Abstract
Advanced glycation end-products (AGEs) and oxidative stress are recognized as central contributors to the pathogenesis of age-related or diabetic cataracts, diabetic retinopathy (DR), and age-related macular degeneration (AMD). These glycation-related diseases are characterized by impaired redox balance and decreased glutathione (GSH) levels. This review aims to examine the mechanistic links between AGEs and GSH depletion across ocular tissues by integrating in vitro, ex vivo, in vivo, and clinical studies relevant to this topic. The multiple levels of evidence highlight GSH homeostasis as both a biomarker and therapeutic target in glycation-related ocular disorders. Therapeutic strategies aimed at restoring GSH homeostasis under glycation stress are categorized into four mechanistic domains: (I) promoting GSH supply and synthesis, (II) enhancing GSH recycling, (III) mitigating glycation stress, and (IV) reducing oxidative and nitrosative stress. Most of these strategies have been explored via different approaches, and experimental findings with various interventions have shown promise in restoring GSH balance and mitigating AGE-induced damage. A pathological link between GSH depletion and vascular endothelial growth factor (VEGF) overexpression is observed in DR and wet AMD. GSH-centered interventions act upstream to modulate redox homeostasis while anti-VEGF therapies target downstream angiogenesis. This study supports the rationale for a dual-targeting strategy that combines redox-based interventions with VEGF inhibition in glycation-related ocular diseases.
1. Introduction
Ocular diseases, such as age-related or diabetic cataracts, diabetic retinopathy (DR), and age-related macular degeneration (AMD), are major causes of vision impairment and blindness worldwide [1,2]. While their etiologies are multifactorial, increasing evidence implicates oxidative stress and metabolic dysfunction as central contributors to their pathogenesis [3]. Among the biochemical culprits, advanced glycation end-products (AGEs) have emerged as key players in the progression of age- and diabetes-related eye diseases [4].
AGEs are formed through the non-enzymatic glycation and oxidation of proteins, lipids, and nucleic acids, particularly under hyperglycemic and oxidative conditions [5,6]. These irreversible modifications disrupt cellular function by altering protein structure, promoting inflammation, and inducing oxidative stress [7,8,9]. In ocular tissues, AGEs accumulate in long-lived proteins of the lens and retina, where they can initiate or exacerbate degenerative processes [10].
One critical target of glycation and oxidation-induced eye disease is glutathione (GSH), a tripeptide composed of glutamate, cysteine, and glycine [11]. GSH not only scavenges reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive carbonyl species (RCS) but also participates in enzymatic detoxification pathways, such as those involving glutathione peroxidase (GPx) and glyoxalase (GLO) systems [12,13]. Dysregulation of GSH homeostasis—either by direct oxidative depletion or by AGE-mediated inactivation of related enzymes—compromises the antioxidant defense of the eye, making it more susceptible to glyco-oxidative damage [14,15].
Several previous review articles have examined the role of oxidative stress and AGEs in diabetes-related complications, including ocular pathologies. Pescosolido et al. reviewed cataractogenesis in the aging lens, noting AGE accumulation and declined GSH levels [16]. Babel and Dandekar provided a broad overview of oxidative stress pathways in diabetic complications, including systemic AGE-GSH interactions [17]. Fan et al. emphasized persistent gaps in our understanding of glutathione dynamics in the ocular lens, underlining the need for targeted strategies to preserve GSH homeostasis [18]. Lim et al. reported that GSH levels in the lens follow a circadian pattern, with maximum antioxidant capacity aligned with core clock gene activity during the early active phase [19]. Despite these contributions, these existing reviews have not comprehensively examined GSH homeostasis across diverse ocular conditions, nor have they emphasized therapeutic strategies aimed at restoring GSH balance in the context of glycation stress.
The present review aims to examine the interplay between AGEs and GSH homeostasis in ocular diseases. Section 2 and Section 3 provide a general background on the cellular and molecular aspects of glycation and GSH homeostasis, respectively. Section 4 summarizes key findings from in vitro, ex vivo, in vivo, and clinical studies that investigated alterations in GSH levels and redox balance under glycation stress in ocular tissues. Section 5 discusses mechanistic insights and therapeutic strategies, including emerging approaches to restore GSH levels and mitigate glycation-related ocular damage. This review highlights the therapeutic importance of maintaining redox homeostasis in ocular pathologies by identifying critical patterns and promising interventions. It is hoped that this work will advance the understanding of GSH homeostasis as both a biomarker and a therapeutic target in glycation-related eye diseases and foster the development of targeted redox-based therapeutic strategies.
2. Cellular and Molecular Aspects of Glycation
This section introduces the mechanisms of glycation stress, the characteristics of AGEs, and the enzymes involved in glycation-related metabolism.
2.1. Glycation Stress
Glycation stress refers to a cellular condition resulting from the accumulation of early and advanced glycation products formed through non-enzymatic reactions between RCS and nucleophilic sites in proteins, lipids, and DNA [5,6,20]. RCS, such as methylglyoxal (MG) and glyoxal (GO), are generated as by-products of glycolysis, lipid peroxidation, and the autoxidation of sugars. Their accumulation is accelerated under hyperglycemic and oxidative conditions.
Initially, a reducing sugar reacts with a free amino group of a protein to form a Schiff base, which subsequently undergoes rearrangement into more stable Amadori products and Heyns products [7,21]. Over time, these early glycation products further degrade through oxidative and dehydration processes, leading to the generation of highly reactive intermediates and AGEs [22,23]. Hyperglycemia, oxidative stress, chronic inflammation, and aging are major factors that accelerate glycation reactions in vivo [24,25,26,27]. The biological consequences of glycation stress include loss of protein function, activation of inflammatory pathways, increased cellular apoptosis, and structural alterations in the extracellular matrix. Glycation and subsequent AGE accumulation contribute to the pathogenesis of various metabolic, cardiovascular, neurodegenerative, ocular, and age-related diseases [28].
2.2. AGEs
AGEs represent a heterogeneous group of complex, stable, and often irreversible adducts formed through prolonged glycation processes [7,29]. Table 1 summarizes various types of AGEs classified by precursor substrates and specific molecular target sites, including amino acid residues (e.g., lysine, arginine, and cysteine) of proteins, collagen, nucleic acids, and lipids.

Table 1.
Classification of advanced glycation end-products (AGEs) by precursor substrate and specific molecular target sites.
AGEs can form either crosslinking structures between proteins or non-crosslinking adducts, depending on the reacting molecules and environmental conditions [35]. Crosslinking AGEs, such as glucosepane and pentosidine, form covalent bonds between amino acid residues (typically lysine and arginine) on long-lived proteins like collagen. Non-crosslinking AGEs, such as Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL), modify individual amino acid residues without forming crosslinks. Cellular responses to AGEs are primarily mediated by specific receptors, including the receptor for advanced glycation end-products (RAGE) and other advanced glycation end-product receptor (AGE-R) family members (AGE-R1, AGE-R2, and AGE-R3) [36]. Activation of RAGE signaling triggers downstream pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, leading to the release of pro-inflammatory cytokines and the amplification of oxidative stress, thereby perpetuating tissue damage [37,38].
RAGE activation triggers NF-κB signaling, leading to pro-inflammatory cytokine release (e.g., tumor necrosis factor (TNF)-α and interleukin (IL)-6), which in turn can induce autophagy [39]. This self-protective process facilitates the degradation of intracellular AGEs and restores redox homeostasis. However, in chronic diseases like diabetes, sustained inflammation often fails to promote regeneration, contributing to prolonged tissue damage and dysfunction.
2.3. Glycation-Related Enzymes
Several enzymes regulate the levels of RCS and glycation products, thereby modulating glycation stress and protecting cellular integrity. The GLO system, comprising GLO1 and GLO2, plays a central role in detoxifying RCS, such as MG and GO [12,40]. This system utilizes GSH to catalyze the conversion of these reactive aldehydes into less harmful products, namely lactate and glycolate, thereby preventing the accumulation of AGEs. In addition, aldehyde dehydrogenase (ALDH) contributes to the detoxification of reactive aldehydes by catalyzing their oxidation into less reactive carboxylic acids [41]. Aldo-keto reductase (AKR) represents a broad superfamily of enzymes that catalyze the NADPH-dependent reduction of diverse carbonyl compounds, including MG and other reactive aldehydes [42]. Through this mechanism, AKR complements other detoxification pathways and supports the maintenance of cellular redox homeostasis.
Among the AKR family, aldose reductase (AR)—encoded by the AKR1B1 gene—is a specialized NADPH-dependent enzyme that primarily catalyzes the reduction of glucose to sorbitol, initiating the polyol pathway [43]. AR plays a dual role under glycation stress: while it may contribute to the reduction of reactive dicarbonyl compounds such as MG and GO, its primary function involves glucose metabolism. Under hyperglycemic conditions, its hyperactivation promotes sorbitol accumulation, osmotic stress, and subsequent lens damage, particularly in diabetic settings. Excessive activation of AR leads to substantial NADPH consumption, thereby limiting its availability for the glutathione reductase (GR)-mediated regeneration of GSH. Additionally, fructose produced via sorbitol dehydrogenase (SDH) in the polyol pathway undergoes glycation reactions at a substantially higher rate than glucose, owing to its greater proportion of open-chain form and the increased electrophilicity of its carbonyl group [44]. Consequently, the hyperactivation of AR not only exacerbates osmotic stress but also intensifies glycation stress, thereby accelerating the accumulation of AGEs [45].
Fructosamine-3-kinase (FR3K) acts at a later stage of the glycation pathway by phosphorylating Amadori products [46]. This modification promotes the degradation of early glycation intermediates, thereby reducing the formation of stable AGEs. Moreover, protein deglycase DJ-1 (Parkinsonism-associated deglycase 7, PARK7) protects cells through antioxidant and deglycation mechanisms [47]. Acting as a redox sensor via the oxidation of its conserved cysteine106 residue, DJ-1 promotes the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2), thereby enhancing the expression of antioxidant enzymes. It also translocates to mitochondria to preserve complex I activity and suppress mitochondrial ROS production. In parallel, DJ-1 reverses early glycation adducts induced by RCS, contributing to the maintenance of proteostasis [48].
3. Cellular and Molecular Aspects of GSH Homeostasis
GSH is the most abundant intracellular thiol and plays crucial roles in cellular defense against oxidative stress, the detoxification of xenobiotics, and the regulation of redox-sensitive signaling pathways [49,50]. This section outlines the homeostasis of GSH, including its synthesis, degradation, and redox cycling, and discusses how GSH metabolism intersects with glycation processes to modulate cellular stress responses.
3.1. GSH Biosynthesis and Degradation
The cystine/glutamate antiporter xCT imports cystine in exchange for glutamate, supporting cysteine availability for GSH biosynthesis in cells [51]. The biosynthesis of GSH occurs in two sequential ATP-dependent enzymatic steps [52]. γ-Glutamylcysteine ligase (GCL) catalyzes the formation of γ-glutamylcysteine from glutamate and cysteine, representing the rate-limiting step. Glutathione synthetase (GS) then adds glycine to form GSH.
GSH degradation occurs through distinct pathways depending on its cellular location. Extracellular GSH is sequentially processed via the γ-glutamyl cycle, where γ-glutamyl transpeptidase (GGT) initiates GSH breakdown by transferring the γ-glutamyl group to acceptor amino acids, facilitating the uptake of precursor amino acids for intracellular GSH resynthesis [53]. The γ-glutamyl amino acids generated are subsequently converted into 5-oxoproline and free amino acids by γ-glutamylcyclotransferase (GGCT), further contributing to amino acid recycling [54]. In contrast, intracellular GSH is directly degraded by ChaC1 (glutathione-specific γ-glutamylcyclotransferase 1), particularly under endoplasmic reticulum (ER) stress conditions, producing 5-oxoproline and cysteinyl-glycine dipeptides [55]. The 5-oxoproline is then converted back to glutamate by 5-oxoprolinase, while cysteinyl-glycine is hydrolyzed by dipeptidases to release cysteine and glycine, both of which can be reutilized for de novo GSH synthesis [56].
3.2. GSH-Mediated Antioxidant Defense and Redox Regulation
GSH plays a central role in cellular antioxidant defense through both enzymatic and non-enzymatic mechanisms. Table 2 summarizes the key enzymatic and non-enzymatic reactions involving GSH, including the relevant enzymes and functional roles.

Table 2.
Glutathione (GSH)-related enzymatic and non-enzymatic reactions.
GPx catalyzes the reduction of hydrogen peroxide and lipid hydroperoxides using GSH, resulting in glutathione disulfide (GSSG) formation [57]. Subsequently, GR regenerates GSH from GSSG using NADPH [58]. Efficient operation of the GSH redox cycle is critical for maintaining the intracellular redox environment and protecting against oxidative stress associated with aging and disease [13].
Glutaredoxin (Grx, also referred to as thioltransferase or TTase) is a small redox enzyme that catalyzes the reversible S-glutathionylation and deglutathionylation of protein thiols [59,60,61]. This post-translational modification regulates redox signaling and protects cysteine residues from irreversible oxidative damage. Grx also functions as a GSH-dependent reductase, reducing both protein–glutathione mixed disulfides and interprotein disulfide bonds using GSH as an electron donor. This reaction is coupled with the GR reaction, constituting a tight redox cycle that maintains thiol homeostasis and protects cells from oxidative stress.
Glutathione S-transferase (GST) mediates the conjugation of GSH to electrophilic compounds, aiding in the detoxification of xenobiotics and endogenous toxicants [62]. Multidrug resistance-associated protein (MRP) 1, MRP1, MRP2, and MRP4 are involved in transporting GSH conjugates and other GSH-bound metabolites out of cells, further supporting cellular detoxification and GSH homeostasis [63].
GSH also functions through non-enzymatic mechanisms by directly scavenging a broad spectrum of ROS, such as hydroxyl radicals (•OH), superoxide anion radicals (O2•−), and singlet oxygen (1O2) [64]. Superoxide dismutase (SOD) effectively dismutates O2•− to hydrogen peroxide (H2O2) [65]. The resulting H2O2 is subsequently detoxified into water by catalase (CAT), GPx, or peroxiredoxin (Prx) [66]. Moreover, GSH functions as a cofactor in both non-enzymatic and enzymatic (via Grx) pathways for the reduction of dehydroascorbate (DHA) back to ascorbate in human cells [67]. Restored ascorbate plays a vital role in scavenging ROS, regenerating other antioxidants, such as vitamin E, and supporting key biosynthetic functions [68,69].
Additionally, GSH neutralizes RNS, including nitric oxide (NO•) and peroxynitrite (ONOO−), with the latter being generated from the reaction of NO• with O2•− [70]. The S-nitrothiol derivative, nitrosoglutathione (GSNO), formed through the reaction of GSH and NO•, is further metabolized by NADH-dependent GSNO reductase (GSNOR) to an unstable intermediate [GSNHOH], which reacts with GSH, producing GSSG and hydroxylamine [71]. These processes help control nitrosative stress and maintain redox homeostasis.
GSH reacts non-enzymatically and reversibly with reactive aldehydes such as MG and GO to form hemithioacetals in solution [12]. Contrary to previous assumptions, hemithioacetals are not freely circulating substrates for GLO1. Instead, GSH and the reactive aldehyde bind sequentially within the active site of GLO1, where the hemithioacetal intermediate is formed in situ and immediately converted into S-lactoylglutathione or S-glycolylglutathione. These thioester intermediates are subsequently hydrolyzed by GLO2, producing lactate or glycolate, with concomitant regeneration of GSH [40].
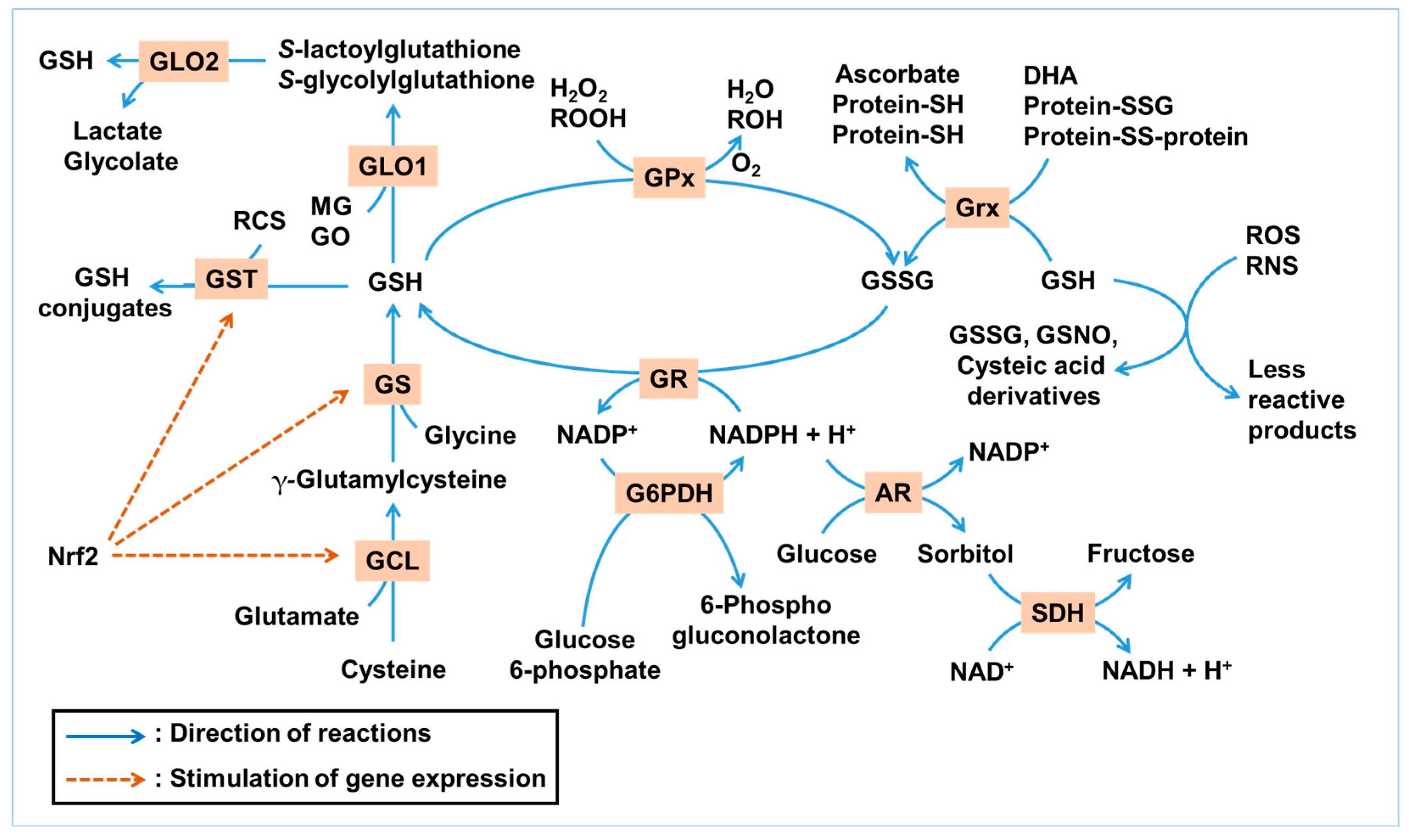
Figure 1 illustrates the GSH-centered redox cycle and its integration with enzymatic pathways that detoxify ROS, RNS, and RCS. This network highlights the interplay between GSH synthesis, utilization, and regeneration under oxidative and glycation stress.
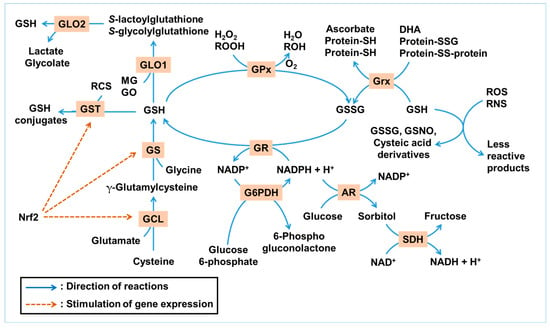
Figure 1.
Glutathione (GSH)-centered antioxidant and antiglycation network. This illustration summarizes the antioxidant and antiglycation roles of GSH under metabolic stress. GSH can directly react non-enzymatically with a range of reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as hydroxyl radicals (•OH), nitric oxide (•NO), and peroxynitrite (ONOO−), resulting in its conversion into GSSG, GSNO, or cysteic acid derivatives, while neutralizing ROS/RNS into less reactive products. Enzymatically, GSH serves as a substrate for glutathione peroxidase (GPx) to reduce hydrogen peroxide (H2O2) and lipid hydroperoxides (ROOH), and for glutaredoxin (Grx) to reduce protein disulfides and dehydroascorbate (DHA). GSH is regenerated from its oxidized form, GSSG, by glutathione reductase (GR) using NADPH. NADPH is mainly regenerated through glucose 6-phosphate dehydrogenase (G6PDH), with contributions from other NADPH-generating enzymes. The GLO system utilizes GSH to detoxify methylglyoxal (MG) and glyoxal (GO). Glutathione S-transferase (GST) conjugates GSH to various electrophilic xenobiotics and reactive carbonyl species (RCS). GSH is synthesized de novo via glutamate–cysteine ligase (GCL) and glutathione synthetase (GS) in two ATP-dependent steps. These enzymes are transcriptionally regulated by nuclear factor erythroid 2-related factor 2 (Nrf2), a master antioxidant response factor. The polyol pathway, initiated by aldose reductase (AR) and followed by sorbitol dehydrogenase (SDH), consumes NADPH during glucose-to-sorbitol conversion, reducing NADPH availability for GSH regeneration. Collectively, this network demonstrates how redox and carbonyl homeostasis in ocular tissues are maintained through a tightly regulated, NADPH-dependent enzymatic system centered on GSH. Blue arrows indicate the directionality of reactions, whereas brown dashed arrows denote the activation of gene expression.
4. GSH Homeostasis in Glycation-Related Eye Diseases
This section provides an integrated overview of findings from in vitro, ex vivo, in vivo, and clinical studies, exploring the impact of glycation stress on GSH homeostasis and therapeutic interventions aimed at preserving or restoring redox balance in ocular tissues. The literature reviewed in this section was primarily retrieved through PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed on 26 April 2025) and Scopus (https://www.scopus.com/, accessed on 26 April 2025) using combinations of keywords, such as ‘advanced glycation end-products (AGEs)’, ‘glutathione’, ‘eyes’, ‘ocular’, ‘oxidative stress’, ‘diabetic’, ‘cataracts’, ‘retinopathy’, and ‘age-related macular degeneration (AMD)’. Relevant in vitro, ex vivo, in vivo, and clinical studies published from 1990 to March 2025 were included.
4.1. In Vitro Studies
Table 3 summarizes studies using in vitro cell culture models to investigate the effects of AGEs or interventions on GSH levels and associated biomarkers in eye-related experimental systems.

Table 3.
Cellular responses to AGEs and effects of different interventions in in vitro models.
AGEs significantly impair antioxidant defenses in ocular cell types, thereby contributing to cellular dysfunction and the development of several eye diseases. Paget et al. observed that exposure to AGE-modified proteins led to divergent effects on CAT and SOD activity depending on the cell type, with pericytes showing increased antioxidant enzyme activity and endothelial cells showing no significant change [72]. These discrepancies highlight the cell-specific susceptibility to AGE-induced oxidative damage and provide insight into early pericyte dropout in DR. Shin et al. reported that glycation induced by high glucose concentrations (50–100 mM) decreased the viability of human lens epithelial cells (HLE-B3) and inactivated key antioxidant enzymes, accompanied by increased lipid peroxidation and DNA damage, suggesting a pro-oxidant shift in redox balance [74].
Several studies highlighted GSH’s central role in mitigating oxidative stress triggered by AGEs. Pigment epithelium-derived factor (PEDF) protected pericytes by decreasing ROS via the upregulation of GSH and SOD, while inhibiting caspase-3 activation through the Src pathway [76]. O-linked N-acetylglucosamine is reversibly added to intracellular proteins by O-linked N-acetylglucosamine transferase (OGT) and removed by O-linked N-acetylglucosaminidase (OGA). In human retinal microvascular endothelial cells, inhibition of OGA by PUGNAc reduced GO-induced ROS and enhanced antioxidant defenses, whereas OGT knockdown using siRNA elevated ROS levels and diminished antioxidant responses [79]. Liu et al. examined the function of TTase in human lens epithelial cells exposed to high glucose and advanced glycation end-products [85]. They found that oxidative stress induced by AGEs decreased CAT and SOD activities and increased the ratio of GSSG to total GSH, suggesting GSH depletion. TTase upregulation was associated with improved redox status, while TTase knockdown exacerbated oxidative damage, highlighting the enzyme’s role in maintaining GSH redox balance.
Numerous bioactive agents and novel compounds demonstrated the potential to prevent or reverse AGE-induced ocular damage through the modulation of oxidative pathways, often restoring GSH levels. Lehman and Ortwerth tested various inhibitors of AGE-associated protein crosslinking, including sulfur-containing compounds like cysteine and GSH, which showed effective inhibition of protein crosslinking processes relevant to ocular tissues [73]. In studies focusing on ocular protein stability, Mantha et al. found that antioxidants like GSH were essential in limiting AGE formation and protein photodegradation under light exposure, reinforcing the significance of GSH in maintaining protein integrity within the eye [83]. Abu-Kheit et al. studied S-allylmercapto-N-acetylcysteine, an antioxidant that increased GSH levels and decreased ROS in AGE-treated bone marrow stromal cells. Although this investigation centered on bone tissue, the study highlighted the systemic antioxidant properties of the compound, suggesting its potential relevance in mitigating AGE-related ocular disorders as well [86].
Fu et al. explored the protective role of berberine, a natural alkaloid compound, on human retinal Müller cells exposed to highly oxidized glycated low-density lipoprotein [81]. They demonstrated that berberine significantly reduced oxidative stress, autophagy, and apoptosis by enhancing adenosine monophosphate-activated protein kinase (AMPK) pathway activity, which included modulation of the GSH-related antioxidant responses. Natural phenolic compounds, such as (−)-epigallocatechin gallate (EGCG), phloretin, 6-shogaol, and 6-gingerol, protected retinal epithelial cells from MGO-induced cytotoxicity by activating Nrf2 and increasing heme oxygenase-1 (HO-1) and GSH expression [80]. Similarly, Wang et al. found that polyphenols like quercetin and cyanidin-3-glucoside not only suppressed AGE-related oxidative stress and photodegradation of a retinal fluorophore A2E (N-retinylidene-N-retinylethanolamine), but also preserved intracellular GSH levels in retinal pigment epithelial cells [82]. Jeon et al. further showed that treatment with caffeic acid significantly decreased AGE-induced oxidative stress and restored GSH levels, suggesting protective effects via β-catenin pathway inhibition [84]. Similarly, moscatilin alleviated oxidative stress in Müller glial cells by inhibiting p38-mitogen-activated protein kinase (MAPK)/c-Jun N-terminal kinase (JNK) and NF-κB signaling, thereby restoring GSH homeostasis and reducing inflammation [88]. Peperomia pellucida extract reduced AGE- and glucose-induced oxidative stress in retinal pigment epithelial cells by increasing GPx expression and modulating NF-κB and peroxisome proliferator-activated receptor gamma (PPAR-γ) signaling pathways [87].
AR inhibitors have been developed as therapeutic agents aimed at restoring intracellular NADPH levels, preserving antioxidant capacity, and mitigating AGE formation [89]. Papastavrou et al. introduced a new class of AR inhibitors with a 1-hydroxypyrazole scaffold, showing improved inhibitory activity and pharmacological profiles, which may complement antioxidant defenses in diabetic ocular pathologies [77]. In addition, silica-based CeCl3 nanoparticles inhibited the glycation of α-crystallin and enhanced chaperone activity while restoring GSH levels under H2O2 stress in lens epithelial cells [78].
The in vitro evidence highlights the crucial role of GSH in defending against AGE-induced oxidative damage across various ocular cell types. AGEs disrupt GSH homeostasis either directly via oxidative mechanisms or indirectly by inhibiting related enzymatic pathways. Therapeutic strategies that restore or enhance GSH levels—through synthetic precursors, natural compounds, enzymatic modulation, or nanomaterial-based delivery—represent promising avenues for mitigating glycation-associated ocular pathologies.
4.2. Ex Vivo Studies
Table 4 summarizes studies using ex vivo organ culture models, such as isolated lenses or retinal tissues, to assess the effects of AGEs or interventions on GSH levels and associated biomarkers.

Table 4.
Glycation-related changes and intervention outcomes in isolated lens and retinal tissues under ex vivo conditions.
Ex vivo lens culture models demonstrated that glycation inducers such as glucose or AGE precursors led to reductions in GSH and increases in AGE accumulation and lens stiffness or opacity. A study analyzing the optical properties of lenses exposed to high glucose found that glucose-induced opacity in human lenses was primarily due to light scattering caused by structural damage [93]. In rat lenses, high-glucose exposure decreased levels of GSH, CAT, and AR while increasing RAGE [93]. The models provide a valuable system for testing agents targeting redox balance and AGE formation.
In the organ culture model, exposure to glyceraldehyde significantly elevated MG and MG-derived AGE levels, leading to a sharp decrease in GSH [90]. Inhibition of GLO1 with S-[N-hydroxy-N-(4-chlorophenyl)carbamoyl]glutathione (HCCG) diester further enhanced MG accumulation [90]. Although GSH was expected to detoxify MG via the GLO pathway, experimental data suggested no consistent correlation between GSH levels and MG-AGE accumulation. This inconsistency may reflect the saturation or inhibition of GLO1 activity under severe glycation stress.
Padival and Nagaraj demonstrated the ability of pyridoxamine (PYM) to inhibit AGE formation in diabetic rat lenses [91]. In both in vivo and organ culture settings, PYM reduced levels of argpyrimidine and pentosidine. Furthermore, PYM restored lens GSH levels and increased GLO1 activity, implicating it as a promising therapeutic agent that not only scavenges dicarbonyl precursors but also enhances endogenous antioxidant defense.
Abdelkader et al. investigated the anticataractogenic mechanisms of carnosine, a naturally occurring dipeptide, in porcine and human lens models [92]. Their in vitro and ex vivo assays showed potent antiglycating properties but weak direct antioxidant and metal-chelating effects. Carnosine inhibited AGE formation induced by galactose in porcine lenses and maintained sulfhydryl group integrity in bovine lens homogenates.
Nandi et al. developed carboxitin, a compound that combines GSH diester with mercaptoethylguanidine, to target AGE-induced protein crosslinking in mouse lenses [94]. Carboxitin replenished GSH levels and inhibited glycation-mediated protein aggregation and lens stiffening. It blocked ascorbate-derived AGE precursors such as 3-deoxythreosone (3-DG), supporting its dual action as a GSH booster and dicarbonyl scavenger.
Overall, these findings suggest that GSH, PYM, carnosine, and carboxitin may help restore antioxidant capacity and attenuate glycation-related damage in eye tissues.
4.3. In Vivo Studies
Table 5 presents in vivo studies evaluating the effects of AGEs and interventions in models of diabetic and age-related eye diseases. Numerous animal studies have explored the complex relationship between glycation stress and GSH depletion, highlighting the pathophysiological mechanisms and testing diverse therapeutic agents.

Table 5.
In vivo effects of AGEs and therapeutic interventions in animal models.
Dietary calorie restriction in Emory mice significantly extended the median lifespan by 40% and delayed cataract progression compared to control-fed animals [97]. It reduced plasma glucose and glycohemoglobin levels, enhanced liver GSH levels, and decreased markers of oxidative stress and glycation, suggesting that the suppression of oxidative and glycation damage contributes to a slowdown in cataract development and other age-associated changes [97].
A 5% or 25% galactose diet administered to pigs resulted in polyol accumulation, a slight depletion of GSH, and increased protein glycation in the lens [95]. While a 5% galactose diet induced only initial cataractogenic changes, the 25% diet caused significant lens damage characterized by inositol loss and enhanced protein glycation. In galactosemic rats, pentosidine accumulation linked to oxidative stress was suppressed by an AR inhibitor, i.e., sorbinil, preventing DC formation [96].
In a chemically (with alloxane) induced diabetes model, lipid peroxidation was elevated while antioxidant enzyme activities (SOD and GPx) and nitric oxide production were diminished [98]. Melatonin supplementation restored these parameters to near-normal levels independent of blood glucose control, suggesting its potent antioxidant role [98].
In streptozotocin (STZ)-induced diabetic rats, early insulin treatment significantly improved hyperglycemia, lipid abnormalities, glycosylated hemoglobin levels, oxidative stress markers, advanced glycation end-product accumulation, and the ratio of GSH to GSSG. Early intervention prevented the development of sensory neuropathy and diabetic cataracts (DCs), whereas late insulin treatment failed to show these protective effects [103]. Additionally, vitamin K1 administration in diabetic rats partially reduced hyperglycemia and normalized lens levels of sorbitol, AGEs, calcium, Ca2+-ATPase activity, and oxidative stress markers [105]. These effects contributed to the inhibition of DC progression, suggesting vitamin K1 as a promising therapeutic agent.
PYM treatment in STZ-induced diabetic rats inhibited the formation of major AGEs such as argpyrimidine and pentosidine in lenses, possibly by scavenging RCS and enhancing AR activity [91]. Although PM decreased AGE accumulation, it did not fully prevent DC development. In hSVCT2 transgenic mice characterized by ascorbate-induced lenticular glycation, nucleophilic compounds, NC-I (i.e., arginine) and NC-II, but not aminoguanidine (AGD), PYM, or penicillamine, reduced AGE levels such as pentosidine, CML, and CEL [75].
Aspirin has been shown to exert antioxidant effects through the inhibition of NF-κB and reduction in AGE formation [100]. Topical administration of carnosine, AGD, or aspirin in diabetic rats delayed DC progression during early stages by inhibiting AGE accumulation and preserving antioxidant enzymes like CAT and GR [100]. However, their protective effect diminished with severe hyperglycemia, suggesting that excessive glycation overwhelms pharmacological interventions at later stages.
Silica-based CeCl3 nanoparticles and S-allylmercapto-N-acetylcysteine significantly restored lens GSH levels and inhibited AGE-induced oxidative stress and protein aggregation [86,106]. Cemtirestat [113], an AR inhibitor, improved GSH redox status in diabetic eyes.
Natural products like nigerloxin (a fungal metabolite) [101,104] and plant-derived compounds, such as curcumin, astaxanthin, and chrysin [99,109,110], displayed protective effects by modulating antioxidant enzyme activities and maintaining redox balance. Berberine and morin were effective in mitigating AGE-induced damage in DC and DR models [111,112]. Moscatilin inhibited the MAPK/NF-κB pathway and inflammation in Müller cells [88]. Rosa damascena hydrosol and extracts of Anemarrhena asphodeloides and Zhujing pill reduced malondialdehyde (MDA) levels, restored GSH or CAT activity, and suppressed AGE accumulation, thereby preserving lens structure and function [102,107,108].
Animal models, particularly galactose-fed and STZ-induced diabetic rats, have provided significant insights into the relationship between AGEs, oxidative stress, and GSH metabolism in the lens and retina. The collected animal studies provide robust evidence that glycation-induced oxidative stress plays a central role in the pathogenesis of ocular diseases, particularly through the depletion of GSH and disruption of redox balance. Interventions using natural antioxidants, plant-based compounds, synthetic molecules, and nanomaterials have shown promise in preserving or restoring GSH levels and preventing glycation-mediated lens and retinal damage. These findings strongly support the therapeutic relevance of targeting glycation and redox imbalance to prevent or treat DC, DR, and other ocular complications.
4.4. Clinical Studies
Table 6 summarizes human clinical studies analyzing the effects of AGEs and interventions in ocular conditions.

Table 6.
Clinical studies on the effects of AGEs and therapeutic interventions in human subjects.
Ahmed et al. demonstrated that CEL accumulates with age in human lens proteins, causing age-related cataracts [114]. In Eales’ disease, with an inflammatory retinal condition, GSH and GPx levels were significantly reduced in both active and healed phases, indicating a chronic redox imbalance [115]. Hashim and Zarina reported that lenses from DC patients showed significantly lower levels of GSH, GPx, GR, and G6PDH compared to non-diabetic cataract patients [116]. In contrast, AR and SDH activities were increased, indicating polyol pathway activation and oxidative imbalance.
Géhl et al. analyzed vitreous humor from patients with proliferative DR and found elevated levels of AGEs and protein carbonyls, confirming oxidative stress [118]. Interestingly, GSH levels were also higher in diabetic samples, possibly reflecting a compensatory antioxidant response to increased oxidative burden. Mynampati et al. examined clear, cortical, and nuclear cataract lenses [119]. They found a significant decrease in GSH in nuclear cataract lenses (a form of age-related cataracts) along with a marked increase in the AGE argpyrimidine, supporting the role of AGEs in promoting redox imbalance and lens opacity.
Babizhayev et al. explored the role of oxidative stress and genetic polymorphisms in antioxidant enzymes in the development of diabetic neuropathy (DN) in type 1 diabetic patients [117]. The study assessed gene variants in CAT, GPx, and GSTs, and found that DN patients exhibited lower levels of GSH and decreased GPx activity compared to those without DN. Specifically, the -262T/T genotype of the CAT gene was associated with higher CAT activity and lower susceptibility to DN. In vitro experiments showed that glycation reactions involving MG generated free radicals, including superoxide, contributing to oxidative stress. The application of SOD-mimetic peptides significantly reduced radical generation, confirming the antioxidant pathway’s protective role. The authors suggested that antioxidant enzyme genotyping may help identify individuals at higher risk for DN and that peptide-based antioxidants may offer a preventive therapeutic approach. Supplementation with antioxidants such as vitamin C and E demonstrated improvements in oxidative defense, while GSH levels varied depending on the disease stage and tissue type [115,120].
Overall, clinical evidence supports the involvement of AGE accumulation and oxidative stress in both age-related and diabetic ocular conditions. However, it is noteworthy that most clinical studies referenced in this review involved relatively small patient cohorts (typically fewer than 100 participants), which may limit the generalizability of the findings.
5. Discussion
5.1. Mechanistic Insights into GSH Depletion and Redox Imbalance in Glycation-Related Eye Diseases
The eye is vulnerable to oxidative and glycation stress due to its high oxygen consumption, continuous exposure to light, and accumulation of long-lived proteins in avascular tissues [121]. Anatomically, ocular structures such as the lens, retina, and vitreous humor are highly susceptible to oxidative insults and glycation-related damage owing to their limited regenerative capacity and low antioxidant enzyme turnover [122]. The avascular nature of the lens and avascular zones of the retina render these compartments especially dependent on cellular redox systems, and GSH homeostasis is increasingly recognized as a key dynamic biomarker in glycation-related ocular diseases.
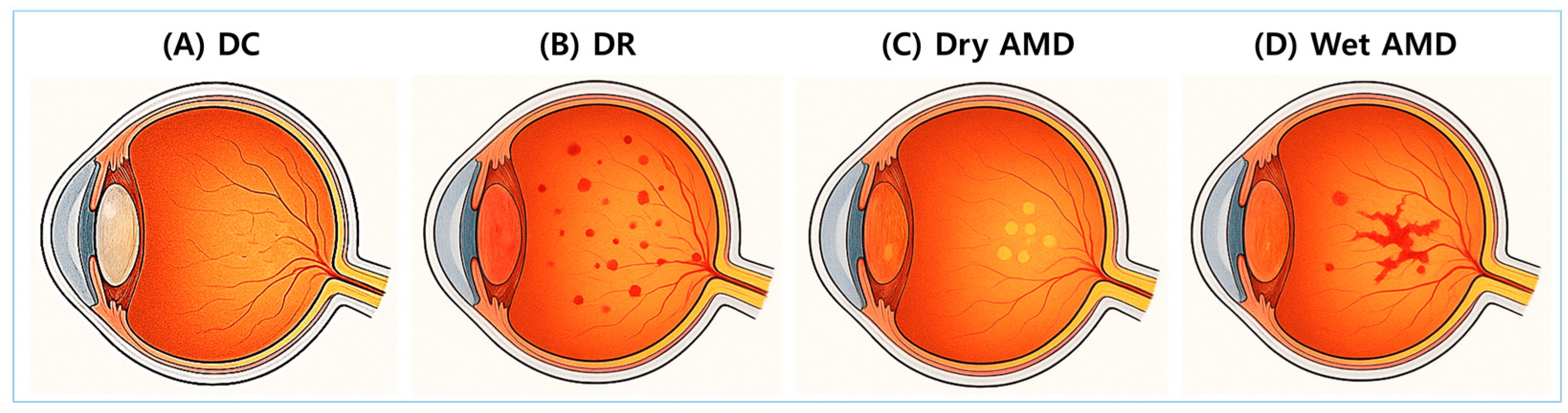
DC, DR, and AMD are representative glycation-related eye diseases. Figure 2 provides anatomical illustrations of DC, DR, dry AMD, and wet AMD, highlighting distinct damage sites and pathological differences.
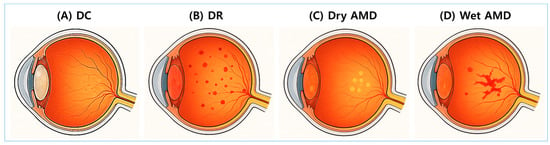
Figure 2.
Anatomical illustrations of major glycation-related eye diseases. Cross-sectional images of the eye illustrate four major ocular complications associated with glycation and oxidative stress. (A) Diabetic cataracts (DCs): A condition characterized by opacification of the lens. (B) Diabetic retinopathy (DR): A diabetes-related complication involving progressive retinal vascular damage. (C) Dry age-related macular degeneration (AMD): A condition characterized by the accumulation of drusen and atrophy of the retinal pigment epithelium (RPE). (D) Wet AMD: A condition marked by abnormal neovascularization beneath the retina and within the subretinal space.
In DC, the lens is primarily affected. The lens contains crystallin proteins that must remain transparent and functionally stable throughout life. These proteins are long-lived and exposed to chronic oxidative and glycation stress, especially under hyperglycemic conditions [123]. The lens epithelium also highly expresses glucose transporters and AR, facilitating flux through the polyol pathway [124]. Activation of the polyol pathway in the lens epithelium leads to the accumulation of sorbitol, which induces osmotic stress and promotes the glycation of crystallin proteins [73,116]. This process is associated with a marked decline in GSH levels and reduced activities of antioxidant enzymes such as GPx and GR, resulting in an elevated GSSG/total GSH ratio [74,78,84,85,119]. Clinically, these biochemical alterations correlate with increased lens opacity and cataract severity, particularly in aging and diabetic populations [78,116,119].
The retina is characterized by intense metabolic activity, rich mitochondrial density, and high oxygen tension, making it a prime target for ROS and AGE accumulation [125]. The outer retina, including photoreceptors and the retinal pigment epithelium (RPE), continuously metabolizes visual cycle by-products and polyunsaturated fatty acids (PUFAs) susceptible to oxidative and glycoxidative damage [126]. A key pathological axis links glycation-induced oxidative stress with pathological angiogenesis: GSH depletion aggravates redox imbalance and activates pro-angiogenic signaling pathways, including NF-κB and MAPK, ultimately upregulating vascular endothelial growth factor (VEGF) expression [87,88]. VEGF functions as both a marker and a mediator of this pathological neovascularization [127]. In DR and wet AMD, angiogenesis is primarily driven by sustained redox imbalance and RAGE signaling, both of which are induced by chronic oxidative and glycation stress. While neovascularization in DR originates from the retinal vasculature and leads to vitreous hemorrhage and fibrovascular proliferation, angiogenesis in wet AMD arises from the choroid, resulting in macular disruption, subretinal fluid accumulation, and rapid central vision loss [128,129].
In DR, retinal vasculature cells, particularly endothelial cells, pericytes, and Müller cells, are damaged. This condition is particularly associated with AGE accumulation in retinal capillaries, leading to pericyte loss, vascular leakage, and neovascularization [130]. Chronic hyperglycemia induces the accumulation of AGEs, which activate RAGE signaling and promote the production of ROS and pro-inflammatory cytokines [76,79,80,85,87,88]. This pathological cascade contributes to GSH depletion in retinal endothelial and Müller cells, along with decreased activities of SOD and GPx, thereby exacerbating oxidative damage [76,79,85]. GSH status in the retina has been proposed as a biomarker for both oxidative stress and inflammatory progression in DR and is a promising target for antioxidant-based therapeutic intervention [80,85,87,88,117].
AMD is a progressive neurodegenerative disorder of the macula that is characterized by chronic inflammation, mitochondrial dysfunction, and progressive loss of photoreceptors [131]. It is a leading cause of vision loss in the elderly and exists in two major forms—dry (non-neovascular) and wet (neovascular)—which differ in their pathological mechanisms and clinical progression [132]. Dry AMD is primarily characterized by the accumulation of drusen—extracellular deposits composed of lipids, proteins, and AGEs—between the RPE and Bruch’s membrane [132]. This form of AMD is marked by RPE atrophy and slow photoreceptor loss, predominantly affecting the central macula. In contrast, wet AMD represents an advanced stage of the disease characterized by the pathological upregulation of VEGF, which promotes the formation of choroidal neovascular membranes. These aberrant vessels breach Bruch’s membrane and invade the subretinal space, resulting in fluid leakage, hemorrhage, and rapid central vision loss.
5.2. Therapeutic Strategies to Restore GSH Homeostasis Under Glycation Stress
The consistent association between GSH depletion, oxidative stress, and AGE accumulation reinforces the rationale for therapeutic strategies that aim to restore GSH levels and enhance endogenous redox capacity. These strategies are categorized into four mechanistic domains: (I) promoting GSH supply and synthesis, (II) enhancing GSH recycling, (III) mitigating glycation stress, and (IV) reducing oxidative and nitrosative stress. Most of these strategies have been experimentally explored with different approaches. Key findings summarized below support the therapeutic efficacy of these strategies in preserving GSH homeostasis under glycation-induced oxidative stress.
5.2.1. GSH Supply and Synthesis
- Approach 1: Supplementation with GSH precursors
Cysteine is the rate-limiting substrate for intracellular GSH biosynthesis, as its availability directly regulates the activity of GCL, the rate-limiting enzyme in the GSH synthetic pathway [52]. N-acetylcysteine and related thiol donors, such as S-allylmercapto-N-acetylcysteine, increased intracellular GSH levels and reduced ROS in AGE-stimulated ocular and non-ocular cells [86].
- Approach 2: Exogenous GSH administration
Direct supplementation with GSH bypasses the need for de novo synthesis and rapidly increases intracellular GSH levels [133,134]. Exogenously administered GSH inhibited AGE-induced crosslinking in lens proteins [73]. S-acetylated GSH analogs improved GSH levels in various experimental systems and showed protective effects against ROS and apoptosis [86].
- Approach 3: Activation of the Nrf2 pathway
Nrf2 activation leads to the transcriptional upregulation of antioxidant response element (ARE)-driven genes, including GCL, GS, and GST [52]. Polyphenolic compounds, such as (−)-EGCG, phloretin, and shogaol, activated Nrf2 signaling in RPE cells, upregulating antioxidant enzymes and increasing intracellular GSH levels in response to dicarbonyl stress [80].
5.2.2. GSH Recycling
- Approach 4: Activation of GR
GR preserves the GSH/GSSG ratio and suppresses ROS accumulation [58]. Grx (also called TTase) overexpression enhanced GSSG reduction and preserved the GSH/GSSG ratio in AGE-stressed ocular models, whereas knockdown exacerbated redox imbalance and ROS accumulation [85].
- Approach 5: Inhibition of AR
As discussed in Section 2.3, AR hyperactivation depletes NADPH and promotes sorbitol accumulation. Inhibiting AR activity has therefore emerged as a strategy to preserve NADPH for GSH regeneration. AR inhibitors, including novel 1-hydroxypyrazole-based compounds and the natural alkaloid berberine, have been shown to effectively suppress polyol pathway flux, preserve NADPH availability, and mitigate GSH depletion in retinal endothelial cells and Müller cells exposed to AGEs [77,81]. In vivo, agents such as berberine, cemtirestat, and stobadine reduced sorbitol levels, decreased AGE and CML accumulation, and improved GSH/GSSG ratios in diabetic rat models [111,113].
- Approach 6: Activation of the pentose phosphate pathway (PPP)
Activation of this pathway, particularly through G6PDH—the rate-limiting enzyme of the PPP—enhances NADPH generation, which is crucial for GSH recycling [135]. In diabetic retinal models, the upregulation of G6PDH activity increased NADPH levels and restored the antioxidant capacity by improving the GSH/GSSG ratio [136].
5.2.3. Mitigation of Glycation Stress
- Approach 7: Activation of GLO system
While the biochemical mechanism of GLO1 has been described in Section 3.2, its therapeutic efficacy varies depending on tissue context and experimental conditions. For example, PYM was shown to enhance GLO1 activity and restore GSH levels in some diabetic and organ culture models, but the extent of its protective effects varied depending on experimental conditions [91]. Intriguingly, the inhibition of GLO1 using HCCG diester led to paradoxical outcomes—elevated MG levels but reduced MG-derived AGEs—suggesting uncoupling between MG availability and AGE formation under certain contexts [90].
- Approach 8: Inhibition of AGE formation
Trapping RCS, such as GO, MG, and glycolaldehyde, represents an effective strategy to suppress AGE formation and reduce GSH consumption [137,138]. AGD reduced CML and CEL formation in diabetic lens models and preserved antioxidant enzyme activity, although its efficacy varied depending on the glycation context [75,99]. PYM and carnosine significantly reduced the levels of pentosidine and argpyrimidine in diabetic and galactose-exposed lenses, respectively, while also preventing GSH depletion and inhibiting protein aggregation in ex vivo systems [92,100]. Carboxitin exhibited dual antiglycation and antioxidant actions by restoring GSH levels, blocking AGE-mediated protein crosslinking, and reducing lens stiffness in ascorbate-accelerated glycation models [94].
5.2.4. Mitigation of Oxidative/Nitrosative Stress
- Approach 9: Direct scavenging of ROS/RNS
Direct scavengers of ROS and RNS reduce oxidative pressure on the intracellular GSH pool and protect ocular tissues from redox imbalance. By neutralizing highly reactive species such as •OH, O2•⁻, •NO, and ONOO⁻, these compounds help prevent lipid peroxidation, protein nitration, and mitochondrial dysfunction [139]. Antioxidants such as astaxanthin have effectively restored GSH levels and mitigated AGE-induced oxidative stress in ocular models [109]. Supplementation of vitamins C and E has been shown to improve ocular oxidative defense in clinical studies [115,120].
- Approach 10: Inhibition of ROS/RNS-generating enzymes
Endogenous production of ROS and RNS is enzymatically mediated by key oxidoreductases, including NADPH oxidases (NOX), xanthine oxidase (XO), nitric oxide synthase (NOS), and myeloperoxidase (MPO) [140,141,142,143]. These enzymes are upregulated in response to glycation stress and chronic inflammation, contributing to GSH depletion and oxidative damage [144]. Pharmacological inhibition of these enzymes reduces intracellular oxidant burden, preserves NADPH availability, and supports GSH recycling [145]. While specific inhibitors of these enzymes have shown efficacy in systemic diseases, their applicability in ocular conditions remains underexplored and requires further investigation.
5.3. Targeting GSH Homeostasis vs. VEGF
Interventions targeting GSH primarily act at upstream levels by alleviating oxidative and carbonyl stress, restoring redox balance, and enhancing intracellular detoxification mechanisms [146]. In contrast, anti-VEGF therapies act on downstream pathophysiological events, particularly by suppressing abnormal angiogenesis and vascular permeability [147]. Despite their mechanistic differences, emerging evidence suggests a pathological link between GSH depletion and VEGF overexpression. This link has been observed in DR and wet AMD, where chronic oxidative stress and GSH deficiency synergistically drive inflammation and neovascularization [148].
Several studies have demonstrated that antioxidant and redox-modulating agents such as morin, cemtirestat, and moscatilin not only restore GSH levels but also suppress VEGF expression in ocular models of glycation stress, indicating that the modulation of GSH homeostasis may indirectly regulate VEGF-driven angiogenic responses [88,112,113]. These findings support the rationale for a dual-targeting strategy that combines redox-based interventions with VEGF inhibition (Figure 3). In advanced stages of DR or wet AMD, where both oxidative damage and neovascularization coexist, such a combined approach may offer synergistic therapeutic benefits [130,132]. Further research is needed to elucidate the cooperative effects of this integrated therapeutic strategy.
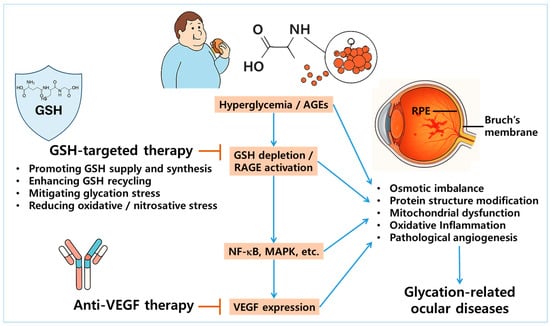
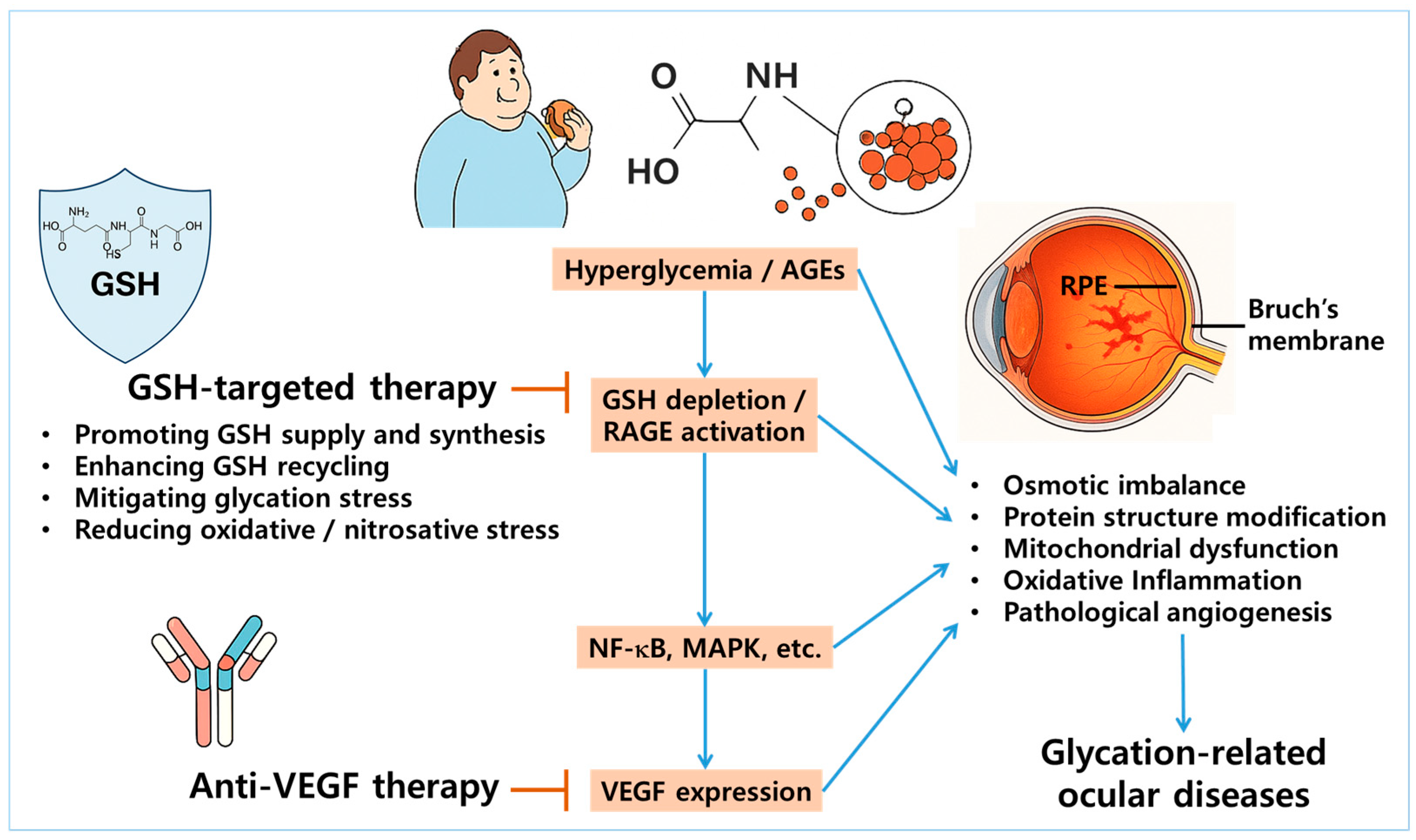
Figure 3.
Rationale for dual targeting of GSH homeostasis and VEGF pathways in glycation-related ocular diseases. Chronic hyperglycemia and accumulation of AGEs promote GSH depletion and activate the receptor for AGEs (RAGE), leading to the downstream stimulation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways. This cascade ultimately results in the overexpression of vascular endothelial growth factor (VEGF), driving pathological angiogenesis in ocular tissues—particularly within the RPE and Bruch’s membrane. The illustration highlights this mechanistic interplay as a therapeutic rationale for dual targeting: restoring GSH homeostasis to mitigate oxidative and glycation-induced stress, while concurrently inhibiting VEGF to suppress neovascularization. Arrows represent stimulation, whereas blunted bars indicate prevention or inhibition.
5.4. Limitations of Current Studies and Future Directions
Despite the encouraging preclinical findings, several limitations are apparent across existing studies. Many investigations have relied predominantly on in vitro or organ culture models, which do not fully replicate the complexity of in vivo ocular environments, including systemic influences and tissue interactions. Significant heterogeneity exists among experimental models, types of glycation inducers, disease stages examined, and intervention protocols, making direct comparisons and generalizations challenging. Clinical translation remains limited; although observational data support the importance of GSH in ocular health, few well-designed clinical trials have specifically evaluated interventions aimed at restoring GSH homeostasis in eye diseases.
Future research should aim to address these gaps by developing targeted ocular delivery systems capable of efficiently delivering GSH precursors, mimetics, or antioxidant molecules to affected tissues. Identifying and optimizing novel Nrf2 activators with improved specificity and safety will be important for enhancing endogenous antioxidant defenses. In parallel, strategies targeting key enzymatic regulators of glycation and redox stress should be further explored. Enhancing GLO system activity may reduce AGE formation while preserving GSH pools, making it a promising therapeutic target in glycation-related ocular damage. Conversely, the inhibition of AR, the first enzyme of the polyol pathway, can prevent excessive NADPH depletion and mitigate downstream glycation and oxidative stress. A deeper mechanistic understanding of the crosstalk between glycation signaling, redox regulation, mitochondrial dysfunction, and inflammation will be critical for refining therapeutic strategies. Large-scale, randomized clinical trials are urgently needed to evaluate the efficacy of GSH-enhancing interventions in preventing or mitigating DC, DR, AMD, and other glycation-related ocular disorders.
Although VEGF inhibitors effectively suppress angiogenesis, they do not address the upstream redox imbalance. GSH-based interventions could act earlier in the pathophysiological cascade, potentially enhancing VEGF inhibitor efficacy and preventing resistance or recurrence. To date, no clinical trials have explicitly tested the combination therapy of VEGF inhibitors with GSH-based interventions in ocular diseases. However, this represents a promising direction for future translational studies.
6. Conclusions
GSH homeostasis plays a central role in maintaining redox balance across ocular tissues, with disease-specific patterns of dysregulation contributing to the pathogenesis of glycation-related eye diseases. In DC, GSH depletion is primarily driven by osmotic imbalance and glycation-induced stress within the lens matrix. In DR, chronic oxidative inflammation perturbs GSH regulation in retinal vascular and glial cells. In AMD, mitochondrial dysfunction and angiogenic stress, particularly in the macular region, further compromise GSH-dependent antioxidant defense. Therapeutic strategies aimed at enhancing GSH synthesis, recycling, or preservation have demonstrated promising results in preclinical models. These findings support the rationale for GSH-targeting redox-modulating interventions that could be used either in combination with or as an alternative to anti-VEGF therapy in certain glycation-related ocular disorders.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: RS-2024-00437643).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
Sincere gratitude is expressed to Min Young Boo of Cheil Eye Hospital in Daegu, Republic of Korea, the city’s sole designated ophthalmology specialty hospital, for his expert clinical review and invaluable advice on this study. Generative AI tools (e.g., ChatGPT) were utilized to assist with language editing and figure generation during the preparation of this manuscript. All AI-generated content was critically reviewed, edited, and validated by the authors. The authors assume full responsibility for the accuracy and integrity of the scientific content presented in this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 3-DG | 3-deoxyglucosone |
| A2E | N-retinylidene-N-retinylethanolamine |
| AGD | aminoguanidine |
| AGE | advanced glycation end-product |
| AGE-R | advanced glycation end-product receptor |
| AKR | aldo-keto reductase |
| ALDH | aldehyde dehydrogenase |
| AMD | age-related macular degeneration |
| AMPK | adenosine monophosphate-activated protein kinase |
| AOPP | advanced oxidation protein product |
| AR | aldose reductase |
| ARE | antioxidant response element |
| BSA | bovine serum albumin |
| CAT | catalase |
| CEL | Nε-(carboxyethyl)lysine |
| ChaC | glutathione-specific γ-glutamylcyclotransferase |
| CML | Nε-(carboxymethyl)lysine |
| DCs | diabetic cataracts |
| dG | deoxyguanosine |
| DHA | dehydroascorbate |
| DN | diabetic neuropathy |
| DNA | deoxyribonucleic acid |
| DR | diabetic retinopathy |
| EMT | epithelial–mesenchymal transition |
| EGCG | epigallocatechin gallate |
| ER | endoplasmic reticulum |
| FL | fructoselysine |
| FR3K | fructosamine-3-kinase |
| G6PDH | glucose-6-phosphate dehydrogenase |
| GCL | γ-glutamylcysteine ligase |
| GFAP | glial fibrillary acidic protein |
| GGT | γ-glutamyl transpeptidase |
| GGCT | γ-glutamylcyclotransferase |
| GLO | glyoxalase |
| GO | glyoxal |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| Grx | glutaredoxin |
| GS | glutathione synthetase |
| GSH | glutathione |
| GSNOR | S-nitrosoglutathione reductase |
| GSSG | glutathione disulfide |
| GST | glutathione S-transferase |
| H1 | hydroimidazolone-1 |
| HO-1 | heme oxygenase-1 |
| HCCG | S-[N-hydroxy-N-(4-chlorophenyl)carbamoyl]glutathione |
| IL | interleukin |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MCP | monocyte chemoattractant protein |
| MDA | malondialdehyde |
| MG | methylglyoxal |
| MMP | matrix metalloproteinase |
| MPO | myeloperoxidase |
| MRP | multidrug resistance-associated protein |
| NC | nucleophilic compound |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOS | nitric oxide synthase |
| NOX | NADPH oxidase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OGA | O-linked N-acetylglucosaminidase |
| OGT | O-linked N-acetylglucosamine transferase |
| PARK | Parkinsonism-associated deglycase |
| PEDF | pigment epithelium-derived factor |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| PPP | pentose phosphate pathway |
| PUFA | polyunsaturated fatty acid |
| PUGNAc | O-(2-Acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate |
| PYM | pyridoxamine |
| Prx | peroxiredoxin |
| RAGE | receptor for advanced glycation end-products |
| RCS | reactive carbonyl species |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelium |
| SDH | sorbitol dehydrogenase |
| siRNA | small interfering RNA |
| SOD | superoxide dismutase |
| STZ | streptozotocin |
| TNF | tumor necrosis factor |
| TTase | thioltransferase |
| VEGF | vascular endothelial growth factor |
| XO | xanthine oxidase |
References
- Lam, N.V. Adult Eye Conditions: Diabetic Retinopathy and Age-Related Macular Degeneration. FP Essent. 2022, 519, 24–28. [Google Scholar] [PubMed]
- Cvekl, A.; Vijg, J. Aging of the eye: Lessons from cataracts and age-related macular degeneration. Ageing Res. Rev. 2024, 99, 102407. [Google Scholar] [CrossRef] [PubMed]
- Castro-Castaneda, C.R.; Altamirano-Lamarque, F.; Ortega-Macias, A.G.; Santa Cruz-Pavlovich, F.J.; Gonzalez-De la Rosa, A.; Armendariz-Borunda, J.; Santos, A.; Navarro-Partida, J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients 2022, 14, 5014. [Google Scholar] [CrossRef]
- Bejarano, E.; Taylor, A. Too sweet: Problems of protein glycation in the eye. Exp. Eye Res. 2019, 178, 255–262. [Google Scholar] [CrossRef]
- Yamagishi, S.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. et Biophys. Acta 2012, 1820, 663–671. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Salazar, J.; Navarro, C.; Ortega, Á.; Nava, M.; Morillo, D.; Torres, W.; Hernández, M.; Cabrera, M.; Angarita, L.; Ortiz, R.; et al. Advanced Glycation End Products: New Clinical and Molecular Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 7236. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Ha, N.G.; Lee, W.J.; Boo, Y.C. Synthetic and Natural Agents Targeting Advanced Glycation End-Products for Skin Anti-Aging: A Comprehensive Review of Experimental and Clinical Studies. Antioxidants 2025, 14, 498. [Google Scholar] [CrossRef]
- Nagaraj, R.H.; Linetsky, M.; Stitt, A.W. The pathogenic role of Maillard reaction in the aging eye. Amino Acids 2012, 42, 1205–1220. [Google Scholar] [CrossRef]
- Giblin, F.J. Glutathione: A vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000, 16, 121–135. [Google Scholar] [CrossRef]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase system: A systematic review of its biological activity, related-diseases, screening methods and small molecule regulators. Biomed. Pharmacother. 2020, 131, 110663. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Lim, J.C.; Jiang, L.; Lust, N.G.; Donaldson, P.J. Minimizing Oxidative Stress in the Lens: Alternative Measures for Elevating Glutathione in the Lens to Protect against Cataract. Antioxidants 2024, 13, 1193. [Google Scholar] [CrossRef]
- Kumar, R.S.; Anthrayose, C.V.; Iyer, K.V.; Vimala, B.; Shashidhar, S. Lipid peroxidation and diabetic retinopathy. Indian. J. Med. Sci. 2001, 55, 133–138. [Google Scholar] [PubMed]
- Pescosolido, N.; Barbato, A.; Giannotti, R.; Komaiha, C.; Lenarduzzi, F. Age-related changes in the kinetics of human lenses: Prevention of the cataract. Int. J. Ophthalmol. 2016, 9, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Babel, R.A.; Dandekar, M.P. A Review on Cellular and Molecular Mechanisms Linked to the Development of Diabetes Complications. Curr. Diabetes Rev. 2021, 17, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Monnier, V.M.; Whitson, J. Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 2017, 156, 103–111. [Google Scholar] [CrossRef]
- Lim, J.C.; Suzuki-Kerr, H.; Nguyen, T.X.; Lim, C.J.J.; Poulsen, R.C. Redox Homeostasis in Ocular Tissues: Circadian Regulation of Glutathione in the Lens? Antioxidants 2022, 11, 1516. [Google Scholar] [CrossRef]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Grune, T. Degradation of oxidized and glycoxidized collagen: Role of collagen cross-linking. Arch. Biochem. Biophys. 2014, 542, 56–64. [Google Scholar] [CrossRef]
- Kinoshita, S.; Mera, K.; Ichikawa, H.; Shimasaki, S.; Nagai, M.; Taga, Y.; Iijima, K.; Hattori, S.; Fujiwara, Y.; Shirakawa, J.I.; et al. N(ω)-(Carboxymethyl)arginine Is One of the Dominant Advanced Glycation End Products in Glycated Collagens and Mouse Tissues. Oxid. Med. Cell. Longev. 2019, 2019, 9073451. [Google Scholar] [CrossRef]
- Uceda, A.B.; Mariño, L.; Casasnovas, R.; Adrover, M. An overview on glycation: Molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophys. Rev. 2024, 16, 189–218. [Google Scholar] [CrossRef]
- Crisan, M.; Taulescu, M.; Crisan, D.; Cosgarea, R.; Parvu, A.; Cãtoi, C.; Drugan, T. Expression of advanced glycation end-products on sun-exposed and non-exposed cutaneous sites during the ageing process in humans. PLoS ONE 2013, 8, e75003. [Google Scholar] [CrossRef] [PubMed]
- Isami, F.; West, B.J.; Nakajima, S.; Yamagishi, S.I. Association of advanced glycation end products, evaluated by skin autofluorescence, with lifestyle habits in a general Japanese population. J. Int. Med. Res. 2018, 46, 1043–1051. [Google Scholar] [CrossRef]
- van de Zande, S.C.; de Vries, J.K.; van den Akker-Scheek, I.; Zwerver, J.; Smit, A.J. A physically active lifestyle is related to a lower level of skin autofluorescence in a large population with chronic-disease (LifeLines cohort). J. Sport Health Sci. 2022, 11, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Bellier, J.; Nokin, M.J.; Lardé, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcène, A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Simm, A.; Wagner, J.; Gursinsky, T.; Nass, N.; Friedrich, I.; Schinzel, R.; Czeslik, E.; Silber, R.E.; Scheubel, R.J. Advanced glycation endproducts: A biomarker for age as an outcome predictor after cardiac surgery? Exp. Gerontol. 2007, 42, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J. Advanced glycation endproducts: What is their relevance to diabetic complications? Diabetes Obes. Metab. 2007, 9, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018, 93, 803–813. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Z.; Lv, C.; Li, M.; Wang, K.; Chen, Z. Advanced Glycation End Products and Health: A Systematic Review. Ann. Biomed. Eng. 2024, 52, 3145–3156. [Google Scholar] [CrossRef]
- Casselmann, C.; Reimann, A.; Friedrich, I.; Schubert, A.; Silber, R.E.; Simm, A. Age-dependent expression of advanced glycation end product receptor genes in the human heart. Gerontology 2004, 50, 127–134. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Munteanu, M.C.; Dinischiotu, A. RAGE and TGF-β1 Cross-Talk Regulate Extracellular Matrix Turnover and Cytokine Synthesis in AGEs Exposed Fibroblast Cells. PLoS ONE 2016, 11, e0152376. [Google Scholar] [CrossRef]
- Takahashi, A.; Takabatake, Y.; Kimura, T.; Maejima, I.; Namba, T.; Yamamoto, T.; Matsuda, J.; Minami, S.; Kaimori, J.Y.; Matsui, I.; et al. Autophagy Inhibits the Accumulation of Advanced Glycation End Products by Promoting Lysosomal Biogenesis and Function in the Kidney Proximal Tubules. Diabetes 2017, 66, 1359–1372. [Google Scholar] [CrossRef]
- Lages, N.F.; Cordeiro, C.; Sousa Silva, M.; Ponces Freire, A.; Ferreira, A.E. Optimization of time-course experiments for kinetic model discrimination. PLoS ONE 2012, 7, e32749. [Google Scholar] [CrossRef]
- Shortall, K.; Djeghader, A.; Magner, E.; Soulimane, T. Insights into Aldehyde Dehydrogenase Enzymes: A Structural Perspective. Front. Mol. Biosci. 2021, 8, 659550. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol. Metab. 2018, 18, 143–152. [Google Scholar] [CrossRef]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Physiological and Pathological Roles of Aldose Reductase. Metabolites 2021, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Amani, S.; Fatima, S. Glycation With Fructose: The Bitter Side of Nature’s Own Sweetener. Curr. Diabetes Rev. 2020, 16, 962–970. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, M.F.A.; Khan, S.; Alouffi, S.; Khan, M.; Prakash, C.; Khan, M.W.A.; Ansari, I.A. Exploring aldose reductase inhibitors as promising therapeutic targets for diabetes-linked disabilities. Int. J. Biol. Macromol. 2024, 280, 135761. [Google Scholar] [CrossRef] [PubMed]
- Van Schaftingen, E.; Collard, F.; Wiame, E.; Veiga-da-Cunha, M. Enzymatic repair of Amadori products. Amino Acids 2012, 42, 1143–1150. [Google Scholar] [CrossRef]
- Mencke, P.; Boussaad, I.; Romano, C.D.; Kitami, T.; Linster, C.L.; Krüger, R. The Role of DJ-1 in Cellular Metabolism and Pathophysiological Implications for Parkinson’s Disease. Cells 2021, 10, 347. [Google Scholar] [CrossRef]
- Advedissian, T.; Deshayes, F.; Poirier, F.; Viguier, M.; Richarme, G. The Parkinsonism-associated protein DJ-1/Park7 prevents glycation damage in human keratinocyte. Biochem. Biophys. Res. Commun. 2016, 473, 87–91. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Lin, H.; Wang, L.; Jiang, X.; Wang, J. Glutathione dynamics in subcellular compartments and implications for drug development. Curr. Opin. Chem. Biol. 2024, 81, 102505. [Google Scholar] [CrossRef]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The Role of Cystine/Glutamate Antiporter SLC7A11/xCT in the Pathophysiology of Cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Et Biophys. Acta-Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Mitrić, A.; Castellano, I. Targeting gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic. Biol. Med. 2023, 208, 672–683. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Sun, X.; Wang, S.; Bai, D.; Zhao, X.; Han, Y.; Hao, P.; Liu, X.S. Ggct (γ-glutamyl cyclotransferase) plays an important role in erythrocyte antioxidant defense and red blood cell survival. Br. J. Haematol. 2021, 195, 267–275. [Google Scholar] [CrossRef]
- Kihira, Y.; Fujimura, Y.; Tomita, S.; Sato, E. Signaling pathways upregulating glutathione-specific γ-glutamylcyclotransferase 1 by 3-(5′hydroxymethyl-2′-furyl)-1-benzylindazole. Mol. Med. Rep. 2023, 28, 216. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Pannala, V.R.; Bazil, J.N.; Camara, A.K.S.; Dash, R.K. A biophysically based mathematical model for the catalytic mechanism of glutathione reductase. Free Radic. Biol. Med. 2013, 65, 1385–1397. [Google Scholar] [CrossRef]
- Xiao, Z.; La Fontaine, S.; Bush, A.I.; Wedd, A.G. Molecular Mechanisms of Glutaredoxin Enzymes: Versatile Hubs for Thiol–Disulfide Exchange between Protein Thiols and Glutathione. J. Mol. Biol. 2019, 431, 158–177. [Google Scholar] [CrossRef]
- Gallogly, M.M.; Mieyal, J.J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr. Opin. Pharmacol. 2007, 7, 381–391. [Google Scholar] [CrossRef]
- Musaogullari, A.; Chai, Y.C. Redox Regulation by Protein S-Glutathionylation: From Molecular Mechanisms to Implications in Health and Disease. Int. J. Mol. Sci. 2020, 21, 8113. [Google Scholar] [CrossRef] [PubMed]
- Aloke, C.; Onisuru, O.O.; Achilonu, I. Glutathione S-transferase: A versatile and dynamic enzyme. Biochem. Biophys. Res. Commun. 2024, 734, 150774. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updates 2021, 54, 100743. [Google Scholar] [CrossRef]
- Jones, D.P. Disruption of mitochondrial redox circuitry in oxidative stress. Chem.-Biol. Interact. 2006, 163, 38–53. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Levine, M. Purification, cloning and expression of dehydroascorbic acid-reducing activity from human neutrophils: Identification as glutaredoxin. Biochem. J. 1996, 315 Pt 3, 931–938. [Google Scholar] [CrossRef]
- Zhitkovich, A. Ascorbate: Antioxidant and biochemical activities and their importance for in vitro models. Arch. Toxicol. 2021, 95, 3623–3631. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Panday, S.; Talreja, R.; Kavdia, M. The role of glutathione and glutathione peroxidase in regulating cellular level of reactive oxygen and nitrogen species. Microvasc. Res. 2020, 131, 104010. [Google Scholar] [CrossRef]
- Barnett, S.D.; Buxton, I.L.O. The role of S-nitrosoglutathione reductase (GSNOR) in human disease and therapy. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Paget, C.; Lecomte, M.; Ruggiero, D.; Wiernsperger, N.; Lagarde, M. Modification of enzymatic antioxidants in retinal microvascular cells by glucose or advanced glycation end products. Free Radic. Biol. Med. 1998, 25, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lehman, T.D.; Ortwerth, B.J. Inhibitors of advanced glycation end product-associated protein cross-linking. Biochim. Et Biophys. Acta 2001, 1535, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.H.; Oh, C.J.; Park, J.W. Glycation-induced inactivation of antioxidant enzymes and modulation of cellular redox status in lens cells. Arch. Pharm. Res. 2006, 29, 577–581. [Google Scholar] [CrossRef]
- Fan, X.; Monnier, V.M. Inhibition of crystallin ascorbylation by nucleophilic compounds in the hSVCT2 mouse model of lenticular aging. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4945–4952. [Google Scholar] [CrossRef]
- Sheikpranbabu, S.; Haribalaganesh, R.; Gurunathan, S. Pigment epithelium-derived factor inhibits advanced glycation end-products-induced cytotoxicity in retinal pericytes. Diabetes Metab. 2011, 37, 505–511. [Google Scholar] [CrossRef]
- Papastavrou, N.; Chatzopoulou, M.; Pegklidou, K.; Nicolaou, I. 1-Hydroxypyrazole as a bioisostere of the acetic acid moiety in a series of aldose reductase inhibitors. Bioorg. Med. Chem. 2013, 21, 4951–4957. [Google Scholar] [CrossRef]
- Yang, J.; Cai, L.; Zhang, S.; Zhu, X.; Zhou, P.; Lu, Y. Silica-based cerium (III) chloride nanoparticles prevent the fructose-induced glycation of alpha-crystallin and H2O2-induced oxidative stress in human lens epithelial cells. Arch. Pharm. Res. 2014, 37, 404–411. [Google Scholar] [CrossRef]
- Liu, G.D.; Xu, C.; Feng, L.; Wang, F. The augmentation of O-GlcNAcylation reduces glyoxal-induced cell injury by attenuating oxidative stress in human retinal microvascular endothelial cells. Int. J. Mol. Med. 2015, 36, 1019–1027. [Google Scholar] [CrossRef]
- Sampath, C.; Zhu, Y.; Sang, S.; Ahmedna, M. Bioactive compounds isolated from apple, tea, and ginger protect against dicarbonyl induced stress in cultured human retinal epithelial cells. Phytomedicine 2016, 23, 200–213. [Google Scholar] [CrossRef]
- Fu, D.; Yu, J.Y.; Connell, A.R.; Yang, S.; Hookham, M.B.; McLeese, R.; Lyons, T.J. Beneficial Effects of Berberine on Oxidized LDL-Induced Cytotoxicity to Human Retinal Muller Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3369–3379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Exp. Eye Res. 2017, 160, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Mantha, N.; Burra, S.; Rajagopal, K.; Sreedhara, A. Protein Stability and Photostability under In Vitro Vitreal Conditions—Implications for Long Acting Delivery of Protein Therapeutics for Ocular Disease. Pharm. Res. 2020, 37, 85. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.Y.; Nam, M.H.; Lee, K.W. Inhibitory effect of caffeic acid on advanced glycation end product-induced renal fibrosis in vitro: A potential therapeutic target. J. Food Sci. 2021, 86, 579–586. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Yan, H. Effect of thioltransferase on oxidative stress induced by high glucose and advanced glycation end products in human lens epithelial cells. Int. J. Ophthalmol. 2021, 14, 965–972. [Google Scholar] [CrossRef]
- Abu-Kheit, R.; Kotev-Emeth, S.; Hiram-Bab, S.; Gabet, Y.; Savion, N. S-allylmercapto-N-acetylcysteine protects bone cells from oxidation and improves femur microarchitecture in healthy and diabetic mice. Exp. Biol. Med. 2022, 247, 1489–1500. [Google Scholar] [CrossRef]
- Ho, K.L.; Yong, P.H.; Wang, C.W.; Lim, S.H.; Kuppusamy, U.R.; Arumugam, B.; Ngo, C.T.; Ng, Z.X. In vitro anti-inflammatory activity and molecular docking of Peperomia pellucida (L.) Kunth extract via the NF-kappaB and PPAR-gamma signalling in human retinal pigment epithelial cells. Bioorg. Chem. 2024, 153, 107969. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, M.; Qu, Z.; Xu, S.; Peng, J.; Jiang, F. Moscatilin alleviates oxidative stress and inflammatory response of Muller cells in diabetic retinopathy through suppressing the p38 mitogen-activated protein kinase/c-Jun N-terminal kinase and nuclear factor kappa-B signaling pathways. J. Cell Commun. Signal. 2025, 19, e12059. [Google Scholar] [CrossRef]
- Bernardoni, B.L.; D’Agostino, I.; Scianò, F.; La Motta, C. The challenging inhibition of Aldose Reductase for the treatment of diabetic complications: A 2019-2023 update of the patent literature. Expert. Opin. Ther. Pat. 2024, 34, 1085–1103. [Google Scholar] [CrossRef]
- Shamsi, F.A.; Sharkey, E.; Creighton, D.; Nagaraj, R.H. Maillard reactions in lens proteins: Methylglyoxal-mediated modifications in the rat lens. Exp. Eye Res. 2000, 70, 369–380. [Google Scholar] [CrossRef]
- Padival, S.; Nagaraj, R.H. Pyridoxamine inhibits maillard reactions in diabetic rat lenses. Ophthalmic Res. 2006, 38, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Longman, M.; Alany, R.G.; Pierscionek, B. On the Anticataractogenic Effects of L-Carnosine: Is It Best Described as an Antioxidant, Metal-Chelating Agent or Glycation Inhibitor? Oxid. Med. Cell. Longev. 2016, 2016, 3240261. [Google Scholar] [CrossRef]
- Alghamdi, A.H.S.; Mohamed, H.; Sledge, S.M.; Borchman, D. Absorbance and Light Scattering of Lenses Organ Cultured with Glucose. Curr. Eye Res. 2018, 43, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Rankenberg, J.; Rakete, S.; Nahomi, R.B.; Glomb, M.A.; Linetsky, M.D.; Nagaraj, R.H. Glycation-mediated protein crosslinking and stiffening in mouse lenses are inhibited by carboxitin in vitro. Glycoconj. J. 2021, 38, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Birlouez-Aragon, I.; Alloussi, S.; Morawiec, M.; Fevrier, C. The effects of 5% and 25% galactose diets on lens polyols, glutathione and protein glycation in male and female pigs. Curr. Eye Res. 1989, 8, 449–457. [Google Scholar] [CrossRef]
- Nagaraj, R.H.; Prabhakaram, M.; Ortwerth, B.J.; Monnier, V.M. Suppression of pentosidine formation in galactosemic rat lens by an inhibitor of aldose reductase. Diabetes 1994, 43, 580–586. [Google Scholar] [CrossRef]
- Taylor, A.; Lipman, R.D.; Jahngen-Hodge, J.; Palmer, V.; Smith, D.; Padhye, N.; Dallal, G.E.; Cyr, D.E.; Laxman, E.; Shepard, D.; et al. Dietary calorie restriction in the Emory mouse: Effects on lifespan, eye lens cataract prevalence and progression, levels of ascorbate, glutathione, glucose, and glycohemoglobin, tail collagen breaktime, DNA and RNA oxidation, skin integrity, fecundity, and cancer. Mech. Ageing Dev. 1995, 79, 33–57. [Google Scholar] [CrossRef]
- Sailaja Devi, M.M.; Suresh, Y.; Das, U.N. Preservation of the antioxidant status in chemically-induced diabetes mellitus by melatonin. J. Pineal Res. 2000, 29, 108–115. [Google Scholar] [CrossRef]
- Suryanarayana, P.; Krishnaswamy, K.; Reddy, G.B. Effect of curcumin on galactose-induced cataractogenesis in rats. Mol. Vis. 2003, 9, 223–230. [Google Scholar]
- Yan, H.; Guo, Y.; Zhang, J.; Ding, Z.; Ha, W.; Harding, J.J. Effect of carnosine, aminoguanidine, and aspirin drops on the prevention of cataracts in diabetic rats. Mol. Vis. 2008, 14, 2282–2291. [Google Scholar]
- Suresha, B.S.; Sattur, A.P.; Srinivasan, K. Beneficial influence of fungal metabolite nigerloxin on eye lens abnormalities in experimental diabetes. Can. J. Physiol. Pharmacol. 2012, 90, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, X.; Wang, J.; Yang, J.; Sun, X.; Li, X.; Zhu, Q.; Li, W. Rhizome of Anemarrhena asphodeloides counteracts diabetic ophthalmopathy progression in streptozotocin-induced diabetic rats. Phytother. Res. 2013, 27, 1243–1250. [Google Scholar] [CrossRef]
- Balakumar, M.; Saravanan, N.; Prabhu, D.; Regin, B.; Reddy, G.B.; Mohan, V.; Rema, M.; Balasubramanyam, M. Benefits of early glycemic control by insulin on sensory neuropathy and cataract in diabetic rats. Indian J. Exp. Biol. 2013, 51, 56–64. [Google Scholar]
- Suresha, B.S.; Srinivasan, K. Antioxidant potential of fungal metabolite nigerloxin during eye lens abnormalities in galactose-fed rats. Curr. Eye Res. 2013, 38, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Sai Varsha, M.K.; Raman, T.; Manikandan, R. Inhibition of diabetic-cataract by vitamin K1 involves modulation of hyperglycemia-induced alterations to lens calcium homeostasis. Exp. Eye Res. 2014, 128, 73–82. [Google Scholar] [CrossRef]
- Yang, J.; Gong, X.; Fang, L.; Fan, Q.; Cai, L.; Qiu, X.; Zhang, B.; Chang, J.; Lu, Y. Potential of CeCl3@mSiO2 nanoparticles in alleviating diabetic cataract development and progression. Nanomedicine 2017, 13, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; He, J.; Ren, C.; Zhou, Y.; Chen, X.; Dou, J. Protective effects of the Chinese herbal medicine prescription Zhujing pill on retina of streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 98, 643–650. [Google Scholar] [CrossRef]
- Demirbolat, I.; Ekinci, C.; Nuhoglu, F.; Kartal, M.; Yildiz, P.; Gecer, M.O. Effects of Orally Consumed Rosa damascena Mill. Hydrosol on Hematology, Clinical Chemistry, Lens Enzymatic Activity, and Lens Pathology in Streptozotocin-Induced Diabetic Rats. Molecules 2019, 24, 4069. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Y.; Zhao, T.; Wang, Z. Effect of astaxanthin on metabolic cataract in rats with type 1 diabetes mellitus. Exp. Mol. Pathol. 2020, 113, 104372. [Google Scholar] [CrossRef]
- Wojnar, W.; Zych, M.; Borymski, S.; Kaczmarczyk-Sedlak, I. Chrysin Reduces Oxidative Stress but Does Not Affect Polyol Pathway in the Lenses of Type 1 Diabetic Rats. Antioxidants 2020, 9, 160. [Google Scholar] [CrossRef]
- Zych, M.; Wojnar, W.; Kielanowska, M.; Folwarczna, J.; Kaczmarczyk-Sedlak, I. Effect of Berberine on Glycation, Aldose Reductase Activity, and Oxidative Stress in the Lenses of Streptozotocin-Induced Diabetic Rats In Vivo-A Preliminary Study. Int. J. Mol. Sci. 2020, 21, 4278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Geng, Q.; Li, T.; Mohammad Firdous, S.; Zhou, X. Morin attenuates STZ-induced diabetic retinopathy in experimental animals. Saudi J. Biol. Sci. 2020, 27, 2139–2142. [Google Scholar] [CrossRef]
- Reihanifar, T.; Sahin, M.; Stefek, M.; Ceylan, A.F.; Karasu, C. Cemtirestat, an aldose reductase inhibitor and antioxidant compound, induces ocular defense against oxidative and inflammatory stress in rat models for glycotoxicity. Cell Biochem. Funct. 2023, 41, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Brinkmann Frye, E.; Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997, 324 Pt 2, 565–570. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Rajesh, M.; Sulochana, K.N. Eales’ disease: Oxidant stress and weak antioxidant defence. Indian J. Ophthalmol. 2007, 55, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Z.; Zarina, S. Osmotic stress induced oxidative damage: Possible mechanism of cataract formation in diabetes. J. Diabetes Complicat. 2012, 26, 275–279. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Strokov, I.A.; Nosikov, V.V.; Savel’yeva, E.L.; Sitnikov, V.F.; Yegorov, Y.E.; Lankin, V.Z. The Role of Oxidative Stress in Diabetic Neuropathy: Generation of Free Radical Species in the Glycation Reaction and Gene Polymorphisms Encoding Antioxidant Enzymes to Genetic Susceptibility to Diabetic Neuropathy in Population of Type I Diabetic Patients. Cell Biochem. Biophys. 2015, 71, 1425–1443. [Google Scholar] [CrossRef]
- Gehl, Z.; Bakondi, E.; Resch, M.D.; Hegedus, C.; Kovacs, K.; Lakatos, P.; Szabo, A.; Nagy, Z.; Virag, L. Diabetes-induced oxidative stress in the vitreous humor. Redox Biol. 2016, 9, 100–103. [Google Scholar] [CrossRef]
- Mynampati, B.K.; Ghosh, S.; Muthukumarappa, T.; Ram, J. Evaluation of antioxidants and argpyrimidine in normal and cataractous lenses in north Indian population. Int. J. Ophthalmol. 2017, 10, 1094–1100. [Google Scholar] [CrossRef]
- Ruamviboonsuk, V.; Grzybowski, A. The Roles of Vitamins in Diabetic Retinopathy: A Narrative Review. J. Clin. Med. 2022, 11, 6490. [Google Scholar] [CrossRef]
- Bohm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidat. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Kumar, P.A.; Kumar, M.S.; Reddy, G.B. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem. J. 2007, 408, 251–258. [Google Scholar] [CrossRef]
- Kubo, E.; Urakami, T.; Fatma, N.; Akagi, Y.; Singh, D.P. Polyol pathway-dependent osmotic and oxidative stresses in aldose reductase-mediated apoptosis in human lens epithelial cells: Role of AOP2. Biochem. Biophys. Res. Commun. 2004, 314, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, N.; Bora, K.; Maurya, M.; Pavlovich, M.C.; Chen, J. Oxidative Stress and Antioxidants in Age-Related Macular Degeneration. Antioxidants 2023, 12, 1379. [Google Scholar] [CrossRef]
- Nowak, J.Z. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304. [Google Scholar] [CrossRef]
- Callan, A.; Heckman, J.; Tah, G.; Lopez, S.; Valdez, L.; Tsin, A. VEGF in Diabetic Retinopathy and Age-Related Macular Degeneration. Int. J. Mol. Sci. 2025, 26, 4992. [Google Scholar] [CrossRef] [PubMed]
- Gáll, T.; Pethő, D.; Erdélyi, K.; Egri, V.; Balla, J.G.; Nagy, A.; Nagy, A.; Póliska, S.; Gram, M.; Gábriel, R.; et al. Heme: A link between hemorrhage and retinopathy of prematurity progression. Redox Biol. 2024, 76, 103316. [Google Scholar] [CrossRef]
- Yamada, K.H. A novel approach in preventing vascular leakage and angiogenesis in wet age-related macular degeneration. Neural Regen. Res. 2022, 17, 1751–1752. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Clark, S.J.; Bishop, P.N. The eye as a complement dysregulation hotspot. Semin. Immunopathol. 2018, 40, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, N.; Capierri, M.; Pascale, A.; Barbieri, A. Different Therapeutic Approaches for Dry and Wet AMD. Int. J. Mol. Sci. 2024, 25, 13053. [Google Scholar] [CrossRef]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P., Jr. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2018, 72, 105–111. [Google Scholar] [CrossRef]
- Li, F.; Cui, L.; Yu, D.; Hao, H.; Liu, Y.; Zhao, X.; Pang, Y.; Zhu, H.; Du, W. Exogenous glutathione improves intracellular glutathione synthesis via the γ-glutamyl cycle in bovine zygotes and cleavage embryos. J. Cell. Physiol. 2019, 234, 7384–7394. [Google Scholar] [CrossRef]
- Tang, B.L. Neuroprotection by glucose-6-phosphate dehydrogenase and the pentose phosphate pathway. J. Cell. Biochem. 2019, 120, 14285–14295. [Google Scholar] [CrossRef] [PubMed]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The Role of the Pentose Phosphate Pathway in Diabetes and Cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Guad, R.M.; Udupa, K.; Fuloria, N.K. A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases. Antioxidants 2020, 9, 1075. [Google Scholar] [CrossRef]
- Davies, S.S.; Zhang, L.S. Reactive Carbonyl Species Scavengers-Novel Therapeutic Approaches for Chronic Diseases. Curr. Pharmacol. Rep. 2017, 3, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2021, 19, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, W.Q.; Broussy, S.; Han, B.; Fang, H. Recent advances of anti-angiogenic inhibitors targeting VEGF/VEGFR axis. Front. Pharmacol. 2023, 14, 1307860. [Google Scholar] [CrossRef]
- Heloterä, H.; Kaarniranta, K. A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration. Cells 2022, 11, 3453. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).