Holtermann, A.; et al. Occupational physical activity, all-cause, cardiovascular disease, and cancer mortality in 349,248 adults: Prospective and longitudinal analyses of the MJ Cohort. J. Sport Health Sci. 2024, 13, 57989. [Google Scholar] [CrossRef]
- Sanchez-Lastra, M.A.; Strain, T.; Ding, D.; Dalene, K.E.; Del Pozo Cruz, B.; Ekelund, U.; Tarp, J. Associations of adiposity and device-measured physical activity with cancer incidence: UK Biobank prospective cohort study. J. Sport Health Sci. 2025, 14, 101018. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gu, S.; Bu, Z.; Liu, Z.; Dong, J.; Shi, J.; Xu, Y. Effect of exercise for patients with advanced lung cancer and cancer-related fatigue: A systematic review and meta-analysis. J. Sport Health Sci. 2025, 14, 101017. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.