Abstract
The analysis of the effectiveness of booster shots compared with primary vaccination is extremely vital. This paper aimed to summarize the results of all available evidence studies on the effectiveness of booster vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Articles published up to 21 June 2022 were systematically searched through PubMed and EMBASE databases. The searched studies were independently assessed for quality using the Newcastle–Ottawa Scale. Results: Seven studies (nine datasets) met the criteria and were included in this study. The pooled results demonstrated a 71% (OR = 0.29, 95% CI = 0.17–0.48) reduction in SARS-CoV-2 infection rates among subjects who received a booster shot compared with those who did not receive a booster shot of coronavirus disease (COVID-19) vaccine. In addition, this analysis emphasized that during the period when the Delta variant was predominant, subjects who received the booster shot showed an 82% (OR = 0.18, 95% CI = 0.13–0.25) reduction in infection rates. Moreover, during the period of dominance of the Omicron variant, subjects who received the booster vaccination displayed a 47% (OR = 0.53, 95% CI = 0.35–0.81) reduction in infection rates. This finding confirmed that booster vaccination against the Omicron variant is significantly less effective than that against the Delta variant. In pandemic periods, correlations between the dominant variant and the efficacy of the COVID-19 vaccine booster should be considered when making vaccine booster plans.
1. Introduction
Coronavirus disease (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was declared a pandemic by the World Health Organization (WHO) on 11 March 2020 [1]. The pandemic has been persisting for more than two years since it started, seriously affecting the health of populations worldwide. However, no effective treatment for COVID-19 is available. Vaccination is considered an effective measure for the prevention and control of COVID-19 and plays a decisive role in the control of the global epidemic.
Although COVID-19 vaccination is fully implemented worldwide, SARS-CoV-2 remains rampant. As with other vaccines, the protective efficacy of the COVID-19 vaccine weakens over time after the completion of vaccination. A meta-analysis [2] showed that the vaccine effectiveness against SARS-CoV-2 infection declined by 21% from one month to six months after primary vaccination. Following booster vaccination, however, a higher level of antibodies will be produced in the recipient’s body, thus providing protection against the virus [3,4]. Therefore, booster doses are currently being administered in various countries to improve the overall immunization level of populations.
The most notable feature of the epidemic is the constant and rapid mutation of SARS-CoV-2. As of 13 August 2022, WHO has identified five variants of concern (VOCs), namely Alpha, identified on 18 December 2020; Beta, identified on 18 December 2020; Gamma, identified on 11 January 2021; Delta, identified on 11 May 2021; and Omicron, identified on 26 November 2021 [5]. Currently, the dominant strain shaping the global outbreak has changed from the Delta variant to Omicron. Compared with the previously prevalent Delta variant, Omicron has more key mutations, including up to 32 mutations in the spike protein, several of which may be associated with immune escape and higher infectiousness. Nevertheless, on the whole, the Omicron variant causes fewer symptoms and significantly fewer cases of severe hospitalization or death than the previous variants. The booster shot has been shown to be effective in current studies. According to the results of domestic and international studies [6,7,8], COVID-19 vaccination significantly reduces the rate of infection, hospitalization, and population mortality caused by SARS-CoV-2 variants and contributes significantly to the prevention and control of outbreaks. Vaccine effectiveness against symptomatic infections caused by omicron was approximately 50% in the first three months after the second dose of vaccine, but vaccine effectiveness against hospitalization and death due to Omicron infection remained high at over 70% after the second dose and above 90% after the booster dose. Overall, the current vaccination still provides protection against the variants.
The effectiveness of a third dose (booster) compared with the two doses in a real-world setting is unknown, and a meta-analysis of the effectiveness of real-world-based booster shots compared with primary vaccination is extremely important. Concerns regarding the possible lower vaccine effectiveness against the Omicron variant compared with that against the Delta variant have emerged. Whether a difference in vaccine effectiveness between the booster doses during the Omicron- and Delta-dominant period should be determined to address both questions. Therefore, this paper aimed to conduct a meta-analysis to summarize the results of all available evidence and studies on the effectiveness of booster vaccination compared with primary vaccination against SARS-CoV-2.
2. Materials and Methods
2.1. Search Strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. This study was prospectively registered on PROSPERO (n.CRD42022337356). Articles published up to 21 June 2022 were systematically searched through PubMed and EMBASE databases. The search keywords included (“SARS-CoV-2” or “COVID-19” or “2019-nCov”) AND (“Vaccine” or “Vaccination”) AND (“booster” or “booster shot” or “third dose” or “additional dose”) AND (“Efficacy” or “Effectiveness”).
2.2. Inclusion and Exclusion Criteria
The inclusion criteria included (1) studies with outcomes of COVID-19 infection; (2) cases defined as individuals who tested positive for SARS-CoV-2 as confirmed by polymerase chain reaction, (3) observational studies, (4) studies published in English; (5) studies that excluded patients who had been infected with COVID-19 prior to their first vaccination.
The exclusion criteria included (1) articles for which the full text could not be found; (2) systematic reviews, commentaries, case reports, letters, and guidelines; (3) studies of non-human subjects, (4) articles for which data could not be extracted; (5) studies considering only hospitalization, serious illness, death and other serious outcomes without considering SARS-CoV-2 infection.
2.3. Data Extraction and Study Quality Assessment
Data extraction and quality assessment were carried out independently by two authors, with disagreements identified by a third author. The following data were extracted from the included articles: name of first author, country, study design, vaccine, dominant variant, age range, and the number of booster and non-booster vaccinations in cases and controls. Table 1 summarizes the extracted data.

Table 1.
Characteristics of the included studies.
The included studies were independently assessed for quality using the Newcastle–Ottawa Scale (NOS), which was designed for observational and non-randomized studies. Scores of 0–3, 4–6, and over 7 stars were considered of low, moderate, and high quality, respectively.
2.4. Data Synthesis and Analysis
The I2 statistic was applied to assess the heterogeneity between studies. If I2 > 50%, which indicates a high heterogeneity, the random-effects model was recommended. Meanwhile, if I2 < 50%, the fixed effects model was normally employed for the analysis. Pooled odds ratio (OR) and 95% confidence interval (95% CI) estimates were calculated to determine the association between COVID-19 vaccine booster shots and infection. Dominant variants were considered for subgroup analysis to further identify the sources of heterogeneity. A two-tailed p < 0.05 was deemed to be statistically significant. Statistical analyses were conducted by STATA/SE version 16.0 (StataCorp, College Station, TX, USA).
3. Results
3.1. Literature Retrieval and Literature Quality Evaluation
A total of 904 records were identified from the literature search. After removing duplicates, 578 articles were identified and subsequently screened by title, abstract, and full text. After excluding the ineligible studies from the title or abstract screening, 35 studies were reviewed in full text. As a result, seven studies (nine datasets) met the criteria and were included in this study. Studies reporting the efficacy of different dominant variants were treated as a separate dataset in the meta-analysis (Figure 1).
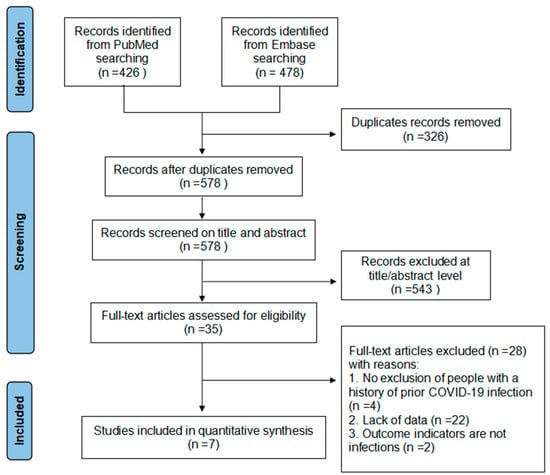
Figure 1.
Flow chart of literature search and screening.
Table 1 shows details on the included studies (n = 7). Most of the included research (n = 4) was conducted in the United States. The meta-analysis included 5,510,606 subjects, of whom 867,361 were infected patients. Of the seven studies, one was a retrospective cohort research study, and the rest were test-negative design studies. All of these studies were published in 2022, and most were conducted on mRNA vaccines. In addition, the quality of all included studies was assessed with the NOS. Four and three studies showed moderate and high quality, respectively.
3.2. Effectiveness of COVID-19 Vaccine Booster versus Non-Booster Doses
Based on the degree of heterogeneity of the included studies (I2 > 50%), the correlation between COVID-19 vaccine booster shots and infections was analyzed using a random-effects model. The pooled results revealed that the COVID-19 vaccine booster was a protective factor against infection relative to the non-booster doses (OR = 0.29, 95% CI = 0.17–0.48). A subgroup analysis was subsequently conducted based on the dominant variant strains: the Delta- and Omicron-dominant phase groups (Figure 2). This analysis emphasized that booster vaccination during the Delta variant-dominant period was more effective in preventing COVID-19 disease compared with the Omicron variant-dominant period (p < 0.001). During the dominance period of the Delta variant, the booster-vaccinated subjects demonstrated a significant reduction in infection rates compared with non-booster-vaccinated subjects (OR = 0.18, 95% CI = 0.13–0.25). During the period of dominance of the Omicron variant, booster-vaccinated subjects also displayed a reduction in infection rates compared with those who did not receive the booster vaccine (OR = 0.53, 95% CI 0.35–0.81). This finding supported the effect of different variant strains on the effectiveness of booster vaccination.
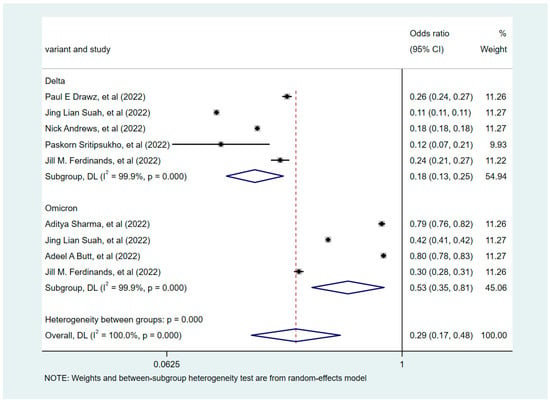
Figure 2.
Forest plots showing pooled risk ratio of COVID-19 vaccine booster shots associated with infections.
3.3. Publication Bias and Sensitivity Analysis
The use of funnel plots and Egger’s test for publication bias assessment was not feasible on account of the small number of studies included in the pooled analysis (n < 10). Sensitivity analysis by excluding one study at a time had no significant effect on the results (Figure 3, Figure 4 and Figure 5).
Figure 3.
Sensitivity analysis (all publications) [9,10,11,12,13,14,15].
Figure 4.
Sensitivity analysis (only Delta) [9,11,13,14,15].
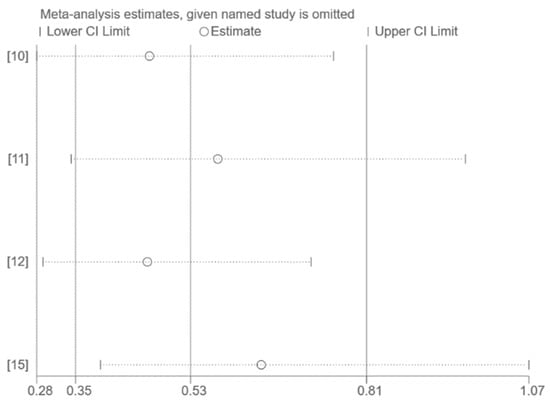
Figure 5.
Sensitivity analysis (only Omicron) [10,11,12,15].
4. Discussion
The COVID-19 vaccine booster provides a further enhancement or restores protection that may have waned over time after the initial series of vaccinations. Furthermore, the booster is one of the most essential means of defending individuals from serious illness or death attributed to COVID-19 [16,17]. In addition, the booster dose is recommended for anyone eligible for vaccination given the presence of the more infectious variants of Delta and Omicron. A systematic review [18] summarized studies associated with the efficacy of the booster dose against the Omicron variant, and all the results reviewed supported the evidence for the efficacy of the booster dose vaccine against SARS-CoV-2 variants, including Omicron.
Vaccine effectiveness is an indicator that measures the effectiveness of immunization in protecting people from consequences, such as infections, symptomatic illnesses, hospitalization, and death. This meta-analysis focused on examining the efficacy and effectiveness of the COVID-19 vaccine booster compared with non-booster doses in reducing infection rates to provide strong evidence for health policy makers in response to ongoing pandemics. Despite the considerable heterogeneity between studies, our findings provide evidence that the booster was less effective against the Omicron variant strain than the Delta variant. Overall, booster vaccination against COVID-19 was effective in reducing the number of COVID-19 cases.
This meta-analysis is the first to report the pooled data on the effectiveness of the COVID-19 vaccine booster shot in comparison with the non-booster shot. Of the included studies, apart from one research study that did not specify an age range, all covered young people and older people aged 18 years and over. Furthermore, the sample sizes of the individual studies ranged from several thousands to several millions. One study was retrospective cohort research, and the other six applied a test-negative design, which is commonly used in vaccine efficacy studies. The results of the studies included in the meta-analysis were consistent. The pooled results demonstrated a 71% (OR = 0.29, 95% CI = 0.17–0.48) reduction in SARS-CoV-2 infection rates among subjects who received a booster shot compared with those who did not receive a booster shot of COVID-19 vaccine. Moreover, this analysis emphasized that during the period when the Delta variant was predominant, subjects who received the booster shot showed an 82% (OR = 0.18, 95% CI = 0.13–0.25) reduction in infection rates. In addition, during the period of dominance of the Omicron variant, subjects who received the booster vaccination displayed a 47% (OR = 0.53, 95% CI= 0.35–0.81) reduction in infection rates. This finding supported the effect of the different variants on the effectiveness of booster vaccination. Booster vaccination during the Delta variant-dominant period was more effective in preventing COVID-19 disease than the booster vaccination during the dominant period of the Omicron variant (I2 = 100.0%, p < 0.001). This result confirmed that booster vaccination against the Omicron variant was significantly less effective than that against the Delta variant. Compared with the Delta variant, the Omicron variant has a larger area of variation, and its “face” is largely different and more infectious than the original strain [19,20,21]. To better control the pandemic, experts must fundamentally redesign the booster vaccine to specifically target the Omicron variant in the new context. At present, a number of companies have developed new generations of COVID-19 vaccines against the Omicron antigen and are carrying out clinical trials.
The present meta-analysis had several limitations. In particular, the analysis of between-study heterogeneity and subgroup analyses failed to reduce the overall heterogeneity. The use of funnel plots and Egger’s test for publication bias assessment was not feasible on account of the small number of studies included in the pooled analysis (n < 10). In addition, given the sparse literature, no distinction was made between different age groups, and data on the effectiveness in preventing severe infections were not available. The potential differences in effectiveness of preventing infection or severe infections between populations could not be compared. Focus on the discrepancies between homologous and heterologous reinforcements was also lacking. Also, in the absence of data, whether there were potential differences associated with the type of vaccine used for the first two doses and for the booster dose was not analyzed. Furthermore, the level of epidemiological dynamics and vaccine policies in each country could also affect the assessment of the effectiveness of booster doses. The major weakness was that the booster shot was administered later compared with the non-booster shot, and the effect of time on the effectiveness of the booster shot was not ruled out. Similarly, in comparison with the Delta strain, the Omicron strain emerged later with an accompanying tendency for the antibodies to fade away. Duration is the most critical confounding factor. Although the time confounder cannot be ruled out, public vaccination did not take place at the same time, and some people were vaccinated after the Omicron epidemic. Therefore, the time of infection with the Delta strain after vaccination and the time of infection with Omicron after vaccination were not totally incomparable. It is merely that information on time was not available due to data limitations. In the same studies included in the meta-analysis, the time of cases and controls were comparable, and hence they were somewhat comparable in the time of cases and controls across the meta. Therefore, despite the fact that time was the most critical confounding factor, the conclusion of this article could provide some suggestive points. More detailed information on the COVID-19 vaccine booster and all the potential factors that can affect the outcome are worthy of attention.
In summary, preliminary data on the COVID-19 vaccine booster suggested that potential dominant strain differences occur in terms of the efficacy. In pandemic periods, correlations between the dominant variant and the efficacy of the COVID-19 vaccine booster should be considered when making vaccine booster plans.
5. Conclusions
This meta-analysis summarized the efficacy of the COVID-19 vaccine booster versus non-booster in reducing infection rates, and the findings supported the comparable effectiveness of the booster. In addition, the booster was less effective against the Omicron variant than the Delta variant. Further studies on the effectiveness of real-world vaccine booster shots are encouraged to explore other sources of heterogeneity that may influence the efficacy of meta-analysis results.
Author Contributions
Conceptualization, D.Z.; Data curation, Y.Z. and S.L.; Formal analysis, Y.Z.; Funding acquisition, D.Z.; Methodology, Y.Z. and S.L.; Writing—original draft, Y.Z. All listed authors meet the ICMJE criteria and all who meet the four criteria are identified as authors. We attest that all authors contributed significantly to the creation of this manuscript, each having fulfilled criteria as established by the ICMJE. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Guangdong Natural Science Foundation Project [grant numbers 2022A1515011012]; Foshan Scientific and Technological Key Project for COVID-19 [grant numbers 2020001000430]; The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the reported results are available on request to the Authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Director-General’s Opening Remarks at the Mission Briefing on COVID-19—12 March 2020[EB/OL]. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-mission-briefing-on-covid-19---12-march-2020 (accessed on 14 July 2022).
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, N.; Li, X.; Peng, Q.; Yiu, C.K.; Huang, H.; Yang, D.; Du, Z.; Kwok, H.Y.; Au, K.K.; et al. Three-Dose Vaccination-induced Immune Responses Protect against SARS-CoV-2 Omicron-BA.2. bioRxiv 2022. [Google Scholar] [CrossRef]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard [EB/OL]. Available online: https://covid19.who.int/ (accessed on 25 July 2022).
- Saban, M.; Myers, V.; Wilf-Miron, R. Changes in infectivity, severity and vaccine effectiveness against delta COVID-19 variant ten months into the vaccination program: The Israeli case. Prev. Med. 2022, 154, 106890. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Coyle, P.; Tang, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Hasan, M.R.; Al-Kanaani, Z.; et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA.1 and BA.2 subvariants in Qatar. Nat. Commun. 2022, 13, 3082. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Drawz, P.E.; DeSilva, M.; Bodurtha, P.; Vazquez Benitez, G.; Murray, A.; Chamberlain, A.M.; Dudley, R.A.; Waring, S.; Kharbanda, A.B.; Murphy, D.; et al. Effectiveness of BNT162b2 and mRNA-1273 Second Doses and Boosters for SARS-CoV-2 infection and SARS-CoV-2 Related Hospitalizations: A Statewide Report from the Minnesota Electronic Health Record Consortium. Clin. Infect Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Oda, G.; Holodniy, M. Effectiveness of mRNA-based vaccines during the emergence of SARS-CoV-2 Omicron variant. Clin. Infect Dis. 2022, ciac325. [Google Scholar] [CrossRef] [PubMed]
- Suah, J.L.; Tng, B.H.; Tok, P.S.; Husin, M.; Thevananthan, T.; Peariasamy, K.M.; Sivasampu, S. Real-world effectiveness of homologous and heterologous BNT162b2, CoronaVac, and AZD1222 booster vaccination against Delta and Omicron SARS-CoV-2 infection. Emerg. Microbes Infect. 2022, 11, 1343–1345. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Talisa, V.B.; Shaikh, O.S.; Omer, S.B.; Mayr, F.B. Relative Vaccine Effectiveness of a SARS-CoV-2 mRNA Vaccine Booster Dose Against the Omicron Variant. Clin. Infect. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Sritipsukho, P.; Khawcharoenporn, T.; Siribumrungwong, B.; Damronglerd, P.; Suwantarat, N.; Satdhabudha, A.; Chaiyakulsil, C.; Sinlapamongkolkul, P.; Tangsathapornpong, A.; Bunjoungmanee, P.; et al. Comparing real-life effectiveness of various COVID-19 vaccine regimens during the delta variant-dominant pandemic: A test-negative case-control study. Emerg. Microbes Infect. 2022, 11, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning 2-Dose and 3-Dose Effectiveness of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 255–263. [Google Scholar]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 Vaccine Booster and Mortality Due to Covid-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Björk, J.; Bonander, C.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Inghammar, M.; Kahn, F. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 Omicron BA.1 and BA.2 subvariants—Surveillance results from southern Sweden, December 2021 to March 2022. Eurosurveillance 2022, 27, 2200322. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Sohan, M.; Hossain, M.J.; Islam, M.R. The SARS-CoV-2 Omicron (B.1.1.529) variant and effectiveness of existing vaccines: What we know so far. J. Med. Virol. 2022, 94, 1796–1798. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Hong, H.; Wang, S.; Ma, L.; Liu, C.; Bai, Y.; Adam, D.C.; Tian, L.; Wang, L.; Lau, E.H.; et al. Reproduction Number of the Omicron Variant Triples That of the Delta Variant. Viruses 2022, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Duong, B.V.; Larpruenrudee, P.; Fang, T.; Hossain, S.I.; Saha, S.C.; Gu, Y.; Islam, M.S. Is the SARS CoV-2 Omicron Variant Deadlier and More Transmissible Than Delta Variant? Int. J. Environ. Res. Public Health 2022, 19, 4586. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).