Could MR Angio Replace Digital Subtraction Angiography for Verification of Occlusion Rate of Cerebral Aneurysms?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Data Storage and GDPR
2.4. Data Processing and Analysis
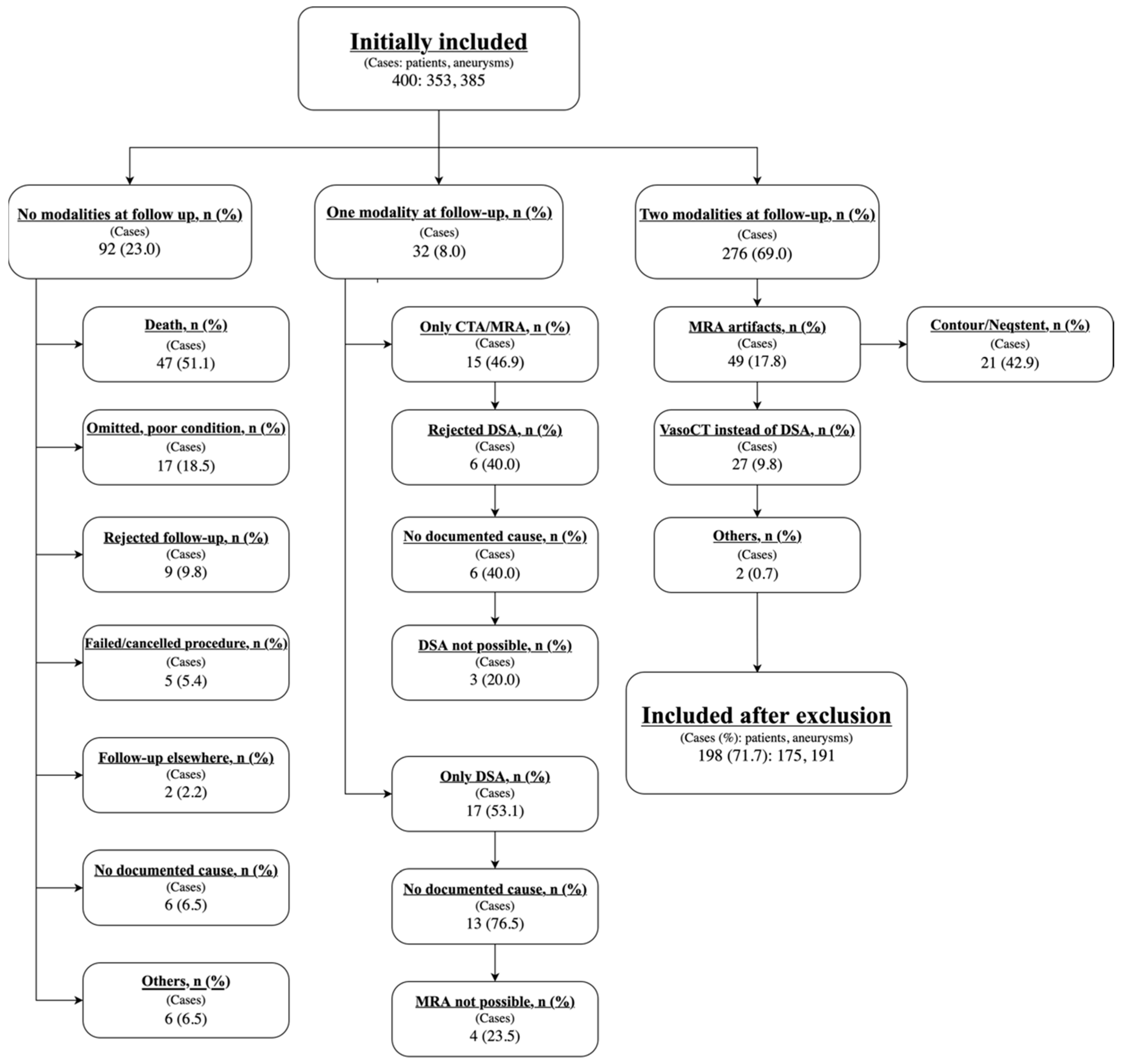
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brisman, J.L.; Song, J.K.; Newell, D.W. Cerebral aneurysms. N. Engl. J. Med. 2006, 355, 928–939. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.; Brown, R.D., Jr.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.E.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Morita, A.; Kirino, T.; Hashi, K.; Aoki, N.; Fukuhara, S.; Hashimoto, N.; Nakayama, T.; Sakai, M.; Teramoto, A.; Tominari, S.; et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [Google Scholar]
- Rehman, S.; Phan, H.T.; Reeves, M.J.; Thrift, A.G.; Cadilhac, D.A.; Sturm, J.; Breslin, M.; Callisaya, M.L.; Vemmos, K.; Parmar, P.; et al. Case-Fatality and Functional Outcome after Subarachnoid Hemorrhage (SAH) in INternational STRoke oUtComes sTudy (INSTRUCT). J. Stroke Cerebrovasc. Dis. 2022, 31, 106201. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, A.J.; Kerr, R.S.; Yu, L.M.; Clarke, M.; Sneade, M.; Yarnold, J.A.; Sandercock, P. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar] [CrossRef]
- Xin, W.Q.; Xin, Q.Q.; Yang, X.Y. Meta-Analysis of Clipping versus Coiling for the Treatment of Unruptured Middle Cerebral Artery Aneurysms: Direct Comparison of Procedure-Related Complications. Neuropsychiatr. Dis. Treat. 2019, 15, 3387–3395. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Hyun, M.K.; Lee, H.J.; Choi, J.E.; Kim, J.H.; Lee, N.R.; Kwon, J.W.; Lee, E. Endovascular coiling versus neurosurgical clipping in patients with unruptured intracranial aneurysm: A systematic review. BMC Neurol. 2012, 12, 99. [Google Scholar] [CrossRef]
- Schaafsma, J.D.; Koffijberg, H.; Buskens, E.; Velthuis, B.K.; van der Graaf, Y.; Rinkel, G.J. Cost-effectiveness of magnetic resonance angiography versus intra-arterial digital subtraction angiography to follow-up patients with coiled intracranial aneurysms. Stroke 2010, 41, 1736–1742. [Google Scholar] [CrossRef]
- Campi, A.; Ramzi, N.; Molyneux, A.J.; Summers, P.E.; Kerr, R.S.; Sneade, M.; Yarnold, J.A.; Rischmiller, J.; Byrne, J.V. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke 2007, 38, 1538–1544. [Google Scholar] [CrossRef]
- Cho, W.S.; Cho, K.I.; Kim, J.E.; Jang, T.S.; Ha, E.J.; Kang, H.S.; Son, Y.J.; Choi, S.H.; Lee, S.; Kim, C.C.; et al. Zirconia-Polyurethane Aneurysm Clip. World Neurosurg. 2018, 115, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, J.D.; Velthuis, B.K.; Majoie, C.B.; van den Berg, R.; Brouwer, P.A.; Barkhof, F.; Eshghi, O.; de Kort, G.A.; Lo, R.T.; Witkamp, T.D.; et al. Intracranial aneurysms treated with coil placement: Test characteristics of follow-up MR angiography--multicenter study. Radiology 2010, 256, 209–218. [Google Scholar] [CrossRef]
- Alakbarzade, V.; Pereira, A.C. Cerebral catheter angiography and its complications. Pract. Neurol. 2018, 18, 393–398. [Google Scholar] [CrossRef]
- Nam, H.H.; Jang, D.K.; Cho, B.R. Complications and risk factors after digital subtraction angiography: 1-year single-center study. J. Cerebrovasc. Endovasc. Neurosurg. 2022, 24, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Levent, A.; Yuce, I.; Eren, S.; Ozyigit, O.; Kantarci, M. Contrast-Enhanced and Time-of-Flight MR Angiographic Assessment of Endovascular Coiled Intracranial Aneurysms at 1.5 T. Interv. Neuroradiol. 2014, 20, 686–692. [Google Scholar] [CrossRef]
- Ledbetter, L.N.; Burns, J.; Shih, R.Y.; Ajam, A.A.; Brown, M.D.; Chakraborty, S.; Davis, M.A.; Ducruet, A.F.; Hunt, C.H.; Lacy, M.E.; et al. ACR Appropriateness Criteria® Cerebrovascular Diseases-Aneurysm, Vascular Malformation, and Subarachnoid Hemorrhage. J. Am. Coll. Radiol. 2021, 18, S283–S304. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.; Vivian, P.; Coulthard, A. Magnetic resonance angiography or digital subtraction catheter angiography for follow-up of coiled aneurysms: Do we need both? J. Med. Imaging Radiat. Oncol. 2015, 59, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Milot, G.; Raymond, J. Endovascular treatment of unruptured aneurysms. Stroke 2001, 32, 1998–2004. [Google Scholar] [CrossRef]
- Lavoie, P.; Gariépy, J.L.; Milot, G.; Jodoin, S.; Bédard, F.; Trottier, F.; Verreault, R. Residual flow after cerebral aneurysm coil occlusion: Diagnostic accuracy of MR angiography. Stroke 2012, 43, 740–746. [Google Scholar] [CrossRef]
- Ayabe, Y.; Hamamoto, K.; Yoshino, Y.; Ikeda, Y.; Chiba, E.; Yuzawa, H.; Oyama-Manabe, N. Ultra-short Echo-time MR Angiography Combined with a Subtraction Method to Assess Intracranial Aneurysms Treated with a Flow-diverter Device. Magn. Reson. Med. Sci. 2023, 22, 117–125. [Google Scholar] [CrossRef]
- van Amerongen, M.J.; Boogaarts, H.D.; de Vries, J.; Verbeek, A.L.; Meijer, F.J.; Prokop, M.; Bartels, R.H. MRA versus DSA for follow-up of coiled intracranial aneurysms: A meta-analysis. AJNR Am. J. Neuroradiol. 2014, 35, 1655–1661. [Google Scholar] [CrossRef]
- Ahmed, S.U.; Mocco, J.; Zhang, X.; Kelly, M.; Doshi, A.; Nael, K.; De Leacy, R. MRA versus DSA for the follow-up imaging of intracranial aneurysms treated using endovascular techniques: A meta-analysis. J. Neurointerv. Surg. 2019, 11, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
| Included in Analysis (n = 198) | |
|---|---|
| Males cases, n (%) | 61 (30.8) |
| Female cases, n (%) | 137 (69.2) |
| Age at treatment, mean (SD) | 56.8 (11.5) |
| Male cases | 55.7 (12.5) |
| Female cases | 57.3 (11.0) |
| Aneurysm rupture status, n (%) | |
| Ruptured aneurysms | 88 (44.4) |
| Unruptured aneurysms | 85 (42.9) |
| Planned retreatment, unruptured | 25 (12.6) |
| Aneurysm size, mm3, median (IQR) | 99.5 [36.0–367.5] |
| Missing or insufficient data | 44 |
| Months from treatment to follow-up, median (IQR) | 11.0 [8.8–17.3] |
| Ruptured aneurysms | 9.2 [8.5–10.3] |
| Unruptured aneurysms | 14.5 [9.3–18.1] |
| Planned retreatment, unruptured | 17.4 [16.3–18.7] |
| Location of treated and retreated aneurysm, n (%) | |
| MCA | 16 (8.1) |
| Basilar bifurcation | 14 (7.1) |
| ICA | 41 (20.7) |
| Posterior communicating | 29 (14.6) |
| Anterior communicating | 64 (32.3) |
| Posterior circulation (others) | 23 (11.6) |
| Anterior circulation (others) | 11 (5.6) |
| Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | |
|---|---|---|---|---|
| Ruptured | 77.8 (52.4–93.6) | 78.6 (67.1–87.5) | 48.3 (35.9–60.9) | 93.2 (85.2–97.1) |
| Unruptured | 58.3 (27.7–84.8) | 83.6 (73.1–91.2) | 36.8 (22.4–54.1) | 92.4 (86.1–96.0) |
| Retreatment | 66.7 (9.4–99.2) | 72.7 (49.8–89.3) | 25.0 (10.4–48.8) | 94.1 (76.0–98.8) |
| Overall | 69.7 (51.3–84.4) | 80.0 (73.1–85.8) | 41.1 (32.3–50.5) | 93.0 (88.7–95.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmager, R.M.; Jensen, J.; Nielsen, T.H.; Munthe, S. Could MR Angio Replace Digital Subtraction Angiography for Verification of Occlusion Rate of Cerebral Aneurysms? J. Clin. Med. 2025, 14, 8221. https://doi.org/10.3390/jcm14228221
Holmager RM, Jensen J, Nielsen TH, Munthe S. Could MR Angio Replace Digital Subtraction Angiography for Verification of Occlusion Rate of Cerebral Aneurysms? Journal of Clinical Medicine. 2025; 14(22):8221. https://doi.org/10.3390/jcm14228221
Chicago/Turabian StyleHolmager, Rasmus Moldt, Jonas Jensen, Troels Halfeld Nielsen, and Sune Munthe. 2025. "Could MR Angio Replace Digital Subtraction Angiography for Verification of Occlusion Rate of Cerebral Aneurysms?" Journal of Clinical Medicine 14, no. 22: 8221. https://doi.org/10.3390/jcm14228221
APA StyleHolmager, R. M., Jensen, J., Nielsen, T. H., & Munthe, S. (2025). Could MR Angio Replace Digital Subtraction Angiography for Verification of Occlusion Rate of Cerebral Aneurysms? Journal of Clinical Medicine, 14(22), 8221. https://doi.org/10.3390/jcm14228221






