Evaluation of Bioactive Properties of Ultrasound-Assisted Extracts from Prokupac Grape Skins for Functional Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Samples of Grape Skin
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. Determination of Total Phenolic Content
2.5. In Vitro Antioxidant Potential Assessment
2.5.1. DPPH Radical Scavenging Assay
2.5.2. ABTS Radical Cation Decolorization Assay
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.4. Measurement of Hydroxyl Radical Scavenging Potential Using Electron Paramagnetic Resonance (EPR) Spectroscopy
2.5.5. Measurement of Ascorbyl Radical Scavenging Potential Using Electron Paramagnetic Resonance (EPR) Spectroscopy
2.6. HPLC Analysis
2.7. Lipoxygenase (LOX) Inhibition Assay
2.8. In Vitro Antimicrobial Assay
2.8.1. Microorganisms Used for Testing
2.8.2. Preparation of Suspensions
2.8.3. Microdilution Method
2.9. Statistical Analysis
3. Results and Discussion
3.1. Extraction and Extraction Optimization
3.2. Spectrophotometric Determination of the Total Phenolic Content
3.3. UV–Vis and EPR Determination of Antioxidant Activity
3.3.1. DPPH Radical Scavenging Activity
3.3.2. ABTS Radical Scavenging Activity
3.3.3. Ferric Reduction Antioxidant Potential
3.3.4. Hydroxyl Radical Reduction Antioxidant Potential
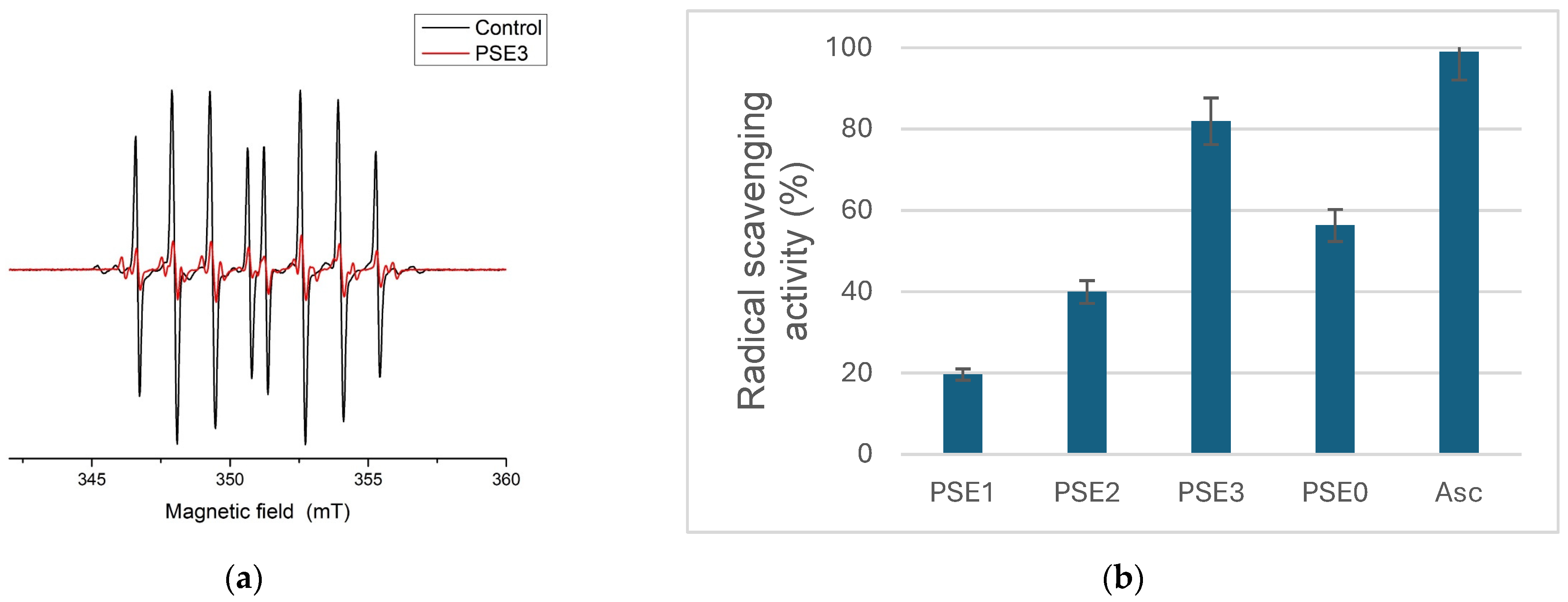
3.3.5. Ascorbyl Radical Reduction Antioxidant Potential
3.3.6. Statistical Analysis of Different Antioxidant Potential Assays
3.4. HPLC Analysis Results
3.5. LOX Inhibition Activity
3.6. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Morré, D.M.; Morré, D.J. Anticancer activity of grape and grape skin extracts alone and combined with green tea infusions. Cancer Lett. 2006, 238, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Nakarada, Đ.; Marković, S.; Popović, M.; Dimitrijević, M.; Rakić, A.; Mojović, M. Redox properties of grape wine skin extracts from the Šumadija region: An electron paramagnetic resonance study. Hosp. Pharmacol.-Int. Multidiscip. J. 2021, 8, 1004–1013. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, M.A.; Lee, Y.; Kim, D.; Jo, J.; Lim, B. Antioxidant Activity and Acetylcholinesterase Inhibition of Grape Skin Anthocyanin (GSA). Molecules 2014, 19, 9403–9418. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Frankel, E.N.; Waterhouse, A.L.; Teissedre, P.L. Principal Phenolic Phytochemicals in Selected California Wines and Their Antioxidant Activity in Inhibiting Oxidation of Human Low-Density Lipoproteins. J. Agric. Food Chem. 1995, 43, 890–894. [Google Scholar] [CrossRef]
- de Moura, R.S.; Viana, F.S.C.; Souza, M.A.V.; Kovary, K.; Guedes, D.C.; Oliveira, E.P.B.; Rubenich, L.M.S.; Carvalho, L.C.R.M.; Oliveira, R.M.; Tano, T.; et al. Antihypertensive, vasodilator and antioxidant effects of a vinifera grape skin extract. J. Pharm. Pharmacol. 2002, 54, 1515–1520. [Google Scholar] [CrossRef]
- Dinicola, S.; Cucina, A.; Antonacci, D.; Bizzarri, M. Anticancer Effects of Grape Seed Extract on Human Cancers: A Review. J. Carcinog. Mutagen. 2014, S8, 5. [Google Scholar] [CrossRef]
- Hudson, T.S.; Hartle, D.K.; Hursting, S.D.; Nunez, N.P.; Wang, T.T.Y.; Young, H.A.; Arany, P.; Green, J.E. Inhibition of Prostate Cancer Growth by Muscadine Grape Skin Extract and Resveratrol through Distinct Mechanisms. Cancer Res. 2007, 67, 8396–8405. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Agarwal, C.; Agarwal, R. Anticancer and Cancer Chemopreventive Potential of Grape Seed Extract and Other Grape-Based Products. J. Nutr. 2009, 139, 1806S–1812S. [Google Scholar] [CrossRef]
- Sun, T.; Chen, Q.Y.; Wu, L.J.; Yao, X.M.; Sun, X.J. Antitumor and antimetastatic activities of grape skin polyphenols in a murine model of breast cancer. Food Chem. Toxicol. 2012, 50, 3462–3467. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.M.; Spigno, G. Grape marc phenolics: Extraction kinetics, quality and stability of extracts. J. Food Eng. 2010, 97, 384–392. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Odabaş, H.İ.; Koca, I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind. Crops Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- Stevanović, S.; Stevanović, S.; Janković-Šoja, S. Regional characteristics of market production of fruit and grapes in Serbia. Ekon. Poljopr. 2018, 65, 201–214. [Google Scholar] [CrossRef]
- Panić, M.; Damjanović, A.; Radošević, K.; Cvjetko Bubalo, M.; Dujmić, F.; Škegro, M.; Radojčić Redovniković, I.; Brnčić, M. Enhanced Preparative-Scale Extraction from Graševina Grape Pomace Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Appl. Sci. 2024, 14, 6185. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, A.; Jovancicevic, B.; Arsic, B.; Radovanovic, B.; Bukarica, L.G. Application of non-supervised pattern recognition techniques to classify Cabernet Sauvignon wines from the Balkan region based on individual phenolic compounds. J. Food Compos. Anal. 2016, 49, 42–48. [Google Scholar] [CrossRef]
- Kandylis, P. Grapes and Their Derivatives in Functional Foods. Foods 2021, 10, 672. [Google Scholar] [CrossRef]
- Fürst, R.; Zündorf, I. Plant-Derived Anti-Inflammatory Compounds: Hopes and Disappointments regarding the Translation of Preclinical Knowledge into Clinical Progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef]
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Kinetics of ultrasound-assisted extraction of antioxidant polyphenols from food by-products: Extraction and energy consumption optimization. Ultrason. Sonochem. 2016, 32, 137–146. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M. Folin-Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 107–115. [Google Scholar] [CrossRef]
- Marcellin-Gros, R.; Hévin, S.; Chevalley, C.; Boccard, J.; Hofstetter, V.; Gindro, K.; Wolfender, J.-L.; Kehrli, P. An advanced metabolomic approach on grape skins untangles cultivar preferences by Drosophila suzukii for oviposition. Front. Plant Sci. 2024, 15, 1435943. [Google Scholar] [CrossRef]
- Antonijević, M.R.; Simijonović, D.M.; Avdović, E.H.; Ćirić, A.; Petrović, Z.D.; Marković, J.D.; Stepanić, V.; Marković, Z.S. Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency. Antioxidants 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Avdović, E.; Antonijević, M.; Simijonović, D.; Grujović, M.; Marković, K.; Milenković, D.; Ćirić, A.; Marković, Z. Ultrasound-Assisted Extraction of Bioactive Phenolics from Marselan and Shiraz Grape Skins: A Step Toward Circular Economy in Food and Pharmaceutical Industry. LWT 2025, 224, 117817. [Google Scholar] [CrossRef]
- Karaman Ersoy, Ş.; Vural, T.; Sözgen Başkan, K.; Tütem, E. Comparison of Total Antioxidant Capacities and Phenolic Constituents of Grapes Cultivated in Turkey for Wine Production. Anal. Lett. 2024, 58, 1464–1478. [Google Scholar] [CrossRef]
- Buettner, G.R. Ascorbate oxidation: UV absorbance of ascorbate and ESR spectroscopy of the ascorbyl radical as assays for iron. Free. Radic. Res. Commun. 1990, 10, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Jaćimović, S.; Kiprovski, B.; Ristivojević, P.; Dimić, D.; Nakarada, Đ.; Dojčinović, B.; Sikora, V.; Teslić, N.; Pantelić, N.Đ. Chemical Composition, Antioxidant Potential, and Nutritional Evaluation of Cultivated Sorghum Grains: A Combined Experimental, Theoretical, and Multivariate Analysis. Antioxidants 2023, 12, 1485. [Google Scholar] [CrossRef]
- Nakarada, Đ.; Pejin, B.; Tommonaro, G.; Mojović, M. Liposomal integration method for assessing antioxidative activity of water insoluble compounds towards biologically relevant free radicals: Example of avarol. J. Liposome Res. 2020, 30, 218–226. [Google Scholar] [CrossRef]
- Savić, A.G.; Mojović, M. Free Radicals Identification from the Complex EPR Signals by Applying Higher Order Statistics. Anal. Chem. 2012, 84, 3398–3402. [Google Scholar] [CrossRef]
- Mollica, A.; Scioli, G.; Della Valle, A.; Cichelli, A.; Novellino, E.; Bauer, M.; Kamysz, W.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Castillo-López, R.; et al. Phenolic Analysis and In Vitro Biological Activity of Red Wine, Pomace and Grape Seeds Oil Derived from Vitis vinifera L. cv. Montepulciano d’Abruzzo. Antioxidants 2021, 10, 1704. [Google Scholar] [CrossRef]
- Serea, D.; Condurache, N.N.; Aprodu, I.; Constantin, O.E.; Bahrim, G.-E.; Stănciuc, N.; Stanciu, S.; Rapeanu, G. Thermal Stability and Inhibitory Action of Red Grape Skin Phytochemicals against Enzymes Associated with Metabolic Syndrome. Antioxidants 2022, 11, 118. [Google Scholar] [CrossRef]
- Simijonović, D.; Vlachou, E.-E.; Petrović, Z.D.; Hadjipavlou-Litina, D.J.; Litinas, Κ.E.; Stanković, N.; Mihović, N.; Mladenović, M.P. Dicoumarol derivatives: Green synthesis and molecular modelling studies of their anti-LOX activity. Bioorg. Chem. 2018, 80, 741–752. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Fernandes, F.; Silva, T.; Planinić, M.; Tišma, M.; Šelo, G.; Šibalić, D.; Pereira, D.M.; Andrade, P.B. Enhancement of the anti-inflammatory properties of grape pomace treated by Trametes versicolor. Food Funct. 2020, 11, 680–688. [Google Scholar] [CrossRef]
- Andrews, J.M. BSAC standardized disc susceptibility testing method (version 4). J. Antimicrob. Chemother. 2005, 56, 60–76. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Muruzović, M.; Mladenović, K.; Stefanović, O.; Vasić, S.; Čomić, L. Extracts of Agrimonia eupatoria L. as sources of biologically active compounds and evaluation of their antioxidant, antimicrobial, and antibiofilm activities. J. Food Drug Anal. 2016, 24, 539–547. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Statistics for Windows, version 24.0; IBM Corporation: Armonk, NY, USA, 2016.
- Čomić, R.L.; Radojević, I.D.; Vasić, S.M.; Mladenović, K.G.; Grujović, M.Ž. Traditionally made red wines produced from an autochthonous grapevine variety as a source of biologically active compounds and their antioxidant potential. J. Food Nutr. Res. 2020, 59, 301–310. [Google Scholar]
- Zdunić, G.; Gođevac, D.; Šavikin, K.; Krivokuća, D.; Mihailović, M.; Pržić, Z.; Marković, N. Grape Seed Polyphenols and Fatty Acids of Autochthonous Prokupac Vine Variety from Serbia. Chem. Biodivers. 2019, 16, 1900053. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Kostić, A.Ž.; Špirović Trifunović, B.D.; Tešić, Ž.L.; Tosti, T.B.; Dramićanin, A.M.; Barać, M.B.; Pešić, M.B. Grape seed flour of different grape pomaces: Fatty acid profile, soluble sugar profile and nutritional value. J. Serbian Chem. Soc. 2020, 85, 305–319. [Google Scholar] [CrossRef]
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]

| Investigated Extract and Standards | Yield (%) | DPPH SC50 (µg/mL) | ABTS SC50 (µg/mL) | FRAP (A700nm) | TPC (mg GAE/g GSE) | LOX IC50 (µg/mL) |
|---|---|---|---|---|---|---|
| PSE1 | 8.9 | 41.9 ± 0.8 | 32.5 ± 0.6 | 0.0744 ± 0.0035 | 3.6 ± 0.1 | 354.9 ± 0.3 |
| PSE2 | 6.3 | 99.4 ± 0.4 | 60.7 ± 0.3 | 0.0579 ± 0.0049 | 2.2 ± 0.1 | >500 |
| PSE3 | 4.9 | 8.3 ± 0.2 | 8.8 ± 0.4 | 0.1636 ± 0.0052 | 9.8 ± 0.5 | 64.7 ± 0.1 |
| PSE0 | 9.8 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.2624 ± 0.0039 | 22.4 ± 0.1 | 7.9 ± 0.1 |
| Trolox | NA | ND | 1.3 ± 0.1 | ND | ND | ND |
| NDGA | NA | 0.5 ± 0.1 | ND | ND | ND | 5.2 ± 0.1 |
| Quercetin | NA | 0.6 ± 0.1 | ND | ND | ND | 43.2 ± 0.5 |
| Ascorbic acid | NA | 18.0 ± 0.1 | 31.1 ± 0.1 | 0.1249 ± 0.0022 | ND | ND |
| Sample | PSE0 | PSE1 | PSE2 | PSE3 | |
|---|---|---|---|---|---|
| Compound | |||||
| Gallic acid | 566 ± 6 | N/A | N/A | 470 ± 6 | |
| 3,4-DHB ** | N/A *** | N/A | N/A | 9 ± 1 | |
| 3,5-DHB | N/A | N/A | N/A | N/A | |
| Chorogenic acid | 302 ± 3 | 23 ± 1 | 32 ± 1 | 315 ± 9 | |
| Caffeic acid | 1051 ± 26 | 10 ± 1 | 14 ± 1 | 31 ± 9 | |
| Syringic acid | 119 ± 1 | 25 ± 1 | 29 ± 1 | 281 ± 6 | |
| Epicatechin | 5415 ± 39 | 34 ± 1 | 42 ± 1 | 440 ± 9 | |
| p-Coumaric acid | 126 ± 5 | 9 ± 1 | 15 ± 1 | 28 ± 1 | |
| Ferulic acid | 142 ± 1 | 18 ± 14 | 68 ± 1 | 93 ± 6 | |
| Sinapic acid | 473 ± 3 | 1 ± 1 | 37 ± 1 | 420 ± 5 | |
| Rutin | 330 ± 3 | 190 ± 1 | 21 ± 1 | 114 ± 7 | |
| Naringin | 3828 ± 54 | 87 ± 1 | 121 ± 5 | 266 ± 9 | |
| Myricetin | 908 ± 12 | 29 ± 1 | 44 ± 1 | 500 ± 5 | |
| Morin | 458 ± 14 | 96 ± 3 | 101 ± 1 | 62 ± 8 | |
| Quercetin | 232 ± 9 | 18 ± 1 | 18 ± 1 | 211 ± 5 | |
| Naringenin | 104 ± 11 | 85 ± 3 | 92 ± 3 | 71 ± 3 | |
| Apigenin | 707 ± 5 | 16 ± 1 | 15 ± 1 | 514 ± 4 | |
| Crysin | 77 ± 1 | 22 ± 1 | 13 ± 1 | 18 ± 5 | |
| Microorganisms | PSE1 1 | PSE2 1 | PSE3 1 | PSE0 2 | Doxycycline/Itraconazole 1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | MIC | MMC | |
| B. subtilis ATCC 6633 | 5 | 5 | 10 | 20 | 2.5 | 2.5 | 12.5 | 25 | 1.953 | 31.25 |
| B. subtilis | >20 | >20 | >20 | >20 | 20 | 20 | 6.25 | 12.5 | 0.112 | 1.953 |
| S. aureus | 5 | >20 | 10 | >20 | 0.625 | 10 | 0.78 | 1.56 | 0.224 | 3.75 |
| S. aureus ATCC 25923 | 5 | >20 | 10 | >20 | >20 | >20 | 1.56 | 1.56 | 0.45 | 7.81 |
| P. mirabilis ATCC 12453 | 0.625 | 1.25 | 5 | 5 | <0.156 | <0.156 | <0.39 | 0.78 | 7.81 | 15.63 |
| P. mirabilis | 2.5 | 2.5 | 5 | 5 | 0.625 | 0.625 | 3.125 | 3.125 | 15.63 | 62.5 |
| P. aeruginosa ATCC 27853 | 5 | 20 | 10 | 20 | 2.5 | 5 | 6.25 | 6.25 | 62.5 | 125 |
| P. aeruginosa | 5 | 20 | 10 | 20 | 2.5 | 5 | 6.25 | 12.5 | 250 | 1000 |
| E. coli | 20 | 20 | 20 | >20 | 10 | 10 | >50 | >50 | 15.63 | 62.5 |
| E. coli ATCC 25922 | 5 | 10 | 10 | 20 | 0.3125 | 1.25 | 12.5 | 25 | 15.63 | 31.25 |
| C. albicans ATCC 10231 | >20 | >20 | >20 | >20 | >20 | >20 | 50 | >50 | 1.95 | 1.95 |
| C. albicans | >20 | >20 | >20 | >20 | >20 | >20 | >50 | >50 | 1.95 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avdović, E.; Dimić, D.; Nakarada, Đ.; Simijonović, D.; Jovičić Milić, S.; Marković, K.; Grujović, M.; Antonijević, M.; Ćirić, A.; Milenković, D.; et al. Evaluation of Bioactive Properties of Ultrasound-Assisted Extracts from Prokupac Grape Skins for Functional Foods. Antioxidants 2025, 14, 733. https://doi.org/10.3390/antiox14060733
Avdović E, Dimić D, Nakarada Đ, Simijonović D, Jovičić Milić S, Marković K, Grujović M, Antonijević M, Ćirić A, Milenković D, et al. Evaluation of Bioactive Properties of Ultrasound-Assisted Extracts from Prokupac Grape Skins for Functional Foods. Antioxidants. 2025; 14(6):733. https://doi.org/10.3390/antiox14060733
Chicago/Turabian StyleAvdović, Edina, Dušan Dimić, Đura Nakarada, Dušica Simijonović, Sandra Jovičić Milić, Katarina Marković, Mirjana Grujović, Marko Antonijević, Andrija Ćirić, Dejan Milenković, and et al. 2025. "Evaluation of Bioactive Properties of Ultrasound-Assisted Extracts from Prokupac Grape Skins for Functional Foods" Antioxidants 14, no. 6: 733. https://doi.org/10.3390/antiox14060733
APA StyleAvdović, E., Dimić, D., Nakarada, Đ., Simijonović, D., Jovičić Milić, S., Marković, K., Grujović, M., Antonijević, M., Ćirić, A., Milenković, D., & Marković, Z. (2025). Evaluation of Bioactive Properties of Ultrasound-Assisted Extracts from Prokupac Grape Skins for Functional Foods. Antioxidants, 14(6), 733. https://doi.org/10.3390/antiox14060733











