Integrated Probiotic Benefits of Bacillus velezensis AAHM-BV2302 Drive Growth, Antioxidant Enhancement, and Immune Protection Against Streptococcus agalactiae in Tilapia (Oreochromis spp.)
Abstract
1. Introduction
2. Methodology
2.1. Bacterial Cultivation and Preparation
2.2. Whole-Genome Sequencing (WGS) and Taxonomic Identification
2.3. Effect of Dietary Supplementation with Probiotic B. velezensis AAHM-BV2302 on Tilapia Growth, Health, and Disease Resistance
2.3.1. Ethical Statement
2.3.2. Fish Husbandry
2.3.3. Bacterial Preparation, Diet Formulation, and Experimental Design
- Control group: fish fed a basal diet top-dressed with 100 mL of PBS per kg of feed.
- Control-TSB group: fish fed a basal diet top-dressed with 100 mL of sterile TSB per kg of feed.
- Probiotic (Cell) group: fish fed a diet supplemented with 100 mL of Bacillus velezensis AAHM-BV2302 suspension (1 × 109 CFU/mL) per kg of feed, yielding an approximate concentration of 1 × 108 CFU/kg of feed.
- Cfs group: fish were fed a diet supplemented with 100 mL of cell-free supernatant per kg of feed, derived from bacterial cultures adjusted to an optical density of OD600 = 1.0 (equivalent to 1 × 109 CFU/mL) after removal of the bacterial cells.
- Cell + Cfs group: fish were fed a diet supplemented with 100 mL per kg of feed of a mixture containing Bacillus velezensis AAHM-BV2302 cells and their corresponding cell-free supernatant, prepared from cultures adjusted to an optical density of OD600 = 1.0 (equivalent to 1 × 109 CFU/mL).
2.3.4. Sample Collection
2.3.5. Growth Performance
- The following formulas were applied.
- Total weight gain (TWG, g/30 or 60 days) = Wt − Wi
- Average daily gain (ADG, g/day) = (Wt − Wi)/t
- 4.
- Feed Conversion Ratio (FCR) was calculated as the ratio of the total feed share intake to the individual weight gain (WG): FCR = Total feed share intake/Weight gain (WG)
2.3.6. Determination of Oxidative Stress and Antioxidant Status in Target Organs
- (1)
- Measurement of malondialdehyde (MDA) levels
- (2)
- Measurement of catalase (CAT) enzyme activity
- (3)
- Determination of glutathione (GSH) content
- (4)
- Measurement of superoxide dismutase (SOD) enzyme activity
2.3.7. Humoral Innate Immune Responses Assays
- (5)
- Lysozyme activity
- (6)
- Bactericidal activity
- (7)
- Total serum IgM
2.3.8. Total RNA Isolation, First-Strand cDNA Synthesis and Gene Expression Analysis by Quantitative Real-Time RT-PCR (qRT-PCR)
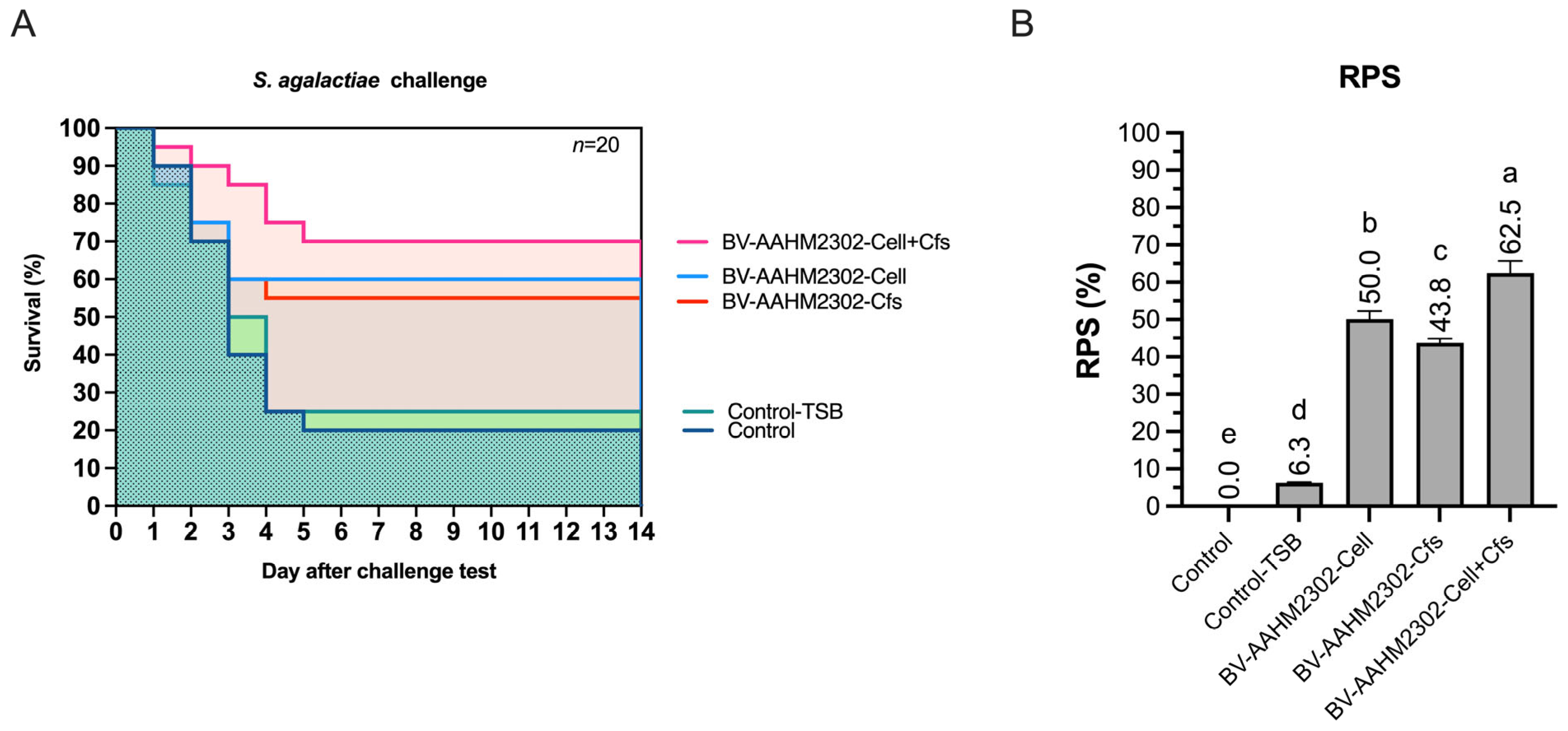
2.3.9. Disease Resistance and Survival Rate (SR) Following Streptococcus agalactiae Challenge
2.3.10. Statistical Data Analysis
3. Results
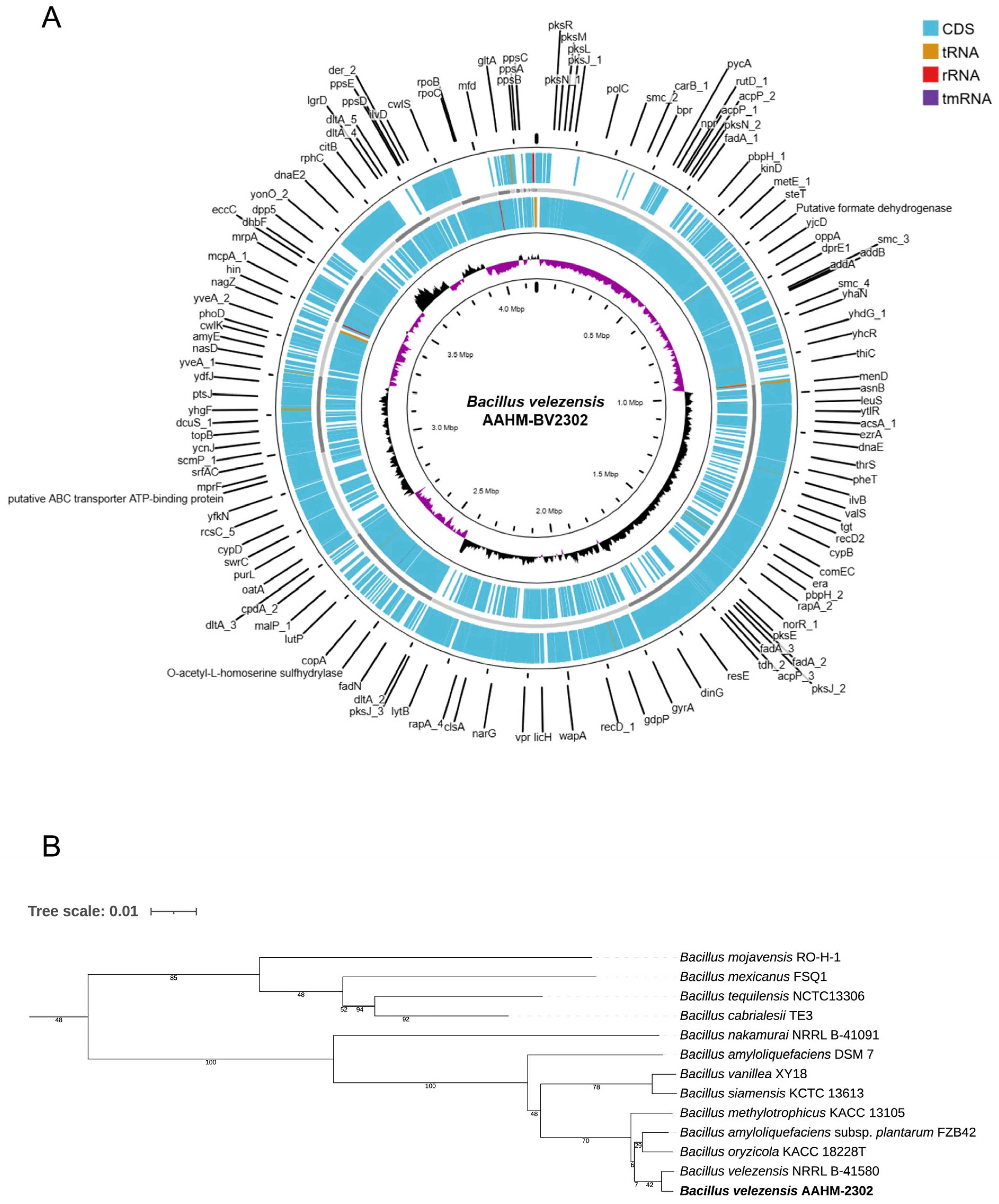
3.1. Genomic Identification and Characterization of Bacillus velezensis AAHM-BV2302
3.2. Growth Parameters and Immune-Growth-Related Gene Expression
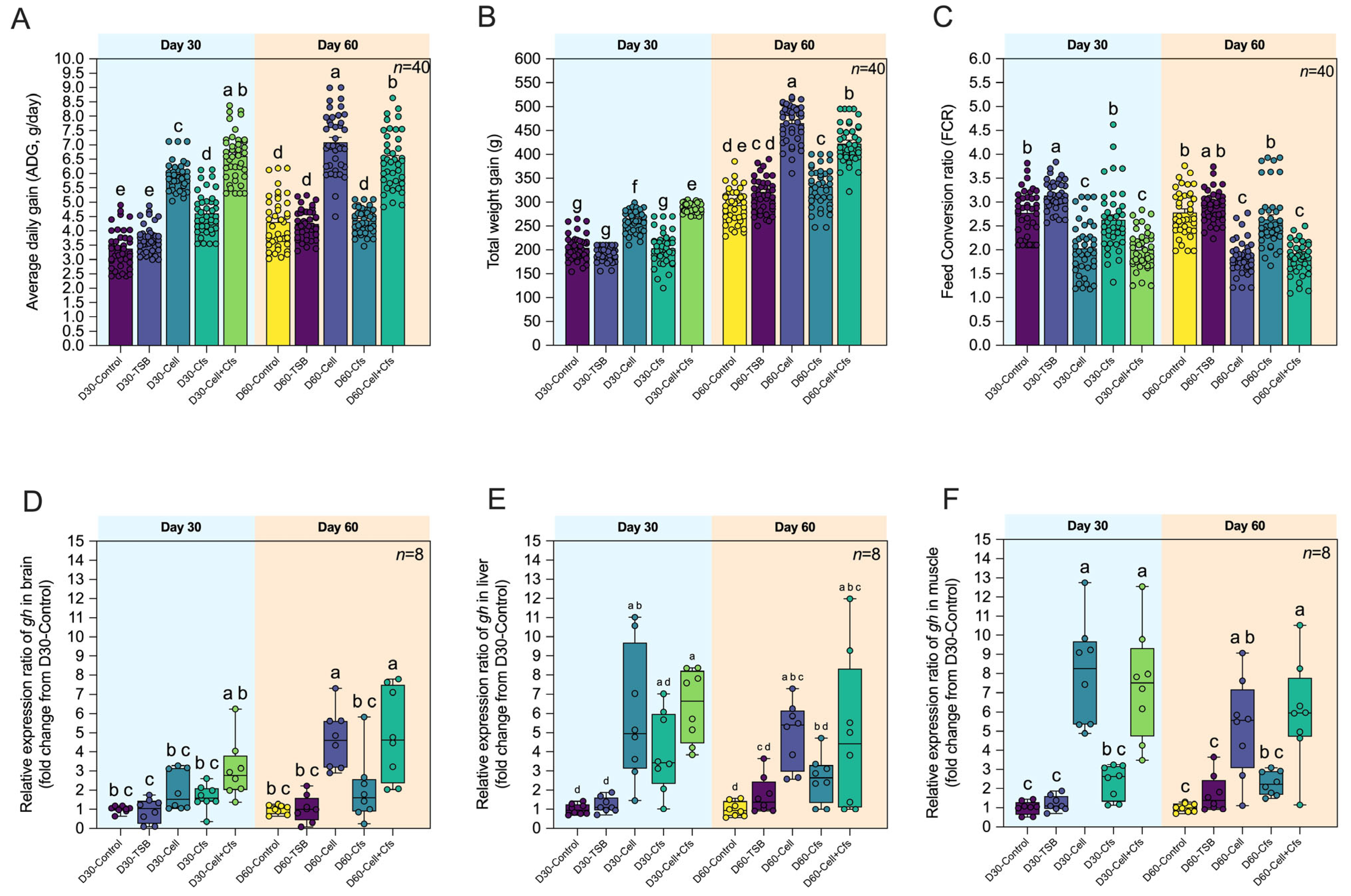
3.2.1. Growth Performance
3.2.2. Expression of Growth-Related Genes
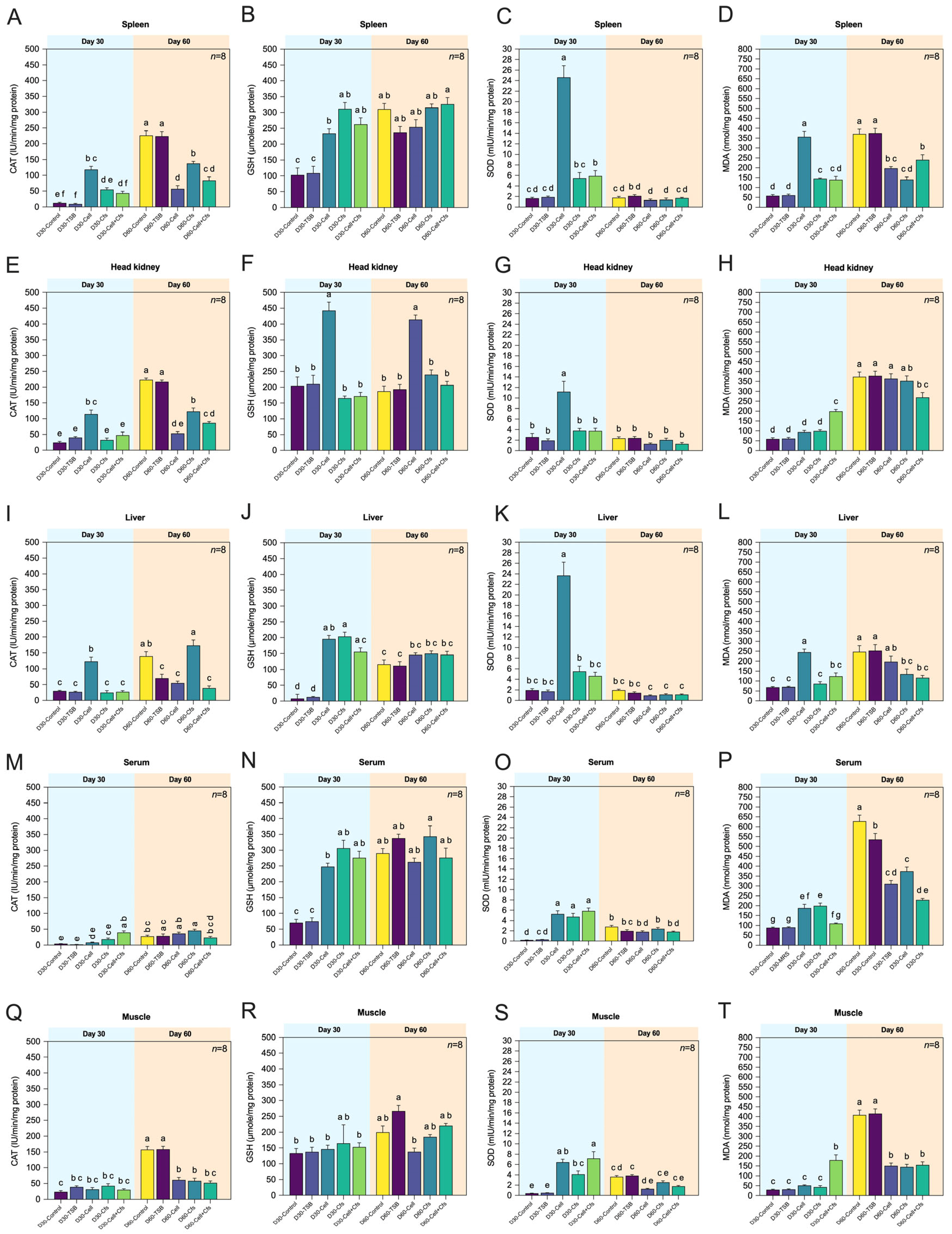
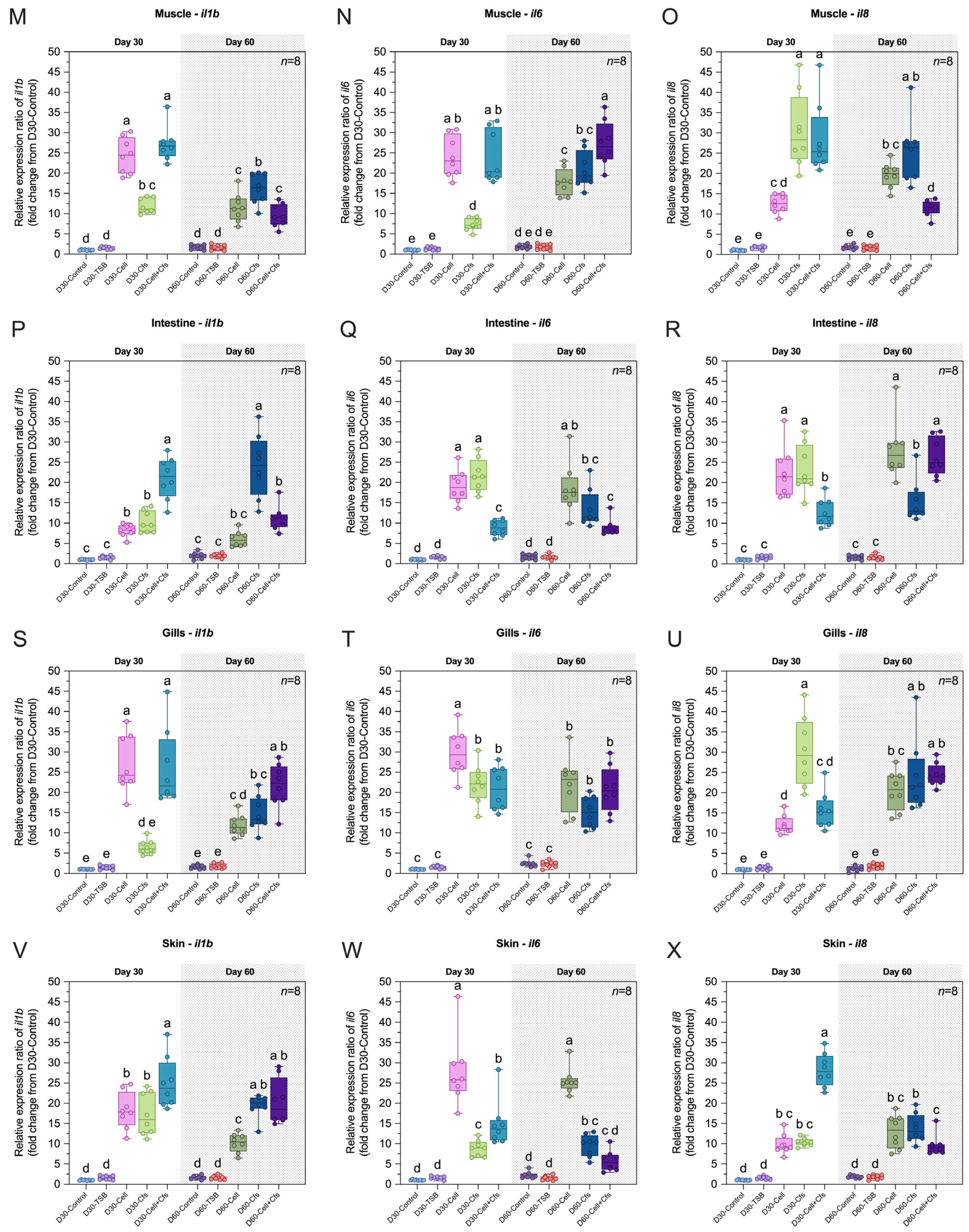
3.3. Determining the Antioxidative Status and Oxidative Stress of Target Organs
3.3.1. Spleen
3.3.2. Head Kidney
3.3.3. Liver
3.3.4. Serum
3.3.5. Muscle
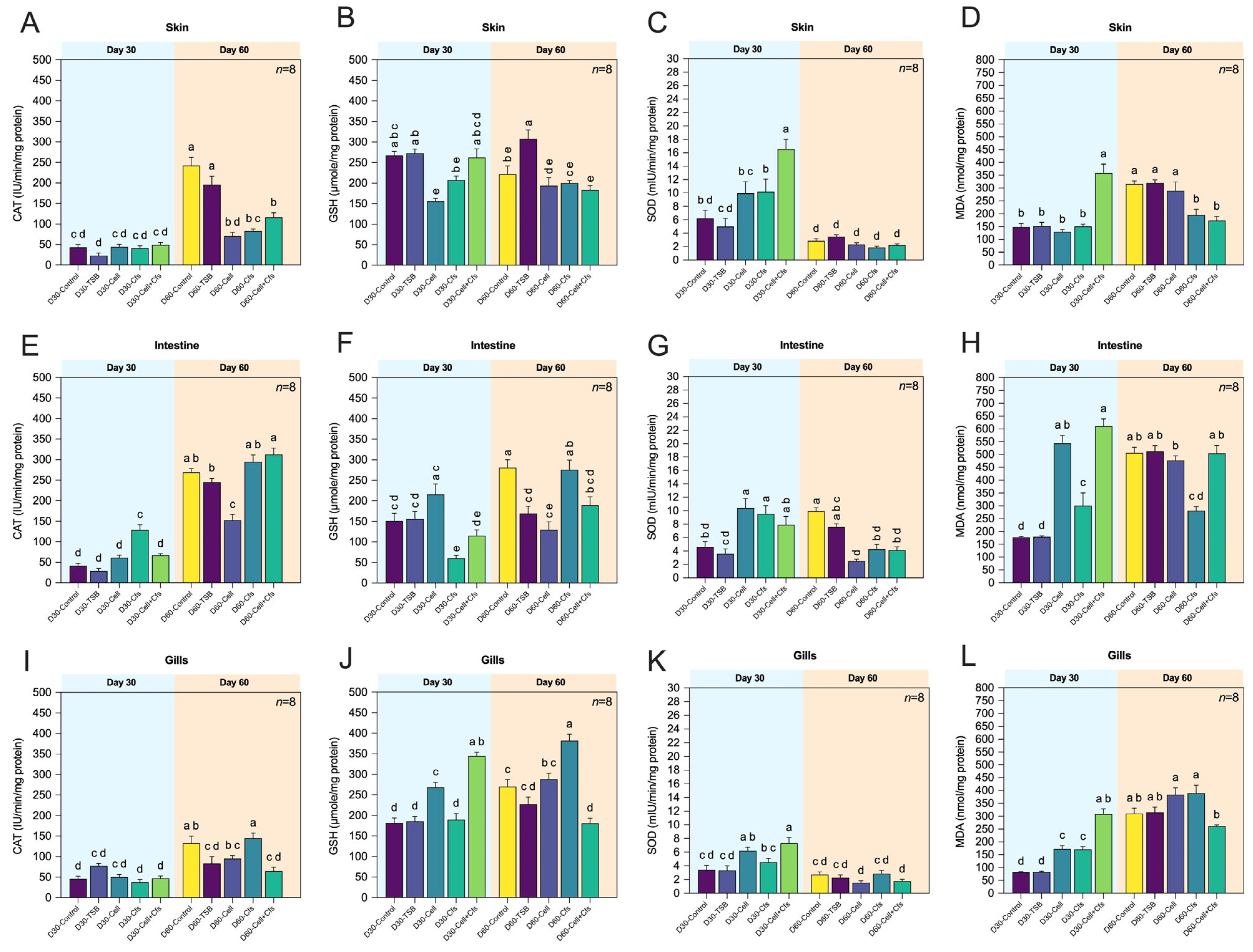
3.4. Assessment of the Antioxidative Status and Oxidative Stress of Organs Associated with Mucosal Immunity
3.4.1. Skin
3.4.2. Intestine
3.4.3. Gills
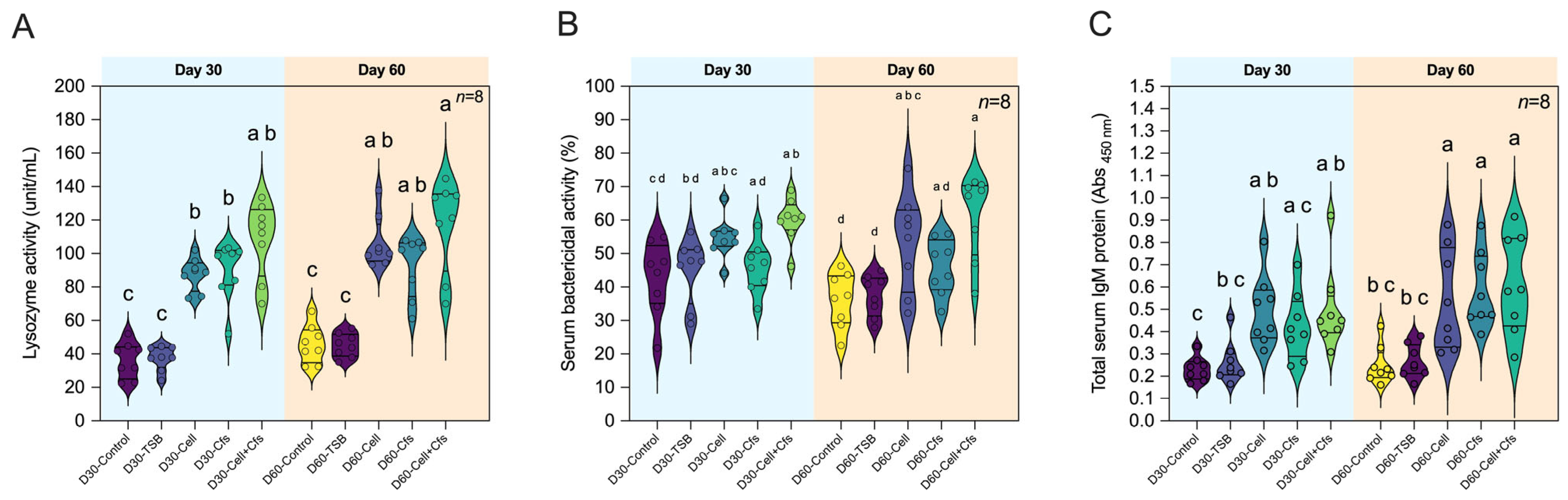
3.5. Effects of Bacillus velezensis AAHM-BV2302 on Humoral Immune Responses and IgM of Tilapia
3.5.1. Lysozyme Activity
3.5.2. Bactericidal Activity
3.5.3. Total Serum IgM
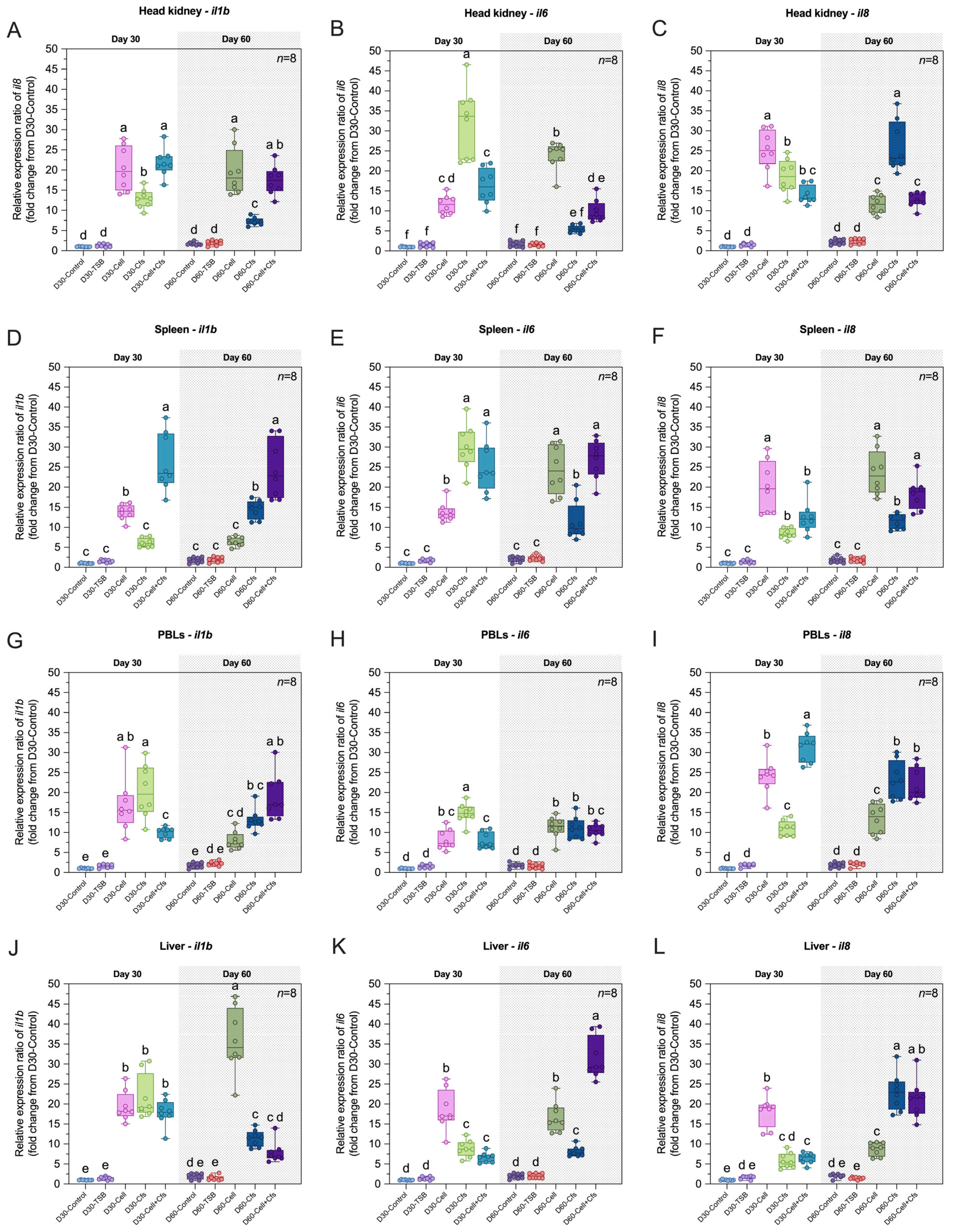
3.6. Effects of Probiotic Supplementation on the Expression of Immune-Related Genes
3.6.1. Expression of il1b Gene
3.6.2. Expression of il6 Gene
3.6.3. Expression of il8 Gene
3.7. Challenge Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Lu, M. Tilapia polyculture: A global review. Aquac. Res. 2016, 47, 2363–2374. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M.; Fitzsimmons, K. From Africa to the world—The journey of Nile tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar] [CrossRef]
- Fimbres-Acedo, Y.E.; Maeda-Martínez, A.N.; Garza-Torres, R. Tilapia diseases reported in Mexico: A systematic review. J. Fish Dis. 2025, 48, e14105. [Google Scholar] [CrossRef]
- Gundi, V.A.; Bogireddy, D.; Vundru, A.K.; Arthala, P.K.; Vadela, M.B.; Karri, S.; Allam, U.S.; Gujjula, M.S.; Kodali, V.P. Microbial pathogens in aquaculture: A review of emerging threats. Acad. Biol. 2025, 3. [Google Scholar] [CrossRef]
- Sukkarun, P.; Kitiyodom, S.; Kamble, M.T.; Bunnoy, A.; Boonanuntanasarn, S.; Yata, T.; Boonrungsiman, S.; Thompson, K.D.; Rodkhum, C.; Pirarat, N. Systemic and mucosal immune responses in red tilapia (Oreochromis sp.) following immersion vaccination with a chitosan polymer-based nanovaccine against Aeromonas veronii. Fish Shellfish Immunol. 2024, 146, 109383. [Google Scholar] [CrossRef]
- Abdallah, E.S.H.; Metwally, W.G.M.; Abdel-Rahman, M.A.M.; Albano, M.; Mahmoud, M.M. Streptococcus agalactiae infection in Nile tilapia (Oreochromis niloticus): A review. Biology 2024, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Surachetpong, W.; Janetanakit, T.; Nonthabenjawan, N.; Tattiyapong, P.; Sirikanchana, K.; Amonsin, A. Outbreaks of tilapia lake virus infection, Thailand, 2015–2016. Emerg. Infect. Dis. 2017, 23, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.Y.; Sherif, A.H.; Kenawy, A.M.; Abdelsalam, M. Phenotypic and molecular characterization of the causative agents of edwardsiellosis causing Nile tilapia (Oreochromis niloticus) summer mortalities. Microb. Pathog. 2022, 169, 105620. [Google Scholar] [CrossRef] [PubMed]
- Sherif, A.H.; Kassab, A.S. Multidrug-resistant Aeromonas bacteria prevalence in Nile tilapia broodstock. BMC Microbiol. 2023, 23, 80. [Google Scholar] [CrossRef]
- Poudyal, S.; Pulpipat, T.; Wang, P.C.; Chen, S.C. Comparison of the pathogenicity of Francisella orientalis in Nile tilapia (Oreochromis niloticus), Asian seabass (Lates calcarifer) and largemouth bass (Micropterus salmoides) through experimental intraperitoneal infection. J. Fish Dis. 2020, 43, 1097–1106. [Google Scholar] [CrossRef]
- Cortés-Sánchez, A.D.J.; Espinosa-Chaurand, L.D.; Díaz-Ramirez, M.; Torres-Ochoa, E. Plesiomonas: A review on food safety, fish-borne diseases, and tilapia. Sci. World J. 2021, 2021, 3119958. [Google Scholar] [CrossRef] [PubMed]
- Thangsunan, P.; Thangsunan, P.; Mahatnirunkul, T.; Buncharoen, W.; Saenphet, K.; Saenphet, S.; Phaksopa, J.; Thompson, K.D.; Srisapoome, P.; Kumwan, B. Development and characterization of an innovative Flavobacterium oreochromis antigen-encapsulated hydrogel bead for enhancing oral vaccine delivery in hybrid red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2025, 165, 110483. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.E.M.; Schreier, H.J.; Lanska, L.; Hale, M.S. The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Lv, Z.; Zhang, Z.; Han, Y.; Liu, Z.; Zhang, H. A review of antibiotics, antibiotic resistant bacteria, and resistance genes in aquaculture: Occurrence, contamination, and transmission. Toxics 2023, 11, 420. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Ringø, E.; Harikrishnan, R.; Soltani, M.; Ghosh, K. The effect of gut microbiota and probiotics on metabolism in fish and shrimp. Animals 2022, 12, 3016. [Google Scholar] [CrossRef]
- Shehata, A.I.; Soliman, A.A.; Ahmed, H.A.; Gewaily, M.S.; Amer, A.A.; Shukry, M.; Abdel-Latif, H.M.R. Evaluation of different probiotics on growth, body composition, antioxidant capacity, and histoarchitecture of Mugil capito. Sci. Rep. 2024, 14, 7379. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Nayak, S.K. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Aquac. 2021, 13, 862–906. [Google Scholar] [CrossRef]
- Fazle Rabbee, M.; Baek, K.H. Antimicrobial activities of lipopeptides and polyketides of Bacillus velezensis for agricultural applications. Molecules 2020, 25, 4973. [Google Scholar] [CrossRef]
- Kaspar, F.; Neubauer, P.; Gimpel, M. Bioactive secondary metabolites from Bacillus subtilis: A comprehensive review. J. Nat. Prod. 2019, 82, 2038–2053. [Google Scholar] [CrossRef] [PubMed]
- Markelova, N.; Chumak, A. Antimicrobial activity of Bacillus cyclic lipopeptides and their role in the host adaptive response to changes in environmental conditions. Int. J. Mol. Sci. 2025, 26, 336. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Nazir, S.; Ijaz, F.; Zahid, M.U.; Mushtaq, M.; Khan, M.; Rahman, A.; Rahman, M.A.U. Dietary supplementation of Bacillus subtilis as probiotic influenced the growth performance, hematological parameters, immune function, antioxidant status, and digestive enzyme activity of Nile tilapia fingerlings (Oreochromis niloticus). Animals 2025, 15, 1256. [Google Scholar] [CrossRef] [PubMed]
- Wiratama, N.; Kumwan, B.; Meachasompop, P.; Adisornprasert, Y.; Srisapoome, P.; Thompson, K.D.; Phrompanya, P.; Thangsunan, P.; Thangsunan, P.; Saenphet, K.; et al. Probiotic and postbiotic effects of Bacillus velezensis AAHM-BV2354 on boosting immunity, growth performance, antioxidant activity and resistance to Edwardsiella tarda infection in pangasius (Pangasianodon hypophthalmus). Fish Shellfish Immunol. 2025, 165, 110561. [Google Scholar] [CrossRef]
- Wiratama, N.; Meachasompop, P.; Kumwan, B.; Adisornprasert, Y.; Srisapoome, P.; Phrompanya, P.; Thangsunan, P.; Thangsunan, P.; Saenphet, K.; Saenphet, S.; et al. Dietary probiotic Bacillus subtilis AAHM-BS2360 and Its postbiotic metabolites enhance growth, immunity, and resistance to edwardsiellosis in Pangasianodon hypophthalmus. Antioxidants 2025, 14, 629. [Google Scholar] [CrossRef]
- Wu, Z.; Qi, X.; Qu, S.; Ling, F.; Wang, G. Dietary supplementation of Bacillus velezensis B8 enhances immune response and resistance against Aeromonas veronii in grass carp. Fish Shellfish Immunol. 2021, 115, 14–21. [Google Scholar] [CrossRef]
- Thorakkattu, P.; Khanashyam, A.C.; Shah, K.; Babu, K.S.; Mundanat, A.S.; Deliephan, A.; Deokar, G.S.; Santivarangkna, C.; Nirmal, N.P. Postbiotics: Current trends in food and pharmaceutical industry. Foods 2022, 11, 3094. [Google Scholar] [CrossRef]
- Tao, L.-T.; Lu, H.; Xiong, J.; Zhang, L.; Sun, W.-W.; Shan, X.-F. The application and potential of postbiotics as sustainable feed additives in aquaculture. Aquaculture 2024, 592, 741237. [Google Scholar] [CrossRef]
- Thakur, K.; Singh, B.; Kumar, S.; Sharma, D.; Sharma, A.K.; Jindal, R.; Kumar, R. Potential of probiotics and postbiotics in aquaculture: Connecting current research gaps and future perspectives. Microbe 2025, 8, 100431. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes de novo assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.-Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Ott, B.D.; Torrans, E.L.; Tucker, C.S. Fish production, water quality, and the role of nitrification as an ammonia removal process in intensively aerated hybrid catfish ponds. J. World Aquac. Soc. 2024, 55, e13094. [Google Scholar] [CrossRef]
- Meachasompop, P.; Bunnoy, A.; Keaswejjareansuk, W.; Dechbumroong, P.; Namdee, K.; Srisapoome, P. Development of immersion and oral bivalent nanovaccines for streptococcosis and columnaris disease prevention in fry and fingerling Asian seabass (Lates calcarifer) nursery farms. Vaccines 2024, 12, 17. [Google Scholar] [CrossRef]
- Paankhao, N.; Sangsawang, A.; Kantha, P.; Paankhao, S.; Promsee, K.; Soontara, C.; Kongsriprapan, S.; Srisapoome, P.; Kumwan, B.; Meachasompop, P. Antioxidant and antibacterial efficiency of the ethanolic leaf extract of Kratom (Mitragyna speciosa (Korth.) Havil) and its effects on growth, health, and disease resistance against Edwardsiella tarda infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2024, 152, 109771. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Maehly, A. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef]
- Jollow, D.; Mitchell, J.; Zampaglione, N.a.; Gillette, J. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Takada, Y.; Noguchi, T.; Okabe, T.; Kajiyama, M. Superoxide dismutase in various tissues from rabbits bearing the Vx-2 carcinoma in the maxillary sinus. Cancer Res. 1982, 42, 4233–4235. [Google Scholar]
- Parry, R.M., Jr.; Chandan, R.C.; Shahani, K.M. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef]
- Uchuwittayakul, A.; Rodkhum, C.; Srisapoome, P. Production of a monoclonal antibody specific to the IgM heavy chain of Asian seabass (Lates calcarifer Bloch, 1790) and its application in assessing health status following vaccination and challenges with Flavobacterium covae and Streptococcus iniae. Aquaculture 2025, 594, 741445. [Google Scholar] [CrossRef]
- Vanichavetin, K.; Uchuwittayakul, A.; Namdee, K.; Srisapoome, P. Oral booster effects of bivalent nanovaccine-primed fingerlings of Asian seabass (Lates calcarifer, Bloch 1790) to prevent streptococcosis and columnaris diseases. Aquaculture 2024, 592, 741165. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Uchida, K.; Moriyama, S.; Breves, J.P.; Fox, B.K.; Pierce, A.L.; Borski, R.J.; Hirano, T.; Gordon Grau, E. cDNA cloning and isolation of somatolactin in Mozambique tilapia and effects of seawater acclimation, confinement stress, and fasting on its pituitary expression. Gen. Comp. Endocrinol. 2009, 161, 162–170. [Google Scholar] [CrossRef]
- Rattanawongwiboon, T.; Paankhao, N.; Buncharoen, W.; Pansawat, N.; Kumwan, B.; Meachasompop, P.; Kantha, P.; Pansiri, T.; Tangthong, T.; Laksee, S.; et al. Characterization and application of synergistically degraded chitosan in aquafeeds to promote immunity, antioxidative status, and disease resistance in Nile tilapia (Oreochromis niloticus). Polymers 2025, 17, 2101. [Google Scholar] [CrossRef]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda. Res. 2010, 1, 274–278. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Wang, Y.; Liu, Z.; Liu, L.; Shi, C. Probiotic effects of the Bacillus velezensis GY65 strain in the mandarin fish, Siniperca chuatsi. Aquac. Rep. 2021, 21, 100902. [Google Scholar] [CrossRef]
- Gao, X.; Chen, A.; Zhou, Y.; Qian, Q.; Qin, L.; Tang, X.; Jiang, Q.; Zhang, X. Genomic characterization and probiotic potency of Bacillus velezensis CPA1-1 reveals its potential for aquaculture applications. Aquaculture 2025, 596, 741852. [Google Scholar] [CrossRef]
- Yang, F.; Jiang, H.; Ma, K.; Wang, X.; Liang, S.; Cai, Y.; Jing, Y.; Tian, B.; Shi, X. Genome sequencing and analysis of Bacillus velezensis VJH504 reveal biocontrol mechanism against cucumber Fusarium wilt. Front. Microbiol. 2023, 14, 1279695. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, R.; Fatima, S.; Komal, W.; Minahal, Q.; Kanwal, Z.; Suleman, M.; Carter, C.G. Effects of Bacillus subtilis as a single strain probiotic on growth, disease resistance and immune response of striped catfish (Pangasius hypophthalmus). PLoS ONE 2024, 19, e0294949. [Google Scholar] [CrossRef] [PubMed]
- El-Son, M.A.M.; Elshopakey, G.E.; Rezk, S.; Eldessouki, E.A.A.; Elbahnaswy, S. Dietary mixed Bacillus strains promoted the growth indices, enzymatic profile, intestinal immunity, and liver and intestinal histomorphology of Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2022, 27, 101385. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, M.; Lin, L.; Wang, J.; Zhang, D.; Wang, Q.; Elsadek, M.M.; Wang, G.; Yao, Q.; Chen, Y. Effects of dietary Bacillus velezensis LSG2-5 on growth, immunity, antioxidant capacity, and disease resistance of Amur minnow (Rhynchocypris lagowskii Dybowski). Aquac. Nutr. 2022, 2022, 7199145. [Google Scholar] [CrossRef]
- Dighiesh, H.S.; Alharbi, N.A.; Awlya, O.F.; Alhassani, W.E.; Hassoubah, S.A.; Albaqami, N.M.; Aljahdali, N.; Abd El-Aziz, Y.M.; Eissa, E.-S.H.; Munir, M.B. Dietary multi-strains Bacillus spp. enhanced growth performance, blood metabolites, digestive tissues histology, gene expression of Oreochromis niloticus, and resistance to Aspergillus flavus infection. Aquac. Int. 2024, 32, 7065–7086. [Google Scholar] [CrossRef]
- Shaheen, A.A.; Eissa, N.; Abou-ElGheit, E.; Yao, H.; Wang, H.-P. Effect of probiotic on growth performance and growth-regulated genes in yellow perch (Perca flavescens). Glob. J. Fish. Aquac. Res. 2014, 1, 1–15. [Google Scholar]
- Vélez-Alavez, M.; De Anda-Montañez, J.A.; Galván-Magaña, F.; Zenteno-Savín, T. Comparative study of enzymatic antioxidants in muscle of elasmobranch and teleost fishes. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 61–65. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The key role of GSH in keeping the redox balance in mammalian cells: Mechanisms and significance of GSH in detoxification via formation of conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Zeng, Y.; Song, Z.; Song, G.; Li, S.; Sun, H.; Zhang, C.; Li, G. Oxidative stress and antioxidant biomarker responses in fish exposed to heavy metals: A review. Environ. Monit. Assess. 2025, 197, 892. [Google Scholar] [CrossRef]
- Ukaegbu, K.; Allen, E.; Svoboda, K.K.H. Reactive oxygen species and antioxidants in wound healing: Mechanisms and therapeutic potential. Int. Wound J. 2025, 22, e70330. [Google Scholar] [CrossRef]
- Salimkumar, A.V.; Sasikumar, A.A.; Rahman, M.S.; Elumalai, P. Bacillus Effects on the Immune System. In Bacillus Probiotics for Sustainable Aquaculture, 1st ed.; CRC Press,: Boca Raton, FL, USA, 2024; p. 20. [Google Scholar]
- Elbahnaswy, S.; Elshopakey, G.E.; Abdelwarith, A.A.; Younis, E.M.; Davies, S.J.; El-Son, M.A.M. Immune protective, stress indicators, antioxidant, histopathological status, and heat shock protein gene expression impacts of dietary Bacillus spp. against heat shock in Nile tilapia, Oreochromis niloticus. BMC Vet. Res. 2024, 20, 469. [Google Scholar] [CrossRef]
- Eissa, E.-S.H.; Abdel Rahman, A.N.; Ahmed, R.A.; Hendam, B.M.; Abd El-Aziz, Y.M.; Dighiesh, H.S.; Eissa, M.E.H.; Okon, E.M.; Ahmed, N.H. Dietary mixtures of Bacillus spp. modulates intestinal morphology, resistance to Vibrio parahaemolyticus, response of immune-antioxidant genes, and growth of Dicentrarchus labrax. Aquac. Rep. 2025, 43, 102930. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Masschalck, B.; Michiels, C.W. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit. Rev. Microbiol. 2003, 29, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Folch, H.; Enríquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Zhang, Y.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Dossou, S.; Wang, W.; Seo, S.; Chen, J.; Zheng, S.; Zhang, X. Effects of dietary supplementation with Bacillus subtilis natto on growth, digestive enzyme activity, immune response, and intestinal microorganisms of red sea bream, Pagrus major. Fishes 2024, 9, 446. [Google Scholar] [CrossRef]
- Giri, S.; Sukumaran, V.; Sen, S.; Jena, P. Effects of dietary supplementation of potential probiotic Bacillus subtilis VSG 1 singularly or in combination with Lactobacillus plantarum VSG 3 or/and Pseudomonas aeruginosa VSG 2 on the growth, immunity and disease resistance of Labeo rohita. Aquac. Nutr. 2014, 20, 163–171. [Google Scholar] [CrossRef]
- Abdelsamad, A.E.M.; Said, R.E.M.; Assas, M.; Gaafar, A.Y.; Hamouda, A.H.; Mahdy, A. Effects of dietary supplementation with Bacillus velezensis on the growth performance, body composition, antioxidant, immune-related gene expression, and histology of Pacific white shrimp, Litopenaeus vannamei. BMC Vet. Res. 2024, 20, 368. [Google Scholar] [CrossRef]
- Lee, C.; Cha, J.H.; Kim, M.G.; Shin, J.; Woo, S.H.; Kim, S.H.; Kim, J.W.; Ji, S.C.; Lee, K.J. The effects of dietary Bacillus subtilis on immune response, hematological parameters, growth performance, and resistance of juvenile olive flounder (Paralichthys olivaceus) against Streptococcus iniae. J. World Aquac. Soc. 2020, 51, 551–562. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An evolving chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef] [PubMed]
- Secombes, C.J.; Wang, T. The innate and adaptive immune system of fish. In Infectious Disease in Aquaculture, 1st ed.; Austin, B., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 3–68. [Google Scholar]
- Shadrack, R.S.; Manabu, I.; Koshio, S.; Yokoyama, S.; Zhang, Y.; Mzengereza, K.; El Basuini, M.F.; Dawood, M.A. Effects of single and mixture probiotic supplements on growth, digestive activity, antioxidative status, immune and growth-related genes, and stress response of juvenile red sea bream (Pagrus major). Aquac. Nutr. 2022, 2022, 8968494. [Google Scholar] [CrossRef]
- Jang, W.J.; Jeon, M.H.; Lee, S.J.; Park, S.Y.; Lee, Y.S.; Noh, D.I.; Hur, S.W.; Lee, S.; Lee, B.J.; Lee, J.M.; et al. Dietary supplementation of Bacillus sp. PM8313 with β-glucan modulates the intestinal microbiota of red sea bream (Pagrus major) to increase growth, immunity, and disease resistance. Front. Immunol. 2022, 13, 960554. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Yun, L.; Liu, Z.; Shen, Y.; Feng, S.; Yang, G.; Meng, X. Antagonistic effects and the underlying mechanisms of Bacillus velezensis and its antibacterial peptide LCI against Aeromonas hydrophila Infection in largemouth bass. Probiotics Antimicrob. Proteins 2025, 17, 2009–2026. [Google Scholar] [CrossRef]
- Yao, Y.-Y.; Zhou, W.-H.; Hu, J.; Yang, Y.-L.; Li, M.; Xia, R.; Ran, C.; Zhang, Z.; Zhou, Z.-G. Strain-specific effects of fish originated Bacillus velezensis on growth, gut health, and disease resistance of zebrafish. Fish Shellfish Immunol. 2025, 163, 110400. [Google Scholar] [CrossRef]
- Hassanin, M.E.; El-Murr, A.; El-Khattib, A.R.; Abdelwarith, A.A.; Younis, E.M.; Metwally, M.M.M.; Ismail, S.H.; Davies, S.J.; Abdel Rahman, A.N.; Ibrahim, R.E. Nano-Bacillus amyloliquefaciens as a dietary intervention in nile tilapia (Oreochromis niloticus): Effects on resistance to Aeromonas hydrophila challenge, immune-antioxidant responses, digestive/absorptive capacity, and growth. Heliyon 2024, 10, e40418. [Google Scholar] [CrossRef]
| Gene Group | Genes | Nucleotide Sequences (5′→3′) | Tm (°C) | Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Reference gene | Actin, beta 1 (actb1) | F: ACAGGATGCAGAAGGAGATCACAG R: GTACTCCTGCTTGCTGATCCACAT | 60 | 101.6 | [38] |
| 18S rRNA (rna18s) | F: GGACACGGAAAGGATTGACAG R: GTTCGTTATCGGAATTAACCAGAC | 60 | 98.9 | [12] | |
| Growth-related gene | Growth hormone (gh) | F: TTACATCATCAGCCCGATCG R: AGATCGACAGCAGCTTCAGGA | 60 | 95.9 | [47] |
| Cytokines and signaling molecules | Interleukin-1β (il1b) | F: GTGCTGAGCACAGAATTCCAGGAT R: GAAGAACCAAGCTCCTCTTTTGGC | 60 | 100.3 | [48] |
| Interleukin-6 (il6) | F: ACAGAGGAGGCGGAGATG R: GCAGTGCTTCGGGATAGAG | 60 | 98.7 | [38] | |
| Interleukin-8 (il8) | F: GCCTCTTCAGGGCTAGAGTCA R: TGAAGCCTGAAGCGCTAAACT | 60 | 99.5 | [38] |
| Organism | Bacillus velezensis AAHM-BV2302 |
| BioProject no. | PRJNA1320929 |
| Accession no. | JBQXGX000000000 |
| Size | 4,156,630 bp |
| GC content | 45.9% |
| CDS | 4103 |
| Gene | 4177 |
| tRNA | 69 |
| rRNA | 4 |
| tmRNA | 1 |
| Reference Genome | ANI | dDDH (d6, in %) | Nucleotide Similarity (%) |
|---|---|---|---|
| Bacillus velezensis NRRL B-41580 | 99.43 | 97.3 | 93.42 |
| Bacillus amyloliquefaciens subsp. plantarum FZB42 | 98.04 | 90.6 | 87.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meachasompop, P.; Kumwan, B.; Chokmangmeepisarn, P.; Phrompanya, P.; Kantha, P.; Thangsunan, P.; Srisapoome, P.; Thangsunan, P.; Kingwascharapong, P.; Imaizumi, K.; et al. Integrated Probiotic Benefits of Bacillus velezensis AAHM-BV2302 Drive Growth, Antioxidant Enhancement, and Immune Protection Against Streptococcus agalactiae in Tilapia (Oreochromis spp.). Antioxidants 2025, 14, 1356. https://doi.org/10.3390/antiox14111356
Meachasompop P, Kumwan B, Chokmangmeepisarn P, Phrompanya P, Kantha P, Thangsunan P, Srisapoome P, Thangsunan P, Kingwascharapong P, Imaizumi K, et al. Integrated Probiotic Benefits of Bacillus velezensis AAHM-BV2302 Drive Growth, Antioxidant Enhancement, and Immune Protection Against Streptococcus agalactiae in Tilapia (Oreochromis spp.). Antioxidants. 2025; 14(11):1356. https://doi.org/10.3390/antiox14111356
Chicago/Turabian StyleMeachasompop, Pakapon, Benchawan Kumwan, Putita Chokmangmeepisarn, Phornphan Phrompanya, Phunsin Kantha, Patcharapong Thangsunan, Prapansak Srisapoome, Pattanapong Thangsunan, Passakorn Kingwascharapong, Kentaro Imaizumi, and et al. 2025. "Integrated Probiotic Benefits of Bacillus velezensis AAHM-BV2302 Drive Growth, Antioxidant Enhancement, and Immune Protection Against Streptococcus agalactiae in Tilapia (Oreochromis spp.)" Antioxidants 14, no. 11: 1356. https://doi.org/10.3390/antiox14111356
APA StyleMeachasompop, P., Kumwan, B., Chokmangmeepisarn, P., Phrompanya, P., Kantha, P., Thangsunan, P., Srisapoome, P., Thangsunan, P., Kingwascharapong, P., Imaizumi, K., Paankhao, N., Saenphet, K., Saenphet, S., Buncharoen, W., & Uchuwittayakul, A. (2025). Integrated Probiotic Benefits of Bacillus velezensis AAHM-BV2302 Drive Growth, Antioxidant Enhancement, and Immune Protection Against Streptococcus agalactiae in Tilapia (Oreochromis spp.). Antioxidants, 14(11), 1356. https://doi.org/10.3390/antiox14111356








