Safety and Immunogenicity of the BBIBP-CorV Vaccine in Adolescents Aged 12 to 17 Years in the Thai Population: An Immunobridging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design and Participants
2.2. Interventions
2.3. Safety
2.4. Immunogenicity
2.5. Outcomes
2.6. Sample Size
2.7. Statistical Methods
3. Results
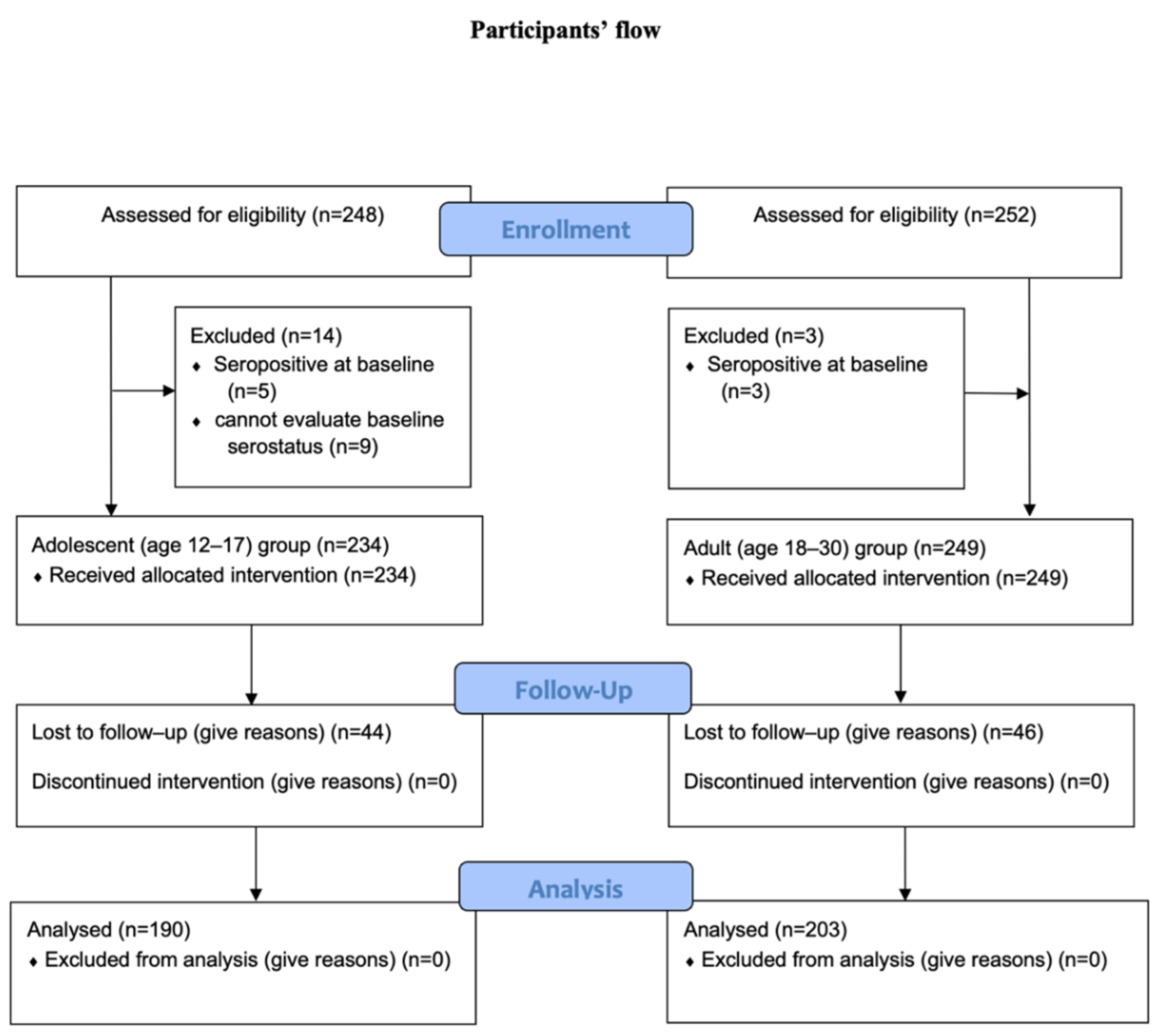
3.1. Participants
3.2. Safety
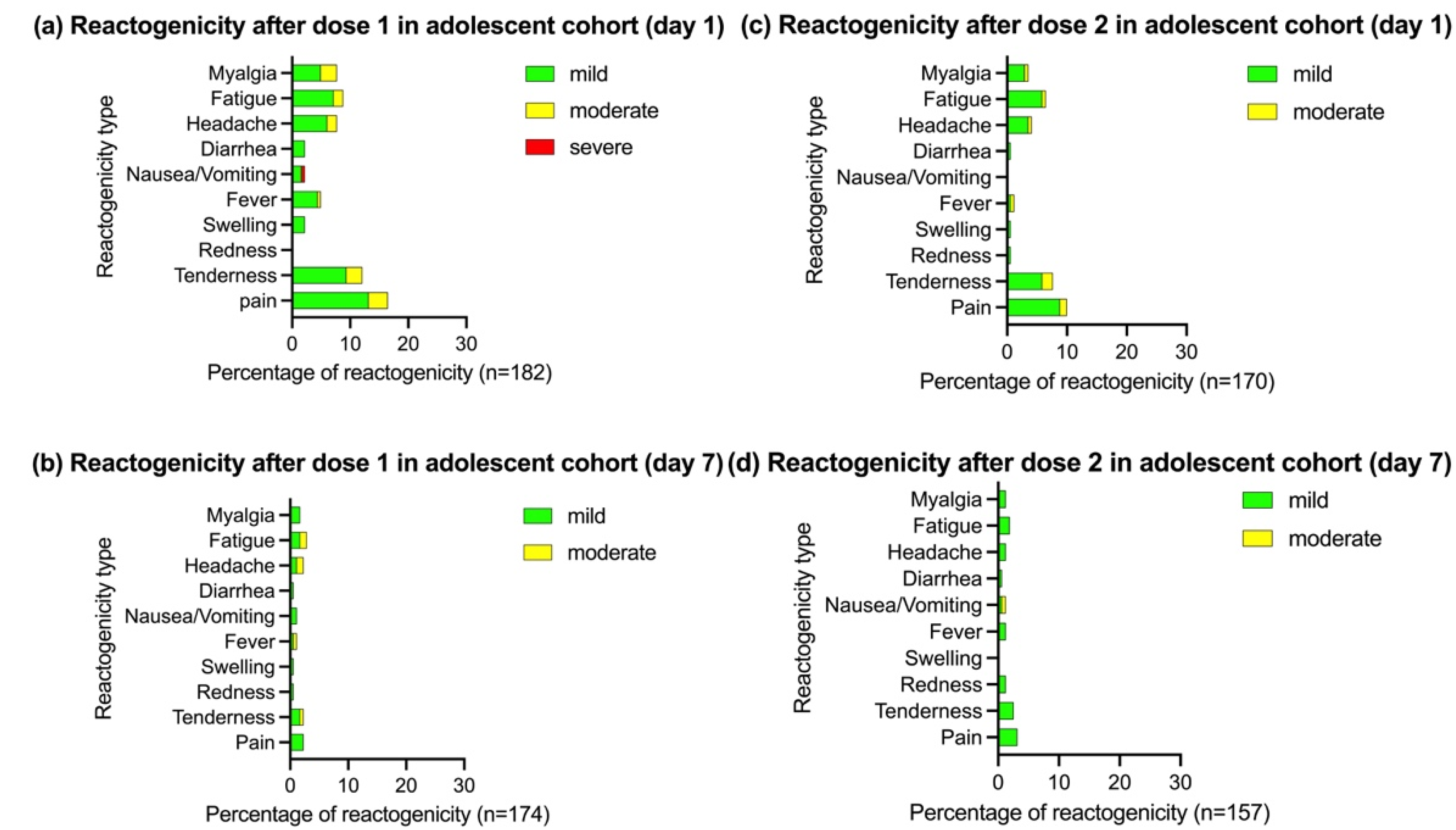
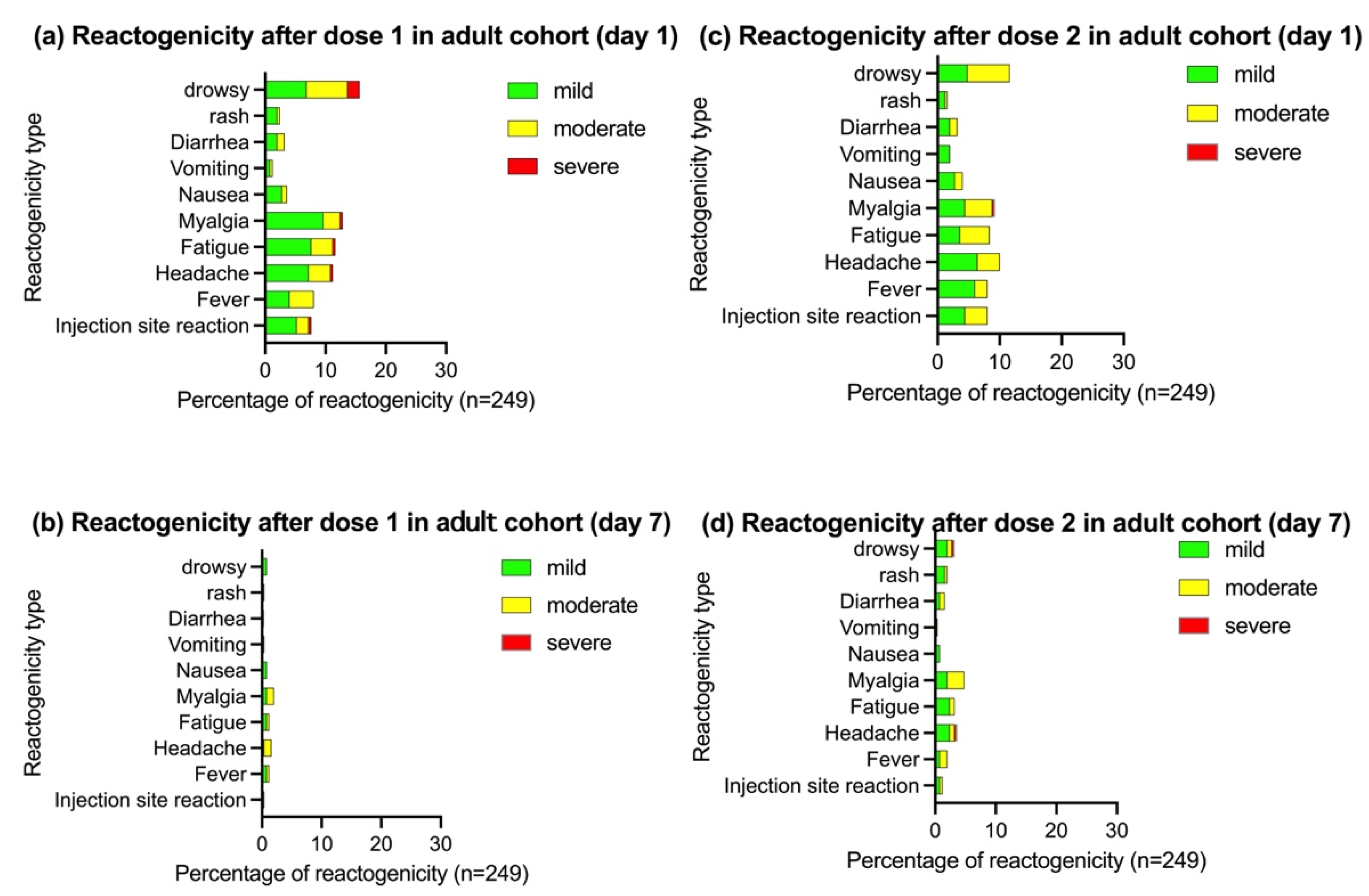
3.2.1. Reactogenicity
3.2.2. Adverse Events
3.3. Immunogenicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castagnoli, R.; Votto, M.; Licari, A.; Brambilla, I.; Bruno, R.; Perlini, S.; Rovida, F.; Baldanti, F.; Marseglia, G.L. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. 2020, 174, 882–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, L.E.; Chevinsky, J.R.; Kompaniyets, L.; Lavery, A.M.; Kimball, A.; Boehmer, T.K.; Goodman, A.B. Characteristics and Disease Severity of US Children and Adolescents Diagnosed With COVID-19. JAMA Netw. Open. 2021, 4, e215298. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Recher, M.; Hubert, H.; Javouhey, E.; Fléchelles, O.; Leteurtre, S.; Angoulvant, F. Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA 2022, 327, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and Outcomes of US Children and Adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) Compared with Severe Acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Kwok, H.F. Review of COVID-19 vaccine clinical trials—A puzzle with missing pieces. Int. J. Biol. Sci. 2021, 17, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (accessed on 22 April 2022).
- World Health Organization. Annexes to the Interim Recommendations for Use of the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm. 2022. Available online: https://apps.who.int/iris/rest/bitstreams/1413987/retrieve (accessed on 27 April 2022).
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e9. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- AlHosani, F.I.; Stanciole, A.E.; Aden, B.; Timoshkin, A.; Najim, O.; Zaher, W.A.; AlDhaheri, F.A.; Al Mazrouie, S.; Rizvi, T.A.; Mustafa, F. Impact of the Sinopharm’s BBIBP-CorV vaccine in preventing hospital admissions and death in infected vaccinees: Results from a retrospective study in the emirate of Abu Dhabi, United Arab Emirates (UAE). Vaccine 2022, 40, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Vaccines WHO EUL Issued. 2021. Available online: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued (accessed on 24 April 2022).
- World Health Organization. Evidence Assessment: Sinopharm/BBIBP COVID-19 Vaccine. 2021. Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/2_sage29apr2021_critical-evidence_sinopharm.pdf (accessed on 5 May 2022).
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and Immunogenicity of an Inactivated COVID-19 Vaccine, BBIBP-Corv, in People younger Than 18 Years: A Randomised, Double-Blind, Controlled, Phase 1/2 Trial. Lancet Infect. Dis. 2022, 22, 196–208. [Google Scholar] [CrossRef]
- Kristiansen, P.A.; Page, M.; Bernasconi, V.; Mattiuzzo, G.; Dull, P.; Makar, K.; Knezevic, I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 2021, 397, 1347–1348. [Google Scholar] [CrossRef]
- Rus, K.R.; Korva, M.; Knap, N.; Županc, T.A.; Poljak, M. Performance of the Rapid High-Throughput Automated Electrochemiluminescence Immunoassay Targeting Total Antibodies to the SARS-CoV-2 Spike Protein Receptor Binding Domain in Comparison to the Neutralization Assay. J. Clin. Virol. 2021, 139, 104820. [Google Scholar] [CrossRef]
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 2241–2251. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Irby, K.; Walker, T.C.; Schwartz, S.P.; Pannaraj, P.S.; et al. Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization Among Persons Aged 12–18 Years—United States, June-September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1483–1488. [Google Scholar] [CrossRef]
- De Beer, P.A.M.; Van den Abbeele, K. Inviting Adolescents Aged 12–17 for COVID-19 Vaccination: The Need for Patience. BMJ 2021, 374, n2172. [Google Scholar] [CrossRef] [PubMed]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis After BNT162b2 mRNA Vaccine Against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis After COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.F.; Ammirati, E.; Adler, E.D.; Cooper, L.T., Jr.; Hong, K.N.; Saponara, G.; Couri, D.; Cereda, A.; Procopio, A.; Cavalotti, C.; et al. Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation 2021, 144, 506–508. [Google Scholar] [CrossRef]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]

| Adolescent Cohort 12–17 Years | Adult Cohort 18–30 Years | |
|---|---|---|
| Participants in safety analysis | 234 | 249 |
| Female sex | 108 (46.2) | 118 (47.4) |
| Age, years | 14 (13–16) | 25 (22–28) |
| Participants in immunogenicity analysis | 190 | 203 |
| Female sex | 90 (47.4) | 97 (47.8) |
| Age, years | 14 (13–16) | 25 (23–28) |
| Underlying disease | 0 | 0 |
| Adverse Event | Adolescents 12–17 Years Old (n = 234) | Adults 18–30 Years Old (n = 249) |
|---|---|---|
| n (%) | n (%) | |
| Any event | 8 (3.4%) | 5 (2.0%) |
| Related | 0 (0%) | 2 (0.8%) |
| Severe | 0 (0%) | 0 (0%) |
| Life-threatening | 0 (0%) | 0 (0%) |
| Any serious adverse event | 0 (0%) | 0 (0%) |
| Related | ||
| Severe | ||
| Life-threatening | ||
| Any adverse event leading to discontinuation | 0 (0%) | 0 (0%) |
| Related | ||
| Severe | ||
| Life-threatening | ||
| Death | 0 (0%) | 0 (0%) |
| 12–17 Years | 18–30 Years | |
|---|---|---|
| Number of participants a | 190 | 203 |
| GMC (95% CI), BAU/mL | 102.9 (91.0–116.4) | 36.9 (30.9–44.0) |
| GMR (95% CI) | 2.79 (2.25–3.46) * | reference |
| Number of participants b | 198 | 203 |
| GMC (95%CI) (BAU/mL) | 102.49 (90.81–115.68) | 36.9 (30.9–44.0) |
| GMR (95%CI) | 2.78 (2.25–3.44) * | reference |
| 12–14 Years | 15–17 Years | 18–30 Years | |
|---|---|---|---|
| Number of participants a | 99 | 91 | 203 |
| GMC (95% CI), BAU/mL | 124.2 (105.8–145.7) | 83.9 (69.8–100.7) | 36.9 (30.9–44.0) |
| GMR (95% CI) | 3.37 (2.59–4.38) * | 2.27 (1.74–2.98) * | reference |
| Number of participants b | 103 | 95 | 203 |
| GMC (95%CI) (BAU/mL) | 125.3 (107.2–146.7) | 82.4 (68.9–98.5) | 36.9 (30.9–44.0) |
| GMR (95%CI) | 3.4 (2.53–4.56) * | 2.23 (1.65–3.02) * | reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawinprai, K.; Siripongboonsitti, T.; Porntharukchareon, T.; Vanichsetakul, P.; Thonginnetra, S.; Niemsorn, K.; Promsena, P.; Tandhansakul, M.; Kasemlawan, N.; Ruangkijpaisal, N.; et al. Safety and Immunogenicity of the BBIBP-CorV Vaccine in Adolescents Aged 12 to 17 Years in the Thai Population: An Immunobridging Study. Vaccines 2022, 10, 807. https://doi.org/10.3390/vaccines10050807
Tawinprai K, Siripongboonsitti T, Porntharukchareon T, Vanichsetakul P, Thonginnetra S, Niemsorn K, Promsena P, Tandhansakul M, Kasemlawan N, Ruangkijpaisal N, et al. Safety and Immunogenicity of the BBIBP-CorV Vaccine in Adolescents Aged 12 to 17 Years in the Thai Population: An Immunobridging Study. Vaccines. 2022; 10(5):807. https://doi.org/10.3390/vaccines10050807
Chicago/Turabian StyleTawinprai, Kriangkrai, Taweegrit Siripongboonsitti, Thachanun Porntharukchareon, Preeda Vanichsetakul, Saraiorn Thonginnetra, Krongkwan Niemsorn, Pathariya Promsena, Manunya Tandhansakul, Naruporn Kasemlawan, Natthanan Ruangkijpaisal, and et al. 2022. "Safety and Immunogenicity of the BBIBP-CorV Vaccine in Adolescents Aged 12 to 17 Years in the Thai Population: An Immunobridging Study" Vaccines 10, no. 5: 807. https://doi.org/10.3390/vaccines10050807
APA StyleTawinprai, K., Siripongboonsitti, T., Porntharukchareon, T., Vanichsetakul, P., Thonginnetra, S., Niemsorn, K., Promsena, P., Tandhansakul, M., Kasemlawan, N., Ruangkijpaisal, N., Banomyong, N., Phattraprayoon, N., Ungtrakul, T., Wittayasak, K., Thonwirak, N., Soonklang, K., Sornsamdang, G., & Mahanonda, N. (2022). Safety and Immunogenicity of the BBIBP-CorV Vaccine in Adolescents Aged 12 to 17 Years in the Thai Population: An Immunobridging Study. Vaccines, 10(5), 807. https://doi.org/10.3390/vaccines10050807






