Abstract
Age-related diseases represent the largest threat to public health. Aging is a degenerative, systemic, multifactorial and progressive process, coupled with progressive loss of function and eventually leading to high mortality rates. Excessive levels of both pro- and anti-oxidant species qualify as oxidative stress (OS) and result in damage to molecules and cells. OS plays a crucial role in the development of age-related diseases. In fact, damage due to oxidation depends strongly on the inherited or acquired defects of the redox-mediated enzymes. Molecular hydrogen (H2) has recently been reported to function as an anti-oxidant and anti-inflammatory agent for the treatment of several oxidative stress and aging-related diseases, including Alzheimer’s, Parkinson’s, cancer and osteoporosis. Additionally, H2 promotes healthy aging, increases the number of good germs in the intestine that produce more intestinal hydrogen and reduces oxidative stress through its anti-oxidant and anti-inflammatory activities. This review focuses on the therapeutic role of H2 in the treatment of neurological diseases. This review manuscript would be useful in knowing the role of H2 in the redox mechanisms for promoting healthful longevity.
1. Introduction
Almost all major human diseases, including atherosclerosis, cancer, cardiovascular disease, metabolic syndrome, dementia, hypertension, and other neurodegenerative diseases have aging, a biosocial concern, as their underlying basis. To help older people maintain their health for as long as possible and to deal with an ever-increasing population, it is essential for healthcare providers to improve the prevention and control of age-related disorders. Diet is a useful and reasonably priced approach to helping seniors live longer and healthier lives [1]. Aging is seen mainly in protected settings as an evolving phenomenon that enables longevity in the wild beyond the normal lifespan. Aging is characterized by the accumulation of nucleic acids, proteins and lipids formed as a result of molecular damage. The free-radical rationale of aging has long been established among the theories explaining the aging process [1]. Aging occurs when several defense mechanisms fail to respond to the damage caused by reactive oxygen species (ROS), particularly in the mitochondria [2]. The key causes of aging-induced damages are the ineffectiveness and inability of the maintenance, repair, and turnover pathways [3]. Aging is related to the propensity for adverse water balance and makes older subjects more vulnerable to dehydration [4]. Water accounts for approximately half the weight of the human body and is necessary for human life and health [5,6]. There is growing evidence that even mild dehydration (determined to be 1–2% body mass loss due to fluid deficit) may lead to various age-related diseases, including arthritis, cataracts, osteoporosis, type 2 diabetes (T2D), hypertension and Alzheimer’s disease (AD) [7]. The type of water supplied as drinking water plays an important role in determining the safety and health issues because tap water quality continues to cause public concern [8,9], with some countries demanding the derogation from European water quality standards [10]. Groundwater is the Earth’s most abundant and important freshwater resource [11]. Although dietary food components have been shown to improve cognitive function in older people [2], the effects of different nutritional compounds on other biomarkers of aging are much less understood. A dietary supplement, which has a direct impact on telomere metabolism, slows telomere decline and reduces aging and might expand life and improve health [3]. Nowadays, molecular hydrogen (H2) can be used safely in the air at body temperature and at concentrations less than 4.7%. H2 selectively quenches toxic ROS and has an anti-apoptotic, anti-oxidant, anti-inflammatory and anti-allergic impact, and it has become a new non-oxidant [12]. H2 has recently been studied in preclinical and clinical studies under various conditions linked to oxidative and inflammatory stress, such as heart failure due to radiation, ischemia-reperfusion (I/R), myocardial infarction, brain infarction, heart storage and heart transplants [13]. Hydrogen-rich water (HRW) has recently come to light as a novel dietary beverage that may improve several aging-related characteristics in interventional trials, reducing different inflammatory responses that may help prevent programmed cell death [4], improve nutrient metabolism, repress wrinkle formation and increase other physiological activities [5]. Japanese centenarians were discovered to have higher levels of H2 gas, which indicated that the intestinal production of H2 gas may have conferred upon them longevity and reduced oxidative stress [6,7]. Increase in longevity was also reported by another study, stating intestinal production of H2 gas as the apparent reason [8]. Cardiovascular and oncological disorders, the primary cause of morbidity and mortality worldwide, are more than 93% [14,15]. Pathological disorders, such as cardiac fibrosis, liver injury, neuronal diseases, and diabetes, causally involving free radicals have been investigated for the protective effects of H2 [13]. Ischemia and subsequent heart reperfusion are other disorders in which a large number of tissue-damaging free radicals are formed [16]. One study revealed that drinking HRW for 6 months favorably affected different age-related features, including general pain, telomere strength and brain metabolism—indices that helped to increase anti-oxidant activity; HRW also enhanced sleep quality [9]. It has been reported that increased H2 gas generation in the intestine depends on the presence of undigested carbohydrates and hydrogen-producing bacteria that are affected by some environmental conditions [10,11]. Over a thousand peer-reviewed study papers have been published thus far, demonstrating the wide-ranging interest in H2 biomedical research.
In this review, we highlight the emerging role of H2 in the prevention of age-related diseases, Alzheimer’s, Parkinson’s, cancer and osteoporosis, etc. This review manuscript would be useful in knowing the role of H2 in the redox mechanisms for promoting healthful longevity.
2. The Mechanism of Action of H2
H2 suppresses the allergic [12] and inflammatory signaling pathways [13]. The anti-oxidative stress effect of H2 was initially thought to be conferred upon by the direct elimination of hydroxyl radicals and peroxynitrite. Subsequent studies reported that H2 activated the system nuclear factor erythroid 2–related factor 2 (Nrf2) [14,15] and its downstream heme oxygenase-1 (HO-1) [16]. Kawamura et al. (2013) suggested that H2 in Nrf2-knockout mice did not relieve hyperoxic lung injury [17]. In addition, at the Medical H2 Symposia in 2012 and 2013, Ohsawa et al. (2012) stated that H2 enhanced mitochondrial functions and induced Nrf2 nuclear translocation. Furthermore, Matsumoto et al. reported that oral H2 water intake increased ghrelin gastric expression and secretion and that the ghrelin receptor-antagonist and ghrelin secretion-antagonist abolished the neuroprotective effects of H2 water [18]. At the 5th Medical H2 Symposium in Nagoya, Japan, in 2015, Ohta et al. demonstrated that H2 affects the free radical chain reaction of unsaturated fatty acids on the cell membrane and modifies the lipid peroxidation process [12]. This irregular oxidation of the phospholipids at low levels of H2 (at least 1.3%) has also been reported indicating that the biological effects of H2 can be explained by the aberrant oxidation of the phospholipids upon exposure to H2. Among the many molecules altered by H2, most molecules were predicted to be “passengers” (downstream regulators), secondarily modulated by the pilot (master regulator) [12]. Proving the effect of H2 in an in vitro setting would be the best way to classify the master regulator. While nothing is known about the lipid peroxidation analysis, the second master control body for H2, next to the radical scavenging effect, may be the free chain response to lipid peroxidation [12]. Intracellular signal transduction systems are modulated by H2, and the downstream gene expression is regulated to alleviate disease processes. Moreover, biologically active substances, which modulate signaling molecules, damage our bodies [19]. Inhaling a gas mixture containing H2 (less than 4%) is effective in protecting against acute oxidative stress, according to one research study [20]. Another research study showed that it is safe and more practical to dissolve H2 in water up to 0.8 mM under atmospheric pressure at room temperature [21]. H2 also mitigates surgery-induced cognitive impairment [22]. Following 4% H2 inhalation, the liver’s H2 concentration rose quickly and reached balance in about 5 min at 20 mol/L. HRW consumption resulted in sporadic availability to H2. One study revealed that, even after 8 h, supersaturated H2 in HRW (1000 mol/L) was maintained at a high content and was still above 600 mol/L [23].
Remarkably, the effects of saturated HW were virtually identical to those of H2 concentrations as low as 0.08 ppm. (1.5 ppmH2). Within 30 min of consuming HW, the majority of H2 in the blood is invisible [24]. Another example would be that the amount of H2 exposed to a 60-kg individual for 24 h as a 2% gas would be at least 104 times greater than what would be consumed by drinking saturated HW. However, HW is sometimes even more efficient than H2 at achieving its goals [25]. Drinking H2-rich water reduced fatigue in healthy people, according to one research [26]. Additionally, blood flow-dependent vasodilatory responses in people were enhanced by H2-rich water [27]. In radiotherapy patients with liver cancer, it helped appetite and taste issues and reduced oxidative stress in the blood [28]. It was reported that H2-rich water improved cognitive impairment [29]. In addition, drinking H2-rich water improved neuropsychiatric and endocrine metabolic disorders in vivo study [29].
3. The Anti-Oxidative Effects of H2 That Extend Life Span
Although H2 has long been assumed to be an inert gas for living organisms, an animal study found that owing to its anti-oxidant properties, inhalation of H2 gas reduced oxidative stress and stifled the brain damage caused by I/R injury. Among several proposed biological activities, the function of H2 as an anti-oxidant has received the greatest attention. Furthermore, even after elimination of H2 from the body, especially at low concentrations, its biological and anti-oxidant benefits continue to exist, implying that the mechanism may include modulation of anti-oxidant signaling rather than actual free radical scavenging [24]. H2 is a specific scavenger of hydroxyl radical and peroxynitrite, powerful oxidants that interact without distinction with nucleic acids, lipids and proteins leading to DNA breakage, lipid peroxidation and protein inactivation [25,26]. In both human diseases and rodent models, H2 administration reduces the expression of various oxidative stress markers, such as myeloperoxidase, malondialdehyde (MDA) and 8-hydroxy-desoxyguanosine (8-OHdG) [27,28]. In addition, H2 can also minimize myeloperoxidase expression [29], decrease the function of mitochondrial oxidoreductase and stabilize the mitochondrial membrane potential to reduce the tissue damage caused by oxidative stress [30]. In 2016, researchers proposed that H2 may reduce the ROS content depending on the endogenous glutathione peroxidase in Ganoderma lucidum [31]. Another study demonstrated that HRW intake affected different aging-related characteristics in aged people, such as extension of telomeres and improvement in DNA methylation [9]. Several studies have shown that H2 is not cytotoxic even at high concentrations [32,33]. H2-water consumption reduced the development of oxidative stress and avoided cognitive decline; therefore, it can play a role in extending the life span [34]. In rats, H2-water stopped the onset and spread of nigrostriatal degeneration [35]. Numerous studies have shown that H2 reduces apoptosis during the treatment of septic injury in rodents [36,37]. Many studies have demonstrated that H2 reduces ROS, increases anti-oxidant enzyme activity and inhibits pro-oxidant enzyme activity to mitigate the tissue damage caused by lipopolysaccharides [38].
4. The Anti-Inflammatory Effects of H2
A study reported that H2 breathing capacity could cure liver inflammation caused by parasites and was the first to demonstrate the anti-inflammatory properties of H2 [39]. Hydrogen has been shown to exhibit anti-inflammatory activity in multiple injury models. H2 is known to prevent the oxidative stress-induced inflammatory tissue damage by downregulating pro-inflammatory and inflammatory cytokines [40], such as interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α) [41]. H2 can also drastically decrease NF-kB expression post-liver damage [42]. In animal models of allergic rhinitis or I/R cerebral injury, H2 also has anti-inflammatory effects via upregulation of regulatory T cells (Tregs), which have an immunosuppressive effect and reduction in the expression of NF-kB [43]. Similarly, a study found that increasing the expression of the heat stress protein Hsp60, which is stimulated at high temperatures to protect itself, may successfully prevent acute pancreatitis in mice in the early stages through pre-inhalation of H2 [44].
5. H2 and Redox Mechanism of Oxidative Stress
The anti-oxidant effects of H2 are primarily expressed in certain ways. First, H2 was discovered to specifically eliminate hydroxyl radicals and peroxynitrite. H2 can readily penetrate biofilms compared to standard anti-oxidants, such as superoxide dismutase (SOD), catalase and alpha-tocopherol, and does not influence the usual metabolic redox reaction, owing to its small molecular weight and anti-oxidant activity, which selectively affects only the strongest oxidant [45]. H2 can also directly downregulate ROS or act as a gas-mediated signal regulator. Recently, a study [46] showed that H2 in the urine, a marker of oxidative stress, can increase quickly and approximately to the same level as that induced by exercise. During cell adaptation, the production of exercise-induced ROS is necessary, and short-term ROS exposure can protect neurons from oxidative stress [47]. H2 can mediate beneficial effects of the mitohormetic effectors of hormone processes on the body [46]. However, the anti-oxidative mechanism of H2 may affect the free radical chain reaction of lipid peroxidation. Many studies have shown that H2 protects cells by preventing the peroxidation of lipids and fatty acids [48]. According to wear-and-tear theory, aging is the slow deterioration of the body’s cells and tissues due to oxidative stress, radiation exposure, exposure to toxins or other deteriorating processes [49]. Denham Harman [50] introduced the free radical theory of aging [51] in the 1950s. Numerous studies have shown that oxidative damage and ROS levels rise with aging [52], that reducing oxidative damage increases lifespan in a variety of model organisms (such as yeast, nematodes, fruit flies, mice, etc.) and that both higher ROS production and oxidative damage have detrimental effects on lifespan [53]. In addition, H2 can reduce myeloperoxidase expression [46], decrease mitochondrial oxidoreductase activity [54] and stabilize mitochondrial membrane potential [55], thus enhancing tissue damage resulting from oxidative stress. The protective mechanism of H2 in treating different age-related diseases is shown in Figure 1.
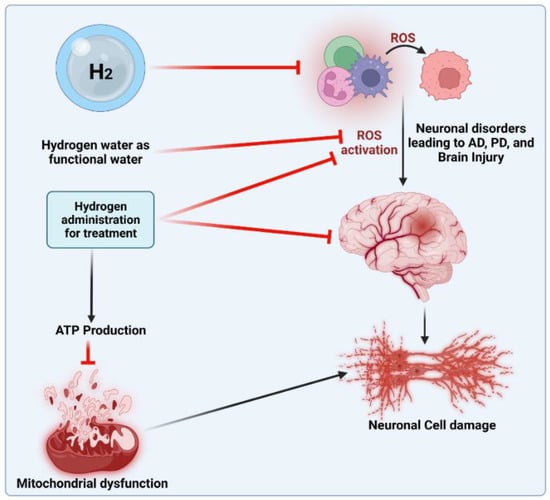
Figure 1.
Protective effects of H2 in treating different age-related diseases.
6. Age-Related Diseases and Redox Mechanisms
Over the last few decades, several models have been suggested to define the relationships and biopathways of aging [56]. The generally accepted theory is the “oxidative stress hypothesis”, which advances and improves upon the free radical aging theory [57,58]. The oxidative stress theory underlines the crucial role of anti-oxidant defenders in the overall redox balance [59]. Ito et al. (2011) performed an open-label H2-water analysis (1.0 L/day) for 12 weeks in 14 patients with muscle disorders, including muscular dystrophy and mitochondrial myopathies. This open-label research showed significant improvements in the lactate: pyruvate ratio, fasting blood glucose, serum matrix metalloproteinase-3 (MMP3) and triglyceride levels [60,61]. In mitochondrial myopathies, the lactate: pyruvate ratio, a responsive biomarker of a weakened mitochondrial electron transportation system, decreased by 28%. In addition, MMP3, the marker of inflammation, decreased by 27% in dermatomyositis. Then, for eight weeks, 22 people with dermatomyositis and mitochondrial myopathies were recruited for a randomized double-blind, placebo-controlled, crossover H2-water or placebo dehydrogenated water (0.5 L/day) examination [60]. H2 may provide an interpretation of multiple energy booster advantages seen in H2 intervention studies that are not due to H2 but which do not control the growth hormone secretagogue receptor (GHS-R1α) in tissues rich in mitochondria (including breast, skeletal muscle, myocardium, testis or liver) [62]. Mitochondria are the most important organelles responsible for energy production via oxidative phosphorylation, which is essential for cellular behavior and adenosine triphosphate (ATP) generation [63]. The formation and oxidation of ROS occurs under normal, healthy conditions in a regulated manner. Changes in the redox state and immune system dysregulation may result in increased systems inflammatory status during aging. Both processes induce inflammatory mediators to stimulate redox imbalances through oxidative stress [64]. The net effects of poor protection by anti-oxidant systems and aggression by reactive species, such as superoxide, hydroxyl radicals, peroxynitrite and H2 peroxide, are most likely to cause age-related redox imbalances [65,66]. Functional shifts may be seen as pathophysiological connections to degenerative disorders correlated with age and unresolved chronic inflammation throughout aging [67]. The functional activities of certain proteins require certain prosthetic groups to be covalently connected to the polypeptide chain. These normally involve the conversion of inactive apoproteins into enzymes through complex organic molecules that, for example, engage in protein activity. In addition, some of the posttranslational changes influence biochemical processes through different enzyme operations [68]. To improve homeostatic cell operation, the preservation of a healthy redox balance is essential for a physiological acid-base buffer system in the body. Modernization of the redox balance would greatly impact transcription and mobile signal pathways because most activations and reactions rely on reduction/oxidation processes [55]. Figure 2 shows the effects of oxidative stress and the associations between aging and age-related diseases [56].
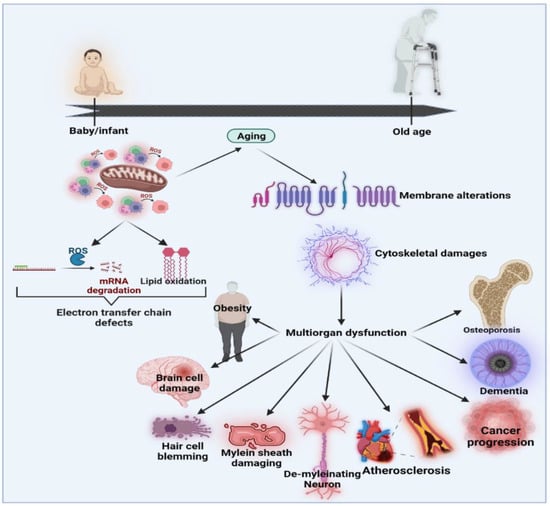
Figure 2.
Effects of oxidative stress and the associations between aging and age-related diseases modified from [56].
7. Effects of Hydrogen Gas on Inflammatory Cytokines
Inflammatory cytokines affect a number of signals, which mediate an innate immune response and can aid dysregulation in many diseases, including cancer [69,70]. Common inflammatory cytokines include white blood cell-secreted ILs and macrophage-secreted TNFs, both of which have been closely correlated with cancer initiation and progression [71,72]; both ILs and TNFs can be blocked by H2 gas [73]. In cancer patients, chemotherapy-induced inflammation not only causes adverse events [74,75], it also promotes cancer metastasis and treatment failure [76]. By regulating inflammation, H2 gas may prevent tumor development, progression and decrease the side effects of chemotherapy and radiation therapy [73].
8. Hydrogen Gas Relieves Adverse Effects of Chemotherapy
The leading methods for treating cancer are chemotherapy and radiotherapy [77,78]. However, cancer patients are frequently fatigued, and their quality of life is compromised [79,80]. During cancer, the generation of ROS skyrockets and contributes to adverse outcomes, which lead to severe oxidative stress and inflammation [81]. Therefore, H2 gas, on account of its anti-oxidant, anti-inflammatory and other cell-defensive characteristics, can be used to suppress these adverse effects. Doxorubicin, a fatal dilated cardiomyopathy and hepatotoxicity causing antibiotic, is also an important cancer antibiotic used in the treatment of various cancers [82,83]. An in vivo study showed that intraperitoneal injections of saline rich in H2 decreased mortality and doxorubicin-led cardiovascular dysfunction. H2 rich water has also been shown to exert renal protective effect against cisplatin-induced nephrotoxicity in rats. Treatment with hydrogen rich water can significantly reverse the toxic effects, and it demonstrated a significantly higher rate of cross-relation by the removal of oxygen radicals [84,85]. In another study, the inhalation of H2 gas (1% hydrogen in air) and the use of water rich in hydrogen (0.8 mM Hydrogen in water) reversed the body-weight loss and the mortality caused by cisplatin due to the antioxidant property of H2 [73]. Similar findings were also reported by Meng et al. (2015), who showed that hydrogen-rich saline could mitigate follicle-stimulated hormone release, increase estrogen levels, improve follicle growth and reduce cisplatin-induced ovarian cortex damage [86]. In a previous study, cisplatin induced higher oxidation levels during therapy and suppressed the activity of antioxidant enzymes. In another study, a six-week intake of H2 rich water in patients with malignant liver tumors minimized reactive oxygen metabolites and increased antioxidant activity [87]. Remarkably, the quality of life during radiotherapy was found to be greatly improved in the H2-rich water consuming group in comparison to the placebo groups. Both groups showed similar tumor reactions to radiation therapy, indicating that the ingestion of water rich in H2 decreased the oxidative stress due to radiation without undermining the antitumor effect of radiation therapy [87]. The various routes of administration, application and mechanisms of action of H2 molecules in cancer treatment are listed in Table 1.

Table 1.
Various routes of administration, application and mechanisms of action of H2 in cancer treatment.
9. Hydrogen and Intestinal Microbiome
In recent years, gut microbiota brain axis (GMBA) has been recommended as an important therapeutic target for neurological disorders affecting the central nervous system, such as AD [97,98]. Several mechanisms play a key role in preventing bacterial overgrowth in the proximal gut, including migrating motor complex, gastric acid, gut immune system and biliary secretions [99]. During fermentation, H2 is produced in the large intestine; this may be excreted through the breath and flatus or metabolized by the flora [100]. Moreover, the proportion of H2 excreted in the breath varies depending on its production rate. In addition, the fermentation of lactulose generated more H2 than that generated by resistant starch or pectin. HRW is a promising functional drink with positive effects on human health. Over the past decade, the publication of approximately 150 papers related to HRW in human trials, have shown multiple advantageous effects of HRW consumption [101]. According to a study, H2 delivered by HRW could affect the gut microbiota, a community of 100 trillion microbial cells that can enhance human metabolism, immune function, nutrition and other physiological activities [102]. A Chinese research team’s first investigation of HRW, released in January 2018, showed that HRW administration in an animal model affected radiation-induced small intestine toxicity [103]. Ikeda et al. investigated the effects of HRW treatment as preventive measure against bacterial translocation in a murine model of sepsis. Zheng et al. (2018) studied the intestinal microbiota response to 25 d oral administration of HRW and lactulose in female piglets fed Fusarium mycotoxin-contaminated maize [104]. The results of this study also showed that HRW treatment affected various intestinal segments, with fewer Escherichia coli and more Bifidobacterium in the HRW group than in the control group. A 15 d HRW therapy reportedly restored the intestinal barrier that had been damaged by permethrin and increased the amount of butyric acid in the feces. Moreover, a first-in-human trial supported HRW consumption and its positive impact on gut microbiota [105]. According to another study, HRW protected against inflammatory bowel disease (IBD) in an animal model [106]. Following oral administration, HRW demonstrated positive effects by decreasing epithelial cell apoptosis in the small intestine, maintaining the intestinal barrier and tight junctions and restoring the protein expression and distribution of CLDN3 in the small intestine of female piglets fed food contaminated with Fusarium toxins [107]. HRW intake improved glucose tolerance that might be decreased in Bacteroides levels [108]. Another clinical study reported that drinking alkaline electrolyzed water for two weeks increased Bifidobacterium in healthy volunteers [109]. Jin et al. reported that H2 released from the gut by hydrogen nanocapsules could induce an abundance of Akkermansia muciniphila and reduce metabolic dysfunction-associated fatty liver diseases [110]. However, the gut microbiota may be the major contributors of the biological effects of exogenous hydrogen consumption. Another study revealed that H2 saline therapy modulated the abundance of Bacteroides and Lactobacillus in feces, which might account for the increase in lipid metabolism in mice fed a high fat diet [111]. A previous study reported that acute exercise augments breathing of H2 after the lactulose test [112], and the results, corroborated through a recent gut-exercise, implied that colonic bacteria are an endogenous H2 source during exercise [113]. The degree of obesity and leanness has a contributory impact on the gut microbiota, and this was observed in the gut flora of bariatric surgery patients [114]. Therefore, HRW might become an upcoming functional water drink that could enhance and adjust endogenous gut microbiota; however, it should be administered as an experimental drink and not suggested for the general population. Interestingly, the function and composition of the intestinal microbiota that routinely produces H2 gas fluctuate throughout the day, and the quantity of H2 produced varies depending on the person and time of day. One study revealed H2S as a new endogenous factor for regulating the circadian clock [115].
10. Protective Effects of H2 on the Cardiovascular System
The essential gas signaling molecule nitric oxide (NO) can usually be recognized for inducing vasodilatation, reducing the production of superoxide, decreasing inflammation and improving the production of mitochondrial energy. I/R lung damage reduces by ventilation during warm ischemia, ex-vivo infusion and post-transplantation with NO nonheart-beating lung grafts [116]. Carbon monoxide (CO) has a high affinity for the heme prosthetic community in laboratory studies and has also been shown to enhance the graft function in combination with preservation solutions [117,118]. Ohsawa et al. (2008) found that oral H2 water prevented the development of atherosclerosis in an apolipoprotein E knockout mouse model [119]. H2S is known to induce smooth muscle relaxation, apoptosis, inflammatory response regulation and oxidative stress relief [120]. Although not a gas transmitter, H2 is now called a gaseous signal molecule [118]. The advantages are similar to those of NO, CO and H2 sulfide (H2S), both physiologically and therapeutically [121,122]. Myocardial damage to the mouse caused by radiation was reduced by H2 water [123]. Inhalation of H2 in a rat model of post-cardiac arrest syndrome also improved survival and functional performance [124]. Researchers have concluded that improved cold-rat ischemia-reperfusion injuries and frequent drinking of H2 water could protect beneficiaries from inflammatory heart and aortic allograft degradation [125]. H2 is beneficial in terms of toxicity; it shows no cytotoxicity even at high concentrations. For inhalation, high levels of H2 gas are defined as high-pressure. In deep-diving gas blends, H2 gas is used to prevent the oxidation and thrombosis of arterial gas [126]. Given that H2 is an inert and nonfunctional gas in the body, it is understandable that it has no toxic effects. As described above, the inhalation of 1–4% H2 gas is highly effective [20]. Basic and clinical research over the past ten years has shown that H2 is a major regulatory pathophysiological factor with anti-oxidative, anti-inflammatory and anti-apoptotic effects on cells and organs [127]. Myocardial transmission releases H2 through inhalation or injection with [128], injection with H2-rich saline [129], drinking H2-rich water [127], taking an H2-rich bath and increasing the development of intestinal H2 through bacteria [130]. Table 2 summarizes the effects of H2 on age-related clinical studies in human diseases.

Table 2.
Effect of H2 on age-related clinical studies in human diseases.
11. Therapeutic Effects of H2 on Parkinson’s Disease (PD) and Co-Relation with Intestinal Microbiome
PD of the substantia nigra with extrapyramidal symptoms is a disorder induced by the degeneration and loss of dopamine-producing cells. However, aggregation of α-synuclein in the intestinal mucosa may be caused by oxidative stress produced by macrophages in the luminal wall due to a hyperpermeable intestinal wall, where the intestinal microbiota significantly affects hyperpermeability-induced oxidative stress that may be linked to synuclein pathology in the enteric nervous system in PD [141]. In a study the breath H2 concentrations were analyzed in 28 healthy controls and 37 PD patients after consumption of 6 g lactulose [142].
The clinical manifestation of PD is associated with oxidative stress [143]. In addition, studies have been conducted on the involvement of PD with mitochondrial dysfunction [12,143]. Both animal PD models and clinical trials have reported the effects of H2 on PD [12]. Oxidative stress was inhibited in the nigrostriatal pathway with the intake of H2-rich- drinking water, and the loss of dopamine cells was reduced. These findings indicated that consuming water rich in H2 may influence the onset of PD [134]. A randomized double-blind study found that 48 weeks of intake of H2-rich water (1000 mL/day) substantially increased the overall Unified PD Rating Scale (UPDRS) score for levodopa-treated PD patients. A double-blind, multicenter H2 water study is currently underway [144]. According to one study, intestinal permeability increased in PD, and its level favorably correlated with intestinal staining for Escherichia coli, nitrotyrosine and other proteins subjected to protein oxidation [145]. However, another study demonstrated that decreased H2 production by the intestinal microbiota is associated with the development and progression of PD [146]. One study also showed how much H2 was produced by the seven bacterial strains representing the main bacterial species and groups in the intestine [146]. According to Scheperjans et al., on analysis of the 16S ribosomal RNA genes of the gut microbiota in 72 PD patients and 72 controls, the degree of postural instability was favorably correlated with the relative abundance of Enterobacteriaceae [147].
12. Therapeutic and Preventive Effect of H2 on AD and Co-Relation with Intestinal Microbiome
The term “gut microbiota” refers to the microbial community that inhabits the gastrointestinal system and may include bacteria, fungi, and protozoans that coexist harmoniously within our intestine [148,149]. This microbiota regulates host homeostasis and many diseases and may play a significant pathogenic role in neurodegenerative disorders, including AD [150,151]. The therapeutic and preventive applications of H2 have been confirmed in more animal and human studies, such as in neurodegeneration [152], inflammation [153], and I/R injuries [154]. The gut microbiota, however, has recently been shown to play a significant role in the development of host immunity, in controlling gut endocrine functions and in controlling other neurological signaling [155]. Moreover, the gut microbiome and H2 consumption relationship is quite limited. One study found that HRW could improve the structural integrity of the butyrate-producing bacteria in the gut, along with the clinical symptoms of disturbed gut microbiota [156]. Another study demonstrated that HRW intake induced a significant increase in the relative abundance of Lactobacillus and a decrease in Bacteroides and Bifidobacterium. Additionally, because the gut microbiota is important for both health and disease, the impact of HRW on the gut microbiota may greatly improve these diseases. One study revealed that patients with AD showed an increased proinflammatory endobacteria species of Escherichia coli and decreased anti-inflammatory taxon, such as E. rectale, which may result in microbiota modification, amyloidosis and peripheral inflammation [152].
The deposition of amyloid beta (Aβ) protein outside nerve cells and the accumulated tau phosphorylated protein inside nerve cells are characteristic of the pathology of Aβ protein deposition. Oxidative stress and neuroinflammation have in recent years been documented to be correlated with AD [157]. To date, studies have focused on the role of oxidative stress in the brain parenchyma [158,159]. Aβ protein accumulation is highly linked to the absence of Aβ clearance, which is intricately linked to AD’s pathogenesis [160,161]. It is understood that Aβ protein removal requires low-density lipoprotein receptor 1 (LRP1). The onset of AD involves LRP dysfunction due to oxidative stress and neuroinflammation [161]. The initiation and progression of AD can be prevented by regulating oxidative stress and neuroinflammation. The effect of H2 on AD prevention has been investigated in several studies [162]. A rat AD model has been identified in the hippocampus and cerebral cortex; herein, memory was enhanced using H2-rich saline (5 mL/kg, i.p., daily) as an inhibitor of oxidative stress, cytokine development and NF-κB production [163]. H2-rich water consumption has also been reported to prevent changes in the brain age and decline in spatial memory [164]. Moreover, H2 water also exhibited the potential to control dementia at the mild cognitive impairment stage of AD [165]. Safety is a primary concern with respect to H2 transportation, storage and administration. H2 is flammable only at temperatures greater than 527 °C and explodes by rapid chain reaction with oxygen in the H2 concentration range of 4–75% (vol/vol) [166,167]. Because inhaling 1–4% H2 has demonstrated great efficacy in medical applications, the use of H2 at such low concentrations has been deemed feasible and safe [168].
13. Effects of H2 in Heart Diseases
Ventricular remodeling contributes to several molecular and cellular pathways in response to pathophysiological stimuli, such as myocardial I/R, hypertension or neurohumoral triggers [169,170]. Endothelin-1 (ET-1) innovations are increased, and Ang II, catecholamines and proinflammatory cytokines activate the receptors and downstream signaling events of their cognates, leading to apoptosis or hypertrophy [169,170]. Until the coronary blood flow was restored in the occluded region, the inhaled H2 was rapidly brought into the ischemic myocardial system, and 2% H2 was inhaled at the time of ischemia and persisted for 60 min after reperfusion decreased the duration of infarction [169]. In H2, for example, the myocardial I/R injury infarction scale is reduced by NO also [169]. In addition to H2 inhalation, intraperitoneal saline injection has been shown to reduce the effects of myocardial I/R and also improve heart activity through its anti-oxidant, anti-apoptotic and anti-inflammatory effects [171]. Inhalation of H2 at low levels in the C57BL/6J left ventricular myocardial mice (1.3 vol/100 vol) decreased transient dyslipidemia caused by hypoxia, oxidative stress and preventive cardiomyocyte and perivascular fibrosis [145]. Neurohumoral activation, such as β-adrenoceptor and Ang II enhancement, not only results in hypertension, but also leads to ischemic heart diseases as well as sleep apnea syndrome [172]. Direct inhibition of NADPH oxidase expression and decrease in mitochondrial damage leads to ROS inhibition and consequent degradation of the downstream signaling ERK1/2, p38, and JNK, leading to the protective effect of H2 [173]. In addition, rats are protected by anti-oxidants and anti-inflammatory activities, such as high-dose ISO-induced acute myocardial infarctions [173]. H2-rich saline spontaneously attenuates left ventricular hypertrophy in hypertensive rats by suppressing the inflammatory mechanisms, minimizing oxidative stress, maintaining mitochondrial production, and locally inhibiting Ang II in the left ventricle [174].
14. Conclusions
H2 is readily available because it has minimal harmful effects and is highly effective against nearly all pathogenic states related to oxidative stress and inflammation. H2 has great potential for protective applications in many diseases, owing to its efficacy. Additionally, H2 gas has proven to be safe in numerous studies, which is crucial for clinical experiments. H2 controls aging primarily through anti-inflammatory and anti-oxidative properties. The treatment of numerous age-related diseases is possible with H2 as promising therapeutic and protective options in the future. In addition, H2-based therapies are anticipated to be novel and revolutionary methods for the prevention of age-related diseases, thereby promoting helpful longevity.
Author Contributions
Conceptualization, K.-J.L.; software, M.H.R.; validation, M.H.R.; writing—original draft preparation, M.H.R., E.-S.J., H.S.Y. and C.-S.K.; writing—review and editing, E.-S.J.; preparation of tables and figures, M.H.R.; visualization, C.-S.K. and H.S.Y.; supervision, K.-J.L. and C.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 8-OHdG | 8-hydroxy-desoxyguanosine |
| AD | Alzheimer’s disease |
| ATP | Adenosine triphosphate |
| Aβ | Amyloid beta |
| BUN | Blood urea nitrogen |
| DNA | Deoxyribonucleic acid |
| ET-1 | Endothelin-1 |
| GHSR1-α | Growth hormone secretagogue receptor |
| GSC | Glioma stem-like cell |
| GSH | Glutathione |
| H2 | Molecular Hydrogen |
| HRW | Hydrogen rich water |
| H2S | Hydrogen sulfide |
| HMGB-1 | High-mobility group box 1 |
| HO-1 | Heme oxygenase-1 |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| LRP1 | Low-density lipoprotein receptor 1 |
| MDA | Malondialdehyde |
| MMP3 | Matrix metalloproteinase 3 |
| MS | Metabolic syndrome |
| NO | Nitric oxide |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| PD | Parkinson’s disease |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| T2D | Type 2 diabetes |
| TNF-α | Tumor necrosis factor-α |
References
- Calder, P.C.; Carding, S.R.; Christopher, G.; Kuh, D.; Langley-Evans, S.C.; McNulty, H. A Holistic Approach to Healthy Ageing: How Can People Live Longer, Healthier Lives? J. Hum. Nutr. Diet. 2018, 31, 439–450. [Google Scholar] [CrossRef]
- Ozawa, H.; Miyazawa, T.; Miyazawa, T. Effects of Dietary Food Components on Cognitive Functions in Older Adults. Nutrients 2021, 13, 2804. [Google Scholar] [CrossRef] [PubMed]
- Vidaček, N.Š.; Nanić, L.; Ravlić, S.; Sopta, M.; Gerić, M.; Gajski, G.; Garaj-Vrhovac, V.; Rubelj, I. Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Sim, M.; Kim, C.S.; Shon, W.J.; Lee, Y.K.; Choi, E.Y.; Shin, D.M. Hydrogen-Rich Water Reduces Inflammatory Responses and Prevents Apoptosis of Peripheral Blood Cells in Healthy Adults: A Randomized, Double-Blind, Controlled Trial. Sci. Rep. 2020, 10, 12130. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi-Fukatsu, A.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Nishida, Y.; Yamamoto, H.; Taketani, Y.; Takeda, E. Natto and Viscous Vegetables in a Japanese-Style Breakfast Improved Insulin Sensitivity, Lipid Metabolism and Oxidative Stress in Overweight Subjects with Impaired Glucose Tolerance. Br. J. Nutr. 2012, 107, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Sasaki, A.; Ebisu, K.; Tajima, K.; Kajimoto, O.; Nojima, J.; Kuratsune, H.; Hori, H.; Watanabe, Y. Hydrogen-Rich Water for Improvements of Mood, Anxiety, and Autonomic Nerve Function in Daily Life. Med. Gas Res. 2017, 7, 247–255. [Google Scholar]
- Hehemann, J.H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of Carbohydrate-Active Enzymes from Marine Bacteria to Japanese Gut Microbiota. Nature 2010, 464, 908–912. [Google Scholar] [CrossRef]
- Aoki, Y. Antioxidant Bioactivity of Molecular Hydrogen Gas Produced by Intestinal Bacteria with Undigested Carbohydrates. Acta Sci. Nutr. Health 2018, 2, 23–25. [Google Scholar]
- Zanini, D.; Todorovic, N.; Korovljev, D.; Stajer, V.; Ostojic, J.; Purac, J.; Kojic, D.; Vukasinovic, E.; Djordjievski, S.; Sopic, M.; et al. The Effects of 6-Month Hydrogen-Rich Water Intake on Molecular and Phenotypic Biomarkers of Aging in Older Adults Aged 70 Years and over: A Randomized Controlled Pilot Trial. Exp. Gerontol. 2021, 155, 111574. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, A.B.; Nezami, B.G.; Gewirtz, A.; Srinivasan, S. Obesity and Its Associated Disease: A Role for Microbiota? Neurogastroenterol. Motil. 2012, 24, 305–311. [Google Scholar] [CrossRef]
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial Biological Effects and the Underlying Mechanisms of Molecular Hydrogen—Comprehensive Review of 321 Original Articles. Med. Gas Res. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Hamada, N.; Terazawa, R.; Ito, M.; Ohno, K.; Ichihara, M.; Nozawa, Y.; Ito, M. Molecular Hydrogen Inhibits Lipopolysaccharide/Interferon γ-Induced Nitric Oxide Production through Modulation of Signal Transduction in Macrophages. Biochem. Biophys. Res. Commun. 2011, 411, 143–149. [Google Scholar] [CrossRef]
- Song, G.; Zong, C.; Zhang, Z.; Yu, Y.; Yao, S.; Jiao, P.; Tian, H.; Zhai, L.; Zhao, H.; Tian, S.; et al. Molecular Hydrogen Stabilizes Atherosclerotic Plaque in Low-Density Lipoprotein Receptor-Knockout Mice. Free Radic. Biol. Med. 2015, 87, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, K.; Chen, H.; Wang, G.; Yu, Y. Hydrogen Gas Inhibits High-Mobility Group Box 1 Release in Septic Mice by Upregulation of Heme Oxygenase 1. J. Surg. Res. 2015, 196, 136–148. [Google Scholar] [CrossRef]
- Stohs, S.J. The Role of Free Radicals in Toxicity and Disease. J. Basic Clin. Physiol. Pharm. 1995, 6, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Wakabayashi, N.; Shigemura, N.; Huang, C.S.; Masutani, K.; Tanaka, Y.; Noda, K.; Peng, X.; Takahashi, T.; Billiar, T.R.; et al. Hydrogen Gas Reduces Hyperoxic Lung Injury via the Nrf2 Pathway in Vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L646–L656. [Google Scholar] [CrossRef]
- Matsumoto, A.; Yamafuji, M.; Tachibana, T.; Nakabeppu, Y.; Noda, M.; Nakaya, H. Oral “hydrogen Water” Induces Neuroprotective Ghrelin Secretion in Mice. Sci. Rep. 2013, 3, 3273. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Redox Mechanisms in Neurodegeneration: From Disease Outcomes to Therapeutic Opportunities. Antioxid. Redox Signal. 2019, 30, 1450–1499. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen Acts as a Therapeutic Antioxidant by Selectively Reducing Cytotoxic Oxygen Radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Qian, L.; Shen, J.; Chuai, Y.; Cai, J. Hydrogen as a New Class of Radioprotective Agent. Int. J. Biol. Sci. 2013, 9, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Seike, T.; Yutsudo, N.; Ohno, M.; Yamada, H.; Yamaguchi, H.; Sakumi, K.; Yamakawa, Y.; Kido, M.A.; Takaki, A.; et al. Hydrogen in Drinking Water Reduces Dopaminergic Neuronal Loss in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model of Parkinson’s Disease. PLoS ONE 2009, 4, e7247. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Y.; Chen, J.; Xue, J.; Zhang, X.; Zhao, M.; Jia, X.; Wang, Y.; Qin, S. Protective Effect of Molecular Hydrogen Following Different Routes of Administration on D-Galactose-Induced Aging Mice. J. Inflamm. Res. 2021, 14, 5541–5550. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.J.; Tang, J.; Zhang, J.H. The Evolution of Molecular Hydrogen: A Noteworthy Potential Therapy with Clinical Significance. Med. Gas Res. 2013, 3, 10. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular Hydrogen: A Preventive and Therapeutic Medical Gas for Various Diseases. Oncotarget 2017, 8, 102653. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef]
- Okamoto, A.; Kohama, K.; Aoyama-Ishikawa, M.; Yamashita, H.; Fujisaki, N.; Yamada, T.; Yumoto, T.; Nosaka, N.; Naito, H.; Tsukahara, K.; et al. Intraperitoneally Administered, Hydrogen-Rich Physiologic Solution Protects against Postoperative Ileus and Is Associated with Reduced Nitric Oxide Production. Surgery 2016, 160, 623–631. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Ito, M.; Oshima, T.; Kojima, S.; Ohno, K. Hydrogen-Rich Water Ameliorates Bronchopulmonary Dysplasia (BPD) in Newborn Rats. Pediatr. Pulmonol. 2016, 51, 928–935. [Google Scholar] [CrossRef]
- Diao, M.; Zhang, S.; Wu, L.; Huan, L.; Huang, F.; Cui, Y.; Lin, Z. Hydrogen Gas Inhalation Attenuates Seawater Instillation-Induced Acute Lung Injury via the Nrf2 Pathway in Rabbits. Inflammation 2016, 39, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxid. Med. Cell. Longev. 2020, 7, 35. [Google Scholar] [CrossRef]
- Ren, A.; Liu, R.; Miao, Z.G.; Zhang, X.; Cao, P.F.; Chen, T.X.; Li, C.Y.; Shi, L.; Jiang, A.L.; Zhao, M.W. Hydrogen-Rich Water Regulates Effects of ROS Balance on Morphology, Growth and Secondary Metabolism via Glutathione Peroxidase in Ganoderma Lucidum. Env. Microbiol. 2017, 19, 566–583. [Google Scholar] [CrossRef] [PubMed]
- Abraini, J.H.; Gardette-Chauffour, M.C.; Martinez, E.; Rostain, J.C.; Lemaire, C. Psychophysiological Reactions in Humans during an Open Sea Dive to 500 m with a Hydrogen-Helium-Oxygen Mixture. J. Appl. Physiol. 1994, 76, 1113–1118. [Google Scholar] [CrossRef]
- Fontanari, P.; Badier, M.; Guillot, C.; Tomei, C.; Burnet, H.; Gardette, B.; Jammes, Y. Changes in Maximal Performance of Inspiratory and Skeletal Muscles during and after the 7.1-MPa Hydra 10 Record Human Dive. Eur. J. Appl. Physiol. 2000, 81, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakashima-Kamimura, N.; Mikami, T.; Ohsawa, I.; Ohta, S. Consumption of Molecular Hydrogen Prevents the Stress-Induced Impairments in Hippocampus-Dependent Learning Tasks during Chronic Physical Restraint in Mice. Neuropsychopharmacology 2009, 34, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ito, M.; Fujita, Y.; Ito, M.; Ichihara, M.; Masuda, A.; Suzuki, Y.; Maesawa, S.; Kajita, Y.; Hirayama, M.; et al. Molecular Hydrogen Is Protective against 6-Hydroxydopamine-Induced Nigrostriatal Degeneration in a Rat Model of Parkinson’s Disease. Neurosci. Lett. 2009, 453, 81–85. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Zhou, W.; Hu, L.; Li, L.; Tu, Q.; Chang, Y.; Liu, Q.; Sun, X.; Wu, M.; et al. The Protective Role of Hydrogen-Rich Saline in Experimental Liver Injury in Mice. J. Hepatol. 2011, 54, 471–480. [Google Scholar] [CrossRef]
- Lu, W.; Li, D.; Hu, J.; Mei, H.; Shu, J.; Long, Z.; Yuan, L.; Li, D.; Guan, R.; Li, Y.; et al. Hydrogen Gas Inhalation Protects against Cigarette Smokeinduced COPD Development in Mice. J. Thorac. Dis. 2018, 10, 3232–3243. [Google Scholar] [CrossRef]
- Xu, X.F.; Zhang, J. Saturated Hydrogen Saline Attenuates Endotoxin-Induced Acute Liver Dysfunction in Rats. Physiol. Res. 2013, 62, 395–403. [Google Scholar] [CrossRef]
- Gharib, B.; Hanna, S.; Abdallahi, O.M.S.; Lepidi, H.; Gardette, B.; De Reggi, M. Anti-Inflammatory Properties of Molecular Hydrogen: Investigation on Parasite-Induced Liver Inflammation. Comptes Rendus L’academie Sci.-Ser. III-Sci. Vie 2001, 324, 719–724. [Google Scholar] [CrossRef]
- Chen, H.; Sun, Y.P.; Li, Y.; Liu, W.W.; Xiang, H.G.; Fan, L.Y.; Sun, Q.; Xu, X.Y.; Cai, J.M.; Ruan, C.P.; et al. Hydrogen-Rich Saline Ameliorates the Severity of l-Arginine-Induced Acute Pancreatitis in Rats. Biochem. Biophys. Res. Commun. 2010, 393, 308–313. [Google Scholar] [CrossRef]
- Shao, A.; Wu, H.; Hong, Y.; Tu, S.; Sun, X.; Wu, Q.; Zhao, Q.; Zhang, J.; Sheng, J. Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-ΚB Pathway and NLRP3 Inflammasome. Mol. Neurobiol. 2016, 53, 3462–3476. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.C.; Xie, F.; Zhang, H.L.; Zhu, Y.L.; Chen, K.; Tan, H.M.; Hu, B.S.; Yang, J.M.; Tan, J.W. Hydrogen-Rich Saline Attenuates Postoperative Liver Failure after Major Hepatectomy in Rats. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, S.; Qin, M.; Mao, Y.; Jin, L.; Che, N.; Liu, S.; Ge, R. Hydrogen-Rich Saline Ameliorates Allergic Rhinitis by Reversing the Imbalance of Th1/Th2 and Up-Regulation of CD4+CD25+Foxp3+Regulatory T Cells, Interleukin-10, and Membrane-Bound Transforming Growth Factor-β in Guinea Pigs. Inflammation 2018, 41, 81–92. [Google Scholar] [CrossRef]
- Li, K.; Yin, H.; Duan, Y.; Lai, P.; Cai, Y.; Wei, Y. Pre-Inhalation of Hydrogen-Rich Gases Protect against Caerulein-Induced Mouse Acute Pancreatitis While Enhance the Pancreatic Hsp60 Protein Expression. BMC Gastroenterol. 2021, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Liu, S.; Liu, K.; Sun, Q.; Liu, W.; Xu, W.; Denoble, P.; Sun, X. Consumption of Hydrogen Water Reduces Paraquat-Induced Acute Lung Injury in Rats. J. Biomed. Biotechnol. 2011, 7, 35. [Google Scholar] [CrossRef]
- Hirayama, M.; Ito, M.; Minato, T.; Yoritaka, A.; Lebaron, T.; Ohno, K. Inhalation of Hydrogen Gas Elevates Urinary 8-Hydroxy-2′-Deoxyguanine in Parkinson’s Disease. Med. Gas Res. 2018, 8, 144–149. [Google Scholar] [CrossRef]
- Bi, Y.; Zhu, Y.; Zhang, M.; Zhang, K.; Hua, X.; Fang, Z.; Zhou, J.; Dai, W.; Cui, Y.; Li, J.; et al. Effect of Shikonin on Spinal Cord Injury in Rats Via Regulation of HMGB1/TLR4/NF-ΚB Signaling Pathway. Cell. Physiol. Biochem. 2017, 43, 481–491. [Google Scholar] [CrossRef]
- Kellogg, E.W.; Fridovich, I. Liposome Oxidation and Erythrocyte Lysis by Enzymically Generated Superoxide and Hydrogen Peroxide. J. Biol. Chem. 1977, 252, 6721–6728. [Google Scholar] [CrossRef]
- Stibich, M. The Wear and Tear Theory of Aging; Verywell Health: New York, NY, USA, 2019. [Google Scholar]
- HARMAN, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Sohal, R.S.; Weindruch, R. Oxidative Stress, Caloric Restriction, and Aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein Oxidation and Aging. Free Radic. Res. 2006, 40, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, T.B.L.; Kowald, A. The Free-Radical Theory of Ageing—Older, Wiser and Still Alive: Modelling Positional Effects of the Primary Targets of ROS Reveals New Support. BioEssays 2012, 34, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, K.; Nishimaki, K.; Kamimura, N.; Ohta, S. Molecular Hydrogen Suppresses Free-Radical-Induced Cell Death by Mitigating Fatty Acid Peroxidation and Mitochondrial Dysfunction. Can. J. Physiol. Pharm. 2019, 97, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T. Therapeutic Efficacy of Molecular Hydrogen: A New Mechanistic Insight. Curr. Pharm. Des. 2019, 25, 946–955. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharm. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Ghezzi, P.; Jaquet, V.; Marcucci, F.; Schmidt, H.H.H.W. The Oxidative Stress Theory of Disease: Levels of Evidence and Epistemological Aspects. Br. J. Pharm. 2017, 174, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Rahman, M.H.; Alim, M.A.; Rahman, M.M.; Khatkar, A.; Al Mamun, A.; Rauf, A.; Mathew, B.; Ashraf, G.Md. Exploring the Multifunctional Neuroprotective Promise of Rasagiline Derivatives for Multi-Dysfunctional Alzheimer’s Disease. Curr. Pharm. Des. 2020, 26, 4690–4698. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Thi, T.T.; Akter, R.; Goh, S.H.; Kim, C.S.; Lee, K.J. Redox Effects of Molecular Hydrogen and Its Therapeutic Efficacy in the Treatment of Neurodegenerative Diseases. Processes 2021, 9, 308. [Google Scholar] [CrossRef]
- Ito, M.; Ibi, T.; Sahashi, K.; Ichihara, M.; Ito, M.; Ohno, K. Open-Label Trial and Randomized, Double-Blind, Placebo-Controlled, Crossover Trial of Hydrogen-Enriched Water for Mitochondrial and Inflammatory Myopathies. Med. Gas Res. 2011, 1, 24. [Google Scholar] [CrossRef]
- Nakayama, M.; Nakano, H.; Hamada, H.; Itami, N.; Nakazawa, R.; Ito, S. A Novel Bioactive Haemodialysis System Using Dissolved Dihydrogen (H2) Produced by Water Electrolysis: A Clinical Trial. Nephrol. Dial. Transplant. 2010, 25, 3026–3033. [Google Scholar] [CrossRef]
- Martins, A.D.; Sá, R.; Monteiro, M.P.; Barros, A.; Sousa, M.; Carvalho, R.A.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Ghrelin Acts as Energy Status Sensor of Male Reproduction by Modulating Sertoli Cells Glycolytic Metabolism and Mitochondrial Bioenergetics. Mol. Cell. Endocrinol. 2016, 434, 199–209. [Google Scholar] [CrossRef]
- Nicolson, G.L. Mitochondrial Dysfunction and Chronic Disease: Treatment with Natural Supplements. Integr. Med. (Boulder) 2014, 13, 35. [Google Scholar]
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular Inflammation: Underpinnings of Aging and Age-Related Diseases. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen Peroxide—Production, Fate and Role in Redox Signaling of Tumor Cells. Cell Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. Mitochondrial Antioxidants and the Maintenance of Cellular Hydrogen Peroxide Levels. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Viola, J.; Soehnlein, O. Atherosclerosis—A Matter of Unresolved Inflammation. Semin. Immunol. 2015, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Lindner, A.B. Protein Posttranslational Modifications: Roles in Aging and Age-Related Disease. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Woods, J.A.; Vieira, V.J.; Keylock, K.T. Exercise, Inflammation, and Innate Immunity. Immunol. Allergy Clin. N. Am. 2009, 29, 381–393. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Miljkovic, M.D.; Conlon, K.C. Interleukin-15 (Dys)Regulation of Lymphoid Homeostasis: Implications for Therapy of Autoimmunity and Cancer. J. Exp. Med. 2020, 217, e20191062. [Google Scholar] [CrossRef]
- Liu, K.Y.P.; Lu, X.J.D.; Zhu, Y.S.; Le, N.; Kim, H.; Poh, C.F. Plasma-Derived Inflammatory Proteins Predict Oral Squamous Cell Carcinoma. Front. Oncol. 2018, 8, 585. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Accardi, G.; Monastero, R.; Nicoletti, F.; Libra, M. Ageing: From Inflammation to Cancer. Immun. Ageing 2018, 15, 1. [Google Scholar] [CrossRef]
- Li, S.; Liao, R.; Sheng, X.; Luo, X.; Zhang, X.; Wen, X.; Zhou, J.; Peng, K. Hydrogen Gas in Cancer Treatment. Front. Oncol. 2019, 9, 696. [Google Scholar] [CrossRef]
- Wardill, H.R.; Mander, K.A.; Van Sebille, Y.Z.A.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Sonis, S.T. Cytokine-Mediated Blood Brain Barrier Disruption as a Conduit for Cancer/Chemotherapy-Associated Neurotoxicity and Cognitive Dysfunction. Int. J. Cancer 2016, 139, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.H.; Shen, L.; Li, J.; Zhou, Z.W.; Liang, H.; Zhang, X.T.; Tang, L.; Xin, Y.; Jin, J.; Zhang, Y.J.; et al. The Chinese Society of Clinical Oncology (CSCO): Clinical Guidelines for the Diagnosis and Treatment of Gastric Cancer. Cancer Commun. 2019, 39, 10. [Google Scholar] [CrossRef]
- Lazzari, C.; Karachaliou, N.; Bulotta, A.; Viganó, M.; Mirabile, A.; Brioschi, E.; Santarpia, M.; Gianni, L.; Rosell, R.; Gregorc, V. Combination of Immunotherapy with Chemotherapy and Radiotherapy in Lung Cancer: Is This the Beginning of the End for Cancer? Adv. Med. Oncol. 2018, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Susanne, K.; Michael, F.; Thomas, S.; Peter, E.; Andreas, H. Predictors of Fatigue in Cancer Patients: A Longitudinal Study. Support. Care Cancer 2019, 27, 3463–3471. [Google Scholar] [CrossRef]
- Razzaghdoust, A.; Mofid, B.; Peyghambarlou, P. Predictors of Chemotherapy-Induced Severe Anemia in Cancer Patients Receiving Chemotherapy. Support. Care Cancer 2020, 28, 155–161. [Google Scholar] [CrossRef]
- Cui, Q.; Wang, J.Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R.; Yang, D.H.; Chen, Z.S. Modulating ROS to Overcome Multidrug Resistance in Cancer. Drug Resist. Updat. 2018, 41, 1–25. [Google Scholar] [CrossRef]
- Shen, B.-Y.; Chen, C.; Xu, Y.-F.; Shen, J.-J.; Guo, H.-M.; Li, H.-F.; Li, X.-N.; Kang, D.; Shao, Y.-H.; Zhu, Z.-P.; et al. Is the Combinational Administration of Doxorubicin and Glutathione a Reasonable Proposal? Acta Pharm. Sin 2019, 40, 699–709. [Google Scholar] [CrossRef]
- Luo, W.; Wen, G.; Yang, L.; Tang, J.; Wang, J.; Wang, J.; Zhang, S.; Zhang, L.; Ma, F.; Xiao, L.; et al. Dual-Targeted and PH-Sensitive Doxorubicin Prodrug-Microbubble Complex with Ultrasound for Tumor Treatment. Theranostics 2017, 7, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, A.; Kobayashi, S.; Matsushita, T.; Fujinawa, H.; Murase, K. Experimental Verification of Protective Effect of Hydrogen-Rich Water against Cisplatin-Induced Nephrotoxicity in Rats Using Dynamic Contrast-Enhanced CT. Br. J. Radiol. 2010, 83, 509–514. [Google Scholar] [CrossRef]
- Matsushita, T.; Kusakabe, Y.; Kitamura, A.; Okada, S.; Murase, K. Investigation of Protective Effect of Hydrogen-Rich Water against Cisplatininduced Nephrotoxicity in Rats Using Blood Oxygenation Level-Dependent Magnetic Resonance Imaging. Jpn. J. Radiol. 2011, 29, 503–512. [Google Scholar] [CrossRef]
- Meng, X.; Chen, H.; Wang, G.; Yu, Y.; Xie, K. Hydrogen-Rich Saline Attenuates Chemotherapy-Induced Ovarian Injury via Regulation of Oxidative Stress. Exp. Med. 2015, 10, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.M.; Kang, Y.N.; Choi, I.B.; Gu, Y.; Kawamura, T.; Toyoda, Y.; Nakao, A. Effects of Drinking Hydrogen-Rich Water on the Quality of Life of Patients Treated with Radiotherapy for Liver Tumors. Med. Gas Res. 2011, 1, 11. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, H.; Fan, Y.; Li, L.; Fang, J.; Yang, W. Hydrogen-Rich Saline Attenuates Cardiac and Hepatic Injury in Doxorubicin Rat Model by Inhibiting Inflammation and Apoptosis. Mediat. Inflamm. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Jin, Z.; Chen, Q.; Yang, T.; Chen, D.; Meng, J.; Lu, X.; Gu, Z.; He, Q. Local Generation of Hydrogen for Enhanced Photothermal Therapy. Nat. Commun. 2018, 9, 4241. [Google Scholar] [CrossRef]
- Liu, M.Y.; Xie, F.; Zhang, Y.; Wang, T.T.; Ma, S.N.; Zhao, P.X.; Zhang, X.; Lebaron, T.W.; Yan, X.L.; Ma, X.M. Molecular Hydrogen Suppresses Glioblastoma Growth via Inducing the Glioma Stem-like Cell Differentiation. Stem. Cell Res. 2019, 10, 145. [Google Scholar] [CrossRef]
- Nakashima-Kamimura, N.; Mori, T.; Ohsawa, I.; Asoh, S.; Ohta, S. Molecular Hydrogen Alleviates Nephrotoxicity Induced by an Anti-Cancer Drug Cisplatin without Compromising Anti-Tumor Activity in Mice. Cancer Chemother. Pharm. 2009, 64, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Xie, F.; Li, J.; Zhang, Y.; Liu, M.; Zhao, P.; Ma, X.; Lebaron, T.W. Therapeutic Potential of Molecular Hydrogen in Ovarian Cancer. Transl. Cancer Res. 2018, 7, 988–995. [Google Scholar] [CrossRef]
- Yang, Q.; Ji, G.; Pan, R.; Zhao, Y.; Yan, P. Protective Effect of Hydrogen-Rich Water on Liver Function of Colorectal Cancer Patients Treated with MFOLFOX6 Chemotherapy. Mol. Clin. Oncol. 2017, 7, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Lin, B.; Wang, P.; Pan, T.; Wang, S.; Chen, W.; Cheng, S.; Liu, S. The Healing Effect of Hydrogen-Rich Water on Acute Radiation-Induced Skin Injury in Rats. J. Radiat. Res. 2019, 60, 17–22. [Google Scholar] [CrossRef]
- Terasaki, Y.; Suzuki, T.; Tonaki, K.; Terasaki, M.; Kuwahara, N.; Ohsiro, J.; Iketani, M.; Takahashi, M.; Hamanoue, M.; Kajimoto, Y.; et al. Molecular Hydrogen Attenuates Gefitinib-Induced Exacerbation of Naphthalene-Evoked Acute Lung Injury through a Reduction in Oxidative Stress and Inflammation. Lab. Investig. 2019, 99, 793–806. [Google Scholar] [CrossRef]
- Kawai, D.; Takaki, A.; Nakatsuka, A.; Wada, J.; Tamaki, N.; Yasunaka, T.; Koike, K.; Tsuzaki, R.; Matsumoto, K.; Miyake, Y.; et al. Hydrogen-Rich Water Prevents Progression of Nonalcoholic Steatohepatitis and Accompanying Hepatocarcinogenesis in Mice. Hepatology 2012, 56, 912–921. [Google Scholar] [CrossRef]
- Provasi, S.; Cattaneo, A.; Cattane, N.; Galluzzi, S.; Lopizzo, N.; Plazzotta, G.; Boccardi, M.; Frisoni, G. Association of Brain Amyloidosis with Pro-Inflammatory Gut Bacterial Strains and Peripheral Inflammation Markers in Cognitively Impaired Elderly. Eur. Neuropsychopharmacol. 2016, 26, S649–S650. [Google Scholar] [CrossRef]
- Simrén, M.; Öhman, L.; Olsson, J.; Svensson, U.; Ohlson, K.; Posserud, I.; Strid, H. Clinical Trial: The Effects of a Fermented Milk Containing Three Probiotic Bacteria in Patients with Irritable Bowel Syndrome—A Randomized, Double-Blind, Controlled Study. Aliment. Pharm. 2010, 31, 218–227. [Google Scholar] [CrossRef]
- Takakura, W.; Pimentel, M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome—An Update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef]
- Marthinsen, D.; Fleming, S.E. Excretion of Breath and Flatus Gases by Humans Consuming High-Fiber Diets. J. Nutr. 1982, 112, 1133–1143. [Google Scholar] [CrossRef]
- Lebaron, T.W.; Singh, R.B.; Fatima, G.; Kartikey, K.; Sharma, J.P.; Ostojic, S.M.; Gvozdjakova, A.; Kura, B.; Noda, M.; Mojto, V.; et al. The Effects of 24-Week, High-Concentration Hydrogen-Rich Water on Body Composition, Blood Lipid Profiles and Inflammation Biomarkers in Men and Women with Metabolic Syndrome: A Randomized Controlled Trial. Diabetes Metab. Syndr. Obes. 2020, 13, 889–896. [Google Scholar] [CrossRef]
- Guinane, C.M.; Cotter, P.D. Role of the Gut Microbiota in Health and Chronic Gastrointestinal Disease: Understanding a Hidden Metabolic Organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.W.; Li, Y.; Luo, D.; Dong, J.L.; Zhou, L.X.; Zhao, S.Y.; Zheng, Q.S.; Wang, H.C.; Cui, M.; Fan, S.J. Hydrogen-Water Ameliorates Radiation-Induced Gastrointestinal Toxicity via Myd88′s Effects on the Gut Microbiota. Exp. Mol. Med. 2018, 50, e433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ji, X.; Zhang, Q.; Yao, W. Intestinal Microbiota Ecological Response to Oral Administrations of Hydrogen-Rich Water and Lactulose in Female Piglets Fed a Fusarium Toxin-Contaminated Diet. Toxins 2018, 10, 246. [Google Scholar] [CrossRef]
- Sha, J.-B.; Zhang, S.S.; Lu, Y.M.; Gong, W.J.; Jiang, X.P.; Wang, J.J.; Qiao, T.L.; Zhang, H.H.; Zhao, M.Q.; Wang, D.P.; et al. Effects of the Long-Term Consumption of Hydrogen-Rich Water on the Antioxidant Activity and the Gut Flora in Female Juvenile Soccer Players from Suzhou, China. Med. Gas Res. 2018, 8, 135–143. [Google Scholar] [CrossRef]
- Shen, N.Y.; Bi, J.B.; Zhang, J.Y.; Zhang, S.M.; Gu, J.X.; Qu, K.; Liu, C. Hydrogen-Rich Water Protects against Inflammatory Bowel Disease in Mice by Inhibiting Endoplasmic Reticulum Stress and Promoting Heme Oxygenase-1 Expression. World J. Gastroenterol. 2017, 23, 1375–1386. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Q.; Zheng, W.; Yao, W. Morphological and Molecular Response of Small Intestine to Lactulose and Hydrogen-Rich Water in Female Piglets Fed Fusarium Mycotoxins Contaminated Diet. J. Anim. Sci. Biotechnol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular Hydrogen Improves Obesity and Diabetes by Inducing Hepatic FGF21 and Stimulating Energy Metabolism in Db/Db Mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kiuchi, M.; Higashimura, Y.; Naito, Y.; Koyama, K. The Effects of Ingestion of Hydrogen-Dissolved Alkaline Electrolyzed Water on Stool Consistency and Gut Microbiota: A Double-Blind Randomized Trial. Med. Gas Res. 2021, 11, 138–144. [Google Scholar] [CrossRef]
- Jin, Z.; Sun, Y.; Yang, T.; Tan, L.; Lv, P.; Xu, Q.; Tao, G.; Qin, S.; Lu, X.; He, Q. Nanocapsule-Mediated Sustained H2 Release in the Gut Ameliorates Metabolic Dysfunction-Associated Fatty Liver Disease. Biomaterials 2021, 276, 121030. [Google Scholar] [CrossRef]
- Qiu, X.; Ye, Q.; Sun, M.; Wang, L.; Tan, Y.; Wu, G. Saturated Hydrogen Improves Lipid Metabolism Disorders and Dysbacteriosis Induced by a High-Fat Diet. Exp. Biol. Med. 2020, 245, 512–521. [Google Scholar] [CrossRef]
- Ehrenpreis, E.D.; Swamy, R.S.; Zaitman, D.; Noth, I. Short Duration Exercise Increases Breath Hydrogen Excretion after Lactulose Ingestion: Description of a New Phenomenon. Am. J. Gastroenterol. 2002, 97, 2798–2802. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, S.K.; Taylor, B.; Muir, J.; Costa, R.J.S. Impact of 24-h High and Low Fermentable Oligo-, Di-, Monosaccharide, and Polyol Diets on Markers of Exercise-Induced Gastrointestinal Syndrome in Response to Exertional Heat Stress. Appl. Physiol. Nutr. Metab. 2020, 45, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ye, F.; Rong, W.; Song, Y.; Wu, F.; Liu, Y.; Zheng, Y.; Siqin, T.; Zhang, K.; Wang, L.; et al. Nomogram to Assist in Surgical Plan for Hepatocellular Carcinoma: A Prediction Model for Microvascular Invasion. J. Gastrointest. Surg. 2019, 23, 2372–2382. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Hydrogen Sulfide Regulates Circadian-Clock Genes in C2C12 Myotubes and the Muscle of High-Fat-Diet-Fed Mice. Arch. Biochem. Biophys. 2019, 672, 108054. [Google Scholar] [CrossRef]
- Dong, B.M.; Abano, J.B.; Egan, T.M. Nitric Oxide Ventilation of Rat Lungs from Non-Heart-Beating Donors Improves Posttransplant Function. Am. J. Transplant. 2009, 9, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.S.; Yoshida, J.; Ueki, S.; Pettigrew, G.L.; Ghonem, N.; Sico, R.M.; Lee, L.Y.; Shapiro, R.; Lakkis, F.G.; Pacheco-Silva, A.; et al. Carbon Monoxide Inhibits Apoptosis during Cold Storage and Protects Kidney Grafts Donated after Cardiac Death. Transpl. Int. 2012, 25, 107–117. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Ohsawa, I.; Nishimaki, K.; Yamagata, K.; Ishikawa, M.; Ohta, S. Consumption of Hydrogen Water Prevents Atherosclerosis in Apolipoprotein E Knockout Mice. Biochem. Biophys. Res. Commun. 2008, 377, 1195–1198. [Google Scholar] [CrossRef]
- Wu, D.; Wang, J.; Li, H.; Xue, M.; Ji, A.; Li, Y. Role of Hydrogen Sulfide in Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef]
- Iida, A.; Nosaka, N.; Yumoto, T.; Knaup, E.; Naito, H.; Nishiyama, C.; Yamakawa, Y.; Tsukahara, K.; Terado, M.; Sato, K.; et al. The Clinical Application of Hydrogen as a Medical Treatment. Acta Med. Okayama 2016, 70, 331–337. [Google Scholar]
- Amilan Jose, D.; Sharma, N.; Sakla, R.; Kaushik, R.; Gadiyaram, S. Fluorescent Nanoprobes for the Sensing of Gasotransmitters Hydrogen Sulfide (H2S), Nitric Oxide (NO) and Carbon Monoxide (CO). Methods 2019, 168, 62–75. [Google Scholar] [CrossRef]
- Qian, L.; Cao, F.; Cui, J.; Wang, Y.; Huang, Y.; Chuai, Y.; Zaho, L.; Jiang, H.; Cai, J. The Potential Cardioprotective Effects of Hydrogenin Irradiated Mice. J. Radiat. Res. 2010, 51, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Sano, M.; Kamimura, N.; Yokota, T.; Suzuki, M.; Ohta, S.; Fukuda, K.; Hori, S. Hydrogen Inhalation during Normoxic Resuscitation Improves Neurological Outcome in a Rat Model of Cardiac Arrest Independently of Targeted Temperature Management. Circulation 2014, 130, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyoda, Y.; Sharma, P.; Evans, M.; Guthrie, N. Effectiveness of Hydrogen Rich Water on Antioxidant Status of Subjects with Potential Metabolic Syndrome—An Open Label Pilot Study. J. Clin. Biochem. Nutr. 2010, 46, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S. Molecular Hydrogen as a Preventive and Therapeutic Medical Gas: Initiation, Development and Potential of Hydrogen Medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, S.; Xu, J.; Wang, T. Hydrogen Therapy in Cardiovascular and Metabolic Diseases: From Bench to Bedside. Cell. Physiol. Biochem. 2018, 47, 1–10. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Sang, H.; Zhang, L.; Li, X.; Yao, S.; Yu, Y.; Zong, C.; Xue, Y.; Qin, S. Hydrogen -Rich Water Decreases Serum LDL-Cholesterol Levels and Improves HDL Function in Patients with Potential Metabolic Syndrome. J. Lipid Res. 2013, 54, 1884–1893. [Google Scholar] [CrossRef]
- Takahashi, H. Application of Hydrogen in Ophthalmology. Curr. Pharm. Des. 2020, 27, 592–594. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sano, M.; Hayashida, K.; Ohsawa, I.; Ohta, S.; Fukuda, K. Are the Effects of α-Glucosidase Inhibitors on Cardiovascular Events Related to Elevated Levels of Hydrogen Gas in the Gastrointestinal Tract? FEBS Lett. 2009, 583, 2157–2159. [Google Scholar] [CrossRef]
- Sakai, T.; Sato, B.; Hara, K.; Hara, Y.; Naritomi, Y.; Koyanagi, S.; Hara, H.; Nagao, T.; Ishibashi, T. Consumption of Water Containing over 3.5 Mg of Dissolved Hydrogen Could Improve Vascular Endothelial Function. Vasc. Health Risk Manag. 2014, 10, 591–597. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Stojanovic, M.D. Hydrogen-Rich Water Affected Blood Alkalinity in Physically Active Men. Res. Sports Med. 2014, 22, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of Hydrogen-Rich Water Improves Lipid and Glucose Metabolism in Patients with Type 2 Diabetes or Impaired Glucose Tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Takanashi, M.; Hirayama, M.; Nakahara, T.; Ohta, S.; Hattori, N. Pilot Study of H2 Therapy in Parkinson’s Disease: A Randomized Double-Blind Placebo-Controlled Trial. Mov. Disord. 2013, 28, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Liu, W.; Zeng, D.; Zhu, L.; Sun, X.; Sun, X. Effect of Hydrogen-Rich Water on Oxidative Stress, Liver Function, and Viral Load in Patients with Chronic Hepatitis B. Clin. Transl. Sci. 2013, 6, 372–375. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Rikitake, M.; Seo, T.; Kurokawa, R.; Hara, Y.; Naritomi, Y.; Hara, H.; Nagao, T. Consumption of Water Containing a High Concentration of Molecular Hydrogen Reduces Oxidative Stress and Disease Activity in Patients with Rheumatoid Arthritis: An Open-Label Pilot Study. Med. Gas Res. 2012, 2, 27. [Google Scholar] [CrossRef]
- Aoki, K.; Nakao, A.; Adachi, T.; Matsui, Y.; Miyakawa, S. Pilot Study: Effects of Drinking Hydrogen-Rich Water on Muscle Fatigue Caused by Acute Exercise in Elite Athletes. Med. Gas Res. 2012, 2, 12. [Google Scholar] [CrossRef]
- Nagatani, K.; Nawashiro, H.; Takeuchi, S.; Tomura, S.; Otani, N.; Osada, H.; Wada, K.; Katoh, H.; Tsuzuki, N.; Mori, K. Safety of Intravenous Administration of Hydrogen-Enriched Fluid in Patients with Acute Cerebral Ischemia: Initial Clinical Studies. Med. Gas Res. 2013, 3, 13. [Google Scholar] [CrossRef]
- Li, Q.; Kato, S.; Matsuoka, D.; Tanaka, H.; Miwa, N. Hydrogen Water Intake via Tube-Feeding for Patients with Pressure Ulcer and Its Reconstructive Effects on Normal Human Skin Cells in Vitro. Med. Gas Res. 2013, 3, 20. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Vukomanovic, B.; Calleja-Gonzalez, J.; Hoffman, J.R. Effectiveness of Oral and Topical Hydrogen for Sports-Related Soft Tissue Injuries. Postgrad. Med. 2014, 126, 188–196. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef]
- Ito, M.; Hirayama, M.; Yamai, K.; Goto, S.; Ito, M.; Ichihara, M.; Ohno, K. Drinking Hydrogen Water and Intermittent Hydrogen Gas Exposure, but Not Lactulose or Continuous Hydrogen Gas Exposure, Prevent 6-Hydorxydopamine-Induced Parkinson’s Disease in Rats. Med. Gas Res. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Dohi, K.; Satoh, K.; Miyamoto, K.; Momma, S.; Fukuda, K.; Higuchi, R.; Ohtaki, H.; Banks, W.A. Molecular Hydrogen in the Treatment of Acute and Chronic Neurological Conditions: Mechanisms of Protection and Routes of Administration. J. Clin. Biochem. Nutr. 2017, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yoritaka, A.; Abe, T.; Ohtsuka, C.; Maeda, T.; Hirayama, M.; Watanabe, H.; Saiki, H.; Oyama, G.; Fukae, J.; Shimo, Y.; et al. A Randomized Double-Blind Multi-Center Trial of Hydrogen Water for Parkinson’s Disease: Protocol and Baseline Characteristics. BMC Neurol. 2016, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased Intestinal Permeability Correlates with Sigmoid Mucosa Alpha-Synuclein Staining and Endotoxin Exposure Markers in Early Parkinson’s Disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ito, M.; Hamaguchi, T.; Mori, H.; Takeda, Y.; Baba, R.; Watanabe, T.; Kurokawa, K.; Asakawa, S.; Hirayama, M.; et al. Quantification of Hydrogen Production by Intestinal Bacteria That Are Specifically Dysregulated in Parkinson’s Disease. PLoS ONE 2018, 13, e0208313. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Sonnenburg, E.D. Vulnerability of the Industrialized Microbiota. Science 2019, 366, eaaw9255. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The Mutual Interplay of Gut Microbiota, Diet and Human Disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef]
- De-Paula, V.d.J.R.; Forlenza, A.S.; Forlenza, O.V. Relevance of Gutmicrobiota in Cognition, Behaviour and Alzheimer’s Disease. Pharm. Res 2018, 136, 29–34. [Google Scholar] [CrossRef]
- Kabir, M.T.; Rahman, M.H.; Shah, M.; Jamiruddin, M.R.; Basak, D.; Al-Harrasi, A.; Bhatia, S.; Ashraf, G.M.; Najda, A.; El-kott, A.F.; et al. Therapeutic Promise of Carotenoids as Antioxidants and Anti-Inflammatory Agents in Neurodegenerative Disorders. Biomed. Pharmacother. 2022, 146, 112610. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer’s Disease Therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef]
- Li, S.; Fujino, M.; Ichimaru, N.; Kurokawa, R.; Hirano, S.; Mou, L.; Takahara, S.; Takahara, T.; Li, X.K. Molecular Hydrogen Protects against Ischemia-Reperfusion Injury in a Mouse Fatty Liver Model via Regulating HO-1 and Sirt1 Expression. Sci. Rep. 2018, 8, 14019. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Hydrogen-Rich Water as a Modulator of Gut Microbiota? J. Funct. Foods 2021, 78, 104360. [Google Scholar] [CrossRef]
- Chen, S.Y.; Gao, Y.; Sun, J.Y.; Meng, X.L.; Yang, D.; Fan, L.H.; Xiang, L.; Wang, P. Traditional Chinese Medicine: Role in Reducing β-Amyloid, Apoptosis, Autophagy, Neuroinflammation, Oxidative Stress, and Mitochondrial Dysfunction of Alzheimer’s Disease. Front. Pharm. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Kuriakose, M.; Younger, D.; Ravula, A.R.; Alay, E.; Rama Rao, K.V.; Chandra, N. Synergistic Role of Oxidative Stress and Blood-Brain Barrier Permeability as Injury Mechanisms in the Acute Pathophysiology of Blast-Induced Neurotrauma. Sci. Rep. 2019, 9, 7717. [Google Scholar] [CrossRef]
- Zhang, P.; Kishimoto, Y.; Grammatikakis, I.; Gottimukkala, K.; Cutler, R.G.; Zhang, S.; Abdelmohsen, K.; Bohr, V.A.; Misra Sen, J.; Gorospe, M.; et al. Senolytic Therapy Alleviates Aβ-Associated Oligodendrocyte Progenitor Cell Senescence and Cognitive Deficits in an Alzheimer’s Disease Model. Nat. Neurosci. 2019, 22, 719–728. [Google Scholar] [CrossRef]
- Erickson, M.A.; Dohi, K.; Banks, W.A. Neuroinflammation: A Common Pathway in CNS Diseases as Mediated at the Blood-Brain Barrier. Neuroimmunomodulation 2012, 19, 121–130. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Liu, Q.; Yang, R.; Zhang, J.H.; Cao, Y.P.; Sun, X.J. Hydrogen-Rich Saline Reduces Oxidative Stress and Inflammation by Inhibit of JNK and NF-ΚB Activation in a Rat Model of Amyloid-Beta-Induced Alzheimer’s Disease. Neurosci. Lett. 2011, 491, 127–132. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Zhang, J.H.; Cai, J.M.; Cao, Y.P.; Sun, X.J. Hydrogen-Rich Saline Improves Memory Function in a Rat Model of Amyloid-Beta-Induced Alzheimer’s Disease by Reduction of Oxidative Stress. Brain Res. 2010, 1328, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Huang, C.S.; Inoue, T.; Yamashita, T.; Ishida, T.; Kang, K.M.; Nakao, A. Drinking Hydrogen Water Ameliorated Cognitive Impairment in Senescence-Accelerated Mice. J. Clin. Biochem. Nutr. 2010, 46, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Nishimaki, K.; Asada, T.; Ohsawa, I.; Nakajima, E.; Ikejima, C.; Yokota, T.; Kamimura, N.; Ohta, S. Effects of Molecular Hydrogen Assessed by an Animal Model and a Randomized Clinical Study on Mild Cognitive Impairment. Curr. Alzheimer Res. 2018, 15, 482–492. [Google Scholar] [CrossRef]
- Ohta, S. Recent Progress toward Hydrogen Medicine: Potential of Molecular Hydrogen for Preventive and Therapeutic Applications. Curr. Pharm. Des. 2011, 17, 2241–2252. [Google Scholar] [CrossRef]
- Ohta, S. Molecular Hydrogen Is a Novel Antioxidant to Efficiently Reduce Oxidative Stress with Potential for the Improvement of Mitochondrial Diseases. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Sano, M.; Ohsawa, I.; Shinmura, K.; Tamaki, K.; Kimura, K.; Endo, J.; Katayama, T.; Kawamura, A.; Kohsaka, S.; et al. Inhalation of Hydrogen Gas Reduces Infarct Size in the Rat Model of Myocardial Ischemia-Reperfusion Injury. Biochem. Biophys. Res. Commun. 2008, 373, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.-J.; Li, H. Targeting Interferon Regulatory Factor for Cardiometabolic Diseases: Opportunities and Challenges. Curr. Drug Targets 2017, 18, 1754–1778. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; He, B.; Xiao, J.; Wang, Z.; Sun, X. Anti-Inflammatory Effect of Hydrogen-Rich Saline in a Rat Model of Regional Myocardial Ischemia and Reperfusion. Int. J. Cardiol. 2011, 148, 91–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Long, Z.; Wang, C.; Wang, L.; Sun, P.; Li, P.; Wang, T. Hydrogen (H2) Inhibits Isoproterenol-Induced Cardiac Hypertrophy via Antioxidative Pathways. Front. Pharm. 2016, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Long, Z.; Xu, J.; Tan, S.; Zhang, N.; Li, A.; Wang, L.; Wang, T. Hydrogen Inhibits Isoproterenol-Induced Autophagy in Cardiomyocytes in Vitro and in Vivo. Mol. Med. Rep. 2017, 16, 8253–8258. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.S.; Zheng, H. Chronic Hydrogen-Rich Saline Treatment Reduces Oxidative Stress and Attenuates Left Ventricular Hypertrophy in Spontaneous Hypertensive Rats. Mol. Cell. Biochem. 2012, 365, 233–242. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).