Robotic Versus Sternotomy Approach for Left Atrial Myxoma Resection: A Retrospective Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Approval
2.3. Robotic Surgical Technique
2.4. Preparation
2.5. Exposition
2.6. Operation
2.7. Completion
2.8. Follow-Up and Study Endpoints
- −
- Stroke: new focal neurological deficit lasting ≥ 24 h and confirmed by cerebral imaging.
- −
- Bleeding: transfusion of >2 units of packed red cells within 24 h or re-exploration for bleeding.
- −
- Acute kidney injury (AKI): AKIN stage ≥ 3 (serum creatinine ≥ 3 × baseline or new dialysis requirement).
- −
- Pneumonia: new radiographic infiltrate associated with fever > 38 °C, leukocytosis or leukopenia, and positive sputum or bronchoalveolar culture.
- −
- Wound or port-site infection: erythema, drainage, or collection requiring antibiotic therapy or surgical revision.
- −
- Tamponade: postoperative pericardial effusion requiring surgical evacuation.
- −
- Transfusion: administration of any allogeneic red blood cell unit postoperatively.
2.9. Statistical Analysis
3. Results
3.1. Study Population
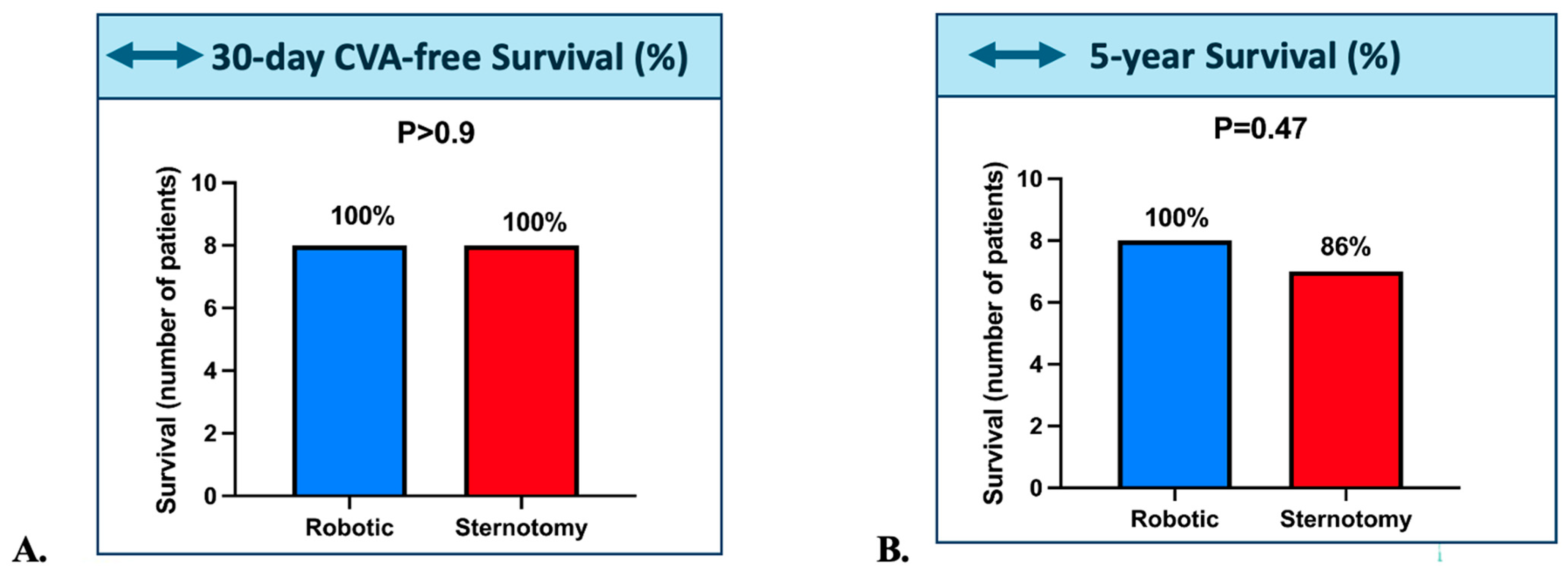
3.2. Survival Outcomes
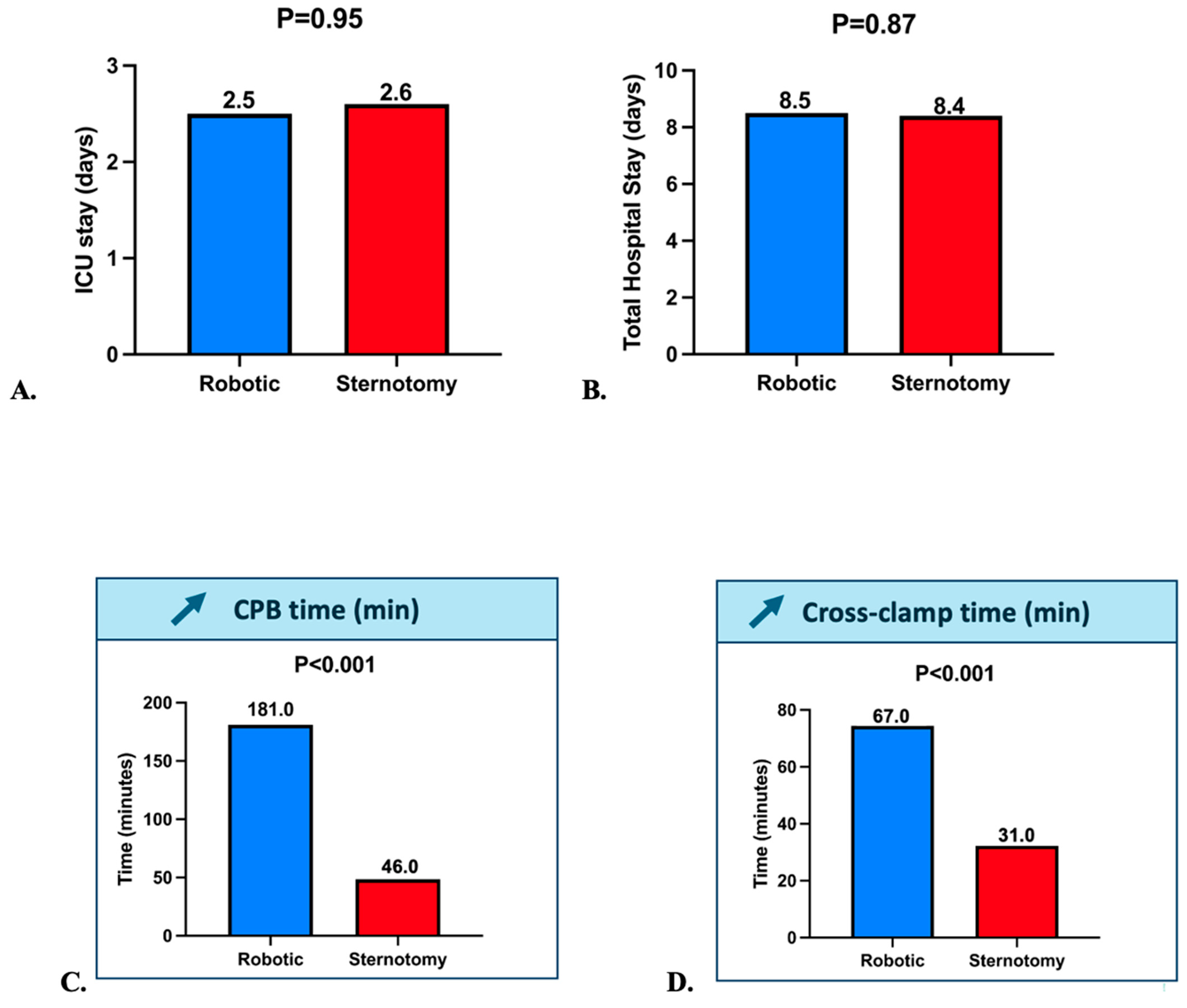
3.3. Intraoperative and Postoperative Outcomes
3.4. Sensitivity Analysis
4. Discussion
4.1. Summary of Findings
4.2. Comparison with Existing Literature
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKIN | Acute Kidney Injury Network |
| ARDS | Acute Respiratory Distress Syndrome |
| CPB | Cardiopulmonary Bypass |
| COPD | Chronic Obstructive Pulmonary Disease |
| CVA | Cerebrovascular Accident |
| HTK (Custodiol®) | Histidine-Tryptophan-Ketoglutarate cardioplegic solution |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| LVEF | Left Ventricular Ejection Fraction |
| MI | Myocardial Infarction |
| NYHA | New York Heart Association (functional classification) |
| PAD | Peripheral Artery Disease |
| PM | Pacemaker |
| sPAP | Systolic Pulmonary Artery Pressure |
| TEE | Transesophageal Echocardiography |
References
- Cerny, S.; Oosterlinck, W.; Onan, B.; Singh, S.; Segers, P.; Bolcal, C.; Alhan, C.; Navarra, E.; Pettinari, M.; Van Praet, F.; et al. Robotic Cardiac Surgery in Europe: Status 2020. Front. Cardiovasc. Med. 2022, 8, 827515. [Google Scholar] [CrossRef]
- Bush, B.; Nifong, L.W.; Chitwood, W.R., Jr. Robotics in cardiac surgery: Past present, and future. Rambam Maimonides Med. J. 2013, 4, e0017. [Google Scholar] [CrossRef]
- Schilling, J.; Engel, A.M.; Hassan, M.; Smith, J.M. Robotic excision of atrial myxoma. J. Card. Surg. 2012, 27, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Kesävuori, R.; Raivio, P.; Jokinen, J.J.; Sahlman, A.; Vento, A. Quality of life after robotically assisted atrial myxoma excision. J. Robot. Surg. 2015, 9, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yang, M.; Wang, G.; Wang, J.; Xiao, C.; Wu, Y.; Li, J. Excision of atrial myxoma using robotic technology. J. Thorac. Cardiovasc. Surg. 2010, 139, 1282–1285. [Google Scholar] [CrossRef]
- Zhuli, Y.; Su, C.; Shen, L.; Yang, F.; Zhou, J. Improved robotic-assisted cardiac surgery outcomes with greater hospital volume: A national representative cohort analysis of 10,543 cardiac surgeries. J. Robot. Surg. 2025, 19, 142. [Google Scholar] [CrossRef]
- Wah, J.N.K. The robotic revolution in cardiac surgery. J. Robot. Surg. 2025, 19, 386. [Google Scholar] [CrossRef]
- Currie, M.E.; Romsa, J.; Fox, S.A.; Vezina, W.C.; Akincioglu, C.; Warrington, J.C.; McClure, R.S.; Stitt, L.W.; Menkis, A.H.; Boyd, W.D.; et al. Long-term angiographic follow-up of robotic-assisted coronary artery revascularization. Ann. Thorac. Surg. 2012, 93, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.P.; Casselman, F.; Bakir, I.; Cammu, G.; Wellens, F.; De Geest, R.; Degrieck, I.; Van Praet, F.; Vermeulen, Y.; Vanermen, H. Endoscopic cardiac tumor resection. Ann. Thorac. Surg. 2007, 83, 2142–2216. [Google Scholar] [CrossRef]
- Gao, C.; Yang, M.; Wang, G.; Wang, J. Totally robotic resection of myxoma and atrial septal defect repair. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Smith, J.M. Robotic assisted excision of a left ventricular myxoma. Interact. Cardiovasc Thorac. Surg. 2012, 14, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.A.; Miller, J.S.; Langford, D.A. Robot-assisted endoscopic excision of left atrial myxomas. J. Thorac. Cardiovasc. Surg. 2005, 130, 596–597. [Google Scholar] [CrossRef]
- Nifong, L.W.; Rodriguez, E.; Chitwood, W.R., Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann. Thorac. Surg. 2012, 94, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Bonaros, N.; Schachner, T.; Wiedemann, D.; Oehlinger, A.; Ruetzler, E.; Feuchtner, G.; Kolbitsch, C.; Velik-Salchner, C.; Friedrich, G.; Pachinger, O.; et al. Quality of life improvement after robotically assisted coronary artery bypass grafting. Cardiology 2009, 114, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.; Peacock, J.C.; Kohmoto, T.; Garrido, M.J.; Schanzer, B.M.; Kherani, A.R.; Vigilance, D.W.; Cheema, F.H.; Kaplan, S.; Smith, C.R.; et al. Robotic techniques improve quality of life in patients undergoing atrial septal defect repair. Ann. Thorac. Surg. 2004, 77, 1328–1333. [Google Scholar] [CrossRef]
- Suri, R.M.; Antiel, R.M.; Burkhart, H.M.; Huebner, M.; Li, Z.; Eton, D.T.; Topilsky, T.; Sarano, M.E.; Schaff, H.V. Quality of life after early mitral valve repair using conventional and robotic approaches. Ann. Thorac. Surg. 2012, 93, 761–769. [Google Scholar] [CrossRef]
- Russo, M.J.; Martens, T.P.; Hong, K.N.; Colman, D.; Voleti, V.; Smith, C.; Argenziano, M. Minimally invasive versus standard approach for excision of atrial masses. Heart Surg. Forum 2007, 10, E50–E54. [Google Scholar] [CrossRef]
- Mihaljevic, T.; Jarrett, C.M.; Gillinov, A.M.; Williams, S.J.; DeVilliers, P.A.; Stewart, W.J.; Svensson, L.G.; Sabik, J.F.; Blackstone, E.H. Robotic repair of posterior mitral valve prolapse versus conventional approaches. J. Thorac. Cardiovasc. Surg. 2011, 141, 72–80.e1-4. [Google Scholar] [CrossRef]
- Mihaljevic, T.; Paul, S.; Byrne, J.G.; Leacche, M.; Farivar, R.S.; Soltesz, E.G.; Rawn, J.D.; Cohn, L.H. Robotic mammary takedown and off-pump bypass surgery. Am. J. Cardiol. 2003, 92, 1222–1224. [Google Scholar] [CrossRef]
- Yamashita, Y.; Torregrossa, G.; Sicouri, S.; Wertan, M.A.C.; Spragan, D.D.; Ramlawi, B.; Sutter, F.P. Robotic-Assisted Redo Coronary Artery Bypass Grafting: A Single-Center Experience. Ann. Thorac. Cardiovasc. Surg. 2025, 31, 25-00026. [Google Scholar] [CrossRef]
- Piperata, A.; Busuttil, O.; d’Ostrevy, N.; Jansens, J.-L.; Taymoor, S.; Cuko, B.; Modine, T.; Pernot, M.; Labrousse, L. Starting A New Robotic Surgery Program for Mitral Valve Repair. Lessons Learned from The First Nine Months. J. Clin. Med. 2021, 10, 5439. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ogihara, Y.; Takagi, S.; Yanagisawa, J.; Okawa, Y. Totally Endoscopic Internal Thoracic Artery Harvesting with Efficient Setup Modifications for Minimally Invasive Direct Coronary Artery Bypass Grafting. Ann. Thorac. Cardiovasc. Surg. 2025, 31, 25-00007. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, E.F.; Maier, R.H.; Hancock, H.C.; Kharatikoopaei, E.; Vale, L.; Fernandez-Garcia, C.; Ogundimu, E.; Wagnild, J.; Mathias, A.; Walmsley, Z.; et al. UK Mini Mitral Trial Investigators. Minithoracotomy vs Conventional Sternotomy for Mitral Valve Repair: A Randomized Clinical Trial. JAMA 2023, 329, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
| Variable | Sternotomy (Median [IQR]) | Robotic (Median [IQR]) | p-Value |
|---|---|---|---|
| Age | 66.6 [62.0–71.0] | 58.0 [53.2–67.8] | 0.312 |
| Weight (kg) | 78.5 [62.5–88.0] | 66.3 [63.0–75.0] | 0.289 |
| Height (cm) | 165.5 [158.8–170.5] | 165.5 [161.5–171.2] | 0.875 |
| Hypertension | 0.0 [0.0–1.0] | 0.0 [0.0–0.5] | 0.777 |
| Dyslipidemia | 0.0 [0.0–0.2] | 0.0 [0.0–0.5] | 0.94 |
| Tobacco use | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.54 |
| NYHA | 0.2 | ||
| I | 1 | 4 | |
| II | 3 | 0 | |
| III | 2 | 4 | |
| IV | 2 | 0 | |
| LVEF (%) | 60.0 [60.0–64.2] | 63.0 [59.0–66.0] | 0.789 |
| sPAP (mmHg) | 25.0 [25.0–26.5] | 24.0 [23.0–25.0] | 0.335 |
| Prior PM | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1 |
| Prior Cardiac Surgery | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| PAD | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Prior Stroke | 0/8 (0.0%) | 1/7 (14.3%) | 0.467 |
| Diabetes | 1/8 (12.5%) | 1/8 (12.5%) | 1 |
| Chronic renal failure | 2/8 (25.0%) | 0/8 (0.0%) | 0.467 |
| Dialysis | 1/8 (12.5%) | 0/8 (0.0%) | 1 |
| COPD | 1/8 (12.5%) | 1/8 (12.5%) | 1 |
| Endocaditis | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| History of MI | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Prior Anticoagulation | 1/8 (12.5%) | 0/8 (0.0%) | 1 |
| Prior Antiaggregation | 4/8 (50.0%) | 2/7 (28.6%) | 0.608 |
| EuroSCORE II (%) | 1.3 [1.0–2.4] | 0.8 [0.6–0.9] | 0.004 |
| CPB Time (min) | 46.0 [34.0–54.0] | 181.0 [169.0–206.0] | 0.001 |
| Cross-clamp time (min) | 31.0 [24.0–39.0] | 67.0 [64.0–74.4] | 0.001 |
| Variable | Sternotomy (Median [IQR]) | Robotic (Median [IQR]) | p-Value |
|---|---|---|---|
| 5-Year Death | 1/8 (12.5%) | 0/8 (0.0%) | 1 |
| Bleeding | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Tamponnade | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Operative site Infection | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Reoperation | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Postoperative MI | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Post-operative PM | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Post-operative stroke | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Renal failure (AKIN 3) | 1/8 (12.5%) | 0/8 (0.0%) | 1 |
| ARDS | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Pneumonia | 0/8 (0.0%) | 0/8 (0.0%) | 1 |
| Postop Transfusion | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1 |
| UTI | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiydoun, G.; Saade, S.; Radu, C.; Bergoend, E.; Folliguet, T. Robotic Versus Sternotomy Approach for Left Atrial Myxoma Resection: A Retrospective Single-Center Study. J. Clin. Med. 2025, 14, 8220. https://doi.org/10.3390/jcm14228220
Saiydoun G, Saade S, Radu C, Bergoend E, Folliguet T. Robotic Versus Sternotomy Approach for Left Atrial Myxoma Resection: A Retrospective Single-Center Study. Journal of Clinical Medicine. 2025; 14(22):8220. https://doi.org/10.3390/jcm14228220
Chicago/Turabian StyleSaiydoun, Gabriel, Saadé Saade, Costin Radu, Eric Bergoend, and Thierry Folliguet. 2025. "Robotic Versus Sternotomy Approach for Left Atrial Myxoma Resection: A Retrospective Single-Center Study" Journal of Clinical Medicine 14, no. 22: 8220. https://doi.org/10.3390/jcm14228220
APA StyleSaiydoun, G., Saade, S., Radu, C., Bergoend, E., & Folliguet, T. (2025). Robotic Versus Sternotomy Approach for Left Atrial Myxoma Resection: A Retrospective Single-Center Study. Journal of Clinical Medicine, 14(22), 8220. https://doi.org/10.3390/jcm14228220






