Clinical Utility of Pan-Immune Inflammation Value (PIV) in Predicting Prognosis of Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
Calculation of Systemic Inflammatory Indices
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EC | Endometrial Cancer |
| PIV | Pan-Immune Inflammation Value |
| SII | Systemic Immune Inflammation Index |
| SIRI | Systemic Inflammation Response Index |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| PLR | Platelet-to-Lymphocyte Ratio |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| LVSI | Lymphovascular Space Invasion |
| PNI | Perineural Invasion |
| FIGO | International Federation of Gynecology and Obstetrics |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| BMI | Body Mass Index |
| RT | Radiotherapy |
| CT | Chemotherapy |
| pT | Pathological T Stage |
| VEGF | Vascular Endothelial Growth Factor |
| POLEmut | Polymerase Epsilon Mutated |
| dMMR | Deficient Mismatch Repair |
| MSI-high | High Microsatellite Instability |
| p53abn | Abnormal p53 Expression |
| NSMP | No Specific Molecular Profile |
| CI | Confidence Interval |
| HR | Hazard Ratio |
| SPSS | Statistical Package for the Social Sciences |
References
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef] [PubMed]
- eBioMedicine. Endometrial cancer: Improving management among increasing incidence rates. eBioMedicine 2024, 103, 105159. [Google Scholar] [CrossRef]
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and management of endometrial cancer. Am. Fam. Physician 2016, 93, 468–474. [Google Scholar]
- Paleari, L.; Pesce, S.; Rutigliani, M.; Greppi, M.; Obino, V.; Gorlero, F.; Vellone, V.G.; Marcenaro, E. New insights into endometrial cancer. Cancers 2021, 13, 1496. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Broaddus, R.R. Endometrial cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Mitric, C.; Bernardini, M.Q. Endometrial cancer: Transitioning from histology to genomics. Curr. Oncol. 2022, 29, 741–757. [Google Scholar] [CrossRef]
- Jamieson, A.; Bosse, T.; McAlpine, J.N. The emerging role of molecular pathology in directing the systemic treatment of endometrial cancer. Ther. Adv. Med. Oncol. 2021, 13, 1–14. [Google Scholar] [CrossRef]
- Njoku, K.; Barr, C.E.; Crosbie, E.J. Current and emerging prognostic biomarkers in endometrial cancer. Front. Oncol. 2022, 12, 890908. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Zhu, Y.; Wu, Q.; Yao, C.; Xia, H.; Li, C. Postoperative systemic immune-inflammation index (SII): A superior prognostic factor of endometrial cancer. Front. Surg. 2021, 8, 704235. [Google Scholar] [CrossRef]
- Abu-Shawer, O.; Abu-Shawer, M.; Hirmas, N.; Alhouri, A.; Massad, A.; Alsibai, B.; Sultan, H.; Hammo, H.; Souleiman, M.; Shebli, Y.; et al. Hematologic markers of distant metastases and poor prognosis in gynecological cancers. BMC Cancer 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.D.; McMillan, D.C. The prevalence of cancer associated systemic inflammation: Implications of prognostic studies using the Glasgow Prognostic Score. Crit. Rev. Oncol. Hematol. 2020, 150, 102962. [Google Scholar] [CrossRef]
- Collde la Rubia, E.; Martinez-Garcia, E.; Dittmar, G.; Gil-Moreno, A.; Cabrera, S.; Colas, E. Prognostic biomarkers in endometrial cancer: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 1900. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Abu-Shawer, M.; Abu-Shawer, O.; Souleiman, M.; Akkawi, M.; Alshakhatreh, O.; Altamimi, T.; Al-Omari, A.; Al-Hussaini, M. Hematologic markers of lung metastasis in stage IV colorectal cancer. J. Gastrointest. Cancer 2019, 50, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.M.; Nakagawa, S.; Toihata, T.; Harada, K.; Iwatsuki, M.M.; Hayashi, H.M.; Miyamoto, Y.M.; Yoshida, N.M.; Baba, H.M. Pan-immune-inflammation value and prognosis in patients with esophageal cancer. Ann. Surg. Open 2022, 3, e113. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Z.; Chen, Y. Prognostic value of pretreatment systemic immune-inflammation index in gastric cancer: A meta-analysis. Front. Oncol. 2021, 11, 537140. [Google Scholar] [CrossRef] [PubMed]
- Jomrich, G.; Gruber, E.S.; Winkler, D.; Hollenstein, M.; Gnant, M.; Sahora, K.; Schindl, M. Systemic immune-inflammation in-dex (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J. Gastrointest. Surg. 2020, 24, 610–618. [Google Scholar] [CrossRef]
- Mouchemore, K.A.; Anderson, R.L.; Hamilton, J.A. Neutrophils, G-CSF and their contribution to breast cancer metastasis. FEBS J. 2018, 285, 665–679. [Google Scholar] [CrossRef]
- Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; Gonzalez-Martin, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO con-sensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Int. J. Gynecol. Cancer 2016, 26, 2–30. [Google Scholar] [CrossRef]
- Liao, W.; Li, J.; Feng, W.; Kong, W.; Shen, Y.; Chen, Z.; Yang, H. Pan-immune-inflammation value: A new prognostic index in epithelial ovarian cancer. BMC Cancer 2024, 24, 1052. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Kilickap, S.; Gambichler, T.; Aksoy, S. The association between the pan-immune-inflammation value and cancer prognosis: A systematic review and meta-analysis. Cancers 2022, 14, 2675. [Google Scholar] [CrossRef]
- Yan, S.; Gong, X.; Liu, R.; Jia, X. Prognostic significance of systemic pan-immune-inflammation value in locally ad-vanced cervical cancer. Front. Oncol. 2024, 14, 1492251. [Google Scholar] [CrossRef]
- Chalabiyev, E.; Ismayilov, R.; Kus, F.; Akyildiz, A.; Guven, D.C.; Yildirim, H.C.; Kirmizigul, B.; Koksal, B.; Kavgaci, G.; Arik, Z. Prognostic and predictive significance of the pan-immune-inflammation value in endometrial cancer patients undergoing adjuvant therapy. Rep. Pract. Oncol. Radiother. 2025, 30, 202–209. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: A meta-analysis. World J. Surg. Oncol. 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.; Merone, L.; Keeble, C.; Burland, L.; Grzelinski, M.; Sutton, K.; Begum, N.; Thacoor, A.; Green, B.; Sarveswaran, J.; et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br. J. Cancer 2015, 113, 311–320. [Google Scholar] [CrossRef]
- Tu, Y.; Jiang, P.; Wang, J.; Huang, Y.; Kong, W.; Li, N.; Zheng, Y.; Zhou, Q.; Gou, S.; Tian, C.; et al. A novel systemic inflammation response index (SIRI) for predicting postoperative survival of patients with endometrial cancer. Front. Oncol. 2022, 12, 919422. [Google Scholar] [CrossRef]
- Templeton, A.J.; Ace, O.; McNamara, M.G.; Al-Mubarak, M.; Vera-Badillo, F.E.; Hermanns, T.; Šeruga, B.; Ocaña, A.; Tannock, I.F.; Amir, E. Prognostic role of platelet to lymphocyte ratio in solid tumors: A systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pandey, A.; Dimri, K.; Sehgal, A.; Bhagat, R.; Gill, G. Endometrial cancer risk factors, treatment, and survival outcomes as per the ESMO-ESGO-ESTRO risk groups and FIGO staging: An experience from developing world. J. Cancer Res. Ther. 2023, 19, 701–707. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Q.; Zhu, L.; Zhang, Y.; Lu, X.; Wu, Y.; Liu, L. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci. Rep. 2019, 9, 3284. [Google Scholar] [CrossRef] [PubMed]

| AUC | Std. Error | 95% CI | Sensitivity | Specificity | Cut-Off Value | p | |
|---|---|---|---|---|---|---|---|
| PIV | 0.776 | 0.06 | 0.659–0.893 | 70.4% | 82.4% | 802 | 0.001 |
| SII | 0.747 | 0.063 | 0.623–0.870 | 63% | 74.5% | 1545 | 0.001 |
| SIRI | 0.718 | 0.068 | 0.585–0.850 | 81.5% | 41.2% | 1.20 | 0.002 |
| NLR | 0.713 | 0.064 | 0.587–0.839 | 85.2% | 45.1% | 2.50 | 0.002 |
| PLR | 0.686 | 0.069 | 0.551–0.820 | 85.2% | 32.3% | 120.5 | 0.007 |
| Variable | Category | Total (n, %) | PIV < 802 (n, %) | PIV ≥ 802 (n, %) | p |
|---|---|---|---|---|---|
| Age | <65 | 48 (61.5) | 33 (66.0) | 15 (53.6) | 0.200 |
| ≥65 | 30 (38.5) | 17 (34.0) | 13 (46.4) | ||
| Menopausal status | Premenopausal | 28 (35.9) | 18 (36.0) | 10 (35.7) | 0.590 |
| Postmenopausal | 50 (64.1) | 32 (64.0) | 18 (64.3) | ||
| ECOG PS | 0–1 | 65 (83.3) | 44 (88.0) | 21 (75.0) | 0.124 |
| ≥2 | 13 (16.7) | 6 (12.0) | 7 (25.0) | ||
| Smoking | No | 59 (75.6) | 38 (76.0) | 21 (75.0) | 0.564 |
| Yes | 19 (24.4) | 12 (24.0) | 7 (25.0) | ||
| Obesity | No | 46 (59.0) | 25 (50.0) | 21 (75.0) | 0.029 |
| Yes | 32 (41.0) | 25 (50.0) | 7 (25.0) | ||
| Comorbidity | No | 29 (37.2) | 18 (36.0) | 11 (39.3) | 0.480 |
| Yes | 49 (62.8) | 32 (64.0) | 17 (60.7) | ||
| Grade | 1 | 32 (41.0) | 22 (44.0) | 10 (35.7) | |
| 2 | 17 (21.8) | 10 (20.0) | 7 (25.0) | 0.581 | |
| 3 | 29 (37.2) | 18 (36.0) | 11 (39.3) | ||
| FIGO stage | I–II | 50 (64.1) | 33 (66) | 17 (60.7) | 0.410 |
| III–IV | 28 (35.9) | 17 (34) | 11 (39.3) | ||
| LVSI | Absent | 57 (73.1) | 37 (74.0) | 20 (71.4) | 0.503 |
| Present | 21 (26.9) | 13 (26.0) | 8 (28.6) | ||
| PNI | Absent | 68 (87.2) | 47 (94.0) | 21 (75.0) | 0.022 |
| Present | 10 (12.8) | 3 (6.0) | 7 (25.0) | ||
| Adjuvant RT | No | 42 (53.8) | 32 (64.0) | 10 (35.7) | 0.015 |
| Yes | 36(46.2) | 18 (36.0) | 18 (64.3) | ||
| Adjuvant CT | No | 51 (65.4) | 35 (70.0) | 16 (57.1) | 0.185 |
| Yes | 27 (34.6) | 15 (30.0) | 12 (42.9) | ||
| Peritoneal involvement | Absent | 65 (83.3) | 42 (84.0) | 23 (82.1) | 0.534 |
| Present | 13 (16.7) | 8 (16.0) | 5 (17.9) | ||
| Distant metastasis | Absent | 63 (80.8) | 40 (80.0) | 23 (82.1) | 0.535 |
| Present | 15 (19.2) | 10 (20.0) | 5 (17.9) | ||
| Risk group | Low | 47 (60.3) | 32 (64.0) | 15 (53.6) | 0.253 |
| High | 31 (39.7) | 18 (36.0) | 13 (46.4) | ||
| Nodal involvement | Absent | 59 (75.6) | 40 (80.0) | 19 (67.9) | 0.177 |
| Present | 19 (24.4) | 10 (20.0) | 9 (32.1) | ||
| pT stage | 1–2 | 50 (64.1) | 33 (66.0) | 17 (60.7) | 0.786 |
| 3–4 | 28 (35.9) | 17 (34.0) | 11 (39.3) | ||
| Stage | I–II | 50 (64.1) | 33 (66.0) | 17 (60.7) | 0.410 |
| III–IV | 28 (35.9) | 17 (34.0) | 11 (39.3) | ||
| SII | <1545 | 53(67.9) | 50 (100.0) | 3 (10.7) | <0.001 |
| ≥1545 | 25 (22.1) | 0 (0.0) | 25 (89.3) | ||
| SIRI | <1.5 | 43 (55.1) | 42 (84.0) | 1 (3.6) | <0.001 |
| ≥1.5 | 35 (44.9) | 8 (16.0) | 27 (96.4) | ||
| NLR | <2.5 | 38 (48.7) | 37 (74.0) | 1 (3.6) | <0.001 |
| ≥2.5 | 40 (51.3) | 13 (26.0) | 27 (96.4) | ||
| PLR | <120.5 | 22 (28.2) | 21 (42.0) | 1 (3.6) | <0.001 |
| ≥120.5 | 56 (71.8) | 29 (58.0) | 27 (96.4) |
| Overall Survival | Progression-Free Survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (≥65 vs. <65) | 2.26 | 1.04–4.93 | 0.040 | 1.98 | 0.89–4.38 | 0.090 |
| Menopausal status | 0.72 | 0.33–1.57 | 0.409 | 0.66 | 0.30–1.47 | 0.313 |
| ECOG (≥2 vs. 0–1) | 0.90 | 0.34–2.39 | 0.825 | 0.73 | 0.25–2.17 | 0.578 |
| Smoking (Yes vs. No) | 0.83 | 0.31–2.19 | 0.703 | 0.87 | 0.33–2.33 | 0.787 |
| Obesity (Yes vs. No) | 0.35 | 0.14–0.86 | 0.023 | 0.38 | 0.16–0.95 | 0.038 |
| Comorbidity (Yes vs. No) | 1.35 | 0.61–2.99 | 0.457 | 1.21 | 0.54–2.72 | 0.634 |
| Tumor grade (2–3 vs. 1) | 2.07 | 1.28–3.33 | 0.003 | 2.07 | 1.29–3.34 | 0.003 |
| LVSI (Present vs. Absent) | 3.12 | 1.38–7.03 | 0.006 | 2.85 | 1.26–6.47 | 0.012 |
| PNI (Present vs. Absent) | 3.89 | 1.53–9.94 | 0.004 | 3.27 | 1.25–8.59 | 0.016 |
| Adjuvant RT (Yes vs. No) | 1.56 | 0.72–3.41 | 0.262 | 1.50 | 0.69–3.29 | 0.301 |
| Adjuvant CT (Yes vs. No) | 4.04 | 1.82–8.99 | 0.001 | 3.58 | 1.62–7.93 | 0.002 |
| Peritoneal involvement | 2.28 | 0.91–5.75 | 0.080 | 2.39 | 0.95–6.06 | 0.065 |
| Distant metastasis | 2.55 | 1.06–6.16 | 0.037 | 2.48 | 1.02–6.01 | 0.044 |
| Risk group (High vs. Low) | 4.83 | 2.14–10.86 | <0.001 | 4.35 | 1.93–9.79 | <0.001 |
| Nodal involvement | 2.18 | 0.93–5.08 | 0.072 | 1.89 | 0.81–4.44 | 0.144 |
| Pathological T stage (pT3–4 vs. pT1–2) | 2.41 | 1.23–4.71 | 0.010 | 2.26 | 1.16–4.44 | 0.017 |
| FIGO stage (III–IV vs. I–II) | 3.08 | 1.41–6.71 | 0.005 | 2.84 | 1.30–6.21 | 0.009 |
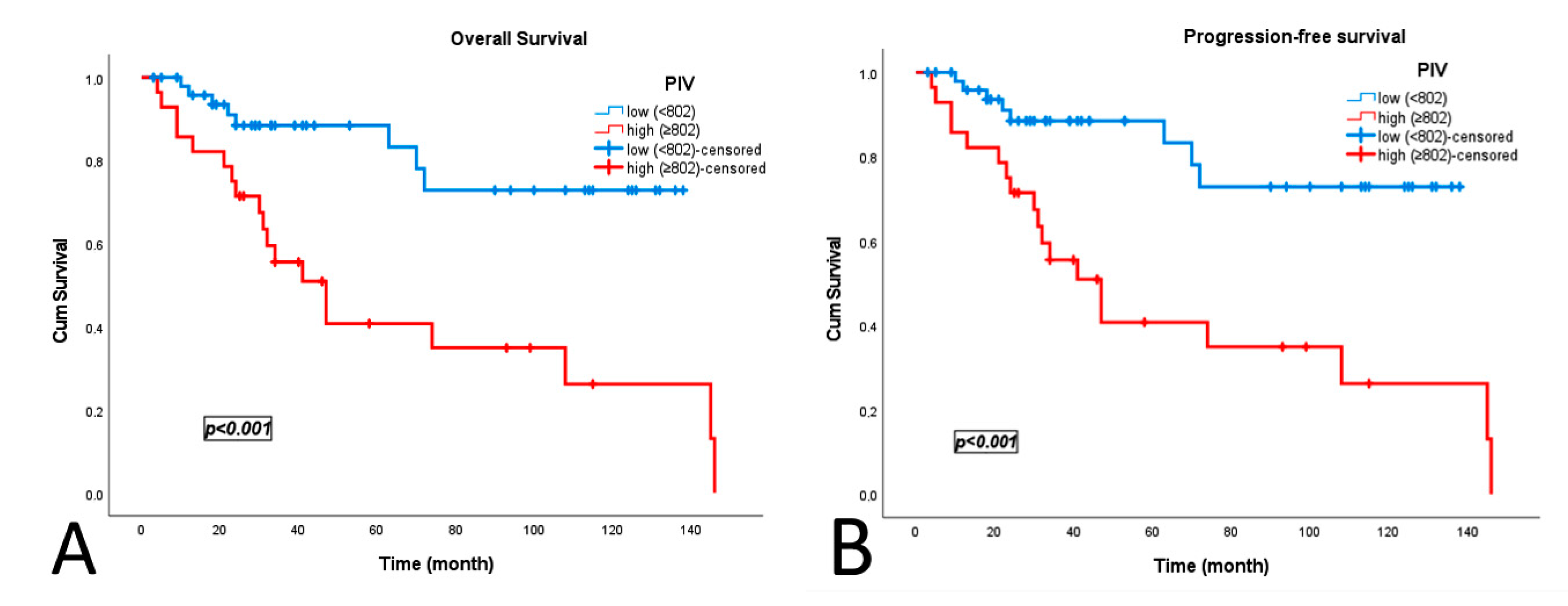
| PIV ≥ 802 | 4.54 | 1.96–10.47 | <0.001 | 4.02 | 1.73–9.32 | 0.001 |
| SII ≥ 1545 | 3.65 | 1.65–8.05 | 0.001 | 3.28 | 1.47–7.30 | 0.004 |
| SIRI ≥ 1.5 | 3.37 | 1.46–7.79 | 0.004 | 2.94 | 1.26–6.82 | 0.012 |
| NLR ≥ 2.5 | 4.03 | 1.60–10.10 | 0.003 | 3.65 | 1.45–9.17 | 0.006 |
| PLR ≥ 120.5 | 2.43 | 0.91–6.48 | 0.077 | 2.22 | 0.83–5.96 | 0.113 |
| Overall Survival | Progression-Free Survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (≥65 vs. <65) | 1.97 | 0.74–5.21 | 0.172 | - | - | - |
| Obesity (Yes vs. No) | 0.82 | 0.28–2.34 | 0.704 | 0.71 | 0.25–2.06 | 0.533 |
| Tumor grade (2–3 vs. 1) | 1.91 | 1.01–3.61 | 0.046 | 1.91 | 1.03–3.55 | 0.040 |
| LVSI (Present vs. Absent) | 0.49 | 0.09–2.57 | 0.398 | 0.50 | 0.10–2.37 | 0.380 |
| PNI (Present vs. Absent) | 0.50 | 0.10–2.39 | 0.385 | 0.62 | 0.15–2.66 | 0.524 |
| Adjuvant CT (Yes vs. No) | 2.82 | 0.75–10.58 | 0.125 | 2.21 | 0.62–7.83 | 0.220 |
| Distant metastasis | 5.28 | 1.47–19.01 | 0.011 | 3.40 | 1.05–11.02 | 0.042 |
| Risk group (High vs. Low) | 1.29 | 0.15–10.81 | 0.815 | 1.73 | 0.26–11.53 | 0.574 |
| Pathological T stage (pT3–4 vs. pT1–2) | 1.80 | 0.13–3.26 | 0.716 | 0.67 | 0.12–1.78 | 0.620 |
| FIGO stage (III–IV vs. I–II) | 1.93 | 0.00–3.50 | 0.640 | 1.88 | 0.01–1.95 | 0.490 |
| PIV ≥ 802 | 5.32 | 0.49–57.10 | 0.168 | 4.58 | 0.46–45.65 | 0.195 |
| SII ≥ 1545 | 1.80 | 0.34–16.08 | 0.580 | 1.19 | 0.32–11.20 | 0.245 |
| SIRI ≥ 1.5 | 1.09 | 0.09–0.97 | 0.042 | 1.12 | 0.08–1.24 | 0.036 |
| NLR ≥ 2.5 | 3.81 | 0.67–21.83 | 0.133 | 4.50 | 0.82–30.36 | 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onal Kalkan, N.; Urakcı, Z.; Mermit Erçek, B.; Bilen, E.; Arvas, H.; Akkuş, M.H. Clinical Utility of Pan-Immune Inflammation Value (PIV) in Predicting Prognosis of Endometrial Cancer. J. Clin. Med. 2025, 14, 7885. https://doi.org/10.3390/jcm14217885
Onal Kalkan N, Urakcı Z, Mermit Erçek B, Bilen E, Arvas H, Akkuş MH. Clinical Utility of Pan-Immune Inflammation Value (PIV) in Predicting Prognosis of Endometrial Cancer. Journal of Clinical Medicine. 2025; 14(21):7885. https://doi.org/10.3390/jcm14217885
Chicago/Turabian StyleOnal Kalkan, Nurhan, Zuhat Urakcı, Berrak Mermit Erçek, Erkan Bilen, Hayati Arvas, and Mehmet Hadi Akkuş. 2025. "Clinical Utility of Pan-Immune Inflammation Value (PIV) in Predicting Prognosis of Endometrial Cancer" Journal of Clinical Medicine 14, no. 21: 7885. https://doi.org/10.3390/jcm14217885
APA StyleOnal Kalkan, N., Urakcı, Z., Mermit Erçek, B., Bilen, E., Arvas, H., & Akkuş, M. H. (2025). Clinical Utility of Pan-Immune Inflammation Value (PIV) in Predicting Prognosis of Endometrial Cancer. Journal of Clinical Medicine, 14(21), 7885. https://doi.org/10.3390/jcm14217885






