Abstract
Polyporus dermoporus mushroom, native to Brazil, is produced under natural conditions in the unexplored reserve of Mata da Estrela-Rio Grande do Norte-RN. These mushrooms were delipidated with chloroform:methanol (2:1 v/v), extracted with water at 100 °C, and fractionated with ethanol (one and three volumes) and then centrifuged. The ethanol precipitation showed a high total sugar level of 64.8% and 1% of protein. This precipitate contained a high glucan level, characterized by chemical methods and by NMR of 13C and 1H and spectroscopy. The 13C NMR spectrum of these mushroom extracts showed the presence of β-glucose by a signal at 103.25 ppm. Studies with these glucans were made to elucidate antioxidant and anti-inflammatory activities. This extract of glucans inhibited the lipid peroxidation (42.9%) and superoxide radicals (83.3%) at 67 μg/mL. However, the inhibition of hydroxyl radical by the extract of this mushroom was 96% at 267 μg/mL. The action of this extract on induced pleurisy showed a 92.5% and 68.7% reduction in polymorphonuclears cells and nitric oxide, respectively, at 30 mg/kg. The glucans reduced the croton oil-induced ear edema by 65.6% at 30 mg/kg.
1. Introduction
Fungal polysaccharides have recently received considerable attention, due to their potential use in a wide variety of industries, including cosmetics, pharmaceuticals and food. [1,2,3]. Several polysaccharides and polysaccharide-protein complexes have been isolated from fungi (mushrooms) and are being used as a source of therapeutic agents [2,3]. Many, if not all, basidiomycete mushrooms contain biologically active polysaccharides in fruit bodies, culture mycelium and culture broth [1,4].
Oxidation is essential to many living organisms for the production of energy to fuel biological processes. Free radicals are produced in normal and/or pathological cell metabolism [5]. However, the uncontrolled production of oxygen-derived free radicals is involved in the onset of many diseases, such as cancer, rheumatoid arthritis, cirrhosis and arteriosclerosis, as well as in degenerative processes associated with aging [5,6]. It has long been recognized that many naturally occurring substances in plants and fungi have antioxidant activities. Several species of mushroom contain a great variety of molecules, scavenger-free radicals or reactive oxygen species, such as polysaccharides and phenolic compounds [6,7,8,9]. This explains why mushrooms have recently become attractive as nutritionally beneficial foods and as a source material for the development of drugs. Several types of inflammatory tissue injury are mediated by reactive oxygen metabolites. The most likely sources of these oxidizing agents are the phagocytic leukocytes (e.g., neutrophils, monocytes, macrophages and eosinophils) that invade the tissue. It is becoming increasingly apparent that in addition to promoting cytotoxicity, reactive oxygen metabolites may also initiate and/or increase inflammation [6,10].
Recent studies have shown that a glucan-protein complex from the fungus Geastrum saccatum has strong anti-inflammatory and antioxidant activity [6,7]. Studies with polysaccharides from the mushroom Polyporus albicans showed that these compounds have powerful stimulating effects on murine lymphocyte proliferation induced by concanavalin A or lipopolysaccharides and that its branches are extremely important for enhancement expression [8].
In this work, chemical features of a β-glucan-protein complex from P. dermoporus, as well as its anti-inflammatory antioxidant and cytotoxic activities were analyzed.
2. Experimental Section
2.1. Mushroom
Fruiting bodies of fungi Polyporus dermoporus Pers. (Polyporaceae) were obtained from a private reserve, Mata da Estrela, of the Atlantic Forest in the state of Rio Grande do Norte (06°22′25″ S, 35°01′24″ W) Brazil in July, 2009. The mushroom was identified by Prof. Iuri Goulart Baseia, Department of Botany, Ecology and Zoology of Universidade Federal do Rio Grande do Norte (UFRN), Natal, Brazil. A voucher specimen was deposited in the herbarium of this department.
2.2. Materials
Thiobarbituric acid, D2O, sodium salicylate, nitroblue tetrazolium, phenazine methosulfate, EDTA, H2O2, indomethacin, croton oil, NADH, Ficoll isopaque, carrageenan, Folin Ciocalteu, gallic acid, ketamine, xylazine and mercaptoethanol were purchased from Sigma Aldrich (St. Louis, MO, USA). RPMI 1640 medium was purchased from Gibco (Grand Island, NY, USA). All other chemicals used were of analytical grade.
2.3. Animals
Male BALBc mice (about 25 g) and male Wistar rats (150–180 g) were housed in temperature-controlled rooms (22–23 °C) until use. Animals were given ad libitum access to food and water. Each experimental group contained 6 animals. The mice were used in the croton oil-induced ear edema test and the rat in pleurisy. All of the experiments were performed in accordance with the guidelines and regulations set forth by the Ethics Committee, Centro de Biociencias, UFRN.
2.4. Extract Preparation
Fruiting bodies of Polyporus dermoporus were dried at 40 °C and powdered. The resulting powder was delipidated with ether and placed in solution in a proportion of 10 g of powder to 100 mL of distilled water at 100 °C for 3 h. Composition analysis of this extract was made [11]. Then, 1 volume of ethanol (50 mL) was added to the supernatant (50 mL). Subsequently, the extract was centrifuged, and 3 volumes of ethanol (150 mL) were added. The precipitate, rich in polysaccharides, was evaporated in a vacuum to obtain a solid extract.
2.5. Chemical Analysis
The total protein of the extract was determined by Spector’s method [12]. Total sugar content was determined by the phenol sulfuric acid colorimetric method for the determination of total sugars (the Dubois method) [13].
2.6. 13C nuclear Magnetic Resonance Spectroscopy (NMR)
13C and 1H NMR spectroscopy experiments were conducted using a 200-MHz Varian Mercury 200 Magneto Oxford spectrometer at 60 °C, and the sample was dissolved in deuterated water (D2O).
2.7. In Vitro Antioxidant Tests
2.7.1. Superoxide Radicals
Superoxide radicals were generated in 3 mL Tris-HCl buffer (16 mM, pH 8.0), which contained 78 μM NADH (reduced form), 50 μM nitroblue tetrazolium, 10 μM phenazine methosulfate [14] and varying concentrations of polysaccharides (67–267 μg/mL). The color reaction of superoxide radical and nitroblue tetrazolium was detected by monitoring the absorbance at 560 nm. In the essential control, NADH was substituted for Tris-HCl buffer [14]. The percentage of scavenging activity on superoxide radicals was determined by this formula: (%) = [(Apositive control − Asample)/Apositive control − Anegative control)] × 100; where Apositive control is positive control absorbance (all reagents without sample), Asample is sample absorbance and Anegative is negative control absorbance (NADH absence).
2.7.2. Hydroxyl Radical Assay
Hydroxyl radicals were generated by an innovation of the Smirnoff and Cumbes 1989 method [15] in sodium phosphate buffer (150 mM, pH 7.4), which contained 0.15 mM FeSO4-EDTA, 2 mM sodium salicylate, 6 mM H2O2 and varying concentrations of polysaccharides (67–267 μg/mL). In the essential control, sodium phosphate buffer replaced H2O2. The solutions were incubated at 37 °C for 1 h and detected by monitoring the absorbance at 510 nm. The inhibition rate (%) = 1 − (Abs1/Abs2) × 100%, where Abs1 is the absorbance obtained from the reaction with the samples and Abs2 is the absorbance of the reaction in which distilled water replaced hydrogen peroxide.
2.7.3. Lipid Peroxides Radical Scavenging Capacity
Liver microsomes were prepared from male Wistar rats according to the method proposed by [16]. The protein content of the microsomes was measured by Spector’s method [12]. The product of microsomal lipid peroxidation was malondialdehyde. Microsomes (200–300 μg/mL) were incubated at 37 °C for 1 h with varying amounts of polysaccharides (67–267 μg/mL), 10 μM FeSO4 and 0.1 mM ascorbic acid in 1.0 mL potassium phosphate buffer solution (0.2 M, pH 7.4). The reaction was stopped by 20% trichloroacetic acid (1.0 mL) and 0.67% 2-thiobarbituric acid (1.5 mL) in succession, and the solution was then heated at 100 °C for 15 min. After precipitation, the proteins were removed by centrifugation. The color reaction of the malondialdehyde-thiobarbituric acid complex was detected by monitoring the absorbance at 532 nm [16]. The essential control did not contain FeSO4 or ascorbic acid. The percentage of antioxidant activity of the samples was evaluated according to the following formula: inhibition rate (%) = (A0 − A)/(A0 − Ae) × 100%; where A0 is the absorbance of the free radical generation system, A is the absorbance of the test sample and Ae is the absorbance of the essential control.
2.8. Assessment Phenolic Compounds
Phenolic compounds extract from P. dermoporus were measured by the Folin Ciocalteu method [17] with few modifications using 0.5 mL ethanol, 2.5 mL distilled water, 0.25 mL Folin Ciocalteu reagent, 0.5 mL sodium carbonate and 0.5 mL of polysaccharides (1 mg/mL). Absorbance was determined at 765 nm against the blank, and a gallic acid calibration curve (0–500 mg/mL) was constructed and used to determine the total phenolic content of the samples, which were expressed as gallic acid equivalents (GAE).
2.9. Croton Oil-Induced Ear Edema Test
The croton oil-induced ear edema test was performed as previously described [18,19]. The animals were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (3:1, i.p.). A total of 20 μL (0.4 μg of croton oil in acetone) was applied to the inner surface of the right ear of each mouse (subcutaneous). After 24 h, the extract, rich in polysaccharide, was intravenously administered at doses of 10, 30 and 50 mg/kg by body weight. The left ear remained untreated. Control animals only received the irritant. The animals were killed by cervical dislocation 24 h after the treatment was removed from both the treated and untreated ear. The difference in the thickness between the two plugs before and after the treatment was measured to determine the edematous response.
2.10. Extract Action in Carrageenan-Induced Pleurisy
The animals were anesthetized intraperitoneally with 20 mg/mL solution of xylazine hydrochloride and a 50 mg/mL solution of ketamine hydrochloride at a 1:2 proportion of 100 mg/kg/weight. The animals had been previously treated with 10, 30 and 50 mg/kg i.p. by body weight of the extract, with the exception of the control group animals (carrageenan (control+) and saline (control−)). After 30 min, the animals were submitted to a skin incision at the sixth intercostal space level. The muscles were dissected, and 0.2 mL of saline containing 1% (w/v) carrageenan were injected into the pleural cavity. The skin incision was closed with a suture, and the animals were allowed to recover. The animals were killed 4 h after carrageenan injection. The pleural cavity was carefully opened and washed with 2 mL of saline solution containing heparin (5 U/mL) and diclofenac (10 μg/mL). The exudate was removed by aspiration. The number of leukocytes for the differential leucocyte count (DLC) in the exudates was counted with an optical microscope after Türk’s staining.
Measurement of Nitrite-Nitrate Concentration in Pleural Exudates
Total nitrite in exudates, an indicator of NO synthesis, was measured as previously described by Cuzzocrea et al. 1998 [19]. Briefly, the nitrate in the samples was first reduced to nitrite by incubation with nitrate reductase (670 mU/mL) and NADPH (160 μM) at room temperature for 3 h. The total nitrite concentration in the samples was then measured using the Griess reaction, by adding 100 μL of Griess reagent ((0.1% (w/v) naphthylethylenediamide dihydrochloride in H2O and 1% (w/v) sulfanilamide in 5% (v/v) concentrated H3PO4(vol 1:1)) to the 100 μL sample [20]. The total nitrite concentration in the samples was then measured using the Griess reaction, by adding 100 μL of Griess reagent. The concentrations were calculated by comparison with the OD550 of standard solutions of sodium nitrite prepared in H2O.
2.11. Histological Examination
For histological examination, ear biopsies were taken 48 h after edema induction by croton oil injection. The tissue slices were fixed in 10% neutral-buffered formaldehyde, embedded in paraffin and sectioned. The sections were stained with hematoxylin and eosin.
2.12. Colorimetric MTT (tetrazolium) Assay
The cytotoxicity of the extract was measured as previously described by Mosmann, 1983 [21]. The leukocytes were obtained from human peripheral blood, previously added with heparin, and centrifuged with Ficoll isopaque (Histopaque-1077). The cells were washed successively with RPMI 1640 medium and supplemented with 50 μM 2-mercaptoethanol and 5%–10% fetal bovine serum, in a 6% CO2 atmosphere. A total of 100 μL of the solution with 1 × 106 cells/well was added. These cells were incubated for 4 h, and different concentrations (0.5, 1.0 and 1.5 μg/mL) of the extract from P. dermoporus and stock MTT solution (10 μL/100 μL medium RPMI) were added to the wells. The plates were incubated at 37 °C for 4 h. Acid-isopropanol (100 μL of 0.04 N HCl) was added to all of the wells and mixed thoroughly to dissolve the dark blue crystals. The plates were read by a MicroElisa reader at 570 nm.
2.13. Statistical Analysis
Values are expressed as the mean ± SEM. Analysis of variance (ANOVA, Tukey–Kramer test) was used to evaluate the data, and p < 0.05 was accepted as statistically significant. The statistical analysis involved the use of the software, GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA).
3. Results and Discussion
3.1. Chemical Analysis
In this work, we investigated the chemical composition of the tissue of this native mushroom. The polysaccharides resulting from the extraction of Polyporus dermoporus and their cytotoxic, antioxidant, and anti-inflammatory action were studied. The results of the analyses of the composition of the tissue showed levels of carbohydrates, proteins, lipids and ash of 51.8%, 21.4%, 1.66% and 10.8%, respectively; assessment by Association of Official Analytical Chemists (A.O.A.C.), 1984 [11]. When submitted to the fractionation with ethanol, the extracts of this mushroom showed 64.8% carbohydrates and 10.0% proteins. Thus, the polysaccharide extract was composed mainly of glucose polymer (64.6%) (see Table 1), which was confirmed by total sugars by the phenol-H2SO4 reaction using d-glucose as the standard [13]. Phenolic compounds have been shown to possess potent antioxidant properties. Several studies have established links between the consumption of foods containing high concentrations of phenolic and antioxidant compounds and a lower incidence of cardiovascular diseases and cancer [5]. However, in extracts of Polyporus dermoporus, the antioxidant properties are not attributed to these bioactive compounds, due to the very low content of phenolic compounds and proteins (Table 1).
3.2. 1H and 13C NMR Spectroscopies of the Extract from Polyporus Dermoporus
The 1H spectra showed a chemical shift in the anomeric region at 4–6 ppm (Figure 1). In this spectrum, signals 4.1 and 4.6 corresponded to the signals obtained for β glucan [22]. According to Chaveau et al., 1996 [23], the chemical shifts correspond to C2 and C6 [7]. We also observed that the regions between 1.4 and 2.5 are related to the glucan-protein structure. These observations are in accord with other studies [6,7].

Table 1.
Chemical composition of natural tissue of mushroom P. dermoporus (g/100 g dry weight) and of the polysaccharides and proteins from the extract of this mushroom.
| Components | % |
|---|---|
| Tissue | |
| Carbohydrates | 51.3 ± 1.32 |
| Proteins | 21.4 ± 2.11 |
| Moisture | 10.01 ± 1.03 |
| Lipids | 1.66 ± 0.51 |
| Ashes | 10.80 ± 1.81 |
| Extract | |
| Polysaccharides | 64.8 ± 5.23 |
| Proteins | 10.2 ± 0.30 |
| Phenolic compounds | 0.90 ± 0.02 |
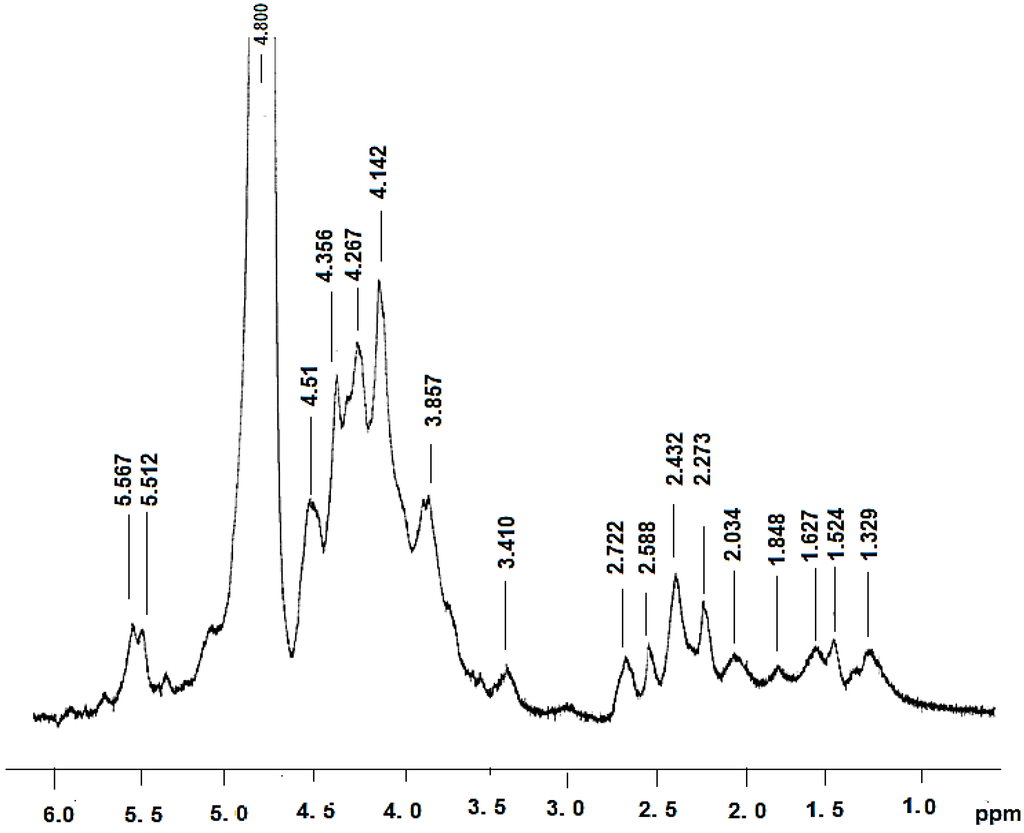
Figure 1.
1 H NMR spectroscopy of Polyporus dermoporus glucan protein.
Figure 1.
1 H NMR spectroscopy of Polyporus dermoporus glucan protein.
The analysis of 13C NMR spectroscopy showed that the polysaccharides o this mushroom contain a high level of glucose. This fact is due to the type of homopolysaccharides (glucans) present in this organism. Figure 2 shows the NMR spectra of 13C of the extracts from Polyporus dermoporus. The presence of glucose can be observed by a signal at 103.2 ppm, characteristic of the β configuration [22,23,24]. Other important signals in the spectra are those in the 60–80 ppm range, where they are related to C2 (73.9 ppm), C3 (84.0 ppm), C4 (80.0 ppm), C5 (76.24 ppm) and C6 (61.7 ppm) of that carbohydrate [25,26,27,28]. A peculiar characteristic of the spectra of β-(1→3) glucans is the presence of at least six signals of similar magnitude (110–60 ppm). The 47.8 ppm signal is related to the –CH2N group of amino acids.
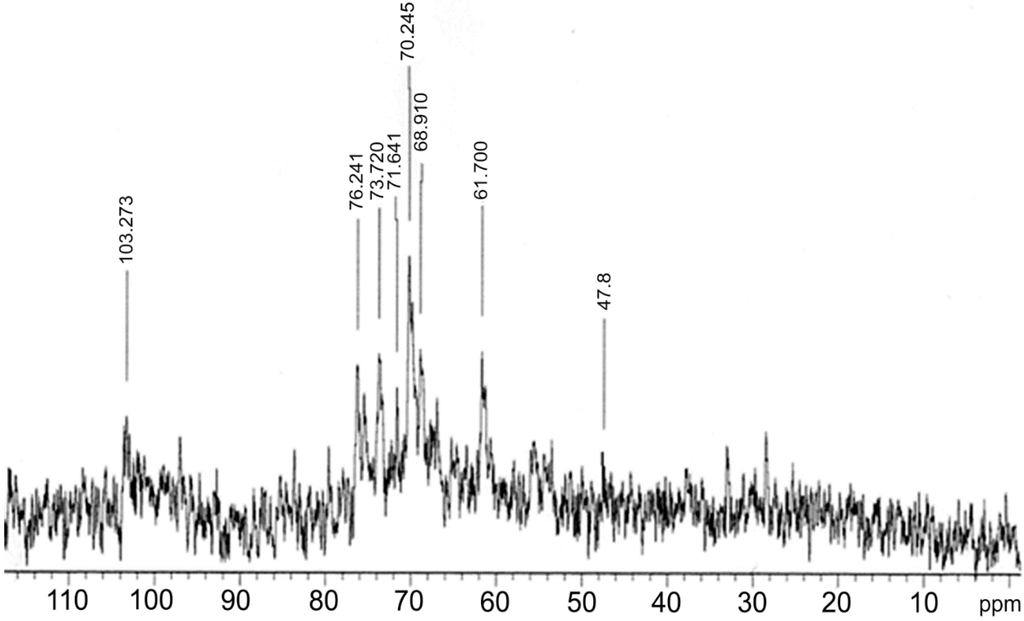
Figure 2.
13 C NMR spectroscopy of Polyporus dermoporus glucan-protein.
Figure 2.
13 C NMR spectroscopy of Polyporus dermoporus glucan-protein.
3.3. Superoxide and Hydroxyl Radicals
Glucans are homopolymers of d-glucose, widely distributed in nature, which may have bioactive properties in various organisms [27,28]. As such, they are highlighted for having a direct influence on the stimulation of phagocytic activity, antitumor properties, antioxidant, immunomodulatory and anti-inflammatory activities [10,26]. The antioxidant effect of the carbohydrates did not correlate with the type of intra-chain linkage, molecular weight or degree of polymer branching. The activity did appear to correlate with the monosaccharide composition of the polymer.
The antioxidant effect did appear to have a relation with the monosaccharide composition of the polymer and the composition of the polymer. Korzaski et al., 2011, in studies with mushroom polysaccharides of the species A. bisporus, A. brasiliensis, G. lucidum and P. linteus, showed that scavenging ability is a correlation that was found between the EC50 values of the chelating and reducing power abilities and the amount of total glucan content in the extracts [25]. The superoxide radicals were generated in a system of NADH-phenazine-methosulfate with different concentrations of the tested polysaccharides; see Zhou and Zheng et al., 1991 [29].
The formation of hydroxyl radicals (•OH) in the biological systems does not occur enzymatically and which need the presence of iron or transition metals [25]. The removing of •OH is important for the protection of living systems. The results obtained (•OH) were expressed as the % inhibition rate; see Table 2. At 267 μg/mL, 96% of the scavenging activity on hydroxyl radicals was exhibited. This shows a high inhibition in the generation of hydroxyl radicals when compared with polysaccharide from C. montagnei (38% at 1000 mg) [11], indicating considerable antioxidant activity in this study.
3.4. Determination of Lipid Peroxidation or Malonaldehyde Formation (LPO)
Combined with the generating system of lipid peroxidation using thiobarbituric acid (TBA), a condensation reaction produces malondialdehyde (MDA)-TBA with a pink coloration that can be subsequently quantified [26,30]. The extract of the mushroom used in this work showed efficient inhibition of lipid peroxidation, varying from 24.3% to 42.91% (Table 2). The process of lipid peroxidation results in the formation and propagation of lipid peroxides that act on the cell membrane and microsomes, producing compounds, such as malondialdehyde (MDA) [30,31]. The maximal effect on inhibition microsomal peroxidation occurred at a dose of 67 μg/μL (42.9%). We observed that the inhibition of superoxide radical and peroxidation of this polysaccharide-protein complex is not dose dependent. Polysaccharide of C. montagnei at 4 mg/mL produced lipidic peroxidation inhibition of 37.6% ± 0.16%, equivalent to 47% of the activity from α-tocopherol at the same concentration [10].

Table 2.
The effect of mushroom polysaccharide extracts from P. dermoporus on superoxide and hydroxyl radical generation and microsomal lipid peroxidation.
| Concentration (μg/mL) | Inhibition Superoxide Radical (%) | Inhibition Hydroxyl Radical (%) | Inhibition Microsomal Peroxidation (%) |
|---|---|---|---|
| 67 | 83.3 | 20.0 | 42.9 |
| 113 | 75.1 | 37.0 | 40.3 |
| 200 | 51.5 | 75.0 | 31.0 |
| 267 | 48.5 | 96.0 | 24.3 |
Each value represents the mean ± S.E.M. (n = 6).
Polysaccharides may be acting similarly to other mechanisms described for chelating, by reducing the redox pro-oxidant potential of metals and stabilizing the oxidized form of the metal, through steric interaction and also by forming soluble and stable complexes with metals, which are eventually excreted in the urine, as related by Ebrahimzadeh et al. 2008 [9]. This is strengthened by the description of the effect of other polysaccharides in the chelation of metal ions [10,29]. This may explain the strong inhibition of the peroxyl radical when observed in incubation in the presence of glucans, in addition to its significant effect in the reduction of lipid peroxidation, showing its strong antioxidant effect at low concentrations. This can result in protection against the development of pathologies, such as inflammation, cancer, oxidative stress, and diabetes.
3.5. Action of the Polysaccharides in Carrageenan-Induced Pleurisy
The results obtained showed a high number of leukocytes cells (2854 cell/mm3) in the pleural infiltrate of the positive control (animals treated with carrageenan). When the animals were submitted to the action of carrageenan and treated previously with the glucans of P. dermoporus (10 and 30 mg/kg by body weight), the cells obtained in the pleural infiltrate were 681 and 264 cell/mm3, respectively (Figure 3). This value corresponds to a reduction of 76% and 92.5% in the number of cells, respectively. At a dose of 50 mg/kg, a 70.6% reduction in infiltrate cells occurs (p < 0.001). The nonsteroidal anti-inflammatory drugs (NSAIDs) used in this experimental was indomethacin; p < 0.001. ANOVA and the Tukey-Kramer test showed that the difference observed in relation to the control group is highly significant; p < 0.001.
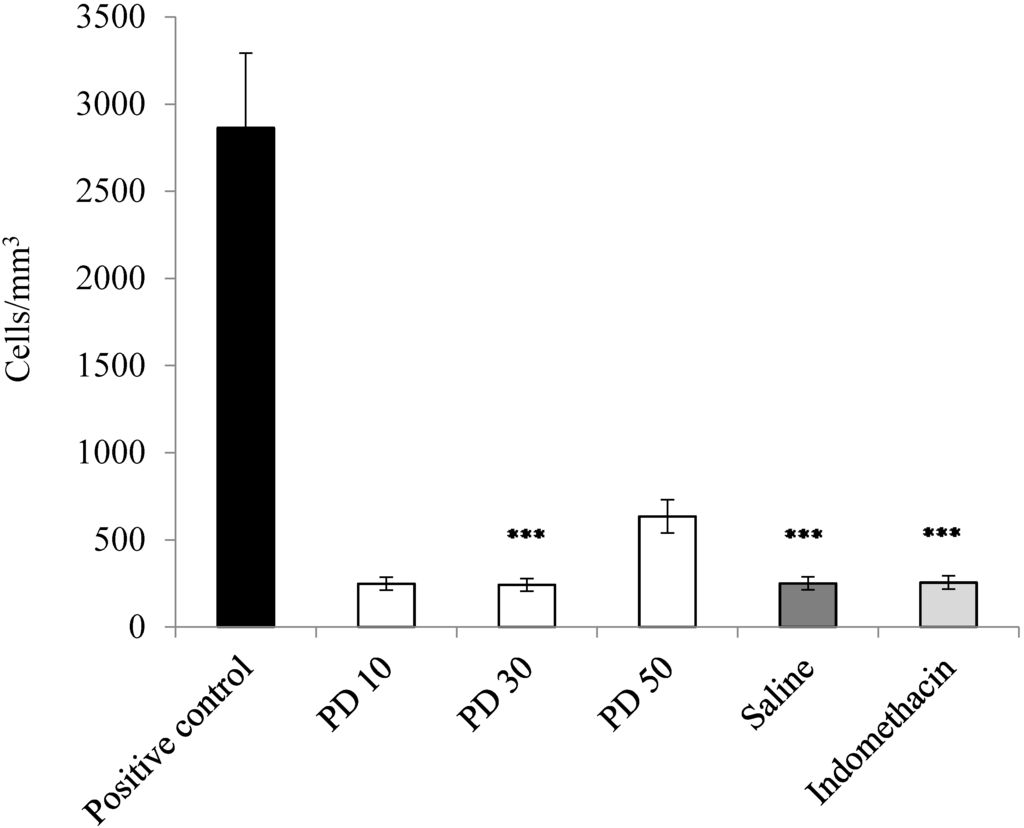
Figure 3.
Anti-inflammatory effect of P. dermoporus extract (PD) on carrageenan-induced pleurisy. The number of pleural exudate leukocytes in carrageenan-induced Wistar rats. The experimental animals were treated with P. dermoporus extract at 10 mg/kg (PD 10), 30 mg/kg (PD 30) and 50 mg/kg (PD 50). Data obtained from animal experiments are expressed as the mean ± SD. The differences between treatment and control were tested by ANOVA. A value of (***) p < 0.001 was considered statistically significant.
Figure 3.
Anti-inflammatory effect of P. dermoporus extract (PD) on carrageenan-induced pleurisy. The number of pleural exudate leukocytes in carrageenan-induced Wistar rats. The experimental animals were treated with P. dermoporus extract at 10 mg/kg (PD 10), 30 mg/kg (PD 30) and 50 mg/kg (PD 50). Data obtained from animal experiments are expressed as the mean ± SD. The differences between treatment and control were tested by ANOVA. A value of (***) p < 0.001 was considered statistically significant.
3.6. Effect of Polysaccharide on Nitric Oxide (NO)
Polysaccharides derived from mushrooms have been considered an important class of bioactive compounds, and their anti-inflammatory potential has been widely studied [25,26,32]. Nitric oxide in most body fluids is rapidly metabolized to stable products, such as nitrite and nitrate. Figure 4 shows the value of nitrate/nitrite produced after carrageenan administration and previous treatments with the extracts of P. dermoporus at doses of 10 mg/kg (PD 10), 30 mg/kg (PD 30) and 50 mg/kg by body weight (PD 50). The positive control (animals with carrageenan) showed 21.39 ± 1.79 nmol of nitrate/nitrite. In the animals that received P. dermoporus (30 mg/kg by body weight), this value was 7.48 ± 0.44 nmol (p < 0.001). We observed that at 10 mg/kg and 50 mg/kg, the results were 12.51 ± 0.81 and 7.32 ± 1.36 nmol of nitrate/nitrite (p < 0.001). In this study, we found the effect of this extract in the modulation of nitric oxide expression in the inflammation model tested.
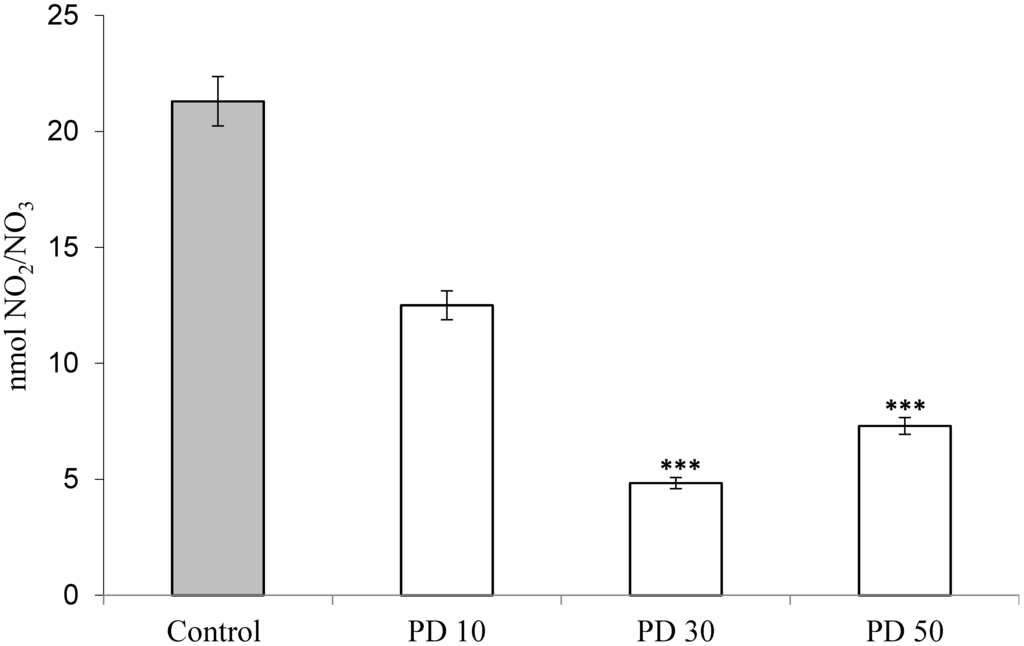
Figure 4.
Effect of P. dermoporus polysaccharides on NO production from the pleural exudate of Wistar rats with carrageenan-induced pleurisy. The animals were treated with 10 mg/kg (PD 10), 30 mg/kg (PD 30) and 50 mg/kg (PD 50) of P. dermoporus extract. Control: Wistar rats with carrageenan-induced pleurisy. Data obtained from animal experiments (n = 7) are expressed as the mean ± SD. The differences between the treatment and control were tested using ANOVA. A value of (***) p < 0.001 was considered statistically significant.
Figure 4.
Effect of P. dermoporus polysaccharides on NO production from the pleural exudate of Wistar rats with carrageenan-induced pleurisy. The animals were treated with 10 mg/kg (PD 10), 30 mg/kg (PD 30) and 50 mg/kg (PD 50) of P. dermoporus extract. Control: Wistar rats with carrageenan-induced pleurisy. Data obtained from animal experiments (n = 7) are expressed as the mean ± SD. The differences between the treatment and control were tested using ANOVA. A value of (***) p < 0.001 was considered statistically significant.
3.7. Croton Oil-Induced Ear Edema Test
The anti-edematogenic activity of the extract from P. dermoporus against croton oil-induced ear edema in rats was assessed. For comparison, the effect of nonsteroidal (indomethacin) anti-inflammatory drug was analyzed. The animals received a subcutaneous injection of croton oil (0.1 mL of 1%) solution in acetone. The ear volume was measured in the groups: positive control rats that received croton oil, saline (negative control) and extracts of P. dermoporus (10, 30, and 50 mg/kg). The extract of P. dermoporus reduced the edema by 65.6% at 30 mg/kg. At 10 mg/kg, the reduction was 58.3%, and at a concentration of 50 mg/kg, the reduction was 55.7% (Figure 5).
3.8. Histological Analysis
To evaluate histological changes, we carried out hematoxylin and eosin (H & E) staining, 200×. Histological examination of ear edema sections (Figure 6A) revealed significant tissue damage to animals treated with croton oil. The slide of animals treated (positive control) presented intense cellular infiltrate, predominantly neutrophilic inflammatory infiltrate (Figure 6A), characteristic of inflammatory reaction. Exposure to saline (Figure 6B) did not lead to significant pathologic alterations. The ear sections of the animals treated with the glucans (Figure 6C–E) were similar to those that received only saline (Figure 6B), showing decreased congestion and infiltration of leukocytes, especially neutrophils into paw tissues.
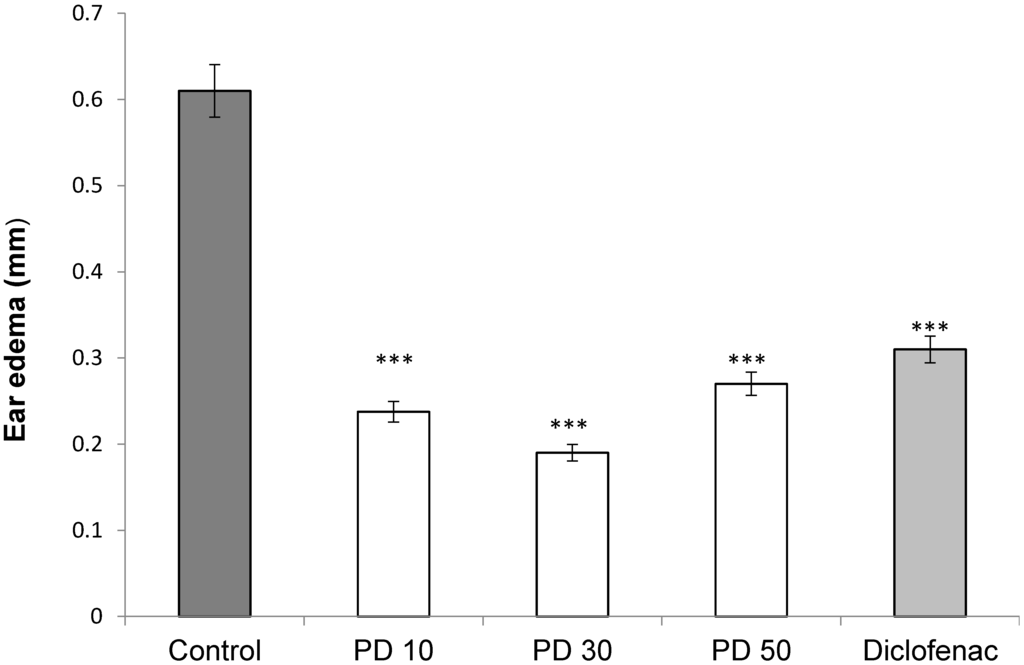
Figure 5.
The effect of P. dermoporus polysaccharides on the croton oil-induced ear edema assay in BALBc mice. The animals were treated with 10 mg/kg (PD 10), 30 mg/kg (PD 30) and 50 mg/kg (PD 50) of P. dermoporus polysaccharides. Data obtained from animal experiments are expressed as the mean ± SD. The differences between treatment and control were tested by ANOVA. A value of (***) p < 0.001 was considered statistically significant.
Figure 5.
The effect of P. dermoporus polysaccharides on the croton oil-induced ear edema assay in BALBc mice. The animals were treated with 10 mg/kg (PD 10), 30 mg/kg (PD 30) and 50 mg/kg (PD 50) of P. dermoporus polysaccharides. Data obtained from animal experiments are expressed as the mean ± SD. The differences between treatment and control were tested by ANOVA. A value of (***) p < 0.001 was considered statistically significant.
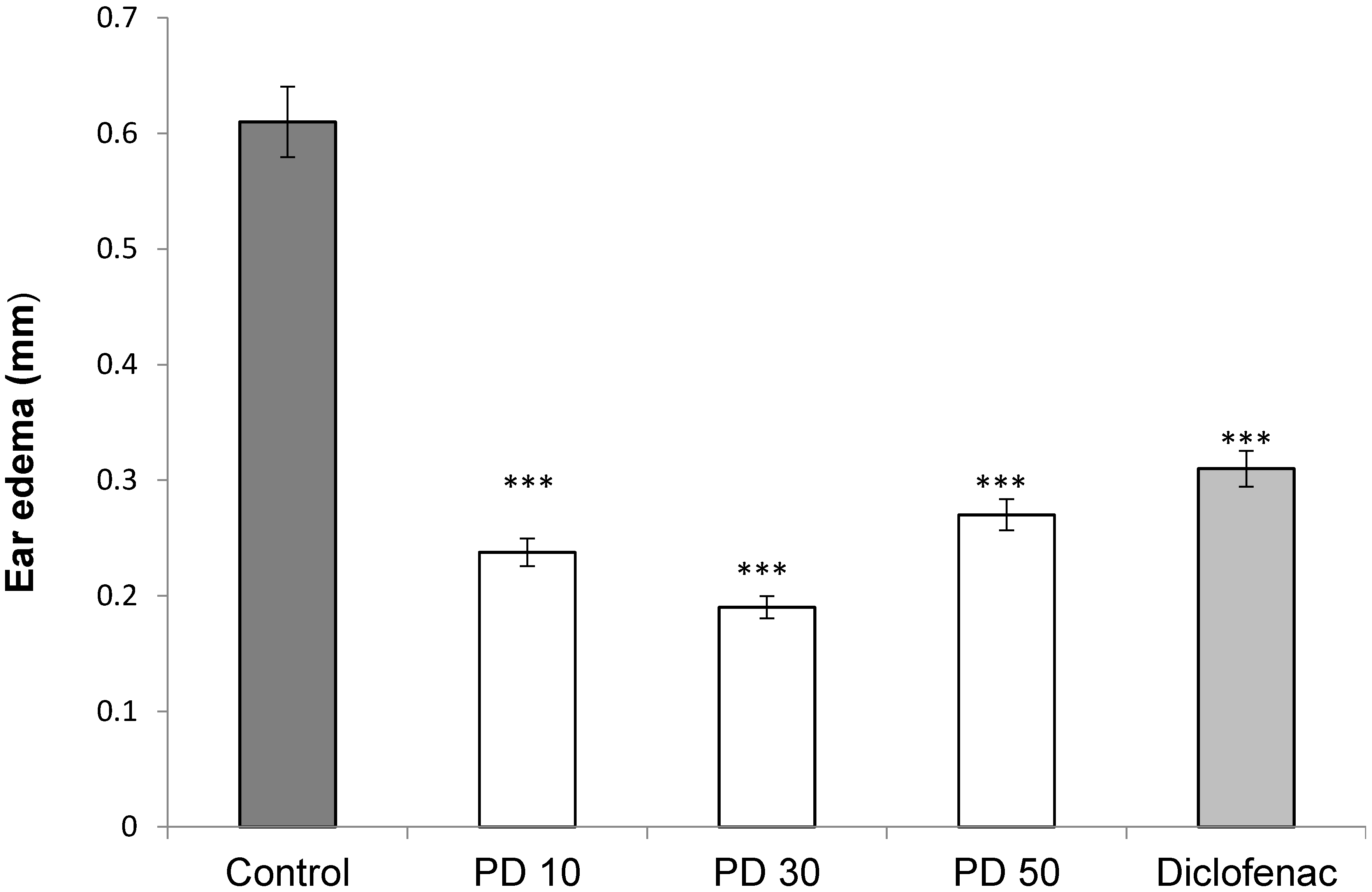
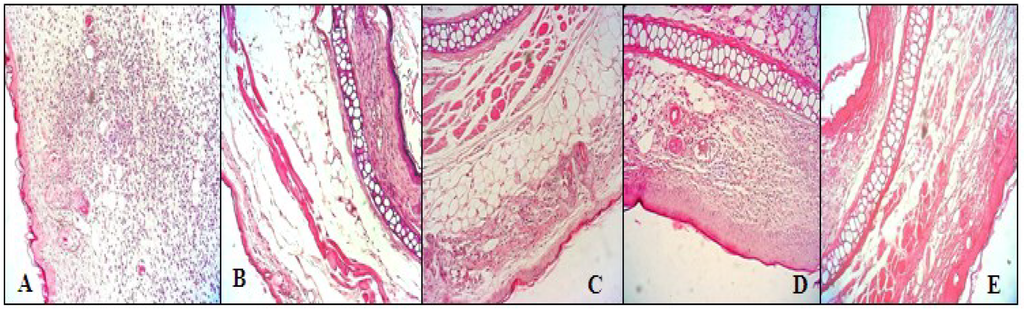
Figure 6.
Histological analysis of ear edema with H & E stain 200× from animals submitted to the croton oil-induced ear edema test and treated with P. dermoporus polysaccharides: (A) positive control (croton oil); (B) negative control (saline); (C) P. dermoporus polysaccharides at 10 mg/kg (PD 10); (D) animals treated with 30 mg/kg (PD 30); (E) animals treated with 50 mg/kg (PD 50).
Figure 6.
Histological analysis of ear edema with H & E stain 200× from animals submitted to the croton oil-induced ear edema test and treated with P. dermoporus polysaccharides: (A) positive control (croton oil); (B) negative control (saline); (C) P. dermoporus polysaccharides at 10 mg/kg (PD 10); (D) animals treated with 30 mg/kg (PD 30); (E) animals treated with 50 mg/kg (PD 50).
3.9. Cytotoxic Test
The cytotoxicity tests are based on the capacity of the cells to convert to tetrazolium (MTT) in a mixture of blue coloration called formazan [21]. However, only live cells have this capacity. The evaluation of the transformation of MTT in formazan in mononuclear cells of the peripheral blood is accomplished by ELISA, reading at 540 and 620 nm. The cells incubated with the extracts of P. dermoporus showed no quantitative difference in relation to the control (p < 0.001). Thus, this polysaccharide had no cytotoxic effect.
4. Conclusions
The results obtained in this paper showed that glucans of Polyporus dermoporus have inhibitory action in the formation of superoxide and hydroxyl radicals. The effect against this radical is dose dependent and inversely proportional to the increase in concentration. In this study, the intrapleural administration of carrageenan produced an acute inflammatory response. The volume and number of cells in pleural fluid increased in the initial 12 h, followed by a decline. A significant decrease in cell numbers was observed in the pleural fluid compared with the control. The value of nitrate/nitrite produced in the pleural cavity of rats by carrageenan administration and previous treatments of P. dermoporus extracts at a concentration of 30 mg/kg (PD 30) showed a 69.5% NO decrease (p < 0.001) in relation to the control group (carrageenan). This decrease suggests an anti-inflammatory effect of the P. dermoporus extract. Overall, this polysaccharide possessed good antioxidant properties and showed anti-inflammatory activity that is not often studied in mushrooms. We suggest that glucans appear to be related to the inhibition of diapedesis after cell migration to the injury site. Analyses conducted in this study demonstrated that the polysaccharide from P. dermoporus has no cytotoxic effect.
Acknowledgments
The authors are grateful for the financial support provided by CAPES and Conselho Nacional de Tecnologia e Pesquisa (CNPq), Process No. 475867/03-3. The authors are grateful to Michael Germain for the English revision.
Author Contributions
Celina Maria P. Guerra Dore participated in the experimental design and completion, as well as interpretation, manuscript design and preparation. Maria da Glória Lima Santos and Monique G. Faustino Alves, Leonardo Augusto R. de Souza participated in the experimental design and data interpretation. Iuri G Baseia collected and classified of mushroom. Finally, Edda Lisboa Leite reviewed all the manuscript and the final version to be submitted participated in manuscript design and preparation. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vaz, J.A.; Barros, L.; Martins, A.; Santos-Buelga, C.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem. 2011, 126, 610–616. [Google Scholar] [CrossRef]
- Chang, S.T. Global impact of edible and medicinal mushrooms on human welfare in the 21st century: Non green evolution. Int. J. Med. Mushrooms 1999, 1, 1–7. [Google Scholar] [CrossRef]
- Wasser, S.P.; Weis, A.L. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Int. J. Med. Mushrooms 1999, 1, 31–62. [Google Scholar] [CrossRef]
- Barros, L.; Cruz, T.P.; Baptista, E.L.M.; Ferreira, I.C.F.R. Wild and commercial mushrooms as source of nutrients and nutraceutical. Food Chem. Tox. 2008, 46, 2742–2747. [Google Scholar] [CrossRef]
- Shirley, R.; Ord, E.N.J.; Work, L.M. Oxidative stress and the use of antioxidants in stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; Azevedo, T.C.G.; Souza, M.C.R.; Rego, L.A.; Dantas, J.C.M.; Silva, F.R.F.; Rocha, H.A.O.; Baseia, I.G.; Leite, E.L. Antiinflamatory, antioxidant and cytotoxic actions of beta-glucan-rich extract from Geastrum saccatum mushroom. Int. Immunopharm. 2007, 7, 1160–1169. [Google Scholar] [CrossRef]
- Gonzaga, M.L.C.; Ricardo, N.M.P.S.; Heatley, F.; Soares, S.A. Isolation and characterization of polysaccharides from Agaricus blazei Murril. Carbohydr. Polym. 2005, 60, 43–49. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, H.; Cai, G.; Guan, S.; Tong, H.; Yang, X.; Liu, J. Sulfated modification of the water-soluble polysaccharides from Polyporus albicans mycelia and its potential biological activities. Int. J. Biol. Macromol. 2009, 44, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Bekhradnia, A.R. Iron chelating activity screening, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotech. 2008, 7, 3188–3192. [Google Scholar]
- Castro, A.J.G.; Castro, L.S.E.P.; Santos, M.S.N.; Faustino, M.G.; Pinheiro, T.S.; Dore, C.M.P.; Baseia, I.G.; Leite, E.L. Anti-inflamatory, anti-angiogenenic and antioxidant activities of polysaccharide-rich extract from fungi Caripia montagnei. Biomed. Prev. Nutr. 2014, 4, 121–129. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (A.O.A.C.). Official Methods Analytical Chemists, 14th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1984; pp. 988–1141. [Google Scholar]
- Spector, J. Refinement of the Coomassie blue method of protein quantification: A simple and linear spectrophotometric assay of 0.5 to 50 mg of protein. Anal. Biochem. 1978, 86, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars, and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ponti, V.; Dianzani, M.U.; Cheeseman, K.; Slater, T.F. Studies on the reduction of nitroblue tetrazolium chloride mediated through the action of NADH and phenazine methosulphate. Chem. Biol. Interact. 1978, 23, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Bueg, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Feischer, S., Packer, L., Eds.; Academic Press: New York, NY, USA, 1978; Volume 52, pp. 302–310. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Tubaro, A.; Dri, P.; Delbello, G.; Zilli, C.; Della Loggia, R. The Croton oil ear test revisited. Agents Actions 1985, 17, 347–349. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Costantino, G.; Zingarelli, B.; Caputi, A.P. Beneficial effects of Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in carrageenan-induced pleurisy. Free Radic. Biol. Med. 1998, 26, 25–33. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immun. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Chakraborty, I.; Mondal, S.; Rout, D.; Islam, S.S. A water-insoluble(1→3)-β-d-glucan from the alkaline extract of an edible mushroom Termitomyces eurhizus. Carbohydr. Res. 2006, 341, 2990–2993. [Google Scholar] [CrossRef]
- Chauveau, C.; Talaga, P.; Wieruszeski, J.M.; Strecker, G.; Chavant, L. A water-soluble beta-d-glucan from Boletus erythropus. Phytochemistry 1996, 43, 413–415. [Google Scholar] [CrossRef]
- Yalin, W.; Yavanjiang, P.; Cuirong, S. Isolation, purification and structural investigation of a water-soluble polysaccharide from Solanum lyratum Thunb. Int. J. Biol. Macromol. 2005, 36, 241–245. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Santos, M.S.N.; Magalhães, J.E.M.; Castro, L.S.E.P.W.; Pinheiro, T.S.; Sabry, D.A.; Nobre, L.T.D.B.; Lima, J.P.M.S.; Baseia, I.G.; Leite, E.L. Effect of Glucans from Caripia montagnei Mushroom on TNBS-Induced Colitis. Int. J. Mol. Sci. 2014, 15, 2368–2385. [Google Scholar] [CrossRef]
- Carbonero, E.R.; Gracher, A.H.P.; Smiderle, F.R.; Rosado, F.R.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. A β-glucan from the fruit bodies of an edible mushrooms Pleurotus eryngii and Pleurotus ostreatoroseus. Carbohydr. Polym. 2006, 66, 252–257. [Google Scholar] [CrossRef]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Zheng, R.L. Phenolic compounds and analog as superoxide anion scavengers and antioxidants. Biochem. Pharm. 1991, 42, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Z.; Hu, Y.; Xu, Z. Inhibition of mouse liver lipid peroxidation by high molecular weight phlorotannins from Sargassum kjellmanianum. J. App. Phycol. 2003, 15, 507–511. [Google Scholar] [CrossRef]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pin. Res. 2008, 1, 343–349. [Google Scholar]
- Zembron-Lacny, A.; Gajewski, M.; Naczk, M.; Siatkowski, I. Effect of shiitake (Lentinus edodes) extract on antioxidant and inflammatory response to prolonged eccentric exercise. J. Physiol. Pharmacol. 2013, 64, 249–254. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
