Phytochemistry, Biological Activities, Molecular Mechanisms, and Toxicity of Saffron (Crocus sativus L.): A Comprehensive Overview
Abstract
1. Introduction
2. Methodology
3. Evolution of Trends in Research with Crocus sativus
4. Crocus sativus Taxonomy
5. Saffron Natural Bioactive Compounds
5.1. Crocin
5.2. Crocetin
5.3. Picrocrocin
5.4. Safranal
6. Benefits of Saffron Stigmas on Human Health and Disease Conditions
6.1. Antioxidant Activity
6.2. Anti-Inflammatory Activity
6.3. Immunomodulatory Effects
6.4. Anti-Cancer Activity
6.5. Protection Against Neurological Disorders
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Crocin (50 mg/kg, i.p.) | PTSD (3 consecutive shocks 0.6 mA, 3 s, along with a sound: 75 dB for 3 s) | Crocin+ extinction learning: ↓ PTSD-like behavior freezing; ↑ BDNF; ↑ pain threshold. | [88] |
| crocin (10, 30, and 50 mg/kg, i.p.) | Maternal Social isolation from PND 30 to 80 (50 days) | Crocin (30 and 50 mg/kg) ↑ (GSK-3beta) in the hippocampus; ↑ locomotion; enhance memory; ↓ anxiety and depressive-like behaviors; ↓ GSK-3beta level | [89] |
| Crocin (0.1 to 100 μM) | MPP+-induced apoptosis to PC12 cells | Inhibition of MPP+ mitochondrial dysfunction; ↓ ER stress by regulation of CHOP-Wnt pathway, ↓ apoptosis induced by MPP+ | [103] |
| crocin (30 mg/kg, i.p.) | Repetitive mild traumatic brain injury (rmTBI) | ↓ cytokine IL-6; ↑ anti-apoptotic cytokine IL-10; ↓caspase3, Bax and P53; ↑ mRNA levels of Bcl-2, ↑ Nrf2; ↑ HO-1 and NQO-1; ↓ NF-κB | [90] |
| Crocin (10, 20, and 30 mg/kg by oral gavage) for 4 weeks | Unpredictable chronic mild stress (UCMS) induced anxiety and depression in rats | ↓ Serum corticosterone levels, MDA, TNF-α, IL-6; ↑ IL-10, SOD, CAT, Thiols, ↑ BDNF | [91] |
| C. sativus (50 mg/kg by oral gavage) | Lead (Pb) induced neurotoxicity in Meriones strains | ↓ Tyrosine Hydroxylase (TH) to normal, restore locomotor activity by 90% | [92] |
| Crocin tablet (30 mg/day, 8 weeks) | chemotherapy-induced peripheral neuropathy (CIPN) | ↓ Grade of sensory, motor, and neuropathic pain; ↓ adverse effects, lower toxicity | [99] |
| saffron methanolic extract/crocin-enrichment | Rotenone (ROT)-induced locomotor neurotoxicity in the Drosophila model | ↑ GSH.THIOLS; ↓ AChE activity and restore dopamine levels; ↑ life span; ↑ locomotor phenotype | [93] |
| saffron extract 60 mg/kg by oral gavage | diode laser burns induced ocular hypertension (OHT) | ↓ microglion, reversed OHT-induced down-regulation of P2RY12; prevented retinal ganglion cell death in OHT eyes; ↓ neuroinflammation; ↑ intraocular pressure | [104] |
| Crocin (30 mg/kg/day; i.p., for 30 days) | rotenone (ROT)-induced Parkinson’s Disease in rats | ↑ phospho-proline-rich Akt, mTOR and p-p70S6K levels; stimulated PI3K/Akt pathway; ↓ caspase-9, attenuating neurodegeneration (↑ TH and DA); Akt/mTOR activation | [94] |
| saffron extract (100 or 200 mg/kg, Ip, for 3 weeks) | cerebral ischemia/reperfusion injury (I/R) in rats | ↓ MDA, ↓ NO and brain natriuretic peptide (BNP); ↑ GSH; ↓ apoptosis (↓ caspase-3 and Bax protein); ↑ vascular endothelial growth factor | [95] |
| Safranal (72.5 and 145 mg/kg, ip) | Focal cerebral ischemia/reperfusion injury model | ↑ total sulfhydryl (SH); ↓ thiobarbituric acid reactive substances (TBARS); ↓ MDA; ↓ infarct volume and hippocampal cell loss, | [96] |
| Crocin (20 mg/kg) | Cortical impact induced traumatic brain injury (TBI) in C57BL/6 mice | ↑ neurological severity score (NSS); ↓ microglial activation; ↓ cell apoptosis; activation of Notch signaling | [105] |
| Safranal (25, 50, 100 and 200 mg/kg, ip, for 3 days) | Laminectomy-induced spinal cord injury (SCI) in rats | ↑ neurons; anti-apoptotic effect; ↓ inflammation; ↓ expression of AQP-4; ↑ IL-10 | [97] |
| Crocin loaded in SLN (25, 50 mg/kg/day; orally taken for 28 days) | pentylenetetrazol (PTZ) induced oxidative damage | ↓ NO; ↑ CAT; ↑ memory; ↓ anxiety; ↓ Nuclear factor kappa B (NF-Κb) | [106] |
| Crocin (150, 300, and 600 nmol, intra-hippocampal (IH)) (5 mg/mL, i.p.) | amyloid β-induced memory deficit | ↑ spatial memory indicators; ↓ Bax/Bcl-2 ratio and cleaved Caspase-3 level; anti-apoptotic effect | [98] |
| saffron aqueous extract and crocin (15 mg twice daily, 12 weeks) | adult patients with schizophrenia | No serious side effects were observed; ↑ white blood cells | [107] |
| CSE (10, 15, and 20 mg/kg orally) | scopolamine-induced cognitive impairment, amyloid beta (Aβ) plaque, and neurofibrillary tangles (NFT) | AChE inhibition; ↓ Aβ plaque and NFT | [108] |
| Stigma of Crocus sativus | fly PD model overexpressing several mutant α-synuclein | ↑ life span; ameliorate retinal degeneration; ↑ climbing ability in the Drosophila | [109] |
| Crocin (10, 20, and 30 mg/kg orally) for weeks | Unpredictable chronic mild stress (UCMS) induced Depression and anxiety in rats | ↓ corticosterone, ↑ antioxidant defenses, ↓ oxidative damage, ↑ brain-derived neurotrophic factor | [91] |
| Safron (50 mg/kg) and crocin (30 mg/kg | weight drop model induced (rmTBI) | ↓ IFN-γ, TNF-α, MDA, and myeloperoxidase activity (MPO), ↑ GSH ↑ neurological, cognitive, motor, and sensorimotor functions. | [110] |
| Saffron extract (50 mg/kg, i.p.) for 5 days | Traumatic brain injury (TBI) in the Zebrafish model | anxiolytic effect, prevent fear; ↑ memory performance | [111] |
| Saffron capsule (15 mg/twice a day) for 12 weeks | Alzheimer’s Disease patients | ↓ IL-1β and MDA; ↑ total antioxidant capacity (TAC) | [100] |
| Safranal (100 mg/kg, 200 mg/kg, or 400 mg/kg) | (PTZ)-induced epileptic seizures in mice | ↓ seizure stage; ↓ hyperactivity of neurons; suppressed the NF-κB signaling pathway; ↓ TNF-α and IL-1β | [102] |
| Crocin (30 mg twice daily) | adult patients with Parkinson’s disease | Enhance daily life activities; attenuate movement disorders | [101] |
6.6. Anti-Obesity and Anti-Dyslipidemic Effects
6.7. Anti-Diabetic Effects
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Saffron (40 and 80 mg/kg) | Streptozotocin-Induced Diabetes in Rats | ↓ blood glucose levels, cholesterol, triglyceride, and LDL; ↑ HDL, SOD, CAT, and GSH; ↓ cognitive deficit | [37] |
| Saffron (120 mg/kg) for 60 days | Tartrazine-induced diabetic male rats | reduce blood glucose level and creatinine | [127] |
| Stigma extract (50 mg/kg) for 3 weeks | Streptozotocin-Induced Diabetes in rats | ↓ blood glucose levels; ↓ total cholesterol and triglyceride; ↓ urea and creatinine; ↓ AST and ALT | [125] |
| Crocin (100 mg/kg, i.p.) for 2 weeks | streptozotocin-induced type-2 diabetic rats | ↑ serum insulin levels; ↑ (SOD, GSH, and CAT); improve fasting glucose levels | [126] |
| Crocin solution | Hyperglycemia. In Vivo Evidence from Zebrafish | ↓ embryo glucose levels; ↑ insulin expression; ↑ expression of phosphoenolpyruvate carboxykinase 1 (pck1) | [134] |
| saffron extract | In vitro alpha-amylase and alpha-glucosidase | high inhibitory activity against α-glucosidase and α-amylase | [135] |
| Saffron extract (84 mg for 6 months) | People with Diabetes Mellitus Type 1 | improves serum triglycerides | [132] |
| Crocin (150 mg/kg orally) 6 weeks | Streptozocin induced type-2 diabetes in rats | ↓ plasma TNF-α and IL-1β levels; ↓ pancreas tissue TNF-α and IFN-γ levels; ↓ inflammation and oxidative stress | [136] |
| Crocin (300 mg/kg in 1 mL PBS) for 8 weeks | Streptozocin induced type-2 diabetes in Sprague Dawley rats | ↓ fasting blood glucose levels, ↓ fat accumulation in the liver, alleviate renal fibrosis, and ↓ blood lipid levels | [128] |
| Saffron extract (0.2–1.2 mg/mL) (400 mg/kg orally) | In vitro inhibition tests of α-amylase and α-glucosidase In vivo Antihyperglycemic Activity in albino Wistar rats | ↓ postprandial hyperglycemia; inhibition activity against α-glucosidase and α-amylase | [129] |
| Crocetin | In vitro biochemical assay In silico | ↑ glucose uptake; GPR40/120 agonist; enhance insulin secretion | [130] |
| Saffron extract capsules twice a day (15 mg) for 8 weeks | A triple-blinded randomized clinical trial | ↓ fasting blood sugar | [133] |
| Crocin (50 mg/kg) for 8 weeks | In vivo investigation of the hypoglycemic effect in db/db mice | ↑ insulin and pyruvate kinase | [131] |
| Saffron extract (15, 200, and 250 mg/kg) for 8 weeks Safranal (15, 20, and 25 mg/kg) for 8 weeks | Alloxan-Induced Diabetes in rats | ↓ MDA; ↑ CAT and GSH; ↑ insulin; beta-cells regeneration | [137] |
6.8. Cardioprotective and Antihypertensive Effects
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Crocin (50 or 10 mg/kg/day) for 1 week | Myocardial Infarction Using Sprague−Dawley rats | Lower arrhythmia score; ↑ expression of connexin 43 (Cx43) mRNA | [152] |
| Crocetin ester (25 and 50 mg/kg) for 14 days | isoproterenol (ISO)-induced acute myocardial ischemia | ↓ TNF-α, IL-1β and IL-6; ↓ creatine kinase (CK) and MDA; ↑ SOD; improve histopathological alteration | [138] |
| Crocin (40 and 80 mg/kg/day i.p.) | Arsenic trioxide-induced cardiotoxicity in rats | ↓ ROS; ↑ SOD, GSH and GPx; ↓ MDA, gamma glutamyl transferase and proinflammatory cytokines; ↓ Caspase-3 and Bcl-2 | [148] |
| Crocin (100 and 200 mg/kg/day i.p.) for 14 days | Isoprenaline-induced myocardial fibrosis in mice | ↓ IL-6, IL-1, TNF-α and NF-κB, ↓ SOD and CAT; ↓ B cell lymphoma-2, Bcl-2-associated X protein, caspase-3, and cleaved caspase-3 expressions | [140] |
| Crocin (10 and 20 mg/kg, orally) for 3 weeks. | Doxorubicin-induced myocardial injury in rats | improve ECG profile; restore the balance between pro-and anti-inflammatory cytokines; reduce Cardiac caspase-3 activity | [146] |
| Crocin (10, 20, and 40 µM) for 1 day | In vitro LPS-induced inflammation in cardiomyocytes | ↓ LPS toxicity (↓ TNF-α, PGE2, IL-β, and IL-6); ↑ thiols; ↓ nitric oxide | [142] |
| Crocin (20 and 40 mg/kg/24 h, for 20 days) | Doxorubicin-induced cardiotoxicity in rats | improved heart damage, structural changes in the myocardium, and ventricular function; it did not affect the in vitro antitumor activity of DOX | [147] |
| Crocin (3, 30, and 300 µM) | In vitro isolated rat cardiomyocytes | reduce Ca2+ flow into cardiomyocytes, resulting in negative inotropic effects on myocardial contractility | [153] |
| Safranal (0, 10, 20, 40, 80, 160, and 320 μM) | ISO-induced myocardial injury | ↓ myocardial apoptosis; downregulating the TNF signaling pathway | [141] |
| Crocin (12.5, 25, 50 mg/kg/day, i.p.) for 4 weeks | Diazinon induced cardiotoxicity | ↓ protein ubiquitylation in heart tissue; ↑ HIF-1α ubiquitylation | [151] |
| Saffron (5, 10, and 20 mg/kg/day orally) for 21 days | Isoproterenol-induced cardiotoxicity in rats | ↑ SOD, GSH, and CAT, ↓ MDA; ↓ left ventricular end-diastolic pressure; ↑ CK-MB and LDH | [139] |
| crocin (50 mg/kg, i.p.) | PM10-induced cardiotoxicity | Restore Hemodynamic parameters; ↓ MDA and xanthine oxidase XOX; ↑ CAT, SOD, and GPx | [149] |
| Safranal (10, 30 µM) | (H/R)-induced cardiomyocyte injury in H9c2 cardiac myoblasts | ↑ cells viability; ↓ ROS; ↓ CK-MB, LDH, MDA and intracellular Ca2; ↓ caspase-3Bax protein; ↑ Bcl-2 protein; ↑ PI3K/AKT/GSK3β. | [143] |
| Saffron extract (60 mg/kg/day orally) for 4 weeks | Ischemia-reperfusion induced myocardial injury in Wild Type and ApoE(-/-) mice | ↑ eNOS, p-Akt, p-ERK1/2, p-44/p-42 and p-GSK3b-Ser9; ↓ IL-6 and iNOS; ↓ MDA and 3-Nitrotyrosine NT; ↓ infractus size | [154] |
| Capsule of crocetin (10 mg) for 2 months | pilot, randomized, double-anonymized, placebo-controlled clinical trial | ↓ (h-FABP), cellular adhesion molecule 1, vascular cell adhesion molecule 1, monocyte chemoattractant protein 1; ↑ HDL, ↓ systolic and diastolic blood pressure | [145] |
| Crocetin diamide derivatives (1, 0.2, and 0.04 μM) | In vitro hypoxia-induced injury in H9c2 cells | ↑ cell and mitochondrial viability; ↓ LDH | [155] |
| Crocin (5 and 7 mM) | Aluminum phosphide-induced cardiotoxicity in Human Cardiac Myocyte | ↓ Protein carbonyl and MDA; ↑ SOD and CAT | [150] |
| Safranal (10 μg/mL) | In vitro doxorubicin and ischemia–reperfusion induced cardiomyocyte injury | ↓ caspase-3 activity; restored contractile proteins expression; inhibited mitochondrial permeability transition pore | [144] |
| Safranal (0.075 and 0.025 mL/kg/day orally) for 9 days | Isoprenaline-induced myocardial ischemia in rats | ↓ CK, LDH, and MDA; ↑ SOD; ↓ intracellular calcium; improve heart morphology | [156] |
6.9. Hepatoprotective Effects
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Crocin (25 and 50 mg/kg orally) | methotrexate-induced liver injury | ↓ MDA, NO, IL-1β, and d TNF-α; ↑ CAT, SOD, GSH, GPx | [158] |
| Crocin (20, 40, and 80 mg/kg orally) for 8 weeks | Carbone tetrachloride-induced liver fibrosis | ↓ nuclear factor-kappa B, IL-6, TNF-α, transforming growth factor β, and α-smooth muscle actin; ↓ caspase 3/7; ↑ peroxisome proliferator-activated receptor γ (PPAR-γ) | [159] |
| Crocin (30 mg/kg) for 14 days | Copper oxide nanoparticles induced hepatic disturbances | ↓ hepatic enzymes activities; ↓ inflammatory biomarkers; repair hepatic alteration | [167] |
| Crocin (10, 20, and 40 mg/kg i.p.) for 4 weeks | Ethanol toxicity in the rat | ↓ MDA; ↑ GSH; restore TNF-α and IL-6 levels; prevent apoptosis | [160] |
| Saffron extract (60 mg/kg i.p.) for 30 days | Copper Nanoparticles Induced hepatoxicity in mice | ↓ MDA, AST, ALT, and ALP; ↑ total antioxidant capacity; partial protection against necrotic cells | [168] |
| Saffron extract by the gastric tube | Silver Nanoparticles caused Hepatotoxicity | ↓ MDA, AST, ALT, and ALP; ↑ GSH | [169] |
| Crocetin (10, 30, and 50 mg/kg orally) | Non-alcoholic fatty liver disease (NAFLD) in mice | ↓ AST, ALT, TC, TG, MDA, CR, UA; ↑ SOD and CAT; suppressed high-fat diet; ↓ TNF-α, IL-6, and IL-1β; ↑ HO-1 and Nrf2 | [165] |
| Crocin (20 mg/kg, orally) for 8 weeks | Leflunomide-induced liver injury | ↓ AST, ALT, ALP, hepatic MDA, nitrite, mTOR gene, PI3K gene, TGF-β; ↑ albumin, total protein, hepatic catalase, and GSH | [161] |
| Safranal (0.025, 0.05 and 0.1 mL/kg/day i.p.) for 14 days | acetaminophen-induced hepatotoxicity in rats | ↓ MDA, IL-6, TNF-α, IL-1β; ↑ GSH, GPx and CAT; ↓ AST, ALT and ALP | [163] |
| Saffron phenolic-enriched fraction nanofibers loaded with C. sativus phenolic | cisplatin-induced hepatotoxicity in mice | ↑ weight; ↓ liver enzymes; ↑ GPx, SOD, CAT; ↓ iNOS and IFN-γ | [162] |
| Saffron, crocin, and safranal (100 mg/kg/day orally) for 7 days | CCL4-Induced Liver Damage | removed histological abnormalities, including necrosis, showing liver injury. | [170] |
| Saffron (150 and 300 mg/kg/day orally) for 28 days | acetaminophen-induced hepatotoxicity | ↓ AST, ALT, ALP, LDH; FXR up-regulation by saffron | [171] |
| Saffron (40 mg/kg/day orally) for 30 days | Oxymetholone-Induced Hepatic Injury in Rats | ↓ in hepatic degenerative changes | [166] |
| Saffron (80 mg/kg i.p.) 10 days | methotrexate-induced liver toxicity in rats | ↓ AST, ALT, ALP, and LDH; ↓ MDA and nitric oxide; ameliorate morphological alterations | [172] |
| Crocin (50 mg/kg/day orally), (100 µM–300 µM) | In Vivo & In Vitro hepatocellular Carcinoma | ↓ C-reactive protein CRP; IL-6; LDH ↓ TNFα, p53, VEGF and NF-κB; anti-tumor effect on HepG2 cells | [164] |
| Stigma extract (50 mg/kg/day) for 14 days | Carbon tetrachloride induced acute liver injury in rats | ↓ AST, ALT, ALP, LDH, creatinine, and MDA; prevent body loss. | [173] |
| Crocin (50, 100, and 250 mg/kg) | sepsis-induced injury in the liver, kidney, and lungs | ↓ IL-1b, TNF-a, IL-6 and IL-10; suppressed p38 MAPK phosphorylation, NF-jB/IjBa and Bcl-2/Bax activation |
6.10. Pulmonary Protective Effects
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Crocin (30 and 60 mg/kg i.p.) | Ovalbumin-sensitized lung tissue in mice | ↓ inflammatory cells (eosinophils, neutrophils, macrophages, and lymphocytes); ↓ Drp1, Pgc1α, and Nrf1 levels; ↑ Mfn2 | [174] |
| Crocin (60 mg/kg orally and 50 mg/kg i.p.) for 2 weeks | acrolein-induced lung injury in albino rats | i.p. crocin ↓ MDA, TNF-α, IL-6, Protein carbonyls, 8-hydroxydeoxy guanosine levels; ↑ GSH | [175] |
| Crocin (7.5, 10, 30 mg/kg) | monocrotaline-induced pulmonary arterial hypertension | ↑ Oxidation resistance 1 and P21 gene expression; ↑SOD, GPx, CAT, TAC | [176] |
| Crocin (25 mg/kg orally) for 28 days | Bleomycin-induced pulmonary fibrosis | ↓ TNF-α, MDA, and NO in lungs; ↑ GSH, CAT, GPx | [177] |
| Crocin (30 and 60 mg/kg) | allergic airway inflammation | Prevent increase in white blood cell; ↓ NF-kB and IL-17; upregulating Nrf2/HO-1 mRNA | [53] |
| Crocin (50 mg/kg i.p.) for 9 days | LPS-induced acute lung injury | ↓ Hemorrhage, inhibition of the HMGB1/TLR4 pathway | [178] |
| Crocin (30 mg/day) for 12 weeks | A Randomized, Double-Blind, Placebo-Controlled Trial | ↓ TOS and NF-κB; ↑ total antioxidant capacity (TAOC); improvement in patients’ exercise capacity | [182] |
| Crocin (15 mg twice a day) for 12 weeks | A Randomized, Double-Blind, Placebo-controlled trial | ↑ pulmonary function tests and walking distance test (6MWD); ↓ TNF-α | [60] |
| Crocin (25 μM) for 48 h | Oxidative stress induced by 2-chloroethyl ethyl sulfide | decline the injury; reduce inflammation and ROS production; ↑ cell survival | [179] |
| Safranal (1 and 10 mg/kg) (10 and 100 ng/mL) | In vivo mouse model of Asthma In vitro cytokines induced stress in bronchial epithelial cells | ↓ NO, iNOS levels, peroxynitrite ion generation and cytochrome c; ↓ airway hyper-responsiveness and airway cellular infiltration to the lungs; ↓ Th2 type cytokine | [180] |
| C. sativus extract (20 and 80 mg/kg/day) | Paraquat-induced lung inflammation in rats | ↑ IFN-γ, IL-10, SOD, CAT, thiol and EC50; ↓ BALF and MDA; ↓ total and differential WBC | [181] |
| Saffron extract (150 and 600 mg/kg/day orally) for 15 days | Paraquat-induced lung injuries | ↑ SOD, CAT, Thiols in bronchoalveolar lavage fluid (BALF); ↓ TNF-α, IL-10, and tracheal responsiveness | [183] |
| C. sativus extract (30 mg/kg and 60 mg/kg i.p.) for 28 days | Ovalbumin (OVA)-induced asthma in rats | ↓ Bronchoalveolar Lavage Fluid; ↓ total protein and albumin in serum, BALF, and lung tissues; ↓ TNF-α, IL-1β, IL-4, IL-13 | [184] |
6.11. Gastrointestinal Protective Effects
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Stigmas extract (0.3 to 10 mg/mL) | In Vitro Assessment of Myorelaxant and Antispasmodic Effects | Dose-dependent antispasmodic and myorelaxant activity; | [185] |
| crocin (7.5, 15, or 30 mg/kg, i.p.) | Indomethacin-induced gastric lesions in rats | ↓ caspase-3 levels and Inos protein expression; Decrease MDA levels and mucus content | [189] |
| Crocin (50 mg/kg/day, i.p.) for 3 days | Ethanol-induced gastric injury in rats | ↑ gastric juice mucin and mucosal prostaglandin E2 (PGE2), IL-6, TNF-α, myeloperoxidase activity; ↑ SOD and glutathione; ↓ caspase-3 activity and mitigated DNA fragmentation | [191] |
| Safranal (0.063, 0.25, and 1 mg/kg) for 7 days | Indomethacin-induced gastric ulcer | Ameliorate histological changes and tissue biochemical alterations (↑ SOD and TAC, ↓ MDA, TNF-α, and caspase-3); reduce gastric mucosa lesions. | [188] |
| crocin (2.50, 10.00 and 40.00 mg/kg i.p.) | Indomethacin induced intestinal ulcer. | Down-regulate intestine weight and organo-somatic index; (↑ SOD; ↓ caspase-3, TNF-α and MDA | [187] |
| Saffron extract (7.5, 15, 20 and 25 mg/kg orally) for 11 days | DSS induced Colitis in C57BL/6 mice | restore body weight, colon length, histology score; ↓ pro-inflammatory macrophages (M1); ↓ anti-inflammatory macrophages (M2) and IL-10 | [192] |
| Saffron extract (100 and 200 mg/kg orally) for 12 days | Acetic Acid-Induced Gastric Ulcer in male Wistar Rats | Reduce prostaglandin E2 (PGE2) and vascular endothelial growth factor (VEGF), ↓ MDA | [190] |
| Crocin (20 mg/kg orally) for 8 days | Acetic Acid-Induced Gastric Ulcer in male Sprague Dawley Rats | ↓ TNF-α, Ca2+ contents, LDH, CRP and Inflammatory cells; Enhance Nrf2 and HO-1 signaling and down-regulate caspase-3 activity; ↑ SOD, GSH and CAT; | [195] |
| Crocin (15 mg/kg, i.p.) for 30 min before induction of injury | Ischemia-reperfusion-induced gastric injury in rats | Decrease area of gastric lesions; ↓ caspase-3 and iNOS | [194] |
| Crocetin (100 and 200 mg/kg) | In vitro burn-induced intestinal injury | ↑ antioxidants enzymes; reduce inflammatory response (IL-6, TNF-α); improve intestinal permeability, and histological changes | [186] |
| Crocin (100 mg/kg/day orally) for 15 days | CCL-4 mediated oxidative stress in rats | Reduce histological lesions; ↓ TOS and MDA; ↑ GSH and TAS | [196] |
| Saffron aqueous extract (10 and 20 mg/kg orally) for 11 days | Dextran sulfate sodium (DSS)-induced colitis in mice | Suppress NF-κB (↓ TNF-α, IL-1β, and IL-6); regulate the composition of gut microbiota; diminishes the susceptibility to colitis reformulate | [197] |
| Crocin (50 mg/kg orally) for 21 days | Acrylamide induced small and large intestine damage in Wistar rats | ↑ GSH and TAS; ↓ MDA, TAS, SOD, and CAT; recover histological damage | [193] |
| Crocetin (10 and 40 mg/kg orally) for 21 days | DSS-Induced Colitis in mice | Promote inflammation, prolong recovery time from colitis, and disturb gut microbiota composition. | [198] |
| Safranal (8 and 16 mM) | DSS and Erwinia carotovora 15 (Ecc15) induced intestinal injury in Drosophila | maintain intestinal homeostasis; ↓ antimicrobial peptide (AMP) and ROS; increase the viability of intestinal epithelial cells | [199] |
| Saffron (50 mg twice daily) for 8 weeks | Open-label, single-center pilot clinical trial | Reduce abdominal pain, diarrhea and rectal bleeding; ↑ IL-10, ↓ TNF-α, IFN-γ, IL-6, IL-2, IL-17A | [200] |
6.12. Effects on the Reproductive System
6.13. Protection Against Skin Diseases
6.14. Bone Regenerative Effects
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Crocin/crocetin (12.5, 25, 50 µM) | In vitro osteogenic differentiation of mesenchymal stem cells | Improve bone regeneration by increasing the number of mesenchymal stem cells (MSCs) mediated osteoblasts | [222] |
| Crocin (40 and 80 µM) | Titanium particles induce inflammation and promote osteogenesis | Downregulate Ti particle-induced inflammation by production of anti-inflammatory cytokines (anti-inflammatory (M2) macrophage polarization); improve osteogenic differentiation | [223] |
| Crocin (10, 20 and 40 mg/kg orally) for 14 days (10, 25 and 50 μmol/L) | In vitro human BMSCs In vivo steroid-induced osteonecrosis of the femoral head | ↑ alkaline phosphatase and calcium nodules; Ameliorate osteonecrosis; ↑ expression levels of RUNX2, COL1A1, and OCN in hBMSCs and femoral head tissues; ↓ GSK-3β phosphorylation | [224] |
| Crocin and bicarbonate de sodium (500 + 80 µg) | Human fetal osteoblast (hFOB) and human osteosarcoma (MG-63) cells; In vivo rat distal femur defects study | Intensify osteoblast proliferation; reduce human osteosarcoma (MG-63) viability by 50%; pro-apoptotic mechanism against osteosarcoma | [229] |
| Crocin (50, 100, 250 and 500 µM) | AlCl3 induced cytotoxicity in rat Bone Marrow Mesenchymal Stem Cells | ↑ mRNA expression of Sox-2 and E-cadherin | [227] |
| Saffron (800 µg/mL) + Pulsed electromagnetic fields (PEMFs) | In vitro osteogenic differentiation of bone marrow mesenchymal stem cells | ↓ dose-dependently, the cell viability, ↑ ALP activity, and synergic effect of saffron and PEMPs on osteogenesis at the initial stage | [225] |
| Crocin (2.5–5 µM) + nanocurcumin (0.3 and 0.7 µM) | In vitro osteogenic differentiation of bone marrow mesenchymal stem cells | Help MSCs proliferate and protect them from apoptosis; increase the expression of OCT4 and SOX2 genes | [230] |
| Crocin (2–10 µM) (20–100 mg/mL) | Methylglyoxal-induced osteoclasts in RAW264.7 cell lines | ↓ osteoclast function and differentiation and bone resorption; ↓gene expression levels of TRAF6, Akt2, ERK1, OSTM1, and MMP-9; ↓bone resorption activity of osteoclasts | [226] |
| Crocin (5 or 10 mg/kg orally) for 12 weeks | Metabolic syndrome-induced osteoporosis in rats | ↓ tartrate-resistant acid phosphatase and C-terminal telopeptide and TNF-α and IL-6 oxidative stress; protect from histological change in bone; ↑ serum alkaline phosphatase and osteocalcin | [228] |
6.15. Nephroprotective Effects
7. Toxicity of Saffron
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative Stress Mitigation by Antioxidants-an Overview on Their Chemistry and Influences on Health Status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Nagasu, H.; Kidokoro, K.; Kashihara, N. Oxidative Stress and the Role of Redox Signalling in Chronic Kidney Disease. Nat. Rev. Nephrol. 2024, 20, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Khatib, S.; Harnafi, M.; Touiss, I.; Bekkouch, O.; Milenkovic, D.; Amrani, S.; Harnafi, H. HPLC–DAD Profiling of a Phenolic Extract from Moroccan Sweet Basil and Its Application as Oxidative Stabilizer of Sunflower Oil. Chem. Pap. 2021, 75, 1907–1917. [Google Scholar] [CrossRef]
- Pinto, T.; Aires, A.; Cosme, F.; Bacelar, E.; Morais, M.C.; Oliveira, I.; Ferreira-Cardoso, J.; Anjos, R.; Vilela, A.; Gonçalves, B. Bioactive (Poly) Phenols, Volatile Compounds from Vegetables, Medicinal and Aromatic Plants. Foods 2021, 10, 106. [Google Scholar] [CrossRef]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of Phenolic Compounds in the Reduction of Risk Factors of Cardiovascular Diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the King of Spices: An Overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- José Bagur, M.; Alonso Salinas, G.L.; Jiménez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef]
- Dai, R.-C.; Nabil, W.N.N.; Xu, H.-X. The History of Saffron in China: From Its Origin to Applications. Chin. Med. Cult. 2021, 4, 228–234. [Google Scholar] [CrossRef]
- Moradi, M.M.; Turhan, S. The Importance of Saffron Plant in Afghanistan’s Agriculture. J. Biol. Environ. Sci. 2017, 11, 165–169. [Google Scholar]
- Leone, S.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Ferrante, C.; Leporini, L.; Brunetti, L.; Menghini, L. Phytotherapic Use of the Crocus sativus L. (Saffron) and Its Potential Applications: A Brief Overview. Phytother. Res. 2018, 32, 2364–2375. [Google Scholar] [CrossRef]
- Kazemi-Shahandashti, S.-S.; Mann, L.; El-Nagish, A.; Harpke, D.; Nemati, Z.; Usadel, B.; Heitkam, T. Ancient Artworks and Crocus Genetics Both Support Saffron’s Origin in Early Greece. Front. Plant Sci. 2022, 13, 834416. [Google Scholar] [CrossRef]
- Baba, S.A.; Ashraf, N. Apocarotenoids of Crocus sativus L.: From Biosynthesis to Pharmacology; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 981-10-1899-5. [Google Scholar]
- Dhar, M.K.; Sharma, M.; Bhat, A.; Chrungoo, N.K.; Kaul, S. Functional Genomics of Apocarotenoids in Saffron: Insights from Chemistry, Molecular Biology and Therapeutic Applications. Brief. Funct. Genom. 2017, 16, 336–347. [Google Scholar] [CrossRef]
- Torricelli, R.; Javan, I.Y.; Albertini, E.; Venanzoni, R.; Hosseinzadeh, Y. Morphological and Molecular Characterization of Italian, Iranian and Spanish Saffron (Crocus sativus L.) Accessions. Appl. Ecol. Environ. Res. 2019, 17, 1875–1887. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Nevárez-Moorillón, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Cid-Pérez, T.S. Detection of Saffron’s Main Bioactive Compounds and Their Relationship with Commercial Quality. Foods 2022, 11, 3245. [Google Scholar] [CrossRef] [PubMed]
- Lage, M.; Cantrell, C.L. Quantification of Saffron (Crocus sativus L.) Metabolites Crocins, Picrocrocin and Safranal for Quality Determination of the Spice Grown under Different Environmental Moroccan Conditions. Sci. Hortic. 2009, 121, 366–373. [Google Scholar] [CrossRef]
- Serrano-Diaz, J.; Sánchez, A.M.; Martinez-Tome, M.; Winterhalter, P.; Alonso, G.L. A Contribution to Nutritional Studies on Crocus sativus Flowers and Their Value as Food. J. Food Compos. Anal. 2013, 31, 101–108. [Google Scholar] [CrossRef]
- Mzabri, I.; Addi, M.; Berrichi, A. Traditional and Modern Uses of Saffron (Crocus sativus). Cosmetics 2019, 6, 63. [Google Scholar] [CrossRef]
- Parizad, S.; Dizadji, A.; Habibi, M.K.; Winter, S.; Kalantari, S.; Movi, S.; Tendero, C.L.; Alonso, G.L.; Moratalla-Lopez, N. The Effects of Geographical Origin and Virus Infection on the Saffron (Crocus sativus L.) Quality. Food Chem. 2019, 295, 387–394. [Google Scholar] [CrossRef]
- Hadavi, R.; Jafari, S.M.; Katouzian, I. Nanoliposomal Encapsulation of Saffron Bioactive Compounds; Characterization and Optimization. Int. J. Biol. Macromol. 2020, 164, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Wenger, T. History of Saffron. Longhua Chin. Med. 2022, 5, 15. [Google Scholar] [CrossRef]
- Mousavi, S.Z.; Bathaie, S.Z. Historical Uses of Saffron: Identifying Potential New Avenues for Modern Research. Avicenna J. Phytomed. 2011, 1, 57–66. [Google Scholar]
- Asgari, M.; Yu, Q.; Abdi, M.; Du, G.-L.; Shen, Y.-H. Survey of the History and Applications of Saffron. Chin. Med. Cult. 2022, 5, 31–38. [Google Scholar] [CrossRef]
- Basker, D.; Negbi, M. Uses of Saffron. Econ. Bot 1983, 37, 228–236. [Google Scholar] [CrossRef]
- Willard, P. Secrets of Saffron: The Vagabond Life of the World’s Most Seductive Spice; Beacon Press: Boston, MA, USA, 2002; ISBN 0-8070-5009-1. [Google Scholar]
- Dalby, A. Dangerous Tastes: The Story of Spices; Univ of California Press: Oakland, CA, USA, 2000; ISBN 0-520-23674-2. [Google Scholar]
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N. Saffron (Crocus sativus L.): A Source of Nutrients for Health and for the Treatment of Neuropsychiatric and Age-Related Diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef]
- Saravask. Saffron Crocus sativus Modern World Production. 2006. Available online: https://commons.wikimedia.org/wiki/File:Saffron_crocus_sativus_modern_world_production.png (accessed on 30 October 2025).
- Bathaie, S.Z.; Farajzade, A.; Hoshyar, R. A Review of the Chemistry and Uses of Crocins and Crocetin, the Carotenoid Natural Dyes in Saffron, with Particular Emphasis on Applications as Colorants Including Their Use as Biological Stains. Biotech. Histochem. 2014, 89, 401–411. [Google Scholar] [CrossRef]
- Marrone, G.; Urciuoli, S.; Di Lauro, M.; Cornali, K.; Montalto, G.; Masci, C.; Vanni, G.; Tesauro, M.; Vignolini, P.; Noce, A. Saffron (Crocus sativus L.) and Its by-Products: Healthy Effects in Internal Medicine. Nutrients 2024, 16, 2319. [Google Scholar] [CrossRef]
- Carmona, M.; Sánchez, A.M.; Ferreres, F.; Zalacain, A.; Tomás-Barberán, F.; Alonso, G.L. Identification of the Flavonoid Fraction in Saffron Spice by LC/DAD/MS/MS: Comparative Study of Samples from Different Geographical Origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main Chemical Compounds and Pharmacological Activities of Stigmas and Tepals of ‘Red Gold’; Saffron. Trends Food Sci. Technol. 2016, 58, 69–78. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F. Ameliorative Effect of Saffron Aqueous Extract on Hyperglycemia, Hyperlipidemia, and Oxidative Stress on Diabetic Encephalopathy in Streptozotocin Induced Experimental Diabetes Mellitus. BioMed Res. Int. 2014, 2014, 920857. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, E.; Kadoglou, N.P.; Kostomitsopoulos, N.; Valsami, G. Saffron: A Natural Product with Potential Pharmaceutical Applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef]
- Ahrazem, O.; Rubio-Moraga, A.; Nebauer, S.G.; Molina, R.V.; Gomez-Gomez, L. Saffron: Its Phytochemistry, Developmental Processes, and Biotechnological Prospects. J. Agric. Food Chem. 2015, 63, 8751–8764. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A. Anticarcinogenic Effect of Saffron (Crocus sativus L.) and Its Ingredients. Pharmacogn. Res. 2014, 6, 99. [Google Scholar] [CrossRef]
- Maggi, L.; Carmona, M.; Zalacain, A.; Kanakis, C.D.; Anastasaki, E.; Tarantilis, P.A.; Polissiou, M.G.; Alonso, G.L. Changes in Saffron Volatile Profile According to Its Storage Time. Food Res. Int. 2010, 43, 1329–1334. [Google Scholar] [CrossRef]
- Cerda-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J. Saffron Bioactives Crocin, Crocetin and Safranal: Effect on Oxidative Stress and Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249. [Google Scholar] [CrossRef]
- Bekkouch, O.; Bekkouch, A.; Elbouny, H.; Touiss, I.; Assri, S.E.; Choukri, M.; Bourhia, M.; Kim, K.; Kang, S.; Choi, M. Combinatorial Effects of Zingiber Officinale and Citrus Limon Juices: Hypolipidemic and Antioxidant Insights from in Vivo, in Vitro, and in Silico Investigations. PLoS ONE 2025, 20, e0319721. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Khoulati, A.; Kadda, S.; Bencheikh, N.; Mamri, S.; Ziani, A.; Baddaoui, S.; Eddabbeh, F.-E.; Lahmass, I.; Benabbes, R. Antioxidant Activity, Metal Chelating Ability and DNA Protective Effect of the Hydroethanolic Extracts of Crocus sativus Stigmas, Tepals and Leaves. Antioxidants 2022, 11, 932. [Google Scholar] [CrossRef]
- Samaha, H.; Chahine, N.; Sobolev, A.P.; Menghini, L.; Makhlouf, H. 1H-NMR Metabolic Profiling and Antioxidant Activity of Saffron (Crocus sativus) Cultivated in Lebanon. Molecules 2021, 26, 4906. [Google Scholar] [CrossRef]
- Gaglio, R.; Gentile, C.; Bonanno, A.; Vintaloro, L.; Perrone, A.; Mazza, F.; Barbaccia, P.; Settanni, L.; Di Grigoli, A. Effect of Saffron Addition on the Microbiological, Physicochemical, Antioxidant and Sensory Characteristics of Yoghurt. Int. J. Dairy Technol. 2019, 72, 208–217. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S. Crocus sativus L. Stigmas and Byproducts: Qualitative Fingerprint, Antioxidant Potentials and Enzyme Inhibitory Activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.T.; Moallem, S.A.; Mahmoudi, M.; Memar, B.; Razavi, B.M.; Hosseinzadeh, H. Effect of Crocus sativus L. Stigma (Saffron) against Subacute Effect of Diazinon: Histopathological, Hematological, Biochemical and Genotoxicity Evaluations in Rats. J. Pharmacopunct. 2018, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xiong, Z.; Yu, C.; Zhou, J.; Shen, Q.; Wang, L.; Xie, X.; Fu, Z. Antidepressant Activity of Crocin-I Is Associated with Amelioration of Neuroinflammation and Attenuates Oxidative Damage Induced by Corticosterone in Mice. Physiol. Behav. 2019, 212, 112699. [Google Scholar] [CrossRef]
- Gudarzi, S.; Jafari, M.; Pirzad Jahromi, G.; Eshrati, R.; Asadollahi, M.; Nikdokht, P. Evaluation of Modulatory Effects of Saffron (Crocus sativus L.) Aqueous Extract on Oxidative Stress in Ischemic Stroke Patients: A Randomized Clinical Trial. Nutr. Neurosci. 2022, 25, 1137–1146. [Google Scholar] [CrossRef]
- Tahvilian, N.; Masoodi, M.; Faghihi Kashani, A.; Vafa, M.; Aryaeian, N.; Heydarian, A.; Hosseini, A.; Moradi, N.; Farsi, F. Effects of Saffron Supplementation on Oxidative/Antioxidant Status and Severity of Disease in Ulcerative Colitis Patients: A Randomized, Double-blind, Placebo-controlled Study. Phytother. Res. 2021, 35, 946–953. [Google Scholar] [CrossRef]
- Zeinali, M.; Zirak, M.R.; Rezaee, S.A.; Karimi, G.; Hosseinzadeh, H. Immunoregulatory and Anti-Inflammatory Properties of Crocus sativus (Saffron) and Its Main Active Constituents: A Review. Iran. J. Basic Med. Sci. 2019, 22, 334. [Google Scholar]
- Jeddi, F.; Zahertar, S.; Bordbar, A.; Salimnejad, R.; Ghobadi, H.; Aslani, M.R. Crocin from Saffron Ameliorates Allergic Airway Inflammation through NF-κB, IL-17, and Nrf2/HO-1 Signaling Pathways in Mice. Iran. J. Basic Med. Sci. 2024, 27, 1624. [Google Scholar]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Immunomodulatory and Antioxidant Effects of Saffron Aqueous Extract (Crocus sativus L.) on Streptozotocin-Induced Diabetes in Rats. Indian Heart J. 2017, 69, 151–159. [Google Scholar] [CrossRef]
- Yosri, H.; Elkashef, W.F.; Said, E.; Gameil, N.M. Crocin Modulates IL-4/IL-13 Signaling and Ameliorates Experimentally Induced Allergic Airway Asthma in a Murine Model. Int. Immunopharmacol. 2017, 50, 305–312. [Google Scholar] [CrossRef]
- Han, S.; Song, R.; Cao, Y.; Yan, X.; Gao, H.; Lian, F. Crocin Mitigates Atherosclerotic Progression in LDLR Knockout Mice by Hepatic Oxidative Stress and Inflammatory Reaction Reduction, and Intestinal Barrier Improvement and Gut Microbiota Modulation. J. Funct. Foods. 2022, 96, 105221. [Google Scholar] [CrossRef]
- Li, J.; Lei, H.; Cao, L.; Mi, Y.-N.; Li, S.; Cao, Y.-X. Crocin Alleviates Coronary Atherosclerosis via Inhibiting Lipid Synthesis and Inducing M2 Macrophage Polarization. Int. Immunopharmacol. 2018, 55, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, Y.; Ma, Z.; Zhao, J. Crocin Exerts Anti-Inflammatory and Anti-Catabolic Effects on Rat Intervertebral Discs by Suppressing the Activation of JNK. Int. J. Mol. Med. 2015, 36, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Chrastina, M.; Dráfi, F.; Pružinská, K.; Poništ, S.; Kamga, K.S.; Khademnematolahi, S.; Bilka, F.; Novák, P.; Pašková, Ľ.; Bauerová, K. Crocus sativus L. Extract (Saffron) Effectively Reduces Arthritic and Inflammatory Parameters in Monotherapy and in Combination with Methotrexate in Adjuvant Arthritis. Nutrients 2023, 15, 4108. [Google Scholar] [CrossRef]
- Aslani, M.R.; Abdollahi, N.; Matin, S.; Zakeri, A.; Ghobadi, H. Effect of Crocin of Crocus sativus L. on Serum Inflammatory Markers (IL-6 and TNF-α) in Chronic Obstructive Pulmonary Disease Patients: A Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Nutr. 2023, 130, 446–453. [Google Scholar] [CrossRef]
- Riahi-Zanjani, B.; Balali-Mood, M.; Mohammadi, E.; Badie-Bostan, H.; Memar, B.; Karimi, G. Safranal as a Safe Compound to Mice Immune System. Avicenna J. Phytomed. 2015, 5, 441. [Google Scholar]
- Farahani, M.H.; Zandieh, M.A.; Torkan, S. The Effects of Crocin from Red Saffron Flower on the Innate and Humoral Immune Systems of Domestic Short Hair Cats: Crocin Supplementation and Immunity of Cats. Lett. Anim. Biol. 2025, 5, 7–11. [Google Scholar] [CrossRef]
- Yousefi, F.; Arab, F.L.; Rastin, M.; Tabasi, N.S.; Nikkhah, K.; Mahmoudi, M. Comparative Assessment of Immunomodulatory, Proliferative, and Antioxidant Activities of Crocin and Crocetin on Mesenchymal Stem Cells. J. Cell. Biochem. 2021, 122, 29–42. [Google Scholar] [CrossRef]
- Tolba, H.A.; Aldawek, A.M.; Eid, R.A.; Aladdin, S.; El-Shaer, N.H. Immune Response and Bacterial Resistance of Oreochromis Niloticus against Bacterial Fish Pathogen with Saffron Diet. Open Vet. J. 2024, 14, 2572. [Google Scholar] [CrossRef]
- Hassanizadeh, S.; Alikiaii, B.; Rouhani, M.H.; Talebi, S.; Mokhtari, Z.; Sharma, M.; Bagherniya, M. The Effects of Saffron Supplementation on Inflammation and Hematological Parameters in Patients with Sepsis: A Randomized Controlled Trial. Nutr. J. 2025, 24, 72. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Nikola, O.; Marka, S.; Koniari, E.; Kakouri, E.; Zografaki, M.-E.; Mavrikou, S.S.; Kanakis, C.; Flemetakis, E.; Chrousos, G.P. An in Vitro Study of Saffron Carotenoids: The Effect of Crocin Extracts and Dimethylcrocetin on Cancer Cell Lines. Antioxidants 2022, 11, 1074. [Google Scholar] [CrossRef]
- Jiang, Z.; Gu, M.; Liu, J.; Li, H.; Peng, J.; Zhang, Y. Anticancer Activity of Crocin against Cervical Carcinoma (HeLa Cells): Bioassessment and Toxicity Evaluation of Crocin in Male Albino Rats. J. Photochem. Photobiol. B 2018, 180, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Guo, J.; Cui, H.; Liu, S. Anticancer Activity of Safranal against Colon Carcinoma Is Due to Induction of Apoptosis and G2/M Cell Cycle Arrest Mediated by Suppression of mTOR/PI3K/Akt Pathway. Off. J. Balk. Union Oncol. 2018, 23, 574–578. [Google Scholar]
- Hassan, P.H.; Elshamy, I.S.; Azmaa, N.E.A. Anticancer Effect of Combined Cinnamon–Saffron versus Its Nanoparticles on Oral Squamous Cell Carcinoma Cell Line. Tanta Dent. J. 2024, 21, 229–236. [Google Scholar] [CrossRef]
- Hire, R.R.; Srivastava, S.; Davis, M.B.; Kumar Konreddy, A.; Panda, D. Antiproliferative Activity of Crocin Involves Targeting of Microtubules in Breast Cancer Cells. Sci. Rep. 2017, 7, 44984. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.A.; Hakkim, F.L.; Sam, S.; Al-Buloshi, M. Assessment of in Vitro Cytotoxicity of Saffron (Crocus sativus L.) on Cervical Cancer Cells (HEp-2) and Their in Vivo Pre-Clinical Toxicity in Normal Swiss Albino Mice. Int. J. Herb. Med. 2016, 4, 80–83. [Google Scholar]
- Khavari, A.; Bolhassani, A.; Alizadeh, F.; Bathaie, S.Z.; Balaram, P.; Agi, E.; Vahabpour, R. Chemo-Immunotherapy Using Saffron and Its Ingredients Followed by E7-NT (Gp96) DNA Vaccine Generates Different Anti-Tumor Effects against Tumors Expressing the E7 Protein of Human Papillomavirus. Arch. Virol. 2015, 160, 499–508. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, J.; Niu, J.; Huang, Y.-F.; Chen, M.; Wang, M.-Z.; Shang, Q.; Lu, W.-Q.; Peng, L.-H.; Jiang, Z.-H. Synthesis, Characterization and Inhibitory Effects of Crocetin Derivative Compounds in Cancer and Inflammation. Biomed. Pharmacother. 2018, 98, 157–164. [Google Scholar] [CrossRef]
- Vago, R.; Trevisani, F.; Vignolini, P.; Vita, C.; Fiorio, F.; Campo, M.; Ieri, F.; Di Marco, F.; Salonia, A.; Romani, A. Evaluation of Anti-Cancer Potential of Saffron Extracts against Kidney and Bladder Cancer Cells. Food Biosci. 2024, 57, 103501. [Google Scholar] [CrossRef]
- Khan, M.; Hearn, K.; Parry, C.; Rashid, M.; Brim, H.; Ashktorab, H.; Kwabi-Addo, B. Mechanism of Antitumor Effects of Saffron in Human Prostate Cancer Cells. Nutrients 2023, 16, 114. [Google Scholar] [CrossRef]
- Feng, S.; Li, S.; Wu, Z.; Li, Y.; Wu, T.; Zhou, Z.; Liu, X.; Chen, J.; Fu, S.; Wang, Z. Saffron Improves the Efficacy of Immunotherapy for Colorectal Cancer through the IL-17 Signaling Pathway. J. Ethnopharmacol. 2025, 337, 118854. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, S.; Hosseinzadeh, H.; Alavizadeh, S.H.; Moghri, M.; Abbasi, A.; Jaafari, M.R. Anti-Tumor Activity of Nanoliposomes Containing Crude Extract of Saffron in Mice Bearing C26 Colon Carcinoma. Nanomed. J. 2021, 8, 290–297. [Google Scholar]
- Gezici, S. Comparative Anticancer Activity Analysis of Saffron Extracts and a Principle Component, Crocetin for Prevention and Treatment of Human Malignancies. J. Food Sci. Technol. 2019, 56, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, M.; Bathaie, S.Z.; Abroun, S.; Azizian, M. Effect of Crocin on Cell Cycle Regulators in N-Nitroso-N-Methylurea-Induced Breast Cancer in Rats. DNA Cell Biol. 2015, 34, 684–691. [Google Scholar] [CrossRef]
- Jabini, R.; Ehtesham-Gharaee, M.; Dalirsani, Z.; Mosaffa, F.; Delavarian, Z.; Behravan, J. Evaluation of the Cytotoxic Activity of Crocin and Safranal, Constituents of Saffron, in Oral Squamous Cell Carcinoma (KB Cell Line). Nutr. Cancer 2017, 69, 911–919. [Google Scholar] [CrossRef]
- Makhlouf, H.; Diab-Assaf, M.; Alghabsha, M.; Tannoury, M.; Chahine, R.; Saab, A.M. In Vitro Antiproliferative Activity of Saffron Extracts against Human Acute Lymphoblastic T-Cell Human Leukemia. Indian J. Trad. Knowl. 2016, 15, 16–21. [Google Scholar]
- Mir, M.A.; Ganai, S.A.; Mansoor, S.; Jan, S.; Mani, P.; Masoodi, K.Z.; Amin, H.; Rehman, M.U.; Ahmad, P. Isolation, Purification and Characterization of Naturally Derived Crocetin Beta-d-Glucosyl Ester from Crocus sativus L. against Breast Cancer and Its Binding Chemistry with ER-Alpha/HDAC2. Saudi J. Biol. Sci. 2020, 27, 975–984. [Google Scholar] [CrossRef]
- Amin, A.; Farrukh, A.; Murali, C.; Soleimani, A.; Praz, F.; Graziani, G.; Brim, H.; Ashktorab, H. Saffron and Its Major Ingredients’ Effect on Colon Cancer Cells with Mismatch Repair Deficiency and Microsatellite Instability. Molecules 2021, 26, 3855. [Google Scholar] [CrossRef]
- Soltani, F.; Ramezani, M.; Amel Farzad, S.; Mokhtarzadeh, A.; Hashemi, M. Comparison Study of the Effect of Alkyl-Modified and Unmodified PAMAM and PPI Dendrimers on Solubility and Antitumor Activity of Crocetin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1356–1362. [Google Scholar] [CrossRef]
- Hafezi Ghahestani, Z.; Alebooye Langroodi, F.; Mokhtarzadeh, A.; Ramezani, M.; Hashemi, M. Evaluation of Anti-Cancer Activity of PLGA Nanoparticles Containing Crocetin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhou, Q.; Hu, J.; Weng, J.; Liu, S.; Yin, L.; Long, L.; Tong, Y.; Tang, K.; Bai, S. Fabrication of Bimetallic Ag@ ZnO Nanocomposite and Its Anti-Cancer Activity on Cervical Cancer via Impeding PI3K/AKT/mTOR Pathway. J. Trace Elem. Med. Biol. 2024, 84, 127437. [Google Scholar] [CrossRef] [PubMed]
- Rahaiee, S.; Hashemi, M.; Shojaosadati, S.A.; Moini, S.; Razavi, S.H. Nanoparticles Based on Crocin Loaded Chitosan-Alginate Biopolymers: Antioxidant Activities, Bioavailability and Anticancer Properties. Int. J. Biol. Macromol. 2017, 99, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Mohamadian, M.; Mostafaei, A.; Rahimi-Danesh, M.; Zargar, M.; Erfani, H.; Sirouskabiri, S.; Vaseghi, S. Crocin Facilitates the Effect of Fear Extinction on Freezing Behavior and BDNF Level in a Rat Model of Fear-Conditioning. Learn. Motiv. 2025, 89, 102095. [Google Scholar] [CrossRef]
- Karimi, H.; Mohamadian, M.; Azizi, P.; Ghasemi, P.; Karimi, M.; Layegh, T.; Rahmatkhah-Yazdi, M.; Vaseghi, S. Crocin Has a Greater Therapeutic Role in the Restoration of Behavioral Impairments Caused by Maternal Social Isolation in Adolescent than in Adult Offspring Probably through GSK-3beta Downregulation. Learn. Motiv. 2024, 88, 102060. [Google Scholar] [CrossRef]
- Salem, M.; Shaheen, M.; Borjac, J. Crocin Suppresses Inflammation-Induced Apoptosis in rmTBI Mouse Model via Modulation of Nrf2 Transcriptional Activity. PharmaNutrition 2022, 21, 100308. [Google Scholar] [CrossRef]
- Abbaszade-Cheragheali, A.; Beheshti, F.; Kakhki, S.; Khatibi, S.R.; Dehnokhalaji, F.; Akbari, E.; Fathi, H.; Farimani, S.S. Crocin, the Main Active Saffron (Crocus sativus L.) Constituent, as a Potential Candidate to Prevent Anxiety and Depressive-like Behaviors Induced by Unpredictable Chronic Mild Stress. Neurosci. Lett. 2022, 791, 136912. [Google Scholar] [CrossRef]
- Tamegart, L.; Abbaoui, A.; Makbal, R.; Zroudi, M.; Bouizgarne, B.; Bouyatas, M.M.; Gamrani, H. Crocus sativus Restores Dopaminergic and Noradrenergic Damages Induced by Lead in Meriones Shawi: A Possible Link with Parkinson’s Disease. Acta Histochem. 2019, 121, 171–181. [Google Scholar] [CrossRef]
- Rao, S.V.; Yenisetti, S.C.; Rajini, P.S. Evidence of Neuroprotective Effects of Saffron and Crocin in a Drosophila Model of Parkinsonism. Neurotoxicology 2016, 52, 230–242. [Google Scholar] [CrossRef]
- Salama, R.M.; Abdel-Latif, G.A.; Abbas, S.S.; Hekmat, M.; Schaalan, M.F. Neuroprotective Effect of Crocin against Rotenone-Induced Parkinson’s Disease in Rats: Interplay between PI3K/Akt/mTOR Signaling Pathway and Enhanced Expression of miRNA-7 and miRNA-221. Neuropharmacology 2020, 164, 107900. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; El Awdan, S.A.; Hegazy, R.R.; Mansour, D.F.; Ogaly, H.; Abdelbaset, M. Neuroprotective Effect of Crocus sativus against Cerebral Ischemia in Rats. Metab. Brain Dis. 2020, 35, 427–439. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Sadeghnia, H.R.; Hosseinzadeh, H.; Khorrami, M.B.; Shaterzadeh, H. P18: Neuroprotective Effect of Safranal, an Active Ingredient of Crocus sativus, in a Rat Model of Transient Cerebral Ischemia. Neurosci. J. Shefaye Khatam 2018, 6, 49. [Google Scholar]
- Zhang, C.; Ma, J.; Fan, L.; Zou, Y.; Dang, X.; Wang, K.; Song, J. Neuroprotective Effects of Safranal in a Rat Model of Traumatic Injury to the Spinal Cord by Anti-Apoptotic, Anti-Inflammatory and Edema-Attenuating. Tissue Cell 2015, 47, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Asadi, F.; Jamshidi, A.H.; Khodagholi, F.; Yans, A.; Azimi, L.; Faizi, M.; Vali, L.; Abdollahi, M.; Ghahremani, M.H.; Sharifzadeh, M. Reversal Effects of Crocin on Amyloid β-Induced Memory Deficit: Modification of Autophagy or Apoptosis Markers. Pharmacol. Biochem. Behav. 2015, 139, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, H.; Ghahremanfard, F.; Motaghi, E.; Zamaemifard, M.; Zamani, M.; Izadi, A. Effectiveness of Crocin of Saffron (Crocus sativus L.) against Chemotherapy-Induced Peripheral Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Ethnopharmacol. 2021, 281, 114511. [Google Scholar] [CrossRef]
- Marzabadi, L.R.; Fazljou, S.M.B.; Araj-Khodaei, M.; Sadigh-Eteghad, S.; Naseri, A.; Talebi, M. Saffron Reduces Some Inflammation and Oxidative Stress Markers in Donepezil-Treated Mild-to-Moderate Alzheimer’s Disease Patients: A Randomized Double-Blind Placebo-Control Trial. J. Herb. Med. 2022, 34, 100574. [Google Scholar] [CrossRef]
- Soleymani, S.M.; Assarzadegan, F.; Habibi, S.A.H.; Mahboubi, A.; Esmaily, H. The Effect of Crocin on Movement Disorders and Oxidative DNA Damage in Parkinson’s Disease: Insights from a Randomized Controlled Trial. Park. Relat. Disord. 2024, 126, 107051. [Google Scholar] [CrossRef]
- Yan, J.; Li, T.; Ji, K.; Zhou, X.; Yao, W.; Zhou, L.; Huang, P.; Zhong, K. Safranal Alleviates Pentetrazole-Induced Epileptic Seizures in Mice by Inhibiting the NF-κB Signaling Pathway and Mitochondrial-Dependent Apoptosis through GSK-3β Inactivation. J. Ethnopharmacol. 2024, 333, 118408. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Zhang, Y.; Zhao, G. Crocin Protects PC12 Cells against MPP+-Induced Injury through Inhibition of Mitochondrial Dysfunction and ER Stress. Neurochem. Int. 2015, 89, 101–110. [Google Scholar] [CrossRef]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; López-Villarín, N.; Salobrar-García, E.; López-Cuenca, I.; Licastro, E.; Inarejos-García, A.M.; Almodóvar, P.; Pinazo-Durán, M.D. Neuroprotective and Anti-Inflammatory Effects of a Hydrophilic Saffron Extract in a Model of Glaucoma. Int. J. Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Rao, W.; Su, N.; Hui, H.; Wang, L.; Peng, C.; Tu, Y.; Zhang, S.; Fei, Z. Neuroprotective Effects of Crocin against Traumatic Brain Injury in Mice: Involvement of Notch Signaling Pathway. Neurosci. Lett. 2015, 591, 53–58. [Google Scholar] [CrossRef]
- Jalili, C.; Gholami, M.; Kakebaraei, S. Protective Effect of Crocin-Loaded Nanoparticles in Rats Following Epileptic Seizures: Biochemical, Behavioral and Histopathological Outcomes. Heliyon 2024, 10, e36122. [Google Scholar] [CrossRef]
- Mousavi, B.; Bathaie, S.Z.; Fadai, F.; Ashtari, Z.; Farhang, S.; Hashempour, S.; Shahhamzei, N.; Heidarzadeh, H. Safety Evaluation of Saffron Stigma (Crocus sativus L.) Aqueous Extract and Crocin in Patients with Schizophrenia. Avicenna J. Phytomed. 2015, 5, 413. [Google Scholar] [PubMed]
- Patel, K.S.; Dharamsi, A.; Priya, M.; Jain, S.; Mandal, V.; Girme, A.; Modi, S.J.; Hingorani, L. Saffron (Crocus sativus L.) Extract Attenuates Chronic Scopolamine-Induced Cognitive Impairment, Amyloid Beta, and Neurofibrillary Tangles Accumulation in Rats. J. Ethnopharmacol. 2024, 326, 117898. [Google Scholar] [CrossRef] [PubMed]
- Inoue, E.; Suzuki, T.; Shimizu, Y.; Sudo, K.; Kawasaki, H.; Ishida, N. Saffron Ameliorated Motor Symptoms, Short Life Span and Retinal Degeneration in Parkinson’s Disease Fly Models. Gene 2021, 799, 145811. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Shaheen, M.; Tabbara, A.; Borjac, J. Saffron Extract and Crocin Exert Anti-Inflammatory and Anti-Oxidative Effects in a Repetitive Mild Traumatic Brain Injury Mouse Model. Sci. Rep. 2022, 12, 5004. [Google Scholar] [CrossRef]
- Chaoul, V.; Awad, M.; Harb, F.; Najjar, F.; Hamade, A.; Nabout, R.; Soueid, J. Saffron Extract Attenuates Anxiogenic Effect and Improves Cognitive Behavior in an Adult Zebrafish Model of Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 11600. [Google Scholar] [CrossRef]
- Al Jaberi, F.M.; Alhawarri, M.B.; Dewa, A.; Zainal, Z.; Zakaria, F. Anti-Obesity, Phytochemical Profiling and Acute Toxicity Study of Ethanolic Extract of Saffron (Crocus sativus L.). Pharmacol. Res.-Mod. Chin. Med. 2024, 11, 100420. [Google Scholar] [CrossRef]
- Mashmoul, M.; Azlan, A.; Mohtarrudin, N.; Yusof, B.N.M.; Khaza’ai, H. Saffron Extract and Crocin Reduced Biomarkers Associated with Obesity in Rats Fed a High-Fat Diet. Malays. J. Nutr. 2017, 23, 117–127. [Google Scholar]
- Xie, X.; Zhang, M.; Sun, L.; Wang, T.; Zhu, Z.; Shu, R.; Wu, F.; Li, Z. Crocin-I Protects against High-Fat Diet-Induced Obesity via Modulation of Gut Microbiota and Intestinal Inflammation in Mice. Front. Pharmacol. 2022, 13, 894089. [Google Scholar] [CrossRef]
- Alipour, R.; Aryaeian, N.; Hajiluian, G.; Soleimani, M.; Barati, M. The Effect of the Saffron Intervention on NAFLD Status and Related Gene Expression in a Rat Model. Med. J. Islam. Repub. Iran 2023, 37, 207–214. [Google Scholar] [CrossRef]
- Mohaqiq, Z.; Moossavi, M.; Hemmati, M.; Kazemi, T.; Mehrpour, O. Antioxidant Properties of Saffron Stigma and Petals: A Potential Therapeutic Approach for Insulin Resistance through an Insulin-Sensitizing Adipocytokine in High-Calorie Diet Rats. Int. J. Prev. Med. 2020, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Gul, T.; Balkhi, H.M.; Haq, E. Inhibition of Adipocyte Differentiation by Crocin in in Vitro Model of Obesity. Saudi J. Life Sci. 2017, 2, 306–311. [Google Scholar]
- Jafari, F.; Emami, S.A.; Javadi, B.; Salmasi, Z.; Tayarani-Najjaran, M.; Tayarani-Najaran, Z. Inhibitory Effect of Saffron, Crocin, Crocetin, and Safranal against Adipocyte Differentiation in Human Adipose-Derived Stem Cells. J. Ethnopharmacol. 2022, 294, 115340. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Luo, L.; Fang, K. Crocin Inhibits Obesity via AMPK-Dependent Inhibition of Adipocyte Differentiation and Promotion of Lipolysis. Biosci. Trends 2018, 12, 587–594. [Google Scholar] [CrossRef]
- Abedimanesh, N.; Bathaie, S.Z.; Abedimanesh, S.; Motlagh, B.; Separham, A.; Ostadrahimi, A. Saffron and Crocin Improved Appetite, Dietary Intakes and Body Composition in Patients with Coronary Artery Disease. J. Cardiovasc. Thorac. Res. 2017, 9, 200. [Google Scholar] [CrossRef]
- Ramli, F.N.; Abu Bakar Sajak, A.; Abas, F.; Mat Daud, Z.A.; Azlan, A. Effect of Saffron Extract and Crocin in Serum Metabolites of Induced Obesity Rats. BioMed Res. Int. 2020, 2020, 1247946. [Google Scholar] [CrossRef]
- Fang, K.; Gu, M. Crocin Improves Insulin Sensitivity and Ameliorates Adiposity by Regulating AMPK-CDK5-PPARγ Signaling. BioMed Res. Int. 2020, 2020, 9136282. [Google Scholar] [CrossRef]
- Hoshyar, R.; Hosseinian, M.; Rajabian Naghandar, M.; Hemmati, M.; Zarban, A.; Amini, Z.; Valavi, M.; Zare Beyki, M.; Mehrpour, O. Anti-Dyslipidemic Properties of Saffron: Reduction in the Associated Risks of Atherosclerosis and Insulin Resistance. Iran. Red Crescent Med. J. 2016, 18, 22. [Google Scholar] [CrossRef]
- Sani, A.; Tajik, A.; Seiiedi, S.S.; Khadem, R.; Tootooni, H.; Taherynejad, M.; Sabet Eqlidi, N.; Alavi dana, S.M.M.; Deravi, N. A Review of the Anti-Diabetic Potential of Saffron. Nutr. Metab. Insights 2022, 15, 11786388221095223. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Lahmass, I.; Bouhrim, M.; Khoulati, A.; Sabouni, A.; Benabbes, R.; Asehraou, A.; Choukri, M.; Bnouham, M.; Saalaoui, E. Antidiabetic Effect of Hydroethanolic Extract of Crocus sativus Stigmas, Tepals and Leaves in Streptozotocin-Induced Diabetic Rats. Physiol. Pharmacol. 2019, 23, 9–20. [Google Scholar]
- Asdaq, S.M.B.; Mannasaheb, B.A.; Orfali, R.; Shaikh, I.A.; Alshehri, A.; Alghamdi, A.; Alrashdi, M.M.; Almadani, M.E.; Abdalla, F.M.A. Antidiabetic and Antioxidant Potential of Crocin in High-Fat Diet plus Streptozotocin-Induced Type-2 Diabetic Rats. Int. J. Immunopathol. Pharmacol. 2024, 38, 03946320231220178. [Google Scholar] [CrossRef] [PubMed]
- Lahmass, I.; Sabouni, A.; Elyoubi, M.; Benabbes, R.; Mokhtari, S.; Saalaoui, E. Anti-Diabetic Effect of Aqueous Extract Crocus sativus L. in Tartrazine Induced Diabetic Male Rats. Physiol. Pharmacol. 2017, 21, 312–321. [Google Scholar]
- Lai, L.-L.; Lu, H.-Q.; Li, W.-N.; Huang, H.-P.; Zhou, H.-Y.; Leng, E.; Zhang, Y. Protective Effects of Quercetin and Crocin in the Kidneys and Liver of Obese Sprague-Dawley Rats with Type 2 Diabetes: Effects of Quercetin and Crocin on T2DM Rats. Hum. Exp. Toxicol. 2021, 40, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Drioiche, A.; Ailli, A.; Handaq, N.; Remok, F.; Elouardi, M.; Elouadni, H.; Al Kamaly, O.; Saleh, A.; Bouhrim, M.; Elazzouzi, H. Identification of Compounds of Crocus sativus by GC-MS and HPLC/UV-ESI-MS and Evaluation of Their Antioxidant, Antimicrobial, Anticoagulant, and Antidiabetic Properties. Pharmaceuticals 2023, 16, 545. [Google Scholar] [CrossRef]
- Zhao, X.; Ahn, D.; Nam, G.; Kwon, J.; Song, S.; Kang, M.J.; Ahn, H.; Chung, S.J. Identification of Crocetin as a Dual Agonist of GPR40 and GPR120 Responsible for the Antidiabetic Effect of Saffron. Nutrients 2023, 15, 4774. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, X.; Liu, D.; Deng, Z.; Hu, W.; Li, Z.; Li, Y. The Hypoglycemic and Renal Protection Properties of Crocin via Oxidative Stress-Regulated NF-κB Signaling in Db/Db Mice. Front. Pharmacol. 2020, 11, 541. [Google Scholar] [CrossRef]
- Giannoulaki, P.; Kotzakioulafi, E.; Nakas, A.; Kontoninas, Z.; Karlafti, E.; Evripidou, P.; Kantartzis, K.; Savopoulos, C.; Chourdakis, M.; Didangelos, T. Effect of Crocus sativus Extract Supplementation in the Metabolic Control of People with Diabetes Mellitus Type 1: A Double-Blind Randomized Placebo-Controlled Trial. Nutrients 2024, 16, 2089. [Google Scholar] [CrossRef]
- Milajerdi, A.; Jazayeri, S.; Hashemzadeh, N.; Shirzadi, E.; Derakhshan, Z.; Djazayeri, A.; Akhondzadeh, S. The Effect of Saffron (Crocus sativus L.) Hydroalcoholic Extract on Metabolic Control in Type 2 Diabetes Mellitus: A Triple-Blinded Randomized Clinical Trial. J. Res. Med. Sci. 2018, 23, 16. [Google Scholar]
- Kakouri, E.; Agalou, A.; Kanakis, C.; Beis, D.; Tarantilis, P.A. Crocins from Crocus sativus L. in the Management of Hyperglycemia. in Vivo Evidence from Zebrafish. Molecules 2020, 25, 5223. [Google Scholar] [CrossRef]
- Zaazaa, L.; Naceiri Mrabti, H.; Ed-Dra, A.; Bendahbia, K.; Hami, H.; Soulaymani, A.; Ibriz, M. Determination of Mineral Composition and Phenolic Content and Investigation of Antioxidant, Antidiabetic, and Antibacterial Activities of Crocus sativus L. Aqueous Stigmas Extracts. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 7533938. [Google Scholar] [CrossRef]
- Hazman, Ö.; Aksoy, L.; Büyükben, A. Effects of Crocin on Experimental Obesity and Type-2 Diabetes. Turk. J. Med. Sci. 2016, 46, 1593–1602. [Google Scholar] [CrossRef]
- Raafat, K.; Aboul-Ela, M.; El-Lakany, A. Phytochemical and Anti-Neuropathic Investigations of Crocus sativus via Alleviating Inflammation, Oxidative Stress and Pancreatic Beta-Cells Regeneration. Chin. Herb. Med. 2020, 12, 47–55. [Google Scholar] [CrossRef]
- Huang, Z.; Nan, C.; Wang, H.; Su, Q.; Xue, W.; Chen, Y.; Shan, X.; Duan, J.; Chen, G.; Tao, W. Crocetin Ester Improves Myocardial Ischemia via Rho/ROCK/NF-κB Pathway. Int. Immunopharmacol. 2016, 38, 186–193. [Google Scholar] [CrossRef]
- Goyal, S.; Arora, S.; Sharma, A.; Joshi, S.; Ray, R.; Bhatia, J.; Kumari, S.; Arya, D. Preventive Effect of Crocin of Crocus sativus on Hemodynamic, Biochemical, Histopathological and Ultrastuctural Alterations in Isoproterenol-Induced Cardiotoxicity in Rats. Phytomedicine 2010, 17, 227–232. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Y.; Xue, Y.; Han, X.; Zhang, X.; Ma, Z.; Sun, S.; Chu, X.; Cheng, J.; Guan, S. Crocin Attenuates Isoprenaline-Induced Myocardial Fibrosis by Targeting TLR4/NF-κB Signaling: Connecting Oxidative Stress, Inflammation, and Apoptosis. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Zhao, J.; Kang, Y.; Liu, L.; He, W.; Xie, Y.; Wang, R.; Shan, L.; Li, X.; Ma, K. Effect and Mechanism of Safranal on ISO-Induced Myocardial Injury Based on Network Pharmacology. J. Ethnopharmacol. 2023, 305, 116103. [Google Scholar] [CrossRef] [PubMed]
- Rahim, V.B.; Khammar, M.T.; Rakhshandeh, H.; Samzadeh-Kermani, A.; Hosseini, A.; Askari, V.R. Crocin Protects Cardiomyocytes against LPS-Induced Inflammation. Pharmacol. Rep. 2019, 71, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, B.; Che, K.; Han, X.; Li, L.; Wang, H.; Liu, Y.; Shi, J.; Sun, S. Protective Effects of Safranal on Hypoxia/Reoxygenation-induced Injury in H9c2 Cardiac Myoblasts via the PI3K/AKT/GSK3β Signaling Pathway. Exp. Ther. Med. 2021, 22, 1400. [Google Scholar] [CrossRef]
- Chahine, N.; Nader, M.; Duca, L.; Martiny, L.; Chahine, R. Saffron Extracts Alleviate Cardiomyocytes Injury Induced by Doxorubicin and Ischemia-Reperfusion in Vitro. Drug Chem. Toxicol. 2016, 39, 87–96. [Google Scholar] [CrossRef]
- Abedimanesh, S.; Bathaie, S.Z.; Ostadrahimi, A.; Jafarabadi, M.A.; Sadeghi, M.T. The Effect of Crocetin Supplementation on Markers of Atherogenic Risk in Patients with Coronary Artery Disease: A Pilot, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Food Funct. 2019, 10, 7461–7475. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Salama, M.F.; Said, E.; El-Sherbiny, M.; Al-Gayyar, M.M. Crocin Protects against Doxorubicin-Induced Myocardial Toxicity in Rats through down-Regulation of Inflammatory and Apoptic Pathways. Chem. Biol. Interact. 2016, 247, 39–48. [Google Scholar] [CrossRef]
- Razmaraii, N.; Babaei, H.; Nayebi, A.M.; Assadnassab, G.; Helan, J.A.; Azarmi, Y. Crocin Treatment Prevents Doxorubicin-Induced Cardiotoxicity in Rats. Life Sci. 2016, 157, 145–151. [Google Scholar] [CrossRef]
- Liang, Y.; Zheng, B.; Li, J.; Shi, J.; Chu, L.; Han, X.; Chu, X.; Zhang, X.; Zhang, J. Crocin Ameliorates Arsenic Trioxide-induced Cardiotoxicity via Keap1-Nrf2/HO-1 Pathway: Reducing Oxidative Stress, Inflammation, and Apoptosis. Biomed. Pharmacother. 2020, 131, 110713. [Google Scholar] [CrossRef] [PubMed]
- Radmanesh, E.; Dianat, M.; Badavi, M.; Goudarzi, G.; Mard, S.A.; Radan, M. Protective Effect of Crocin on Hemodynamic Parameters, Electrocardiogram Parameters, and Oxidative Stress in Isolated Hearts of Rats Exposed to PM10. Iran. J. Basic Med. Sci. 2022, 25, 460. [Google Scholar] [PubMed]
- Naddafi, M.; Eghbal, M.A.; Ghazi-Khansari, M.; Sattari, M.R.; Azarmi, Y. Study of the Cardioprotective Effects of Crocin on Human Cardiac Myocyte Cells and Reduction of Oxidative Stress Produced by Aluminum Phosphide Poisoning. J. Pharm. Pharmacol. 2021, 73, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.; Hosseinzadeh, H.; Imenshahidi, M.; Malekian, M.; Ramezani, M.; Abnous, K. Evaluation of Protein Ubiquitylation in Heart Tissue of Rats Exposed to Diazinon (an Organophosphate Insecticide) and Crocin (an Active Saffron Ingredient): Role of HIF-1α. Drug Res. 2015, 65, 561–566. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Wang, J.; Afzal, O.; Altamimi, A.S.; Nasar Mir Najib Ullah, S.; Shilbayeh, S.A.R.; Ibrahim, A.A.; Khan, S. Analysis of Anti-Arrhythmic Impacts of Crocin through Estimation of Expression of Cx43 in Myocardial Infarction Using a Rat Animal Model. ACS Omega 2022, 7, 37164–37169. [Google Scholar] [CrossRef]
- Christodoulou, E.; Kadoglou, N.; Stasinopoulou, M.; Konstandi, O.; Kenoutis, C.; Kakazanis, Z.; Rizakou, A.; Kostomitsopoulos, N.; Valsami, G. Crocus sativus L. Aqueous Extract Reduces Atherogenesis, Increases Atherosclerotic Plaque Stability and Improves Glucose Control in Diabetic Atherosclerotic Animals. Atherosclerosis 2018, 268, 207–214. [Google Scholar] [CrossRef]
- Efentakis, P.; Rizakou, A.; Christodoulou, E.; Chatzianastasiou, A.; López, M.; León, R.; Balafas, E.; Kadoglou, N.; Tseti, I.; Skaltsa, H. Saffron (Crocus sativus) Intake Provides Nutritional Preconditioning against Myocardial Ischemia–Reperfusion Injury in Wild Type and ApoE (−/−) Mice: Involvement of Nrf2 Activation. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 919–929. [Google Scholar] [CrossRef]
- Gao, J.; Chen, M.; Ren, X.-C.; Zhou, X.-B.; Shang, Q.; Lu, W.-Q.; Luo, P.; Jiang, Z.-H. Synthesis and Cardiomyocyte Protection Activity of Crocetin Diamide Derivatives. Fitoterapia 2017, 121, 106–111. [Google Scholar] [CrossRef]
- Xue, Y.; Jin, W.; Xue, Y.; Zhang, Y.; Wang, H.; Zhang, Y.; Guan, S.; Chu, X.; Zhang, J. Safranal, an Active Constituent of Saffron, Ameliorates Myocardial Ischemia via Reduction of Oxidative Stress and Regulation of Ca2+ Homeostasis. J. Pharmacol. Sci. 2020, 143, 156–164. [Google Scholar] [CrossRef]
- Elshibani, F.A.; Alamami, A.D.; Mohammed, H.A.; Rasheed, R.A.; El Sabban, R.M.; Yehia, M.A.; Abdel Mageed, S.S.; Majrashi, T.A.; Elkaeed, E.B.; El Hassab, M.A. A Multidisciplinary Approach to the Antioxidant and Hepatoprotective Activities of Arbutus Pavarii Pampan Fruit; in Vitro and in Vivo Biological Evaluations, and in Silico Investigations. Enzym. Inhib. Med. Chem. 2024, 39, 2293639. [Google Scholar] [CrossRef] [PubMed]
- Kalantar, M.; Kalantari, H.; Goudarzi, M.; Khorsandi, L.; Bakhit, S.; Kalantar, H. Crocin Ameliorates Methotrexate-Induced Liver Injury via Inhibition of Oxidative Stress and Inflammation in Rats. Pharmacol. Rep. 2019, 71, 746–752. [Google Scholar] [CrossRef]
- Chhimwal, J.; Sharma, S.; Kulurkar, P.; Patial, V. Crocin Attenuates CCl4-Induced Liver Fibrosis via PPAR-γ Mediated Modulation of Inflammation and Fibrogenesis in Rats. Hum. Exp. Toxicol. 2020, 39, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Rezaee-Khorasany, A.; Razavi, B.M.; Taghiabadi, E.; Yazdi, A.T.; Hosseinzadeh, H. Effect of Crocin, an Active Saffron Constituent, on Ethanol Toxicity in the Rat: Histopathological and Biochemical Studies. Iran. J. Basic Med. Sci. 2020, 23, 51. [Google Scholar]
- Sokar, S.S.; Alkabbani, M.A.; Akool, E.-S.; Abu-Risha, S.E.-S. Hepatoprotective Effects of Carvedilol and Crocin against Leflunomide-Induced Liver Injury. Int. Immunopharmacol. 2022, 113, 109297. [Google Scholar] [CrossRef] [PubMed]
- Kowsarkhizi, A.S.; Abedi, S.; Karimi, E.; Ghorani, B.; Oskoueian, E. Nanofiber-Based Delivery of Crocus sativus Phenolic Compounds to Ameliorate the Cisplatin-Induced Hepatotoxicity in Mice. J. Funct. Foods 2024, 120, 106334. [Google Scholar] [CrossRef]
- Alayunt, N.Ö.; Parlak, A.E.; Türkoğlu, S.; Taş, F. Hepatoprotective Effects of Safranal on Acetaminophen-Induced Hepatotoxicity in Rats. Open Chem. 2024, 22, 20240029. [Google Scholar] [CrossRef]
- Abdu, S.; Juaid, N.; Amin, A.; Moulay, M.; Miled, N. Therapeutic Effects of Crocin Alone or in Combination with Sorafenib against Hepatocellular Carcinoma: In Vivo & in Vitro Insights. Antioxidants 2022, 11, 1645. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, S.; Gong, J.; Feng, P.; Cao, Y.; Li, Q.; Jiang, Y.; You, Y.; Tong, Y.; Wang, P. Exploring the Protective Effects and Mechanism of Crocetin from Saffron against NAFLD by Network Pharmacology and Experimental Validation. Front. Med. 2021, 8, 681391. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish, R.; Saberi, M.; Azizi, S.; Khakdan, R.; Kordzadeh Kermani, Z. The Effect of Saffron (Crocus sativus) on Oxymetholone-Induced Hepatic and Renal Injury in Rats. Iran. J. Toxicol. 2021, 15, 233–240. [Google Scholar] [CrossRef]
- Yousef, D.M.; Hassan, H.A.; Nafea, O.E.; El Fattah, E.R.A. Crocin Averts Functional and Structural Rat Hepatic Disturbances Induced by Copper Oxide Nanoparticles. Toxicol. Res. 2022, 11, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.A.; Ramdan, H.S.; Al-Eisa, R.A.; Adle Fadle, B.O.; El-Shenawy, N.S. Effect of Saffron Extract on the Hepatotoxicity Induced by Copper Nanoparticles in Male Mice. Molecules 2021, 26, 3045. [Google Scholar] [CrossRef]
- Taher, G.N. Effectiveness of Saffron against Hepatotoxicity Caused by Silver Nanoparticles in Vivo. Pak. J. Med. Health Sci. 2022, 16, 361. [Google Scholar] [CrossRef]
- Aras, I.; Bayram, I.; Oto, G.; Erten, R.; Almali, A.; Ilik, Z. Saffron and Saffron Ingredients like Safranal and Crocin’s Cytoprotective Effects on Carbon Tetrachloride Induced Liver Damage. East. J. Med. 2022, 27, 424–431. [Google Scholar] [CrossRef]
- Jamshidi, V.; Hashemi, S.A.; Khalili, A.; Fallah, P.; Ahmadian-Attari, M.M.; Beikzadeh, L.; Mazloom, R.; Najafizadeh, P.; Bayat, G. Saffron Offers Hepatoprotection via Up-Regulation of Hepatic Farnesoid-X-Activated Receptors in a Rat Model of Acetaminophen-Induced Hepatotoxicity. Avicenna J. Phytomed. 2021, 11, 622. [Google Scholar]
- Hoshyar, R.; Sebzari, A.; Balforoush, M.; Valavi, M.; Hosseini, M. The Impact of Crocus sativus Stigma against Methotrexate-Induced Liver Toxicity in Rats. J. Complement. Integr. Med. 2020, 17, 20190201. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Touiss, I.; Khoulati, A.; Lahmass, I.; Mamri, S.; Meziane, M.; Elassri, S.; Bencheikh, N.; Benabbas, R.; Asehraou, A. Hepatoprotective Effects of Hydroethanolic Extracts of Crocus sativus Tepals, Stigmas and Leaves on Carbon Tetrachloride Induced Acute Liver Injury in Rats. Physiol. Pharmacol. 2021, 25, 178–188. [Google Scholar] [CrossRef]
- Nair, P.; Prabhavalkar, K. Anti-Asthmatic Effects of Saffron Extract and Salbutamol in an Ovalbumin-Induced Airway Model of Allergic Asthma. Sinusitis 2021, 5, 17–31. [Google Scholar] [CrossRef]
- Rashad, W.A.; Sakr, S.; Domouky, A.M. Comparative Study of Oral versus Parenteral Crocin in Mitigating Acrolein-Induced Lung Injury in Albino Rats. Sci. Rep. 2022, 12, 10233. [Google Scholar] [CrossRef]
- Dianat, M.; Radan, M.; Mard, S.A.; Sohrabi, F.; Saryazdi, S.S.N. Contribution of Reactive Oxygen Species via the OXR1 Signaling Pathway in the Pathogenesis of Monocrotaline-Induced Pulmonary Arterial Hypertension: The Protective Role of Crocin. Life Sci. 2020, 256, 117848. [Google Scholar] [CrossRef]
- Mehrabani, M.; Goudarzi, M.; Mehrzadi, S.; Siahpoosh, A.; Mohammadi, M.; Khalili, H.; Malayeri, A. Crocin: A Protective Natural Antioxidant against Pulmonary Fibrosis Induced by Bleomycin. Pharmacol. Rep. 2020, 72, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, T.; Akın, A.; Kaymak, E.; Varinli, Ş.; Toluk, A. Crocin Suppresses Inflammatory Response in LPS-Induced Acute Lung Injury (ALI) Via Regulation of HMGB1/TLR4 Inflammation Pathway. J. Basic Clin. Health Sci. 2024, 8, 271–278. [Google Scholar] [CrossRef]
- Jamshidi, V.; Nobakht, B.F.; Bagheri, H.; Saeedi, P.; Ghanei, M.; Halabian, R. Metabolomics to Investigate the Effect of Preconditioned Mesenchymal Stem Cells with Crocin on Pulmonary Epithelial Cells Exposed to 2-Chloroethyl Ethyl Sulfide. J. Proteom. 2024, 308, 105280. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.I.; Pattnaik, B.; Rayees, S.; Kaul, S.; Dhar, M.K. Safranal of Crocus sativus L. Inhibits Inducible Nitric Oxide Synthase and Attenuates Asthma in a Mouse Model of Asthma. Phytother. Res. 2015, 29, 617–627. [Google Scholar] [CrossRef]
- Memarzia, A.; Ghasemi, S.Z.; Behrouz, S.; Boskabady, M.H. The Effects of Crocus sativus Extract on Inhaled Paraquat-Induced Lung Inflammation, Oxidative Stress, Pathological Changes and Tracheal Responsiveness in Rats. Toxicon 2023, 235, 107316. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, H.; Abdollahi, N.; Madani, H.; Aslani, M.R. Effect of Crocin from Saffron (Crocus sativus L.) Supplementation on Oxidant/Antioxidant Markers, Exercise Capacity, and Pulmonary Function Tests in COPD Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 2022, 13, 884710. [Google Scholar] [CrossRef]
- Memarzia, A.; Beigoli, S.; Ghalibaf, M.H.E.; Ghasemi, S.Z.; Abbasian, A.; Mahzoon, E.; Toosi, A.N.; Roshan, N.M.; Boskabady, M.H. The Preventive Effectiveness of Crocus sativus Extract in Treating Lung Injuries Caused by Inhaled Paraquat in Rats. J. Ethnopharmacol. 2025, 337, 118767. [Google Scholar] [CrossRef]
- Soozangar, N.; Amani, M.; Jeddi, F.; Salimnejad, R.; Aslani, M.R. The Suppressive Effects of Crocin from Saffron on Allergic Airway Inflammation through Drp1/Nfr1/Mfn2/Pgc1-Alpha Signaling Pathway in Mice. J. Ethnopharmacol. 2025, 337, 118862. [Google Scholar]
- Ouahhoud, S.; Marghich, M.; Makrane, H.; Karim, A.; Khoulati, A.; Mamri, S.; Addi, M.; Hano, C.; Benabbes, R.; Aziz, M. In Vitro Assessment of Myorelaxant and Antispasmodic Effects of Stigmas, Tepals, and Leaves Hydroethanolic Extracts of Crocus sativus. J. Food Biochem. 2023, 2023, 4165305. [Google Scholar] [CrossRef]
- Zhou, C.; Bai, W.; Chen, Q.; Xu, Z.; Zhu, X.; Wen, A.; Yang, X. Protective Effect of Crocetin against Burn-Induced Intestinal Injury. J. Surg. Res. 2015, 198, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ghafarzadeh, S.; Hobbenaghi, R.; Tamaddonfard, E.; Farshid, A.A.; Imani, M. Crocin Exerts Improving Effects on Indomethacin-Induced Small Intestinal Ulcer by Antioxidant, Anti-Inflammatory and Anti-Apoptotic Mechanisms. Vet. Res. Forum 2019, 10, 277–284. [Google Scholar] [PubMed]
- Tamaddonfard, E.; Erfanparast, A.; Farshid, A.A.; Imani, M.; Mirzakhani, N.; Salighedar, R.; Tamaddonfard, S. Safranal, a Constituent of Saffron, Exerts Gastro-Protective Effects against Indomethacin-Induced Gastric Ulcer. Life Sci. 2019, 224, 88–94. [Google Scholar] [CrossRef]
- Mard, S.A.; Pipelzadeh, M.H.; Teimoori, A.; Neisi, N.; Mojahedin, S.; Khani, M.Z.S.; Ahmadi, I. Protective Activity of Crocin against Indomethacin-Induced Gastric Lesions in Rats. J. Nat. Med. 2016, 70, 62–74. [Google Scholar] [CrossRef]
- Mohammadifard, M.; Javdani, H.; Khalili-Tanha, G.; Farahi, A.; Foadoddini, M.; Hosseini, M. Evaluation of Therapeutic Efficacy of Stigma and Petal Extracts of Crocus sativus L. on Acetic Acid-Induced Gastric Ulcer in Rats. Tradit. Integr. Med. 2021, 6, 216–228. [Google Scholar] [CrossRef]
- El-Maraghy, S.A.; Rizk, S.M.; Shahin, N.N. Gastroprotective Effect of Crocin in Ethanol-Induced Gastric Injury in Rats. Chem. Biol. Interact. 2015, 229, 26–35. [Google Scholar] [CrossRef]
- Singh, G.; Haileselassie, Y.; Ji, A.R.; Maecker, H.T.; Sinha, S.R.; Brim, H.; Habtezion, A.; Ashktorab, H. Protective Effect of Saffron in Mouse Colitis Models through Immune Modulation. Dig. Dis. Sci. 2022, 67, 2922–2935. [Google Scholar] [CrossRef]
- Gedik, S.; Erdemli, M.; Gul, M.; Yigitcan, B.; Gozukara Bag, H.; Aksungur, Z.; Altinoz, E. Investigation of the Protective Effects of Crocin on Acrylamide Induced Small and Large Intestine Damage in Rats. Biotech. Histochem. 2018, 93, 267–276. [Google Scholar] [CrossRef]
- Mard, S.A.; Nikraftar, Z.; Farbood, Y.; Mansouri, E. A Preliminary Study of the Anti-Inflammatory and Anti-Apoptotic Effects of Crocin against Gastric Ischemia-Reperfusion Injury in Rats. Braz. J. Pharm. Sci. 2015, 51, 637–642. [Google Scholar] [CrossRef]
- Khodir, A.E.; Said, E.; Atif, H.; ElKashef, H.A.; Salem, H.A. Targeting Nrf2/HO-1 Signaling by Crocin: Role in Attenuation of AA-Induced Ulcerative Colitis in Rats. Biomed. Pharmacother. 2019, 110, 389–399. [Google Scholar] [CrossRef]
- Cosgun, B.E.; Erdeml, M.E.; Gul, M.; Gul, S.; Bag, H.G.; Aksungur, Z.; Altinoz, E. Crocin Protects Intestine Tissue against Carbon Tetrachloride-Mediated Oxidative Stress in Rats. Gen. Physiol. Biophys. 2018, 37, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Brim, H.; Kwon, Y.H.; Singh, G.; Sinha, S.R.; Wang, H.; Khan, W.I.; Ashktorab, H. Saffron Pre-Treatment Promotes Reduction in Tissue Inflammatory Profiles and Alters Microbiome Composition in Experimental Colitis Mice. Molecules 2021, 26, 3351. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Li, Q.; Liu, L.; Wang, S.; Wu, Z.; Tao, Y.; Huang, P.; Wang, P. Crocetin Prolongs Recovery Period of DSS-Induced Colitis via Altering Intestinal Microbiome and Increasing Intestinal Permeability. Int. J. Mol. Sci. 2022, 23, 3832. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Zhou, Z.; Wang, S.; Jin, L.H. The Protective Effect of Safranal against Intestinal Tissue Damage in Drosophila. Toxicol. Appl. Pharmacol. 2022, 439, 115939. [Google Scholar] [CrossRef]
- Ashktorab, H.; Oppong-Twene, P.; Maecker, H.T.; Chirumamilla, L.; Kibreab, A.; Nabi, E.; Laiyemo, A.; Brim, H. Saffron as an Adjuvant Therapy in Ulcerative Colitis Patients. Inflamm. Bowel Dis. 2022, 28, S18. [Google Scholar] [CrossRef]
- Davoodi, M.; Bouri, S.Z.; Ghahfarokhi, S.D. Antioxidant Effects of Aerobic Training and Crocin Consumption on Doxorubicin-Induced Testicular Toxicity in Rats. J. Fam. Reprod. Health 2021, 15, 28. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Wen, T.; Deng, H.; Zhang, Y.; Guo, H.; Chang, H.; Xu, H.; Zhang, W. Quantification of Cisplatin Encapsulated in Nanomedicine: An Overview. Biosensors 2025, 15, 293. [Google Scholar] [CrossRef]
- Mesbahzadeh, B.; Hassanzadeh-Taheri, M.; Aliparast, M.; Baniasadi, P.; Hosseini, M. The Protective Effect of Crocin on Cisplatin-Induced Testicular Impairment in Rats. BMC Urol. 2021, 21, 117. [Google Scholar] [CrossRef]
- Abdelwahab, O.A.; Alsemeh, A.E.; Ahmed Refaay, N.E. Possible Protective Effect of Crocin on Cisplatin-Induced Changes in the Testis of Prepubertal Albino Rats: Histological, Immunohistochemical, and Morphometric Study. Egypt. Soc. Clin. Toxicol. J. 2023, 11, 53–71. [Google Scholar] [CrossRef]
- Zaeem, S.N.; Nasrabadi, M.H.; Salehipour, M.; Ehtsham, S. Effects of Letrozole and Crocin Combination on Sperm Motility and Biochemical Markers in Azoospermia: Insights from Gene Expression Analysis. Animals 2024, 15, 697. [Google Scholar]
- El-Beshbishy, H.A.; Waggas, D.S.; Ali, R.A. Rats’ Testicular Toxicity Induced by Bisphenol A Is Lessened by Crocin via an Antiapoptotic Mechanism and Bumped P-Glycoprotein Expression. Toxicon 2024, 241, 107674. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.R.; Khazaei, M.; Jalili, C.; Keivan, M. Crocin Improves Damage Induced by Nicotine on a Number of Reproductive Parameters in Male Mice. Int. J. Fertil. Steril. 2016, 10, 71. [Google Scholar] [PubMed]
- Ganjiani, V.; Ahmadi, N.; Divar, M.R.; Sharifiyazdi, H.; Meimandi-Parizi, A. Protective Effects of Crocin on Testicular Torsion/Detorsion in Rats. Theriogenology 2021, 173, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, A.A.; Raheem Hussein Al-Fartwsy, A.; Parham, A. Effect of Crocin on the Spermatogenesis Indices of Mice Testis: A Histopathological and Histomorphological Study. Egypt. J. Vet. Sci. 2023, 54, 297–308. [Google Scholar] [CrossRef]
- Rossi, G.; Placidi, M.; Castellini, C.; Rea, F.; D’Andrea, S.; Alonso, G.L.; Gravina, G.L.; Tatone, C.; Di Emidio, G.; D’Alessandro, A.M. Crocetin Mitigates Irradiation Injury in an in Vitro Model of the Pubertal Testis: Focus on Biological Effects and Molecular Mechanisms. Molecules 2021, 26, 1676. [Google Scholar] [CrossRef]
- Kandil, E.H.; Elmasady, F.A.; Abdeen, A.M.; Ali, D.A.; Elagamy, S.A. Assessment of the Protective Effect of Saffron against Khat Induced Testicular Dysfunction in Male Rats. Egypt. J. Basic Appl. Sci. 2024, 11, 318–333. [Google Scholar] [CrossRef]
- Khorasani, M.K.; Ahangarpour, A.; Khorsandi, L. Effects of Crocin and Metformin on Methylglyoxal-Induced Reproductive System Dysfunction in Diabetic Male Mice. Clin. Exp. Reprod. Med. 2021, 48, 221. [Google Scholar] [CrossRef]
- Hamidi, H.; Tofighi, A.; Azar, J.T.; Khaki, A.A.; Razi, M. Effect of Crocin and Treadmill Exercise on Spermatogenesis and Testis Structure in Streptozotocin-Induced Diabetic Rats: An Experimental Study. Crescent J. Med. Biol. Sci. 2023, 10, 98–104. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Z.; Zang, Z.; Jia, B.; Zhou, Y.; Zhang, H.; Fu, Q. Saffron Extract Alleviates D-Gal-Induced Late-Onset Hypogonadism by Activating the PI3K-Akt-Nrf2 Signaling Pathway. J. Ethnopharmacol. 2025, 340, 119273. [Google Scholar] [CrossRef]
- Hashemi-Shahri, S.H.; Golshan, A.; Mohajeri, S.A.; Baharara, J.; Amini, E.; Salek, F.; Sahebkar, A.; Tayarani-Najaran, Z. ROS-Scavenging and Anti-Tyrosinase Properties of Crocetin on B16F10 Murine Melanoma Cells. Anticancer Agents Med. Chem. 2018, 18, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Li, D.; Zhang, Y.; Zhou, G.; Liu, W.; Cao, Y.; Zhang, W. Protective Effect of Crocin on Ultraviolet B-induced Dermal Fibroblast Photoaging. Mol. Med. Rep. 2018, 18, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Grace, M.H.; Kobayashi, H.; Lila, M.A. Evaluation of Saffron Extract Bioactivities Relevant to Skin Resilience. J. Herb. Med. 2023, 37, 100629. [Google Scholar] [CrossRef]
- Madan, K.; Nanda, S. In-Vitro Evaluation of Antioxidant, Anti-Elastase, Anti-Collagenase, Anti-Hyaluronidase Activities of Safranal and Determination of Its Sun Protection Factor in Skin Photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, R.; Han, Y.; Xia, L.; Lin, K.; Fu, W.; Zhong, K.; Ye, Y. NAMPT-NAD+ Is Involved in the Senescence-delaying Effects of Saffron in Aging Mice. Exp. Ther. Med. 2024, 27, 123. [Google Scholar] [CrossRef]
- Fagot, D.; Pham, D.; Laboureau, J.; Planel, E.; Guerin, L.; Nègre, C.; Donovan, M.; Bernard, B. Crocin, a Natural Molecule with Potentially Beneficial Effects against Skin Ageing. Int. J. Cosmet. Sci. 2018, 40, 388–400. [Google Scholar] [CrossRef]
- Habibi, Z.; Hoormand, M.; Banimohammad, M.; Ajami, M.; Amin, G.; Amin, M.; Pazoki-Toroudi, H. The Novel Role of Crocus sativus L. in Enhancing Skin Flap Survival by Affecting Apoptosis Independent of mTOR: A Data-Virtualized Study. Aesthet. Plast. Surg. 2022, 46, 3047–3062. [Google Scholar] [CrossRef]
- Kalalinia, F.; Ghasim, H.; Farzad, S.A.; Pishavar, E.; Ramezani, M.; Hashemi, M. Comparison of the Effect of Crocin and Crocetin, Two Major Compounds Extracted from Saffron, on Osteogenic Differentiation of Mesenchymal Stem Cells. Life Sci. 2018, 208, 262–267. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, C.; Dai, H.; Li, J.; Liu, W.; Luo, Y.; Zhang, X.; Wang, Q. Crocin Inhibits Titanium Particle-Induced Inflammation and Promotes Osteogenesis by Regulating Macrophage Polarization. Int. Immunopharmacol. 2019, 76, 105865. [Google Scholar] [CrossRef]
- Li, B.; Qin, K.; Wang, B.; Liu, B.; Yu, W.; Li, Z.; Zhao, D. Crocin Promotes Osteogenesis Differentiation of Bone Marrow Mesenchymal Stem Cells. Vitr. Cell. Dev. Biol.-Anim. 2020, 56, 680–688. [Google Scholar] [CrossRef]
- Ramezani, T.; Mousavi, M.; Asadi-Samani, M. Synergistic Effect of Pulsed Electromagnetic Fields and Saffron Extract on Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. J. Herbmed. Pharmacol. 2015, 6, 27–32. [Google Scholar]
- Suh, K.S.; Chon, S.; Jung, W.-W.; Choi, E.M. Crocin Attenuates Methylglyoxal-Induced Osteoclast Dysfunction by Regulating Glyoxalase, Oxidative Stress, and Mitochondrial Function. Food Chem. Toxicol. 2019, 124, 367–373. [Google Scholar] [CrossRef]
- Amini, E.; Baharvand, Z.; Niknejad, A.; Tabari, Y.; Shemshadi, S. The Protective Effect of Crocin on Rat Bone Marrow Mesenchymal Stem Cells Exposed to Aluminum Chloride as an Endocrine Disruptor. Avicenna J. Med. Biotechnol. 2024, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Algandaby, M.M. Crocin Attenuates Metabolic Syndrome-induced Osteoporosis in Rats. J. Food Biochem. 2019, 43, e12895. [Google Scholar] [CrossRef] [PubMed]
- Koski, C.; Sarkar, N.; Bose, S. Cytotoxic and Osteogenic Effects of Crocin and Bicarbonate from Calcium Phosphates for Potential Chemopreventative and Anti-Inflammatory Applications in Vitro and in Vivo. J. Mater. Chem. B 2020, 8, 2048–2062. [Google Scholar] [CrossRef] [PubMed]
- Sabouni, N.; Mohammadi, M.; Boroumand, A.R.; Palizban, S.; Afshari, J.T. Stimulating Effect of Nanocurcumin and Crocin on Proliferation and Pluripotency of Bone Marrow-Derived Mesenchymal Stem Cells. Iran. J. Basic Med. Sci. 2024, 27, 1187. [Google Scholar]
- Sarwar, R.; Ahmad, B.; Al-Qaaneh, A.M.; Rauf, S.; Ahmad, L.; Bekhit, M.M.; Hassan, S.; Ullah, N.; Zengin, G.; Farid, A. Nephroprotective Effects of Fraxinus Hookeri Wenz. Against Renal Toxicity and DNA Oxidative Damages Induced by CCl4 in Rats. ChemistryOpen 2025, 14, 2400515. [Google Scholar] [CrossRef]
- Hussain, M.A.; Abogresha, N.M.; AbdelKader, G.; Hassan, R.; Abdelaziz, E.Z.; Greish, S.M. Antioxidant and Anti-inflammatory Effects of Crocin Ameliorate Doxorubicin-induced Nephrotoxicity in Rats. Oxid. Med. Cell Longev. 2021, 2021, 8841726. [Google Scholar] [CrossRef]
- Kamran, M.; Khan, N.; Jabeen, H.; Iqbal, S.; Siddiqi, A.M. Comparison of Antioxidant Effect of Crocus sativus and Vitamin E on Amakicin Induced Nephrotoxicity in Albino Rats. Ann. Abbasi Shaheed Hosp. Karachi Med. Dent. Coll. 2022, 27, 31–39. [Google Scholar] [CrossRef]
- Radmehr, V.; Ahangarpour, A.; Mard, S.A.; Khorsandi, L. Crocin Attenuates Endoplasmic Reticulum Stress in Methylglyoxal-Induced Diabetic Nephropathy in Male Mice: MicroRNAs Alterations and Glyoxalase 1-Nrf2 Signaling Pathways. Iran. J. Basic Med. Sci. 2022, 25, 1341. [Google Scholar]
- Mazani, M.; Mahdavifard, S.; Koohi, A. Crocetin Ameliorative Effect on Diabetic Nephropathy in Rats through a Decrease in Transforming Growth Factor-β and an Increase in Glyoxalase-I Activity. Clin. Nutr. ESPEN 2023, 58, 61–66. [Google Scholar] [CrossRef]
- Amir, S.; Abid, M.; Nadeem, H.; Tipu, M.K.; Irshad, N. The Nephroprotective Potential of Selected Synthetic Compound against Gentamicin Induced Nephrotoxicity. BMC Pharmacol. Toxicol. 2024, 25, 68. [Google Scholar] [CrossRef] [PubMed]
- Dogan, T.; Yildirim, B.A.; Kapakin, K.A.T. Investigation of the Effects of Crocin on Inflammation, Oxidative Stress, Apoptosis, NF-κB, TLR-4 and Nrf-2/HO-1 Pathways in Gentamicin-Induced Nephrotoxicity in Rats. Environ. Toxicol. Pharmacol. 2024, 106, 104374. [Google Scholar] [CrossRef] [PubMed]
- Ouahhoud, S.; Bencheikh, N.; Khoulati, A.; Kadda, S.; Mamri, S.; Ziani, A.; Baddaoui, S.; Eddabbeh, F.-E.; Elassri, S.; Lahmass, I. Crocus sativus L. Stigmas, Tepals, and Leaves Ameliorate Gentamicin-induced Renal Toxicity: A Biochemical and Histopathological Study. Evid.-Based Complement. Altern. Med. 2022, 2022, 7127037. [Google Scholar] [CrossRef] [PubMed]
- Abdelsatar, A.A.; Ghada, N.O.; Hassan, D.M.; Mousa, A.A. Possible Protective Role of Saffron Against Gentamicin-Induced Renal Toxicity in Adult Male Albino Rats: A Histological and Immunohistochemical and Biochemical Study. Med. J. Cairo Univ. 2022, 90, 2285–2296. [Google Scholar] [CrossRef]
- Altinoz, E.; Cetinavci, D.; Abdulkareem Aljumaily, S.A.; Elbe, H.; Cengil, O.; Bicer, Y. Crocin, the Compound of the Dried Stigma of Crocus sativus L (Saffron), Restores Doxorubicin-Induced Disturbances in Kidney Functioning, Oxidative Stress, Inflammation, Renal Tissue Morphology and TGF-β Signalling Pathways. Nat. Prod. Res. 2024, 39, 4609–4622. [Google Scholar] [CrossRef]
- Yarijani, Z.M.; Pourmotabbed, A.; Pourmotabbed, T.; Najafi, H. Crocin Has Anti-Inflammatory and Protective Effects in Ischemia-Reperfusion Induced Renal Injuries. Iran. J. Basic Med. Sci. 2017, 20, 753. [Google Scholar]
- Naderi, R.; Pardakhty, A.; Abbasi, M.F.; Ranjbar, M.; Iranpour, M. Preparation and Evaluation of Crocin Loaded in Nanoniosomes and Their Effects on Ischemia–Reperfusion Injuries in Rat Kidney. Sci. Rep. 2021, 11, 23525. [Google Scholar] [CrossRef]
- Rajabalizadeh, R.; Rahbardar, M.G.; Razavi, B.M.; Hosseinzadeh, H. Renoprotective Effects of Crocin against Colistin-Induced Nephrotoxicity in a Rat Model. Iran. J. Basic Med. Sci. 2024, 27, 151. [Google Scholar]
- Liu, H.; Cheng, H.; Wang, H.; Wang, Q.; Yuan, J. Crocin Improves the Renal Autophagy in Rat Experimental Membranous Nephropathy via Regulating the SIRT1/Nrf2/HO-1 Signaling Pathway. Ren. Fail. 2023, 45, 2253924. [Google Scholar] [CrossRef]
- Harchegani, A.B.; Sohrabiyan, M.; Kaboutaraki, H.B.; Shirvani, H.; Shahriary, A. The Protective Effects of Saffron Stigma Alcoholic Extract against Vincristine Sulfate Drug-Induced Renal Toxicity in Rat: Saffron Extract Mitigate Vincristine-Induced Renal Toxicity. J. Pharm. Sci. 2019, 15, 83–94. [Google Scholar]
- Jalili, C.; Ghanbari, A.; Roshankhah, S.; Salahshoor, M.R. Toxic Effects of Methotrexate on Rat Kidney Recovered by Crocin as a Consequence of Antioxidant Activity and Lipid Peroxidation Prevention. Iran. Biomed. J. 2019, 24, 39. [Google Scholar] [CrossRef]
- Bakshi, H.A.; Quinn, G.A.; Nasef, M.M.; Mishra, V.; Aljabali, A.A.; El-Tanani, M.; Serrano-Aroca, Á.; Webba Da Silva, M.; McCarron, P.A.; Tambuwala, M.M. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways. Cells 2022, 11, 1502. [Google Scholar] [CrossRef]
- Esalatmanesh, S.; Biuseh, M.; Noorbala, A.A.; Mostafavi, S.-A.; Rezaei, F.; Mesgarpour, B.; Mohammadinejad, P.; Akhondzadeh, S. Comparison of Saffron and Fluvoxamine in the Treatment of Mild to Moderate Obsessive-Compulsive Disorder: A Double Blind Randomized Clinical Trial. Iran. J. Psychiatry 2017, 12, 154. [Google Scholar]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. Affron® a Novel Saffron Extract (Crocus sativus L.) Improves Mood in Healthy Adults over 4 Weeks in a Double-Blind, Parallel, Randomized, Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef]
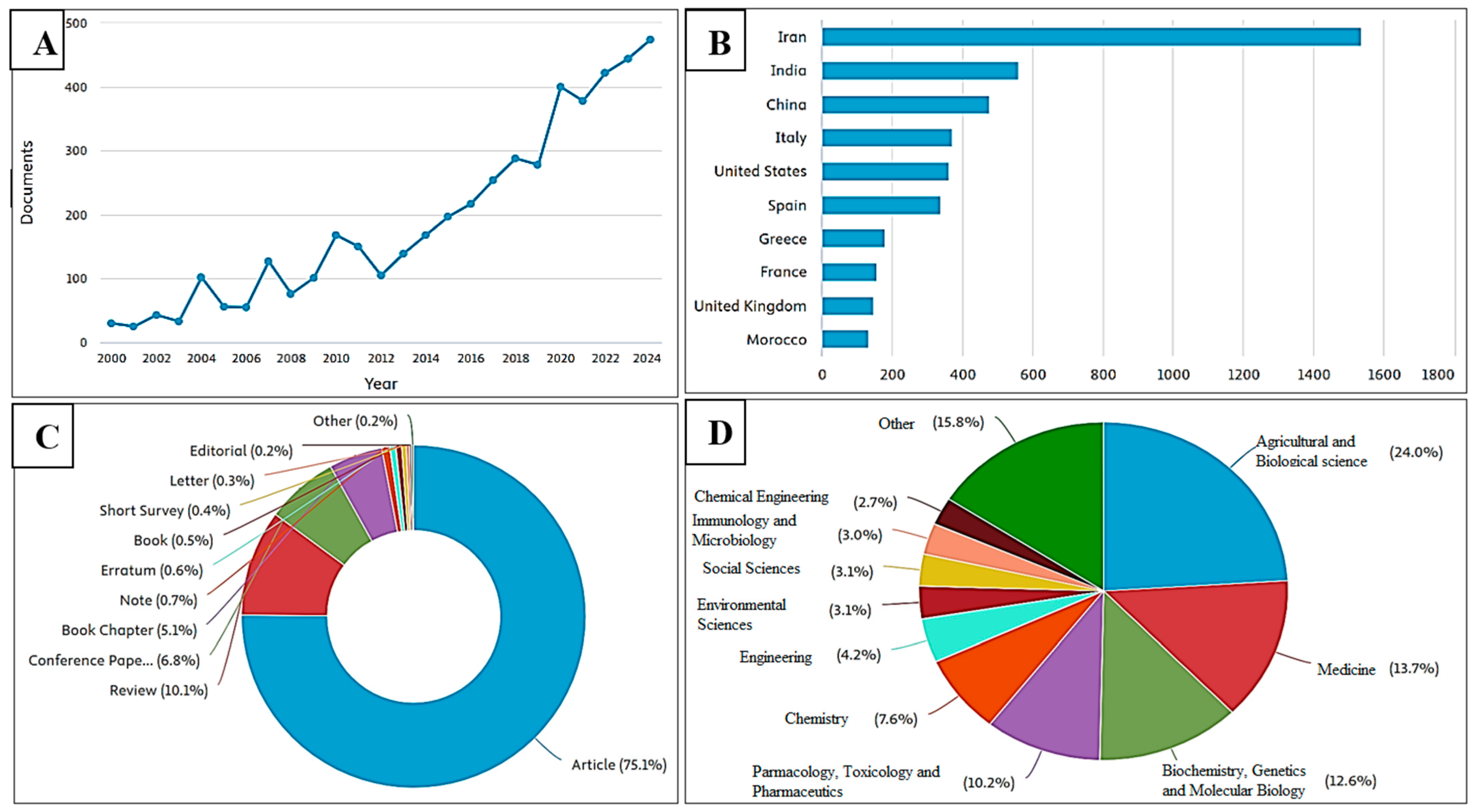

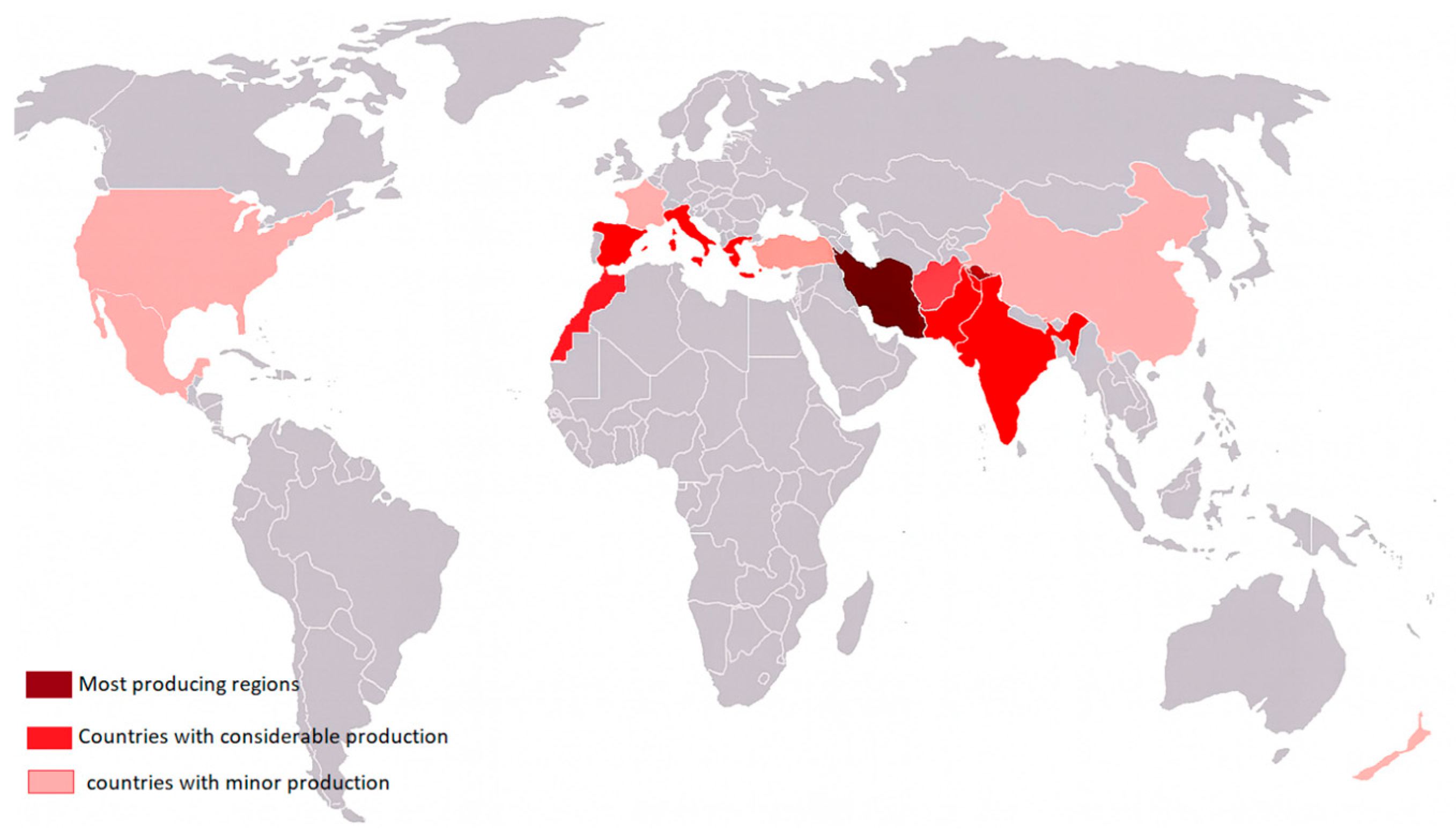
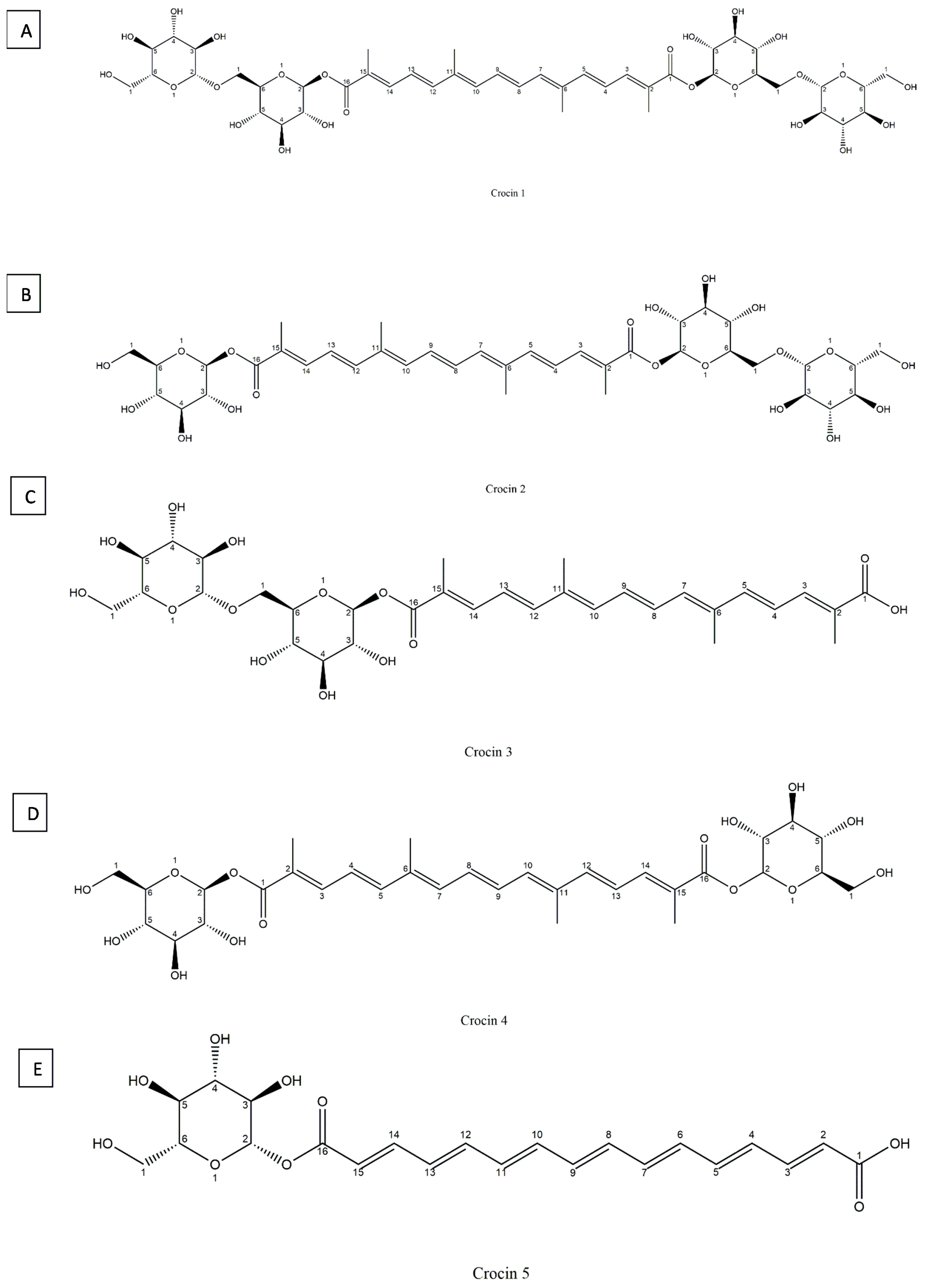
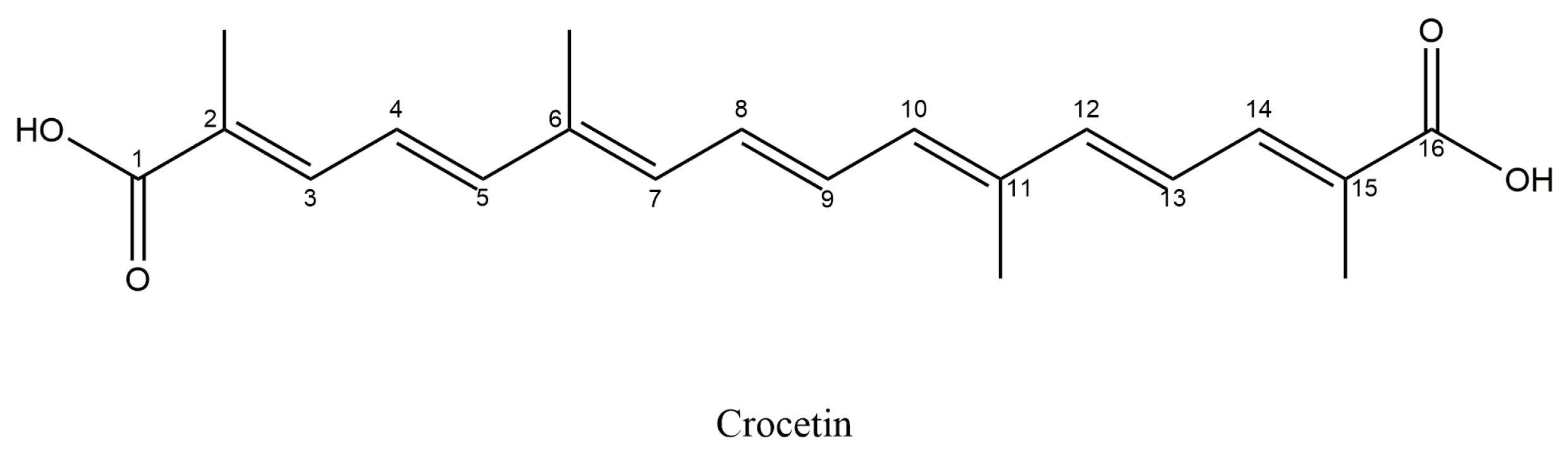
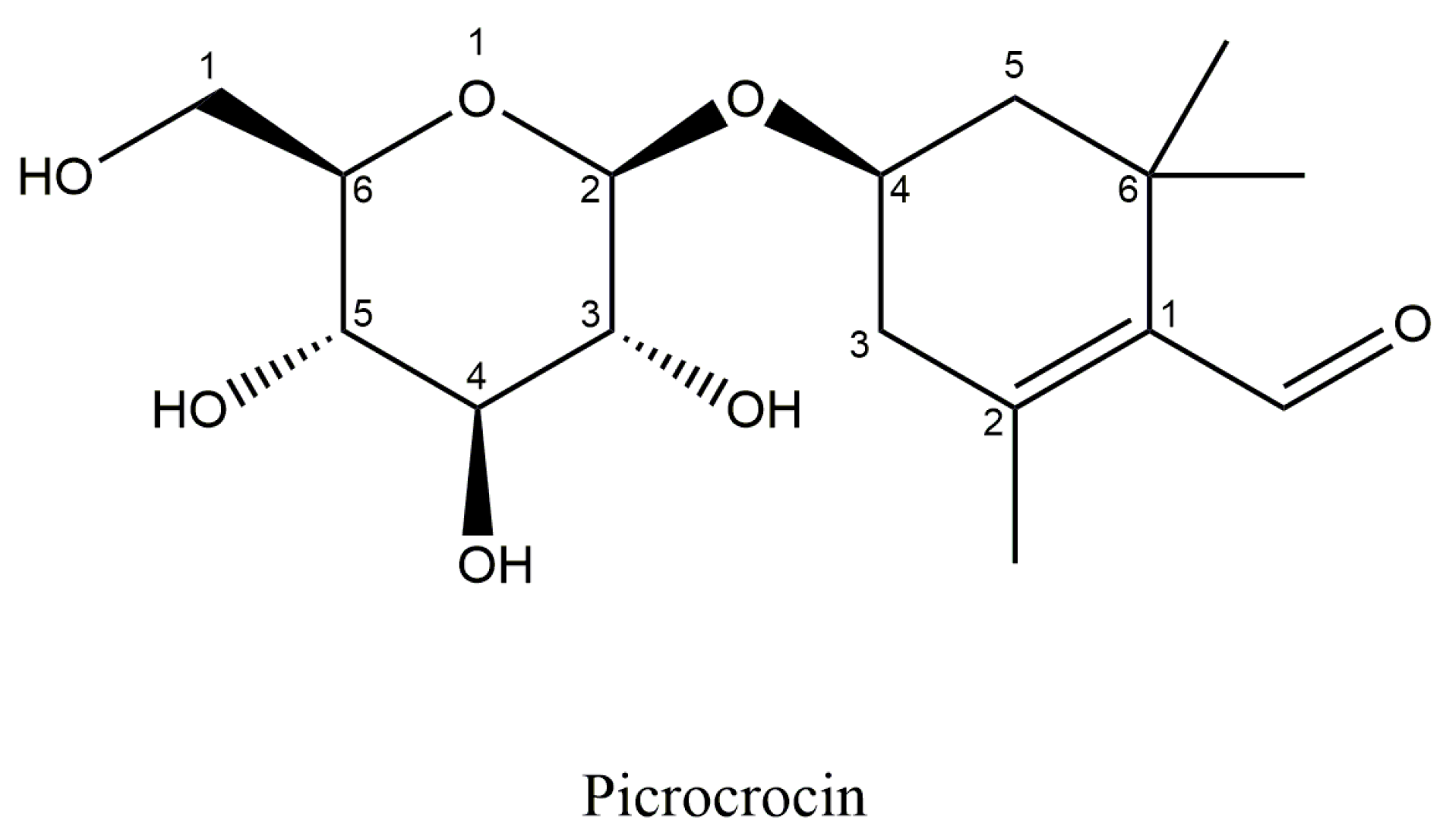

| Kingdom | Plantae |
|---|---|
| Division | Magnoliophyta |
| Class | Liliopsida |
| Order | Asparagales |
| Family | Iridaceae |
| Genus | Crocus |
| Species | Crocus sativus |
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Crocin and Dimethyl crocetin (22.85 to 0.18 and 11.43 to 0.09 mg/mL) | Glioblastoma A172 and rhabdomyosarcoma TE671cells lines | dose-time dependent cytotoxicity; ↑ regulation of BAX and BID, ↓ regulation of MYCN and BCL-2, SOD1, GSTM1 | [66] |
| Crocin (25, 50, 75, 100, and 125 mg/L) | Cervical carcinoma (HeLa cells) | ↓ cell viability | [67] |
| Safranal (1.6 to 200 µM) | Colon carcinoma colo-205 | Cytotoxic effect with an IC50 of 20 µM (↑ ROS and ↓ mitochondrial membrane potential (MMP); ↑ expression of Bax, ↓ Bcl-2; G2/M cell cycle arrest | [68] |
| cinnamon–saffron extract | oral squamous cell carcinoma SCC-25 | Dose-time dependent cytotoxicity; antimigratory effects; ↑ wound healing | [69] |
| Crocin (1, 20, and 50 μM) | Breast Cancer Cells HCC70, HCC1806, HeLa, and CCD1059sk | Cytotoxic effect in different types of cancer; it inhibited microtubule assembly but induced aggregation of tubulin at higher concentrations; perturbed the mitotic phase in cancerous cells | [70] |
| Saffron extract (25, 50, 75, and 100 µg/mL) | Cervical cancer cells (HEp-2) | dose-dependent cytotoxicity on (HEp-2); lost the original form and their adherence | [71] |
| Crocin and picrocrocin (0.5 to 4 mM) | malignant TC-1 and non-malignant COS-7 cell lines | Stimulates apoptosis and prevents cell growth (crocin is more cytotoxic than picrocrocin) | [72] |
| SWE, SEE, and Crocetin (0 to 1000 µg/mL) | Human lung carcinoma (A549), breast adenocarcinoma (MCF-7), cervical cancer (HeLa) | Dose-time dependent cytotoxicity only in malignant cells; ↑ LDH | [78] |
| Crocin (200 mg/kg i.p.) for 7 days | N-Nitroso-N-Methylurea-Induced Breast Cancer in Rats | ↓ tumor volumes; down regulation of cyclin D1 and p21Cip1; stimulate cell cycle arrest | [79] |
| saffron extract and safranal (0.5 mM crocin, 0.15 mM) | Kidney Caki-1 and bladder cancer RT4 and RT112 cell lines | ↓ cell viability (mixture of crocin and safranal) of tumoral cells only | [74] |
| Crocin and safranal (0.05–4 and 0.2–3.2 mM) | Oral Squamous Cell Carcinoma (KB Cell Line) | apoptotic effects in the KB cell line | [80] |
| Saffron extract (0 to 400 µg/mL) | Lymphoblastic T-cell leukemia (Jurkat cell line) | ↓ cell growth in a dose-dependent manner; a mixture of safranal and crocin has a lower IC50 | [81] |
| crocetin (β-D-glucosyl) ester (31 to 1000 mg/mL) | human breast adeno-carcinoma cell model (MCF-7) | ↓ cell proliferation; inhibit estrogen receptor alpha | [82] |
| Saffron extract (0 to 4 mg/mL) | Human Prostate Cancer Cells line MDA-PCa-2b | inhibit cell proliferation by apoptotic pathways in dose-dependent effects; a down-regulation of DNA methyltransferases and DNA repair intermediates in a time-dependent manner | [75] |
| Saffron extract, Crocin And Safranal | Colon cancer cell lines CT26 and HCT116 In vivo, CT26 cells induced colon cancer in mice | ↑ body mass, ↓tumor weight; downregulating the expression of inflammatory factors; saffron enhances immunotherapy efficacy (↑ Th 17 cells differentiation, modulates CD4+/CD8+T ratio); suppresses the proliferation of CT26 and HCT116 | [76] |
| Saffron extract, Crocin And Safranal (up to 8 mg/mL) | human colorectal cancer cell lines | ↓ in cellular proliferation (upregulation of caspase 3 and 7); ↓ wound area | [83] |
| Nanoliposome with saffron in vitro (25 mg/mL) In vivo (75 and 300 mg/kg tail vein injections) | In vitro and in vivo C26 colon carcinoma | Higher cytotoxic effect in the formulation than aqueous extract; nanoliposome reduces tumor volume at 300 mg/kg due to the site-directed drug delivery | [77] |
| crocetin/dendrimers complex (PPI and PAMAM) (0.8 to 50 µM) | human breast cancer MCF-7 cells | ↑ percentage of apoptotic cells in encapsulated crocetin due to higher cellular uptake | [84] |
| Crocetin/poly (lactic-co-glycolic acid) (PLGA) | human breast cancer MCF-7 cells | Encapsulation increases cytotoxicity; IC50 of PLGA–crocetin NPs was 84.73 µM, free crocetin showed higher IC50 values 589.65 µM | [85] |
| Saffron and silver/zinc oxide nanocomposites (0 to 8 µg/mL) | HeLa cervical cancer cell line | ↑ ROS, ↓mitochondrial membrane potential; ↑ apoptotic signals by reducing Cyclin D, PCNA, and CDK expression; | [86] |
| Crocin-loaded chitosan-alginate | human breast cancer MCF-7 cells | Crocin reduces viability in a dose- and time-dependent manner. | [87] |
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Stigma extract (93.75–1500 mg/L) | high-fat diet-induced zebrafish model | 100 and 200 mg/L ↓ body mass index 1500 mg/L is highly toxic | [112] |
| Crocin (10–50 μM) | In vitro model of obesity | ↓ cell viability of adipocyte; ↓ adipocyte differentiation; ↑ expression levels of AMP-activated protein Kinase | [117] |
| Saffron extract (1.25, 5, 10, 20, 40, and 80 μg/mL) | In vitro cytotoxicity in adipocyte differentiation in human adipose-derived stem cells (ADSCs) | ↓ adipocyte differentiation with no cytotoxicity; ↓ PPARγ, GAPDH, and FAS proteins | [118] |
| saffron aqueous extract (30 mg), crocin (30 mg) for 8 weeks | Double-blind and placebo-controlled trial on a patient with coronary artery disease | ↓ body mass index (BMI), suppresses appetite | [120] |
| Saffron extract (40 and 80 mg/kg/day) | High-fat diet induced Obesity in rats | ↓ plasma glucose levels; ↓ insulin; ↑ adiponectin and ghrelin; ↓ leptine | [113] |
| Crocin-I (20 mg/kg/day) for 10 weeks orally | high-fat diet (HFD) -induced obese mice | ↓ body and liver weight; ↑ glucose resistance; ↓ lipid accumulation in liver; ↓ intestinal microbial disorders; ↓ F/B ratio; repaired altered intestinal barrier functioning; ↓ intestinal inflammation | [114] |
| Saffron (250 mg/kg) for 7 weeks | high-fat, high-sugar diet in a rat model | ↓ body weight and food intake; ↓ insulin; lower total antioxidants activities | [115] |
| Saffron (20 mg/kg) 20 μM crocin in vitro | Differentiation of adipocytes in vitro/in vivo adipose tissue in db/db mice | inhibits adipogenesis and promotes lipolysis via activation of AMPK | [119] |
| Saffron extract and crocin (40 and 80 mg/kg) for 8 weeks | High-fat diet-induced obesity rat model | ↑ glucose, pyruvate, betaine, and taurine; ↓ lactate, alanine, and creatinine; ↓ Trimethylamine N-oxide | [121] |
| Crocin (20 mg/kg orally) for 12 weeks | Streptozotocin and high-fat diet-induced obesity and type 2 diabetes in mice | Activation of AMPK (inhibition of adipose formation) inhibits the changes of glucose metabolic parameters and serum lipid profiles in wild-type diabetic mice. | [122] |
| Saffron extract (40 and 80 mg/kg orally) for 3 weeks | high-fat diet-induced in rats | ↓ total cholesterol, triglyceride and LDL; ↑ HDL; ↓ atherosclerosis-index and liver enzymes; ↓ insulin, leptin, resistin; ↑ adiponectin; ↓ MDA | [123] |
| Saffron extract (40 and 80 mg/kg) for 4 weeks | Insulin-sensitizing adipokine in high-calorie diet rats | ↓ body weight, ↑ insulin, and adiponectin | [116] |
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Crocin (10 and 50 mg/kg/day orally) for 8 weeks | Doxorubicin-Induced Testicular Toxicity in Rats | ↑ GPx, TAC, and protein carbonyl (PC) levels in testicular tissue | [201] |
| Crocetin (50 µM) | In vitro Irradiation Injury of the Pubertal Testis | ↓ testis injury; did not restore levels of p62 and LC3-II; ↑ SOD2, CAT and HuR; restore PARP1 and PCNA | [210] |
| Saffron (100 mg/kg orally) | Khat induced testicular dysfunction in male rats | improve in testicular histology, histochemistry, and biochemical results (SOD, CAT, MDA) | [211] |
| Crocin (12.5, 25 and 50 mg/kg i.p.) 4 weeks | Nicotine-induced damage to reproductive parameters in mice | ↑ testosterone, sperm, count, viability, and motility; testis weight | [207] |
| Crocin (4, 20 and 100 mg/kg orally) for 6 weeks | Crocin impact on spermatogenesis | At high doses ↓, the diameter of seminiferous tubules and the number of sperm in seminiferous tubules are affected by ↓ Sertoli cells. | [209] |
| Crocin (15, 30 and 60 mg/kg/day orally) for 31 days | Methylglyoxal-induced reproductive system dysfunction in mice | Improve testicular morphology, testosterone, and sperm count; ↓ Luteinizing hormone | [212] |
| Crocin (15 mg i.p.) | Busulfan-induced Azoospermia in rats | improve sperm motility and testosterone levels, ↑ total sperm count; ↓ Total Oxidant Status (TOS), reduce DNA damage | [205] |
| Crocin (200 mg/kg i.p.) for 4 days | Cisplatin-induced changes in the testis in rats | Slightly improve body weights, testicular weights, and serum testosterone levels; ↓ apoptotic cells. | [204] |
| Crocin (50 and 100 mg/kg i.p.) | Ischemia-Reperfusion followed by torsion induced testicular injury | ↑ SOD, GPx activity, and testosterone level; ↓ MDA; improve the histopathological parameters | [208] |
| Crocin (200 mg/kg/day, i.p.) for 14 days | Bisphenol induced testicular toxicity | ↓ apoptosis, caspase-3; ↓ TBARS; ↑ sperm motility and sperm count ↑ FSH, LH, and testosterone, ↑ in the P-gp expression | [206] |
| Crocin (6.25–100 mg/kg i.p.) 16 days | cisplatin-induced testicular impairment in rats | ↑ testis weight, testosterone level, SOD; ↓ lipid peroxidation, ↑ germinal layer area | [203] |
| Crocin (50 mg/kg) | Streptozotocin-Induced Diabetic Rats | Recover testicular tissue damage, restore sperm parameters | [213] |
| Saffron extract (10 and 20 mg/kg orally) for 30 days | D-gal-induced late-onset hypogonadism | improve Leydig cell resistance and escape apoptosis; ↑ testosterone; modulate the PI3K-Akt-Nrf2 signaling pathway | [214] |
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Saffron extract (40 and 80 mg/kg orally) for 7 days | Skin flap surgery in rats | ↓ flap necrosis; improve histological healing; ↑ Bax and Bcl-2; ↓ MPO and MDA | [221] |
| Crocetin (0.5 to 32 µM) | In vitro ROS-scavenging and Anti-tyrosinase Properties of Crocetin | Inhibition of tyrosinase activity; ↓melanin in B16 melanoma cells; ↓ tyrosinase and MITF; ↓ ROS | [215] |
| Crocin (12.5, 50, 100 µM) | Ultraviolet β-induced dermal fibroblast photoaging | ↓ cell proliferation; restore cell cycle arrest; anti-aging (↓ SA-β-gal-positive cells); ↑in Col-1 expression | [216] |
| Saffron extract (80 mg/kg/day intragastrically) | The anti-aging activity of saffron in old mice | Anti-aging activity by ameliorating the thickness of the skin | [219] |
| Safranal (2 to 100 µg/mL) | In vitro biochemical assays (dermal enzymes inhibition activities) | Inhibit dermal enzyme (elastase, collagenase, and hyaluronidase) | [218] |
| Saffron extract (100 and 200 µg/mg) | In vitro biochemical assays (tyrosinase and collagenase inhibition activities) | Inhibit tyrosinase and collagenase; ↓ ROS and NO in macrophage cells; ↑ collagen and hyaluronic acid synthesis; improve wound healing | [217] |
| Crocin (0.3 mM and 1 mM) (10 to 100 µM) | In vitro peroxidation and inflammation on NHEKs and HDF | ↓ release of pro-inflammatory mediators; ↑ antioxidant defense; downregulation of NF-κB signaling pathway in NHEKs | [220] |
| Treatment | Methods of Analysis | Major Findings | References |
|---|---|---|---|
| Crocin (100 mg/kg body i.p.) for 3 weeks | Doxorubicin-Induced Nephrotoxicity in rats | ↑ kidney functions; upregulate creatinine clearance; ↓ MDA, NF-κB, iNOS, COX2, and TNFα; ↑ SOD; Area of renal proximal and distal convoluted tubules and distal convoluted tubules | [232] |
| Saffron extract (50 mg/mL orally) for 3 weeks | Amikacin-Induced Nephrotoxicity in Albino Rats | ↑ nuclei number; ↓ MDA in renal tissue; ↑ number of intact proximal tubules | [233] |
| Crocetin (100 mg/kg i.p.) for 3 months | STZ-induced diabetic nephropathy in rats | ↓ Proteinuria, Creatinine, glomerulosclerosis, diverse glycation, oxidative stress, and inflammatory markers | [235] |
| Crocin (15, 30 and 60 mg/kg/day orally) for 2 weeks | methylglyoxal-induced diabetic nephropathy in mice | ↓ MDA, proteinuria, blood urea nitrogen, plasma creatinine, and Nrf2, miR-204, miR-192 expression; ↑ SOD, CAT, and glutathione glyoxalase 1 | [234] |
| Crocin (0, 100, 200 and 400 mg/kg i.p.) | Ischemia-reperfusion-induced renal injuries | ↓ creatinine, urea-nitrogen, MDA; ↓ leukocyte infiltration; ↓ ICAM-1 and TNF-α mRNA expression levels in a dose-dependent manner | [241] |
| Crocin (100 mg/kg i.p.) for 30 days | passive Heymann nephritis (PHN) induced by anti-Fx1A antiserum in rats | ↓ creatinine, proteinuria, total cholesterol; ↑ in urinary creatinine clearance, ↑ SOD, CAT, GSH; ↓ MDA; activated the Sirt1/Nrf2/HO-1 pathways; attenuated the renal histopathological changes | [244] |
| Crocin (40 mg/kg i.p.) 14 days | doxorubicin-induced disturbances in the kidney | ↑ SOD and CAT, GSH, prevent inflammation and oxidative stress; stabilize cellular redox homeostasis, reducing renal fibrosis | [240] |
| Stigmas extract (50 mg/kg orally) for 14 days | Gentamicin-Induced Renal Toxicity | Protect against weight loss; ↓ plasma creatinine, ↑ urine creatinine clearance, ↑ plasma potassium levels, ↓ and MDA. | [238] |
| Crocin (25 and 50 mg/kg i.p.) for 8 days | gentamicin-induced nephrotoxicity in rats | ↓ kidney weight; ↑ in uric acid, creatinine, and urea levels; ↓ pro-inflammatory biomarkers; decrease in COX-2, NF-κB, and TLR-4; up-regulate Bcl-2, Nrf-2, and HO-1 | [237] |
| Saffron extract (80 mg/kg i.p.) for 7 days | Gentamicin-Induced Renal Toxicity in Albino Rats | ↑ GPx and SOD; ↓ NF-κB, TNF-α, Creatinine, and urea | [239] |
| Crocin-loaded naniosomes intravenous injection | Ischemia–reperfusion injuries in the rat kidney | Reduce histopathological changes in sick rats; ↑ SOD; ↓ MDA; creatinine and urea. | [242] |
| Crocin (40 mg/kg, i.p.) for 10 days | Colistin-induced nephrotoxicity in a rat | ↓ in BUN and creatinine, ↑ GSH levels, and ameliorated the histopathological alterations | [243] |
| Saffron extract | Oxymetholone-Induced Hepatic and Renal Injury in Rats | ↓ renal degenerative changes | [166] |
| Crocin (50 mg/kg orally) for 8 weeks | db/db mice | Protect renal structure; ↓ phospho-IkBa and NF-kB; ↑ nuclear respiratory factor 2, manganese SOD 1, heme oxygenase-1, and catalase | [131] |
| Crocin (0.5 mg/kg orally) for 8 weeks | Vincristine Sulfate Drug-Induced renal toxicity | ↓ blood urea nitrogen, creatinine, and uric acid; improvement in serum TAC content. | [245] |
| Crocin (12.5, 25, and 50 mg/kg i.p.) for 28 days | Methotrexate induced renal toxicity | ↓ MDA, creatinine; NO levels; enhance the antioxidant capacity | [246] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziani, A.; Bekkouch, O.; Ouahhoud, S.; Baddaoui, S.; Ben’Mbarek, S.; Bekkouch, A.; Khoulati, A.; Jaouadi, B.; Choi, J.; Choi, M.; et al. Phytochemistry, Biological Activities, Molecular Mechanisms, and Toxicity of Saffron (Crocus sativus L.): A Comprehensive Overview. Antioxidants 2025, 14, 1433. https://doi.org/10.3390/antiox14121433
Ziani A, Bekkouch O, Ouahhoud S, Baddaoui S, Ben’Mbarek S, Bekkouch A, Khoulati A, Jaouadi B, Choi J, Choi M, et al. Phytochemistry, Biological Activities, Molecular Mechanisms, and Toxicity of Saffron (Crocus sativus L.): A Comprehensive Overview. Antioxidants. 2025; 14(12):1433. https://doi.org/10.3390/antiox14121433
Chicago/Turabian StyleZiani, Anas, Oussama Bekkouch, Sabir Ouahhoud, Sanae Baddaoui, Soufiane Ben’Mbarek, Ayoub Bekkouch, Amine Khoulati, Bassem Jaouadi, Jinwon Choi, Min Choi, and et al. 2025. "Phytochemistry, Biological Activities, Molecular Mechanisms, and Toxicity of Saffron (Crocus sativus L.): A Comprehensive Overview" Antioxidants 14, no. 12: 1433. https://doi.org/10.3390/antiox14121433
APA StyleZiani, A., Bekkouch, O., Ouahhoud, S., Baddaoui, S., Ben’Mbarek, S., Bekkouch, A., Khoulati, A., Jaouadi, B., Choi, J., Choi, M., Kim, H. J., Benabbes, R., Asehraou, A., Park, M. N., Kim, B., & Saalaoui, E. (2025). Phytochemistry, Biological Activities, Molecular Mechanisms, and Toxicity of Saffron (Crocus sativus L.): A Comprehensive Overview. Antioxidants, 14(12), 1433. https://doi.org/10.3390/antiox14121433








