Dietary Antioxidants Influence IER5 Activation and DNA Repair: Implications for Radioprotection and Healthy Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Radioprotective Compounds
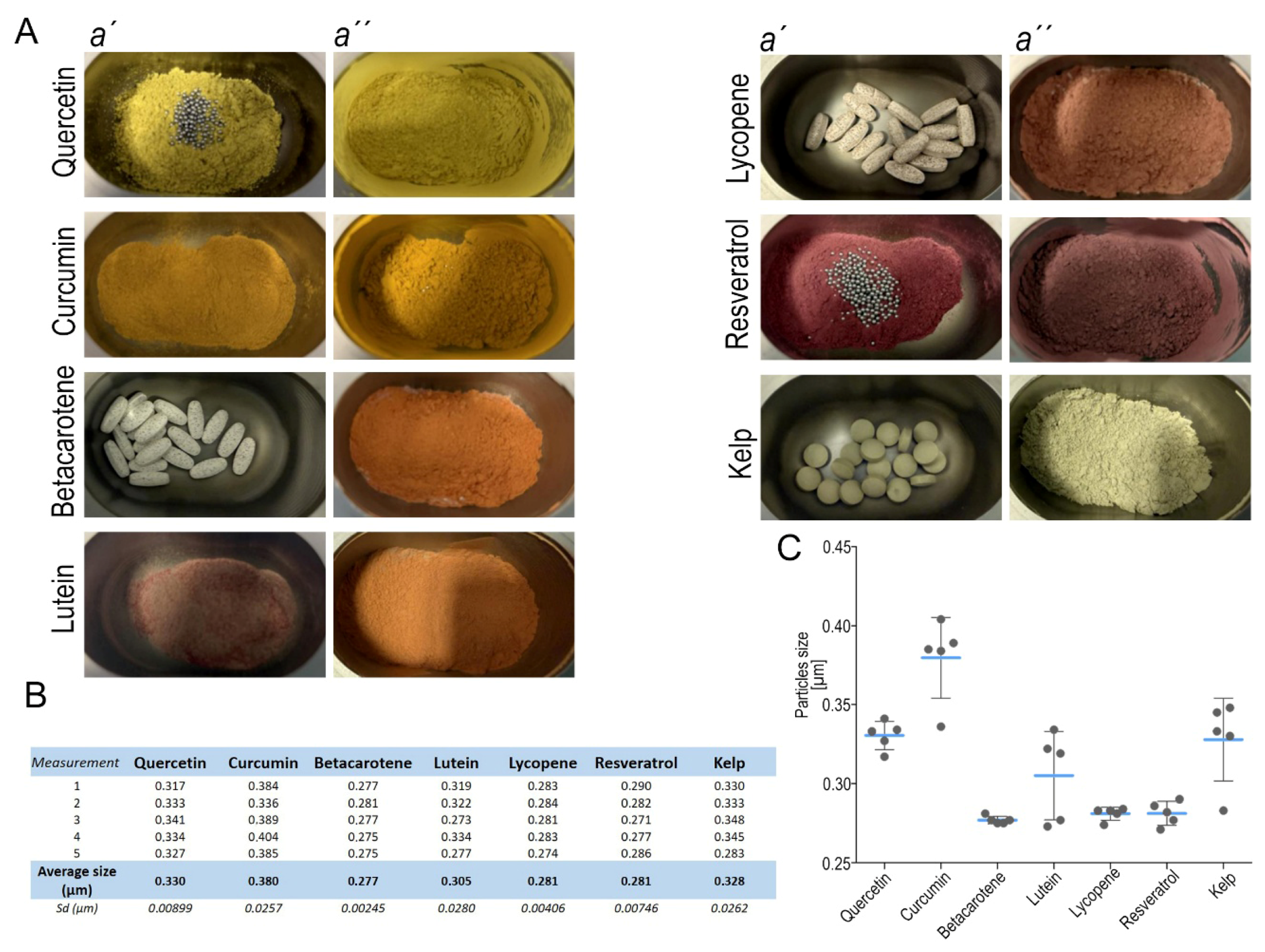
2.2. Micronization by Nanomilling
2.3. Stock Solutions of Radioprotective Compounds
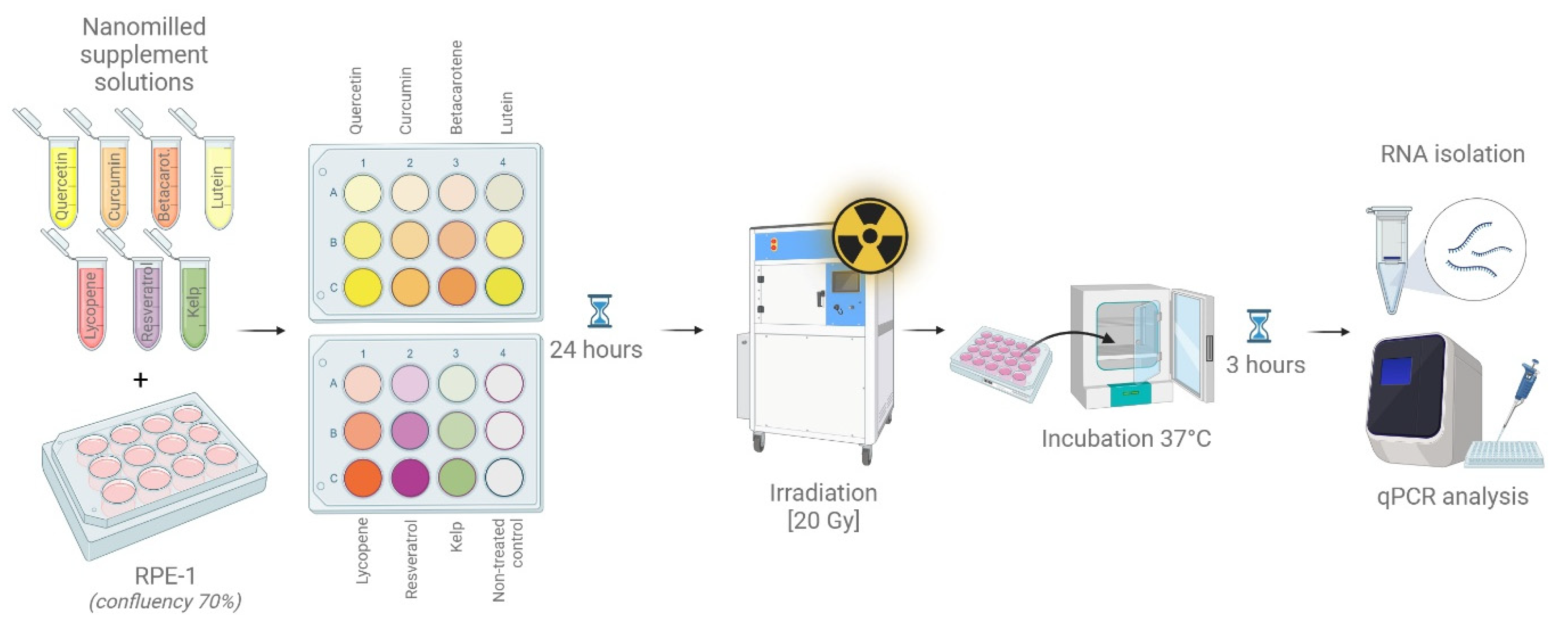
2.4. Cell Line and Induction of DNA Damage by Irradiation
2.5. Microscopy
2.6. RNA Isolation and qPCR
2.7. Testing of Antioxidant Activity
3. Results
3.1. DNA Damage Induced by Gamma Radiation and Radioprotective Defense
3.2. Protective Potential of Selected Natural Compounds Against Gamma Radiation
3.3. Micronization of the Compounds Increases Biological Availability
3.4. Physiological Effect of Compounds on the Cells
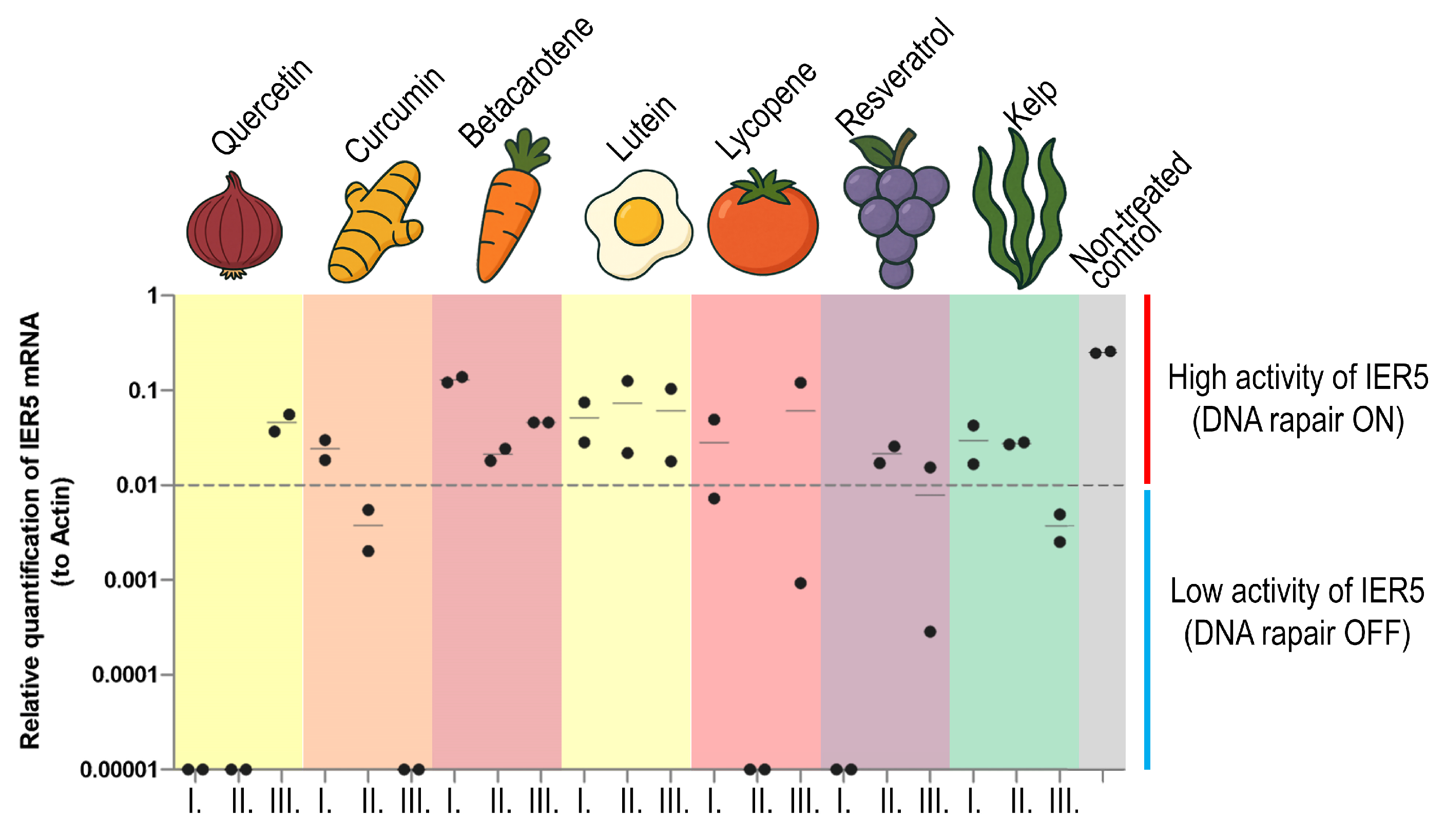
3.5. Assessment of IER5 and γH2AX Activation as an Early Indicator of DNA Damage and Repair
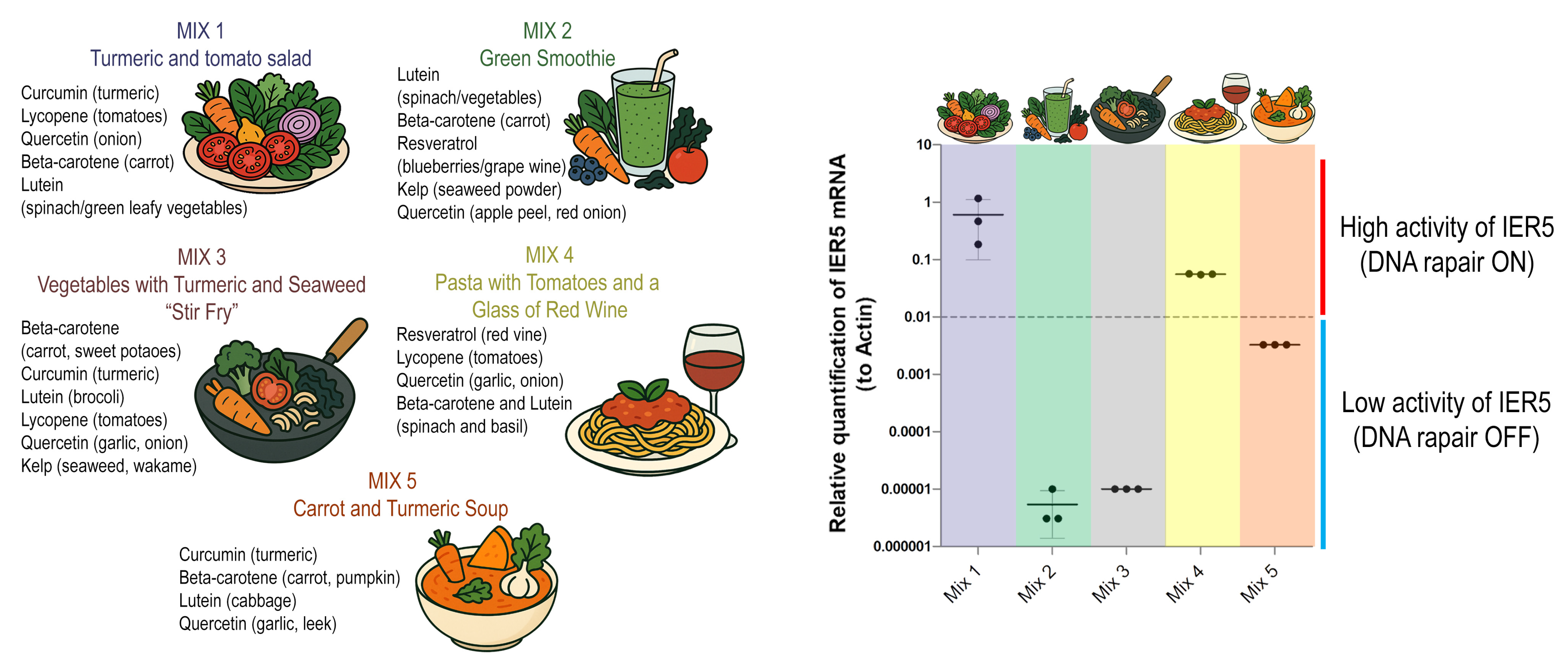
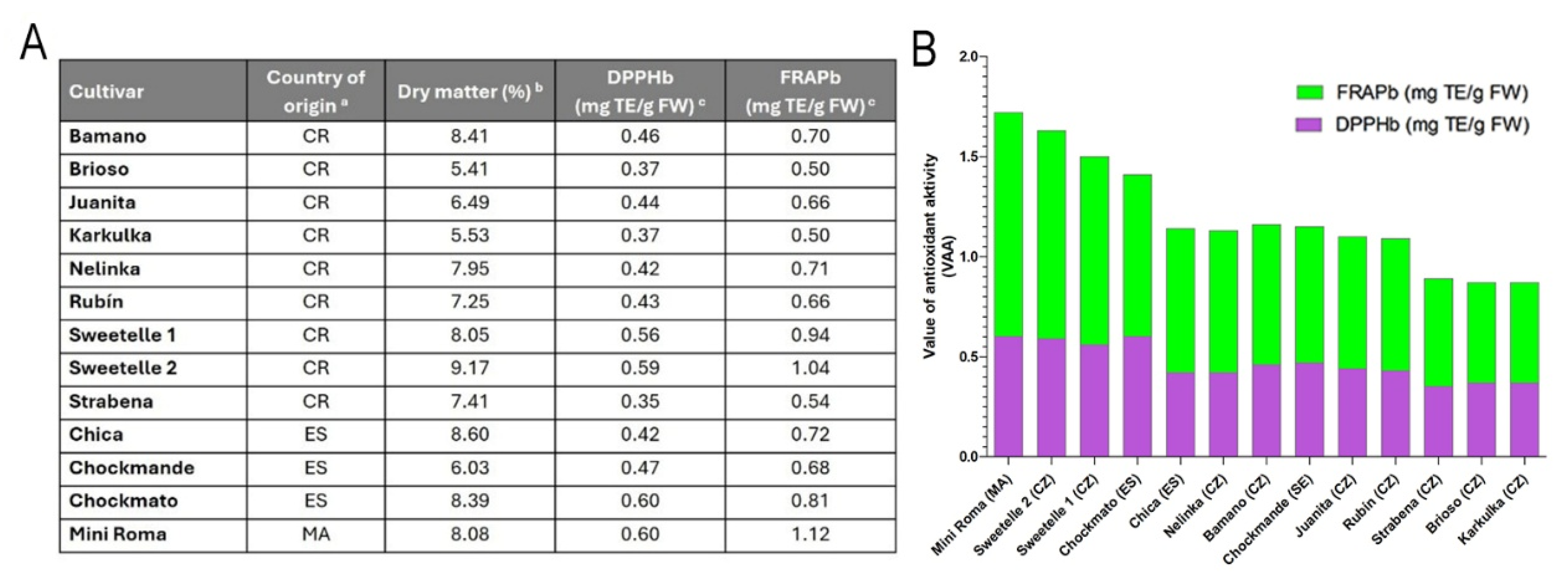
3.6. Evaluation of the Effectiveness of Quercetin and Lycopene in Commonly Consumed Foods Available on the Czech Market
3.6.1. Lycopene in Tomatoes
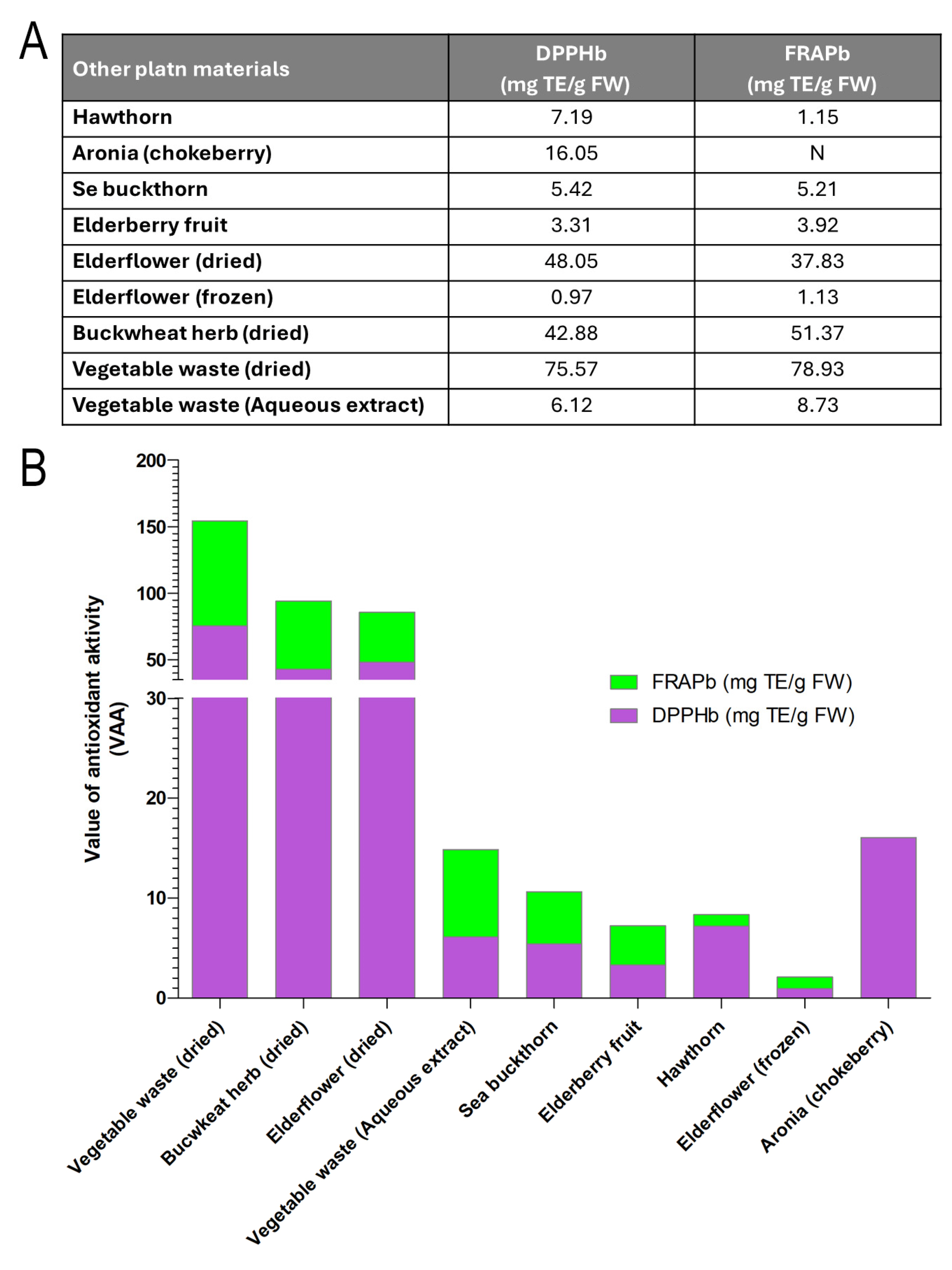
3.6.2. Quercetin from Garlic and Other Natural Sources
4. Discussion
4.1. Potential of Combinatory Antioxidants for Healthy Aging
4.1.1. Quercetin
4.1.2. Lycopene
4.1.3. Curcumin and Resveratrol
4.2. Availability of Consumed Foods on the European Market
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IER5 | Immediate early response 5 |
| ROS | Reactive oxygen species |
| DSBs | DNA double-strand breaks |
| hTERT RPE-1 | Human retinal pigment epithelial cell line |
| DMEM | Dulbecco’s modified eagle medium |
| FBS | Fetal bovine serum |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FRAP | Ferric reducing antioxidant power |
| Fe3+-TPTZ | Ferric-tripyridyl triazine (Fe3+-TPTZ) |
| NF-κB | Nuclear factor-kappa B |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| Gy | Gray |
| γH2AX | Phosphorylated form of histone variant H2AX |
| SOD | Superoxide Dismutase |
| p53 | Tumor protein p53 |
| UV | Ultraviolet radiation |
| UVB | Ultraviolet B radiation |
| VAA | Combined antioxidant activity |
| ATM | Ataxia-telangiectasia mutated |
| ATR | Ataxia-telangiectasia and Rad3-related protein |
| 53BP1 | Tumor suppressor p53-binding protein 1 |
| GSH | γ-l-glutamyl-l-cysteinyl-glycine |
| ARE | Antioxidant response element |
| AMPK | Adenosine monophosphate-activated protein kinase |
| SIRT7 | Sirtuin 7 |
| HMGB1 | High-mobility group box 1 |
References
- Smith, T.A.; Kirkpatrick, D.R.; Smith, S.; Smith, T.K.; Pearson, T.; Kailasam, A.; Herrmann, K.Z.; Schubert, J.; Agrawal, D.K. Radioprotective Agents to Prevent Cellular Damage Due to Ionizing Radiation. J. Transl. Med. 2017, 15, 232. [Google Scholar] [CrossRef]
- Javadi, A.; Nikhbakht, M.R.; Ghasemian Yadegari, J.; Rustamzadeh, A.; Mohammadi, M.; Shirazinejad, A.; Azadbakht, S.; Abdi, Z. In-Vivo and in Vitro Assessments of the Radioprotective Potential Natural and Chemical Compounds: A Review. Int. J. Radiat. Biol. 2023, 99, 155–165. [Google Scholar] [CrossRef]
- Johnke, R.M.; Sattler, J.A.; Allison, R.R. Radioprotective Agents for Radiation Therapy: Future Trends. Future Oncol. 2014, 10, 2345–2357. [Google Scholar] [CrossRef]
- Akpolat, M.; Oz, Z.S.; Gulle, K.; Hamamcioglu, A.C.; Bakkal, B.H.; Kececi, M. X Irradiation Induced Colonic Mucosal Injury and the Detection of Apoptosis through PARP-1/P53 Regulatory Pathway. Biomed. Pharmacother. 2020, 127, 110134. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Cao, F.; Liu, H. Radiation-Induced Cell Death and Its Mechanisms. Health Phys. 2022, 123, 376. [Google Scholar] [CrossRef] [PubMed]
- Storer, J.B. Radiation Carcinogenesis; Spring Nature: Berlin/Heidelberg, Germany, 1975; pp. 453–483. [Google Scholar] [CrossRef]
- Williams, D. Radiation Carcinogenesis: Lessons from Chernobyl. Oncogene 2008, 27, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, L.; Passante, M.; Cameli, N.; Cristaudo, A.; Patruno, C.; Nisticò, S.P.; Silvestri, M. Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering 2021, 8, 153. [Google Scholar] [CrossRef]
- Ryan, J.L. Ionizing Radiation: The Good, the Bad, and the Ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Pei, Y.; Wang, Z.; Wang, C.; Hua, D. Recent Advances on Macromolecular Medicinal Materials for Radioprotection. J. Drug Deliv. Sci. Technol. 2023, 81, 104224. [Google Scholar] [CrossRef]
- Du, J.; Zhang, P.; Cheng, Y.; Liu, R.; Liu, H.; Gao, F.; Shi, C.; Liu, C. General Principles of Developing Novel Radioprotective Agents for Nuclear Emergency. Radiat. Med. Prot. 2020, 1, 120–126. [Google Scholar] [CrossRef]
- Hosseinimehr, S.J. Trends in the Development of Radioprotective Agents. Drug Discov. Today 2007, 12, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Chen, W.; Hu, W.; Zhu, M.; Liu, T.; Wang, J.; Zhou, G. GANRA-5 Protects Both Cultured Cells and Mice from Various Radiation Types by Functioning as a Free Radical Scavenger. Free Radic. Res. 2014, 48, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Gielecińska, A.; Kciuk, M.; Yahya, E.B.; Ainane, T.; Mujwar, S.; Kontek, R. Apoptosis, Necroptosis, and Pyroptosis as Alternative Cell Death Pathways Induced by Chemotherapeutic Agents? Biochim. Biophys. Acta BBA—Rev. Cancer 2023, 1878, 189024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Si, J.; Zhang, H.; Wang, Z.; Li, J.; Zhou, X.; Gan, L.; Liu, Y. The Effects of X-Ray Radiation on the Eye Development of Zebrafish. Hum. Exp. Toxicol. 2014, 33, 1040–1050. [Google Scholar] [CrossRef]
- Brand, M.; Sommer, M.; Ellmann, S.; Wuest, W.; May, M.S.; Eller, A.; Vogt, S.; Lell, M.M.; Kuefner, M.A.; Uder, M. Influence of Different Antioxidants on X-Ray Induced DNA Double-Strand Breaks (DSBs) Using γ-H2AX Immunofluorescence Microscopy in a Preliminary Study. PLoS ONE 2015, 10, e0127142. [Google Scholar] [CrossRef]
- Chivte, V.; Rathod, A.; Rathod, M.; Rokade, S.; Kale, A. Nutraceuticals: A New Growth of Pharmaceuticals. Int. J. Multidiscip. Res. 2024, 5, 1. [Google Scholar]
- Manful, C.F.; Fordjour, E.; Subramaniam, D.; Sey, A.A.; Abbey, L.; Thomas, R. Antioxidants and Reactive Oxygen Species: Shaping Human Health and Disease Outcomes. Int. J. Mol. Sci. 2025, 26, 7520. [Google Scholar] [CrossRef]
- Tu, Y.S.; Fu, J.W.; Sun, D.M.; Zhang, J.J.; Yao, N.; Huang, D.E.; Shi, Z.Q. Preparation, Characterisation and Evaluation of Curcumin with Piperine-Loaded Cubosome Nanoparticles. J. Microencapsul. 2014, 31, 551–559. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle Encapsulation Improves Oral Bioavailability of Curcumin by at Least 9-Fold When Compared to Curcumin Administered with Piperine as Absorption Enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Salovska, B.; Kondelova, A.; Pimkova, K.; Liblova, Z.; Pribyl, M.; Fabrik, I.; Bartek, J.; Vajrychova, M.; Hodny, Z. Peroxiredoxin 6 Protects Irradiated Cells from Oxidative Stress and Shapes Their Senescence-Associated Cytokine Landscape. Redox Biol. 2022, 49, 102212. [Google Scholar] [CrossRef]
- Oike, T.; Uchihara, Y.; Permata, T.B.M.; Gondhowiardjo, S.; Ohno, T.; Shibata, A. Quantitative Volumetric Analysis of the Golgi Apparatus Following X-Ray Irradiation by Super-Resolution 3D-SIM Microscopy. Med. Mol. Morphol. 2021, 54, 166–172. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.K.; Miao, L.; St. Clair, D.K.; St. Clair, W.H. Redox-Modulated Phenomena and Radiation Therapy: The Central Role of Superoxide Dismutases. Antioxid. Redox Signal. 2014, 20, 1567–1589. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.A.; Gius, D.; Wink, D.A.; Krishna, M.C.; Russo, A.; Mitchell, J.B. Oxidative Stress, Redox, and the Tumor Microenvironment. Semin. Radiat. Oncol. 2004, 14, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Macià i Garau, M.; Lucas Calduch, A.; López, E.C. Radiobiology of the Acute Radiation Syndrome. Rep. Pract. Oncol. Radiother. 2011, 16, 123. [Google Scholar] [CrossRef]
- Gourmelon, P.; Benderitter, M.; Bertho, J.M.; Huet, C.; Gorin, N.C.; De Revel, P. European Consensus on the Medical Management of Acute Radiation Syndrome and Analysis of the Radiation Accidents in Belgium and Senegal. Health Phys. 2010, 98, 825–832. [Google Scholar] [CrossRef]
- Neuwahl, J.; Neumann, C.A.; Fitz, A.C.; Biermann, A.D.; Magel, M.; Friedrich, A.; Sellin, L.; Stork, B.; Piekorz, R.P.; Proksch, P.; et al. Combined Inhibition of Class 1-PI3K-Alpha and Delta Isoforms Causes Senolysis by Inducing p21WAF1/CIP1 Proteasomal Degradation in Senescent Cells. Cell Death Dis. 2024, 15, 373. [Google Scholar] [CrossRef]
- Mishra, K.; Alsbeih, G. Appraisal of Biochemical Classes of Radioprotectors: Evidence, Current Status and Guidelines for Future Development. 3 Biotech 2017, 7, 292. [Google Scholar] [CrossRef]
- Nishimura, M.; Muro, T.; Kobori, M.; Nishihira, J. Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients 2019, 12, 91. [Google Scholar] [CrossRef]
- Skoczylas, J.; Jędrszczyk, E.; Dziadek, K.; Dacewicz, E.; Kopeć, A. Basic Chemical Composition, Antioxidant Activity and Selected Polyphenolic Compounds Profile in Garlic Leaves and Bulbs Collected at Various Stages of Development. Molecules 2023, 28, 6653. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Xie, A.; Yue, X.; Li, M.; Zhou, Q. Garlic Peel Extract as an Antioxidant Inhibits Triple—Negative Breast Tumor Growth and Angiogenesis by Inhibiting Cyclooxygenase—2 Expression. Food Sci. Nutr. 2024, 12, 6886. [Google Scholar] [CrossRef]
- Zbikowska, H.M.; Antosik, A.; Szejk, M.; Bijak, M.; Nowak, P. A Moderate Protective Effect of Quercetin against γ-Irradiation- and Storage-Induced Oxidative Damage in Red Blood Cells for Transfusion. Int. J. Radiat. Biol. 2014, 90, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.R.; Chakraborty, A.; Sinha, M.; Manna, K.; Mukherjee, D.; Chakraborty, A.; Bhattacharjee, S.; Dey, S. Modulatory Role of Quercetin against Gamma Radiation-Mediated Biochemical and Morphological Alterations of Red Blood Cells. Int. J. Radiat. Biol. 2013, 89, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Rohn, S.; Böhm, V.; Despotovic, M.; de Lera, A.R.; Krezel, W.; Kucuk, O.; Bánáti, D.; Rühl, R. Vitamin A5: Evidence, Definitions, Gaps, and Future Directions. Nutrients 2025, 17, 2317. [Google Scholar] [CrossRef] [PubMed]
- Lazdiņa, D.; Miķelsone, I.; Mišina, I.; Dukurs, K.; Benítez-González, A.M.; Stinco, C.M.; Meléndez-Martínez, A.J.; Górnaś, P. Species- and Age-Dependent Prenyllipid Accumulation in Hypericum Species’ Leaves. Plants 2025, 14, 2239. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Tsekeris, P.; Kyritsis, A.P.; Alexiou, G.A. Radiosensitization and Radioprotection by Curcumin in Glioblastoma and Other Cancers. Biomedicines 2022, 10, 312. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Thekkekkara, D.; Basavan, D.; Chandna, S.; Nanjan, M.J. A Combination of Resveratrol and 3,3′-Diindolylmethane, a Potent Radioprotector. Int. J. Radiat. Biol. 2018, 94, 558–568. [Google Scholar] [CrossRef]
- Vasudeva, V.; Tenkanidiyoor, Y.S.; Peter, A.J.; Shetty, J.; Lakshman, S.P.; Fernandes, R.; Patali, K.A. Radioprotective Efficacy of Lutein in Ameliorating Electron Beam Radiation-Induced Oxidative Injury in Swiss Albino Mice. Iran. J. Med. Sci. 2018, 43, 41. [Google Scholar]
- Küpper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.-M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide Accumulation Provides Kelp with an Inorganic Antioxidant Impacting Atmospheric Chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef]
- Alsabeelah, N.; Kumar, V. Formulation and Optimization of Quercetin Nanoemulsion for Enhancing Its Dissolution Rate, Bioavailability and Cardioprotective Activity. J. Clust. Sci. 2023, 34, 1893–1906. [Google Scholar] [CrossRef]
- Shakori Poshteh, S.; Alipour, S.; Varamini, P. Harnessing Curcumin and Nanotechnology for Enhanced Treatment of Breast Cancer Bone Metastasis. Discov. Nano 2024, 19, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-Based Nanoparticles: Advancements and Challenges in Tumor Therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Randomized, Double-Blind Pilot Study of Micronized Resveratrol (SRT501) in Patients with Hepatic Metastases—Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.P.; Wu, Y.M.; Liu, Y.; Tian, M.; Wang, J.D.; Ding, K.K.; Ma, T.; Zhou, P.K. IER5 Is Involved in DNA Double-Strand Breaks Repair in Association with PAPR1 in Hela Cells. Int. J. Med. Sci. 2017, 14, 1292–1300. [Google Scholar] [CrossRef]
- Ding, K.K.; Shang, Z.F.; Hao, C.; Xu, Q.Z.; Shen, J.J.; Yang, C.J.; Xie, Y.H.; Qiao, C.; Wang, Y.; Xu, L.L.; et al. Induced Expression of the IER5 Gene by γ-Ray Irradiation and Its Involvement in Cell Cycle Checkpoint Control and Survival. Radiat. Environ. Biophys. 2009, 48, 205–213. [Google Scholar] [CrossRef]
- Hosking, H.; Pederick, W.; Neilsen, P.; Fenning, A. Considerations for the Use of the DNA Damage Marker γ-H2AX in Disease Modeling, Detection, Diagnosis, and Prognosis. Aging Cancer 2024, 5, 62–69. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Kuttikrishnan, S.; Ahmad, N.; Habeeba, U.; Mariyam, Z.; Suleman, M.; Bhat, A.A.; Uddin, S. H2AX: A Key Player in DNA Damage Response and a Promising Target for Cancer Therapy. Biomed. Pharmacother. 2024, 175, 116663. [Google Scholar] [CrossRef]
- Aghelan, Z.; Bakhtiari, N.; Karami, F.; Ebrahimi, A.; Khodarahmi, R. Comparative Investigation of Antioxidant Effects in Different Binary Systems: Does Quercetin Stabilize Ascorbic Acid/Resveratrol in Their Binary Mixtures? J. Rep. Pharm. Sci. 2024, 13, e156437. [Google Scholar] [CrossRef]
- Marcu, I.; Radu, G.-L.; Dăncilă, A.M. Analysis of the Antioxidant Capacity of Whole-Fruit Tomato Powder Using the Ferric Reducing Antioxidant Power (FRAP) Assay—An Eco-Friendly Approach for the Valorization of Horticultural Products. Horticulturae 2025, 11, 1145. [Google Scholar] [CrossRef]
- Baranowska, M.; Koziara, Z.; Suliborska, K.; Chrzanowski, W.; Wormstone, M.; Namieśnik, J.; Bartoszek, A. Interactions between Polyphenolic Antioxidants Quercetin and Naringenin Dictate the Distinctive Redox-Related Chemical and Biological Behaviour of Their Mixtures. Sci. Rep. 2021, 11, 12282. [Google Scholar] [CrossRef]
- Debnath, I.; Ghosh, S.; Jha, S.K.; Bhunia, S.; Nayak, A.; Basak, S.; Nandi, S.; Bhattacharjee, S. Mechanistic Insights and Therapeutic Potential of Quercetin in Neuroprotection: A Comprehensive Review of Pathways and Clinical Perspectives. BIO Integr. 2025, 6, 978. [Google Scholar] [CrossRef]
- Rosiak, N.; Cielecka-Piontek, J.; Skibiński, R.; Lewandowska, K.; Bednarski, W.; Zalewski, P. Do Rutin and Quercetin Retain Their Structure and Radical Scavenging Activity after Exposure to Radiation? Molecules 2023, 28, 2713. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORAC(FL))) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Güçlü, K.; Kıbrıslıoğlu, G.; Özyürek, M.; Apak, R. Development of a Fluorescent Probe for Measurement of Peroxyl Radical Scavenging Activity in Biological Samples. J. Agric. Food Chem. 2014, 62, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Trh s Rajčaty v ČR. Available online: https://www.erstegroup.com/en/research/report/cz/SR439779 (accessed on 11 August 2025).
- Patil, S.L.; Swaroop, K.; Kakde, N.; Somashekarappa, H.M. In Vitro Protective Effect of Rutin and Quercetin against Radiation-Induced Genetic Damage in Human Lymphocytes. Indian J. Nucl. Med. 2017, 32, 289–295. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Y.; Yue, L.; Zhou, X.; Li, J.; Zhang, Y.; Yu, Z.; Liu, Y.; Xu, Y.; Wu, L.; et al. Quercetin Mitigates Radiation-Induced Intestinal Injury and Promotes Intestinal Regeneration via Nrf2-Mediated Antioxidant Pathway1. Radiat. Res. 2023, 199, 252–262. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kang, M.Y.; Lim, Y.K.; Kim, J.H.; Kim, J.K. Effects of Quercetin on Ionizing Radiation-Induced Cellular Responses in HepG2 Cells. Int. J. Radiat. Res. 2017, 15, 229–239. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic Application of Quercetin in Aging-Related Diseases: SIRT1 as a Potential Mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Gajowik, A.; Dobrzyńska, M.M. The Evaluation of Protective Effect of Lycopene against Genotoxic Influence of X-Irradiation in Human Blood Lymphocytes. Radiat. Environ. Biophys. 2017, 56, 413–422. [Google Scholar] [CrossRef]
- Qu, M.; He, Q.; Guo, B. Lycopene Protects against Ionizing Radiation—Induced Testicular Damage by Inhibition of Apoptosis and Mitochondrial Dysfunction. Food Sci. Nutr. 2023, 12, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Kostova, N.; Staynova, A.; Popova-Hadjiiska, L.; Georgieva, D.; Ivanova, I.; Aneva, N.; Atanasova, M.; Hristova, R. Effect of Curcumin on γ–Ray-Induced Cell Response. J. Radiat. Res. 2023, 64, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.S.; Abouelella, A.M.; Shahein, Y.E. Curcumin Protection Activities against γ-Rays-Induced Molecular and Biochemical Lesions. BMC Res. Notes 2013, 6, 375. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.M.; Yu, M.; Lee, K.-S.; Park, S.; Jin, Y.W.; Min, K.-J. Curcumin Mitigates Accelerated Aging after Irradiation in Drosophila by Reducing Oxidative Stress. BioMed Res. Int. 2015, 2015, 425380. [Google Scholar] [CrossRef]
- Koohian, F.; Shanei, A.; Shahbazi-Gahrouei, D.; Hejazi, S.H.; Moradi, M.-T. The Radioprotective Effect of Resveratrol Against Genotoxicity Induced by γ-Irradiation in Mice Blood Lymphocytes. Dose-Response 2017, 15, 1559325817705699. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, X.; Liang, X.; Liu, J.; Liu, J.; Han, Z.; Lu, Q.; Wang, K.; Meng, B.; Zhang, C.; et al. Resveratrol Rescues Cutaneous Radiation-Induced DNA Damage via a Novel AMPK/SIRT7/HMGB1 Regulatory Axis. Cell Death Dis. 2023, 13, 847. [Google Scholar] [CrossRef]
- Denissova, N.G.; Nasello, C.M.; Yeung, P.L.; Tischfield, J.A.; Brenneman, M.A. Resveratrol Protects Mouse Embryonic Stem Cells from Ionizing Radiation by Accelerating Recovery from DNA Strand Breakage. Carcinogenesis 2012, 33, 149–155. [Google Scholar] [CrossRef]
- Danihelová, M.; Veverka, M.; Šturdík, E.; Jantová, S. Antioxidant Action and Cytotoxicity on HeLa and NIH-3T3 Cells of New Quercetin Derivatives. Interdiscip. Toxicol. 2013, 6, 209–216. [Google Scholar] [CrossRef]
- Konopacka, M.; Widel, M.; Rzeszowska-Wolny, J. Modifying Effect of Vitamins C, E and Beta-Carotene against Gamma-Ray-Induced DNA Damage in Mouse Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 417, 85–94. [Google Scholar] [CrossRef]
- Fukui, M.; Choi, H.J.; Zhu Bao Ting, B.T. Mechanism for the Protective Effect of Resveratrol against Oxidative Stress-Induced Neuronal Death. Free Radic. Biol. Med. 2010, 49, 800–813. [Google Scholar] [CrossRef]
- Frede, K.; Ebert, F.; Kipp, A.P.; Schwerdtle, T.; Baldermann, S. Lutein Activates the Transcription Factor Nrf2 in Human Retinal Pigment Epithelial Cells. J. Agric. Food Chem. 2017, 65, 5944–5952. [Google Scholar] [CrossRef]
- Stefaniak–Vidarsson, M.M.; Gudjónsdóttir, M.; Marteinsdottir, G.; Omarsdottir, S.; Bravo, E.; Sigurjonsson, O.E.; Kristbergsson, K. Determination of Bioactive Properties of Food Grade Extracts from Icelandic Edible Brown Seaweed Sugar Kelp (Saccharina latissima) with in Vitro Human Cell Cultures (THP-1). Funct. Foods Health Dis. 2019, 9, 1–15. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotný, P.; Laknerová, I.; Jakubek, M.; Petrusová, J. Dietary Antioxidants Influence IER5 Activation and DNA Repair: Implications for Radioprotection and Healthy Aging. Antioxidants 2025, 14, 1357. https://doi.org/10.3390/antiox14111357
Novotný P, Laknerová I, Jakubek M, Petrusová J. Dietary Antioxidants Influence IER5 Activation and DNA Repair: Implications for Radioprotection and Healthy Aging. Antioxidants. 2025; 14(11):1357. https://doi.org/10.3390/antiox14111357
Chicago/Turabian StyleNovotný, Petr, Ivana Laknerová, Milan Jakubek, and Jana Petrusová. 2025. "Dietary Antioxidants Influence IER5 Activation and DNA Repair: Implications for Radioprotection and Healthy Aging" Antioxidants 14, no. 11: 1357. https://doi.org/10.3390/antiox14111357
APA StyleNovotný, P., Laknerová, I., Jakubek, M., & Petrusová, J. (2025). Dietary Antioxidants Influence IER5 Activation and DNA Repair: Implications for Radioprotection and Healthy Aging. Antioxidants, 14(11), 1357. https://doi.org/10.3390/antiox14111357







