Abstract
“Athlete’s heart” is a spectrum of morphological and functional changes which occur in the heart of people who practice physical activity. When athlete’s heart occurs with its most marked expression, it may overlap with a differential diagnosis with certain structural cardiac diseases, including cardiomyopathies, valvular diseases, aortopathies, myocarditis, and coronary artery anomalies. Identifying the underlying cardiac is essential to reduce the potential for sudden cardiac death. For this purpose, a spectrum of imaging modalities, including rest and exercise stress echocardiography, speckle tracking echocardiography, cardiac magnetic resonance, computed tomography, and nuclear scintigraphy, can be undertaken. The objective of this review article is to provide to the clinician a practical step-by-step approach, aiming at distinguishing between extreme physiology and structural cardiac disease during the athlete’s cardiovascular evaluation.
1. Introduction
The term “athlete’s heart” refers to a spectrum of electrical, morphological, and functional heart adaptations of the heart to athletic training [1,2].
When athlete’s heart occurs in its most marked expression, it can overlap with mild phenotypes of cardiac diseases. Therefore, it is important for the clinician to be able to differentiate this extreme form of physiological adaptation from pathology (such as hypertrophic, dilated, arrhythmogenic, and left ventricular (LV) non-compaction cardiomyopathy). For this purpose, different modern imaging modalities, including traditional and novel echocardiographic techniques, cardiac magnetic resonance (CMR), and computed tomography (CT), can be used. Identifying the pathologic cardiovascular condition which may be hidden behind athlete’s heart is crucial to prevent sudden cardiac death [3,4].
The objective of this review article is to provide physicians with a practical, diagnostic step-by-step approach aiming at distinguishing between extreme physiology and structural cardiac disease during the cardiovascular evaluation of athletes.
2. Clinical and Electrocardiographic Evaluation
Clinical history, physical examination, and the standard 12-lead electrocardiogram (ECG) represent the first step of the athlete’s evaluation.
Clinical history should be focused on the type and volume of physical activity practiced by the athlete: it is possible to hypothesize an “athlete’s heart” only if training loads are really heavy. Additionally, the use of performance-enhancing drugs should be investigated and taken into account during history collection.
Athlete’s heart is commonly (up to 80%) associated with adaptative ECG changes [5,6]. Sinus bradycardia, first-degree atrioventricular (AV) block, and early repolarization pattern are training-related ECG findings due to increased vagal tone and/or reduced sympathetic activity. Sinus bradycardia <40 beats per minute, Mobitz type 1 second degree AV block, and junctional rhythm are not uncommon and do not warrant further investigation in asymptomatic athletes. Finally, highly trained athletes’ ECGs often exhibit signs of physiologic cardiac chambers’ remodeling, such as pure QRS voltage criteria for LV hypertrophy (e.g., isolated Sokolow–Lyon criterion). Voltage criteria for right ventricular (RV) hypertrophy, right or left axis deviation, right and/or left atrial enlargement require further evaluation just in presence of symptoms or abnormal physical examination.
Table 1 summarizes all the athletes’ clinical and electrocardiographic features which are not considered training-related or physiologic adaptations to exercise, and therefore require further investigation.

Table 1.
Athlete’s clinical and electrocardiographic features requiring further investigation.
3. Echocardiography
According to the current recommendation of the European Association of Preventive Cardiology (EAPC) and the European Association of Cardiovascular Imaging (EACVI), echocardiography is a second-line investigative tool for the differential diagnosis between the athlete’s physiological adaptation to exercise and underlying malignant cardiac conditions [1,7,8]. However, in contrast with contemporary recommendations, standard echocardiography is often used as a first-line screening tool in the pre-participation cardiovascular evaluation of professional and amateur athletes, even in the setting of a normal clinical and electrocardiographic evaluation [7,9].
The physiological hypertrophy of athlete’s heart is characterized by a harmonic and symmetric wall thickening that is homogeneously distributed and involves all the cardiac chambers (Figure 1). The exercise-induced chamber thickening is proportional to the type of sport participated (mainly in combined power and endurance disciplines) and it is reversible after temporary (3 months) detraining [10]. A physiological enlargement of both ventricles is usually observed (mostly among endurance athletes), together with a proportional atrial enlargement [11,12,13,14,15,16]. Despite the cardiac chambers’ hypertrophy and enlargement, cardiac systolic function is not altered in athletes, with no significant differences compared to untrained subjects [12,17,18,19]. Likewise, LV diastolic function is normal and an increased contribution of early filling velocity at rest (E/A > 2) can be observed [20,21,22,23,24]. Finally, aortic root diameters are, generally, normal in athletes [25,26,27].
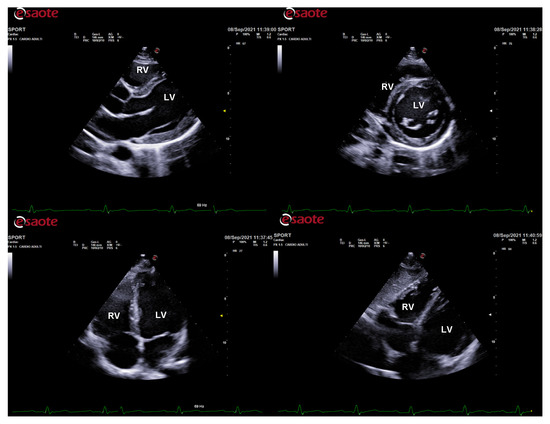
Figure 1.
Standard echocardiography in an endurance athlete (cyclist). The physiological hypertrophy of the athlete’s heart is characterized by harmonic and symmetric wall thickening which is homogeneously distributed and involves all the cardiac chambers. In parasternal long-axis (top left), short-axis (top right), apical 4-chamber (bottom left), and subcostal (bottom right) views, both left and right chamber dilatation is evident. LV: left ventricle; RV: right ventricle.
Table 2 reports the echocardiographic features which have to be considered non-physiological in athletes, and thus should raise suspicion of underlying cardiovascular disease.

Table 2.
Athlete’s echocardiographic findings suggestive of cardiovascular disease.
3.1. Exercise Stress Echocardiography
Exercise stress echocardiography (ESE) is a reliable, safe, non-invasive imaging test, well-tolerated by the athletes, which can be combined with clinical and electrocardiographic information to detect cardiac abnormalities (Table 2).
A typical indication of ESE is the detection of exercise-induced ischemia in athletes with chest pain and/or ECG anomalies, and suspicion of coronary artery disease or congenital coronary artery anomalies [1].
In endurance athletes with LV and/or RV dilatation and mildly reduced ejection fraction at rest, ESE may be used to assess contractile reserve during exercise. A significant improvement in contractility during physical exertion (e.g., ΔLV ejection fraction > 5%) suggests a physiological cardiac remodeling; conversely, an absent or subnormal improvement is in favor of a pathological condition (e.g., dilated cardiomyopathy, LV non-compaction, arrhythmogenic cardiomyopathy) [2,31,32,33]. Similarly, exercise-induced ventricular arrhythmias support the hypothesis of underlying cardiac disease.
An LV outflow tract gradient >50 mmHg during or immediately after exercise in athletes with LV hypertrophy complaining symptoms (syncope, shortness of breath) can be suggestive of hypertrophic cardiomyopathy [1,32].
Finally, ESE may be useful in athletes with valvular heart disease, where it provides information about exercise tolerance, biventricular contractile reserve, changes in hemodynamics (LV filling pressure, pulmonary pressure), and in valvular functional parameters (transvalvular gradients, regurgitation entity) [32].
3.2. New Echocardiographic Technologies
Speckle tracking echocardiography (STE) is a relatively recent echocardiographic technique of deformation imaging that has provided new insights into the characterization of the athletes’ myocardial properties [34]. It is able to detect subclinical ventricular systolic function in an early-stage cardiac disease when LVEF is still normal.
LV global longitudinal strain (GLS) obtained by STE is the most used parameter in clinical practice, and it is not significantly different between athletes and healthy controls. Therefore, a reduction in longitudinal strain in athletes has to be considered as a subclinical sign of LV contractile dysfunction and should raise the suspicion of myocardial disease, particularly in the presence of doubtful LV hypertrophy or dilatation [35,36,37,38,39,40,41,42]. A blunted increase in GLS (ΔGLS < 2%) during ESE, meaning a limited contractile reserve, favors the diagnosis of cardiomyopathy rather than athlete’s heart [32].
Data regarding the interpretation of RV, STE-derived parameters in athletes are still controversial [43,44,45,46,47,48,49]. Strenuous and chronic exercise training seems to have a detrimental effect on RV function, with reduction of RV strain immediately after the endurance race, followed by complete recovery [33,50]. Finally, RV strain imaging can be useful to distinguish between physiology and pathology, given its ability in identifying regional wall motion abnormalities in patients with arrhythmogenic cardiomyopathy [51].
During the last decades, STE has also been applied to the evaluation of left and right atrial function [15,46,52,53,54]. In both athlete’s heart and cardiomyopathy, atrial enlargement can be found, but atrial deformation indices are reduced only in the latter.
Myocardial work (MW) is a novel, less load-dependent ultrasonographic index of LV contractile function, which corrects STE-derived parameters for afterload, by using systolic blood pressure [55]. In different physiologic and pathologic conditions, an increased afterload may lead to strain impairment, with preserved or increased MW indices. This may be important for athletes with variable blood pressure and loading conditions from exam to exam and in different phases of the training program (Figure 2).
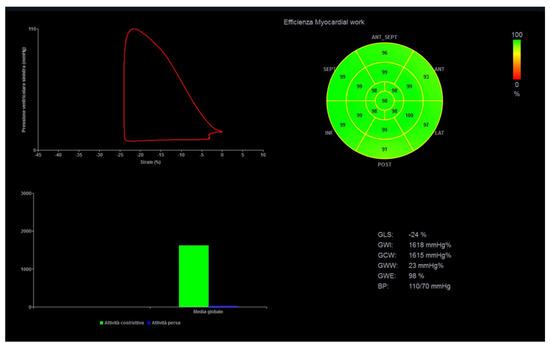
Figure 2.
Myocardial work analysis in a power athlete (bodybuilder). On the left, left ventricular pressure–strain loop, showing the relationship between left ventricular systolic pressure and global longitudinal strain. On the right, the 17-segment bull’s-eye representation of myocardial work efficiency, showing homogeneous areas of high efficiency coded in green.
ColorDoppler flow mapping is an advanced echocardiographic tool that evaluates LV function through the analysis of intracardiac flows [56]. LV vortex flow study may provide new insights into the characterization of athlete’s heart properties and its differences with normal subjects and patients with cardiomyopathies (Figure 3).
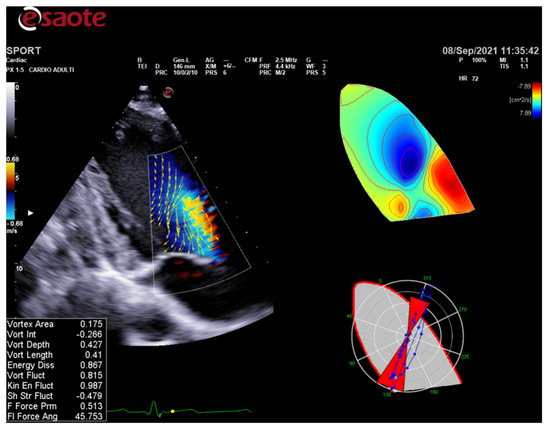
Figure 3.
HyperDoppler flow analysis in an endurance athlete. On the left, the flow velocity vector map shows the blood flow that circulates towards the direction of the left ventricular outflow tract. On the right, the circulation parametric map brings to light the formation of a pair of vortices immediately below the aortic valve.
Finally, three-dimensional (3D) echocardiography improves the diagnostic capability of cardiac ultrasound in evaluating cardiac anatomy, ventricular function, valvular disease, and blood flow velocity. It allows quantifying LV volume and mass, provides data on LV remodeling and function, and can show, in detail, the heart chambers’ morphological features [57].
4. Cardiac Magnetic Resonance
Cardiac magnetic resonance (CMR) is the second most valuable imaging method for the differential diagnosis between physiology and pathology in athletes. It can help discriminate health from disease where echocardiography leaves doubts.
CMR is the gold standard for the definition of myocardial morphology, wall motion assessment, heart chambers size, and tissue characterization. It evaluates, with high accuracy and reproducibility, the heart chambers’ volume and mass, as well as global and regional contractile function [58,59,60]. It is the method of choice for the accurate evaluation of right ventricle morphology and function (Figure 4 and Figure 5).
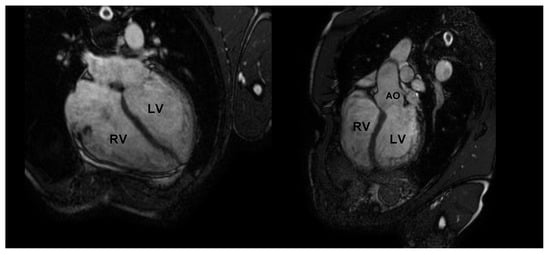
Figure 4.
Cardiac magnetic resonance in an endurance athlete (long-distance swimmer). A symmetric dilatation of both right and left ventricular chambers is clearly depicted in 4-chamber (left panel) and 5-chamber (right panel) views. AO: aorta; LV: left ventricle; RV: right ventricle.
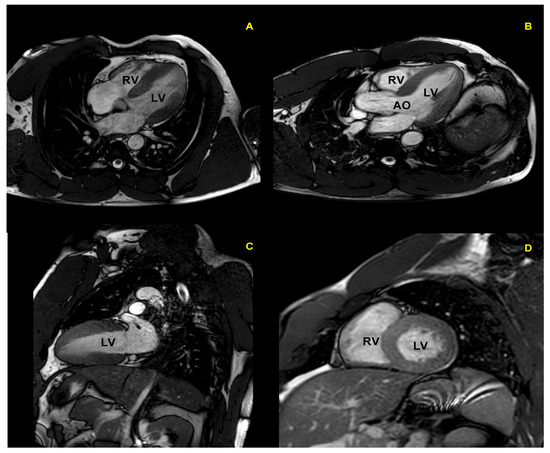
Figure 5.
Cardiac magnetic resonance in a top-level power athlete (weightlifter). A significant harmonic and symmetric wall thickening is documented in 4-chamber (A), 5-chamber (B), 2-chamber (C), and short-axis (D) views. AO: aorta; LV: left ventricle; RV: right ventricle.
CMR represents the superior method to identify myocardial fibrosis and its distribution pattern, through the assessment of late gadolinium enhancement (LGE), native T1, and extracellular volume (ECV) mapping [61,62,63,64,65]. Moreover, CMR can identify the presence of edema and fat in myocardial walls. The newer techniques such as LGE, native T1 and ECV mapping (for replacement and diffuse fibrosis), and T2 mapping (for edema) are emerging as valuable tools for the differential diagnosis between athlete’s heart and cardiomyopathies [65,66,67,68]. In particular, the identification of myocardial fibrosis can allow differentiation of athlete’s heart and pathological LV hypertrophy, since fibrosis generally occurs with cardiac remodeling due to pathology [69]. Classically, in hypertrophic cardiomyopathy, mid-wall fibrosis is predominantly found in areas of maximum hypertrophy, although it can be present in non-hypertrophied segments too.
Myocardial fibrosis has also been observed in athletes with a higher prevalence than in non-athlete healthy populations. In healthy athletes, myocardial fibrosis involves less than 3% of the myocardium. It shows a large variation in quantity, location, and pattern, but it is generally found in the right ventricle (particularly in right ventricle insertion points) or in the interventricular septum. The prevalence of myocardial fibrosis in athlete’s heart appears greater when there is a long-standing history of exercise training, particularly of the endurance type [68]. The prognostic significance of myocardial fibrosis in athlete’s heart is unknown. As for diffuse interstitial fibrosis, studies comparing ECV in athletes and controls have reported similar or lower values of ECV in athletes [70,71].
Heart valve morphology and function, and great vessel structure can be well evaluated by CMR.
Coronary magnetic resonance angiography shows high accuracy for the non-invasive detection and definition of anomalous coronary arteries, thanks to its ability in identifying the proximal coronary course and generating tomographic images in any orientation [72].
Stress CMR, usually with exercise, can be used to identify a reduced functional reserve and early-stage cardiomyopathy when resting functional assessment is mildly abnormal [73]. However, further studies are necessary to evaluate the cost-effectiveness of stress CMR imaging in this setting.
Finally, the possible role of CMR-based deformation imaging in distinguishing athlete’s heart from pathological phenotypes is being studied in different disease settings [66,74,75].
Table 3 summarizes the CMR features of the athletes which orient towards underlying cardiovascular disease.

Table 3.
Athlete’s CMR findings suggestive of cardiovascular disease.
Limitations of CMR include high cost, less availability than echocardiography, incompatibility with some metallic devices (though this is rarely an issue in athlete populations), the need for evaluation of renal function prior to administration of gadolinium, and claustrophobia.
5. Cardiac Computed Tomography and Other Imaging Modalities
Cardiac computed tomography (CCT) shows high accuracy in the evaluation of coronary arteries’ origin, course (including intramural), and termination [1] (Figure 6). Therefore, the main indication to perform a coronary CT angiography (CTA) is the suspicion of coronary artery anomalies (generally under 35 years of age) or atherosclerotic coronary artery disease (over 35), raising from clinical markers (exertional syncope, angina, or arrhythmias), or abnormal exercise test [76].

Figure 6.
Cardiac computed tomography of an endurance athlete (runner) symptomatic for syncope. The figure shows an intramural course of the left anterior descending coronary artery. LAD: left anterior descending.
All athletes with indeterminate, suspected, or confirmed anomalous coronary artery anatomy following echocardiography should undergo CTA or CMR, according to institutional preferences and expertise.
In the case of suspected coronary atherosclerotic disease, CTA is a valuable tool for coronary artery calcium scoring and non-invasive coronary angiography.
If dilatation of aortic root or ascending aorta is suspected or confirmed, at least one comprehensive aortic tomographic assessment (with CTA or CMR according to preference and local expertise) is indicated [76].
Recent technological advances have extended the role of CCT beyond coronary and great vessels evaluation. Morphological and functional assessment of the left ventricle is performed using a retrospective ECG-gated scanning protocol. The evaluation of LV volumes, stroke volume, ejection fraction, and mass have shown excellent correlation with CMR assessment [77].
LV functional evaluation by CCT is particularly useful in claustrophobic patients, unable to undergo CMR, or if contraindications to CMR exist (albeit rare among athletes). Otherwise, CCT cannot be recommended as a first-line imaging technique for functional evaluation of the LV in athletes, given the higher radiation exposure required [73,77].
The use of iodine contrast medium allows the evaluation of ECV and myocardial fibrosis (with the analysis of late iodine enhancement, LIE) by CCT, even if not routinely used in clinical practice, with good agreement with the same evaluations performed by contrast-enhanced CMR [73].
Nuclear scintigraphy can be used for the research of exercise-induced ischemia—as an alternative or integration to ESE—when coronary disease is suspected, with the same indications as in the non-athlete population.
6. Conclusions
A broad spectrum of imaging modalities is available for pre-participation cardiovascular evaluation of the athletes. The clinician should adopt a step-by-step approach, with the aim to distinguish between the physiologic, exercise-induced remodeling and structural cardiac disease. This practical approach is based on the periodic use of first-line screening tools (clinical history, physical examination, and ECG), possibly integrated by standard echocardiography, which is safe and not very time-consuming. Third-line imaging modalities (e.g., stress echocardiography, CMR, cardiac CT, etc.) should be selected when the previous evaluation is still doubtful. Speckle tracking echocardiography provides new insights into the characterization of the athlete’s myocardial properties. However, further accumulation of evidence is needed to refine STE diagnostic performance and make it part of the standard diagnostic workup.
Author Contributions
Conceptualization, A.D. and E.B.; writing—original draft preparation, S.S.; writing—review and first editing, G.B. and F.R.; visualization and following editing, S.S., V.R., F.D., F.I., S.P., F.G. and G.L.; supervision, A.D. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. Internal reviewers for EAPC and EACVI. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cardim, N.; D’Andrea, A.; Bruder, O.; Cosyns, B.; Davin, L.; Donal, E.; Edvardsen, T.; Freitas, A.; Habib, G.; et al. The multi-modality cardiac imaging approach to the Athlete’s heart: An expert consensus of the European Association of Cardiovascular Imaging. Eur. Hear. J. Cardiovasc. Imaging 2015, 16, 353–353r. [Google Scholar] [CrossRef] [PubMed]
- Radmilovic, J.; D’Andrea, A.; D’Amato, A.; Tagliamonte, E.; Sperlongano, S.; Riegler, L.; Scarafile, R.; Forni, A.; Muscogiuri, G.; Pontone, G.; et al. Echocardiography in Athletes in Primary Prevention of Sudden Death. J. Cardiovasc. Echogr. 2019, 29, 139–148. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Mele, D.; Palermi, S.; Rizzo, M.; Campana, M.; Di Giannuario, G.; Gimelli, A.; Khoury, G.; Moreo, A. A nome dell’Area Cardioimaging dell’Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO). Le “zone grigie” degli adattamenti cardiovascolari all’esercizio fisico: Come orientarsi nella valutazione ecocardiografica del cuore d’atleta [Grey zones in cardiovascular adaptations to physical exercise: How to navigate in the echocardiographic evaluation of the athlete’s heart]. G. Ital. Cardiol. 2020, 21, 457–468. (In Italian) [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International Recommendations for Electrocardiographic Interpretation in Athletes. J. Am. Coll. Cardiol. 2017, 69, 1057–1075. [Google Scholar] [CrossRef]
- Corrado, D.; Pelliccia, A.; Heidbuchel, H.; Sharma, S.; Link, M.; Basso, C.; Biffi, A.; Buja, G.; Delise, P.; Gussac, I.; et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur. Hear. J. 2009, 31, 243–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascenzi, F.; Anselmi, F.; Mondillo, S.; Finocchiaro, G.; Caselli, S.; La Garza, M.S.-D.; Schmied, C.; Adami, P.E.; Galderisi, M.; Adler, Y.; et al. The use of cardiac imaging in the evaluation of athletes in the clinical practice: A survey by the Sports Cardiology and Exercise Section of the European Association of Preventive Cardiology and University of Siena, in collaboration with the European Association of Cardiovascular Imaging, the European Heart Rhythm Association and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Prev. Cardiol. 2020, 28, 1071–1077. [Google Scholar] [CrossRef]
- Niederseer, D.; Rossi, V.A.; Kissel, C.; Scherr, J.; Caselli, S.; Tanner, F.C.; Bohm, P.; Schmied, C. Role of echocardiography in screening and evaluation of athletes. Heart 2020, 107, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Palermi, S.; Serio, A.; Vecchiato, M.; Sirico, F.; Gambardella, F.; Ricci, F.; Iodice, F.; Radmilovic, J.; Russo, V.; D’Andrea, A. Potential role of an athlete-focused echocardiogram in sports eligibility. World J. Cardiol. 2021, 13, 271–297. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; De Luca, R.; Di Paolo, F.M.; Spataro, A.; Culasso, F. Remodeling of Left Ventricular Hypertrophy in Elite Athletes After Long-Term Deconditioning. Circulation 2002, 105, 944–949. [Google Scholar] [CrossRef] [Green Version]
- Pelliccia, A.; Maron, B.J.; Spataro, A.; Proschan, M.A.; Spirito, P. The Upper Limit of Physiologic Cardiac Hypertrophy in Highly Trained Elite Athletes. N. Engl. J. Med. 1991, 324, 295–301. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Pisicchio, C.; Caselli, S.; Di Paolo, F.M.; Spataro, A.; Pelliccia, A. RV Remodeling in Olympic Athletes. JACC Cardiovasc. Imaging 2017, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Ghani, S.; Sharma, R.; Oxborough, D.; Panoulas, V.F.; Sheikh, N.; Gati, S.; Papadakis, M.; Sharma, S. Physiological Right Ventricular Adaptation in Elite Athletes of African and Afro-Caribbean Origin. Circulation 2013, 127, 1783–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; La Gerche, A.; Golia, E.; Teske, A.J.; Bossone, E.; Russo, M.G.; Calabrò, R.; Baggish, A.L. Right Heart Structural and Functional Remodeling in Athletes. Echocardiography 2014, 32, S11–S22. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Maron, B.J.; Di Paolo, F.M.; Biffi, A.; Quattrini, F.M.; Pisicchio, C.; Roselli, A.; Caselli, S.; Culasso, F. Prevalence and Clinical Significance of Left Atrial Remodeling in Competitive Athletes. J. Am. Coll. Cardiol. 2005, 46, 690–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; Riegler, L.; Cocchia, R.; Scarafile, R.; Salerno, G.; Gravino, R.; Golia, E.; Vriz, O.; Citro, R.; Limongelli, G.; et al. Left atrial volume index in highly trained athletes. Am. Hear. J. 2010, 159, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; Di Paolo, F.M.; Pisicchio, C.; Di Pietro, R.; Quattrini, F.M.; Di Giacinto, B.; Culasso, F.; Pelliccia, A. Three-Dimensional Echocardiographic Characterization of Left Ventricular Remodeling in Olympic Athletes. Am. J. Cardiol. 2011, 108, 141–147. [Google Scholar] [CrossRef]
- Pluim, B.M.; Zwinderman, A.H.; van der Laarse, A.; van der Wall, E.E. The Athlete’s Heart. Circulation 2000, 101, 336–344. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, A.; Riegler, L.; Golia, E.; Cocchia, R.; Scarafile, R.; Salerno, G.; Pezzullo, E.; Nunziata, L.; Citro, R.; Cuomo, S.; et al. Range of right heart measurements in top-level athletes: The training impact. Int. J. Cardiol. 2013, 164, 48–57. [Google Scholar] [CrossRef]
- Caselli, S.; Di Paolo, F.M.; Pisicchio, C.; Pandian, N.G.; Pelliccia, A. Patterns of Left Ventricular Diastolic Function in Olympic Athletes. J. Am. Soc. Echocardiogr. 2015, 28, 236–244. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Limongelli, G.; Caso, P.; Sarubbi, B.; Della Pietra, A.; Brancaccio, P.; Cice, G.; Scherillo, M.; Limongelli, F.; Calabrò, R. Association between left ventricular structure and cardiac performance during effort in two morphological forms of athlete’s heart. Int. J. Cardiol. 2002, 86, 177–184. [Google Scholar] [CrossRef]
- Cardim, N.; Oliveira, A.; Longo, S.; Ferreira, T.; Pereira, A.; Reis, R.P.; Correia, J.M. Doppler tissue imaging: Regional myocardial function in hypertrophic cardiomyopathy and in athlete’s heart. J. Am. Soc. Echocardiogr. 2003, 16, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Vinereanu, D.; Florescu, N.; Sculthorpe, N.; Tweddel, A.C.; Stephens, M.R.; Fraser, A.G. Differentiation between pathologic and physiologic left ventricular hypertrophy by tissue doppler assessment of long-axis function in patients with hypertrophic cardiomyopathy or systemic hypertension and in athletes. Am. J. Cardiol. 2001, 88, 53–58. [Google Scholar] [CrossRef]
- D’Andrea, A.; Cocchia, R.; Riegler, L.; Scarafile, R.; Salerno, G.; Gravino, R.; Golia, E.; Pezzullo, E.; Citro, R.; Limongelli, G.; et al. Left Ventricular Myocardial Velocities and Deformation Indexes in Top-Level Athletes. J. Am. Soc. Echocardiogr. 2010, 23, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Di Paolo, F.M.; De Blasiis, E.; Quattrini, F.M.; Pisicchio, C.; Guerra, E.; Culasso, F.; Maron, B.J. Prevalence and Clinical Significance of Aortic Root Dilation in Highly Trained Competitive Athletes. Circulation 2010, 122, 698–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; Cocchia, R.; Riegler, L.; Scarafile, R.; Salerno, G.; Gravino, R.; Vriz, O.; Citro, R.; Limongelli, G.; Di Salvo, G.; et al. Aortic Root Dimensions in Elite Athletes. Am. J. Cardiol. 2010, 105, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, A.; Thompson, P.D. A Meta-Analysis of Aortic Root Size in Elite Athletes. Circulation 2013, 127, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Spirito, P.; Pelliccia, A.; Proschan, M.A.; Granata, M.; Spataro, A.; Bellone, P.; Caselli, G.; Biffi, A.; Vecchio, C.; Maron, B.J. Morphology of the “athlete’s heart” assessed by echocardiography in 947 elite athletes representing 27 sports. Am. J. Cardiol. 1994, 74, 802–806. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Basso, C.; Badano, L.P.; Bucciarelli-Ducci, C.; Cardim, N.; Gaemperli, O.; Galderisi, M.; Habib, G.; Knuuti, J.; Lancellotti, P.; et al. Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy—An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Hear. J. Cardiovasc. Imaging 2017, 18, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed Modification of the Task Force Criteria. Eur. Hear. J. 2010, 31, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Claessen, G.; La Gerche, A.; Voigt, J.-U.; Dymarkowski, S.; Schnell, F.; Petit, T.; Willems, R.; Claus, P.; Delcroix, M.; Heidbuchel, H. Accuracy of Echocardiography to Evaluate Pulmonary Vascular and RV Function During Exercise. JACC Cardiovasc. Imaging 2016, 9, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Hear. J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [Green Version]
- Millar, L.M.; Fanton, Z.; Finocchiaro, G.; Sanchez-Fernandez, G.; Dhutia, H.; Malhotra, A.; Merghani, A.; Papadakis, M.; Behr, E.R.; Bunce, N.; et al. Differentiation between athlete’s heart and dilated cardiomyopathy in athletic individuals. Heart 2020, 106, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Caselli, S.; Solari, M.; Pelliccia, A.; Cameli, M.; Focardi, M.; Padeletti, M.; Corrado, D.; Bonifazi, M.; Mondillo, S. Novel echocardiographic techniques for the evaluation of athletes’ heart: A focus on speckle-tracking echocardiography. Eur. J. Prev. Cardiol. 2015, 23, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, F.; Toncelli, L.; Cappelli, B.; De Luca, A.; Stefani, L.; Maffulli, N.; Galanti, G. Adaptative or maladaptative hypertrophy, different spatial distribution of myocardial contraction. Clin. Physiol. Funct. Imaging 2010, 30, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; Montesanti, D.; Autore, C.; Di Paolo, F.M.; Pisicchio, C.; Squeo, M.R.; Musumeci, B.; Spataro, A.; Pandian, N.G.; Pelliccia, A. Patterns of Left Ventricular Longitudinal Strain and Strain Rate in Olympic Athletes. J. Am. Soc. Echocardiogr. 2015, 28, 245–253. [Google Scholar] [CrossRef]
- Galderisi, M.; Lomoriello, V.S.; Santoro, A.; Esposito, R.; Olibet, M.; Raia, R.; Di Minno, M.; Guerra, G.; Mele, D.; Lombardi, G. Differences of Myocardial Systolic Deformation and Correlates of Diastolic Function in Competitive Rowers and Young Hypertensives: A Speckle-Tracking Echocardiography Study. J. Am. Soc. Echocardiogr. 2010, 23, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Nottin, S.; Doucende, G.; Schuster-Beck, I.; Dauzat, M.; Obert, P. Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete’s heart. J. Physiol. 2008, 586, 4721–4733. [Google Scholar] [CrossRef]
- Simsek, Z.; Tas, M.H.; Degirmenci, H.; Yazıcı, A.G.; Ipek, E.; Duman, H.; Gundogdu, F.; Karakelleoglu, S.; Senocak, H. Speckle Tracking Echocardiographic Analysis of Left Ventricular Systolic and Diastolic Functions of Young Elite Athletes with Eccentric and Concentric Type of Cardiac Remodeling. Echocardiography 2013, 30, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Soullier, C.; Obert, P.; Doucende, G.; Nottin, S.; Cade, S.; Perez-Martin, A.; Messner-Pellenc, P.; Schuster, I. Exercise Response in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2012, 5, 324–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, R.B.; Hutter, A.M.; Wang, F.; Kim, J.; Weyman, A.E.; Wood, M.J.; Picard, M.; Baggish, A.L. The Impact of Endurance Exercise Training on Left Ventricular Torsion. JACC: Cardiovasc. Imaging 2010, 3, 1001–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butz, T.; Van Buuren, F.; Mellwig, K.-P.; Langer, C.; Plehn, G.; Meissner, A.; Trappe, H.-J.; Horstkotte, D.; Faber, L. Two-dimensional strain analysis of the global and regional myocardial function for the differentiation of pathologic and physiologic left ventricular hypertrophy: A study in athletes and in patients with hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2010, 27, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Stefani, L.; Pedrizzetti, G.; De Luca, A.; Mercuri, R.; Innocenti, G.; Galanti, G. Real-time evaluation of longitudinal peak systolic strain (speckle tracking measurement) in left and right ventricles of athletes. Cardiovasc. Ultrasound 2009, 7, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxborough, D.; Sharma, S.; Shave, R.; Whyte, G.; Birch, K.; Artis, N.; Batterham, A.M.; George, K. The Right Ventricle of the Endurance Athlete: The Relationship between Morphology and Deformation. J. Am. Soc. Echocardiogr. 2012, 25, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.J.; Prakken, N.H.; De Boeck, B.W.; Velthuis, B.K.; Martens, E.P.; Doevendans, P.A.; Cramer, M.J. Echocardiographic tissue deformation imaging of right ventricular systolic function in endurance athletes. Eur. Hear. J. 2008, 30, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Bauce, B.; Frigo, G.; Benini, G.; Michieli, P.; Basso, C.; Folino, A.F.; Rigato, I.; Mazzotti, E.; Daliento, L.; Thiene, G.; et al. Differences and similarities between arrhythmogenic right ventricular cardiomyopathy and athlete’s heart adaptations. Br. J. Sports Med. 2008, 44, 148–154.e1. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Burns, A.T.; D’Hooge, J.; MacIsaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise Strain Rate Imaging Demonstrates Normal Right Ventricular Contractile Reserve and Clarifies Ambiguous Resting Measures in Endurance Athletes. J. Am. Soc. Echocardiogr. 2012, 25, 253–262. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Kouidi, E.; Efthimiadis, G.K.; Deligiannis, A.; Geleris, P.; Vassilikos, V. Right Atrial and Ventricular Adaptations to Training in Male Caucasian Athletes: An Echocardiographic Study. J. Am. Soc. Echocardiogr. 2013, 26, 1344–1352. [Google Scholar] [CrossRef]
- Esposito, R.; Galderisi, M.; M.D., V.S.; M.D., A.S.; De Palma, D.; Ippolito, R.; M.D., R.M.; Santoro, C.; Guerra, G.; Cameli, M.; et al. Nonsymmetric Myocardial Contribution to Supranormal Right Ventricular Function in the Athlete’s Heart: Combined Assessment by Speckle Tracking and Real Time Three-Dimensional Echocardiography. Echocardiography 2013, 31, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Oxborough, D.; Shave, R.; Warburton, D.; Williams, K.; Oxborough, A.; Charlesworth, S.; Foulds, H.; Hoffman, M.D.; Birch, K.; George, K. Dilatation and Dysfunction of the Right Ventricle Immediately After Ultraendurance Exercise. Circ. Cardiovasc. Imaging 2011, 4, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.J.; Cox, M.G.; De Boeck, B.W.; Doevendans, P.A.; Hauer, R.N.; Cramer, M.J. Echocardiographic Tissue Deformation Imaging Quantifies Abnormal Regional Right Ventricular Function in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. J. Am. Soc. Echocardiogr. 2009, 22, 920–927. [Google Scholar] [CrossRef]
- Paraskevaidis, I.A.; Panou, F.; Papadopoulos, C.; Farmakis, D.; Parissis, J.; Ikonomidis, I.; Rigopoulos, A.; Iliodromitis, E.K.; Kremastinos, D.T. Evaluation of left atrial longitudinal function in patients with hypertrophic cardiomyopathy: A tissue Doppler imaging and two-dimensional strain study. Heart 2008, 95, 483–489. [Google Scholar] [CrossRef]
- Roşca, M.; Popescu, B.A.; Beladan, C.C.; Călin, A.; Muraru, D.; Popa, E.C.; Lancellotti, P.; Enache, R.; Coman, I.M.; Jurcuţ, R.; et al. Left Atrial Dysfunction as a Correlate of Heart Failure Symptoms in Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2010, 23, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, L.; Enríquez, A.; Córdova, S.; Yáñez, F.; Godoy, I.; Corbalán, R. Assessment of left atrial function in hypertrophic cardiomyopathy and athlete’s heart: A left atrial myocardial deformation study. Echocardiography 2012, 29, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: A non-invasive index of myocardial work. Eur. Hear. J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, D.; Smarrazzo, V.; Pedrizzetti, G.; Capasso, F.; Pepe, M.; Severino, S.; Luisi, G.A.; Maglione, M.; Ferrari, R. Intracardiac Flow Analysis: Techniques and Potential Clinical Applications. J. Am. Soc. Echocardiogr. 2019, 32, 319–332. [Google Scholar] [CrossRef]
- D’Andrea, A.; Bossone, E.; Radmilovic, J.; Caso, P.; Calabrò, R.; Russo, M.G.; Galderisi, M. The role of new echocardiographic techniques in athlete’s heart. F1000Research 2015, 4, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scharf, M.; Brem, M.H.; Wilhelm, M.; Schoepf, U.J.; Uder, M.; Lell, M.M. Atrial and Ventricular Functional and Structural Adaptations of the Heart in Elite Triathletes Assessed with Cardiac MR Imaging. Radiology 2010, 257, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Bluemke, D.A.; Krupinski, E.A.; Ovitt, T.; Gear, K.; Unger, E.; Axel, L.; Boxt, L.M.; Casolo, G.; Ferrari, V.; Funaki, B.; et al. MR Imaging of Arrhythmogenic Right Ventricular Cardiomyopathy: Morphologic Findings and Interobserver Reliability. Cardiology 2003, 99, 153–162. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Anselmi, F.; Piu, P.; Fiorentini, C.; Carbone, S.F.; Volterrani, L.; Focardi, M.; Bonifazi, M.; Mondillo, S. Cardiac Magnetic Resonance Normal Reference Values of Biventricular Size and Function in Male Athlete’s Heart. JACC: Cardiovasc. Imaging 2018, 12, 1755–1765. [Google Scholar] [CrossRef]
- Kim, R.J.; Fieno, D.S.; Parrish, T.; Harris, K.; Chen, E.-L.; Simonetti, O.; Bundy, J.; Finn, J.P.; Klocke, F.J.; Judd, R.M. Relationship of MRI Delayed Contrast Enhancement to Irreversible Injury, Infarct Age, and Contractile Function. Circulation 1999, 100, 1992–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fieno, D.S.; Kim, R.J.; Chen, E.-L.; Lomasney, J.W.; Klocke, F.J.; Judd, R.M. Contrast-enhanced magnetic resonance imaging of myocardium at risk: Distinction between reversible and irreversible injury throughout infarct healing. J. Am. Coll. Cardiol. 2000, 36, 1985–1991. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Reed, E.; Sheppard, M.; Elkington, A.G.; Ho, S.; Burke, M.; Petrou, M.; Pennell, D.J. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2004, 43, 2260–2264. [Google Scholar] [CrossRef] [PubMed]
- Breuckmann, F.; Möhlenkamp, S.; Nassenstein, K.; Lehmann, N.; Ladd, S.; Schmermund, A.; Sievers, B.; Schlosser, T.; Jöckel, K.-H.; Heusch, G.; et al. Myocardial Late Gadolinium Enhancement: Prevalence, Pattern, and Prognostic Relevance in Marathon Runners. Radiology 2009, 251, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Maestrini, V.; Torlasco, C.; Hughes, R.; Moon, J.C. Cardiovascular Magnetic Resonance and Sport Cardiology: A Growing Role in Clinical Dilemmas. J. Cardiovasc. Transl. Res. 2020, 13, 296–305. [Google Scholar] [CrossRef]
- Gastl, M.; Lachmann, V.; Christidi, A.; Janzarik, N.; Veulemans, V.; Haberkorn, S.; Holzbach, L.; Jacoby, C.; Schnackenburg, B.; Berrisch-Rahmel, S.; et al. Cardiac magnetic resonance T2 mapping and feature tracking in athlete’s heart and HCM. Eur. Radiol. 2021, 31, 2768–2777. [Google Scholar] [CrossRef]
- Mordi, I.; Carrick, D.; Bezerra, H.G.; Tzemos, N. T1andT2mapping for early diagnosis of dilated non-ischaemic cardiomyopathy in middle-aged patients and differentiation from normal physiological adaptation. Eur. Hear. J. Cardiovasc. Imaging 2015, 17, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Caruso, M.R.; Garg, L.; Martinez, M.W. Cardiac Imaging in the Athlete: Shrinking the “Gray Zone”. Curr. Treat. Options Cardiovasc. Med. 2020, 22, 5. [Google Scholar] [CrossRef]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological Ventricular Remodeling. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef] [Green Version]
- Augustine, D.X.; Howard, L. Left Ventricular Hypertrophy in Athletes: Differentiating Physiology From Pathology. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 96. [Google Scholar] [CrossRef]
- Missenard, O.; Gabaudan, C.; Astier, H.; Desmots, F.; Garnotel, E.; Massoure, P.-L. Absence of cardiac damage induced by long-term intensive endurance exercise training: A cardiac magnetic resonance and exercise echocardiography analysis in masters athletes. Am. J. Prev. Cardiol. 2021, 7, 100196. [Google Scholar] [CrossRef] [PubMed]
- Stuber, M.; Weiss, R.G. Coronary magnetic resonance angiography. J. Magn. Reson. Imaging 2007, 26, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Fogante, M.; Agliata, G.; Basile, M.; Compagnucci, P.; Volpato, G.; Falanga, U.; Stronati, G.; Guerra, F.; Vignale, D.; Esposito, A.; et al. Cardiac Imaging in Athlete’s Heart: The Role of the Radiologist. Medicina 2021, 57, 455. [Google Scholar] [CrossRef] [PubMed]
- Czimbalmos, C.; Csecs, I.; Dohy, Z.; Toth, A.; Suhai, F.I.; Müssigbrodt, A.; Kiss, O.; Geller, L.; Merkely, B.; Vago, H. Cardiac magnetic resonance based deformation imaging: Role of feature tracking in athletes with suspected arrhythmogenic right ventricular cardiomyopathy. Int. J. Cardiovasc. Imaging 2018, 35, 529–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Małek, Ł.A.; Mazurkiewicz, Ł.; Marszałek, M.; Barczuk-Falęcka, M.; Simon, J.; Grzybowski, J.; Miłosz-Wieczorek, B.; Postuła, M.; Marczak, M. Deformation Parameters of the Heart in Endurance Athletes and in Patients with Dilated Cardiomyopathy—A Cardiac Magnetic Resonance Study. Diagnostics 2021, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L.; Battle, R.W.; Beaver, T.A.; Border, W.L.; Douglas, P.S.; Kramer, C.M.; Martinez, M.W.; Mercandetti, J.H.; Phelan, D.; Singh, T.K.; et al. Recommendations on the Use of Multimodality Cardiovascular Imaging in Young Adult Competitive Athletes: A Report from the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Computed Tomography and the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2020, 33, 523–549. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.M.; Hwang, S.H.; Lee, H.-J. Role of Cardiac Computed Tomography in the Diagnosis of Left Ventricular Myocardial Diseases. J. Cardiovasc. Imaging 2019, 27, 73–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).