Lactobacillus fermentum ZC529 Protects Intestinal Epithelial Barrier Integrity by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the NF-κB Signaling Pathway
Abstract
1. Introduction
2. Methods and Materials
2.1. Culture and Preparation of Lactobacillus fermentum ZC529
2.2. Fly Husbandry and Survival Assay
2.3. Smurf Assay
2.4. Excretion Assay
2.5. Cell Pretreatment
2.6. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels in Cells
2.7. Real-Time Quantitative PCR (RT-qPCR)
2.8. Anti-Oxidative Activity Measurement in Flies
2.9. Anti-Oxidative Activity Measurement in IPEC-J2 Cells
2.10. Statistical Analyses
3. Results
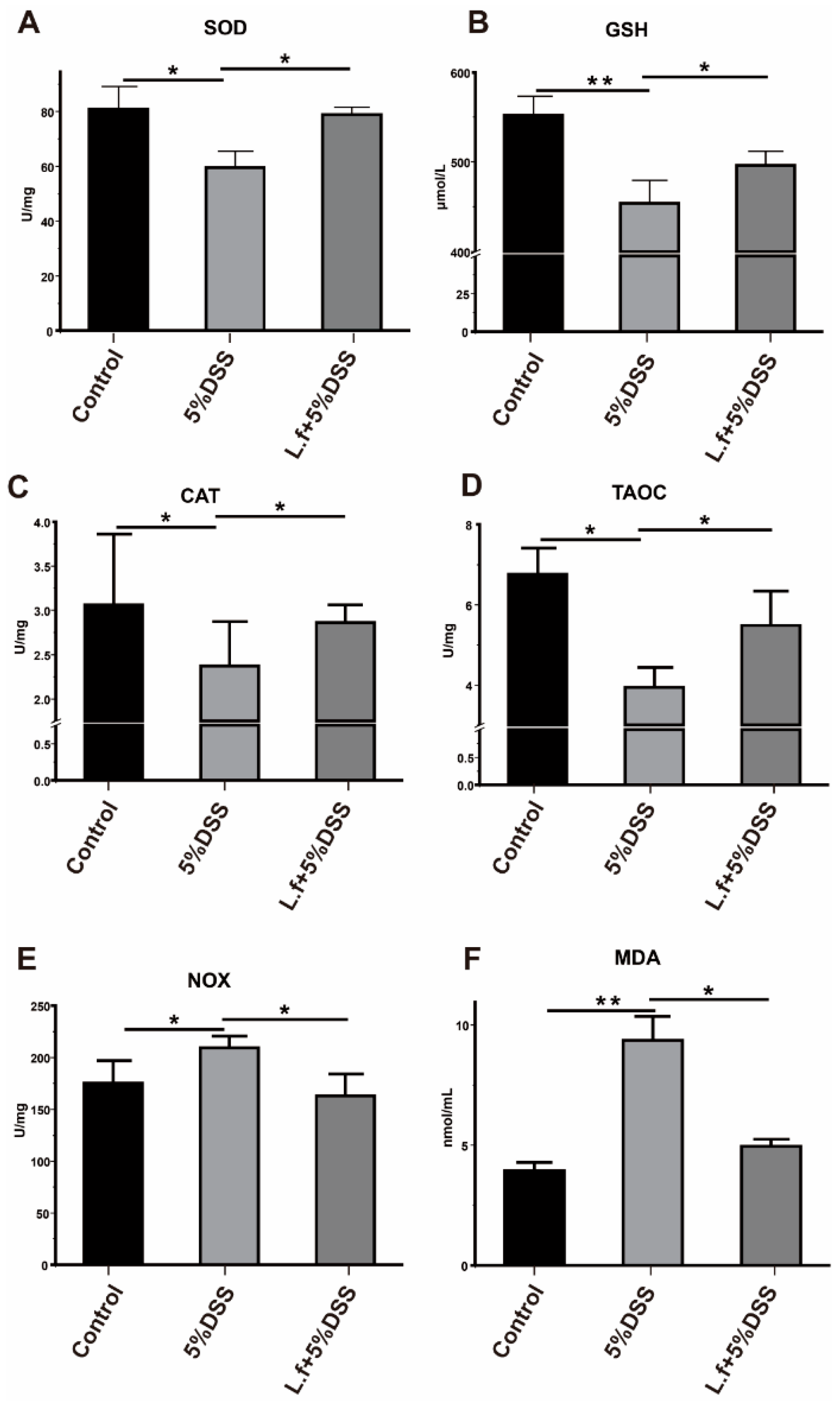
3.1. L.f ZC529 Alleviates DSS-Induced Intestinal Oxidative Stress in Drosophila
3.2. L.f ZC529 Mitigates ROS Generation by Activation of the CncC Pathway
3.3. L.f ZC529 Prevents Flies from DSS-Induced Intestinal Epithelium Damage and Barrier Disruption
3.4. L.f ZC529 Alleviates DSS-Induced Intestinal Inflammation and Restores the Lifespan of Drosophila
3.5. L.f ZC529 Ameliorates DSS-Induced Oxidative Stress in IPEC-J2 Cells via Activating the Keap1-Nrf2 Pathway
3.6. L.f ZC529 Alleviates DSS-Induced Inflammatory Response in IPEC-J2 Cells via Inhibiting the TLR4-NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Jia, Z. Microbiome modulates intestinal homeostasis against inflammatory diseases. Vet. Immunol. Immunopathol. 2018, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Okumura, R.; Takeda, K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu. Rev. Immunol. 2020, 38, 23–48. [Google Scholar] [CrossRef]
- Wang, J.; Song, H.; Huang, Y.; Yang, C.; Wu, Y.; Lin, R.; Lin, W. Protective effect of crocin on peroxidation-induced oxidative stress and apoptosis in IPEC-J2 cells. Environ. Toxicol. 2024, 39, 3537–3547. [Google Scholar] [CrossRef] [PubMed]
- Geertsema, S.; Bourgonje, A.R.; Fagundes, R.R.; Gacesa, R.; Weersma, R.K.; van Goor, H.; Faber, K.N. The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol. Med. 2023, 29, 830–842. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Yu, C.; Wang, D.; Yang, Z.; Wang, T. Pharmacological Effects of Polyphenol Phytochemicals on the Intestinal Inflammation via Targeting TLR4/NF-κB Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 6939. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Guan, X.; Zhu, J.; Sun, H.; Zhao, X.; Yang, M.; Huang, Y.; Zhao, S. Analysis of Gut Microbiota and Metabolites in Diannan Small Ear Sows at Diestrus and Metestrus. Front. Microbiol. 2022, 13, 826881. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.; Li, B.; Zhang, C.; Tang, X.; Tan, B.; Jiang, Q. Screening of Macadamia Green Peel Fermentation Strain and Improvement of Its Feeding Value after Fermentation. Chin. J. Anim. Nutr. 2024, 36, 1292–1302. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Liu, B.; Tang, X.; Tan, B.; Jiang, Q.; Yin, Y. Optimizing Cellulase-Limosilactobacillus fermentum ZC529 Synergy Fermentation for Preserving Macadamia integrifolia Pericarp’s Potential Use as Antioxidants. Antioxidants 2024, 13, 783. [Google Scholar] [CrossRef]
- Wang, A.N.; Yi, X.W.; Yu, H.F.; Dong, B.; Qiao, S.Y. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. J. Appl. Microbiol. 2009, 107, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Staats, S.; Lüersen, K.; Wagner, A.E.; Rimbach, G. Drosophila melanogaster as a versatile model organism in food and nutrition research. J. Agric. Food Chem. 2018, 66, 3737–3753. [Google Scholar] [CrossRef]
- Baenas, N.; Wagner, A.E. Drosophila melanogaster as an alternative model organism in nutrigenomics. Genes. Nutr. 2019, 14, 14. [Google Scholar] [CrossRef]
- Liegeois, S.; Ferrandon, D. Sensing microbial infections in the Drosophila melanogaster genetic model organism. Immunogenetics 2022, 74, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Ip, Y.T. Pathogenic stimulation of intestinal stem cell response in Drosophila. J. Cell Physiol. 2009, 220, 664–671. [Google Scholar] [CrossRef]
- Hochmuth, C.E.; Biteau, B.; Bohmann, D.; Jasper, H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 2011, 8, 188–199. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, X.; Zhang, Z.; Guo, J.; Guan, L.; Li, J.; Tong, D. Differentially expressed non-coding RNAs induced by transmissible gastroenteritis virus potentially regulate inflammation and NF-κB pathway in porcine intestinal epithelial cell line. BMC Genom. 2018, 19, 747. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Li, X.; Yin, Y. 4-Phenylbutyric acid accelerates rehabilitation of barrier function in IPEC-J2 cell monolayer model. Anim. Nutr. 2021, 7, 1061–1069. [Google Scholar] [CrossRef]
- Livingston, D.B.H.; Patel, H.; Donini, A.; MacMillan, H.A. Active transport of brilliant blue FCF across the Drosophila midgut and Malpighian tubule epithelia. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 239, 110588. [Google Scholar] [CrossRef] [PubMed]
- Cognigni, P.; Bailey, A.P.; Miguel-Aliaga, I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011, 13, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2011, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Spellman, P.; Rubin, G.; Lemaitre, B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc. Natl. Acad. Sci. USA 2001, 98, 12590–12595. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Osman, D. All for one and one for all: Regionalization of the Drosophila intestine. Insect Biochem. Mol. Biol. 2001, 67, 2–8. [Google Scholar] [CrossRef]
- Sykiotis, G.; Bohmann, D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yan, E.; He, J.; Zhong, X.; Zhang, L. Dietary Supplemented Curcumin Improves Meat Quality and Antioxidant Status of Intrauterine Growth Retardation Growing Pigs via Nrf2 Signal Pathway. Animals 2020, 10, 539. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef]
- Zanchi, C.; Lindeza, A.S.; Kurtz, J. Comparative mortality and adaptation of a smurf assay in two species of tenebrionid beetles exposed to Bacillus thuringiensis. Insects 2020, 11, 261. [Google Scholar] [CrossRef]
- Jones, R.M.; Desai, C.; Darby, T.M.; Luo, L.; Wolfarth, A.A.; Scharer, C.D.; Neish, A.S. Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep. 2015, 12, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yang, C.; Li, P.; Zhang, M.; Xie, X.; Xie, X.; Luo, X. Astragaloside IV inhibits AOM/DSS-induced colitis-associated tumorigenesis via activation of PPARγ signaling in mice. Phytomedicine 2023, 121, 155116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Aardsma, M.A.; Duttlinger, A.W.; Kpodo, K.R. Early life thermal stress: Impact on future thermotolerance, stress response, behavior, and intestinal morphology in piglets exposed to a heat stress challenge during simulated transport. J. Anim. Sci. 2018, 96, 1640–1653. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Wensley, M.R.; Tokach, M.D.; Woodworth, J.C.; Goodband, R.D.; Gebhardt, J.T.; DeRouchey, J.M.; McKilligan, D. Maintaining continuity of nutrient intake after weaning. I. Review of pre-weaning strategies. Transl. Anim. Sci. 2021, 5, txab021. [Google Scholar] [CrossRef]
- Zappaterra, M.; Faucitano, L.; Nanni Costa, L. Road Transport: A Review of Its Effects on the Welfare of Piglets. Animals 2023, 13, 1604. [Google Scholar] [CrossRef]
- Carvajal-Yepes, M.; Himmelsbach, K.; Schaedler, S.; Ploen, D.; Krause, J.; Ludwig, L.; Hildt, E. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J. Biol. Chem. 2011, 286, 8941–8951. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, Z.; Ding, Y.; Li, X.; Zhang, Z.; Yang, L.; Guo, R. EGFR-Targeted Immunotoxin Exerts Antitumor Effects on Esophageal Cancers by Increasing ROS Accumulation and Inducing Apoptosis via Inhibition of the Nrf2-Keap1 Pathway. J. Immunol. Res. 2018, 2018, 1090287. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Ding, L.; Zhao, P.; Zhang, C.; Wang, H.; Liu, Z. Alleviating effect of quercetin on cadmium-induced oxidative damage and apoptosis by activating the Nrf2-keap1 pathway in BRL-3A cells. Front. Pharmacol. 2022, 13, 969892. [Google Scholar] [CrossRef]
- Edamitsu, T.; Taguchi, K.; Okuyama, R.; Yamamoto, M. AHR and NRF2 in skin homeostasis and atopic dermatitis. Antioxidants 2022, 11, 227. [Google Scholar] [CrossRef]
- Xing, H.; Li, R.; Ying, B.; Qin, Y. Biomaterial-based osteoimmunomodulatory strategies via the TLR4-NF-κB signaling pathway: A review. Appl. Mater. Today 2021, 22, 100969. [Google Scholar] [CrossRef]
- Ma, J.; Yin, G.; Lu, Z.; Xie, P.; Zhou, H.; Liu, J.; Yu, L. Casticin prevents DSS induced ulcerative colitis in mice through inhibitions of NF-κB pathway and ROS signaling. Phytother. Res. 2018, 32, 1770–1783. [Google Scholar] [CrossRef] [PubMed]
- Boza, P.; Ayala, P.; Vivar, R.; Humeres, C.; Cáceres, F.T.; Muñoz, C.; Díaz-Araya, G. Expression and function of toll-like receptor 4 and inflammasomes in cardiac fibroblasts and myofibroblasts: IL-1β synthesis, secretion, and degradation. Mol. Immunol. 2016, 74, 96–105. [Google Scholar] [CrossRef]
- Block, M.S.; Vierkant, R.A.; Rambau, P.F.; Winham, S.J.; Wagner, P.; Traficante, N.; Goode, E.L. MyD88 and TLR4 Expression in Epithelial Ovarian Cancer. Mayo Clin. Proc. 2018, 93, 307–320. [Google Scholar] [CrossRef]
- Su, C.-H.; Lin, C.-Y.; Tsai, C.-H.; Lee, H.-P.; Lo, L.-c.; Huang, W.C.; Tang, C.-H. Betulin suppresses TNF-α and IL-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κB pathway. J. Funct. Foods 2021, 86, 104729. [Google Scholar] [CrossRef]
- Gitlin, A.D.; Maltzman, A.; Kanno, Y.; Heger, K.; Reja, R.; Schubert, A.F.; Dixit, V.M. N4BP1 coordinates ubiquitin-dependent crosstalk within the IκB kinase family to limit Toll-like receptor signaling and inflammation. Immunity 2024, 57, 973–986.e977. [Google Scholar] [CrossRef]
- Kucinski, I.; Dinan, M.; Kolahgar, G.; Piddini, E. Chronic activation of JNK JAK/STAT and oxidative stress signalling causes the loser cell status. Nat. Commun. 2017, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, H.; Nanthirudjanar, T.; Kume, T.; Izumi, Y.; Park, S.-B.; Kitamura, N.; Sugawara, T. 10-Oxo-trans-11-octadecenoic acid generated from linoleic acid by a gut lactic acid bacterium Lactobacillus plantarum is cytoprotective against oxidative stress. Toxicol. Appl. Pharmacol. 2016, 296, 1–9. [Google Scholar] [CrossRef]
- Mu, G.; Li, H.; Tuo, Y.; Gao, Y.; Zhang, Y. Antioxidative effect of Lactobacillus plantarum Y44 on 2, 2′-azobis (2-methylpropionamidine) dihydrochloride (ABAP)-damaged Caco-2 cells. J. Dairy Sci. 2019, 102, 6863–6875. [Google Scholar] [CrossRef]
- Zheng, J.; Ahmad, A.A.; Yang, C.; Liang, Z.; Shen, W.; Liu, J.; Dong, P. Orally Administered Lactobacillus rhamnosus CY12 Alleviates DSS-Induced Colitis in Mice by Restoring the Intestinal Barrier and Inhibiting the TLR4-MyD88-NF-κB Pathway via Intestinal Microbiota Modulation. J. Agric. Food Chem. 2024, 72, 9102–9116. [Google Scholar] [CrossRef]





| Drosophila | Primers Sequence (5′–3′) | Accession No. | Length (bp) | References |
|---|---|---|---|---|
| Rp49 | F:ATCGGTTACGGATCGAACAAGC R: GTAAACGCGGTTCTGCATGAGC | Y13939.1 | 148 | [24,25,26] |
| CAT | F: TTCCTGTGGGCAAAATGGTG R: ATCTTCACCTTGTACGGGCA | NM_080483.3 | 134 | |
| SOD | F: CAAGGGCACGGTTTTCTTC R: CCTCACCGGAGACCTTCAC | NM_057387.5 | 120 | |
| gstd1 | F: CATCGCGAGTTTCACAACAG R: GTTGAGCAGCTTCTTGTTCAG | NM_001038953.2 | 141 | |
| keap1 | F: CAAGGAGTCGGAGATGTCG R: GTAGAGGATGCGTGACATGG | DQ372684.1 | 150 | |
| Attacin A | F: GCATCCTAATCGTGGCCCT R: AGCGGGATTGGAGGTTAAGG | NM_079021.5 | 132 | |
| Diptericin | F: CTCAATCTTCAGGGAGGCGG R: AGGTGCTTCCCACTTTCCAG | NM_057460.4 | 125 | |
| Imd | F: GCTCCGTCTACAACTTCAACC R: CCACAATGCTGACCGTTTTG | NM_133166.4 | 140 | |
| Relish | F: GGTCCAGCTGCTGAAGAATG R: ACGGAATCCTCGTCCTTTGT | NM_057746.4 | 128 | |
| Mmp1 | F: AGGACTCCAAGGTAGACACAC R: TTGCCGTTCTTGTAGGTGAACGC | NM_001259570.2 | 138 | |
| MtnA | F: TGCAAATGCGCCAGCCAG R: TCGGAGCAGCCGCAGG | NM_079575.2 | 142 | |
| upd2 | F: CGGAACATCACGATGAGCGAAT R: TCGGCAGGAACTTGTACTCG | NM_001370039.1 | 129 | |
| upd3 | F: ATCCCACCAATCCCCTGAAG R: AGATTGCAGGTGTTCTCCCA | NM_001103544.2 | 130 | |
| STAT92E | F: AGTTCTACTCAAAGCGTCAAGATCC R: CAGTTGCATGCTTTCCTGAGC | NM_001275833.1 | 146 |
| IPEC-J2 | Primers Sequence (5′–3′) | Accession No. | Length (bp) | References |
|---|---|---|---|---|
| β-actin | F: CCAGGTCATCACCATCGGCAAC R: CAGCACCGTGTTGGCGTAGAG | DQ845171.1 | 143 | [27,28] |
| SOD2 | F: GGCCTACGTGAACAACCTGA R: TGATTGATGTGGCCTCCACC | NM_214127.2 | 135 | |
| CAT | F: AGCCTACGTCCTGAGTCTCTGC R: TCCATATCCGTTCATGTGCCTGTG | NM_214301.2 | 126 | |
| Nrf2 | F: AGCCTACGTCCTGAGTCTCTGC R: TCCATATCCGTTCATGTGCCTGTG | MH101365.1 | 119 | |
| Keap1 | F: CGTGGAGACAGAAACGTGGA R: CAATCTGCTTCCGACAGGGT | NM_001114671.1 | 131 | |
| HO-1 | F: TGATGGCGTCCTTGTACCAC R: GACCGGGTTCTCCTTGTTGT | NM_001004027.1 | 115 | |
| NQO-1 | F: CATGGCGGTCAGAAAAGCAC R: ATGGCATACAGGTCCGACAC | NM_001159613.1 | 129 | |
| ZO-1 | F: CAGAGACCAAGAGCCGTCC R: TGCTTCAAGACATGGTTGGC | AJ318101.1 | 140 | |
| Occludin | F: TCAGGTGCACCCTCCAGATT R: AGGAGGTGGACTTTCAAGAGG | NM_001163647.2 | 137 | |
| TLR4 | F: GACAGCAATAGCTTCTCCAGC R: GGTTTGTCTCAACGGCAACC | NM_001113039.2 | 132 | |
| MyD88 | F: GTGCCGTCGGATGGTAGTG R: TCTGGAAGTCACATTCCTTGCTT | EU056737.1 | 145 | |
| MAPK8 (JNK1) | F: CAGCCGATTCGGAGCACAACA R: GGTGGTGGAGCTTCAGCTTCAG | NM_001143717.1 | 132 | |
| NF-κB p65 (RelA) | F: AAGCAGAGCCGCACAGCATTC R: CCAGACCAACAACAACCCCTTCC | EU399817.1 | 138 | |
| TNF-α | F: AACCTCAGATAAGCCCGTCG R: ACCACCAGCTGGTTGTCTTT | JF831365.1 | 141 | |
| RegⅢγ | F: GGCTTGGAACCAAATGCTGG R: TAGCCAGGGTATGAGCTGGT | NM_001144847.1 | 133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Huang, L.; Hu, Z.; Deng, J.; Duan, Y.; Jiang, Q.; Tan, B.; Ma, X.; Zhang, C.; Tang, X. Lactobacillus fermentum ZC529 Protects Intestinal Epithelial Barrier Integrity by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the NF-κB Signaling Pathway. Antioxidants 2025, 14, 732. https://doi.org/10.3390/antiox14060732
Yuan Z, Huang L, Hu Z, Deng J, Duan Y, Jiang Q, Tan B, Ma X, Zhang C, Tang X. Lactobacillus fermentum ZC529 Protects Intestinal Epithelial Barrier Integrity by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the NF-κB Signaling Pathway. Antioxidants. 2025; 14(6):732. https://doi.org/10.3390/antiox14060732
Chicago/Turabian StyleYuan, Zian, Lang Huang, Zhenguo Hu, Junhao Deng, Yehui Duan, Qian Jiang, Bi’e Tan, Xiaokang Ma, Chen Zhang, and Xiongzhuo Tang. 2025. "Lactobacillus fermentum ZC529 Protects Intestinal Epithelial Barrier Integrity by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the NF-κB Signaling Pathway" Antioxidants 14, no. 6: 732. https://doi.org/10.3390/antiox14060732
APA StyleYuan, Z., Huang, L., Hu, Z., Deng, J., Duan, Y., Jiang, Q., Tan, B., Ma, X., Zhang, C., & Tang, X. (2025). Lactobacillus fermentum ZC529 Protects Intestinal Epithelial Barrier Integrity by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the NF-κB Signaling Pathway. Antioxidants, 14(6), 732. https://doi.org/10.3390/antiox14060732









