Exploring the Potential of Olive Flounder Processing By-Products as a Source of Functional Ingredients for Muscle Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. General Composition Analysis of OFB
2.4. Cell Culture
2.5. MTT Assay, Glucose Uptake, and Cell Proliferation Measurement
2.6. ROS Production in C2C12 Myotube
2.7. Immunoblotting
2.8. Immunofluorescence Staining
2.9. Zebrafish Experiments
2.10. Statistical Analysis
3. Results
3.1. Screening of Nutritional Components and the Features of Enzymatic Hydrolysis Application in OFB
3.2. Determination of the Low-Molecularization Rate OFBEs
3.3. Evaluation of the Muscle Regenerative Potential of OFBEs Using C2C12 Myoblasts
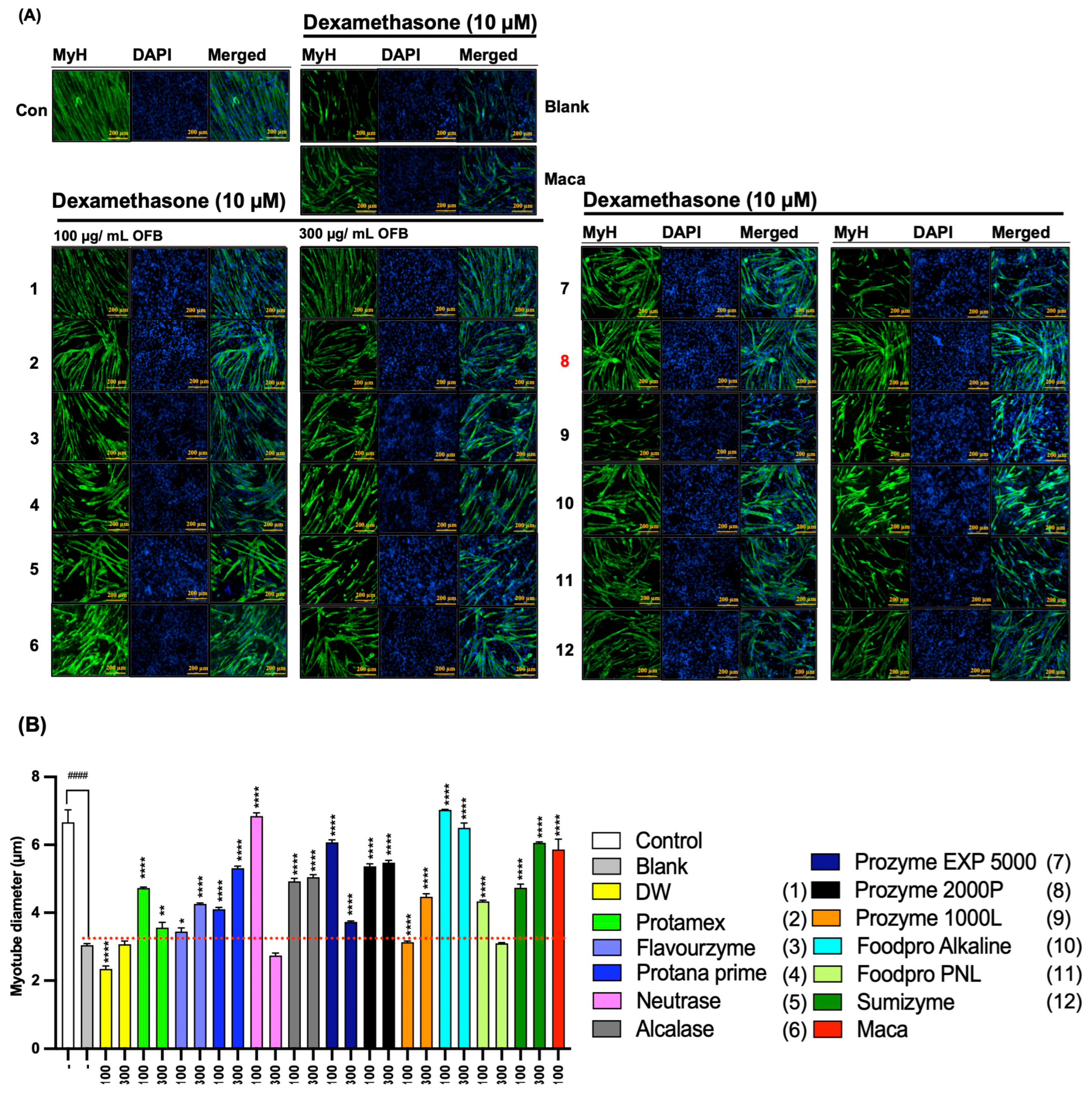
3.4. The Protective Effect of OFBEs in Dex-Induced Myotube Atrophy in C2C12 Cells
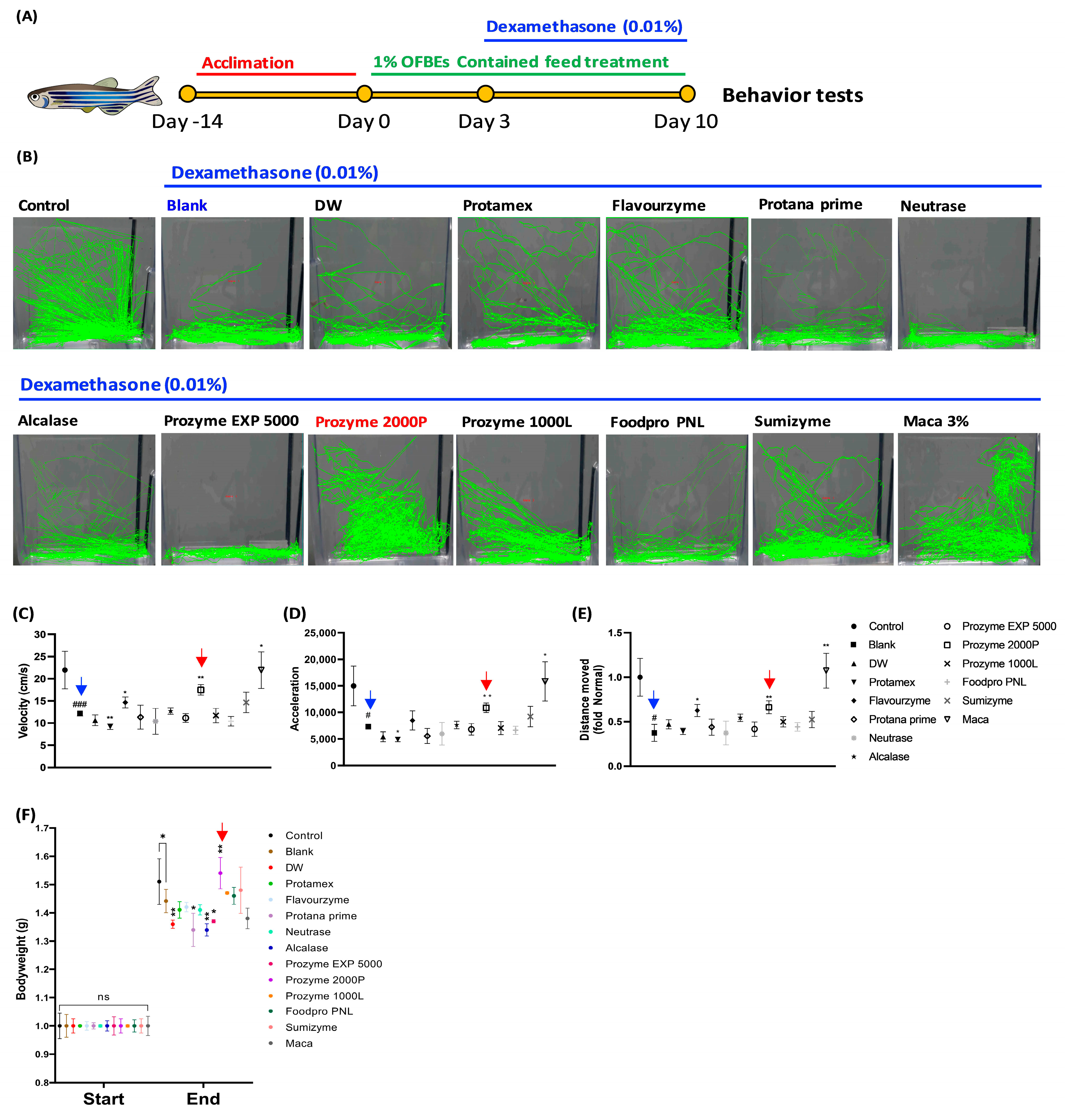
3.5. Restoration of Dex-Induced Muscle Atrophied Zebrafish by Treatment with OFBEs
3.6. Improvement of C2C12 Myoblast Differentiation by OFBP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maulu, S.; Nawanzi, K.; Abdel-Tawwab, M.; Khalil, H.S. Fish Nutritional Value as an Approach to Children’s Nutrition. Front. Nutr. 2021, 8, 780844. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Nam, J. The determinants of live fish consumption frequency in South Korea. Food Res. Int. 2019, 120, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.; Kim, K. Nutrition knowledge and eating behaviors of elementary school children in Seoul. Korean J. Community Nutr. 2009, 14, 55–66. [Google Scholar]
- Kim, K.H.; Moon, H.N.; Noh, Y.H.; Yeo, I.K. Influence of Osmolality and Acidity on Fertilized Eggs and Larvae of Olive Flounder (Paralichthys olivaceus). Dev. Reprod. 2020, 24, 19–30. [Google Scholar] [CrossRef]
- Jayawardhana, H.H.A.C.K.; Oh, J.Y.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Liyanage, N.M.; Nagahawatta, D.P.; Hyun, J.; Son, K.-T.; Jeon, Y.-J.; Park, J. Protective Effect of Fish Gut Hydrolysates from Olive Flounder (Paralichthys olivaceus) Surimi Byproducts Against AAPH-Induced Oxidative Stress in In Vitro and In Vivo Zebrafish Models. J. Aquat. Food Prod. Technol. 2022, 31, 924–938. [Google Scholar] [CrossRef]
- Dent, T.; Maleky, F. Pulse protein processing: The effect of processing choices and enzymatic hydrolysis on ingredient functionality. In Critical Reviews in Food Science and Nutrition; Taylor & Francis: Abingdon, UK, 2022; pp. 1–12. [Google Scholar]
- Morgan, P.T.; Breen, L. The role of protein hydrolysates for exercise-induced skeletal muscle recovery and adaptation: A current perspective. Nutr. Metab. 2021, 18, 44. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachex-Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Robinson, S.; Cooper, C.; Aihie Sayer, A. Nutrition and sarcopenia: A review of the evidence and implications for preventive strategies. J. Aging Res. 2012, 2012, 510801. [Google Scholar] [CrossRef]
- Phillips, S.M. A brief review of critical processes in exercise-induced muscular hypertrophy. Sport. Med. 2014, 44, 71–77. [Google Scholar] [CrossRef]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle full effect after oral protein: Time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Box, J.D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- Bradstreet, R.B. Kjeldahl Method for Organic Nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Noë, S.; Corvelyn, M.; Willems, S.; Costamagna, D.; Aerts, J.-M.; Van Campenhout, A.; Desloovere, K. The Myotube Analyzer: How to assess myogenic features in muscle stem cells. Skelet. Muscle 2022, 12, 12. [Google Scholar] [CrossRef]
- Park, G.-H.; Cho, J.-H.; Lee, D.; Kim, Y. Association between Seafood Intake and Cardiovascular Disease in South Korean Adults: A Community-Based Prospective Cohort Study. Nutrients 2022, 14, 4864. [Google Scholar] [CrossRef]
- Alhussain, M.H.; ALshammari, M.M. Association between fish consumption and muscle mass and function in middle-age and older adults. Front. Nutr. 2021, 8, 746880. [Google Scholar] [CrossRef]
- Gao, R.; Yang, Z.; Yan, W.; Du, W.; Zhou, Y.; Zhu, F. Protein intake from different sources and cognitive decline over 9 years in community-dwelling older adults. Front. Public Health 2022, 10, 1016016. [Google Scholar] [CrossRef]
- Irianto, H.; Dewi, A.; Giyatmi, G. Prospective Utilization of Fishery By-Products in Indonesia; Springer: New York, NY, USA, 2014; pp. 21–34. [Google Scholar]
- Gham, M.; Um, M.; Kye, S. Evaluation of dietary quality and nutritional status based on nutrition quotient and health functional food intake in the Korea elderly. J. Korean Soc. Food Cult. 2019, 34, 474–485. [Google Scholar]
- Farid, M.; Kodama, K.; Arato, T.; Okazaki, T.; Oda, T.; Ikeda, H.; Sengoku, S. Comparative study of functional food regulations in Japan and globally. Glob. J. Health Sci 2019, 11, 132. [Google Scholar] [CrossRef]
- van der Velden, V.H.; Hulsmann, A.R. Peptidases: Structure, function and modulation of peptide-mediated effects in the human lung. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 1999, 29, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, L.A.; Rudnicki, M.A. The molecular regulation of myogenesis. Clin. Genet. 2000, 57, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, K.M.; Binet, E.R.; Collao, N.; De Lisio, M. Nutritional Regulation of Muscle Stem Cells in Exercise and Disease: The Role of Protein and Amino Acid Dietary Supplementation. Front. Physiol. 2022, 13, 915390. [Google Scholar] [CrossRef]
- Arnold, H.H.; Braun, T. Targeted inactivation of myogenic factor genes reveals their role during mouse myogenesis: A review. Int. J. Dev. Biol. 1996, 40, 345–353. [Google Scholar]
- Dedieu, S.; Mazères, G.; Cottin, P.; Brustis, J.J. Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int. J. Dev. Biol. 2002, 46, 235–241. [Google Scholar]
- Yamauchi, Y.; Ferdousi, F.; Fukumitsu, S.; Isoda, H. Maslinic Acid Attenuates Denervation-Induced Loss of Skeletal Muscle Mass and Strength. Nutrients 2021, 13, 2950. [Google Scholar] [CrossRef]
- Yi, D.; Yoshikawa, M.; Sugimoto, T.; Tomoo, K.; Okada, Y.; Hashimoto, T. Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells. Int. J. Mol. Sci. 2022, 23, 6825. [Google Scholar] [CrossRef]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S.J.E.o.o.d.s. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Fappi, A.; Neves, J.C.; Sanches, L.N.; Massaroto, E.S.P.V.; Sikusawa, G.Y.; Brandão, T.P.C.; Chadi, G.; Zanoteli, E. Skeletal Muscle Response to Deflazacort, Dexamethasone and Methylprednisolone. Cells 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.-H.; Hsiao, A.W.-T.; Wong, N.; Chen, Y.-F.; Lee, C.-W.; Lee, W.Y.W. Is dexamethasone-induced muscle atrophy an alternative model for naturally aged sarcopenia model? J. Orthop. Transl. 2023, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Je, J.G.; Jeon, Y.J.; Yang, H.W. Zebrafish Model for Studying Dexamethasone-Induced Muscle Atrophy and Preventive Effect of Maca (Lepidium meyenii). Cells 2021, 10, 2879. [Google Scholar] [CrossRef] [PubMed]
- Hulmi, J.J.; Lockwood, C.M.; Stout, J.R. Effect of protein/essential amino acids and resistance training on skeletal muscle hypertrophy: A case for whey protein. Nutr. Metab. 2010, 7, 51. [Google Scholar] [CrossRef]
- Phillips, S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr. Metab. 2016, 13, 64. [Google Scholar] [CrossRef]
- Gusso, D.; Altenhofen, S.; Fritsch, P.M.; Rübensam, G.; Bonan, C.D. Oxytetracycline induces anxiety-like behavior in adult zebrafish. Toxicol. Appl. Pharmacol. 2021, 426, 115616. [Google Scholar] [CrossRef]
- Chang, K.-V.; Hsu, T.-H.; Wu, W.-T.; Huang, K.-C.; Han, D.-S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017, 46, 738–746. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, S.; Dong, W.; Huang, S.; Jiao, Z.; Hu, Z.; Dai, S.; Yi, Y.; Gong, X.; Li, K.; et al. Prenatal dexamethasone exposure induces anxiety- and depressive-like behavior of male offspring rats through intrauterine programming of the activation of NRG1-ErbB4 signaling in hippocampal PV interneurons. Cell Biol. Toxicol. 2021, 39, 657–678. [Google Scholar] [CrossRef]
- Chen, C.; Yang, J.S.; Lu, C.C.; Chiu, Y.J.; Chen, H.C.; Chung, M.I.; Wu, Y.T.; Chen, F.A. Effect of Quercetin on Dexamethasone-Induced C2C12 Skeletal Muscle Cell Injury. Molecules 2020, 25, 3267. [Google Scholar] [CrossRef]
- Distefano, G.; Standley, R.A.; Zhang, X.; Carnero, E.A.; Yi, F.; Cornnell, H.H.; Coen, P.M. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J. Cachexia Sarcopenia Muscle 2018, 9, 279–294. [Google Scholar] [CrossRef]
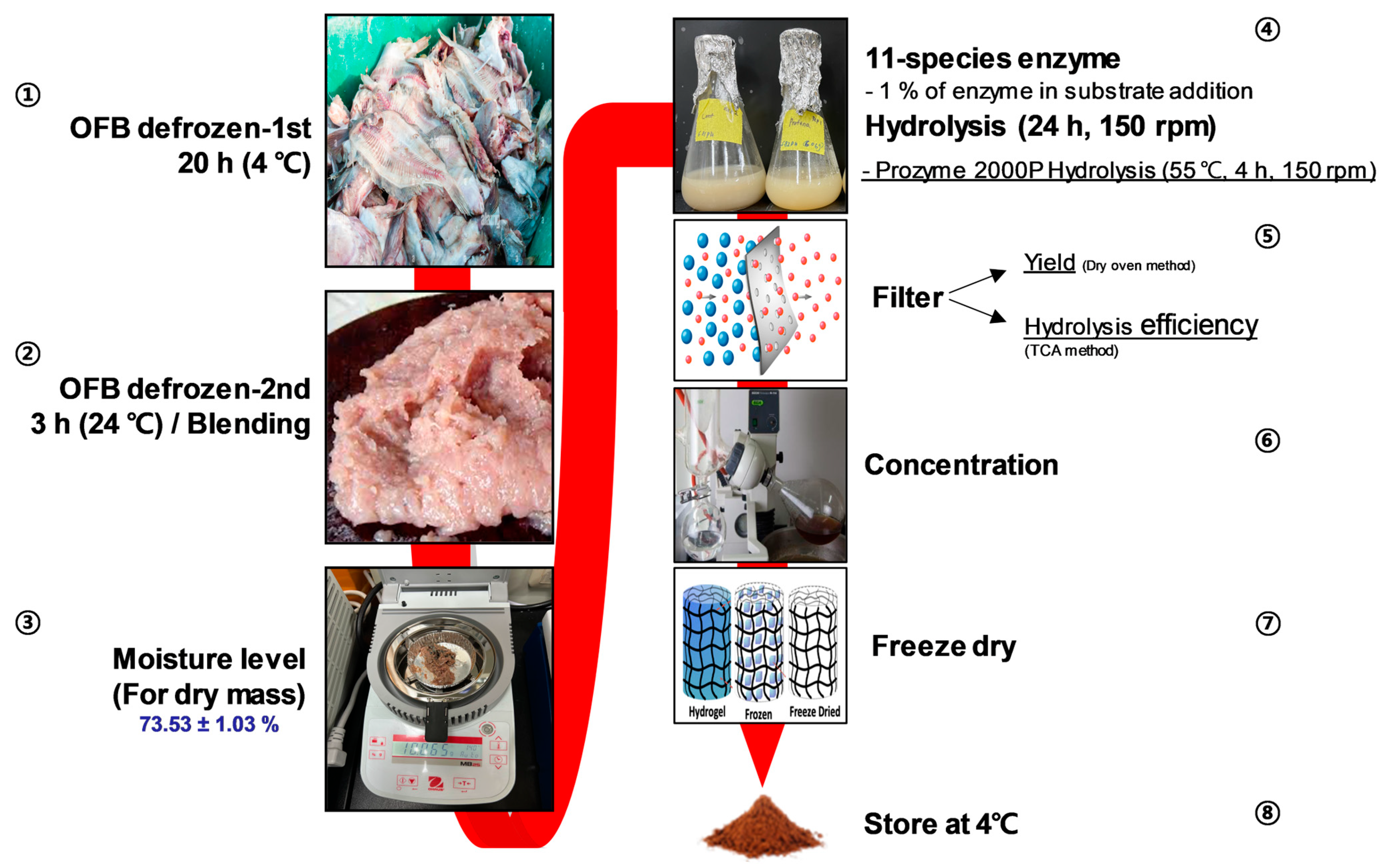
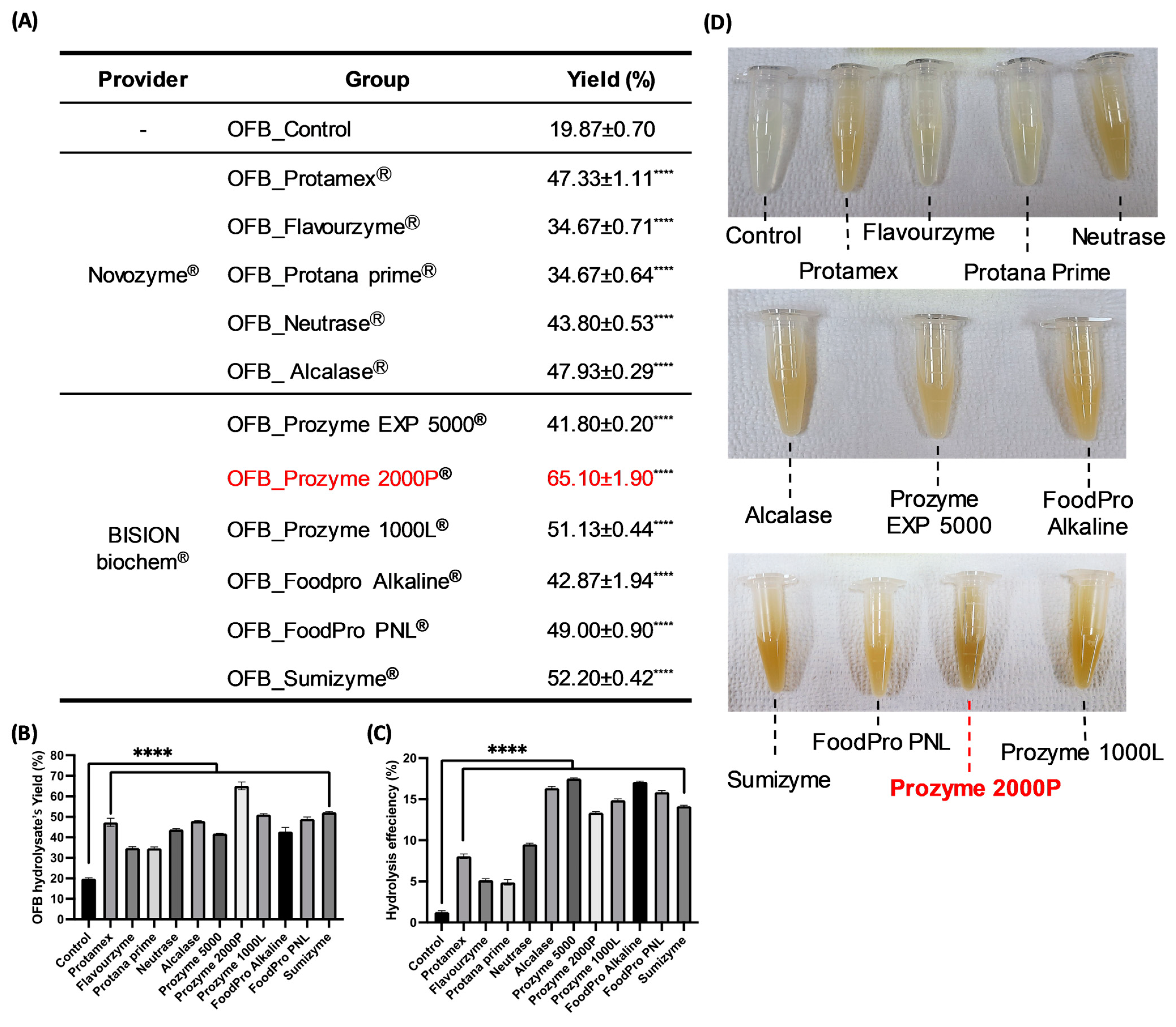



| Sample | Moisture (%) | Carbohydrate (%) | Protein (%) | Lipid (%) | Ash (%) |
|---|---|---|---|---|---|
| OFB (Bone, Skin, Head) | 73.53 ± 1.03 | 3.98 ± 1.01 | 56.51 ± 1.16 | 20.37 ± 0.32 | 18.13 ± 1.65 |
| No. | Protein Hydrolysis Enzyme | Available Strengths (Range) | Hydrolysis Action | Generation of Peptides or Single Amino Acids | Working pH Range | Working Temperature Range (°C) | Quality Grade |
|---|---|---|---|---|---|---|---|
| 1 | Protamex® | 1.5 AU-A/g | Endo-peptidase | Peptides | 6.0–9.0 | 30–65 | Food |
| 2 | Flavourzyme® | 500–100 LAPU/g | Endo-/Exo-peptidase blend | Peptides and Amino acids | 4.0–8.0 | 30–65 | Food |
| 3 | Protana® Prime | 1067 LAPU/g 979 CPDU(A)/g | Leucyl Exo-peptidase | Small peptides and amino acids | 3.0–7.0 | 20–55 | Food |
| 4 | Neutrase® | 0.8–1.5 AU-N/g | Endo-peptidase | Peptides | 6.0–9.0 | 30–35 | Food |
| 5 | Alcalase® | 2.4–4.0 AU-A/g | Serine endo-peptidase | Peptides | 6.5–10.0 | 60–75 | Food |
| 6 | Prozyme EXP 5000® | 5000 LAPU/g | Leucyl exo-peptidase | Small peptides and amino acids | 6.0–12.0 | 55–75 | Food |
| 7 | Prozyme 2000P® | 2000 LAPU/g | Leucyl exo-peptidase | Small peptides and amino acids | 6.0–9.0 | 50–60 | Food |
| 8 | Prozyme 1000L® | 200,000 PC/g | Endo-peptidase | Peptides | 7.0–8.0 | 50–55 | Food |
| 9 | FoodPro PNL® | 1600 AZO/g | Endo-peptidase | Peptides | 6.0–8.0 | 50–55 | Food |
| 10 | FoodPro Alkaline® | 580,000 DU/g | Alkaline Serine Endo-peptidase | Peptides | 8.5–9.0 | 60–70 | Food |
| 11 | Sumizyme DPP-G® | 100 U/g | Dipeptidyl-aminopeptidase | Peptides | 7.0–7.5 | 55–65 | Food |
| Provider | Samples | General Composition (%) | |||
|---|---|---|---|---|---|
| Carbohydrate | Protein | Lipid | Ash | ||
| - | OFB_Control (DW) | 0.66 ± 0.11 | 93.83 ± 2.84 | 0.69 ± 2.37 | 4.82 ± 0.40 |
| Novozyme® | OFB_Protamex® | 7.27 ± 0.25 **** | 63.56 ± 0.95 ** | 25.64 ± 0.98 * | 3.53 ± 0.19 * |
| OFB_Flavourzyme® | 7.43 ± 0.42 **** | 56.00 ± 0.95 ** | 32.82 ± 1.07 ** | 3.75 ± 0.15 * | |
| OFB_Protana prime® | 12.72 ± 0.19 **** | 47.48 ± 1.89 ** | 36.33 ± 1.57 ** | 3.47 ± 0.14 * | |
| OFB_Neutrase® | 3.26 ± 0.50 ** | 65.46 ± 2.84 * | 28.37 ± 3.10 * | 2.91 ± 1.05 | |
| OFB_Alcalase® | 11.60 ± 0.31 **** | 68.29 ± 1.89 * | 14.56 ± 2.15 * | 5.55 ± 0.84 | |
| BISION-Biochem® | OFB_Prozyme 5000P® | 8.15 ± 0.00 **** | 77.75 ± 0.00 * | 9.56 ± 0.42 | 4.54 ± 0.59 |
| OFB_Prozyme 2000P® | 4.20 ± 0.19 **** | 85.72 ± 6.62 | 6.38 ± 4.99 | 3.70 ± 0.25 | |
| OFB_Prozyme 1000L® | 9.78 ± 0.15 **** | 57.89 ± 0.95 ** | 29.25 ± 0.82 ** | 3.08 ± 0.06 ** | |
| OFB_Foodpro Alkaline® | 9.03 ± 0.13 **** | 62.62 ± 1.89 * | 24.76 ± 1.51 * | 3.59 ± 0.11 * | |
| OFB_FoodPro PNL® | 0.63 ± 0.13 | 62.62 ± 7.57 | 33.61 ± 5.54 * | 3.14 ± 0.14 ** | |
| OFB_Sumizyme® | 1.67 ± 0.08 *** | 58.83 ± 1.89 ** | 36.53 ± 1.54 ** | 2.97 ± 0.21 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, J.; Kang, S.-I.; Lee, S.-W.; Amarasiri, R.P.G.S.K.; Nagahawatta, D.P.; Roh, Y.; Wang, L.; Ryu, B.; Jeon, Y.-J. Exploring the Potential of Olive Flounder Processing By-Products as a Source of Functional Ingredients for Muscle Enhancement. Antioxidants 2023, 12, 1755. https://doi.org/10.3390/antiox12091755
Hyun J, Kang S-I, Lee S-W, Amarasiri RPGSK, Nagahawatta DP, Roh Y, Wang L, Ryu B, Jeon Y-J. Exploring the Potential of Olive Flounder Processing By-Products as a Source of Functional Ingredients for Muscle Enhancement. Antioxidants. 2023; 12(9):1755. https://doi.org/10.3390/antiox12091755
Chicago/Turabian StyleHyun, Jimin, Sang-In Kang, Sang-Woon Lee, R. P. G. S. K. Amarasiri, D. P. Nagahawatta, Yujin Roh, Lei Wang, Bomi Ryu, and You-Jin Jeon. 2023. "Exploring the Potential of Olive Flounder Processing By-Products as a Source of Functional Ingredients for Muscle Enhancement" Antioxidants 12, no. 9: 1755. https://doi.org/10.3390/antiox12091755
APA StyleHyun, J., Kang, S.-I., Lee, S.-W., Amarasiri, R. P. G. S. K., Nagahawatta, D. P., Roh, Y., Wang, L., Ryu, B., & Jeon, Y.-J. (2023). Exploring the Potential of Olive Flounder Processing By-Products as a Source of Functional Ingredients for Muscle Enhancement. Antioxidants, 12(9), 1755. https://doi.org/10.3390/antiox12091755







