Jatrorrhizine Suppresses Murine-Norovirus-Triggered N-GSDMD-Dependent Pyroptosis in RAW264.7 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus
2.2. MNV Infection and JAT Treatment
2.3. Cell Viability Assay
2.4. Western Blotting Analysis
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) of IL-1β and IL-18 in Cell Culture Supernatants
2.6. Viral RNA Isolation and qRT-PCR Analysis
2.7. Statistical Analysis
3. Results
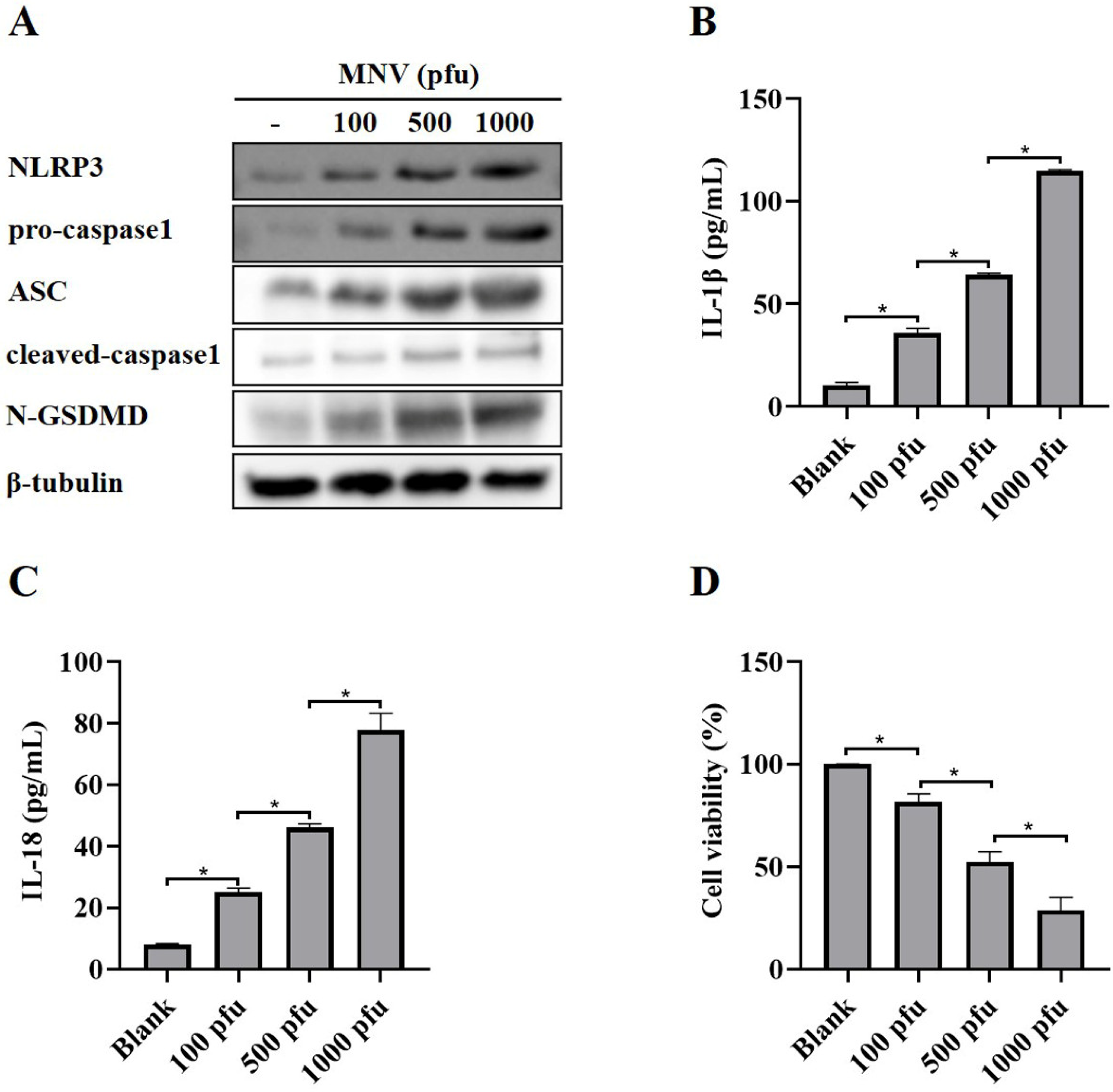
3.1. MNV Induces Activation of NLRP3 Inflammasome and Pyroptosis
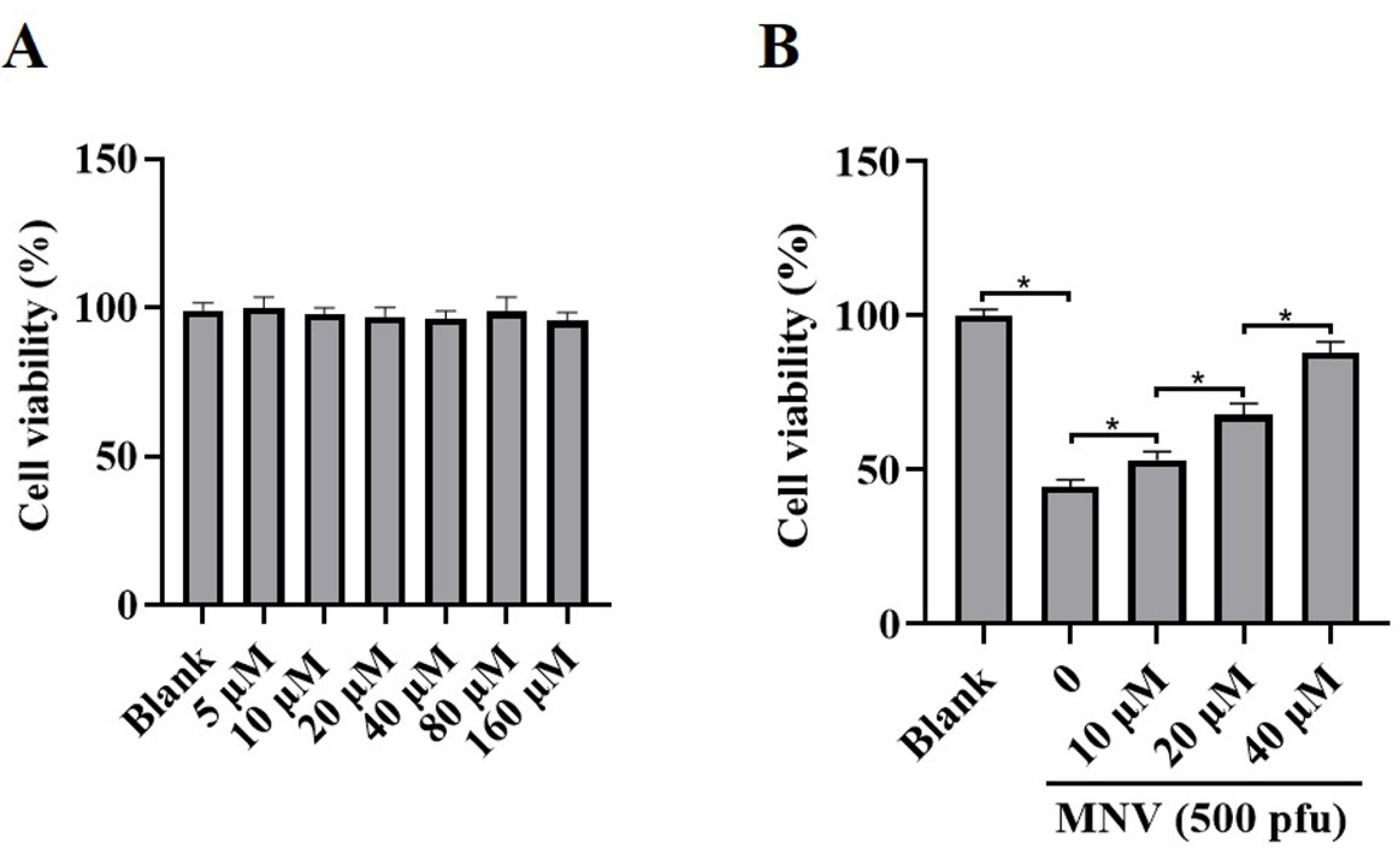
3.2. JAT Decreases Cytotoxicity Caused by MNV
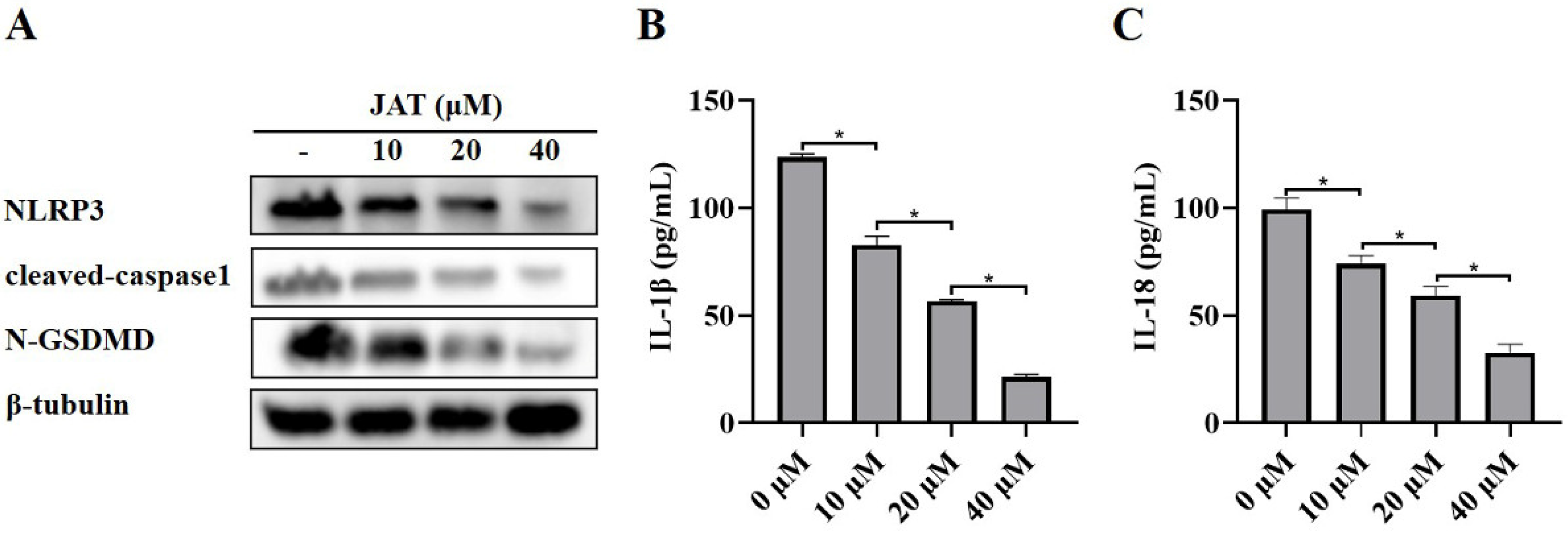
3.3. JAT Suppresses GSDMD Cleavage through Inhibiting NLRP3 Inflammasome Activation
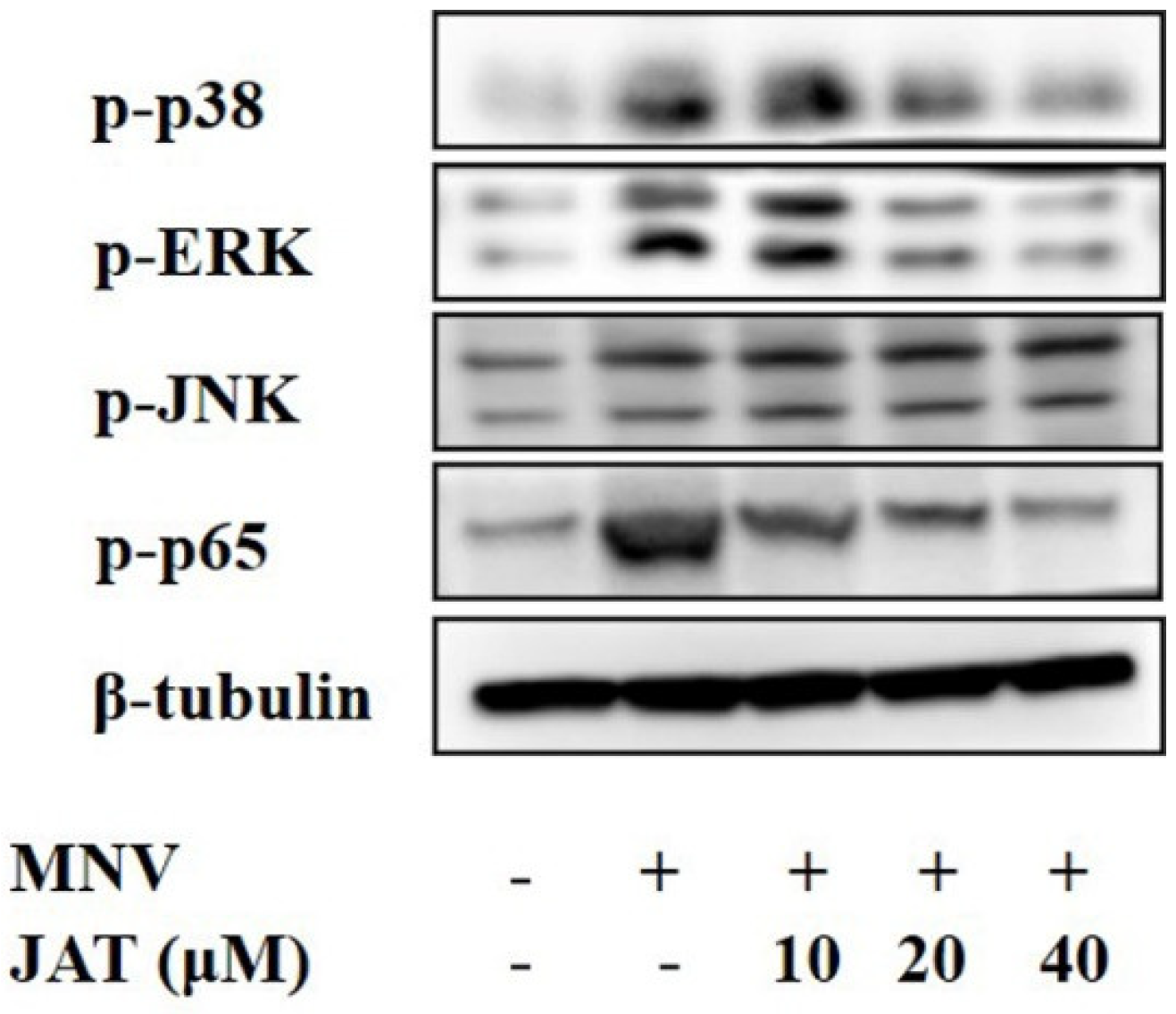
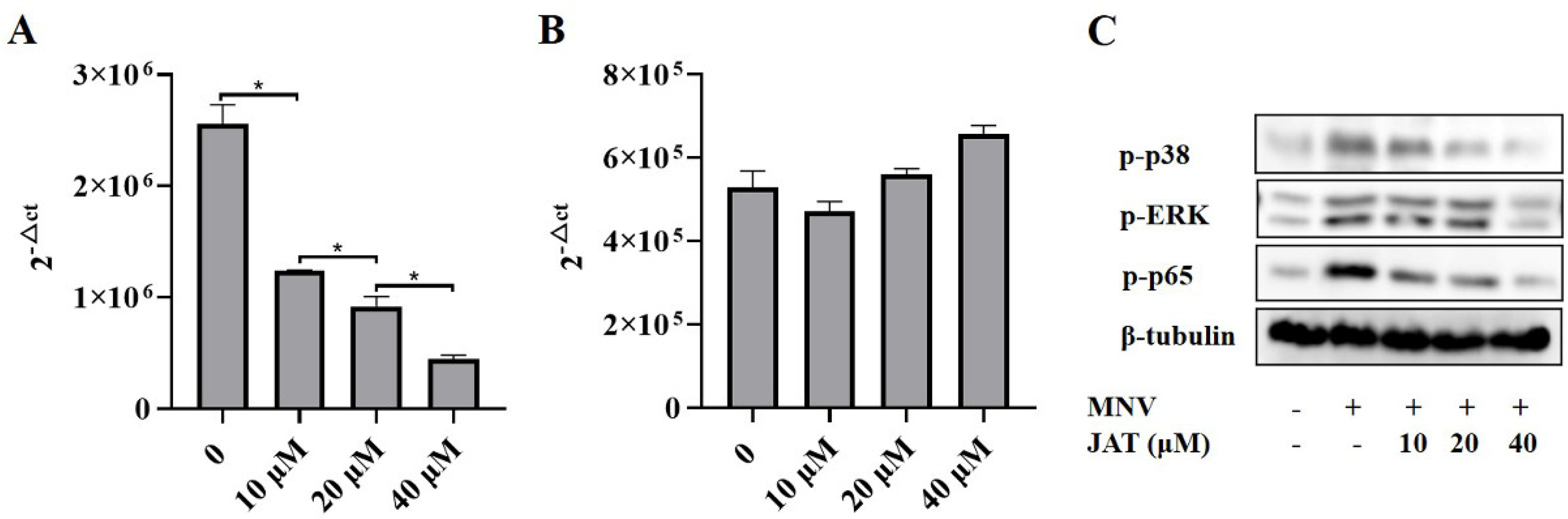
3.4. JAT Inhibits MNV-Induced Activation of MAPKs/NF-κB Signaling
3.5. JAT Inhibits the Replication of MNV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, R.; Neill, J.D.; Noel, J.S.; Hutson, A.M.; Glass, R.I.; Estes, M.K.; Prasad, B.V.V. Inter- and Intragenus Structural Variations in Caliciviruses and Their Functional Implications. J. Virol. 2004, 78, 6469–6479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, L.; Fu, Y.; Ji, L.; Wu, X.; Xu, D.; Han, J. Clinical and molecular analyses of norovirus-associated sporadic acute gastroenteritis: The emergence of GII.17 over GII.4, Huzhou, China, 2015. BMC Infect. Dis. 2016, 16, 717. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, C.; Du, Y.; Yan, D.; Jiang, D.; Liu, X.; Yang, M.; Ding, C.; Lan, L.; Hecht, R.; et al. Global Burden and Trends of Norovirus-Associated Diseases From 1990 to 2019: An Observational Trend Study. Front. Public Health 2022, 10, 905172. [Google Scholar] [CrossRef]
- Esposito, S.; Principi, N. Norovirus Vaccine: Priorities for Future Research and Development. Front. Immunol. 2020, 11, 1383. [Google Scholar] [CrossRef]
- Atmar, R.L.; Opekun, A.R.; Gilger, M.A.; Estes, M.K.; Crawford, S.E.; Neill, F.H.; Graham, D.Y. Norwalk Virus Shedding after Experimental Human Infection. Emerg. Infect. Dis. 2008, 14, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, L.; Hewitt, J.; Barclay, L.; Ahmed, S.M.; Lake, R.; Hall, A.J.; Lopman, B.; Kroneman, A.; Vennema, H.; Vinjé, J.; et al. Norovirus Genotype Profiles Associated with Foodborne Transmission, 1999–2012. Emerg. Infect. Dis. 2015, 21, 592–599. [Google Scholar] [CrossRef]
- Pouillot, R.; Van Doren, J.M.; Woods, J.; Plante, D.; Smith, M.; Goblick, G.; Roberts, C.; Locas, A.; Hajen, W.; Stobo, J.; et al. Meta-Analysis of the Reduction of Norovirus and Male-Specific Coliphage Concentrations in Wastewater Treatment Plants. Appl. Environ. Microbiol. 2015, 81, 4669–4681. [Google Scholar] [CrossRef]
- Trivedi, T.K.; Desai, R.; Hall, A.J.; Patel, M.; Parashar, U.D.; Lopman, B.A. Clinical characteristics of norovirus-associated deaths: A systematic literature review. Am. J. Infect. Control. 2013, 41, 654–657. [Google Scholar] [CrossRef]
- Xerry, J.; Gallimore, C.I.; Cubitt, D.; Gray, J.J. Tracking Environmental Norovirus Contamination in a Pediatric Primary Immunodeficiency Unit. J. Clin. Microbiol. 2010, 48, 2552–2556. [Google Scholar] [CrossRef]
- Wobus, C.E.; Karst, S.M.; Thackray, L.B.; Chang, K.-O.; Sosnovtsev, S.V.; Belliot, G.; Krug, A.; Mackenzie, J.M.; Green, K.Y.; Virgin, H.W., IV. Replication of Norovirus in Cell Culture Reveals a Tropism for Dendritic Cells and Macrophages. PLoS Biol. 2004, 2, e432. [Google Scholar] [CrossRef] [PubMed]
- Wobus, C.E.; Thackray, L.B.; Virgin, H.W. Murine Norovirus: A Model System To Study Norovirus Biology and Pathogenesis. J. Virol. 2006, 80, 5104–5112. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Aggeletopoulou, I.; Konstantakis, C.; Triantos, C. Role of NLRP3 inflammasome in inflammatory bowel diseases. World J. Gastroenterol. 2019, 25, 4796–4804. [Google Scholar] [CrossRef]
- Abderrazak, A.; Syrovets, T.; Couchie, D.; El Hadri, K.; Friguet, B.; Simmet, T.; Rouis, M. NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015, 4, 296–307. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.-D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Christgen, S.; Kanneganti, T.-D. Inflammasomes and the fine line between defense and disease. Curr. Opin. Immunol. 2019, 62, 39–44. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.-K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Dubois, H.; Sorgeloos, F.; Sarvestani, S.T.; Martens, L.; Saeys, Y.; Mackenzie, J.; Lamkanfi, M.; Van Loo, G.; Goodfellow, I.; Wullaert, A. Nlrp3 inflammasome activation and Gasdermin D-driven pyroptosis are immunopathogenic upon gastrointestinal norovirus infection. PLOS Pathog. 2019, 15, e1007709. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Chen, Y.; Chen, J.; Liao, H.; Li, Y.; Ma, Y. Jatrorrhizine: A Review of Sources, Pharmacology, Pharmacokinetics and Toxicity. Front. Pharmacol. 2022, 12, 783127. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, C.; Zhang, Y.; Du, H.; Xu, T.; Xu, X.; Zhang, J.; Kuang, T.; Lai, X.; Fan, G.; et al. 1H NMR-Based Metabolomics Coupled With Molecular Docking Reveal the Anti-Diabetic Effects and Potential Active Components of Berberis vernae on Type 2 Diabetic Rats. Front. Pharmacol. 2020, 11, 932. [Google Scholar] [CrossRef]
- Du, W.; Jin, L.; Li, L.; Wang, W.; Zeng, S.; Jiang, H.; Zhou, H. Development and Validation of a HPLC-ESI-MS/MS Method for Simultaneous Quantification of Fourteen Alkaloids in Mouse Plasma after Oral Administration of the Extract of Corydalis yanhusuo Tuber: Application to Pharmacokinetic Study. Molecules 2018, 23, 714. [Google Scholar] [CrossRef] [PubMed]
- Abdykerimova, S.; Sakipova, Z.; Nakonieczna, S.; Koch, W.; Biernasiuk, A.; Grabarska, A.; Malm, A.; Kozhanova, K.; Kukula-Koch, W. Superior Antioxidant Capacity of Berberis iliensis—HPLC-Q-TOF-MS Based Phytochemical Studies and Spectrophotometric Determinations. Antioxidants 2020, 9, 504. [Google Scholar] [CrossRef]
- Xian, Y.-F.; Mao, Q.-Q.; Ip, S.-P.; Lin, Z.-X.; Che, C.-T. Comparison on the anti-inflammatory effect of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J. Ethnopharmacol. 2011, 137, 1425–1430. [Google Scholar] [CrossRef]
- Mbaoji, F.N.; Nweze, J.A. Antioxidant and hepatoprotective potentials of active fractions of Lannea barteri Oliv. (Anarcadiaceae) in rats. Heliyon 2020, 6, e04099. [Google Scholar] [CrossRef]
- Xie, Q.; Li, H.; Ma, R.; Ren, M.; Li, Y.; Li, J.; Chen, H.; Chen, Z.; Gong, D.; Wang, J. Effect of Coptis chinensis franch and Magnolia officinalis on intestinal flora and intestinal barrier in a TNBS-induced ulcerative colitis rats model. Phytomedicine 2022, 97, 153927. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, L.; Wang, M.; Jin, M.; Zhang, X.; Liu, D.; Wang, Z.; Yang, L.; Xu, X. Berberine inhibits intestinal epithelial barrier dysfunction in colon caused by peritoneal dialysis fluid by improving cell migration. J. Ethnopharmacol. 2020, 264, 113206. [Google Scholar] [CrossRef]
- Mori-Quiroz, L.M.; Hedrick, S.L.; Santos, A.R.D.L.; Clift, M.D. A Unified Strategy for the Syntheses of the Isoquinolinium Alkaloids Berberine, Coptisine, and Jatrorrhizine. Org. Lett. 2018, 20, 4281–4284. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Sun, Q.; Chen, G.; Sun, S.; Ma, X.; Qiu, H.; Liu, X.; Xu, L.; Liu, M. Jatrorrhizine Hydrochloride Suppresses RANKL-Induced Osteoclastogenesis and Protects against Wear Particle-Induced Osteolysis. Int. J. Mol. Sci. 2018, 19, 3698. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Sun, S.; Ma, X.; Cui, C.; Chen, G.; Liu, Z.; Li, H.; Liu, M. Jatrorrhizine Hydrochloride Suppresses Proliferation, Migration, and Secretion of Synoviocytes In Vitro and Ameliorates Rat Models of Rheumatoid Arthritis In Vivo. Int. J. Mol. Sci. 2018, 19, 1514. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Jing, M.; Wen, J.; Wei, S.; Li, H.; Li, X.; Ma, X.; Zhao, Y. Jatrorrhizine Alleviates DSS-Induced Ulcerative Colitis by Regulating the Intestinal Barrier Function and Inhibiting TLR4/MyD88/NF-κB Signaling Pathway. Evid. Based Complement Altern. Med. 2022, 2022, 3498310. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Barroso-Gutierrez, C.; Surprenant, A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol. 2008, 180, 7147–7157. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Xiao, Y.; Du, T.; Hu, H.; Ni, F.; Hu, K.; Hu, Q. Fusion Proteins CLD and CLDmut Demonstrate Potent and Broad Neutralizing Activity against HIV-1. Viruses 2022, 14, 1365. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, C.; Ding, H.; Wang, Z.; Liu, X.; Chai, Y.; Jiang, W.; Han, Y.; Zeng, H. Maf1 Ameliorates Sepsis-Associated Encephalopathy by Suppressing the NF-kB/NLRP3 Inflammasome Signaling Pathway. Front. Immunol. 2020, 11, 594071. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, L.; Cai, C.; Gong, X. Caffeine Inhibits NLRP3 Inflammasome Activation by Suppressing MAPK/NF-kappaB and A2aR Signaling in LPS-Induced THP-1 Macrophages. Int. J. Biol. Sci. 2019, 15, 1571–1581. [Google Scholar] [CrossRef]
- Bok, K.; Prikhodko, V.G.; Green, K.Y.; Sosnovtsev, S.V. Apoptosis in Murine Norovirus-Infected RAW264.7 Cells Is Associated with Downregulation of Survivin. J. Virol. 2009, 83, 3647–3656. [Google Scholar] [CrossRef]
- Boozari, M.; Hosseinzadeh, H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytotherapy Res. 2020, 35, 864–876. [Google Scholar] [CrossRef]
- Cao, J.; Forrest, J.C.; Zhang, X. A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Antivir. Res. 2015, 114, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Min, J.S.; Jang, M.S.; Lee, J.Y.; Shin, Y.S.; Park, C.M.; Song, J.H.; Kim, H.R.; Kim, S.; Jin, Y.-H.; et al. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells. Biomolecules 2019, 9, 696. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, M.; Chen, N.; Zhou, Y.; Chen, S.; Xu, W.; Gong, S.; Geng, L. Jatrorrhizine Suppresses Murine-Norovirus-Triggered N-GSDMD-Dependent Pyroptosis in RAW264.7 Macrophages. Vaccines 2023, 11, 164. https://doi.org/10.3390/vaccines11010164
Fu M, Chen N, Zhou Y, Chen S, Xu W, Gong S, Geng L. Jatrorrhizine Suppresses Murine-Norovirus-Triggered N-GSDMD-Dependent Pyroptosis in RAW264.7 Macrophages. Vaccines. 2023; 11(1):164. https://doi.org/10.3390/vaccines11010164
Chicago/Turabian StyleFu, Ming, Nini Chen, Yanhe Zhou, Sidong Chen, Wanfu Xu, Sitang Gong, and Lanlan Geng. 2023. "Jatrorrhizine Suppresses Murine-Norovirus-Triggered N-GSDMD-Dependent Pyroptosis in RAW264.7 Macrophages" Vaccines 11, no. 1: 164. https://doi.org/10.3390/vaccines11010164
APA StyleFu, M., Chen, N., Zhou, Y., Chen, S., Xu, W., Gong, S., & Geng, L. (2023). Jatrorrhizine Suppresses Murine-Norovirus-Triggered N-GSDMD-Dependent Pyroptosis in RAW264.7 Macrophages. Vaccines, 11(1), 164. https://doi.org/10.3390/vaccines11010164





