Does Sex or Age Impact the Prognostic Value of a Zero Coronary Artery Calcium Score?
Abstract
1. Introduction
2. Materials and Methods
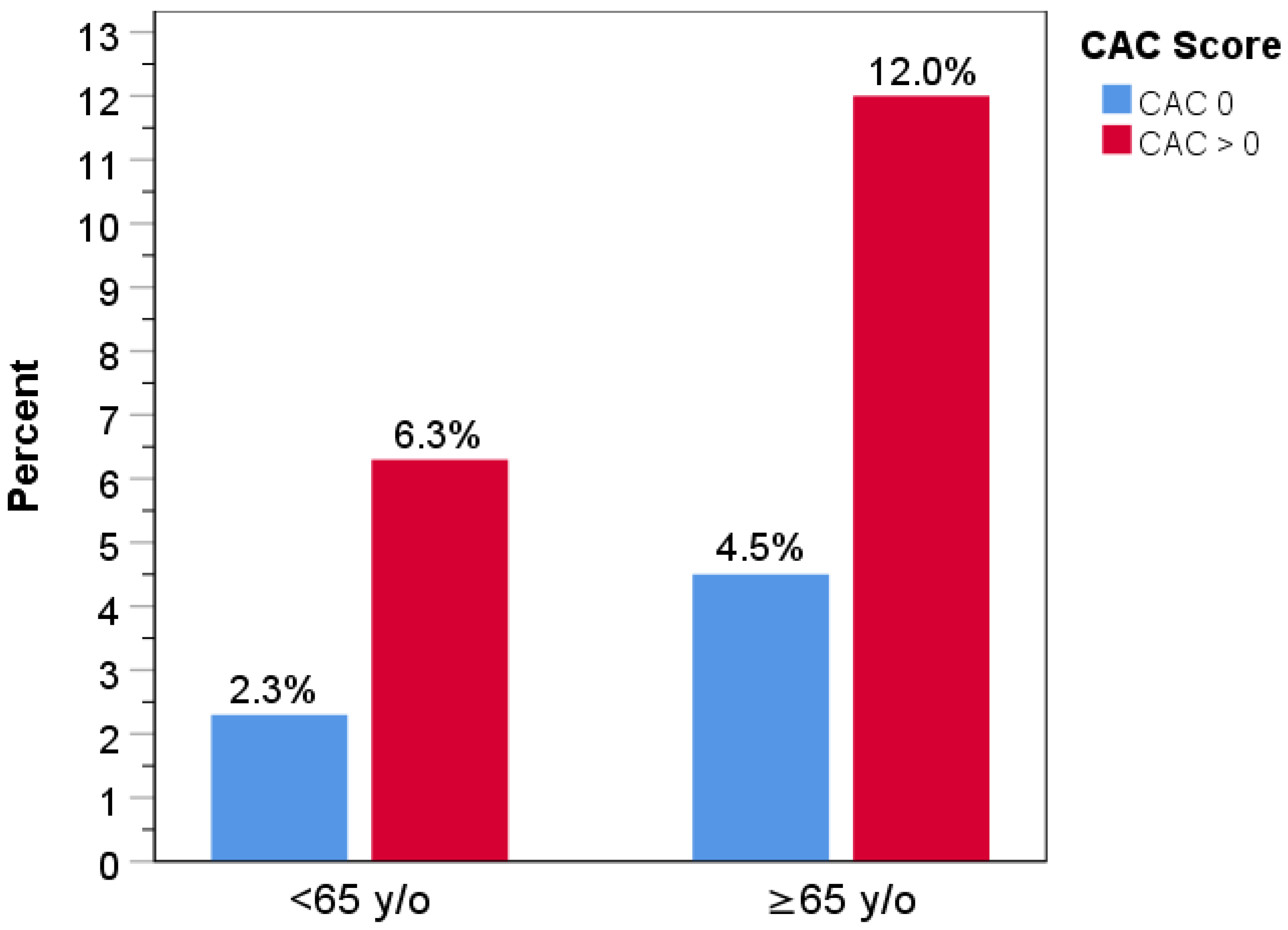
3. Results
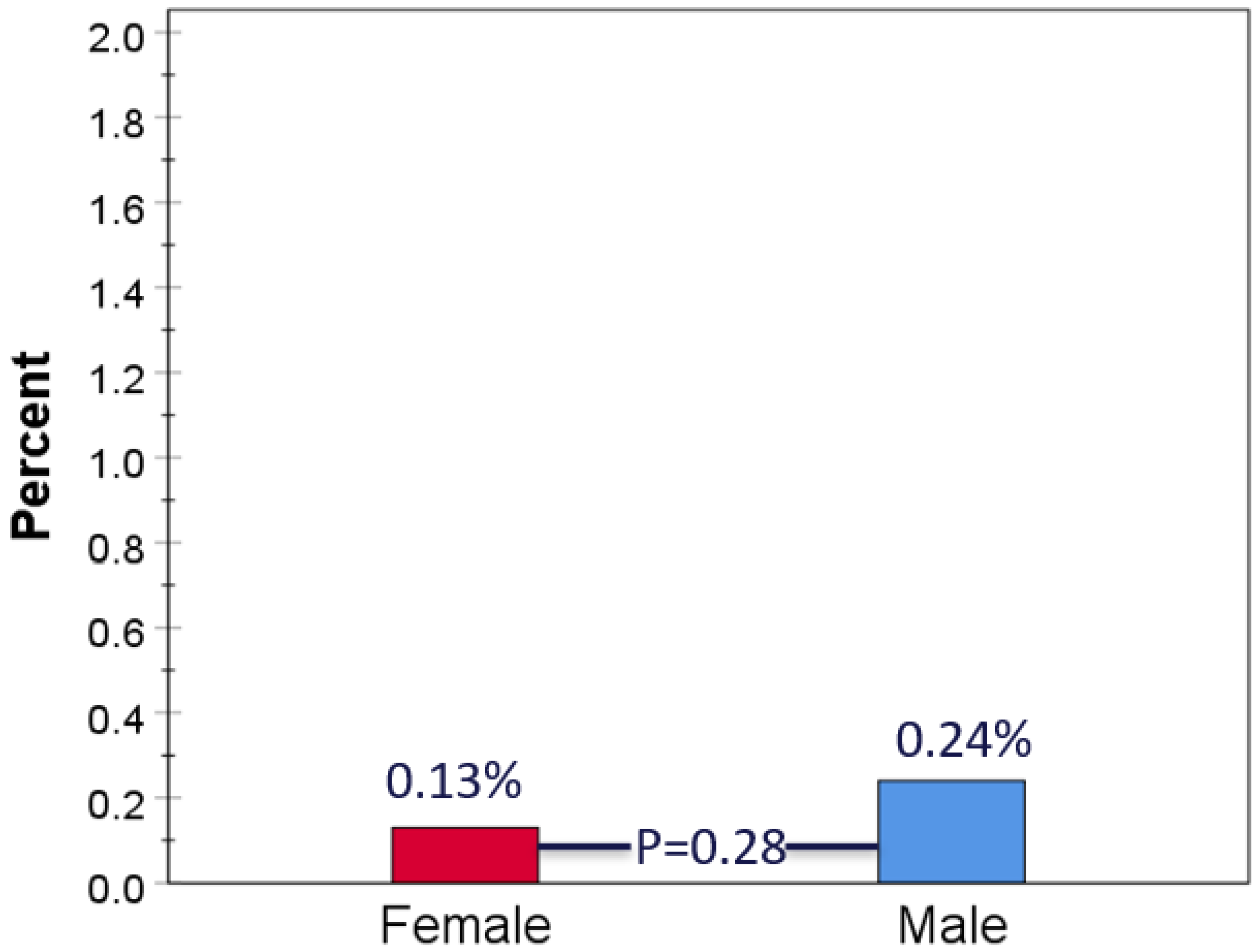
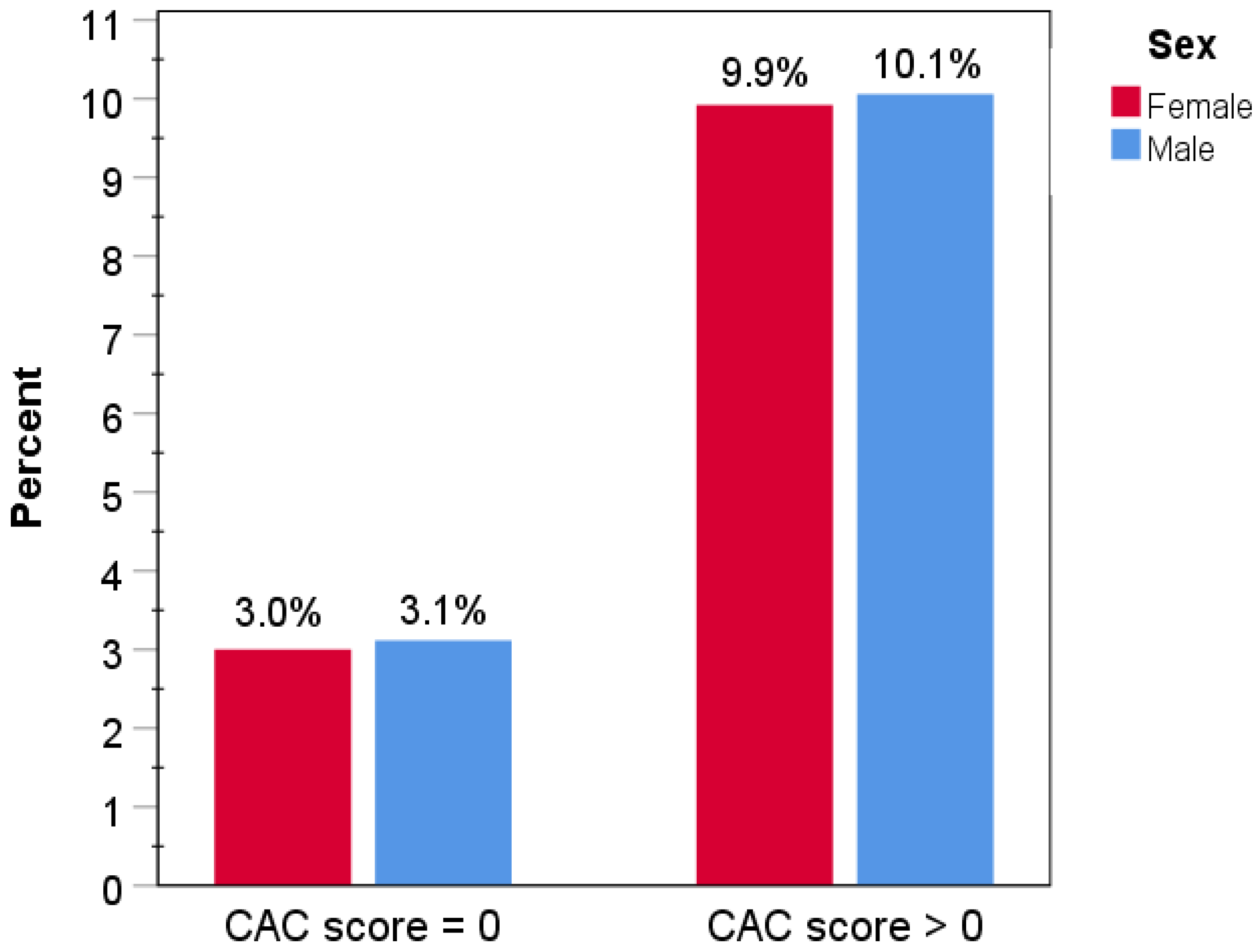
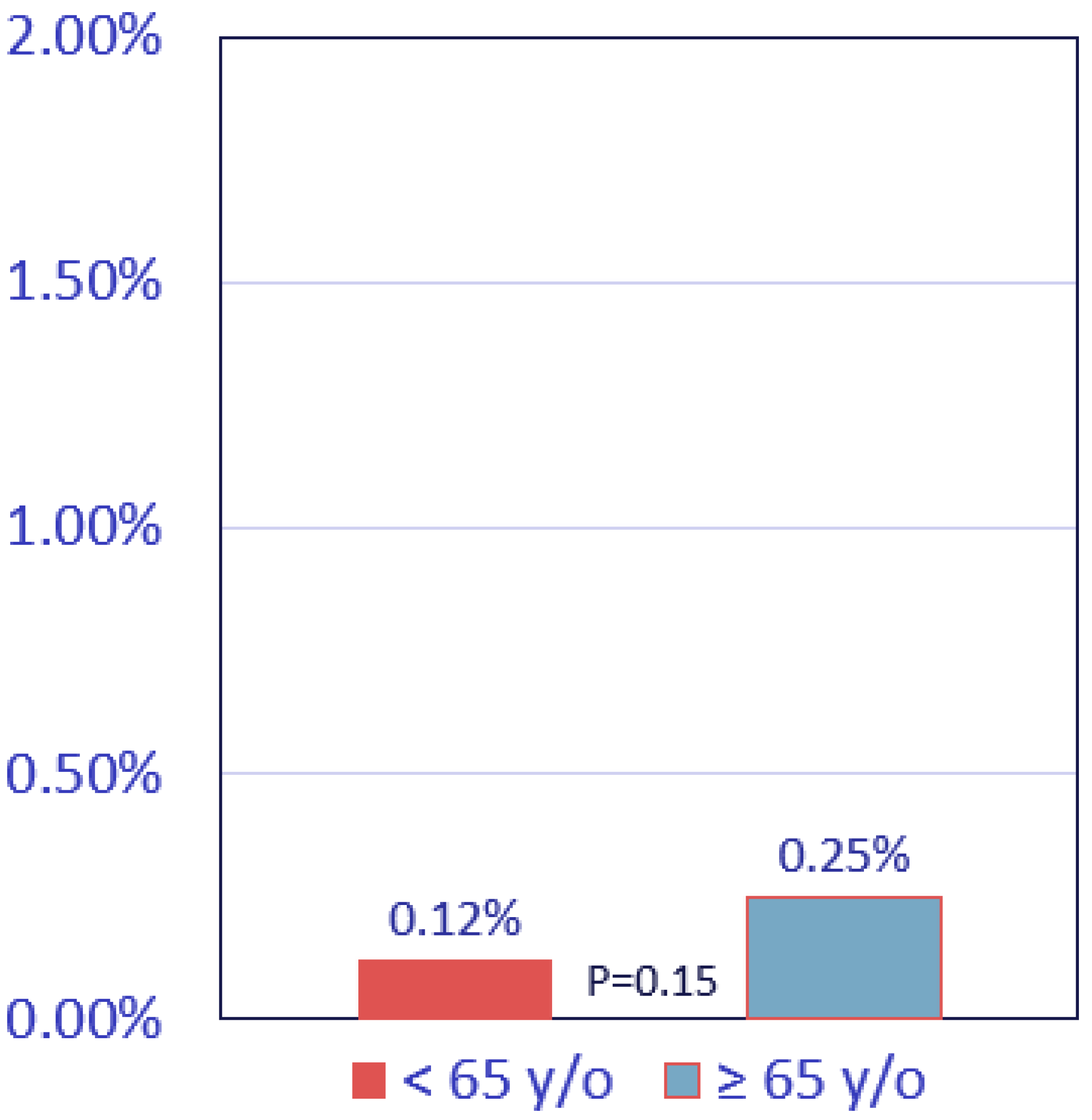
3.1. Study Population and Baseline Characteristics
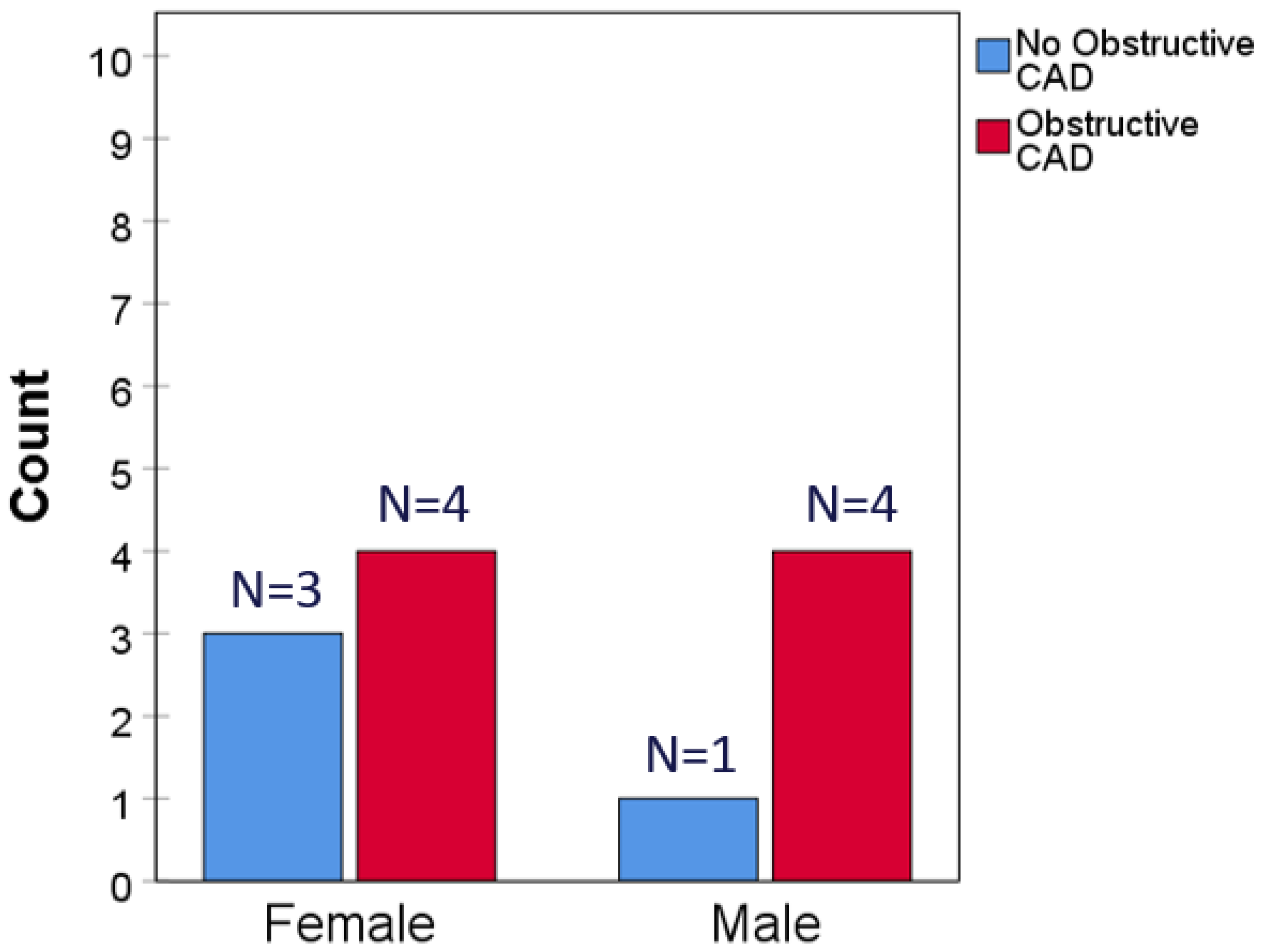
3.2. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAC = 0 | Coronary artery calcium score of zero |
| CAC | Coronary artery calcium |
| CAD | Coronary artery disease |
| PET/CT | Positron emission tomography/computed tomography |
| CV | Cardiovascular |
| MI | Myocardial infarction |
| eMR | Electronic medical records |
| MINOCA | MI with no obstructive CAD |
| ANOCA | Angina with no obstructive coronary artery disease |
References
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.L.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Schenker, M.P.; Dorbala, S.; Hong, E.C.T.; Rybicki, F.J.; Hachamovitch, R.; Kwong, R.Y.; Di Carli, M.F. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease. Circulation 2008, 117, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [PubMed]
- Bavishi, C.; Argulian, E.; Chatterjee, S.; Rozanski, A. CACS and the frequency of stress-induced myocardial ischemia during MPI. A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.M.; Clerc, O.F.; Honegger, U.; Amrein, M.; Thommen, K.; Caobelli, F.; Haaf, P.; Müller, C.E.; Zellweger, M.J. The power of zero calcium in 82-Rubidium PET irrespective of sex and age. J. Nucl. Cardiol. 2023, 30, 1514–1527. [Google Scholar] [CrossRef]
- Blaha, M.J.; Cainzos-Achirica, M.; Greenland, P.; McEvoy, J.W.; Blankstein, R.; Budoff, M.J.; Dardari, Z.; Sibley, C.T.; Burke, G.L.; Kronmal, R.A.; et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016, 133, 849–858. [Google Scholar] [CrossRef]
- Hayes, S.N.; Tweet, M.S.; Adlam, D.; Kim, E.S.; Gulati, R.; Price, J.E.; Rose, C.H. Spontaneous coronary artery dissection: JACC State-of the Art Review. J. Am. Coll. Cardiol. 2020, 76, 961–984. [Google Scholar] [CrossRef]
- Samuels, B.D.; Shah, S.M.; Widmer, R.J.; Kobayashi, Y.; Miner, S.E.; Taqueti, V.R.; Jeremias, A.; Albadri, A.; Blair, J.A.; Kearney, K.E.; et al. Comprehensive management of ANOCA. Part 1—Definition, patient population, and diagnosis: JACC State-of-the Art Review. J. Am. Coll. Cardiol. 2023, 82, 1245–1263. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Prasad, M.; Widmer, R.J.; Toleva, O.; Quesada, O.; Sutton, N.R.; Lerman, A.; Reynolds, H.R.; Kesarwani, M.; Savage, M.P.; et al. Comprehensive management of ANOCA, Part 2—Program development, treatment, and research initiatives: JACC State-of-the Art Review. J. Am. Coll. Cardiol. 2023, 82, 1264–1278. [Google Scholar] [CrossRef]
- Pepine, C.J. ANOCA/INOCA/MINOCA: Open artery ischemia. Am. Heart J. 2023, 26, 100260. [Google Scholar] [CrossRef]
- Karmali, K.N.; Goff, D.C.; Ning, H.; Lloyd-Jones, D.M. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol. 2014, 64, 959–968. [Google Scholar] [CrossRef]
- Sadiya, F.K.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Surapaneni, A.; Blaha, M.J.; Carson, A.P.; Chang, A.R.; Ciemins, E.; et al. Development and validation of the American Heart Association’s PREVENT equations. Circulation 2024, 149, 430–449. [Google Scholar]
- Sheppard, J.P.; Lakshmanan, S.; Lichtenstein, S.J.; Budoff, M.J.; Roy, S.K. Age and the power of zero CAC in cardiac risk assessment: Overview of the literature and a cautionary case. Br. J. Cardiol. 2022, 29, 89–94. [Google Scholar] [CrossRef]
- Meredith, K.G.; Dhar, R.; Mason, S.; Knight, S.; Bruno, D.; McCubrey, R.; Le, V.; Revenaugh, J.R.; Miner, E.; Lappe, D.; et al. Impact of transitioning from SPECT to PET on myocardial ischemia detection: Experience from a high volume “Real World” practice. J. Am. Coll. Cardiol. 2015, 65, A299. [Google Scholar] [CrossRef]
- Knight, S.; Min, D.B.; Le, V.T.; Meredith, K.G.; Dhar, R.; Biswas, S.; Jensen, K.R.; Mason, S.M.; Ethington, J.-D.; Lappe, D.L.; et al. Implementation of a cardiac PET stress program: Comparison of outcomes to the preceding SPECT era. JCI Insight 2018, 3, e120949. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Coll. Cardiol. Am. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Arad, Y.; Spadaro, L.A.; Goodman, K.; Lledo-Perez, A.; Sherman, S.; Lerner, G.; Guerci, A.D. Predictive value of electron beam computed tomography of the coronary arteries. 19-month follow-up of 1173 asymptomatic subjects. Circulation 1996, 93, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [PubMed]
- Muhlestein, J.B.; Knowlton, K.U.; Le, V.T.; Lappe, D.L.; May, H.T.; Min, D.B.; Johnson, K.M.; Cripps, S.T.; Schwab, L.H.; Braun, S.B.; et al. Coronary artery calcium versus pooled cohort equation for primary prevention guidance: Randomized feasibility trial. JACC Cardiovasc. Imaging 2022, 15, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: Results from the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef]
- Villines, T.C.; Hulten, E.A.; Shaw, L.J.; Goyal, M.; Dunning, A.; Achenbach, S.; Al-Mallah, M.; Berman, D.S.; Budoff, M.J.; Cademartiri, F.; et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography. J. Am. Coll. Cardiol. 2011, 58, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Mittal, T.K.; Pottle, A.; Nicol, E.; Barbir, M.; Ariff, B.; Mirsadraee, S.; Dubowitz, M.; Gorog, D.A.; Clifford, P.; Firoozan, S.; et al. Prevalence of obstructive coronary artery disease and prognosis in patients with stable symptoms and a zero-coronary calcium score. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Le, E.P.V.; Rajani, N.K.; Hudson-Peacock, N.; Pavey, H.; Tarkin, J.M.; Babar, J.; Williams, M.C.; Gopalan, D.; Rudd, J.H.F. A zero coronary artery calcium score in patients with stable chest pain is associated with a good prognosis, despite risk of non-calcified plaques. Open Heart 2019, 6, e000945. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Peri-Okonny, P.A.; Qarajeh, R.; Patel, F.S.; Sperry, B.W.; McGhie, A.I.; Thompson, R.C.; Kennedy, K.F.; Chan, P.S.; Spertus, J.A.; et al. Prognostic relationship between coronary artery calcium score, perfusion defects, and myocardial blood flow reserve in patients with suspected coronary artery disease. Circ. Cardiovasc. Imaging 2022, 15, e012599. [Google Scholar] [CrossRef]
- Zheutlin, A.R.; Chokshi, A.K.; Wilkins, J.T.; Stone, N.J. Coronary artery calcium testing—Too early, too late, too often. JAMA Cardiol. 2025, 10, 503–509. [Google Scholar] [CrossRef]
- Blaha, M.J.; Cainzoso-Achirica, M.; Dardari, Z.; Blankstein, R.; Shaw, L.J.; Rozanski, A.; Rumberger, J.A.; Dzaye, O.; Michos, E.D.; Berman, D.S.; et al. All-cause and cause-specific mortality in individuals with zero and minimal coronary artery calcium: A long-term, competing risk analysis in the coronary artery calcium consortium. Atherosclerosis 2020, 294, 72–79. [Google Scholar] [CrossRef]
- Cortiana, V.; Vaghela, H.; Bakhle, R.; Santhosh, T.; Kaiwan, O.; Tausif, A.; Goel, A.; Suhail, M.K.; Patel, N.; Akram, O.; et al. Beyond the heart: The predictive role of coronary artery calcium scoring in non-cardiovascular disease risk stratification. Diagnostics 2024, 14, 2349. [Google Scholar] [CrossRef]
- Achim, A.; Peter, O.A.; Coci, M.; Serban, A.; Mot, S.; Dadarlat-Pop, A.; Nemes, A.; Ruzsa, Z. Correlation between coronary artery disease with other arterial systems: Similar, albeit separate, underlying pathophysiologic mechanisms. J. Cardiovasc. Dev. Dis. 2023, 10, 210. [Google Scholar] [CrossRef]
- Bell, C.F.; Lei, X.; Haas, A.; Baylis, R.A.; Gao, H.; Luo, L.; Giordano, S.H.; Wehner, M.R.; Nead, K.T.; Leeper, N.J. Risk of cancer after diagnosis of cardiovascular disease. JACC CardioOncol. 2023, 5, 431–440. [Google Scholar] [CrossRef]
- Malmborg, M.; Christiansen, C.B.; Schmiegelow, M.D.; Torp-Pedersen, C.; Gislason, G.; Schou, M. Incidence of new onset cancer in patients with a myocardial infarction—A nationwide cohort study. BMC Cardiovasc. Disord. 2018, 18, 198. [Google Scholar] [CrossRef]
- Anderson, J.L.; May, H.T.; Winslow, T.; Knight, S.; Le, V.T.; Iverson, L.K.; Bair, T.L.; Knowlton, K.U.; Muhlestein, J.B. CorCal outcomes: A randomized trial using the pooled cohort equation or coronary artery calcium to select statin therapy in primary prevention patients. Baseline characteristics and statin recommendations. J. Am. Coll. Cardiol. 2025, 85, 521. [Google Scholar] [CrossRef]
| Characteristic | Women with CAC = 0 | Men with CAC = 0 | p-Value |
|---|---|---|---|
| Number of subjects, n (%) | 5400 (67.8) | 2567 (32.2) | <0.001 |
| Age, mean (SD) | 60.5 (12.0) | 53.8 (12.6) | <0.001 |
| Race, n (%) | |||
| White/Caucasian | 4872 (90.2) | 2234 (87.0) | <0.001 |
| African American (Black) | 48 (0.9) | 67 (2.6) | <0.001 |
| Asian | 77 (1.4) | 33 (1.3) | 0.69 |
| American Indian/AK native | 64 (1.2) | 16 (0.6) | 0.03 |
| Multiple | 13 (0.2) | 12 (0.5) | 0.14 |
| Pacific Islander | 60 (1.1) | 36 (1.4) | 0.32 |
| Unknown | 266 (4.9) | 169 (6.6) | 0.003 |
| Family history of heart disease, n (%) | 2766 (55.2) | 1052 (44.4) | <0.001 |
| Medical History, n (%) | |||
| Hyperlipidemia | 3551 (65.8) | 1551 (60.4) | <0.001 |
| Hypertension | 3653 (67.6) | 1750 (68.2) | 0.66 |
| Diabetes | 964 (18.5) | 454 (18.5) | 1.0 |
| Smoking history | 1335 (24.7) | 964 (37.6) | <0.001 |
| Atrial fibrillation | 753 (13.9) | 403 (15.7) | 0.04 |
| COPD | 555 (10.3) | 235 (9.2) | 0.13 |
| Depression | 2510 (46.5) | 720 (28.0) | <0.001 |
| Heart failure | 920 (17.0) | 522 (20.3) | <0.001 |
| Renal failure | 1028 (19.0) | 558 (21.7) | 0.005 |
| Statins at discharge | 536 (9.9) | 282 (11.0) | 0.16 |
| Stroke | 664 (12.3) | 228 (8.9) | <0.001 |
| Myocardial infarction | 259 (4.8) | 160 (6.2) | 0.01 |
| Characteristic | Patients Aged < 65 y old | Patients Aged ≥ 65 y old | p-Value |
|---|---|---|---|
| Number of subjects, n (%) | 5185 (65.1) | 2782 (34.9) | <0.001 |
| Age, median (IQR) | 52.0 (14) | 70.0 (8) | <0.001 |
| Male, n (%) | 2014 (38.8) | 553 (19.9) | <0.001 |
| Female, n (%) | 3171 (61.2) | 2229 (80.1) | <0.001 |
| Race, n (%) | |||
| White/Caucasian | 4507 (86.9) | 2599 (93.4) | <0.001 |
| African American (Black) | 96 (1.9) | 19 (0.7) | <0.001 |
| Asian | 75 (1.4) | 35 (1.3) | 0.56 |
| American Indian/AK native | 65 (1.3) | 15 (0.5) | 0.003 |
| Multiple | 16 (0.3) | 9 (0.3) | 1.0 |
| Pacific Islander | 83 (1.6) | 13 (0.5) | <0.001 |
| Unknown | 343 (6.6) | 92 (3.3) | <0.001 |
| Family history of heart disease | 2539 (53.3) | 1279 (48.8) | 0.01 |
| Medical history, n (%) | |||
| Hyperlipidemia | 2999 (57.8) | 2103 (75.6) | <0.001 |
| Hypertension | 3328 (64.2) | 2075 (74.6) | <0.001 |
| Diabetes | 983 (19.9) | 435 (16.1) | <0.001 |
| Smoking history | 1712 (33.0) | 587 (21.1) | <0.001 |
| Atrial Fibrillation | 499 (9.6) | 657 (23.6) | <0.001 |
| COPD | 433 (8.4) | 357 (12.8) | <0.001 |
| Depression | 2137 (41.2) | 1093 (39.3) | 0.10 |
| Heart Failure | 853 (16.5) | 589 (21.2) | <0.001 |
| Renal failure | 818 (15.8) | 768 (27.6) | <0.001 |
| Statins at discharge | 546 (10.5) | 272 (9.8) | 0.31 |
| Stroke | 405 (7.8) | 487 (17.5) | <0.001 |
| Myocardial infarction | 265 (5.1) | 154 (5.5) | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, J.L.; Collingridge, D.S.; Le, V.T.; Iverson, L.; Muhlestein, J.B.; Bair, T.L.; Knight, S.; Mason, S.M.; Knowlton, K.U. Does Sex or Age Impact the Prognostic Value of a Zero Coronary Artery Calcium Score? J. Clin. Med. 2025, 14, 6260. https://doi.org/10.3390/jcm14176260
Anderson JL, Collingridge DS, Le VT, Iverson L, Muhlestein JB, Bair TL, Knight S, Mason SM, Knowlton KU. Does Sex or Age Impact the Prognostic Value of a Zero Coronary Artery Calcium Score? Journal of Clinical Medicine. 2025; 14(17):6260. https://doi.org/10.3390/jcm14176260
Chicago/Turabian StyleAnderson, Jeffrey L., Dave S. Collingridge, Viet T. Le, Leslie Iverson, Joseph B. Muhlestein, Tami L. Bair, Stacey Knight, Steve M. Mason, and Kirk U. Knowlton. 2025. "Does Sex or Age Impact the Prognostic Value of a Zero Coronary Artery Calcium Score?" Journal of Clinical Medicine 14, no. 17: 6260. https://doi.org/10.3390/jcm14176260
APA StyleAnderson, J. L., Collingridge, D. S., Le, V. T., Iverson, L., Muhlestein, J. B., Bair, T. L., Knight, S., Mason, S. M., & Knowlton, K. U. (2025). Does Sex or Age Impact the Prognostic Value of a Zero Coronary Artery Calcium Score? Journal of Clinical Medicine, 14(17), 6260. https://doi.org/10.3390/jcm14176260







