Abstract
Current routine high-sensitivity cardiac troponin assays are the criterion standard for the laboratory diagnosis of myocardial injury due to their high analytical sensitivity and specificity. However, in daily clinical practice, unexpectedly elevated cardiac troponin test results without an obvious clinical correlate are becoming more frequent compared with previous cardiac troponin assay generations. In these patients, myocardial injury may sometimes be undetected by imaging techniques, including cardiac magnetic resonance imaging. This has led to an increased interest in the pathophysiology of cardiac troponin release, particularly with regard to whether troponin can be released in the absence of myocardial necrosis and thereby resulting in an increase in cardiac troponin in the systemic circulation. Although there is in vitro evidence that cardiac biomarkers are released from reversibly injured cultured cardiomyocytes, there is still a lack of evidence for cardiac troponin release apart from different forms of cell death (i.e., apoptosis or necrosis) in animal experiments. Conversely, various circulating cardiac troponin forms have been identified in human blood samples using different analytical methods, raising the question of whether the cause of myocardial injury can be reliably determined by measuring specific circulating cardiac troponin forms. Preliminary clinical data suggests that testing for specific circulating troponin forms could increase the specificity of cardiac troponin for diagnosing acute myocardial infarctions caused by an acute coronary syndrome. This review aims to provide an up-to-date overview of these current cardiac troponin research topics with their potential clinical implications. Typical clinical cases illustrate how to interpret cTn in the individual patient and how to derive a correct diagnosis.
1. Background
Since the 1950s, alongside the electrocardiogram (ECG), laboratory parameters for the detection of myocardial injury have been a cornerstone in the clinical assessment of patients with suspected acute myocardial infarction (AMI) [1,2,3]. However, the landscape of cardiac biomarkers has evolved dramatically since then. There has been an ongoing search for more sensitive and cardiac-specific laboratory parameters for the diagnosis of AMI, finally resulting in the development of immunoassays for the detection of cardiac troponins (cTn) [1]. To improve the early diagnosis of AMI, the analytical sensitivity of cTn assays has steadily been increased with each new assay generation, while maintaining their cardiospecificity. This has led to the development of so-called “high-sensitivity” (hs) cTn routine assays, which can detect circulating cTn in the majority of healthy individuals [2]. Thus, for the diagnosis of myocardial injury, cardiac troponin I (cTnI) and cardiac troponin T (cTnT) have become the preferred laboratory parameters, as they are currently the most sensitive and cardiac-specific available for routine laboratories [1,2,3,4,5,6]. However, due to the high analytical sensitivity of current hs-cTn assays, the positive predictive value of hs-cTn for AMI is markedly lower than that of previous cTn assay generations [2,4,5]. Therefore, unexpectedly elevated cTn test results without an obvious clinical correlate are becoming increasingly common and challenging in daily clinical practice [4,5,6]. In most of these patients, these cTn elevations are due to acute or chronic myocardial injury related to various cardiac or non-cardiac pathologies involving the heart, but which are not an AMI [3,4,5,6]. In some of these patients, myocardial injury may even go undetected by imaging due to the higher sensitivity of hs-cTn assays, which can detect cTn in blood samples at femtomolar concentrations [3,4,5,6]. The huge variety of causes of myocardial injury is summarized in Table 1 [1,2,3,4,5,6,7]. These mechanisms include myocardial ischemia caused by an acute coronary syndrome (ACS), which is caused by coronary plaque rupture or erosion with intracoronary thrombus formation (type 1 AMI); myocardial ischemia or hypoxia unrelated to an ACS (type 2 AMI); and myocardial injury unrelated to myocardial ischemia or hypoxia, such as inflammation (e.g., myocarditis), increased myocardial wall stress (e.g., heart failure), toxic injury to the myocardium and trauma (e.g., cardiac contusion) [4,5]. Analytically false-positive test results are very rare [6,8,9].

Table 1.
Differential diagnosis of cardiac troponin increases in peripheral blood samples: cardiac and non-cardiac diseases leading to myocardial injury.
However, many aspects of cTn degradation within the myocardium, its release, and its subsequent degradation and clearance from the human circulation are still not fully understood. This review aims to provide an update on the current knowledge on cTn degradation, release, and on circulating human cTnI and cTnT forms with their clinical implications. It highlights gaps in current knowledge and the potential future application of measuring different circulating cTn forms for the differential diagnosis of diseases leading to myocardial injury. The problems of cTn interpretation in patients presenting with specific clinical scenarios in daily routine are summarized. Typical clinical cases are presented (mostly as supplemental figures) to illustrate how to interpret cTn in the individual patient and how to derive a correct diagnosis.
2. The Pathophysiology of Cardiac Troponin Release from Injured Myocardium
The cTn complex is part of the thin filaments of the myocardium, and its biochemistry and pathophysiology have been reviewed extensively previously [10,11,12]. In summary, the cTn complex plays a central role in the calcium-dependent regulation of actin thin filament function, and it is, therefore, essential for controlling striated muscle contraction. Only two of the three troponins are encoded as cardiac-specific isoforms (cTnI and cTnT) in humans (see Table 2): troponin I (TnI, about 24 kDa), the actomyosin adenosine triphosphatase inhibitory subunit, which confers calcium sensitivity to striated muscle contraction; and troponin T (TnT), the tropomyosin-binding subunit (about 35–36 kDa depending on the additional cTnT isoform variation; the latter is a result of alternative splicing). cTnT and cTnI have a cardiac-specific N-terminal extension. However, troponin C (TnC, about 18 kDa), the calcium-binding subunit, which initiates the sequence of conformational changes on the thin filament, is also expressed in slow-twitch skeletal muscle fibers [13,14,15,16,17].

Table 2.
The biochemistry of cardiac troponin isoforms in humans.
Initially, small “cytosolic” pools of cTnI and cTnT (approximately 5% of the total content) were reported in human myocardium [18,19,20]. However, given the preparation protocols employed by the researchers and the poor solubility of cTnI and cTnT in the hydrophilic sarcoplasma, a more fitting term would be “rapidly degraded, loosely bound, early releasable pool”. When not incorporated into myofilaments, cTnT, for example, is rapidly degraded within cardiomyocytes by enzymes such as caspase or µ-calpain to avoid toxic effects [21,22,23,24]. µ-calpain is activated by increased cytoplasmic calcium, an important and early feature of cell injury [21,22,23,24]. Increased cytoplasmic calcium also activates phospholipases and endonucleases [25]. pH-dependent dissociation of the troponin complex may be another important factor in early cTn release from injured myocardium via a temporarily leaky plasma membrane (see Figure 1) [26]. Subsequent data suggest that the proportion of cTnT that can dissociate rapidly from myofibrils in vivo is substantially higher than the 5–10% previously reported [27]. cTnI is also a substrate of caspase-3, which has a key role in apoptosis; it targets the N-terminal region of cTnT (see Figure 2) [24]. Therefore, all cTn released is likely to be of myofibrillar origin. The pathophysiological background of the limited N-terminal intramyocardial proteolysis of cTn by caspase and calpain may be that cTns are involved in the sensitivity of the myocardium to decreases in intracellular pH, and thus, this may be an early specific functional adaptive response to myocyte injury rather than a simple destructive degradation [21,22,23,24]. Intramyocardial posttranslational modifications of cTn, such as oxidation or phosphorylization, may enhance or inhibit the susceptibility to proteolysis [21,23,24,28,29,30].
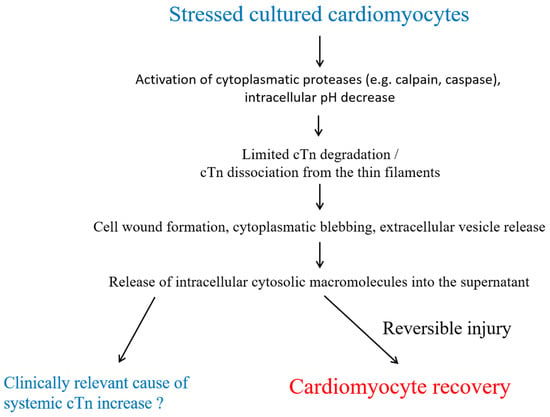
Figure 1.
Potential mechanisms of cardiac troponin release from reversibly injured cardiomyocytes. Degraded and dissociated cTn is released into the supernatant via cell wounds, cytoplasmic blebbing, and the formation of extracellular vesicles through a temporarily leaky plasma membrane. Abbreviations: cardiac troponin (cTn).
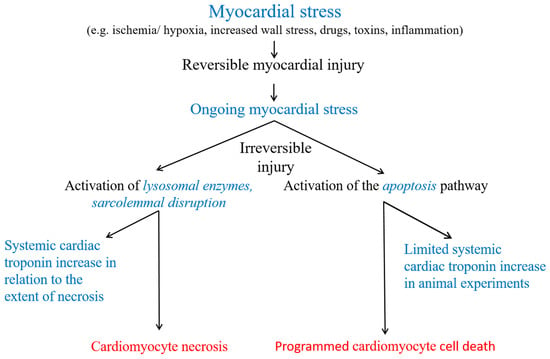
Figure 2.
Potential cardiac troponin release mechanisms from injured myocardium. Cardiomyocyte necrosis is the only generally accepted cause of a cTn increase into the systemic circulation in humans. In animal experiments, cardiomyocyte apoptosis was associated with cTn increases into the systemic circulation.
Cell death is preceded by a substantial reversible pre-lethal phase (see Figure 1) [26]. However, ongoing myocardial stress, depending on the mechanism and extent of myocardial stress, may lead to different forms of cell death (see Figure 2). When myocardial necrosis occurs, lysosomal enzymes degrade the contractile filaments, and the integrity of the plasmalemma is disrupted. Cardiomyocytes rapidly release intracellular macromolecules into the interstitial space. cTn shows a rapid increase after myocardial injury, comparably fast to cytosolic proteins [19,20,31,32,33,34]. There, additional proteolysis of troponins may occur, e.g., by matrix metalloproteinase-2 or thrombin [35,36]. Notably, however, the currently commercially available routine hs-cTnT assay, which utilizes antibodies targeting the amino acid fragment 125–147, is unaffected by thrombin-mediated cTnT degradation [37]. Local blood and lymphatic flow also influence the onset of the subsequent cTn increase in the systemic circulation. For example, early reperfusion of the infarct-related coronary artery results in more rapid extraction and clearance of cTn from damaged myocardium [38,39,40].
2.1. Experimental Data on the Release of cTn from Reversibly Injured Cardiomyocytes or in the Absence of Myocardial Necrosis
There is evidence from in vitro experimental models, that macromolecules, including cTn, are released in the absence of histological evidence of cardiomyocyte necrosis [26,41,42,43,44,45]. For example, cytoplasmic blebbing may already occur during the reversible phase of cell injury [26]. These blebs contain intracellular macromolecules and detach with resealing of the plasma membrane without cell death in cell culture experiments. This may therefore be responsible for limited cTn release from cardiomyocytes [26,41]. Furthermore, it has been reported in cell culture experiments, that tachycardia may stimulate integrins, thereby triggering the release of cTnI in the absence of necrosis [42]. Additionally, the cTn content of cardiomyocytes decreases prior to necrosis [46].
Recent large-animal experiments in pigs by Weil et al. found cTnI increases in peripheral blood samples in the absence of myocardial necrosis [47,48]. cTnI increased after applying left anterior descending artery (LAD) occlusion of only 10 min, which was associated with reversible myocardial dysfunction but without histological evidence of myocardial necrosis in tissue analysis [47]. Apart from ischemia or hypoxia, mechanical stretch in response to pressure or volume overload may trigger the activation of proteases associated with intracellular troponin degradation and the release of troponin fragments from injured cardiomyocytes without evidence of necrosis despite reversible ventricular dysfunction [48]. In this model, an intravenous infusion of phenylepinephrine led to preload-induced myocardial injury by acutely increasing wall tension. While these experiments demonstrate cTn release in response to myocardial ischemia or increased myocardial wall tension in the absence of myocardial necrosis, these results do not prove release from reversibly injured myocardium, as apoptosis, another form of cell death, was detected.
2.2. Clinical Data Suggesting the Release of cTn in the Absence of Myocardial Necrosis
Since the publication of the first research radioimmunoassay for cTnI detection [49], the analytical sensitivity of current hs-cTn assays has improved dramatically from 500 ng/L to approximately 1–3 ng/L [37]. It has been postulated that for every 1 µg of human myocardium injured, the cTnI and cTnT concentrations increase by approximately 4 ng/L in peripheral blood samples [50]. This advancement in the analytical performance of cTn assays, while maintaining cardiospecificity, has led to a dramatic improvement in the clinical sensitivity for detecting myocardial injury, that was previously unimaginable at the beginning of clinical cTn research. It is higher than all currently available imaging modalities [51,52].
Initially, cTn could not be detected in healthy individuals; however, with hs-cTn testing, cTn can now be detected in over 50% of healthy individuals [1,2,37,49,53,54]. As analytical interferences have been ruled out [53,54], detectable hs-cTn concentrations in humans with normal hearts suggest a constant limited turnover of cTn and/or cardiomyocytes with cTn being released into the systemic circulation. In a functioning sarcomere, protein synthesis, processing, and degradation occur continuously as a part of physiological turnover [55,56]. cTnT undergoes rapid turnover with a half-life of approximately 3.5 days within cardiomyocytes [56]. If not incorporated into myofilaments, cTnT is rapidly degraded to avoid toxic effects [18,55].
It is probably impossible to prove the release of cTn from reversibly injured myocardium in humans in the absence of different forms of cell death. However, there are increasing numbers of reports in the literature on cTn release in clinical scenarios in which myocardial necrosis is highly improbable [57,58,59,60,61]. For instance, a very sensitive assay demonstrated cTnI release in humans following nuclear perfusion scintigraphy with peak concentrations associated with the extent of myocardial ischemia during stress testing [57]. Furthermore, it has been reported that even normal individuals exhibit elevated circulating cTn levels in response to dobutamine stress or exercise testing [58,59]. Small, but significant cTn concentration increases in coronary sinus blood samples were found within 30 min after brief episodes of incremental rapid atrial pacing using a protocol with comparable myocardial stress to moderate brief physical exercise [60]. This cTnT release in coronary sinus blood was subsequently mirrowed in a delayed, small but significant hs-cTnT concentration increase in peripheral blood samples after 3 h (doubling to tripling of baseline values), even in the subgroups without significant coronary artery disease (CAD) or without net myocardial lactate production proving myocardial ischemia. In all patients without significant CAD and no net myocardial lactate production, hs-cTnT concentrations remained within the upper reference limit (URL).
2.2.1. Clinical Implications: Challenges in Interpreting hs-cTn Concentrations Following Elective Percutaneous Coronary Interventions
cTn increases are found in almost all patients, even those with completely uneventful procedures, after elective percutaneous coronary interventions (PCI) (see Figure 3). This makes it difficult to interpret cTn increases in individual symptomatic patients without knowing their baseline values. When measured with hs-cTn assays, cTn increased significantly in peripheral venous blood samples in individuals with angiographically normal coronary arteries, even after LAD occlusion of only 30 s duration, a setting in which myocardial necrosis is highly unlikely [61]. cTn increases started as early as 15–30 min after balloon occlusion. The highest cTn values were found at the end of the blood sampling period at 4 h after LAD occlusion, with the highest relative increases found after a 90 s lasting LAD occlusion (all participants developed angina, and assay-dependent increases ranged from about 3- to 8-fold baseline values).
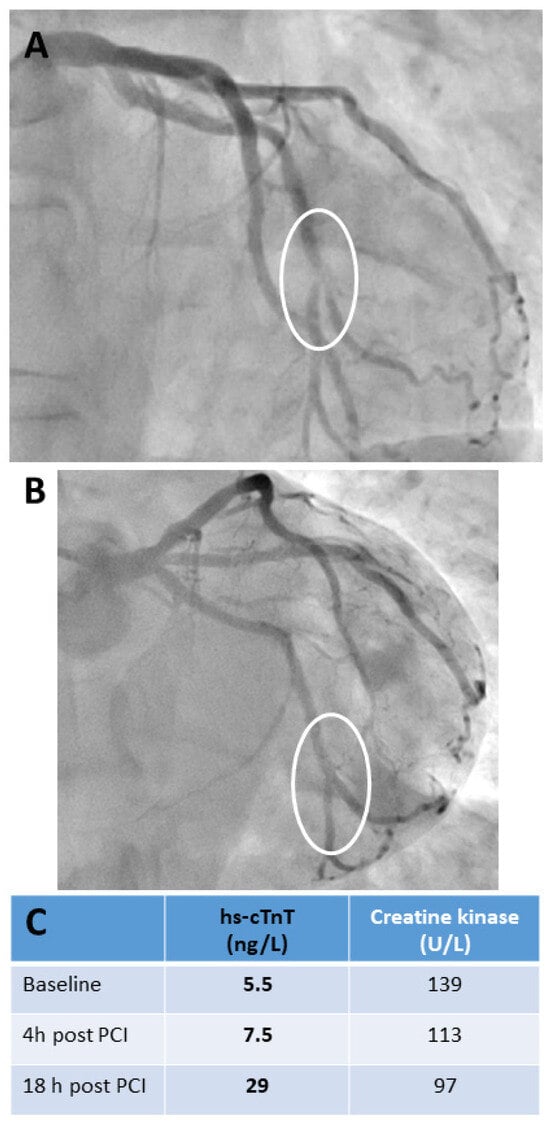
Figure 3.
Cardiac biomarker release in a patient undergoing uncomplicated bifurcation percutaneous transluminal coronary intervention for unstable angina. This case clearly demonstrates the importance of specifying exactly when a proposed cTn decision limit should be used to diagnose peri-interventional AMI after elective PCI. A 55-year-old male with a history of CAD was admitted with symptoms of unstable angina. He underwent PCI of the left circumflex coronary artery 11 years ago. Serial ECGs before and after PCI showed no signs of acute myocardial ischemia. Serial hs-cTnT testing in the emergency department before PCI revealed concentrations within the normal range without a significant change. The criteria for AMI were not met. Urgent coronary angiography revealed a subtotal in-stent restenosis at the distal end of the stent involving the distal circumflex artery bifurcation (see (A), marked with a circle). There was no other significant stenosis. PCI was performed with an excellent primary result without any complications (see (B), marked with circle). The biomarker time courses are listed in (C). hs-cTnT showed no significant increase within 4 h after PCI, but increased significantly above the upper reference limit of 14 ng/L the following morning. Creatine kinase activities remained within the reference limit even showing a constant decline; the patient was a tunnel-building construction worker with physically demanding work. Abbreviations: high-sensitivity troponin T (hs-cTnT), percutaneous coronary intervention (PCI), circumflex artery (RCX), coronary artery disease (CAD), acute myocardial infarction (AMI).
Baseline cTn values correlate with the severity and complexity of CAD and may already be increased due to repeated episodes of myocardial ischemia or comorbidities, such as left ventricular hypertrophy or dysfunction. This can lead to increased wall tension in the left ventricle and reduced subendocardial myocardial perfusion (see Supplemental Figure S1) [7,60,62]. In general, the increase in cTn after elective PCI is related to the severity of CAD and the complexity of the interventions performed (see Supplemental Figure S1) [7,62].
Apart from the obvious cases of peri-interventional AMI with complications evident on coronary angiography (see Supplemental Figure S2), its diagnosis is not always straightforward in clinical practice. Applying of the criteria of the 4th Universal Definition of Myocardial Infarction in clinical practice can be challenging, as it requires careful consideration of cTn increase together with clinical symptoms, and evidence for acute myocardial ischemia from angiography, ECG, or imaging [3,7]. However, it has been shown that diagnosing peri-interventional type 4a AMI complicating elective PCI has prognostic implications [63]. It was associated with an about 2-fold higher cardiovascular 1-year event rate and an about 3-fold higher, albeit still low 1-year mortality rate (3%) in patients with normal cTn baseline concentrations [63,64]. Other societies (see Table 3) have opted for a simpler approach based on cTn release, involving markedly higher cTn decision limits in combination with gross ECG evidence for AMI occurrence (e.g., the development of new pathological Q waves) [65,66]. A major limitation of all suggested cTn decision limits is that they do not consider the time-dependence of cTn release after myocardial injury (see Figure 3). No clear criteria are given in all current recommendations on blood sampling regimens or the point in time after PCI at which these decision limits are to be used [3,65,66]. Therefore, all recommendations must be revised to address these issues, although data on the optimal blood sampling regimen after elective PCI is still limited. From a practical point of view, it is suggested that cTn is tested at baseline, which is also useful for risk stratification [7,62,63,64], and, if clinically indicated, 4–6 h after PCI, and in the morning of the next day, if the patient is staying overnight in the hospital. Current clinical practice is usually to only test cTn after PCI when symptoms or complications arise during or after the procedure.

Table 3.
Suggested criteria for diagnosing peri-procedural AMI in elective PCI.
2.2.2. Clinical Implications: Challenges in Interpreting cTn Test Results in Symptomatic Athletes After Competitions or Heavy Endurance Exercise Training Sessions
Even greater debate surrounds the potential clinical significance of cTn increases in asymptomatic endurance athletes (e.g., marathon runners) after competitions or heavy training sessions, with hs-cTn concentrations above the URL and rising and/or falling patterns [59,67,68,69,70,71,72]. Peak concentrations usually do not exceed 3 times the URL, occurring several hours after exercise, with concentrations returning below the URL within 48–72 h [69]. The frequent release of cTn in asymptomatic athletes makes interpreting cTn concentrations in athletes who develop symptoms during or after competitions or training sessions very challenging. A representative clinical case that illustrates the problems encountered in cTn interpreting and deriving the correct diagnosis in symptomatic endurance athletes is presented in Supplemental Figure S3. Depending on the presenting key symptoms, the symptomatic athlete should be assessed as any other high-risk patient admitted to the emergency department. However, the usual post-exercise increase in cTn makes its interpretation more difficult. Serial cTn testing, considering the magnitude of cTn increase and the rate of change, is essential to rule out acute myocardial ischemia as the cause of symptoms, particularly in older (>35 years) male recreational athletes. In the absence of unequivocal ECG findings of acute myocardial ischemia. Performing imaging, in particular coronary computed tomography angiography (CCTA), is also essential.
Elevated cTn concentrations are very common in healthy individuals after extreme endurance exercise [59,68,69,70,71,72]. Following prolonged endurance events, such as marathons, triathlons, ultramarathons, post-race mild and transient acute reversible systolic dysfunction of the right ventricle, but not the left ventricle, has typically been described [69,73,74]. There was no close correlation between post-race cTnI concentrations and decreases in the right ventricular ejection fraction [73]. In marathon runners, the extent of cTn release did not correlate with myocardial injury in imaging [69,73], and the time course of cTn concentrations differed from that of AMI [69,73]. In athletes, post-exercise cTn release is not associated with worse prognosis [69,73,74,75]. However, this appears to be different in physically active elderly individuals from the general population [76]. Experimental data supports the possibility of cTn release from reversibly injured myocardium in athletes. In a rat animal model of an endurance exercise challenge, cTnT release was only associated with reversible changes in cardiomyocyte structure [67]. This finding is corroborated by the observation of minimal cTnT release during and following a marathon in a group of healthy, asymptomatic humans exercising on a motorized treadmill [68]. cTnT increased in all nine participants during running, at completion or within 1 h; subsequently, cTnT returned to baseline values. All but one participant showed a further small increase within a 24 h recovery period, which started several hours after finishing. Five participants still had increased cTnT concentrations 24 h after exercise. The release of cTnT within the first 60 min of running suggests that exercise-induced cTn release is not restricted solely to prolonged endurance exercise. It is highly unlikely that these minor cTn increases reflect myocardial necrosis in these healthy, extremely fit individuals. However, apoptosis cannot be ruled out. A high training volume and long training duration appear to protect against post-exercise cTn release [72]. However, exercise-induced cTn release itself appears to be related to the overall cardiac workload (duration, and in particular, the intensity) of the competition or training session [69]. There was no significant difference in exercise-induced cTn release between patients with a CAD rule-out (calcium score 0 and no plaques in CCTA) and patients with signs of coronary arteriosclerosis in CCTA but without evidence of exercise-induced myocardial ischemia. These individuals were therefore cleared to perform exercise before the study [70]. Nevertheless, despite being clinically asymptomatic, the exercise-induced cTnI release was reported to be higher in individuals with hemodynamically significant CAD [77].
Although cardiac magnetic resonance imaging (MRI) studies of marathon participants found no evidence of myocardial edema or late gadolinium enhancement despite increased cTn concentrations after exercise [69,73,74], its limited sensitivity does not rule out necrosis as the cause of cTn release from the heart. It has been speculated, that long-term exercise training could cause myocardial damage through repetitive high-intensity exercise sessions, which could explain why lifelong endurance athletes exhibit more late gadolinium enhancement compared with their physically less active peers [69,78]. There was also a relation between the extent of late gadolinium enhancement with the number of races competed in and years of training [78].
3. Clearance of cTnI and cTnT from the Circulation in Humans
Circulating proteins in the blood can be eliminated in three ways: enzymatic degradation within the circulation; metabolism in organs with a high metabolic rate, such as the liver, pancreas, kidneys, and skeletal muscles; and endocytosis by the reticuloendothelial system. Elimination can also occur via glomerular filtration and excretion in urine. Thus, in cases of impaired clearance from the blood (e.g., renal failure, hypothyroidism) increased concentrations of biomarker may be observed for longer than usual, and baseline values may be higher than normal.
Most proteins released from injured myocardium, including cTn, appear to be catabolized in tissues with a high metabolic rate, particularly in the liver, probably via scavenger receptor-mediated endocytosis and subsequent degradation in lysosomes [79]. In this respect, the reports of cTnT degradation by thrombin [36], a rather indiscriminate serine protease, which cleaves cTnT between R68 and S69, add interesting information and may explain the heparin interference of a previous generation of cTnT assays [36,80]. However, the role of thrombin in cTnT elimination remains to be shown. Small molecules, such as myoglobin, pass through the glomerular filtration membranes of the kidneys and can be found in the urine [81]. These proteins are mainly reabsorbed and subsequently metabolized in the tubular epithelial cells. Thus, prolonged increases in such biomarkers are found in the presence of impaired renal clearance from blood. Intact free cTnT is too large to be filtered by the glomerula, but smaller cTnT fragments are small enough to be cleared by the kidneys. By contrast, cTnI appears to be mainly found in the blood as part of complexes (see below) which are too large for glomerular filtration. At high cTnT concentrations (e.g., after AMI), extra-renal clearance of cTnT dominates [79], but at low concentrations (<100 ng/L), such as in patients with chronic cardiac diseases, renal clearance appears to dominate, as demonstrated in experiments where renal perfusion was reduced [82]. Recent data suggest that, as cTn drops to lower concentrations such as those often observed in patients with stable cTn elevations, cTnT clearance becomes slower and more renal-dependent [82]. Smaller, circulating, degraded cTn fragments, which are predominantly found in the blood in the case of chronic myocardial injury (see below), are small enough for glomerular filtration [82]. However, local degradation and tubular reabsorption could prevent the detection of cTnT and cTnI in urine. At these low steady-state concentrations, cTnT levels are roughly twice as high if kidney function is reduced by 50% [82]. Nevertheless, other mechanisms such as increased myocardial stress and wall tension related to various causes may also contribute to the cTnT elevations observed in patients with chronic renal failure [6,8].
When cTn enters the bloodstream, it follows an exponential two-phase model comprising a distribution and an elimination phase. Once cTnT and cTnI enter the circulation, they are initially cleared with a short half-life of around 0.5 h in dogs and rats [83]. A recent, carefully designed clinical study [84] involving autologous re-transfusion of plasma, which was obtained by plasmapheresis during the subacute phase of AMI, several weeks later, found that the median half-life of cTnT calculated in humans was about 2 h with a clearance of about 80 mL/min. The half-life of cTnI was longer, at about 4 h, with a clearance rate of about 40 mL/min. This study design avoids the problem of ongoing release of cTn from damaged myocardium. However, different cTn fragments may have different half-lives [82,85]. Recently, the half-life of high-molecular-mass (long-form) cTnT was reported to be approximately 9 h as calculated from the analyis of concentration time courses in AMI patients [85]. However, this approach may overestimate the true half-life due to ongoing cTn release from damaged myocardium.
4. Circulating Forms of Cardiac Troponin in Human Blood
The biochemical and pathophysiological background includes post-translational modifications, such as phosphorylation and oxidation, intramyocardial N-terminal proteolysis of cTnI and cTnT by caspase and calpain, and the dissociation of the cTn subunits from the cTn complexes, resulting in cytoplasmatic cTn forms, that can be released in response to myocardial injury (see above [10,11,12,28,29,30,45,46,47,48]). Released cTn may undergo further degradation in the interstitial space and the blood [35,36]. Serum or plasma is a complex matrix containing many abundant proteins, such as albumin, and comparably low concentrations of cTn. This presents a significant analytical challenge when trying to characterize circulating cTn forms using standard proteomics techniques, such as mass spectrometry [86,87,88], the criterion standard in proteomics, or gel filtration, as highly sensitive methods are required. In principal, there are two primary approaches: antibody-based techniques and physical separation techniques, such as Western blotting, gel filtration chromatography, and mass spectrometry. All these techniques have their own strengths and limitations (e.g., denaturing conditions and analytical sensitivity). For example, specific sample preparation and purification procedures are required to eliminate this background noise caused by abundant blood proteins to enrich cTn in the sample for detection by mass spectrometry [86,87]. Nevertheless, its analytical sensitivity remains limited, requiring very high cTn concentrations in samples [87]. Denaturing techniques are not suitable for distinguishing between the different forms and sizes of the ternary cTnTIC or cTnIC complexes.
A more straightforward approach would be to develop specific and sensitive immunoassays for detecting particular circulating cTn forms [85], once the relevant circulating cTn forms released in response to different causes of myocardial injury have been characterized using more sophisticated analytical techniques. For practical reasons, therefore, sensitive and specific sandwich immunoassays are the preferred method for research and potential future routine use [85,89,90]. These assays have the potential to be adopted for routine diagnosis on automated, high-throughput platforms.
Table 4 summarizes the currently available data on circulating cTn forms. These data are based on different analytical approaches, mainly Western blotting, gel filtration chromatography, and sandwich immunoassays, each with their own method-specific limitations. The data are also mainly based on analysis of stored samples. Testing of stored samples has limitations compared to testing of freshly drawn blood samples as storage conditions and freeze–thaw cycles may affect in vitro stability and consequently the circulating degradation forms within samples. Additionally, thrombin generation in serum samples may affect in particular cTnT forms. In EDTA plasma samples, the anticoagulant may affect cTn complexes [91], and ideally, the reported results on cTn complexes should be confirmed by testing fresh heparinized plasma samples.

Table 4.
A summary of the current knowledge regarding circulating cardiac troponin complexes and cardiac troponin forms in relation to the cause of myocardial injury.
The published results [92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] can be summarized as follows: intact cTn forms disappear rapidly from the circulation in the hours immediately following AMI. After AMI, mainly truncated binary cTnIC with varying quantities of mostly truncated ternary cTnICT complexes are found [92,93,94,96,97]. So-called “large size” cTnIC and cTnTIC complexes are only found early after AMI. Between 75 and 100% of the total cTnI content in the serum of post-AMI patients is part of a complex [92,93,94,100,102,106]. By contrast, cTnT also circulates in significant amounts as a free unbound form [101,103,104]. Intact cTnT is rapidly degraded at the N-terminal end. Early after AMI, it has mainly been described as a high-molecular-mass (HMM, ≥29 kDa), “long” form, including complex-bound cTnT [97,104,105]. Over time, the amount of heavily truncated cTnT forms (14–16 kDa) increases, probably due to degradation at both the C-terminal and N-terminal ends [101,103,104]. In AMI patients, cTnI primarily exists as truncated cTnIC and truncated cTnTIC complexes. The data on cTnI degradation is less consistent, but degradation at both ends has been reported as well [102,106,107]. Unlike the N- and C-terminal regions, the central portions of cTnI and cTnT appear to be stable [94,97,102,104,109]. These epitopes are the best targets for developing a routine cTn immunoassay with high analytical sensitivity and high in vitro stability [37].
Interestingly, the cause of myocardial injury may affect the circulating cTn forms (see Table 4). In the acute phase of AMI, HMM (long) cTnT forms predominate, whereas in chronic end-stage renal disease or in individuals who have performed strenous endurance exercise (e.g., marathon running or triathlons), only LMM cTnT forms could be detected [101,104,105]. These observations suggest that the detection of cTn composition in blood samples could be used in the future to define the cause of myocardial injury and to increase the specificity of cTn testing for AMI.
4.1. Commercially Available Routine hs-cTn Assays Aim to Measure Total cTnI or cTnT
The practical relevance of complex formation, post-translational modifications of cTn and cTn degradation is that these processes may alter the availability of specific epitopes, resulting in different cTn variant recoveries of various cTn assays. The antibodies in currently commercially available hs-cTn assays target epitopes within the stable central regions of the cTnI and cTnT molecules [37]. Consequently, most of these assays detect total cTnI or cTnT, including intact cTn (free or complex-bound), as well as a comprehensive but varying mixture of cTn degradation products [37].
4.2. Research cTn Assays That Detect Specific Forms of Circulating cTn
Recently, immunoassays have been developed to quantify intact or only moderately degraded cTnT (referred to as the “long-form”) [85,89,90], which do not detect heavily degraded cTnT, referred to as the “small-form”. In pilot studies, the ratio of “long-form” to total cTnT has shown potential for discriminating acute from chronic myocardial injury, as well as between different etiologies of myocardial injury [90,105,110,111,112,113,114]. Similar findings have not yet been reported for cTnI [106]. Nevertheless, a hs-cTnI/hs-cTnT ratio in blood samples could exhibit comparable discriminatory capabilities for distinguishing type 1 AMI from other causes of myocardial injury [115,116]. The promising results of these pilot studies of testing cTn composition, however, must be confirmed in large-scale clinical studies using fresh blood samples.
5. Are There Any Clinically Relevant Differences Between cTnI and cTnT?
When critically reviewing the published literature on this topic, it must be acknowledged, that cTnI and cTnT assays are neither standardized nor harmonized, meaning absolute values are difficult to compare. The only way to make meaningful comparisons is to transform absolute values into multiples of the URL of each assay. However, even then, there may still be a bias if one cTn assay has a markedly higher analytical sensitivity than the other. Therefore, URLs should be used that were calculated from the same reference population (such as the American Association of Clinical Chemistry and Laboratory Medicine reference population biobank) using the same statistical method for URL calculation. In addition, comparisons were primarily based on stored samples, so differences in the in vitro stabilities of cTns must be ruled out as a reason for the observed discrepancies. In many studies, samples were stored for months or even years before measurement. Therefore, further information is often required, such as immediate cTn measurements without sample storage, and studies using cardiac MRI imaging, which is the most sensitive imaging modality currently available for assessing myocardial injury, to confirm the reported differences in the clinical sensitivities and specificities of cTnI and cTnT in specific clinical scenarios. Examples include patients with chronic skeletal muscle myopathies or patients with severe chronic renal failure.
5.1. Time Courses of cTnT and cTnI Concentrations After an AMI
Although cTnT has a broader diagnostic window after AMI than cTnI [31,39,40,117], both are considered equally effective in terms of diagnostic performance during the acute phase of AMI [40,118,119]. No clinically relevant differences in their early diagnostic sensitivity for AMI have yet been published. Both cTns generally detect myocardial damage equally well [120].
Following an AMI with occluded coronary arteries, the cTn time courses are influenced by whether or not early tissue reperfusion of the infarcted myocardium has occurred [38,39,40]. Following early reperfusion of the infarct-related coronary artery, cTnT and cTnI peaks’ concentrations are typically found around 12 h after the onset of symptoms (see Supplemental Figure S4). Both troponins exhibit a biphasic release pattern, with a second peak value several days after AMI. However, this feature is much more pronounced in cTnT concentration time courses (see Supplemental Figure S4), with cTnT increases typically lasting a couple of days longer than cTnI increases [40]. These second cTnI peaks are much lower than the initial peaks (see Supplemental Figure S5) and may be overlooked if blood samples are taken infrequently (e.g., only daily) [40]. In AMI patients with ST-elevations (STEMI) who underwent successful primary PCI, they were even absent [117]. If early tissue reperfusion of the infarcted myocardium fails, cTnI peaks occur around 24 h after symptom onset, with the maximum of cTnT concentrations found several days after AMI (see Supplemental Figure S6) [38,39,40]. The cTn concentration time courses of AMI patients without ST-segment elevation (non-STEMI) resemble those in STEMI patients with successful primary PCI [121]. cTn peaks and release correlate with infarct size [33,122,123,124]. Early cTn concentration time courses do not differ significantly between STEMI and non-STEMI patients. Admission concentrations and the rate of increase within the first 1–2 h—although usually higher in STEMI—do not permit a reliable discrimination [120,121]. Similarly, they do not allow an accurate discrimination between AMI patients and patients with myocardial injury unrelated to myocardial ischemia [120].
Despite the lack of differences in early diagnostic sensitivity for AMI [40,118,119], it has been hypothesized that cTnI is released more quickly following myocardial damage [125]. However, as mentioned above, although absolute concentrations of hs-cTnI may be markedly higher, this conclusion is not possible, as the observed differences in absolute values in in vitro and animal experiments may simply be related to differences of cTn assay cross-reactivities with cTn of animals using cTn assays, given that these assays were designed to detect human cTn. The freeze–thawed human myocardial tissue damage model used in one study [125] is highly unphysiological. From a pathophysiological point of view, it is likely that cTnI and cTnT are released from the thin filaments equimolarly in equal amounts after myocardial damage. However, theoretically, cTnT could re-bind to a larger extent to insoluble structures of cardiomyocytes, which remains to be demonstrated.
5.2. cTn Discrepancies in Patients with Chronic Skeletal Muscle Diseases
One potential explanation for the commonly reported increased cTnT and normal cTnI concentrations in patients with chronically stressed skeletal muscle [126,127,128,129,130,131,132,133] is that, during fetal development, cTnT is expressed in cardiac and embryonic and neonatal skeletal muscles [16,134,135,136,137]. A representative patient with Becker’s muscular dystrophy is presented and discussed in Supplemental Figure S7. cTnT is down-regulated in skeletal muscle during development and gradually disappears after birth. Healthy adult human skeletal muscle does not contain cTnT. In contrast, cTnI has not yet been reported to be expressed outside the heart so far [10,15]. Chronic injury to human skeletal muscle, as experienced by patients with chronic skeletal muscle myopathies, recapitulates embryonic myogenesis with the re-expression of fetal proteins, which may include cTnT isoforms [134]. The re-expression of these proteins is likely to be dependent on the severity of the disease. These re-expressed proteins can be released into the circulation from damaged skeletal muscles, as occurs in patients with chronic myositis, myopathies, or storage diseases.
The presence of messenger ribonucleic acid (mRNA) coding for cTnT in biopsies from patients with chronic skeletal muscle diseases has been repeatedly reported in the literature [128,130,132,133,138,139,140,141]. Data on the re-expression of cTnT in humans at the protein level in humans are primarily derived from immunohistochemistry and Western blotting [128,133,138,139]. However, given the inherent problems of specificity of these technologies, these observations are not a definitive proof of re-expression. More recently, cTnT expression in skeletal muscle biopsy specimens from patients with Pompe disease was demonstrated by the detection of cTnT fragments using nanoflow LC-MS mass spectrometry, alongside the simultaneous detection of cTnT mRNA [130]. In contrast, cTnT was not detected in the skeletal muscle of healthy controls. Wens et al. [130] detected the cTnT isoform 6, which is the cTnT isoform expressed in healthy hearts, in the skeletal muscle of some patients with Pompe disease. This suggests that skeletal muscle could be a source of circulating cTnT in these patients if cardiac involvement is ruled out. However, Schmid et al. [131] found no evidence of cTnT re-expression in skeletal muscle biopsies from patients with myopathies and myositis using mass spectrometry. Nevertheless, significantly more patients had elevated peripheral blood cTnT concentrations compared to cTnI (see also Supplemental Figure S7). These conflicting results may be explained by differences in cTnT concentrations within skeletal muscle dependent on diseases, and/or differences in the analytical sensitivities of mass spectrometry-based analytical methods used in both studies. The study of Schmid et al. [131] did not test for cTnT mRNA expression in skeletal muscle specimens.
In summary, accumulating evidence suggests that cTnT is re-expressed in several chronic human skeletal muscle diseases. cTnI is the most cardiac-specific marker in these rare patient populations. Although it is easier to interpret when AMI is suspected in these patients, serial testing is still required to confirm acute myocardial injury by a significant change in cTnI concentrations. Furthermore, the frequency of cardiac involvement increases with the age of patients with skeletal muscle myopathies, which may lead to chronic increases in both cTnT and cTnI concentrations.
5.3. cTnI and cTnT in Patients with Chronic Renal Failure
As already discussed in above, discrepancies in cTnI and cTnT concentrations are common in patients with advanced renal failure [142,143,144]. This may be due, at least in part, to differences in the renal clearance of cTn fragments from the blood [79,82]. In hemodialysis patients, changes in cTns during hemodialysis depend on the membrane used and the dialysis modality [145,146]. Both influence the permeabilities and clearances of proteins including cTn fragments. In addition, cTn complex and cTn fragment adherence to the dialyzer membrane may differ and be membrane-dependent. The recovery of cTn fragments is assay-dependent as well. Furthermore, myocardial stress due to various causes (e.g., CAD, hypertension, left ventricular hypertrophy, and heart failure) in this patient population may lead to a stable, chronic increase in both cTnI and cTnT. However, cTnI increases are less frequent [142,143,144]. Ricchiuti et al. [140] reported cTnT mRNA expression and cTnT protein expression by Western blot analysis, with no cTnI expression in approximately 50% of skeletal muscle specimens taken from the abdominal wall, back muscles, and arm muscles of hemodialysis patients. It was not reported which muscle specimens tested positive. In contrast, Haller et al. [147] reported the absence of cTnT expression in the skeletal muscle biopsies taken from abdominal wall or back skeletal muscles of five patients with end-stage renal failure. However, truncal skeletal muscles are not usually affected by uremic skeletal myopathy. Despite cTnT increases being more frequently observed and irrespective of the mechanisms of increase, both hs-cTnT and hs-cTnI maintain their prognostic value in patients with chronic renal failure [142]. As with chronic skeletal muscle diseases, cTnI appears to be the most cardiac-specific biomarker for the detection of AMI in this patient population, although serial testing of cTnI is also required to document a significant change in cTnI concentrations.
6. Summary and Conclusions
This systematic literature review aims to assist with the routine interpretation of cTn and to provide insight into potential release mechanisms and future developments of cTn testing. It offers practical guidance on cTn interpretation and describes how to derive a correct diagnosis in the individual patient by including discussion of routine clinical cases. The key messages of this review can be summarized as follows:
1. Currently, cTnI and cTnT remain the most accurate laboratory parameters for the routine laboratory diagnosis of myocardial injury, and they will be difficult to replace.
2. An unexpectedly elevated cTn test result should not be ignored, as analytical interferences resulting in false-positive test results are very rare.
3. cTn degradation in response to myocardial injury starts within cardiomyocytes, and cTnI and cTnT are rapidly released after myocardial injury, at a comparable rate to cytosolic proteins.
3. Even very mild forms of myocardial injury, such as those resulting from brief periods of myocardial ischemia or intense myocardial workload, can be reliably detected using hs-cTn assays.
4. Currently, the only generally accepted mechanism of cTn release into the systemic circulation in humans is myocardial necrosis.
5. Other potential mechanisms demonstrated in cultured cardiomyocytes (reversible cell injury) or in animal models (apoptosis, another form of cell death) are very difficult, if not impossible, to prove in humans in the absence of histological tissue analysis.
6. In general, there are no clinically significant differences in the diagnostic performance of cTnI and cTnT. However, after AMI, cTnT tends to remain elevated for a longer period of time than cTnI.
7. In specific, albeit rare, patient populations with chronic skeletal muscle diseases or chronic renal failure, cTnI has a higher clinical specificity than cTnT for the detection of acute myocardial injury.
8. The extreme analytical sensitivity of hs-cTn assays comes at the cost of clinical specificity for AMI.
9. One promising approach to improving the specificity for Type 1 AMI is to measure specific cTn degradation forms (e.g., the so-called “long-form” of cTnT or calculating a cTnI/cTnT ratio). However, these initial results must be confirmed in larger, unselected patient populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm14124241/s1, Figure S1: Peri-interventional cTnT interpretation in a 60-year-old male with uncomplicated chronic total occlusion (CTO)-PCI; Figure S2: A patient with angiographically documented peri-interventional type 4a AMI; Figure S3: A symptomatic marathon cyclist; Figure S4: Time course of cTnT concentrations in a patient with successful primary PCI; Figure S5: Time courses of cardiac biomarkers in a 45-year old male with STEMI; Figure S6: Time course of cTnT concentrations in a patient with STEMI and no reflow after primary PCI; Figure S7: cTnI, cTnT, and creatine kinase in an asymptomatic young male with Becker’s dystrophy.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author reports research collaboration on cardiac biomarker point-of-care diagnostics with Siemens Healthineers, The Netherlands.
References
- Thygesen, K.; Mair, J.; Katus, H.; Plebani, M.; Venge, P.; Collinson, P.; Lindahl, B.; Giannitsis, E.; Hasin, Y.; Galvani, M.; et al. Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care; Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur. Heart J. 2010, 31, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Mair, J.; Giannitsis, E.; Mueller, C.; Lindahl, B.; Blankenberg, S.; Huber, K.; Plebani, M.; Biasucci, L.M.; Tubaro, M.; et al. The Study Group on Biomarkers in Cardiology of the ESC Working group on Acute Cardiac Care; How to use high-sensitivity cardiac troponins in acute cardiac care. Eur. Heart J. 2012, 33, 2252–2257. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.B.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar]
- Mair, J.; Cullen, L.; Giannitsis, E.; Hammarsten, O.; Huber, K.; Jaffe, A.; Mills, N.; Möckel, M.; Müller, C.; Thygesen, K.; et al. Application of the fourth universal definition of myocardial infarction in clinical practice. Biomarkers 2020, 25, 322–330. [Google Scholar] [CrossRef]
- Lindahl, B.; Baron, T.; Albertucci, M.; Prati, F. Myocardial infarction with non-obstructive coronary artery disease. Eurointervention 2021, 17, e875–e887. [Google Scholar] [CrossRef]
- Mair, J.; Lindahl, B.; Müller, C.; Giannitsis, E.; Huber, K.; Möckel, M.; PLebani, M.; Thygesen, K.; Jaffe, A.S. What to do when you question cardiac troponin values. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 577–586. [Google Scholar] [CrossRef]
- Mair, J.; Jaffe, A.; Lindahl, B.; Mills, N.; Möckel, M.; Cullen, L.; Giannitsis, E.; Hammarsten, O.; Huber, K.; Krychtiuk, K.; et al. The clinical approach to diagnosing peri-procedural myocardial infarction after percutaneous coronary interventions according to the fourth universal definition of myocardial infarction—From the study group on biomarkers of the European Society of Cardiology (ESC) Association for Acute CardioVascular Care (ACVC). Biomarkers 2022, 27, 407–417. [Google Scholar]
- Mair, J.; Giannitsis, E.; Mills, N.L.; Mueller, C.; Study Group on Biomarkers of the European Society of Cardiology Association for Acute CardioVascular Care. How to deal with unexpected cardiac troponin results. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Hammarsten, O. Potential analytical interferences in cardiac troponin immunoassays. J. Lab. Precis. Med. 2023, 8, 12. [Google Scholar] [CrossRef]
- Parmacek, M.S.; Solaro, R.J. Biology of troponin complex in cardiac myocytes. Prog. Cardiovasc. Dis. 2004, 47, 159–176. [Google Scholar] [CrossRef]
- Katrukha, I.A. Human cardiac troponin complex. Structure and functions. Biochemistry 2013, 73, 1447–1465. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Jin, J.P. Troponin T isoforms and posttranscriptional modifications: Evolution, regulation and function. Arch. Biochem. Biophys. 2011, 505, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yamashita, A.; Maeda, K.; Maeda, Y. Structure of the core domain of human cardiac troponin in the Ca2+ saturated form. Nature 2003, 424, 35–41. [Google Scholar] [CrossRef]
- Schreier, T.; Kedes, L.; Gahlmann, R. Cloning, structural analysis, and expression of the human slow twitch skeletal muscle/cardiac troponin C gene. J. Biol. Chem. 1990, 265, 21247–21253. [Google Scholar] [CrossRef]
- Vallins, W.J.; Brand, N.J.; Dabhade, N.; Butler-Browne, G.; Yacoub, M.H.; Barton, P.J. Molecular cloning of human cardiac troponin I using polymerase chain reaction. FEBS Lett. 1990, 270, 57–61. [Google Scholar] [CrossRef]
- Mesnard, L.; Samson, F.; Espinasse, I.; Durand, J.; Neveux, J.Y.; Mercadier, J.J. Molecular cloning and developmental expression of human cardiac troponin T. FEBS Lett. 1993, 328, 139–144. [Google Scholar] [CrossRef]
- Anderson, P.A.W.; Greig, A.; Mark, T.M.; Malouf, N.N.; Oakeley, A.E.; Ungerleider, R.M.; Allen, P.D.; Kay, B.K. Molecular basis of human cardiac troponin T isoforms expressed in developing, adult, anf failing heart. Circ. Res. 1995, 76, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.F. Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin I. J. Biol. Chem. 1981, 256, 964–968. [Google Scholar] [CrossRef]
- Remppis, A.; Scheffold, T.; Greten, J.; Haas, M.; Greten, T.; Kübler, W.; Katus, H.A. Intracellular compartmentation of troponin T: Release kinetics after global ischemia and calcium paradox in the isolated perfused rat heart. J. Mol. Cell. Cardiol. 1995, 27, 793–803. [Google Scholar] [CrossRef]
- Bleier, J.; Vorderwinkler, K.P.; Falkensammer, J.; Mair, P.; Dapunt, O.; Puschendorf, B.; Mair, J. Different intracellular compartmentations of cardiac troponins and myosin heavy chains: A causal connenction to their different early release after myocardial damage. Clin. Chem. 1998, 44, 1912–1918. [Google Scholar] [CrossRef]
- De Lisa, F.; De Tullio, R.; Salamino, F.; Barbato, R.; Melloni, E.; Siliprandi, N.; Schiaffino, S.; Pontremoli, S. Specific degradation of troponin T and I by µ-calpain and its modulation by substrate phosphorylation. Biochem. J. 1995, 308, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Communal, C.; Sumandea, M.; de Tombe, P.; Narula, J.; Solaro, R.J.; Hajjar, R.J. Functional consequences of caspase activation in cardiac myocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 6252–6256. [Google Scholar] [CrossRef]
- Martin-Garrido, A.; Biesiadecki, B.J.; Sakhi, H.E.; Shaifta, Y.; G Dos Remiedios, C.; Ayaz-Guner, S.; Wenxuan, C.; Ge, Y.; Avkiran, M. Monophosphorylation of cardiac troponin-I at Ser-23/24 is sufficient to regulate cardiac myofibrillar Ca2+ sensitivity and calpain-induced proteolysis. J. Biol. Chem. 2018, 293, 8588–8599. [Google Scholar] [CrossRef]
- Ke, L.; Qi, X.Y.; Dijkhuis, A.J.; Chartier, D.; Nattel, S.; Henning, R.H.; Kampinga, H.H.; Brundel, B.J.J.M. Calpain mediates cardiac troponin degradation and contractile dysfunction in atrial fibrillation. J. Mol. Cell. Cardiol. 2008, 45, 685–693. [Google Scholar] [CrossRef]
- Trump, B.F.; Berezesky, I.K. The role of cytosolic Ca2+ in cell injury, necrosis and apoptosis. Curr. Opin. Cell Biol. 1992, 2, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Piper, H.M.; Schwartz, P.; Spahr, R.; Hütter, J.F.; Spieckermann, G. Early enzyme release from myocardial cells is not due to irreversible cell damage. J. Mol. Cell. Cardiol. 1984, 16, 385–388. [Google Scholar] [CrossRef]
- Starnberg, K.; Jeppsson, A.; Lindahl, B.; Hammarsten, O. Revision of the troponin T release mechanism from damaged human myocardium. Clin. Chem. 2014, 60, 1098–1104. [Google Scholar] [CrossRef]
- Burkart, E.M.; Sumandea, M.P.; Kobayashi, T.; Nili, M.; Martin, A.F.; Homsher, E.; Solaro, R.J. Phosphorylation or Glutamic Acid Substitution at Protein Kinase C Sites on Cardiac Troponin I Differentially Depress Myofilament Tension and Shortening Velocity. J. Biol. Chem. 2003, 278, 11265–11272. [Google Scholar] [CrossRef] [PubMed]
- Sumandea, M.P.; Vahebi, S.; Sumandea, C.A.; Garcia-Cazarin, M.L.; Staidle, J.; Homsher, E. Impact of Cardiac Troponin T N-Terminal Deletion and Phosphorylation on Myofilament Function. Biochemistry 2009, 48, 7722–7731. [Google Scholar] [CrossRef]
- Mahmud, Z.; Zahran, S.; Liu, P.B.; Reiz, B.; Chan, B.Y.H.; Roczkowsky, A.; McCartney, C.-S.E.; Davies, P.L.; Li, L.; Schulz, R.; et al. Structure and proteolytic susceptibility of the inhibitory C-terminal tail of cardiac troponin I. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 661–671. [Google Scholar] [CrossRef]
- Asayama, J.; Yamahara, Y.; Ohta, B.; Miyazaki, H.; Tatsumi, T.; Matsumoto, T.; Inoue, D.; Nakagawa, M. Release kinetics of cardiac troponin T in coronary effluent from isolated rat hearts during hypoxia and reoxygenation. Basic Res. Cardiol. 1992, 87, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Bertinchant, J.P.; Polge, A.; Robert, E.; Sabbah, N.; Fabbo-Paray, P.; Poirey, S.; Laprade, M.; Pau, B.; Juan, J.M.; Bali, J.P.; et al. Time-course of cardiac troponin I release from isolated perfused rat hearts during hypoxia/reoxygenation and ischemia/reperfusion. Clin. Chim. Acta 1999, 283, 43–56. [Google Scholar] [CrossRef]
- Metzler, B.; Hammerer-Lercher, A.; Jehle, J.; Dietrich, H.; Pachinger, O.; Xu, Q.; Mair, J. Plasma cardiac troponin T closely correlates with infarct size in a mouse model of acute myocardial infarction. Clin. Chim. Acta. 2002, 325, 87–90. [Google Scholar] [CrossRef]
- Mair, J.; Morandell, D.; Genser, N.; Lechleitner, P.; Dienstl, F.; Puschendorf, B. Equivalent early sensitivitties of myoglobin, creatine kinase MB mass, creatine kinase isoform ratios and cardiac troponin I and T after acute myocardial infarction. Clin. Chem. 1995, 41, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.N.; Cheng, C.C.; Lee, Y.F.; Chen, J.-S.; Ho, S.-P.; Chiu, Y.-T. Early activation of myocardial matrix metalloproteinases and degradation of cardiac troponin I after experimental subarachnoid haemorrhage. J. Surg. Res. 2013, 179, e41–e48. [Google Scholar] [CrossRef] [PubMed]
- Katrukha, I.A.; Kogan, A.E.; Vylegzhanina, A.V.; Serebryakova, M.V.; Koshkina, E.V.; Bereznikova, A.V.; Katrukha, A.G. Thrombin-mediated degradation of human cardiac troponin T. Clin. Chem. 2017, 63, 1094–1100. [Google Scholar] [CrossRef]
- Committee on Clinical Applications of Cardiac Biomarkers (C-CB). High-Sensitivity Cardiac Troponin I and T Assay Analytical Characteristics Designated by Manufacturer v062024. Available online: https://ifccfiles.com/2024/03/High-Sensitivity-Cardiac-Troponin-I-and-T-Assay-Analytical-Characteristics-Designated-By-Manufacturer-v062024.pdf (accessed on 25 April 2025).
- Zabel, M.; Hohnloser, S.H.; Köster, W.; Prinz, M.; Kasper, W.; Just, H. Analysis of creatine kinase; CK-MB, myoglobin, and troponin T time-activity curves for early assessment of coronary artery reperfusion after intravenous thrombolysis. Circulation 1993, 87, 1542–1550. [Google Scholar] [CrossRef]
- Katus, H.A.; Remppis, A.; Scheffold, T.; Diederich, K.W.; Kuebler, W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am. J. Cardiol. 1991, 67, 1360–1367. [Google Scholar] [CrossRef]
- Mair, J.; Thome-Kromer, B.; Wagner, I.; Lechleitner, P.; Dienstl, F.; Puschendorf, B.; Michel, G. Concentration time courses of troponin and myosin subunits after acute myocardial infarction. Coron. Artery Dis. 1994, 5, 865–872. [Google Scholar]
- Cooper, S.T.; McNeil, P.L. Membrane Repair: Mechanisms and Pathophysiology. Physiol. Rev. 2015, 95, 1205–1440. [Google Scholar] [CrossRef]
- Hessel, M.H.; Michielsen, E.C.; Atsma, D.E.; Schalij, M.J.; van der Valk, E.J.; Bax, W.H.; Hermens, W.T.; van Dieijen-Visser, M.; van der Laarse, A. Release kinetics of intact and degraded troponin I and T after irreversible cell damage. Exp. Mol. Pathol. 2008, 85, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Piper, H.M.; Schwartz, P.; Hutter, J.F.; Spieckermann, P.G. Energy metabolism and enzyme release of cultured adult rat heart muscle cells during anoxia. J. Mol. Cell. Cardiol. 1984, 16, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Saffitz, J.E.; Mealman, T.L.; Grace, A.M.; Roberts, R. Reversible myocardial ischemic injury is not associated with increased creatine kinase activity in plasma. Clin. Chem. 1997, 43, 467–475. [Google Scholar] [CrossRef]
- Hammersten, O.; Mair, J.; Möckel, M.; Lindahl, B.; Jaffe, A.S. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018, 23, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Streng, A.S.; Jacobs, L.H.; Schwenk, R.W.; Cardinaels, E.P.; Meex, S.J.; Glatz, J.F.; Wodzig, W.K.W.H.; van Dieijen-Visser, M.P. Cardiac troponin in ischemic cardiomyocytes: Intracellular decrease before onset of cell death. Exp. Mol. Pathol. 2014, 96, 339–345. [Google Scholar] [CrossRef]
- Weil, B.R.; Young, R.F.; Shen, X.; Suzuki, G.; Qu, J.; Malhotra, S.; Canty, J.M., Jr. Brief Myocardial Ischemia Produces Cardiac Troponin I Release and Focal Myocyte Apoptosis in the Absence of Pathological Infarction in Swine. J. Am. Coll. Cardiol. Basic Transl. Sci. 2017, 2, 105–114. [Google Scholar] [CrossRef]
- Weil, B.R.; Suzuki, G.; Young, R.F.; Iyer, V.; Canty, J.M., Jr. Troponin Release and Reversible Left Ventricular Dysfunction After Transient Pressure Overload. J. Am. Coll. Cardiol. 2018, 71, 2906–2916. [Google Scholar] [CrossRef]
- Cummins, B.; Auckland, M.L.; Cummins, P. Cardiac-specific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction. Am. Heart J. 1987, 113, 1333–1344. [Google Scholar] [CrossRef]
- Marjot, J.; Kaier, T.E.; Martin, E.D.; Reji, S.S.; Copeland, O.; Iqbal, M.; Goodson, B.; Harding, S.E.; Marber, S. Quantifying the Release of Biomarkers of Myocardial Necrosis from Cardiac Myocytes and Intact Myocardium. Clin. Chem. 2017, 63, 990–996. [Google Scholar] [CrossRef]
- Nassenstein, K.; Breuckmann, F.; Bucher, C.; Kaiser, K.; Konzora, T.; Schäfer, L.; Konietzka, I.; de Greiff, A.; Heusch, G.; Erbel, R.; et al. How much myocardial damage is necessary to enable detection of focal gadolinium enhancement at cardiac MR imaging? Radiology 2008, 249, 829–835. [Google Scholar] [CrossRef]
- Salatzki, J.; Giannitsis, E.; Hegenbarth, A.; Mueller-Hennessen, M.; André, F.; Frey, N.; Biener, M. Absence of visible infarction on cardiac magnetic resonance imaging despite the established diagnosis of myocardial infarction by 4th Universal Definition of Myocardial Infarction. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Giannitsis, E.; Kurz, K.; Hallermayer, K.; Jarausch, J.; Jaffe, A.S.; Katus, H.A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin. Chem. 2010, 56, 254–261. [Google Scholar] [CrossRef]
- Krintus, M.; Kozinski, M.; Boudry, P.; Capell, N.E.; Köller, U.; Lackner, K.; Lefevre, G.; Lennartz, L.; Lotz, J.; Herranz, A.M.; et al. European multicenter analytical evaluation of the Abbott Architect STAT high sensitivity troponin I assay. Clin. Chem. Lab. Med. 2014, 52, 1657–1665. [Google Scholar] [PubMed]
- Portbury, A.L.; Willis, M.S.; Patterson, C. Tearin’ Up My Heart: Proteolysis in the Cardiac Sarcomere. J. Biol. Chem. 2011, 286, 9929–9934. [Google Scholar] [CrossRef] [PubMed]
- Canty J., J. M. Myocardial injury, troponin release, and cardiomyocyte death in brief ischemia, failure, and ventricular remodeling. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H1–H15. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Morrow, D.A.; de Lermos, J.A.; Jarolim, P.; Braunwald, E. Detection of acute changes in circulating troponin in the setting of transient stress test-induced myocardial ischemia using an ultrasensitive assay: Results from TIMI 35. Eur. Heart J. 2009, 30, 162–169. [Google Scholar] [CrossRef]
- Siriwardena, M.; Campbell, V.; Richards, A.M.; Pemberton, C.J. Cardiac Biomarker Responses to Dobutamine Stress Echocardiography in Healthy Volunteers and Patients with Coronary Artery Disease. Clin. Chem. 2012, 58, 1492–1494. [Google Scholar] [CrossRef]
- Jannssen, S.L.J.E.; de Fries, F.; Mingels, A.M.A.; Kleinnibbelink, G.; Hopman, M.T.E.; Mosterd, A.; Velthuis, B.K.; Aengevaeren, V.L.; Eijsvogels, T.M.H. Exercise-induced cardiac troponin release in athletes with versus without coronary atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2024, 326, H1045–H1052. [Google Scholar] [CrossRef]
- Turer, A.T.; Addo, T.A.; Martin, J.L.; Sabatine, M.S.; Lewis, G.D.; Gerszten, R.E.; Keeley, E.C.; Cigarroa, J.E.; Lange, R.A.; Hills, D.; et al. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: Insights from a coronary sinus sampling study. J. Am. Coll. Cardiol. 2011, 57, 2398–2405. [Google Scholar] [CrossRef]
- Arnadottir, A.; Pedersen, S.; Bo Hasselbalch, R.; Goetze, J.P.; Friis-Hansen, L.J.; Bloch-Munster, A.M.; Jensen, J.S.; Bundgaard, H.; Iversen, K. Temporal Release of High-Sensitivity Cardiac Troponin T and I and Copeptin After Brief Induced Coronary Artery Balloon Occlusion in Humans. Circulation 2021, 143, 1095–1104. [Google Scholar] [CrossRef]
- Oemrawsingh, R.M.; Cheng, J.M.; Garcia-Garcia, H.M.; van Schaik, R.H.N.; Regar, E.; van Geuns, R.-J.; Serruys, P.W.; Boersma, E.; Akkerhuis, K.M. High-sensitivity troponin T in relation to coronary plaque characteristics in patients with stable coronary artery disease; results of the ATHEROREMO-IVUS study. Atherosclerosis 2016, 247, 135–141. [Google Scholar] [CrossRef]
- Silvain, J.; Zeitouni, M.; Paradies, V.; Zheng, H.L.; Ndrepepa, G.; Cavallini, C.; Feldman, D.N.; Sharma, S.K.; Mehilli, J.; Gili, S.; et al. Cardiac procedural myocardial injury, infarction, and mortality in patients undergoing elective percutaneous coronary intervention: A pooled analysis of patient-level data. Eur. Heart J. 2021, 42, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zeitouni, M.; Silvain, J.; Guedeney, P.; Kerneis, M.; Yan, Y.; Overtchouk, P.; Barthelemy, O.; Hauguel-Moreau, M.; Choussat, R.; Helft, G.; et al. ACTION Study Group; Periprocedural myocardial infarction and injury in elective coronary stenting. Eur. Heart J. 2018, 39, 1100–1109. [Google Scholar] [CrossRef]
- Moussa, I.D.; Klein, L.W.; Shah, B.; Mehran, R.; Mack, M.J.; Brilakis, E.S.; Reilly, J.P.; Zoghbi, G.; Holper, E.; Stone, G.W. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: An expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J. Am. Coll. Cardiol. 2013, 62, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.-A.; van Es, G.-A.; Zuckerman, B.; et al. Academic Research ConsortiumStandardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Eur. Heart J. 2018, 39, 2192–2207. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; George, K.; Duan, F.; Tong, T.K.; Tian, Y. Histological evidence for reversible cardiomyocyte changes and serum cardiac troponin T elevations after exercise in rats. Physiol. Rep. 2016, 4, e13083. [Google Scholar] [CrossRef]
- Middleton, N.; George, K.; Whyte, G.; Gaze, D.; Collinson, P.; Shave, R. Cardiac troponin T release is stimulated by endurance exercise in healthy humans. J. Am. Coll. Cardiol. 2008, 52, 1813–1814. [Google Scholar] [CrossRef]
- Aengevaeren, V.L.; Baggish, A.L.; Chung, E.H.; George, K.; KLeiven, O.; Mingels, A.M.A.; Orn, S.; Shave, R.E.; Thompson, P.D.; Eijsvogels, T.M.H. Exercise-induced cardiac troponin elevation: From underlying mechanisms to clinical relevance. Circulation 2021, 144, 1955–1972. [Google Scholar] [CrossRef]
- Marshall, L.; Lee, K.K.; Stewart, S.D.; Wild, A.; Fujisawa, T.; Ferry, A.V.; Stables, C.L.; Kithgow, H.; Chapman, A.R.; Anand, A.; et al. Effects of exercise intensity and duration on cardiac troponin release. Circulation 2020, 141, 83–85. [Google Scholar] [CrossRef]
- Scharhag, J.; Urhausen, A.; Schneider, G.; Hermann, M.; Schuhmacher, K.; Haschke, M.; Krieg, A.; Meyer, T.; Herrmann, W.; Kindermann, W. Reproducibility and clinical significance of exercise-induced increases in cardiac troponins and N-terminal N-termional pro brain natriuretic peptide in endurance athletes. Eur. J. Cardiovasc. Prev. Rehabil. 2006, 13, 388–397. [Google Scholar] [CrossRef]
- Mehta, R.; Gaze, D.; Mohan, S.; Williams, K.L.; Sprung, V.; George, K.; Jeffries, R.; Hudson, Z.; Perry, M.; Shave, R. Post-exercise cardiac troponin release is related to exercise training history. Int. J. Sports Med. 2012, 33, 333–337. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Burns, A.T.; Mooney, D.J.; Inder, W.J.; Taylor, A.J.; Bogaert, J.; Macisaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur. Heart J. 2012, 33, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Shave, R.; Oxborough, D. Exercise-induced cardiac injury: Evidence from novel imaging techniques and highly sensitive cardiac troponin assays. Prog. Cardiovasc. Dis. 2012, 54, 407–415. [Google Scholar] [CrossRef]
- Möhlenkamp, S.; Leineweber, K.; Lehman, N.; Braun, S.; Roggenbuck, U.; Perrey, M.; Broecker-Preuss, M.; Budde, T.; Halle, M. Coronary atherosclerosis burden, but not transient troponin elevation, predicts long-term outcome in recreational marathon runners. Basic Res. Cardiol. 2014, 109, 391. [Google Scholar] [CrossRef] [PubMed]
- Aengevaeren, V.L.; Hopman, M.T.E.; Thompson, P.D.; Bakker, E.A.; George, K.P.; Thijssen, D.H.J.; Eijsvogels, T.M.H. Exercise-induced cardiac troponin I increase and incident mortality and cardiovascular events. Circulation 2019, 140, 804–814. [Google Scholar] [CrossRef]
- Skadberg, O.; Kleiven, O.; Bjorkavoll-Bergeth, M.; Melberg, T.; Bergseth, R.; Selvag, J.; Auestad, B.; Greve, O.J.; Dickstein, K.; Aaarsland, T.; et al. Highly increased troponin I levels following high-intensity endurance cycling may detect subclinical coronary artery disease in presumably healthy leisure sport cyclists: The North Sea Race Endurance Exercise Study (NEEDED) 2013. Eur. J. Prev. Cardiol. 2017, 24, 885–894. [Google Scholar] [CrossRef]
- van de Schoor, F.R.; Aengevaeren, V.L.; Hopman, M.T.; Oxborough, D.L.; George, K.P.; Thompson, P.D.; Eijsvogels, T.M.H. Myocardial fibrosis in athletes. Mayo Clin. Proc. 2016, 91, 1617–1631. [Google Scholar] [CrossRef]
- Muslimovic, A.; Friden, V.; Tenstad, O.; Starnberg, K.; Nystrom, S.; Wesen, E.; Esnjörner, E.K.; Granholm, K.; Lindahl, B.; Hammarsten, O. The liver and kidneys mediate clearance of cardiac troponin in the rat. Sci. Rep. 2020, 10, 6791. [Google Scholar] [CrossRef]
- Streng, A.S.; de Boer, D.; van Doorn, W.P.; Kocken, J.M.; Bekers, O.; Wodzig, W.K. Cardiac troponin T degradation in serum is catalysed by human thrombin. Biochem. Biophys. Res. Commun. 2016, 481, 165–168. [Google Scholar] [CrossRef]
- Klocke, F.J.; Copley, D.P.; Krawczyk, J.A.; Reichlin, M. Rapid renal clearance of immunoreactive canine plasma myoglobin. Circulation 1982, 65, 1522–1528. [Google Scholar] [CrossRef]
- Friden, V.; Starnberg, K.; Muslimovic, A.; Ricksten, S.-E.; Bjurman, C.; Forsgard, N.; Wickman, A.; Hammarsten, O. Clearance of cardiac troponin T with and without kidney function. Clin. Biochem. 2017, 50, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.E.; Coluccio, G.; Hirkaler, I.; Mikaelian, R.; Nicklaus, R.; Lipschutz, S.E.; Doessegger, L.; Reddy, M.; Singer, T.; Geng, W.; et al. The complete pharmacokinetic profile of serum cardiac troponin I in the rat and dog. Toxicol. Sci. 2011, 123, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.H.; Hasselbalch, R.B.; Strandkjaer, N.; Jorgensen, N.; Ostergaard, M.; Moller-Sorensen, P.H.; Nilsson, J.C.; Afzal, S.; Kamstrup, P.R.; Dahl, M.; et al. Half-Life and Clearance of Cardiac Troponin I and Troponin T in Humans. Circulation 2024, 150, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Salonen, S.M.; Tuominen, T.J.K.; Raiko, K.I.S.; Vasankari, T.; Aalto, R.; Hellman, T.A.; Lahtinen, S.E.; Soukka, T.; Airaksinen, K.E.J.; Wittfooth, S.T. Highly sensitive immunoassay for long form of cardiac troponin T using upconversion luminescence. Clin. Chem. 2024, 70, 1037–1045. [Google Scholar]
- Hammerer-Lercher, A.; Halfinger, B.; Sarg, B.; Mair, J.; Puschendorf, B.; Griesmacher, A.; Guzman, N.A.; Lindner, H.H. Analysis of circulating forms of proBNP and NT-proBNP in patients with severe heart failure. Clin. Chem. 2008, 54, 858–865. [Google Scholar] [CrossRef]
- Amplatz, B.; Sarg, B.; Faserl, K.; Hammerer-Lercher, A.; Mair, J.; Lindner, H.H. Exposing the High Heterogeneity of Circulating Pro B-Type Natriuretic Peptide Fragments in Healthy Individuals and Heart Failure Patients. Clin. Chem. 2020, 66, 1200–1209. [Google Scholar] [CrossRef]
- Peronnet, E.; Becquart, L.; Poirier, F.; Cubizolles, M.; Choquet-Kastylevsky, G.; Jolivet-Reynaud, C. SELDI-TOF MS analysis of the cardiac troponin I forms present in plasma from patients with myocardial infarction. Proteomics 2006, 6, 6288–6299. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Katrukha, I.A.; Zhang, L.; Shu, X.; Xu, A.; Yang, J.; Wu, Y.; Jing, Y.; Ni, T.; et al. Design and analytical evaluation of novel cardiac troponin assays targeting multiple forms of the cardiac troponin I–cardiac troponin T–troponin C complex and fragmentation forms. Clin. Chem. 2025, 71, 387–395. [Google Scholar] [CrossRef]
- Airaksinen, K.E.J.; Aalto, R.; Hellman, T.; Vasankari, T.; Lahtinen, A.; Wittfooth, S. Novel Troponin Fragmentation Assay to Discriminate Between Troponin Elevations in Acute Myocardial Infarction and End-Stage Renal Disease. Circulation 2022, 146, 1408–1410. [Google Scholar] [CrossRef]
- Riabkova, N.S.; Kogan, A.E.; Katrukha, I.A.; Vylegzhanina, A.V.; Bogomolova, A.P.; Alieva, A.K.; Pevzner, D.V.; Bereznikova, A.V.; Katrukha, A.G. Influence of Anticoagulants on the Dissociation of Cardiac Troponin Complex in Blood Samples. Int. J. Mol. Sci. 2024, 25, 8919. [Google Scholar] [CrossRef]
- Katrukha, A.G.; Bereznikova, A.V.; Esakova, T.V.; Petterson, K.; Lövgren, T.; Severina, M.E.; Pulkki, K.; Vuopio-Pulkki, L.M.; Gusev, N.B. Troponin I is released in blood stream of patients with acute myocardial infarction not in free form but as complex. Clin. Chem. 1997, 43, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.B.; Feng, Y.J.; Moore, R.; Apple, F.S.; McPherson, P.H.; Buechler, K.F.; Bodor, G. Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. Clin. Chem. 1998, 44, 1198–1208. [Google Scholar] [CrossRef]
- McDonough, J.L.; Arrell, D.K.; Van Eyk, J.E. Troponin I degradation and covalent complex formation accompanies myocardial ischemia/reperfusion injury. Circ. Res. 1999, 84, 9–20. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.L.; Labugger, R.; Pickett, W.; Tse, M.Y.; MacKenzie, S.; Pang, S.C.; Atar, G.; Ropchan, G.; van Eyk, J.E. Cardiac troponin I is modified in the myocardium of bypass patients. Circulation 2001, 103, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, I.; Bertinchant, J.P.; Granier, C.; Laprade, M.; Chocron, S.; Toubin, G.; Etievent, J.P.; Larue, C.; Trinquier, S. Determination of cardiac troponin I forms in the blood of patients with acute myocardial infarction and patients receiving crystalloid or cold blood cardioplegia. Clin. Chem. 1999, 45, 213–222. [Google Scholar] [CrossRef]
- Labugger, R.; Organ, L.; Collier, C.; Atar, D.; van Eyk, J.E. Extensive troponin I and troponin T modification detected in serum from patients with acute myocardial infarction. Circulation 2000, 102, 1221–1226. [Google Scholar] [CrossRef]
- Giuliani, I.; Bertinchant, J.P.; Lopez, M.; Coquelin, H.; Granier, C.; Laprade, M.; Pau, B.; Larue, C. Determination of cardiac troponin I forms in the blood of patients with unstable angina pectoris. Clin. Biochem. 2002, 35, 111–117. [Google Scholar] [CrossRef]
- Fahie-Wilson, M.N.; Carmichael, D.J.; Delaney, M.P.; Stevens, P.E.; Hall, E.M.; Lamb, E.J. Cardiac troponin T circulates in the free, intact form in patients with kidney failure. Clin. Chem. 2006, 52, 414–420. [Google Scholar] [CrossRef]
- Madsen, L.H.; Christensen, G.; Lund, T.; Serebruany, V.L.; Granger, C.B.; Hoen, I.; Grieg, Z.; Alexander, J.H.; Jaffe, A.S.; van Eyk, J.E.; et al. Time course of degradation of cardiac troponin I in patients with acute ST-elevation myocardial infarction: The ASSENT-2 troponin substudy. Circ. Res. 2006, 99, 1141–1147. [Google Scholar] [CrossRef]
- Michielsen, E.C.; Diris, J.H.; Kleijnen, V.W.; Wodzig, W.K.; Van Dieijen-Visser, M.P. Investigation of release and degradation of cardiac troponin T in patients with acute myocardial infarction. Clin. Biochem. 2007, 40, 851–855. [Google Scholar] [CrossRef]
- Bates, K.J.; Hall, E.M.; Fahie-Wilson, M.N.; Kindler, H.; Bailey, C.; Lythall, D.; Lamb, E.J. Circulating immunoreactive cardiac troponin forms determined by gel filtration chromatography after acute myocardial infarction. Clin. Chem. 2010, 56, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Cardinaels, E.P.; Mingels, A.M.; van Rooij, T.; Collinson, P.O.; Prinzen, F.W.; van Dieijen-Visser, M.P. Time-dependent degradation pattern of cardiac troponin T following myocardial infarction. Clin. Chem. 2013, 59, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Streng, A.S.; de Boer, D.; van Doorn, W.P.T.M.; Bouwman, F.G.; Mariman, E.C.M.; Bekers, O.; van Dieijen-Visser, M.P.; Wodzig, W.K.W.H. Identification and characterization of cardiac troponin T fragments in serum of patients suffering from acute myocardial infarction. Clin. Chem. 2017, 63, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Mingels, A.M.; Cardinaels, E.P.; Broers, N.J.; van Sleeuwen, A.; Streng, A.S.; van Dieijen-Visser, M.P.; Kooman, J.P.; Bekers, O. Cardiac Troponin T: Smaller Molecules in Patients with End-Stage Renal Disease than after Onset of Acute Myocardial Infarction. Clin. Chem. 2017, 63, 683–690. [Google Scholar] [CrossRef]
- van Wijk, X.M.R.; Claassen, S.; Enea, N.S.; Li, P.; Yang, S.; Brouwer, M.A.; Cramer, G.E.; Zuk, R.; Lynch, K.L.; Wu, A.H.B. Cardiac troponin I is present in plasma of type 1 myocardial infarction patients and patients with troponin I elevations due to other etiologies as complex with little free I. Clin. Biochem. 2019, 73, 3543. [Google Scholar] [CrossRef]
- Vylegzhanina, A.V.; Kogan, A.E.; Katrukha, I.A.; Koshkina, E.V.; Bereznikova, A.V.; Filatov, V.L.; Bloshchitsyna, M.N.; Bogomolova, A.P.; Katrukha, A.G. Full-size and partially truncated cardiac troponin complexes in the blood of patients with acute myocardial infarction. Clin. Chem. 2019, 65, 882–892. [Google Scholar] [CrossRef]
- Damen, S.A.J.; Cramer, G.E.; Dieker, H.-J.; Gehlmann, H.; Ophuis, T.J.M.O.; Aengevaeren, W.R.M.; Fokkert, M.; Verheugt, F.W.A.; Suryapranata, H.; Wu, A.H.B.; et al. Cardiac troponin composition characterization after non ST-elevation myocardial infarction: Relation with culprit artery, ischemic time window, and severity of injury. Clin. Chem. 2021, 67, 227–236. [Google Scholar] [CrossRef]
- Katrukha, I.A.; Riabkova, N.S.; Kogan, A.E.; Vylegzhanina, A.V.; Mukharyamova, K.S.; Bogomolova, A.P.; Zabolotskii, A.I.; Koshinka, E.V.; Bereznikova, A.V.; Katrukha, A.G. Fragmentation of human cardiac troponin T after acute myocardial infarction. Clin. Chim. Acta 2023, 542, 117281. [Google Scholar] [CrossRef]
- Vroemen, W.H.M.; Mezger, S.T.P.; Masotti, S.; Clerico, A.; Bekers, O.; de Boer, D.; Mingels, A. Cardiac troponin T: Only small molecules in recreational runners after marathon completion. J. Appl. Lab. Med. 2019, 3, 909–911. [Google Scholar] [CrossRef]
- Airaksinen, K.E.J.; Paana, T.; Vasankari, T.; Salonen, S.; Tuominen, T.; Linko-Parvinen, A.; Pallari, H.-M.; Hellmann, T.; Teppo, K.; Heinonen, O.J.; et al. Composition of cardiac troponin release differs after marathon running and myocardial infarction. Open Heart 2024, 11, e002954. [Google Scholar] [CrossRef]
- Airaksinen, K.E.J.; Tuominen, T.; Paana, T.; Hellman, T.; Vasankari, T.; Salonen, S.; Junes, H.; Linko-Parvinen, A.; Pallari, H.M.; Strandberg, M.; et al. Novel troponin fragmentation assay to discriminate between Takotsubo syndrome and acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2024, 13, 782–788. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Katrukha, I.A.; Zhang, L.; Shu, X.; Xu, A.; Yang, J.; Wu, Y.; Jing, Y.; Wang, H.; et al. Characterization of cardiac troponin fragment composition reveals potential for differentiating etiologies of myocardial injury. Clin. Chem. 2025, 71, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Damen, S.A.J.; Vroemen, W.H.M.; Brouwer, M.A.; Mezger, S.T.P.; Suryapranata, H.; van Royen, N.; Bekers, O.; Meex, S.J.R.; Wodzig, W.K.W.H.; Verheugt, F.W.A.; et al. Multi-Site Coronary Vein Sampling Study on Cardiac Troponin T Degradation in Non-ST segment-Elevation Myocardial Infarction: Toward a More Specific Cardiac Troponin T Assay. J. Am. Heart Assoc. 2019, 8, e012602. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Hammarsten, O.; Lindahl, B. Differences between high-sensitivity cardiac troponin T and I in stable populations: Underlying causes and clinical implications. Clin. Chem. Lab. Med. 2023, 61, 380–387. [Google Scholar] [CrossRef]
- Eggers, K.M.; Hammarsten, O.; Aldous, S.J.; Cullen, L.; Greenslade, J.H.; Lindahl, B.; Parsonage, W.A.; Pemberton, C.J.; Pickering, J.W.; Richards, A.M.; et al. Diagnostic and prognostic performance of the ratio between high-sensitivity cardiac troponin I and troponin T in patients with chest pain. PLoS ONE 2022, 17, e0276645. [Google Scholar] [CrossRef] [PubMed]
- Laugaudin, G.; Kuster, N.; Petiton, A.; Leclercq, F.; Gervasoni, R.; Macia, J.-C.; Cung, T.-T.; Dupuy, A.-M.; Solecki, K.; Lattuca, B.; et al. Kinetics of high-sensitivity cardiac troponin T and I differ in patients with ST-segment elevation myocardial infarction treated by primary coronary intervention. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 354–363. [Google Scholar] [CrossRef]
- Cullen, L.; Aldous, S.; Than, M.; Greenslade, J.H.; Tate, J.R.; George, P.M.; Hammett, C.J.; Richards, A.M.; Ungerer, J.P.J.; Troughton, R.W.; et al. Comparison of high sensitivity troponin T and I assays in the diagnosis of non-ST elevation acute myocardial infarction in emergency patients with chest pain. Clin. Biochem. 2014, 47, 321–326. [Google Scholar] [CrossRef]
- Rubini Gimenez, M.; Twerenbold, R.; Reichlin, T.; Wildi, K.; Haaf, P.; Schaefer, M.; Zellweger, C.; Moehring, B.; Stallone, F.; Sou, S.M.; et al. Direct comparison of high-sensitivity-cardiac troponin I vs. T for the early diagnosis of acute myocardial infarction. Eur. Heart J. 2014, 35, 2303–2311. [Google Scholar] [CrossRef]
- Wereski, R.; Kimenai, D.M.; Taggart, C.; Doudesis, D.; Lee, K.K.; Lowry, M.T.H.; Bularga, A.; Lowe, D.J.; Fujisawa, T.; Apple, F.S.; et al. Cardiac Troponin Thresholds and Kinetics to Differentiate Myocardial Injury and Myocardial Infarction. Circulation 2021, 144, 528–538. [Google Scholar] [CrossRef]
- van Doorn, W.; Vroemen, W.H.M.; Smulders, M.W.; van Suijlen, J.D.; van Cauteren, Y.J.M.; Bekkers, S.; Bekers, O.; Meex, S.J.R. High-Sensitivity Cardiac Troponin I and T Kinetics after Non-ST-Segment Elevation Myocardial Infarction. J. Appl. Lab. Med. 2020, 5, 239–241. [Google Scholar] [CrossRef]
- Reinstadler, S.J.; Feistritzer, H.J.; Klug, G.; Mair, J.; Minh-Duc Tu, A.; Kofler, M.; Henninger, B.; Franz, W.-M.; Metzler, B. High-sensitivity troponin T for prediction of left ventricular function and infarct size one year following ST-elevation myocardial infarction. Int. J. Cardiol. 2016, 202, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Wagner, I.; Morass, B.; Fridrich, L.; Lechleitner, P.; Dienstl, F.; Calcolari, C.; Larue, C.; Puschendorf, B. Cardiac troponin I release correlates with myocardial infarction size. Eur. J. Clin. Chem. Clin. Biochem. 1995, 33, 869–872. [Google Scholar]
- Wagner, I.; Mair, J.; Fridrich, L.; Artner-Dworzak, E.; Lechleitner, P.; Morass, B.; Dienstl, F.; Puschendorf, B. Cardiac troponin T release in acute myocardial infarction is associated with scintigraphic estimates of myocardial scar. Coron. Artery Dis. 1993, 4, 537–544. [Google Scholar] [CrossRef]
- Starnberg, K.; Friden, V.; Muslimovic, A.; Ricksten, S.-V.; Nystrom, S.; Forsgard, N.; Lindahl, B.; Vukusic, K.; Sandstedt, J.; Dellgren, G.; et al. A possible mechanism behind faster clearance and higher peak concentrations of cardiac troponin I compared with troponin T in acute myocardial infarction. Clin. Chem. 2020, 66, 333–341. [Google Scholar] [CrossRef]
- Hammerer-Lercher, A.; Erlacher, P.; Bittner, R.; Korinthenberg, R.; Skladal, D.; Sorichter, S.; Sperl, W.; Puschendorf, B.; Mair, J. Clinical and experimental results on cardiac troponin expression in Duchenne muscular dystrophy. Clin. Chem. 2001, 47, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Erlacher, P.; Lercher, A.; Falkensammer, J.; Nassanov, M.I.; Shtutman, V.Z.; Puschendorf, B.; Mair, J. Cardiac troponin and beta-type myosin heavy chain concentrations in patients with polymyositis or dermatomyositis. Clin. Chim. Acta 2001, 306, 27–33. [Google Scholar] [CrossRef]
- Jaffe, A.S.; Vasile, V.C.; Milone, M.; Saenger, A.K.; Olson, K.N.; Apple, F.S. Diseased skeletal muscle. A noncardiac source of increased circulating concentrations of cardiac troponin T. J. Am. Coll. Cardiol. 2011, 58, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Rittoo, D.; Jones, A.; Lecky, B.; Neithercut, D. Elevation of cardiac troponin T but not cardiac troponin I in patients with neuromuscular diseases: Implications for the diagnosis of myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 2411–2420. [Google Scholar] [CrossRef]
- Wens, S.C.A.; Schaaf, G.J.; Michels, M.; Kruijshaar, M.E.; van Gestel, T.J.M.; In‘t Groen, S.; Pijnenburg, J.; Dekkers, D.H.; Demmers, J.A.; Verdijk, L.B.; et al. Elevated plasma cardiac troponin T levels caused by skeletal muscle damage in Pompe disease. Circ. Cardiovasc. Genet. 2016, 9, 6–13. [Google Scholar] [CrossRef]
- Schmid, J.; Liesinger, L.; Birner-Gruenberger, R.; Stojakovic, T.; Scharnagl, H.; Dieplinger, B.; Asslaber, M.; Radl, R.; Beer, M.; Polacin, M.; et al. Elevated cardiac troponin T in patients with skeletal myopathies. J. Am. Coll. Cardiol. 2018, 71, 1540–1549. [Google Scholar] [CrossRef]
- Du Fay de Lavallaz, J.; Prepoudis, A.; Wendebourg, M.J.; Kesenheimer, E.; Kyburz, D.; Daikeler, T.; Haaf, P.; Wanschitz, J.; Löscher, W.N.; Schreiner, B.; et al. Skeletal muscle disorders: A non-cardiac source of cardiac troponin T. Circulation 2022, 145, 1764–1779. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.H.; Heckmann, M.B.; Bailly, G.; Finke, D.; Procureur, A.; Power, J.R.; Stein, F.; Bretagne, M.; Ederhy, S.; Fenioux, C.; et al. Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis. Circulation 2023, 148, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Saggin, L.; Gorza, L.; Ausoni, S.; Schiaffino, S. Cardiac troponin T in developing, regenerating and denervated rat skeletal muscle. Development 1990, 110, 547–554. [Google Scholar] [CrossRef]
- Anderson, P.A.W.; Malouf, N.N.; Oakeley, A.E.; Pagani, E.D.; Allen, P.D. Troponin T isoform expression in humans: A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ. Res. 1991, 69, 1226–1233. [Google Scholar] [CrossRef]
- Sabry, M.A.; Dhoot, G.K. Identification of and pattern of transitions of cardiac, adult slow and slow skeletal musle-like embryonic isoforms of troponin T in developing rat and human skeletal muscles. J. Muscle Res. Cell Motil. 1991, 12, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.P. Alternative RNA splicing-generated cardiac troponin T isoform switching: A non-heart-restricted genetic programming synchronized in developing cardiac and skeletal muscles. Biochem. Biophys. Res. Commun. 1996, 225, 883–889. [Google Scholar] [CrossRef]
- McLaurin, M.D.; Apple, F.S.; Voss, E.M.; Herzog, C.A.; Sharkey, S.W. Cardiac troponin I, cardiac troponin T, and creatine kinase MB in dialysis patients without ischemic heart disease: Evidence of cardiac troponin T reexpression in skeletal muscle. Clin. Chem. 1997, 43, 976–982. [Google Scholar] [CrossRef]
- Bodor, G.S.; Survant, L.; Voss, E.M.; Smith, S.; Porterfield, D.; Apple, F.S. Cardiac troponin T composition in normal and regenerating human skeletal muscle. Clin. Chem. 1997, 43, 476–484. [Google Scholar] [CrossRef]
- Ricchiuti, V.; Apple, F.S. RNA expression of cardiac troponin T isoforms in diseased human skeletal muscle. Clin. Chem. 1999, 45, 2129–2135. [Google Scholar] [CrossRef]
- Messner, B.; Baum, H.; Fischer, P.; Quasthoff, S.; Neumeier, D. Expression of messenger RNA of cardiac isoforms of troponin T and I in myopathic skeletal muscle. Am. J. Clin. Pathol. 2000, 114, 544–549. [Google Scholar] [CrossRef]
- Hickman, P.E.; McGill, D.; Potter, J.M.; Koerbin, G.; Apple, F.S.; Talaulikar, G. Multiple biomarkers including cardiac troponin I and T measured by high-sensitivity assays, as predictors of long-term mortality in patients with chronic renal failure who underwend dialysis. Am. J. Cardiol. 2015, 115, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Buiten, M.S.; de Bie, M.K.; Rotmans, J.I.; Dekker, F.W.; van Buren, M.; Rabelink, T.J.; Cobbaert, C.M.; Schalij, M.J.; van der Laarse, A.; Jukema, J.W. Serum cardiac troponin I is superior to troponin T as a marker for left ventricular dysfunction in clinically stable patients with end-stage renal disease. PLoS ONE 2015, 10, e0134245. [Google Scholar] [CrossRef] [PubMed]
- Twerenbold, R.; Wildi, K.; Jaeger, C.; Rubini Gimenez, M.; Reiter, M.; Reichlin, M.; Walukiewicz, A.; Gugula, M.; Krivoshei, L.; Marti, N.; et al. Optimal cutoff levels of more sensitive cardiac troponin assays for early diagnosis of myocardial infarction in patients with renal dysfunction. Circulation 2015, 131, 2041–2050. [Google Scholar] [CrossRef]
- Kolland, M.; Amenitsch, J.; Schreiber, N.; Ginthör, N.; Schuller, M.; Riedl, R.; Rainer, P.P.; Schneditz, D.; Niedrist, T.; Eller, K.; et al. Changes in cardiac troponin during hemodialysis depend on hemodialysis membrane and modality: A randomized crossover trial. Clin. Kidney J. 2023, 17, sfad297. [Google Scholar] [CrossRef] [PubMed]
- Gaze, D.C.; Collinson, P.O. Cardiac troponin I but not cardiac troponin T adheres to polysulfone dialyser membranes in an in vitro hemodialysis model: Explanation for lower cTnI concentrations following dialysis. Open Heart 2014, 1, e000108. [Google Scholar] [CrossRef]
- Haller, C.; Zehelein, J.; Remppis, A.; Müller-Bardorff, M.; Katus, H.A. Cardiac troponin T in patients with end stage renal disease: Absence of expression in truncal skeletal muscle. Clin. Chem. 1998, 44, 930–938. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).