Sex-Based Disparities in Clinical Burden and Diagnostic Delay in COPD: Insights from Primary Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Eligibility Criteria
2.4. Data Collection and Measurement
2.5. Variable Selection and Definitions
- Diagnostic Inertia Index 1 = MDO/(Total Interactions PreDx + 1)
- 2.
- Diagnostic Inertia Index 2 = Diagnostic Delay (days)/(CAT + mMRC + AVD + 1)
- 3.
- Symptom Intensity Score = First principal component derived from a Principal Component Analysis (PCA) of CAT, mMRC, AVD, and COPD-PS. PCA is a dimensionality reduction technique that identifies a unified symptom score by capturing the most relevant shared variance among these four indicators. The first component explained 46% of the total variance, showed high internal consistency, and was used as a unified symptom index.
- 4.
- DOSE Index = Sum of z-scores for mMRC − FEV1 %, pack-years, and exacerbation frequency.
- 5.
- Diagnosis Complexity Score = Diagnostic Delay + Number of Visits + MDO + 5 × ICS Initiation
2.6. Sample Size Calculation and Statistical Power
2.7. Ethical Considerations
2.8. Statistical Analysis
3. Results
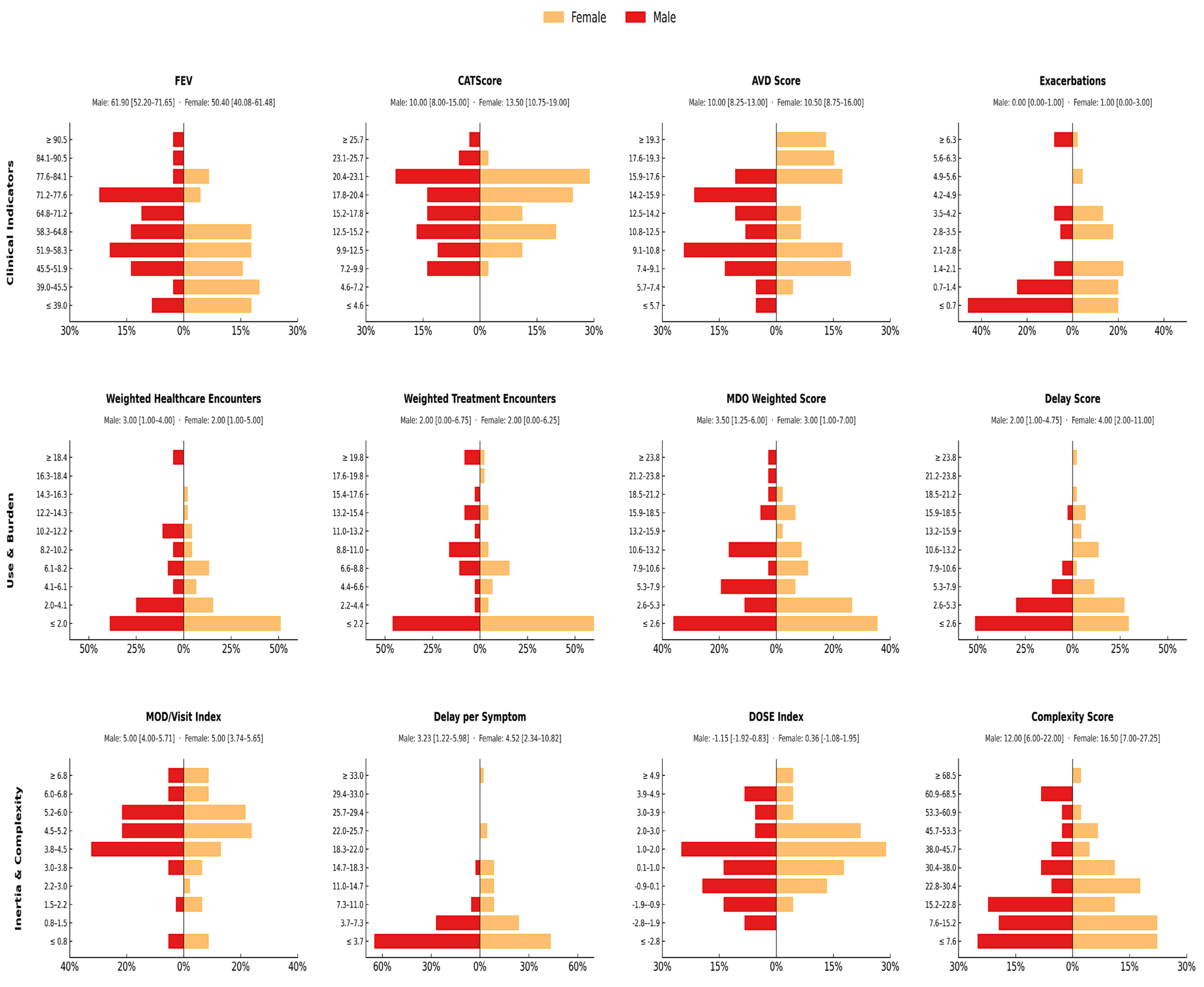
3.1. Clinical and Functional Profile at Diagnosis by Sex
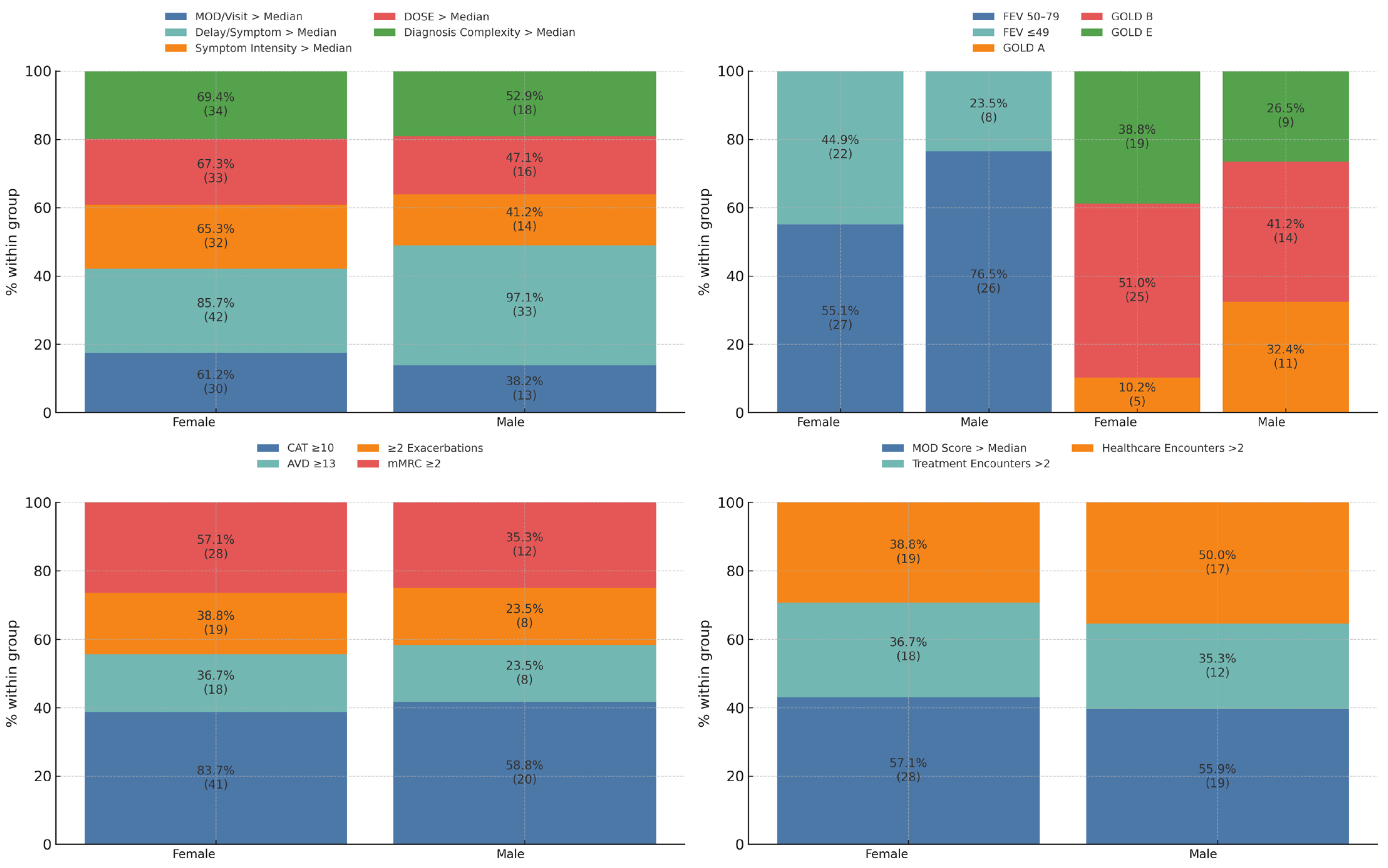
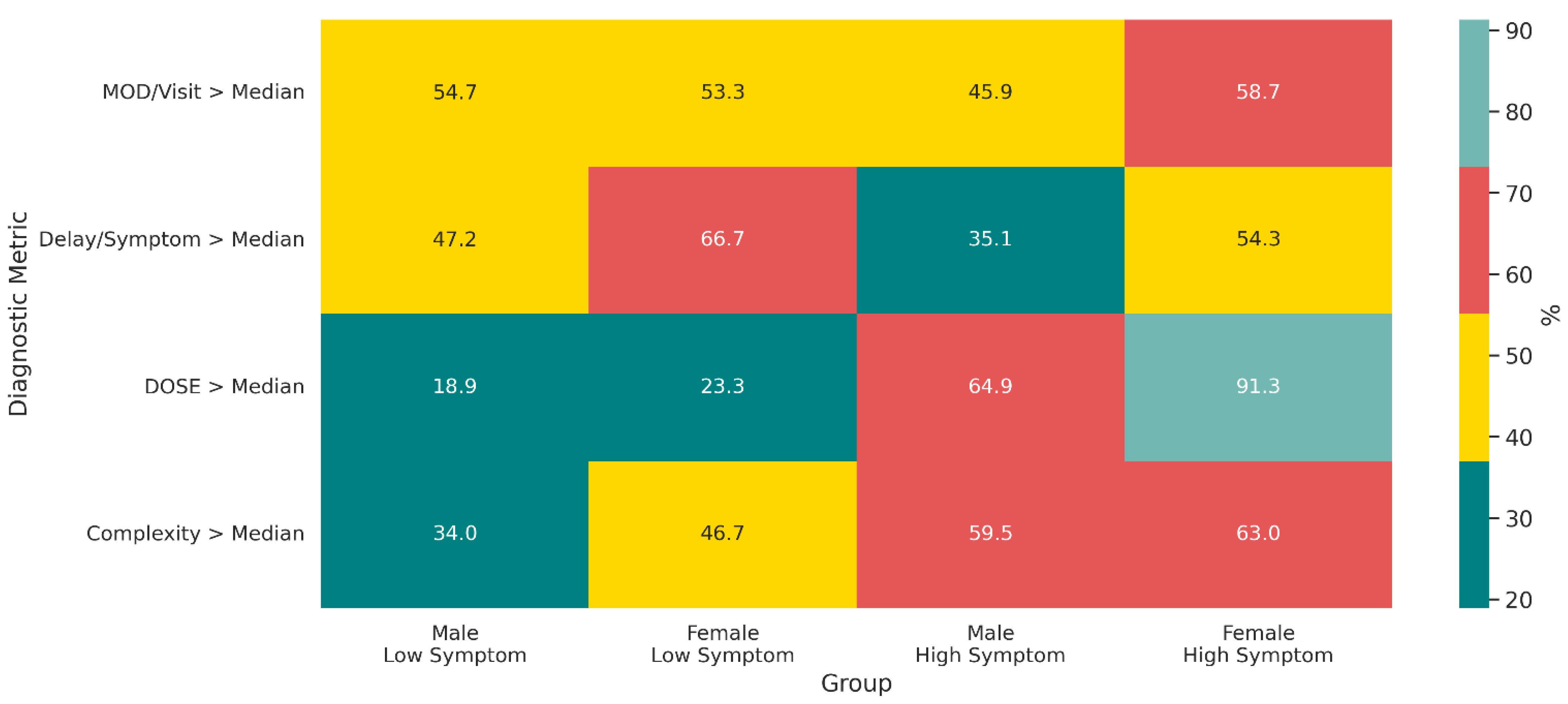
3.2. Stratified Diagnostic, Clinical, and Systemic Burden
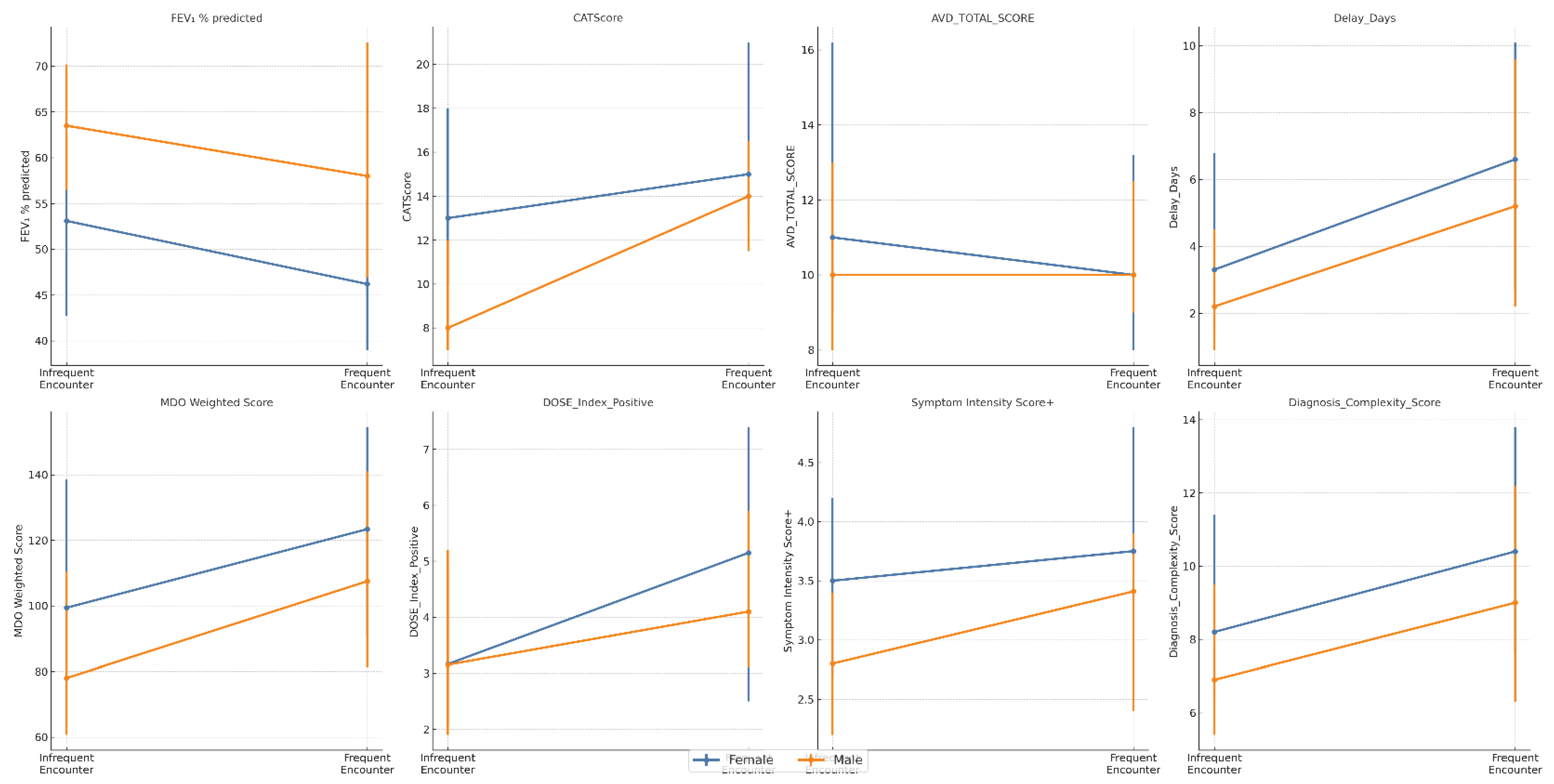
3.3. Healthcare Contact Frequency and Diagnostic Yield
3.4. Independent Predictors of Diagnostic Delay and Missed Opportunities
4. Discussion
4.1. Diagnostic Underrecognition in COPD and Need for Multidimensional Assessment
4.2. Sex-Based Differences in Clinical Burden, Diagnostic Delay, and Composite Efficiency Indicators
4.3. Interpretation of Sex-Based Disparities in Light of Prior Evidence
4.4. Sex-Specific Insights from Composite Indices of Diagnostic Appropriateness
4.5. Limitations
4.6. Clinical Implications and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MET | Metabolic Equivalent of Task |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| BMI | Body Mass Index |
| CAT | COPD Assessment Test score |
| mMRC | Modified Medical Research Council Dyspnea Scale |
| GesEPOC | Spanish COPD guidelines |
| AVD | Activity Limitation Score |
| IPAQ | International Physical Activity Questionnaire |
| MDO | Missed Diagnostic Opportunities |
| AIC | Akaike Information Criterion |
| PCA | Principal Component Analysis |
| LAMA | Long-Acting Muscarinic Antagonist |
| LABA | Long-Acting Beta-Agonist |
| ICS | Inhaled Corticosteroids |
| COPD-PS | COPD Population Screener |
Appendix A
Appendix A.1. Statistical Power Analysis
| Regression Model (Dependent Variable) | Sample Size (n) | α | Power (1 − β) | No. of Predictors | Minimum Detectable Effect Size (f2) |
|---|---|---|---|---|---|
| log(Diagnostic Delay + 1) | 166 | 0.05 | 0.80 | 7 | 0.081 |
| log(Weighted MDO + 1) | 166 | 0.05 | 0.80 | 8 | 0.086 |
Appendix A.2. Multicollinearity Diagnostics
| Predictor Variable | VIF (Delay Model) | VIF (MDO Model) |
|---|---|---|
| Female sex (ref = Male) | 1.53 | 1.59 |
| Age (years) | 1.07 | 1.08 |
| FEV1 % predicted | 1.20 | 1.20 |
| Cumulative smoking (pack-years) | 1.20 | 1.20 |
| Symptom Intensity Score | 1.24 | 1.89 |
| log(Delay days + 1) | — | 1.08 |
| GOLD Class B (ref = A) | — | 2.07 |
| GOLD Class E (ref = A) | — | 2.52 |
Appendix A.3. Sensitivity Analysis and Construct Validation
Appendix A.3.1. Construction of the Symptom Intensity Index
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| CAT | 0.614 | −0.075 | −0.378 | −0.689 |
| mMRC | 0.623 | 0.090 | −0.307 | 0.714 |
| AVD | 0.472 | −0.247 | 0.846 | −0.016 |
| COPD-PS | 0.111 | 0.962 | 0.216 | −0.125 |
Appendix A.3.2. Correlation Analysis of Composite Indices
| Composite Index | Target Outcome | Rho (ρ) | p-Value |
|---|---|---|---|
| Symptom Intensity | delay_days | 0.097 | 0.214 |
| Symptom Intensity | mod_weighted | 0.232 | 0.003 |
| DOSE Index | delay_days | 0.159 | 0.041 |
| DOSE Index | mod_weighted | 0.398 | <0.001 |
| Diagnosis Complexity | delay_days | 0.409 | <0.001 |
| Diagnosis Complexity | mod_weighted | 0.845 | <0.001 |
| MDOs per visit | delay_days | 0.109 | 0.164 |
| MDOs per visit | mod_weighted | 0.432 | <0.001 |
Appendix A.3.3. Model Robustness: Key Interaction Test
| Dependent Variable and Predictor | β (Coefficient) | 95% CI | p-Value |
|---|---|---|---|
| log(Diagnostic Delay + 1) | |||
| Female Sex × Symptom Intensity Score | 0.052 | [−0.250, 0.354] | 0.735 |
| log(Weighted MDO + 1) | |||
| Female Sex × Symptom Intensity Score | −0.038 | [−0.281, 0.205] | 0.759 |
Appendix A.3.4. Empirical Justification of the Cut-Off Point for Diagnostic Delay
| Delay Threshold (days) | n (%) in the Delayed group | Odds Ratio (95% CI) for GOLD E | p-Value |
|---|---|---|---|
| >30 | 102 (61.4%) | 2.85 (1.31–6.20) | 0.008 |
| >60 | 85 (51.2%) | 2.41 (1.14–5.10) | 0.021 |
| >90 | 73 (44.0%) | 1.98 (0.92–4.26) | 0.081 |
| >120 | 66 (39.8%) | 1.75 (0.80–3.83) | 0.159 |
Appendix B. Supplementary Analyses
Appendix B.1. Evaluation of the “Survivor Population” Hypothesis
| Comorbidity Group | Women (n = 76) | Men (n = 90) | χ2 (df) | p-Value |
|---|---|---|---|---|
| Cardiovascular 1 | 17 (22.4%) | 8 (8.9%) | 4.86 (1) | 0.028 |
| Smoking-related 2 | 19 (25.0%) | 12 (13.3%) | 2.97 (1) | 0.085 |
Appendix B.2. Analysis of Asthma as a Potential Confounder
| Variable | Women (n = 76) | Men (n = 90) | Fisher’s p |
|---|---|---|---|
| Asthma history | 18 (23.7%) | 5 (5.6%) | 0.0012 |
| ICS use | 11 (14.5%) | 6 (6.7%) | 0.125 |
| Characteristic | Women with Asthma (n = 18) | Women without Asthma (n = 58) | p-Value |
|---|---|---|---|
| Diagnostic Delay (days), median [IQR] | 230.5 [108.5–515.5] | 122.5 [59.0–243.5] | 0.041 |
| CAT Score, median [IQR] | 19.5 [15.0–23.5] | 12.5 [9.5–18.0] | 0.004 |
| Predictor | Baseline Model (β [95% CI]) | Model + Asthma/ICS (β [95% CI]) |
|---|---|---|
| Sex (male vs. female) | −0.926 [−1.525 to −0.326] | −0.888 [−1.494 to −0.282] |
| p-Value | 0.003 | 0.004 |
Appendix B.3. Sensitivity Analysis: Unweighted Missed Diagnostic Opportunities
| Predictor | β | SE | 95% CI | p | %Δ MDO |
|---|---|---|---|---|---|
| Intercept | 6.716 | 0.251 | [6.222, 7.211] | <0.001 | 82,483.3 |
| Sex (male vs. female) | −0.075 | 0.074 | [−0.222, 0.071] | 0.311 | −7.3 |
| Age (years) | −0.002 | 0.003 | [−0.008, 0.004] | 0.562 | −0.2 |
| FEV1 % predicted | −0.015 | 0.002 | [−0.020, −0.011] | <0.001 | −1.5 |
| Pack-years | 0.003 | 0.002 | [−0.001, 0.007] | 0.179 | 0.3 |
| Symptom Intensity | 0.008 | 0.024 | [−0.040, 0.056] | 0.734 | 0.8 |
| Healthcare encounters (count) | −0.008 | 0.015 | [−0.039, 0.022] | 0.582 | −0.8 |
| Treatment encounters (count) | 0.009 | 0.026 | [−0.042, 0.060] | 0.739 | 0.9 |
| Predictor | β | SE | 95% CI | p | %Δ MDO |
|---|---|---|---|---|---|
| Intercept | 6.646 | 0.250 | [6.152, 7.139] | <0.001 | 76,850.4 |
| Sex (male vs. female) | −0.051 | 0.074 | [−0.196, 0.095] | 0.491 | −5.0 |
| Age (years) | −0.001 | 0.003 | [−0.007, 0.005] | 0.641 | −0.1 |
| FEV1 % predicted | −0.015 | 0.002 | [−0.020, −0.011] | <0.001 | −1.5 |
| Pack-years | 0.003 | 0.002 | [−0.001, 0.007] | 0.167 | 0.3 |
| Symptom Intensity | −0.017 | 0.026 | [−0.069, 0.034] | 0.506 | −1.7 |
| Healthcare encounters (count) | −0.005 | 0.015 | [−0.035, 0.025] | 0.732 | −0.5 |
| Treatment encounters (count) | 0.004 | 0.026 | [−0.046, 0.055] | 0.872 | 0.4 |
| Asthma (yes vs. no) | 0.257 | 0.096 | [0.067, 0.447] | 0.008 | 29.3 |
| ICS use (yes vs. no) | 0.006 | 0.100 | [−0.192, 0.204] | 0.954 | 0.6 |
| Predictor | β | SE | 95% CI | p | %Δ MDO |
|---|---|---|---|---|---|
| Intercept | 5.840 | 0.054 | [5.732, 5.947] | <0.001 | 34,261.0 |
| Sex (male vs. female) | −0.183 | 0.069 | [−0.319, −0.047] | 0.009 | −16.7 |
| Asthma (yes vs. no) | 0.217 | 0.099 | [0.021, 0.412] | 0.030 | 24.2 |
Appendix B.4. Sensitivity Analysis: Environmental Exposure
| Predictor | β | SE | 95% CI | p | %Δ Delay (expβ − 1) |
|---|---|---|---|---|---|
| Intercept | 4.450 | 1.041 | [2.393, 6.507] | <0.001 | +8463% |
| Sex (male vs. female) | −0.926 | 0.305 | [−1.528, −0.324] | 0.003 | −60.4% |
| Age (years) | 0.005 | 0.013 | [−0.020, 0.030] | 0.679 | +0.5% |
| FEV1 % predicted | −0.004 | 0.009 | [−0.022, 0.015] | 0.701 | −0.4% |
| Cumulative smoking (pack-years) | 0.005 | 0.008 | [−0.012, 0.022] | 0.549 | +0.5% |
| Symptom Intensity Score | −0.043 | 0.101 | [−0.242, 0.156] | 0.673 | −4.2% |
| Weighted healthcare encounters | −0.021 | 0.037 | [−0.095, 0.053] | 0.579 | −2.1% |
| Weighted treatment encounters | 0.029 | 0.034 | [−0.038, 0.096] | 0.397 | +2.9% |
| Environmental exposure (Yes vs. No) | −0.013 | 0.434 | [−0.869, 0.844] | 0.977 | −1.2% |
| Predictor | β | SE | 95% CI | p | %Δ MDO (expβ − 1) |
|---|---|---|---|---|---|
| Intercept | 0.812 | 0.274 | [0.270, 1.354] | 0.0036 | +125.3% |
| Sex (male vs. female) | 0.032 | 0.080 | [−0.127, 0.190] | 0.694 | +3.2% |
| Age (years) | 0.000 | 0.003 | [−0.007, 0.007] | 0.987 | 0.0% |
| FEV1 % predicted | 0.001 | 0.002 | [−0.004, 0.006] | 0.796 | +0.1% |
| Cumulative smoking (pack-years) | −0.002 | 0.002 | [−0.007, 0.002] | 0.298 | −0.2% |
| Symptom Intensity Score | 0.046 | 0.027 | [−0.006, 0.099] | 0.083 | +4.7% |
| Weighted healthcare encounters | 0.122 | 0.010 | [0.103, 0.142] | <0.001 | +13.0% |
| Weighted treatment encounters | 0.050 | 0.009 | [0.032, 0.067] | <0.001 | +5.1% |
| Environmental exposure (Yes vs. No) | −0.002 | 0.114 | [−0.228, 0.224] | 0.988 | −0.2% |
References
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- Patel, K.; Smith, D.J.; Huntley, C.C.; Jordan, R.E.; Purdy, S.; Culliford, D. Exploring the causes of COPD misdiagnosis in primary care: A mixed methods study. PLoS ONE 2024, 19, e0298432. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, E.; Bierbrier, J.; Whitmore, G.A.; Van der Veen, M.; Singh, A.; Vandemheen, K.L. Impact of undiagnosed chronic obstructive pulmonary disease and asthma on symptoms, quality of life, healthcare use, and work productivity. Am. J. Respir. Crit. Care Med. 2023, 208, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Aaron, S.D.; Vandemheen, K.L.; Whitmore, G.A.; McCormack, D.G.; Hernandez, P.; Maltais, F. Early diagnosis and treatment of COPD and asthma—A randomized, controlled trial. N. Engl. J. Med. 2024, 390, 2061–2073. [Google Scholar] [CrossRef]
- Roberts, N.; Patel, I.S.; Partridge, M.R. The diagnosis of COPD in primary care: Gender differences and the role of spirometry. Respir. Med. 2016, 111, 60–63. [Google Scholar] [CrossRef]
- Aryal, S.; Diaz-Guzman, E.; Mannino, D.M. Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int. J. Chronic Obstruct. Pulmon. Dis. 2014, 9, 1145–1154. [Google Scholar] [CrossRef]
- Milne, K.M.; Mitchell, R.A.; Ferguson, O.N.; Hind, A.S.; Guenette, J.A. Sex-differences in COPD: From biological mechanisms to therapeutic considerations. Front. Med. 2024, 11, 1289259. [Google Scholar] [CrossRef]
- Jones, R.C.; Donaldson, G.C.; Chavannes, N.H.; Wedzicha, J.A. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease: The DOSE index. Am. J. Respir. Crit. Care Med. 2009, 180, 1189–1195. [Google Scholar] [CrossRef]
- Martinez, C.H.; Raparla, S.; Plauschinat, C.A.; Giardino, N.D.; Rogers, B.; Beresford, J. Gender differences in symptoms and care delivery for chronic obstructive pulmonary disease. J. Women’s Health 2012, 21, 1267–1274. [Google Scholar] [CrossRef]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, M. Development and first validation of the COPD Assessment Test (CAT). Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Martinez, F.J.; Raczek, A.E.; Seifer, F.D.; Conoscenti, C.S.; Curtice, T.G.; D’Eletto, T.; Phillips, A.L. Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS). J. Chronic Obstr. Pulm. Dis. 2008, 5, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, Y.; Wen, Q.; Ouyang, Y.; Shen, Y.; Yu, H.; Wen, F. Performance of COPD Population Screener Questionnaire in COPD screening: A validation study and meta-analysis. Ann. Med. 2021, 53, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gutiérrez, F.J.; Miravitlles, M.; Calle, M.; Gobartt, E.; López, F.; Martín, A. Impacto de la EPOC en la vida diaria de los pacientes. Resultados del estudio multicéntrico EIME. Arch. Bronconeumol. 2007, 43, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Oja, P. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Aljama, C.; Esquinas, C.; Loeb, E.; Granados, G.; Nuñez, A.; Lopez Gonzalez, A.; Miravitlles, M.; Barrecheguren, M. Demographic and clinical characteristics of mild, young and early COPD: A cross sectional analysis of 5468 patients. J. Clin. Med. 2024, 13, 7380. [Google Scholar] [CrossRef]
- Haroon, S.M.; Jordan, R.E.; O’Beirne Elliman, J.; Adab, P. Effectiveness of case finding strategies for COPD in primary care: A systematic review and meta analysis. npj Prim. Care Respir. Med. 2015, 25, 15056. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Abadi, E.; Anzueto, A.; Bodduluri, S.; Casaburi, R.; Castaldi, P.J.; Cho, M.H.; Comellas, A.P.; Conrad, D.J.; Curtis, J.L.; et al. A multidimensional diagnostic approach for chronic obstructive pulmonary disease. JAMA 2025, 323, 2164–2175. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Barr, R.G.; Bleecker, E.R.; Christenson, S.A.; Couper, D.; Curtis, J.L.; Han, M.K. Clinical significance of symptoms in smokers with preserved pulmonary function. N. Engl. J. Med. 2016, 374, 1811–1821. [Google Scholar] [CrossRef]
- Abad-Arranz, M.; Moran-Rodriguez, A.; Mascarós Balaguer, E.; Quintana Velasco, C.; Abad Polo, L.; Núñez Palomo, S.; Lopez-Campos, J.L. Quantification of inaccurate diagnosis of COPD in primary care medicine: An analysis of the COACH clinical audit. Int. J. Chronic Obstruct. Pulmon. Dis. 2019, 14, 1187–1194. [Google Scholar] [CrossRef]
- Corlateanu, A.; Pahontu, A.; Cortaleanu, O.; Botnaru, V.; Bikov, A.; Mathioudakis, A.G.; Siafakas, N. Multidimensional indices in the assessment of chronic obstructive pulmonary disease. Respir. Med. 2021, 185, 106519. [Google Scholar] [CrossRef]
- Souto-Miranda, S.; van ‘t Hul, A.J.; Vaes, A.W.; Antons, J.C.; Djamin, R.S.; Janssen, D.J.; Spruit, M.A. Differences in pulmonary and extra-pulmonary traits between women and men with chronic obstructive pulmonary disease. J. Clin. Med. 2022, 11, 3680. [Google Scholar] [CrossRef]
- Dai, Z.; Ma, Y.; Zhan, Z.; Chen, P.; Chen, Y. Analysis of diagnostic delay and its influencing factors in patients with chronic obstructive pulmonary disease: A cross-sectional study. Sci. Rep. 2021, 11, 14213. [Google Scholar] [CrossRef]
- Jenkins, C. Differences Between Men and Women with Chronic Obstructive Pulmonary Disease. Clin. Chest Med. 2021, 42, 443–456. [Google Scholar] [CrossRef]
- Perez, T.A.; Castillo, E.G.; Ancochea, J.; Sanz, M.T.P.; Almagro, P.; Martínez-Camblor, P.; Soriano, J.B. Sex Differences Between Women and Men with COPD: A New Analysis of the 3CIA Study. Respir. Med. 2020, 172, 106105. [Google Scholar] [CrossRef]
- Roberts, M.; Smith, T.; Wheatley, J.; Cho, J.G. Symptom Burden of Patients with Chronic Obstructive Pulmonary Disease Attending the Westmead Breathlessness Service: Prevalence, Associations, and Sex-Related Differences. Int. J. Chronic Obstruct. Pulmon. Dis. 2023, 18, 2285–2287. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.L.; Nyberg, A.; Maltais, F.; Saey, D. Functional Tests in Chronic Obstructive Pulmonary Disease, Part 2: Measurement Properties. Ann. Am. Thorac. Soc. 2017, 14, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Pinto, L.M.; Morogan, A.; Bourbeau, J. The COPD Assessment Test: A Systematic Review. Eur. Respir. J. 2014, 44, 873–884. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, J.C.; Rosenfeld, A.G.; Abel, W.M.; Braun, L.T.; Burke, L.E.; Daugherty, S.L.; Reckelhoff, J.F. Preventing and Experiencing Ischemic Heart Disease as a Woman: State of the Science: A Scientific Statement from the American Heart Association. Circulation 2016, 133, 1302–1331. [Google Scholar] [CrossRef]
- Pepine, C.J.; Ferdinand, K.C.; Shaw, L.J.; Light-McGroary, K.A.; Shah, R.U.; Gulati, M.; ACC CVD in Women Committee. Emergence of Nonobstructive Coronary Artery Disease: A Woman’s Problem and Need for Change in Definition on Angiography. J. Am. Coll. Cardiol. 2015, 66, 1918–1933. [Google Scholar] [CrossRef]
- Baller, J.G.; Rosmalen, J.G.M.; Olde Hartman, T.C. Differences Between Women and Men Are Present in the Rate of Diagnosed Diseases After a Diagnostic Intervention Is Conducted in Primary Care. J. Am. Board Fam. Med. 2022, 35, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Mustofa, N.; Sayed, A.; Hamed, M.; Dervis, M.; Almaadawy, O.; Baqal, O. Gender Disparities in Delayed Angina Diagnosis: Insights from 2001–2020 NHANES Data. BMC Public Health 2022, 25, 1197. [Google Scholar] [CrossRef] [PubMed]
- McCallister, J.W.; Holbrook, J.T.; Wei, C.Y.; Parsons, J.P.; Benninger, C.G.; Dixon, A.E.; American Lung Association Asthma Clinical Research Centers. Sex Differences in Asthma Symptom Profiles and Control in the American Lung Association Asthma Clinical Research Centers. Respir. Med. 2013, 107, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Stolfo, D.; Anderson, L.; Abdelhamid, M.; Adamo, M.; Bauersachs, J.; Coats, A.J. Differences in Presentation, Diagnosis and Management of Heart Failure in Women. A Scientific Statement of the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2024, 26, 1669–1686. [Google Scholar] [CrossRef]
- Riley, C.M.; Sciurba, F.C. Diagnosis and outpatient management of chronic obstructive pulmonary disease: A review. JAMA 2019, 321, 786–797. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Y.; Qi, W.; Lazzeri, C. Prognostic value of composite inflammatory markers in patients with chronic obstructive pulmonary disease: A retrospective cohort study based on the MIMIC-IV database. PLoS ONE 2025, 20, e0316390. [Google Scholar] [CrossRef]
- Delgado, A.; Saletti-Cuesta, L.; López-Fernández, L.A.; Gil-Garrido, N.; Luna Del Castillo, J.D. Gender Inequalities in COPD Decision-Making in Primary Care. Respir. Med. 2016, 114, 91–96. [Google Scholar] [CrossRef][Green Version]
- Jordan, R.E.; Miller, M.R.; Lam, K.B.; Cheng, K.K.; Marsh, J.; Adab, P. Sex, susceptibility to smoking and chronic obstructive pulmonary disease: The effect of different diagnostic criteria. Analysis of the Health Survey for England. Thorax 2012, 67, 600–605. [Google Scholar] [CrossRef]

| Variable | Female (n = 76) | Male (n = 90) | p-Value | Mann–Whitney U | Z | Effect Size (r) |
|---|---|---|---|---|---|---|
| Age (years) | 65.50 [57.00, 73.00] | 69.00 [61.00, 74.00] | 0.013 | 2778.5 | −2.08 | 0.162 |
| BMI (kg/m2) | 25.81 [23.37, 33.16] | 27.33 [23.92, 30.10] | 0.531 | 3296 | −0.40 | 0.031 |
| Cumulative smoking (pack-years) | 30.00 [20.00, 36.00] | 40.00 [30.00, 45.00] | <0.001 | 2024.5 | −4.54 | 0.352 |
| FEV1 % predicted | 50.40 [39.95, 61.55] | 61.40 [52.10, 71.50] | <0.001 | 2027 | −4.52 | 0.350 |
| FVC % predicted | 92.70 [84.00, 100.00] | 100.00 [87.00, 116.10] | 0.002 | 2477 | −3.06 | 0.237 |
| FEV1/FVC ratio | 0.59 [0.48, 0.65] | 0.63 [0.59, 0.67] | <0.001 | 2359 | −3.44 | 0.267 |
| CAT score | 13.50 [10.50, 19.00] | 10.00 [8.00, 15.00] | 0.005 | 2411.5 | −3.27 | 0.254 |
| AVD total score | 10.50 [8.50, 16.00] | 10.00 [8.00, 13.00] | 0.011 | 2904.5 | −1.68 | 0.130 |
| COPD-PS | 7.00 [6.00, 8.00] | 6.00 [5.00, 7.00] | 0.002 | 2562 | −2.86 | 0.222 |
| mMRC | 2.00 [1.00, 2.00] | 1.00 [1.00, 2.00] | 0.001 | 2522.5 | −3.12 | 0.242 |
| Number of exacerbations | 1.00 [0.00, 3.00] | 0.00 [0.00, 1.00] | 0.001 | 2486.5 | −3.29 | 0.255 |
| Total MET-minutes/week (IPAQ) | 1260.00 [655.00, 1650.00] | 1080.00 [630.00, 1420.00] | 0.723 | 2977 | −1.44 | 0.112 |
| IPAQ MET-min/week | 1386.00 [704.50, 1768.00] | 1080.00 [630.00, 1420.00] | 0.599 | 2986.5 | −1.41 | 0.109 |
| Walking ≥ 10 min—Days/week | 7.00 [5.00, 7.00] | 7.00 [7.00, 7.00] | 0.086 | 2654 | −3.13 | 0.243 |
| Walking—Minutes/day | 32.00 [30.00, 60.00] | 50.00 [30.00, 60.00] | 0.666 | 2698 | −1.95 | 0.151 |
| Variable | Female (n = 76) | Male (n = 90) | p-Value | χ2 (df) | Cramer’s V |
|---|---|---|---|---|---|
| Current smoker | 50 (65.8%) | 45 (50.0%) | 0.043 | 4.20 (1) | 0.159 |
| Former smoker | 26 (34.2%) | 45 (50.0%) | — | — | — |
| Occupational Risk Exposure | 9 (11.8%) | 6 (6.7%) | 0.285 | 1.34 (1) | 0.090 |
| Diagnostic delay (>median) | 49 (64.5%) | 34 (37.8%) | 0.001 | 11.75 (1) | 0.266 |
| W. MOD Score > Median | 42 (55.3%) | 51 (56.7%) | 0.290 | 0.03 (1) | 0.064 |
| CAT ≥ 10 | 61 (80.3%) | 48 (53.3%) | <0.001 | 13.25 (1) | 0.283 |
| Frequent Exacerbations (≥2/year) | 32 (42.1%) | 16 (17.8%) | 0.001 | 11.86 (1) | 0.267 |
| Exacerbations (≥1/year) | 46 (60.5%) | 20 (22.2%) | <0.001 | 25.24 (1) | 0.390 |
| Dyspnea (mMRC ≥ 2) | 42 (55.3%) | 30 (33.3%) | 0.005 | 8.07 (1) | 0.220 |
| Dyspnea presence (mMRC ≥ 1) | 51 (67.1%) | 31 (34.4%) | <0.001 | 17.58 (1) | 0.325 |
| Asthma History | 18 (23.7%) | 5 (5.6%) | 0.001 | 11.35 (1) | 0.261 |
| Emphysema Signs | 28 (36.8%) | 18 (20.0%) | 0.023 | 5.83 (1) | 0.187 |
| COPD_PS ≥ 5 | 62 (81.6%) | 66 (73.3%) | 0.141 | 1.59 (1) | 0.098 |
| AVD ≥ 13 | 29 (38.2%) | 24 (26.7%) | 0.079 | 2.50 (1) | 0.143 |
| AVD Tertile | — | — | 0.216 | 3.07 (2) | 0.136 |
| Low Limitation | 29 (38.2%) | 36 (40.0%) | — | — | — |
| Moderate Limitation | 18 (23.7%) | 30 (33.3%) | — | — | — |
| Severe Limitation | 29 (38.2%) | 24 (26.7%) | — | — | — |
| Comorbidity Category | 0.482 | 2.46 (3) | 0.122 | ||
| None | 39 (51.3%) | 47 (52.2%) | — | — | — |
| 1 | 27 (35.5%) | 30 (33.3%) | — | — | — |
| 2 | 9 (11.8%) | 8 (8.9%) | — | — | — |
| 3 | 1 (1.3%) | 5 (5.6%) | — | — | — |
| GOLD Class | <0.001 | 15.89 (2) | 0.309 | ||
| A | 12 (15.8%) | 36 (40.0%) | — | — | — |
| B | 32 (42.1%) | 37 (41.1%) | — | — | — |
| E | 32 (42.1%) | 17 (18.9%) | — | — | — |
| FEV1 Categories | 0.001 | 14.05 (2) | 0.291 | ||
| ≥80% | 0 (0.0%) | 7 (7.8%) | — | — | — |
| 50–79% | 41 (53.9%) | 64 (71.1%) | — | — | — |
| ≤49% | 34 (44.7%) | 19 (21.1%) | — | — | — |
| GESEPOC Risk | 0.354 | 0.92 (1) | 0.075 | ||
| High Risk | 42 (55.3%) | 43 (47.8%) | — | — | — |
| Low Risk | 34 (44.7%) | 47 (52.2%) | — | — | — |
| Symptom Intensity Category | 0.013 | 6.21 (1) | 0.193 | ||
| Low Symptom Intensity | 30 (39.5%) | 53 (58.9%) | — | — | — |
| High Symptom Intensity | 46 (60.5%) | 37 (41.1%) | — | — | — |
| LAMA | 75 (98.7%) | 90 (100.0%) | 0.458 | 1.19 (1) | 0.085 |
| LABA | 25 (32.9%) | 24 (26.7%) | 0.398 | 0.77 (1) | 0.068 |
| ICS | 11 (14.5%) | 6 (6.7%) | 0.125 | 2.73 (1) | 0.128 |
| Sports | <0.001 | 19.69 (3) | 0.621 | ||
| A little | 9 (11.8%) | 23 (25.6%) | — | — | — |
| A lot | 41 (53.9%) | 19 (21.1%) | — | — | — |
| None | 5 (6.6%) | 9 (10.0%) | — | — | — |
| Some | 21 (27.6%) | 39 (43.3%) | — | — | — |
| Physical Activity | 0.020 | 9.89 (3) | 0.244 | ||
| A little | 17 (22.4%) | 32 (35.6%) | — | — | — |
| A lot | 16 (21.1%) | 7 (7.8%) | — | — | — |
| None | 2 (2.6%) | 7 (7.8%) | — | — | — |
| Some | 41 (53.9%) | 44 (48.9%) | — | — | — |
| Social | 0.003 | 14.04 (3) | 0.291 | ||
| A little | 26 (34.2%) | 57 (63.3%) | — | — | — |
| A lot | 2 (2.6%) | 1 (1.1%) | — | — | — |
| None | 34 (44.7%) | 23 (25.6%) | — | — | — |
| Some | 14 (18.4%) | 9 (10.0%) | — | — | — |
| Family | 0.043 | 8.15 (3) | 0.222 | ||
| A little | 31 (40.8%) | 56 (62.2%) | — | — | — |
| A lot | 2 (2.6%) | 1 (1.1%) | — | — | — |
| None | 28 (36.8%) | 19 (21.1%) | — | — | — |
| Some | 15 (19.7%) | 14 (15.6%) | — | — | — |
| Sleep | 0.002 | 14.33 (3) | 0.294 | ||
| A little | 25 (32.9%) | 55 (61.1%) | — | — | — |
| A lot | 1 (1.3%) | 0 (0.0%) | — | — | — |
| None | 35 (46.1%) | 27 (30.0%) | — | — | — |
| Some | 15 (19.7%) | 8 (8.9%) | — | — | — |
| Housework | 0.003 | 13.65 (3) | 0.287 | ||
| A little | 29 (38.2%) | 56 (62.2%) | — | — | — |
| A lot | 5 (6.6%) | 0 (0.0%) | — | — | — |
| None | 22 (28.9%) | 20 (22.2%) | — | — | — |
| Some | 20 (26.3%) | 14 (15.6%) | — | — | — |
| Sexual | 0.043 | 6.67 (3) | 0.200 | ||
| A little | 22 (28.9%) | 36 (40.0%) | — | — | — |
| A lot | 4 (5.3%) | 0 (0.0%) | — | — | — |
| None | 33 (43.4%) | 33 (36.7%) | — | — | — |
| Some | 17 (22.4%) | 21 (23.3%) | — | — | — |
| Variable | Female Median [IQR] | Male Median [IQR] | U | Z | p-Value | r |
|---|---|---|---|---|---|---|
| Weighted MDO per Visit | 12.00 [10.00, 15.69] | 11.91 [10.00, 15.00] | 3212 | −0.683 | 0.494 | 0.053 |
| Delay per Symptom | 4.52 [2.33, 11.42] | 3.23 [1.20, 6.00] | 2554 | −2.807 | 0.005 | 0.218 |
| Symptom Intensity Score (z-score) | 0.13 [−0.57, 1.66] | −0.67 [−1.17, −0.42] | 2255 | −3.776 | <0.001 | 0.293 |
| DOSE Index (z-score) | 0.36 [−1.09, 1.96] | −1.15 [−1.97, −0.84] | 2450 | −3.144 | 0.004 | 0.244 |
| Diagnosis Complexity Score | 403.50 [264.50, 529.00] | 272.0 [224.3, 428.0] | 2450.5 | −3.142 | <0.001 | 0.244 |
| Weighted Total Interactions (PreDx) | 2.00 [1.00, 5.00] | 3.00 [1.00, 5.00] | 3331 | −0.293 | 0.716 | 0.023 |
| Unscheduled Primary Care Visits | 1.00 [1.00, 2.00] | 2.00 [1.00, 3.00] | 3099.5 | −1.066 | 0.160 | 0.083 |
| Primary Care ER Visits | 0.00 [0.00, 1.00] | 0.00 [0.00, 1.00] | 3270 | −0.598 | 0.793 | 0.046 |
| Hospital ER Visits | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 3380 | −0.184 | 0.876 | 0.014 |
| Hospital Admissions | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 3335.5 | −0.635 | 0.635 | 0.049 |
| Weighted Total Treatment (PreDx) | 3.00 [0.00, 8.00] | 3.00 [0.00, 8.00] | 3409.0 | −0.037 | 0.905 | 0.014 |
| Antibiotic Courses (past year) | 1.00 [0.00, 1.00] | 1.00 [0.00, 2.00] | 3343 | −0.265 | 0.735 | 0.021 |
| Systemic Steroid Courses (past year) | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 3412.5 | −0.033 | 0.852 | 0.003 |
| Diagnostic Delay (days) | 133.00 [63.50, 330.50] | 66.50 [30.00, 3136.00] | 2275.5 | −3.710 | <0.001 | 0.288 |
| MDO Weighted Score | 3.00 [1.00, 7.00] | 3.50 [1.00, 6.00] | 3319.5 | −0.328 | 0.687 | 0.025 |
| χ2(df) | Cramer’sV | p | OR | |||
| Frequent Healthcare or Treatment Encounter | 24 (31.58%) | 31 (34.4%) | 0.153 (1) | 0.030 | 0.412 | 1.20 |
| Frequent Healthcare and Treatment Encounter | 45 (59.2%) | 50 (55.6%) | 0.225 (1) | 0.037 | 0.376 | 0.878 |
| Predictor | β | SE | 95% CI | p | %Δ Delay (expβ − 1) |
|---|---|---|---|---|---|
| Model 1—Sex only | |||||
| Intercept | 4.773 | 0.178 | [4.421, 5.124] | <0.001 | +118.7% |
| Sex (male vs. female) | −0.863 | 0.242 | [−1.341, −0.386] | <0.001 | −57.8% |
| Model fit: Adj. R2 = 0.066; AIC = 619.3; n = 166 | |||||
| Model 2—+ Age, FEV1 %, Pack-years | |||||
| Intercept | 4.553 | 0.998 | [2.583, 6.524] | <0.001 | +94.8% |
| Sex (male vs. female) | −0.874 | 0.279 | [−1.425, −0.324] | 0.002 | −58.2% |
| Age (years) | 0.005 | 0.012 | [−0.019, 0.030] | 0.671 | +0.5% |
| FEV1 % predicted | −0.004 | 0.009 | [−0.023, 0.014] | 0.627 | −0.4% |
| Pack-years | 0.004 | 0.008 | [−0.012, 0.020] | 0.650 | +0.4% |
| Model fit: Adj. R2 = 0.053; AIC = 624.5; n = 166 | |||||
| Model 3—+ Symptom Intensity | |||||
| Intercept | 4.542 | 1.002 | [2.564, 6.520] | <0.001 | +93.8% |
| Sex (male vs. female) | −0.902 | 0.298 | [−1.491, −0.313] | 0.003 | −59.0% |
| Age (years) | 0.005 | 0.012 | [−0.020, 0.030] | 0.673 | +0.5% |
| FEV1 % predicted | −0.004 | 0.009 | [−0.023, 0.015] | 0.642 | −0.4% |
| Pack-years | 0.004 | 0.008 | [−0.012, 0.020] | 0.617 | +0.4% |
| Symptom Intensity Score | −0.026 | 0.097 | [−0.217, 0.165] | 0.788 | −2.6% |
| Model fit: Adj. R2 = 0.048; AIC = 626.5; n = 166 | |||||
| Model 4—+ Encounters, Exposures, Asthma, ICS, GOLD Classes | |||||
| Intercept | 4.383 | 1.054 | [2.302, 6.464] | <0.001 | +79.7% |
| Sex (male vs. female) | −0.888 | 0.308 | [−1.497, −0.279] | 0.005 | −59.2% |
| Age (years) | 0.005 | 0.013 | [−0.020, 0.030] | 0.681 | +0.5% |
| FEV1 % predicted | −0.003 | 0.009 | [−0.022, 0.015] | 0.715 | −0.3% |
| Pack-years | 0.005 | 0.008 | [−0.011, 0.020] | 0.565 | +0.5% |
| Symptom Intensity Score | −0.084 | 0.109 | [−0.299, 0.131] | 0.442 | −8.1% |
| Environmental exposure (Yes vs. No) | −0.003 | 0.435 | [−0.858, 0.852] | 0.994 | −0.3% |
| Asthma (Yes vs. No) | 0.522 | 0.402 | [−0.269, 1.313] | 0.196 | +68.6% |
| ICS use (Yes vs. No) | −0.278 | 0.419 | [−1.102, 0.546] | 0.509 | −24.2% |
| GOLD Class B (vs. A) | −0.155 | 0.211 | [−0.572, 0.262] | 0.463 | −14.4% |
| GOLD Class E (vs. A) | 0.096 | 0.225 | [−0.347, 0.539] | 0.669 | +10.1% |
| Model fit: Adj. R2 = 0.039; AIC = 634.8; n = 166 |
| Predictor | β | SE | 95% CI | p | %Δ MDO (expβ − 1) |
|---|---|---|---|---|---|
| Model 1—Sex only | |||||
| Intercept | 1.442 | 0.103 | [1.238, 1.645] | <0.001 | +142.7% |
| Sex (male vs. female) | 0.024 | 0.140 | [−0.252, 0.300] | 0.863 | +2.4% |
| Model fit: Adj. R2 = −0.006; AIC = 437.0; n = 166 | |||||
| Model 2—+ Diagnostic Delay | |||||
| Intercept | 1.435 | 0.136 | [1.167, 1.702] | <0.001 | +143.6% |
| Sex (male vs. female) | 0.028 | 0.147 | [−0.262, 0.317] | 0.851 | +2.8% |
| Diagnostic Delay (days) | 0.00003 | 0.000 | [−0.001, 0.001] | 0.936 | 0.0% |
| Model fit: Adj. R2 = −0.012; AIC = 438.9; n = 166 | |||||
| Model 3—+ Clinical covariates | |||||
| Intercept | 2.091 | 0.566 | [0.973, 3.208] | <0.001 | +708.9% |
| Sex (male vs. female) | 0.321 | 0.170 | [−0.015, 0.657] | 0.061 | +37.9% |
| Diagnostic Delay (days) | −0.00007 | 0.000 | [−0.001, 0.001] | 0.862 | 0.0% |
| Age (years) | −0.001 | 0.007 | [−0.015, 0.013] | 0.883 | −0.1% |
| FEV1 % predicted | −0.009 | 0.005 | [−0.019, 0.001] | 0.086 | −0.9% |
| Pack-years | −0.006 | 0.005 | [−0.015, 0.003] | 0.180 | −0.6% |
| Symptom Intensity Score | 0.179 | 0.054 | [0.072, 0.285] | 0.001 | +19.6% |
| Model fit: Adj. R2 = 0.044; AIC = 433.4; n = 166 | |||||
| Model 4—+ GOLD, Environmental exposure, Asthma | |||||
| Intercept | 2.590 | 0.512 | [1.580, 3.600] | <0.001 | +233.8% |
| Sex (male vs. female) | 0.112 | 0.132 | [−0.149, 0.373] | 0.395 | +11.8% |
| Diagnostic Delay (days) | −0.00001 | 0.000 | [−0.001, 0.001] | 0.934 | 0.0% |
| Age (years) | −0.002 | 0.006 | [−0.014, 0.010] | 0.712 | −0.2% |
| FEV1 % predicted | −0.007 | 0.004 | [−0.015, 0.002] | 0.124 | −0.7% |
| Pack-years | −0.004 | 0.004 | [−0.012, 0.004] | 0.308 | −0.4% |
| Symptom Intensity Score | −0.041 | 0.057 | [−0.153, 0.072] | 0.474 | −4.0% |
| Environmental exposure (Yes vs. No) | −0.047 | 0.119 | [−0.282, 0.188] | 0.691 | −4.6% |
| Asthma (Yes vs. No) | 0.058 | 0.109 | [−0.156, 0.272] | 0.598 | +6.0% |
| GOLD Class B (vs. A) | −0.983 | 0.142 | [−1.263, −0.703] | <0.001 | −62.6% |
| GOLD Class E (vs. A) | −1.390 | 0.195 | [−1.774, −1.006] | <0.001 | −75.0% |
| Model fit: Adj. R2 = 0.289; AIC = 389.6; n = 166 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calle Rubio, M.; Esmaili, S.; Esmaili, I.; Gómez Martín-Caro, L.; Ayat Ortiz, S.; Rodríguez Hermosa, J.L. Sex-Based Disparities in Clinical Burden and Diagnostic Delay in COPD: Insights from Primary Care. J. Clin. Med. 2025, 14, 6258. https://doi.org/10.3390/jcm14176258
Calle Rubio M, Esmaili S, Esmaili I, Gómez Martín-Caro L, Ayat Ortiz S, Rodríguez Hermosa JL. Sex-Based Disparities in Clinical Burden and Diagnostic Delay in COPD: Insights from Primary Care. Journal of Clinical Medicine. 2025; 14(17):6258. https://doi.org/10.3390/jcm14176258
Chicago/Turabian StyleCalle Rubio, Myriam, Soha Esmaili, Iman Esmaili, Lucia Gómez Martín-Caro, Sofia Ayat Ortiz, and Juan Luis Rodríguez Hermosa. 2025. "Sex-Based Disparities in Clinical Burden and Diagnostic Delay in COPD: Insights from Primary Care" Journal of Clinical Medicine 14, no. 17: 6258. https://doi.org/10.3390/jcm14176258
APA StyleCalle Rubio, M., Esmaili, S., Esmaili, I., Gómez Martín-Caro, L., Ayat Ortiz, S., & Rodríguez Hermosa, J. L. (2025). Sex-Based Disparities in Clinical Burden and Diagnostic Delay in COPD: Insights from Primary Care. Journal of Clinical Medicine, 14(17), 6258. https://doi.org/10.3390/jcm14176258







